10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(2):536-551. doi:10.7150/ijbs.64537 This issue Cite
Review
The Landscape Of Alpha Fetoprotein In Hepatocellular Carcinoma: Where Are We?
1. Department of Hepatobiliary and Pancreatic Surgery, The Center for Integrated Oncology and Precision Medicine, Affiliated Hangzhou First People's Hospital, Zhejiang University School of Medicine, Hangzhou 310006, China.
2. Zhejiang University Cancer Center, Hangzhou, 310058, China.
3. Department of Hepatobiliary and Pancreatic Surgery, The First Affiliated Hospital, Zhejiang University School of Medicine, 79 Qingchun Road, Hangzhou, 310003, China.
4. Institute of Organ Transplantation, Zhejiang University, Hangzhou, 310003, China.
* These authors contributed equally to this work.
Received 2021-7-4; Accepted 2021-10-15; Published 2022-1-1
Abstract
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and has been acknowledged as a leading cause of death among cirrhosis patients. Difficulties in early diagnosis and heterogeneity are obstacles to effective treatment, especially for advanced HCC. Liver transplantation (LT) is considered the best therapy for HCC. Although many biomarkers are being proposed, alpha-fetoprotein (AFP), which was identified over 60 years ago, remains the most utilized. Recently, much hope has been placed in the immunogenicity of AFP to develop novel therapies, such as AFP vaccines and AFP-specific adoptive T-cell transfer (ACT). This review summarizes the performance of AFP as a biomarker for HCC diagnosis and prognosis, as well as its correlation with molecular classes. In addition, the role of AFP in LT is also described. Finally, we highlight the mechanism and application prospects of two immune therapies (AFP vaccine and ACT) for HCC. In general, our review points out the prevalence of AFP in HCC, accompanied by some controversies and novel directions for future research.
Keywords: Alpha-fetoprotein, Hepatocellular carcinoma, Liver transplantation, Chimeric antigen receptor (CAR) T-cell therapy, T-cell receptor-engineered T-cell therapy
Introduction
Hepatocellular carcinoma (HCC), which is closely related to chronic liver disease, accounts for most primary liver cancers (representing 70 to 85%)1. It has the sixth-highest incidence among all cancers and is the third leading cause of cancer-related death globally2-4. The 5-year survival rate of HCC is only approximately 15%5, 6. Its high mortality is considered to be a result of late detection, therapy resistance, a high recurrence rate after treatment and significant molecular heterogeneity7.
Progress has been made in drug chemotherapy, radiotherapy and interventional therapy due to further understanding of the etiology pathogenesis of HCC. However, treatments for patients with advanced HCC are still limited, and liver transplantation (LT) remains the best curative method for HCC8. For example, sorafenib, a multikinase inhibitor, demonstrated an increased survival rate accompanied by an increased incidence of adverse events9. The heterogeneity of HCC is an obstacle to the precise diagnosis and treatment. Given the individual differences, the achievement of early diagnosis and therapy requires specific biomarkers, an understanding of molecular subtyping, precise criteria for candidate selection for various therapies and the development of immunotherapy.
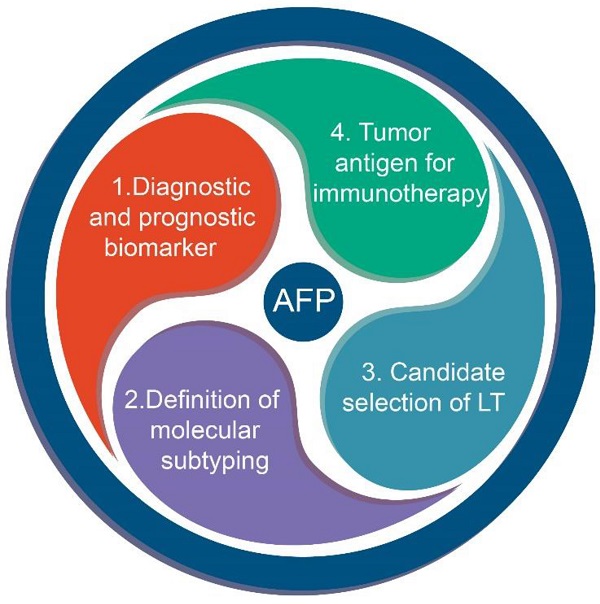
Current studies are aiming at selecting biomarkers to improve early diagnosis and prolong the survival of patients with HCC. The most common serologic marker of HCC is alpha-fetoprotein (AFP)10, 11. Identified in human fetal sera by Bergstrand and Czar in 1956, AFP acts as a transporter for several ligands, such as bilirubin, fatty acids and possibly some drugs12. Normally, its levels drop sharply after birth and remain at a low level thereafter. It has been used for screening, diagnosis, prognostication and therapeutic evaluation of HCC since it was identified as an oncofetal biomarker. In addition, it is also applied as an indicator in some new criteria for the selection of LT recipients, such as the Hangzhou criteria13. Over the past decade, some progress has been made in the use of AFP based on clinical and basic studies. In addition to being a biomarker for HCC and LT, it might be employed for immune therapy14 as well as for defining the HCC molecular classes15 (Figure 1).
The role of AFP in HCC.
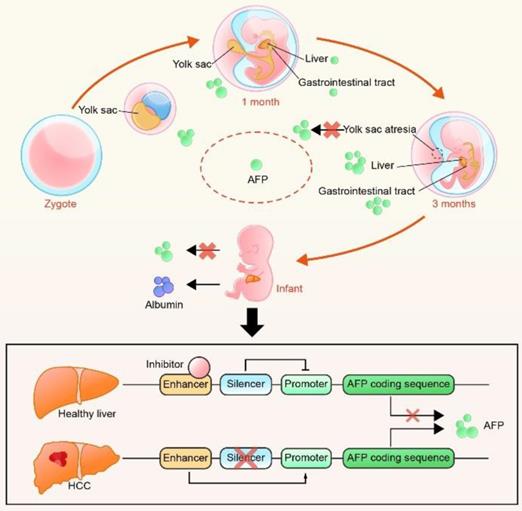
AFP is overexpressed in HCC
AFP is produced by the yolk sac during the first trimester of pregnancy. Then, as the sac becomes atretic, the production of AFP decreases rapidly. After the fourth week of pregnancy, the fetal liver and gastrointestinal tract begin to secrete AFP, which is sustained throughout the embryonic development period16. In healthy individuals, AFP is maintained at a low level throughout the lifespan but it is aberrantly expressed in HCCs. The AFP gene, which belongs to the albumin gene family, is located on the long arm of chromosome 4 of humans (4q11-q13), and it has two independent enhancer and silencer regions17. Several studies have indicated that a block of enhancer inhibition and deletion of the silencer leads to the restoration of promoter activity, resulting in the overexpression of AFP8, 18 (Figure 2).
AFP is used as a biomarker in HCC
Although many scientists are now seeking new biomarkers due to the controversy regarding the utility of AFP, it remains the most universally used biomarker for HCC. It has been confirmed that persistently increased AFP level, which has been proven to be associated with an aggressive histological morphology (vascular invasion, poorly differentiated and satellitosis), are a hazardous factor for HCC2, 8, 19, 20. Current studies have discussed the critical role of AFP as a biomarker in HCC for surveillance, diagnosis and prognostication4, 7, 8. However, the fact that AFP could also be elevated in other benign liver diseases sparked controversy about the use of AFP for HCC surveillance21-23. Given these reasons, recent studies have tried to combine AFP with other factors. In a meta-analysis, Tzartzeva et al. compared the efficiency of surveillance imaging with or without AFP for the early detection of HCC in patients with cirrhosis and found that the former improved the sensitivity from 45% to 63%24. Based on the other meta-analysis, a score based on AFP, AFP-L3 and DCP was also confirmed to have a superior ability for early diagnosis25, 26. Other studies have proposed a combination of AFP with platelets and age27, CEA and CA-19928, microRNAs29, 30 and protein induced by vitamin K absence/antagonist-II (PIVKA-II)31. The satisfactory results of these studies have led to recommendations that AFP should be integrated with other factors by some guidelines for HCC screening10, 32, 33. For predicting patient outcomes, baseline AFP levels and dynamic AFP monitoring could reflect the prognosis and the response to different treatments. Three phase III studies identified high AFP as a prognostic factor of a worse overall survival (OS)34, 35. However, Giannini et al. pointed out that AFP had no prognostic significance in those with well-compensated cirrhosis and a single, small HCC (≤ 3 cm) treated with curative intent36. Moreover, the use of AFP was found to be valid in the therapeutic evaluation of drug treatment (lenvatinib35, regorafenib37, cabozantinib38 and ramucirumab39). For example, lenvatinib showed a superior curative effect than sorafenib when the cutoff value of AFP was 200 ng/mL (HR: 0.78, 95% CI: 0.63-0.98)35. There is no doubt that AFP has utility in HCC screening and prognostication, but additional studies need to be conducted to explore its appropriate usage and scope of application.
It was reported that 30% of HCC patients remained AFP-negative (<20 ng/mL)40, and many institutions no longer recommend the use of AFP during HCC surveillance10, 41, 42. Compared with AFP-positive patients, AFP-negative patients might have smaller tumor sizes, lower recurrence rates, superior liver function and a better Edmondson-Steiner grade with complete neoplasm capsules43, 44. In addition, AFP negativity was found to be a favorable predictor of LT eligibility, which means that these patients would benefit more from LT45. Currently, for patients with a significant increase in AFP, a liver biopsy can be performed directly to confirm the diagnosis. Therefore, most studies have focused on distinguishing AFP-negative HCC from benign liver diseases (liver cirrhosis (LC), chronic hepatitis and so on) and normal groups with no significant increase in AFP. Several potential biomarkers and laboratory tests have been identified for the diagnosis and prognostication of AFP-negative HCC (Table 1).
Stable, detectable serological biomarkers for AFP-negative HCC have been widely explored, and most of them are proteins46-50 and genes51-53. Liu et al. concluded that des-gamma-carboxyprothrombin (DCP) can distinguish AFP-negative HBV-related HCC from chronic HBV infection (AUC = 0.731) or LC (AUC = 0.685)48. Several studies have attempted to combine multiple biomarkers54-61 or serological examinations62-66. With the help of proteomics technology, researchers have identified some abnormally expressed proteins that were verified in various cancers67-70 to construct a logistic regression model, which had good performance in distinguishing AFP-negative HCC 60. A logistic regression model consisting of LHPP71-associated microRNAs (miR‐363‐5p and miR‐765) and PIVKA-II exhibited a high identification value with an AUC of 0.93057. The ratio of fucosylated serum paraoxonase 1 to the total serum paraoxonase 1 (Fuc-PON1)58 as well as the combination of fibrinogen to prealbumin ratio (FPR) and gamma-glutamyl transpeptidase to platelet ratio (GPR)59 were proven to have diagnostic potential (AUC = 0.78, sensitivity = 62.2%, specificity = 67.7% and AUC = 0.98, sensitivity = 91.1%, specificity = 96.5%, respectively). Moreover, Wang et al. constructed a nomogram including body mass index (BMI), oncology indicators and liver function indicators, while Huang et al. applied cirrhosis, alkaline phosphatase (ALP), tumor size, microvascular invasion, satellite lesions and tumor differentiation to build a nomogram64, 65. These models had a more accurate predictive and superior discriminative power relative to the conventional method, with C-indexes for OS prediction of 0.807 (95% CI: 0.770-0.844) and 0.742 (95% CI: 0.684‐0.800), respectively.
The origin of AFP in different periods and the hypothesis of AFP overexpression in HCC. AFP is produced by the yolk sac from zygote to three months of pregnancy and by fetal liver and gastrointestinal tract from the fourth week of pregnancy. After birth, AFP is gradually replaced by albumin. The re-secretion of AFP in HCC is thought to be a coaction of enhancers and silencers.
Biomarkers and methods of ANAC for early diagnosis.
| Content | Year | Type | AUC | Sensitivity/Specificity | Population | Ref. |
|---|---|---|---|---|---|---|
| Cmi | 2015 | microRNAs | 0.83 | - | Asian | 52 |
| AFP-L3 | 2015 | protein | 0.61 | 50.0%/97.5% | Asian | 56 |
| GP73 | 2015 | protein | 0.78 | 66.0%/96.2% | Asian | 56 |
| Midkine | 2016 | protein | 0.70 | 70.9%/62.2% | Asian/Africa | 47 |
| FAHB-M | 2016 | regression model | 0.88 | 80.3%/82.9% | Asian | 63 |
| Fuc-PON1 | 2017 | protein | 0.78 | 62.2%/67.7% | Asian | 58 |
| TEMs | 2017 | monocytes | 0.69 | 80.0%/65.5% | Asian | 49 |
| NPM1 + 14-3-3zeta + MDM2 | 2017 | autoantibody | - | 30.4%/91.6% | Asian | 55 |
| metabolomic profiles | 2019 | SCMs | >0.80 | - | Asian | 61 |
| hematological parameters | 2019 | regression model | 0.92 | 83.0%/93.1% | Asian | 66 |
| PA + D-Dimer + Fibrinogen | 2020 | protein | 0.94 | 93.4%/80.8% | Asian | 54 |
| miR-363-5p + miR-765 + PIVKA-II | 2020 | regression model | 0.93 | 79.4%/95.4% | Asian | 57 |
| FPR + GPR | 2020 | protein, platelet | 0.98 | 91.1%/96.5% | Asian | 59 |
| PT/Fbg system | 2020 | clinical examination | 0.68 | - | Asian | 62 |
| DCP | 2020 | protein | 0.73 | 50.6%/91.7% | Asian | 48 |
| P53 +MSH2 + Tm-4 + inflammatory factors + life-history traits | 2020 | regression model | 0.91 | 85.2%/88.3% | Asian | 60 |
ANHC: AFP-negative hepatic carcinoma; Cmi: miRNA classifier; GP73: golgi protein 73; FAHB-M: fluorescence intensity, alpha-fetoprotein, hepatic function test results and blood cell analyses with the model; Fuc-PON1: the ratio of fucosylated serum paraoxonase 1 to the total serum serum paraoxonase 1; TEMs: Tie2-expressing monocytes; SCMs: significantly changed metabolites; PA: pre-albumin; PIVKA-II: vitamin K deficiency or antagonist-II; FPR: fibrinogen to prealbumin ratio; GPR: gamma-glutamyl transpeptidase to platelet ratio; PT: plasma prothrombin time; Fbg: fibrinogen; DCP: des-gamma-carboxyprothrombin; MSH2: MutS homologs 2; Tm-4: tropomyosin-4.
Recently, many studies have pointed out the lack of an accurate diagnosis when using AFP, but its isoforms were found to be a specific alternative. There are three various AFP isoforms (AFP-L1, AFP-L2, and AFP-L3) based on the binding capacity of lens culinaris agglutinin (LCA). Among them, AFP-L3, also known as lens culinaris-reactive AFP, is the main isoform in HCC patients, especially in small HCCs (< 3 cm)72. AFP-L3 was identified to be related to poorly differentiated and advanced HCC73. It can be detected in early-stage HCC, especially when it is supplied by the hepatic artery, and AFP-L3-positive HCC is more likely to have an early metastasis and rapid growth74. Currently, many studies have applied AFP L3 as an adjuvant marker to improve the accuracy and completeness of early diagnosis of HCC26, 75, 76.
To date, most of these results were acquired from retrospective, single-center studies with small samples, and there is a lack of prospective, large-sample and multicenter studies to confirm their value.
AFP is associated with HCC molecular classes
As a heterogeneous disease, patients diagnosed with HCC have diverse clinical features and disease progression levels15, 77. With the continuous development of bioinformatics, especially the progress in gene sequencing technology, the classification of HCC is no longer limited to the histopathological level. Several new molecular classifications defined by AFP combined with other indicators have been successively discovered and validated. These distinct classifications are associated with different morphological phenotypes and clinical characteristics, which are linked to specific genetic mutations and signaling pathways78, 79.
AFP is used to define novel classes
The expression of epithelial cell adhesion molecule (EpCAM) is positive in the majority of hepatocytes in the embryonic liver. However, in adults, it is negative in hepatocytes and positive in the bile duct epithelium80. EpCAM+ HCC exhibits hepatic cancer stem cell-like, highly invasive and tumorigenic features81, 82. Yamashita et al. classified HCC into four subtypes by EpCAM and AFP (EpCAM- AFP-, EpCAM- AFP+, EpCAM+ AFP- and EpCAM+ AFP+ HCC) with the name of mature hepatocyte-like HCC, hepatocytic progenitor-like HCC, bile duct epithelium-like HCC and hepatic stem cell-like HCC83, 84. Apparent differences existed in the transcriptome of these subtypes, and AFP+ HCC (EpCAM- AFP+ and EpCAM+ AFP+ HCC) was more likely to have a poor prognosis, advanced TNM stages and vascular invasion84. The S2 subclass identified by Hoshida et al. showed that increased AFP levels were also distinctly enriched in a signature of EpCAM positivity85. Recently, some scientists have further explored the molecular mechanisms and potential therapeutic targets of EpCAM+ AFP+ HCC. Wei et al. discovered that MAGE-A9 (a specific cancer testis antigen), whose anomalous expression was correlated with enhanced tumor proliferation and metastases, was increased in EpCAM+ AFP+ HCC characterized by hepatic stem/progenitor cells, indicating that MAGE-A9 might perform a role in regulating stem cell-like feature and act as an underlying therapeutic target79. Furthermore, Takai et al. conducted a genome-wide RNAi screen to explore genes with a synthetic lethal interaction with EpCAM and filtered out PMPCB, which encodes proteins to maintain the function of mitochondria as a potential target82. Moreover, based on the expression of AFP and CD133 (a typical stem cell marker), Dai et al. classified HCC into four groups (CD133+AFP+, CD133-AFP-, CD133+AFP- and CD133-AFP+ HCC) with significantly distinct clinicopathological features and prognosis78.
AFP is abnormally expressed in several classes
Apart from defining the novel classes, AFP was also proven to be increased or decreased in several molecular classifications (Table 2).
HCC mutated with CTNNB1
CTNNB1 involved in the Wnt/β-catenin signaling pathway is a prevalent mutation gene in HCC86-89. Calderaro et al. indicated that CTNNB1 mutations defined a specific cholestatic, low inflammatory infiltrate levels and a well-differentiated subtype of HCC with a lower expression of AFP compared with the nonmutation group15. Another study found that an HCC subtype overexpressing AFP (median serum level, 472 ng/mL) exhibited tyrosine kinase activation (IGF1R, RPS6 and Akt phosphorylation), decreased frequencies of CTNNB1 exon 3 mutation and 6q loss, increased frequencies of 4q and 13q loss and significant macrovascular invasion90.
HCC mutated with TP53 and a novel subtype (MTM-HCC)
As a hallmark in DNA repair, genomic stability and apoptosis regulation, TP53 mutation was found to be correlated with AFP positivity, as there were 50.00% (12/24) of AFP-positive HCC in the TP53 mutation group and 20.69% (6/29) in the wild type (p < 0.05)91. Another study identified a prognostic protein biomarker, ADH1A (oxidoreductase activity), associated with metabolic reprogramming, and HCC with high ADH1A showed reduced TP53 mutations and lower AFP levels92. Similarly, Yang et al. pointed out that the low-AFP subclass C1 had numerous enriched metabolism-associated biological processes (especially the urea cycle), a significantly lower mutation frequency of TP53, and notable cabozantinib resistance93. In addition, TP53 mutation was proven to be associated with a novel histological subtype called "macrotrabecular-massive HCC (MTM-HCC)", which was designated by Calderaro et al. and characterized by a predominant macrotrabecular architecture involving more than 50% of the tumor, high AFP serum levels (AFP > 100 ng/ml, P < 0.02) and poor recurrence-free survival15, 94. Logistic and multivariable cox regression analyses were performed and found that a high serum AFP levels was an independent feature and predictor (OR: 4.4, 95% [CI]: 1.3, 16; P = 0.02) of the MTM-HCC subtype95, 96.
Other classes
Six robust subgroups of HCC (G1-G6) were identified by Boyault et al. after investigating 57 HCCs by global transcriptome analysis, and HCCs involved in G1-G3, which are known to be characterized by chromosomal instability and high cell proliferation, were correlated with elevated AFP levels (AFP > 100 ng/mL; P < 0.001)15, 97. Glypican-3 (GPC3), a protein that can stimulate the proliferation and migration of tumor cells through the activation of Wnt signaling in HCC98, was applied by Xue et al. to divide 316 patients into GPC3+ and GPC3- phenotypes99. The results revealed that there was a significant difference in serum AFP levels between the two groups99.
Elevated levels of AFP indicate aggressive tumor pathologic characteristics and a poor prognosis85, 100, 101. Currently, some genes recognized as signatures in novel molecular classifications have been identified. Combined with these genes or their coding proteins, the alteration of AFP levels might show better performance in defining new subtypes.
The change of AFP levels in several classes.
| Subtype | AFP level | Relevant Characteristic | Signal pathway | Population | Ref. |
|---|---|---|---|---|---|
| CTNNB1 mutation | low | large size, well-differentiated, intact tumor capsule, microtrabecular and pseudoglandular chistological patterns, tumor cholestasis, a lack of inflammatory infiltrates | IL6/JAK/STAT, Wnt/β-catenin | European/ North American | 15, 90, 97 |
| TP53 mutation (MTM-HCC) | high | poor differentiation, macrovascular and microvascular invasion, compact histological pattern, foci of sarcomatous changes, pleomorphic and multinucleated cells, a lack of tumor cholestasis | PI3K/AKT | European/Asian | 15, 91-97 |
| G1/G2/G3 subclasses | high | high cell proliferation, chromosomal instability, female gender, hemochromatosis, HBV infection | Cell cycle, proliferation, DNA metabolism | European | 97 |
| S2 | high | large size, poor-differentiated, high proliferation | MYC and AKT | Asian | 85, 100 |
| GPC3+ | high | thick trabecular pattern and compact variants, vascular invasion, distant metastasis, short survival time | - | Asian | 99 |
MTM-HCC: macrotrabecular-massive subtype of HCC; OS: overall survival; GPC3: Glypican-3.
Applying AFP for candidate selection and predicting the recurrence of LT
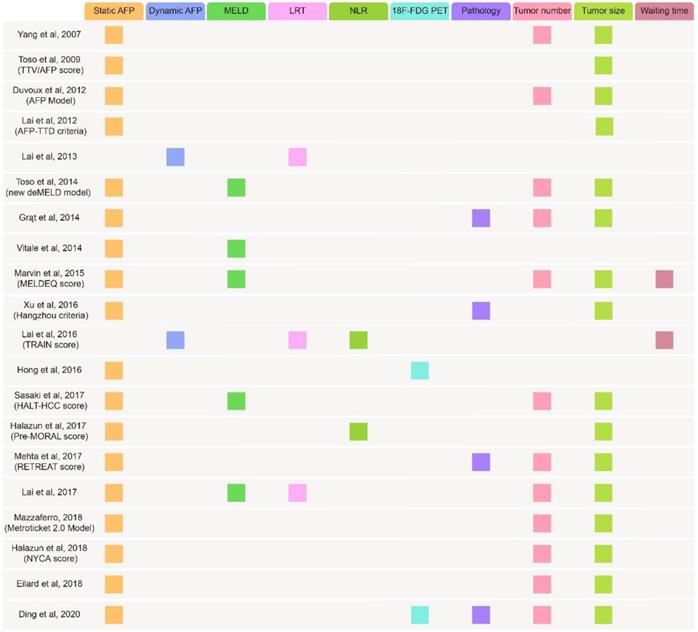
Currently, LT remains the best treatment for HCC because it eliminates carcinogenic background. Whether patients obtain effective disease mitigation after LT relies on the use of accurate criteria for candidate selection. At present, the Milan criteria (MC) (single tumor nodule, tumor diameter < 5 cm or no more than three tumor nodules, none exceeding 3 cm in diameter) is the most widely used in 95% of countries to select suitable candidates102, 103. However, several studies confirmed that patients beyond the MC had comparable post-LT survival rates, suggesting that MC might preclude access to LT for those who might benefit13, 104, 105. In addition, researchers have found a powerful predictive ability of some biomarkers for LT outcomes, especially AFP. Distinct evidence has shown that the post-LT survival rate declines with increasing AFP levels103, 106, 107. Hence, scientists have employed AFP in candidate selection and prognostication to relax the criteria and expand the donor pool (Table 3).
AFP with tumor morphology
AFP is most commonly used in combination with tumor morphology. A Korean group created a revised scoring system based on tumor size, tumor number and pretransplant AFP levels (< or =20, 20.1 to 200, 200.1 to 1000, >1000 ng/mL), allowing an expansion for candidate selection without adverse outcomes108. Similarly, another two criteria, named the "Model of Recurrence After Liver Transplantation" (MORAL) and New York/California (NYCA) scores developed by Halazun et al., provided highly accurate tools for candidate selection and forecasting recurrence109, 110.
Several studies have focused on total tumor diameter (TTD) or total tumor volume (TTV) rather than single tumor features. Zheng et al. designed the Hangzhou criteria, which included AFP, TTD and histopathologic grade, for candidate selection, indicating the possibility of LT for those who were beyond MC but fulfilled the Hangzhou criteria and pointing out that AFP >100 ng/mL was an independent prognostic factor among them13. In some cases, the Hangzhou criteria was also considered as a downstaging criteria for HCC patients before LT to lower the threshold for LT111. Then, a team from Italy proposed a score containing AFP and TTD (the AFP-TTD score) but no histopathologic features, which simplified the Hangzhou criteria112. With no need for a tumor biopsy, it could avoid bleeding, tumor seeding and unnecessary surgery. Besides, by utilizing the same three characteristics, Duvoux et al. proposed an AFP model whose cutoff values were 100 ng/ml and 1000 ng/ml113. Its superiority of strong predictability has been validated in different populations114-116. Notably, this model was innovatively used to predict the recurrence rate in patients with viral hepatitis-related cirrhosis who had received LT for HCC107. Other scores combining AFP with TTD or TTV were also proposed117-119. Interestingly, Mazzaferro et al. applied the sum of the number and size of tumors (in centimeters) to replace TTV/TTD to build a Metroticket 2.0 Model, expanding the idea of tumor morphology120.
AFP with the model for end-stage liver disease (MELD)
The end-stage liver disease (MELD) model is used for evaluating liver function reserve and prognosis in patients with chronic liver disease. Integrating MELD into the evaluation system allowed for a complete assessment of patients' preoperative status since many patients had a background of cirrhosis. In this situation, some models including MELD were designed120-125. Among them, Vitale et al. established a model using transplant benefit as the common endpoint to re-establish allocation equity in patients with and without HCC123. They created a "MELD equivalent" that matches HCC patients to non-HCC patients by the same numerical MELD score and developed the equation: HCC-MELD (1.27∗MELD - 0.51∗logAFP+4.59), whereby the same transplant benefit between the two groups was achieved.
AFP with modified Response Evaluation Criteria in Solid Tumors (mRECIST)
Attention to locoregional therapy (LRT) has increased because effective preoperative LRT predicts a low recurrence rate. Complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) (MRECIST) is widely applied to measure the response to LRT126, 127. The Time-Radiological-response-Alpha-fetoprotein-Inflammation (TRAIN) score regards LRT as one of the risk variables in its formula128. Another study pointed out that the Metroticket 2.0 criteria120 affiliated with mRECIST enhanced its prediction ability129.Moreover, Lai et al. made use of pre-LT LRT to stratify the survival rate of LT and to improve the equity of liver allocation124, 130. In general, patients with a good response to LTR are likely to gain better post-LT prognostics, suggesting that LRT is a valuable factor for LT decisions.
The role of AFP in LT.
| Study | No. | AFP cut value | Type | Population | Ref. |
|---|---|---|---|---|---|
| Yang et al, 2007 | 63 | ≤ 20, 20.1 to 200, 200.1 to 1000, > 1000 ng/mL | candidate selection | Asian | 108 |
| Toso et al, 2009 (TTV/AFP score) | 6478 | 400 ng/mL | candidate selection | North American | 117 |
| Duvoux et al, 2012 (AFP Model) | 537 | log10AFP (Simplified: AFP ≤ 100, 100 to 100, > 1000 ng/mL) | candidate selection | European | 113 |
| Lai et al, 2012 (AFP-TTD criteria) | 158 | 400 ng/mL | candidate selection | European | 112 |
| Lai et al, 2013 | 422 | AFP slope: 15 ng/mL/month | Prediction | European | 130 |
| Toso et al, 2014 (new deMELD model) | 49026 | 400 ng/mL | candidate selection | North American | 122 |
| Grąt et al, 2014 (combination of UCSF and Up-to-7 criteria) | 121 | 100 ng/ml; 200mg/ml | candidate selection | European | 134 |
| Vitale et al, 2014 | 4399 | 100, 100 to 100, > 1000 ng/mL | candidate selection | European | 123 |
| Marvin et al, 2015 | 41801 | Log AFP: 0 to 1.61, 1.61 to 2.48, 2.48 to 3.93, 3.93 to 10.9 (MELDCALC-EQ = 1.143MELD + 1.324 (log AFP) + 1.438 (TumorNum) + 1.194(MaxTumorSize) + c(t), where c(t) = -2/0.146 if t < 6 months and c(t) = -1/0.146 if t ≥ 6 months) | candidate selection | North American | 121 |
| Xu et al, 2016 (Hangzhou criteria) | 6012 | 400 ng/mL | candidate selection | Asian | 13 |
| Lai et al, 2016 (TRAIN score) | 179 | AFP slope: 15 ng/mL/month | prediction | European | 128 |
| Hong et al, 2016 | 123 | 200 ng/ml | prediction | Asian | 131 |
| Sasaki et al, 2017 (HALT-HCC score) | 420 | HALT-HCC = (1·27 × TBS) + (1·85 × lnAFP) + (0·26 × MELD-Na) | prediction | North American | 125 |
| Halazun et al, 2017 (Pre-MORAL score) | 339 | 200 ng/ml | prediction | North American | 109 |
| Mehta et al, 2017 (RETREAT score) | 721 | 0-20, 21-99, 100-999, ≥1000 ng/ml | prediction | North American | 118 |
| Lai et al, 2017 | 2103 | 20 ng/ml, 1000 ng/ml | candidate selection | European | 124 |
| Mazzaferro, 2018 (Metroticket 2.0 Model) | 1018 | <200, 200-400 ng/mL, 400-1000, >1000 ng/ml | candidate selection | European | 120 |
| Halazun et al, 2018 (NYCA score) | 1450 | <200, 200-1000, >1000 ng/ml | candidate selection | North American | 110 |
| Eilard et al, 2018 | 336 | <99, 100-999, >1000 ng/ml | candidate selection | European | 135 |
| Ding et al, 2020 | 93 | 144ng / ml | prediction | Asian | 132 |
LT: liver transplantation; AFP: alpha‐fetoprotein; TTV: total tumor volume; TTD: total tumor diameter; MELD: model for end‐stage liver disease; deMELD: dropout equivalent calculated equivalent Model for End-Stage Liver Disease; UCSF: University of California: San Francisco; MELDCALC-EQ: calculated equivalent Model for End-Stage Liver Disease; TRAIN: time-radiological-response-alpha-fetoprotein-inflammation; LRT: loco-regional treatment; NLR: neutrophil-to-lymphocyte ratio; 18F-FDG PET/CT: 18F-fluorodeoxyglucose positron emission tomography/computed tomography; HALT-HCC: Hazard Associated with Liver Transplantation for Hepatocellular Carcinoma; TBS: tumor burden score; MELD-Na: MELD-sodium; MORAL: model of recurrence after liver transplant; RETREAT: risk estimation of tumor recurrence after transplant; NYCA: New York/California.
AFP with other factors
Some novel indicators have been investigated for integration with AFP, such as 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET)131, 132 and plasma metabolomics profiling133. In addition, several criteria established and validated previously were combined with AFP for the purpose of minimizing the risk of post-LT tumor recurrence134, 135. Grąt et al. cited University of California, San Francisco (UCSF) criteria, Up-to-7 criteria and AFP levels <100 ng/ml to build a score134. It exhibited the superior predictive power since patients fulfilling two criteria with AFP levels <100 ng/ml showed an excellent 5-year recurrence-free survival (100.0%). This score, named the Warsaw proposal, was verified in a total of 240 HCC patients136.
The value of dynamic AFP
The variation in AFP from pre-LT to post-LT might be better to evaluate disease progression. Post-LT AFP levels not decreasing to 20 ng/ml were proved to be a risk factor for recurrence by Xu et al137. Lai et al. conducted a retrospective study on 422 HCC patients who underwent LT, confirming that an AFP slope > 15 ng/mL/month was a unique independent predictive factor for HCC outcome130. A similar result was also found in another study128. Later, an AFP slope >7.5 was shown to be significantly related to HCC recurrence (HR, 3.0; P=0.03) and was also associated with microvascular invasion (OR, 6.8; P=0.008)138.
Since multiple studies have confirmed the predictive value of the AFP level and offered several reliable criteria (Figure 3) containing it139, 140, fairness of liver allocation and prediction of the outcome of LT have been significantly improved.
Summary of several metrics used in proposed criteria. TTV: total tumor volume; TTD: total tumor diameter; deMELD: dropout equivalent calculated equivalent Model for End-Stage Liver Disease; MELDEQ: equivalent Model for End-Stage Liver Disease; TRAIN: time-radiological-response-alpha-fetoprotein-inflammation; HALT-HCC: Hazard Associated with Liver Transplantation for Hepatocellular Carcinoma; MORAL: model of recurrence after liver transplant; RETREAT: risk estimation of tumor recurrence after transplant; NYCA: New York/California; MELD: model for end‐stage liver disease; LRT: loco-regional treatment; NLR: neutrophil-to-lymphocyte ratio; 18F-FDG PET: 18F-fluorodeoxyglucose positron emission tomography.
The role of AFP in immune therapy
AFP serves as the biomarker for checkpoint inhibitor
In the process of tumor occurrence and development, immune checkpoint has become one of the main reasons for immune tolerance. Immune-checkpoint inhibitor (ICI) promotes the host to recognize tumor antigen and to generate an immune response by ceasing the co-inhibitory signaling141. The arrival of ICI as a new milestone for HCC treatment has led to a conceptual transform of therapeutic strategy. Recently, several studies have proved that the change of AFP could accurately reflect the therapeutic effect of ICI. Spahn et al. conducted a study contained 67 patients received nivolumab and 32 patients received pembrolizumab to explore the biomarkers to predict response to ICI142. They pointed that the patients whose AFP < 400 µg/L at the beginning of ICI treatment were more likely to have complete response. Besides, AFP < 400 µg/L was related to a longer median progress-free and overall survival. Similarly, Post-treatment decline in serum AFP levels were also proved to be a predictor of prognosis143-145.
AFP performs as a tumor antigen for immune therapy
Increasing evidence has shown that infiltrating immune cells in HCC tissue, which form the tumor immune microenvironment, play an important role in tumor proliferation and metastasis146-148. HCCs belonging to different immune-specific classifications and immune cell infiltrations might refer to distinct outcomes of therapies. Kurebayashi et al. identified that patients in a cytokeratin 19+-associated immune-high subtype had a better prognosis147. Hence, developing various immunotherapies aiming at different HCC classifications or using biomarkers to select appropriate patients is particularly important.
Immunotherapy efficiency depends on the recognition of tumor-specific antigens by the autoimmune system. The re-expression of AFP is observed in approximately 70%-80% of HCC patients but is not observed in healthy individuals after birth14, 149. In addition, AFP has been proven to promote tumor proliferation through the initiation of the cyclic AMP-protein kinase A pathway, Ca2+ influx and apoptotic signal transduction mediated by caspase‐3150-152. AFP can also mediate HCC immune escape by altering the proportion of CD4+ T/CD8+ T cells153 and inhibiting dendritic cells (DCs)154 and natural killer (NK) cells155. These features make AFP itself an appropriate therapeutic target. However, immune tolerance results in a low immune response to AFP despite the immune system being exposed to high plasma levels of AFP150, 156. The crucial point of mounting effective antitumor immunity is to ameliorate the low affinity of the immune system to AFP. Many approaches containing recombinant plasmid DNA, adoptive transfer of tumor-specific T cells and chimeric virus-like particles have been proposed to improve the immune response14, 157-159.
AFP-based cancer vaccine
HCC vaccines are designed to target tumor-specific antigens to induce an effective immune response, aiming to prevent tumor proliferation and even eliminate it. AFP is considered a favorable target due to its immunogenicity and specificity. The AFP vaccine presents the AFP epitope polypeptides to antigen presenting cells (APCs), generating multiple AFP-specific cytotoxic T lymphocytes (CTLs) to induce tumor immunity. At present, a variety of AFP vaccines have been created, such as DC vaccines160-163, DNA vaccines164 and peptide vaccines165-167, which have been continuously applied to HCC mouse models and clinical trials.
DC vaccines exhibit favorable application prospects due to their specificity and effectiveness for immunotherapy of HCC. Vollmer et al. first reported genetically engineered and AFP-transduced DCs that were injected into C57BL/6 mice and elicited effective T-cell immune responses160. More recently, scientists have attempted to boost the antigen-presenting function of DCs. Methods such as zoledronic acid stimulation168, DC-derived exosomes (DEXs)161, 162 and coculture with IL-2 and GM-CSF169 could promote the secretion of valid interferons (IL9, IL15 and TNF) to enhance tumor immunity.
It has been proven that exosomes are involved in the biological behavior. DEXs were then discovered to express major histocompatibility complex class I and II (MHC I and II) and costimulatory molecules170, 171. Therefore, Lu et al. monitored the tumor growth and immune microenvironment of three HCC mouse models after using exosomes derived from AFP-expressing DCs (DEXAFP)161. It induced more powerful antigen-specific immune responses, which were demonstrated by the prevention of tumor proliferation, a prolonged survival time and an ameliorative tumor microenvironment (increased levels of IFN-γ, IL-2 and CD8+ T lymphocytes). Later, the same conclusions were found when Li et al. stimulated naive T cells with DEXs generated by peripheral blood-derived DCs loaded with the recombinant adeno-associated viral vector (rAAV) -carrying AFP gene162. In addition, two researchers applied tumor antigen-pulsed dendritic cells as an immunotherapy to treat HCC patients and obtained encouraging therapeutic effects163, 172. However, it is worth noting that DEXs may transfer their immunogenicity to other APCs due to secretion and uptake of exosomes, leading to antigen cross-presentation among APCs. In brief, DEXs are the novel idea for a cell-free vaccine, and their combination with DCs might be feasible.
Polypeptide vaccines can be synthesized in vitro without the involvement of viral vectors, rendering them safe and easy to produce. Compared to oligopeptides, the higher relative molecular weight and the stronger immunogenicity of polypeptides could make CTLs more efficacious [192]. Tam et al. described a multiple antigen peptide (MAP) system to synthesize a peptide-antigen matrix by a solid-phase method173. Recently, two phase I clinical studies have been conducted to investigate the safety and efficacy of AFP peptide vaccination for patients with advanced HCC166, 167. Nakagawa et al. injected AFP-derived peptides (AFP357 and AFP403) into 15 patients and found that one patient had a complete remission, eight patients had tumor suppression, and none had adverse events166. Another study employed a combination of peptide vaccination and radiotherapy, showing a 33% response rate and 66% disease control rate with no side effects167. To boost T-cell responses, Li et al. made use of heat shock protein 72 (HSP72) and AFP epitope peptide (AFP-P) to construct a peptide vaccine and then immunized BALB/C mice174. Compared to those immunized with AFP-P or HSP72 alone, mice immunized with HSP72/AFP-P developed more IFN-γ-producing CD8+ T cells and their tumor volume was smaller. Similar results were also found when crosslinking the AFP epitope peptide with heat shock protein 70 functional peptide or glycoprotein 96165, 175, 176.
AFP as a target for Chimeric antigen receptor (CAR) T-cell and T cell receptor (TCR) T-cell therapy
CAR T-cell therapy, which grafts genetically engineered receptors onto host T cells to target tumor-associated antigen (TAA), represents a remarkable advance in immunotherapy for cancer (Figure 4). It made modified T cells MHC-unrestricted. The FDA has approved two CAR-T therapies targeting CD19 antigen (Kymriah and Yescarta) for the treatment of acute lymphoblastic leukemia (ALL) and diffuse large B-cell lymphoma (DLBCL) due to their powerful antitumor effects177, 178. Liu et al. generated a novel CAR (ET1402L1) that specifically bonded to the AFP158-166 peptide presented by HLA-A*02:01179. T cells could suppress HLA-A*02:01+/AFP+ tumor growth in vivo and in vitro after being transduced by this AFP-CAR. This result also suggested that local injection of AFP-CAR T cells promoted a more intense and sustained immune response179 so that local treatment may be a better method. The AFP-CAR could bind to the peptide-MHC complex, intracellular antigens and secreted protein products that could not be recognized by traditional CAR.
The process of AFP performing as a tumor antigen in CAR T-cell or TCR T-cell therapy. CAR: Chimeric antigen receptor; TCR: T cell receptor; MHC: major histocompatibility complex.
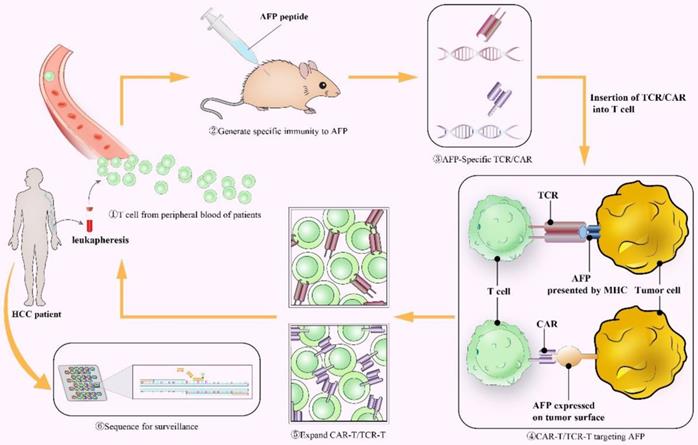
Some ideal TAAs, which are expressed on all tumor cells but hardly express on normal tissues, are found inside the cell and must be presented to the cell surface by the MHC to activate the immune response180, 181. The TCR utilizes heterodimers to recognize intracellular or cell surface MHC-restricted TAAs, while traditional CAR cannot (Figure 4). Thus, the first and most critical step of TCR T-cell therapy is to engineer a TCR that specifically binds to the AFP peptide-MHC complex. Recently, several studies have identified optimized TCRs that can recognize AFP/HLA-A*02+ tumor cells182-184. Zhu et al. immunized HLA-A2 transgenic AAD mice with the AFP158 epitope peptide to generate AFP158-specific CD8+ T cells with TCR diversity and transduced three pairs of TCR genes into human T cells182. The results showed that both mouse CD8+ T cells and engineered human T cells could kill HLA-A2+ AFP+ HepG2 tumor cells without targeting normal primary hepatocytes in vitro. Then, Luo et al. excluded two of the above three TCRs182 due to their underlying cross-reactivity, and the remaining TCR with optimal affinity, efficiency and safety was applied to an early clinical trial (NCT03971747)185. Similar findings (an increased number of IFN-γ secretion T cells and cytotoxicity toward tumor cells) were achieved when Sun et al. infected nonspecific T cells with a lentiviral vector constructed by cloned TCR genes of AFP-specific CTLs183. Furthermore, on the basis of AFP-specific TCRs, Docta et al. employed a combination of physicochemical and cell biology methods to adjust the TCR affinity184. These TCRs were validated among normal and malignant cells in different tissues, cell types and HLA alleles. Instead of HLA-A * 02: 01, HLA-A * 24: 02 was found to be more common in Asian populations, so Li et al. distinguished the HLA-A*24: 02-restricted peptide KWVESIFLIF (AFP2-11) to create a specific TCR (KWV3.1)186.
CAR T-cell therapy has high specificity and effectiveness, as it is not MHC restricted, but it cannot recognize intracellular antigens, while TCR T-cell therapy has a wider range of targets but is limited by MHC molecules. Studies have proven their remarkable antitumor effect. However, multiple TAAs, including AFP, were not 100% tumor-specific, and promiscuous recognition of unassociated epitopes of normal proteins might cause off-target reactivity of both therapies, which could cause serious systemic toxicity. Hence, Cai et al. measured the off-target cross-reactivity of three AFP-specific TCRs187. Several other peptides (ENPP1436 and RCL1215) were able to cross-activate these TCRs, but they required higher concentrations (approximately 250 times and 10,000 times, respectively) than AFP to fulfill the same level of response. Making CAR or TCR recognize multiantigen complexes simultaneously188 or inserting suicide genes that could be activated when off-target reactivity occurs189 is considered a remedial action to overcome side effects. Additional studies are needed to create TCR/CAR with an ideal affinity to target high densities of AFP on HCC while not targeting low expression on nonmalignant cells.
Conclusion and future prospective
The heterogeneity of HCC caused by multiple pathogenic mechanisms and various risk factors gives rise to limitations in diagnosis and treatment. Identified more than 60 years ago, AFP has become one of the most frequently used biomarkers in HCC and is a critical element to select patients who are suitable for LT. Several researchers have also identified its function in HCC classes. In addition, some studies have paid attention to the role of AFP as a tumor antigen to treat HCC due to its immunogenicity and universality.
Although AFP is widely used in the diagnosis and treatment of HCC, improvements are required in many fields. Does HCC molecular classification defined by AFP have therapeutic benefits? Which combination with AFP can improve its performance in LT candidate selection? What is the optimal cut value of AFP in HCC diagnosis and prognosis? Currently, quite a few expectations have been placed regarding AFP as an antigen, and some AFP vaccines, CAR-T and TCR-T are being verified in clinical trials. However, a low immune response to AFP caused by immune tolerance and off-target reactivity in CAR-T and TCR-T become obstacles. The development of original engineered AFP peptides and an understanding of the mechanisms regulating immune escape might offer a superior therapeutic effect. Besides, how to increase the affinity of AFP epitopes for CAR and TCR should be considered.
Current applications of AFP in HCC have been widely accepted, and future challenges lie in confirming its effectiveness in clinical trials. With rapid progress in research in the future, the use of AFP will be more accurate and widespread.
Abbreviations
HCC: Hepatocellular carcinoma; LT: liver transplantation; AFP: alpha-fetoprotein; PIVKA-II: protein induced by vitamin K absence/antagonist-II; OS: overall survival; DCP: des-gamma-carboxyprothrombin; Fuc-PON1: fucosylated serum paraoxonase 1 to the total serum paraoxonase 1; FPR: fibrinogen to prealbumin ratio; GPR: gamma-glutamyl transpeptidase to platelet ratio; BMI: body mass index; LDH: lactate dehydrogenase; GGT: gamma-glutamyl transpeptidase; ALB: albumin; ALP: alkaline phosphatase; LCA: lens culinaris agglutinin; EpCAM: epithelial cell adhesion molecule; TERT: telomerase reverse transcriptase; MTM-HCC: macrotrabecular-massive HCC; GPC-3: Glypican-3; MC: Milan criteria; MORAL: Model of Recurrence After Liver Transplantation; NYCA: New York/California; TTD: total tumor diameter; TTV: total tumor volume; MELD: model of end-stage liver disease; LRT: locoregional therapy; MRECIST: complete response, partial response, stable disease and progressive disease; TRAIN: Time-Radiological-response-Alpha-fetoprotein-Inflammation; WT: waiting time; 18F-FDG PET: 18F-fluorodeoxyglucose positron emission tomography; UCSF: University of California, San Francisco; DCs: dendritic cells; NK cells: natural killer cells; APCs: antigen presenting cells; CTLs: cytotoxic T lymphocytes; DEXs: DC-derived exosomes; MHC: major histocompatibility complex; DEXAFP: AFP-expressing DCs; rAAV: recombinant adeno-associated viral vector; MAP: multiple antigen peptide; HSP72: heat shock protein 72; AFP-P: AFP epitope peptide; CAR: Chimeric antigen receptor; TCR: T cell receptor; TAA: tumor-associated antigen; ALL: acute lymphoblastic leukemia; DLBCL: diffuse large B-cell lymphoma.
Acknowledgements
This work was supported by National Natural Science Funds for Distinguished Young Scholar of China (No. 81625003): Key Program: National Natural Science Foundation of China (No. 81930016) and Key Research & Development Plan of Zhejiang Province (No. 2019C03050 and No. 2021C03118).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450-1462
2. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314
3. McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis. 2015;19:223-38
4. Zheng Y, Zhu M, Li M. Effects of alpha-fetoprotein on the occurrence and progression of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2020;146:2439-2446
5. Jepsen P, Andersen MW, Villadsen GE. et al. Time-trends in incidence and prognosis of hepatocellular carcinoma in Denmark: A nationwide register-based cohort study. Liver Int. 2017;37:871-878
6. Kim DY, Han KH. Epidemiology and surveillance of hepatocellular carcinoma. Liver Cancer. 2012;1:2-14
7. Trevisani F, Garuti F, Neri A. Alpha-fetoprotein for Diagnosis, Prognosis, and Transplant Selection. Semin Liver Dis. 2019;39:163-177
8. Galle PR, Foerster F, Kudo M. et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 2019;39:2214-2229
9. Llovet JM, Ricci S, Mazzaferro V. et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-90
10. EASL Clinical Practice Guidelines. Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236
11. Huang A, Yang XR, Chung WY. et al. Targeted therapy for hepatocellular carcinoma. Signal Transduct Target Ther. 2020;5:146
12. Mizejewski GJ. Alpha-fetoprotein structure and function: relevance to isoforms, epitopes, and conformational variants. Exp Biol Med (Maywood). 2001;226:377-408
13. Xu X, Lu D, Ling Q. et al. Liver transplantation for hepatocellular carcinoma beyond the Milan criteria. Gut. 2016;65:1035-41
14. Wang X, Wang Q. Alpha-Fetoprotein and Hepatocellular Carcinoma Immunity. Can J Gastroenterol Hepatol. 2018;2018:9049252
15. Calderaro J, Couchy G, Imbeaud S. et al. Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification. J Hepatol. 2017;67:727-738
16. Gitlin D, Perricelli A, Gitlin GM. Synthesis of -fetoprotein by liver, yolk sac, and gastrointestinal tract of the human conceptus. Cancer Res. 1972;32:979-82
17. Nakabayashi H, Hashimoto T, Miyao Y. et al. A position-dependent silencer plays a major role in repressing alpha-fetoprotein expression in human hepatoma. Mol Cell Biol. 1991;11:5885-93
18. Lazarevich NL. Molecular mechanisms of alpha-fetoprotein gene expression. Biochemistry (Mosc). 2000;65:117-33
19. Tsukuma H, Hiyama T, Tanaka S. et al. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med. 1993;328:1797-801
20. Peng SY, Chen WJ, Lai PL. et al. High alpha-fetoprotein level correlates with high stage, early recurrence and poor prognosis of hepatocellular carcinoma: significance of hepatitis virus infection, age, p53 and beta-catenin mutations. Int J Cancer. 2004;112:44-50
21. Di Bisceglie AM, Sterling RK, Chung RT. et al. Serum alpha-fetoprotein levels in patients with advanced hepatitis C: results from the HALT-C Trial. J Hepatol. 2005;43:434-41
22. Fouad R, Elsharkawy A, Abdel Alem S. et al. Clinical impact of serum α-fetoprotein and its relation on changes in liver fibrosis in hepatitis C virus patients receiving direct-acting antivirals. Eur J Gastroenterol Hepatol. 2019;31:1129-1134
23. Gamil M, Alboraie M, El-Sayed M. et al. Novel scores combining AFP with non-invasive markers for prediction of liver fibrosis in chronic hepatitis C patients. J Med Virol. 2018;90:1080-1086
24. Tzartzeva K, Obi J, Rich NE. et al. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology. 2018;154:1706-1718.e1
25. Best J, Bilgi H, Heider D. et al. The GALAD scoring algorithm based on AFP, AFP-L3, and DCP significantly improves detection of BCLC early stage hepatocellular carcinoma. Z Gastroenterol. 2016;54:1296-1305
26. Wang X, Zhang Y, Yang N. et al. Evaluation of the Combined Application of AFP, AFP-L3%, and DCP for Hepatocellular Carcinoma Diagnosis: A Meta-analysis. Biomed Res Int. 2020;2020:5087643
27. El-Serag HB, Kanwal F, Davila JA. et al. A new laboratory-based algorithm to predict development of hepatocellular carcinoma in patients with hepatitis C and cirrhosis. Gastroenterology. 2014;146:1249-55.e1
28. Edoo MIA, Chutturghoon VK, Wusu-Ansah GK. et al. Serum Biomarkers AFP, CEA and CA19-9 Combined Detection for Early Diagnosis of Hepatocellular Carcinoma. Iran J Public Health. 2019;48:314-322
29. Wang Y, Zhang C, Zhang P. et al. Serum exosomal microRNAs combined with alpha-fetoprotein as diagnostic markers of hepatocellular carcinoma. Cancer Med. 2018;7:1670-1679
30. Peng C, Ye Y, Wang Z. et al. Circulating microRNAs for the diagnosis of hepatocellular carcinoma. Dig Liver Dis. 2019;51:621-631
31. Hemken PM, Sokoll LJ, Yang X. et al. Validation of a novel model for the early detection of hepatocellular carcinoma. Clin Proteomics. 2019;16:2
32. Kudo M, Izumi N, Kokudo N. et al. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29:339-64
33. Heimbach JK, Kulik LM, Finn RS. et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380
34. Bruix J, Cheng AL, Meinhardt G. et al. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: Analysis of two phase III studies. J Hepatol. 2017;67:999-1008
35. Kudo M, Finn RS, Qin S. et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173
36. Giannini EG, Marenco S, Borgonovo G. et al. Alpha-fetoprotein has no prognostic role in small hepatocellular carcinoma identified during surveillance in compensated cirrhosis. Hepatology. 2012;56:1371-9
37. Bruix J, Qin S, Merle P. et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66
38. Abou-Alfa GK, Meyer T, Cheng AL. et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med. 2018;379:54-63
39. Zhu AX, Park JO, Ryoo BY. et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16:859-70
40. Baig JA, Alam JM, Mahmood SR. et al. Hepatocellular carcinoma (HCC) and diagnostic significance of A-fetoprotein (AFP). J Ayub Med Coll Abbottabad. 2009;21:72-5
41. Burak KW, Sherman M. Hepatocellular carcinoma: Consensus, controversies and future directions. A report from the Canadian Association for the Study of the Liver Hepatocellular Carcinoma Meeting. Can J Gastroenterol Hepatol. 2015;29:178-84
42. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-2
43. An SL, Xiao T, Wang LM. et al. Prognostic Significance of Preoperative Serum Alpha- fetoprotein in Hepatocellular Carcinoma and Correlation with Clinicopathological Factors: a Single-center Experience from China. Asian Pac J Cancer Prev. 2015;16:4421-7
44. Xu J, Liu C, Zhou L. et al. Distinctions between clinicopathological factors and prognosis of alpha-fetoprotein negative and positive hepatocelluar carcinoma patients. Asian Pac J Cancer Prev. 2012;13:559-62
45. Singal AG, Chan V, Getachew Y. et al. Predictors of liver transplant eligibility for patients with hepatocellular carcinoma in a safety net hospital. Dig Dis Sci. 2012;57:580-6
46. Zhu WW, Guo JJ, Guo L. et al. Evaluation of midkine as a diagnostic serum biomarker in hepatocellular carcinoma. Clin Cancer Res. 2013;19:3944-54
47. Vongsuvanh R, van der Poorten D, Iseli T. et al. Midkine Increases Diagnostic Yield in AFP Negative and NASH-Related Hepatocellular Carcinoma. PLoS One. 2016;11:e0155800
48. Liu Z, Wu M, Lin D. et al. Des-gamma-carboxyprothrombin is a favorable biomarker for the early diagnosis of alfa-fetoprotein-negative hepatitis B virus-related hepatocellular carcinoma. J Int Med Res. 2020;48:300060520902575
49. Mao L, Wang Y, Wang D. et al. TEMs but not DKK1 could serve as complementary biomarkers for AFP in diagnosing AFP-negative hepatocellular carcinoma. PLoS One. 2017;12:e0183880
50. Lu LH, Wei W, Kan A. et al. Novel Value of Preoperative Gamma-Glutamyltransferase Levels in the Prognosis of AFP-Negative Hepatocellular Carcinoma. Dis Markers. 2020;2020:4269460
51. Guo X, Lv X, Lv X. et al. Circulating miR-21 serves as a serum biomarker for hepatocellular carcinoma and correlated with distant metastasis. Oncotarget. 2017;8:44050-44058
52. Lin XJ, Chong Y, Guo ZW. et al. A serum microRNA classifier for early detection of hepatocellular carcinoma: a multicentre, retrospective, longitudinal biomarker identification study with a nested case-control study. Lancet Oncol. 2015;16:804-15
53. Zhang L, Wang K, Deng Q. et al. Identification of Key Hydroxymethylated Genes and Transcription Factors Associated with Alpha-Fetoprotein-Negative Hepatocellular Carcinoma. DNA Cell Biol. 2019;38:1346-1356
54. Jing W, Peng R, Zhu M. et al. Differential Expression and Diagnostic Significance of Pre-Albumin, Fibrinogen Combined with D-Dimer in AFP-Negative Hepatocellular Carcinoma. Pathol Oncol Res. 2020;26:1669-1676
55. Wang T, Liu M, Zheng SJ. et al. Tumor-associated autoantibodies are useful biomarkers in immunodiagnosis of α-fetoprotein-negative hepatocellular carcinoma. World J Gastroenterol. 2017;23:3496-3504
56. Zhang Z, Zhang Y, Wang Y. et al. Alpha-fetoprotein-L3 and Golgi protein 73 may serve as candidate biomarkers for diagnosing alpha-fetoprotein-negative hepatocellular carcinoma. Onco Targets Ther. 2016;9:123-9
57. Tian Z, Yu T, Wei H. et al. Clinical value of LHPP-associated microRNAs combined with protein induced by vitamin K deficiency or antagonist-II in the diagnosis of alpha-fetoprotein-negative hepatocellular carcinoma. J Clin Lab Anal. 2020;34:e23071
58. Shu H, Li W, Shang S. et al. Diagnosis of AFP-negative early-stage hepatocellular carcinoma using Fuc-PON1. Discov Med. 2017;23:163-168
59. Huang L, Mo Z, Hu Z. et al. Diagnostic value of fibrinogen to prealbumin ratio and gamma-glutamyl transpeptidase to platelet ratio in the progression of AFP-negative hepatocellular carcinoma. Cancer Cell Int. 2020;20:77
60. Gong X, Huang A. Differential expression and diagnostic significance of P53, MutS homologs 2, tropomyosin-4 in alpha-fetoprotein-negative hepatocellular carcinoma. J Clin Lab Anal. 2020;34:e23353
61. Sun J, Zhao Y, Qin L. et al. Metabolomic Profiles for HBV Related Hepatocellular Carcinoma Including Alpha-Fetoproteins Positive and Negative Subtypes. Front Oncol. 2019;9:1069
62. Mao M, Wang X, Song Y. et al. Novel Prognostic Scores Based on Plasma Prothrombin Time and Fibrinogen Levels in Patients With AFP-Negative Hepatocellular Carcinoma. Cancer Control. 2020;27:1073274820915520
63. Wang T, Zhang KH, Hu PP. et al. Simple and robust diagnosis of early, small and AFP-negative primary hepatic carcinomas: an integrative approach of serum fluorescence and conventional blood tests. Oncotarget. 2016;7:64053-64070
64. Wang X, Mao M, He Z. et al. Development and Validation of a Prognostic Nomogram in AFP-negative hepatocellular carcinoma. Int J Biol Sci. 2019;15:221-228
65. Huang J, Liu FC, Li L. et al. Nomograms to predict the long-time prognosis in patients with alpha-fetoprotein negative hepatocellular carcinoma following radical resection. Cancer Med. 2020;9:2791-2802
66. Luo CL, Rong Y, Chen H. et al. A Logistic Regression Model for Noninvasive Prediction of AFP-Negative Hepatocellular Carcinoma. Technol Cancer Res Treat. 2019;18:1533033819846632
67. Wang Y, Zhu W, Chen X. et al. Selenium-binding protein 1 transcriptionally activates p21 expression via p53-independent mechanism and its frequent reduction associates with poor prognosis in bladder cancer. J Transl Med. 2020;18:17
68. Phermthai T, Pokathikorn P, Wichitwiengrat S. et al. P53 Mutation and Epigenetic Imprinted IGF2/H19 Gene Analysis in Mesenchymal Stem Cells Derived from Amniotic Fluid, Amnion, Endometrium, and Wharton's Jelly. Stem Cells Dev. 2017;26:1344-1354
69. Sameer AS, Nissar S, Fatima K. Mismatch repair pathway: molecules, functions, and role in colorectal carcinogenesis. Eur J Cancer Prev. 2014;23:246-57
70. Yang R, Zheng G, Ren D. et al. The clinical significance and biological function of tropomyosin 4 in colon cancer. Biomed Pharmacother. 2018;101:1-7
71. Hindupur SK, Colombi M, Fuhs SR. et al. The protein histidine phosphatase LHPP is a tumour suppressor. Nature. 2018;555:678-682
72. AlSalloom AA. An update of biochemical markers of hepatocellular carcinoma. Int J Health Sci (Qassim). 2016;10:121-36
73. Hayashi K, Kumada T, Nakano S. et al. Usefulness of measurement of Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein as a marker of prognosis and recurrence of small hepatocellular carcinoma. Am J Gastroenterol. 1999;94:3028-33
74. Wong RJ, Ahmed A, Gish RG. Elevated alpha-fetoprotein: differential diagnosis - hepatocellular carcinoma and other disorders. Clin Liver Dis. 2015;19:309-23
75. Kim H, Sohn A, Yeo I. et al. Clinical Assay for AFP-L3 by Using Multiple Reaction Monitoring-Mass Spectrometry for Diagnosing Hepatocellular Carcinoma. Clin Chem. 2018;64:1230-1238
76. Choi J, Kim GA, Han S. et al. Longitudinal Assessment of Three Serum Biomarkers to Detect Very Early-Stage Hepatocellular Carcinoma. Hepatology. 2019;69:1983-1994
77. Goossens N, Sun X, Hoshida Y. Molecular classification of hepatocellular carcinoma: potential therapeutic implications. Hepat Oncol. 2015;2:371-379
78. Dai XM, Yang SL, Zheng XM. et al. CD133 expression and α-fetoprotein levels define novel prognostic subtypes of HBV-associated hepatocellular carcinoma: A long-term follow-up analysis. Oncol Lett. 2018;15:2985-2991
79. Wei Y, Wang Y, Gong J. et al. High expression of MAGE-A9 contributes to stemness and malignancy of human hepatocellular carcinoma. Int J Oncol. 2018;52:219-230
80. de Boer CJ, van Krieken JH, Janssen-van Rhijn CM. et al. Expression of Ep-CAM in normal, regenerating, metaplastic, and neoplastic liver. J Pathol. 1999;188:201-6
81. Terris B, Cavard C, Perret C. EpCAM, a new marker for cancer stem cells in hepatocellular carcinoma. J Hepatol. 2010;52:280-1
82. Yamashita T, Ji J, Budhu A. et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136:1012-24
83. Llovet JM, Montal R, Sia D. et al. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15:599-616
84. Yamashita T, Forgues M, Wang W. et al. EpCAM and alpha-fetoprotein expression defines novel prognostic subtypes of hepatocellular carcinoma. Cancer Res. 2008;68:1451-61
85. Hoshida Y, Nijman SM, Kobayashi M. et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 2009;69:7385-92
86. Ahn SM, Jang SJ, Shim JH. et al. Genomic portrait of resectable hepatocellular carcinomas: implications of RB1 and FGF19 aberrations for patient stratification. Hepatology. 2014;60:1972-82
87. Zucman-Rossi J, Villanueva A, Nault JC. et al. Genetic Landscape and Biomarkers of Hepatocellular Carcinoma. Gastroenterology. 2015;149:1226-1239.e4
88. Schulze K, Imbeaud S, Letouzé E. et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505-511
89. Nault JC, Calderaro J, Di Tommaso L. et al. Telomerase reverse transcriptase promoter mutation is an early somatic genetic alteration in the transformation of premalignant nodules in hepatocellular carcinoma on cirrhosis. Hepatology. 2014;60:1983-92
90. Chiang DY, Villanueva A, Hoshida Y. et al. Focal gains of VEGFA and molecular classification of hepatocellular carcinoma. Cancer Res. 2008;68:6779-88
91. Sasaki M, Sato Y, Nakanuma Y. Mutational landscape of combined hepatocellular carcinoma and cholangiocarcinoma, and its clinicopathological significance. Histopathology. 2017;70:423-434
92. Gao Q, Zhu H, Dong L. et al. Integrated Proteogenomic Characterization of HBV-Related Hepatocellular Carcinoma. Cell. 2019;179:561-577.e22
93. Yang C, Huang X, Liu Z. et al. Metabolism-associated molecular classification of hepatocellular carcinoma. Mol Oncol. 2020;14:896-913
94. Ziol M, Poté N, Amaddeo G. et al. Macrotrabecular-massive hepatocellular carcinoma: A distinctive histological subtype with clinical relevance. Hepatology. 2018;68:103-112
95. Calderaro J, Ziol M, Paradis V. et al. Molecular and histological correlations in liver cancer. J Hepatol. 2019;71:616-630
96. Mulé S, Galletto Pregliasco A, Tenenhaus A. et al. Multiphase Liver MRI for Identifying the Macrotrabecular-Massive Subtype of Hepatocellular Carcinoma. Radiology. 2020;295:562-571
97. Boyault S, Rickman DS, de Reyniès A. et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology. 2007;45:42-52
98. Gao W, Kim H, Feng M. et al. Inactivation of Wnt signaling by a human antibody that recognizes the heparan sulfate chains of glypican-3 for liver cancer therapy. Hepatology. 2014;60:576-87
99. Xue R, Feng J, Meng Q. et al. The significance of glypican-3 expression profiling in the tumor cellular origin theoretical system for hepatocellular carcinoma progression. J Gastroenterol Hepatol. 2017;32:1503-1511
100. Nishioka ST, Sato MM, Wong LL. et al. Clinical and molecular sub-classification of hepatocellular carcinoma relative to alpha-fetoprotein level in an Asia-Pacific island cohort. Hepatoma Res. 2018 4
101. Ke K, Chen G, Cai Z. et al. Evaluation and prediction of hepatocellular carcinoma prognosis based on molecular classification. Cancer Manag Res. 2018;10:5291-5302
102. Mazzaferro V, Regalia E, Doci R. et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-9
103. Tschuor C, Ferrarese A, Kuemmerli C. et al. Allocation of liver grafts worldwide - Is there a best system? J Hepatol. 2019;71:707-718
104. Yao FY, Ferrell L, Bass NM. et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394-403
105. Morgul MH, Felgendreff P, Kienlein A. et al. Milan criteria in the MELD era-is it justifiable to extend the limits for orthotopic liver transplantation? World J Surg Oncol. 2020;18:158
106. Berry K, Ioannou GN. Serum alpha-fetoprotein level independently predicts posttransplant survival in patients with hepatocellular carcinoma. Liver Transpl. 2013;19:634-45
107. Notarpaolo A, Layese R, Magistri P. et al. Validation of the AFP model as a predictor of HCC recurrence in patients with viral hepatitis-related cirrhosis who had received a liver transplant for HCC. J Hepatol. 2017;66:552-559
108. Yang SH, Suh KS, Lee HW. et al. A revised scoring system utilizing serum alphafetoprotein levels to expand candidates for living donor transplantation in hepatocellular carcinoma. Surgery. 2007;141:598-609
109. Halazun KJ, Najjar M, Abdelmessih RM. et al. Recurrence After Liver Transplantation for Hepatocellular Carcinoma: A New MORAL to the Story. Ann Surg. 2017;265:557-564
110. Halazun KJ, Tabrizian P, Najjar M. et al. Is it Time to Abandon the Milan Criteria?: Results of a Bicoastal US Collaboration to Redefine Hepatocellular Carcinoma Liver Transplantation Selection Policies. Ann Surg. 2018;268:690-699
111. Zhan QF, Ling SB, Deng YN. et al. Hangzhou criteria as downstaging criteria in hepatocellular carcinoma before liver transplantation: A multicenter study from China. Hepatobiliary Pancreat Dis Int. 2020;19:349-357
112. Lai Q, Avolio AW, Manzia TM. et al. Combination of biological and morphological parameters for the selection of patients with hepatocellular carcinoma waiting for liver transplantation. Clin Transplant. 2012;26:E125-31
113. Duvoux C, Roudot-Thoraval F, Decaens T. et al. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012;143:986-94.e3 quiz e14-5
114. Varona MA, Soriano A, Aguirre-Jaime A. et al. Risk factors of hepatocellular carcinoma recurrence after liver transplantation: accuracy of the alpha-fetoprotein model in a single-center experience. Transplant Proc. 2015;47:84-9
115. Piñero F, Anders M, Boin IF, et al. Liver transplantation for hepatocellular carcinoma: impact of expansion criteria in a multicenter cohort study from a high waitlist mortality region.". Transpl Int. 2020
116. Menahem B, Duvoux C, Ganne N. et al. Liver Resection for Solitary Transplantable Hepatocellular Carcinoma: The Role of AFP-Score. World J Surg. 2019;43:221-229
117. Toso C, Asthana S, Bigam DL. et al. Reassessing selection criteria prior to liver transplantation for hepatocellular carcinoma utilizing the Scientific Registry of Transplant Recipients database. Hepatology. 2009;49:832-8
118. Mehta N, Heimbach J, Harnois DM. et al. Validation of a Risk Estimation of Tumor Recurrence After Transplant (RETREAT) Score for Hepatocellular Carcinoma Recurrence After Liver Transplant. JAMA Oncol. 2017;3:493-500
119. Toso C, Meeberg G, Hernandez-Alejandro R. et al. Total tumor volume and alpha-fetoprotein for selection of transplant candidates with hepatocellular carcinoma: A prospective validation. Hepatology. 2015;62:158-65
120. Mazzaferro V, Sposito C, Zhou J. et al. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology. 2018;154:128-139
121. Marvin MR, Ferguson N, Cannon RM. et al. MELDEQ: An alternative Model for End-Stage Liver Disease score for patients with hepatocellular carcinoma. Liver Transpl. 2015;21:612-22
122. Toso C, Majno P, Berney T. et al. Validation of a dropout assessment model of candidates with/without hepatocellular carcinoma on a common liver transplant waiting list. Transpl Int. 2014;27:686-95
123. Vitale A, Volk ML, De Feo TM. et al. A method for establishing allocation equity among patients with and without hepatocellular carcinoma on a common liver transplant waiting list. J Hepatol. 2014;60:290-7
124. Lai Q, Vitale A, Iesari S. et al. Intention-to-treat survival benefit of liver transplantation in patients with hepatocellular cancer. Hepatology. 2017;66:1910-1919
125. Sasaki K, Firl DJ, Hashimoto K. et al. Development and validation of the HALT-HCC score to predict mortality in liver transplant recipients with hepatocellular carcinoma: a retrospective cohort analysis. Lancet Gastroenterol Hepatol. 2017;2:595-603
126. Mazzaferro V. Squaring the circle of selection and allocation in liver transplantation for HCC: An adaptive approach. Hepatology. 2016;63:1707-17
127. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60
128. Lai Q, Nicolini D, Inostroza Nunez M. et al. A Novel Prognostic Index in Patients With Hepatocellular Cancer Waiting for Liver Transplantation: Time-Radiological-response-Alpha-fetoprotein-INflammation (TRAIN) Score. Ann Surg. 2016;264:787-796
129. Cucchetti A, Serenari M, Sposito C. et al. Including mRECIST in the Metroticket 2.0 criteria improves prediction of hepatocellular carcinoma-related death after liver transplant. J Hepatol. 2020;73:342-348
130. Lai Q, Avolio AW, Graziadei I. et al. Alpha-fetoprotein and modified response evaluation criteria in solid tumors progression after locoregional therapy as predictors of hepatocellular cancer recurrence and death after transplantation. Liver Transpl. 2013;19:1108-18
131. Hong G, Suh KS, Suh SW. et al. Alpha-fetoprotein and (18)F-FDG positron emission tomography predict tumor recurrence better than Milan criteria in living donor liver transplantation. J Hepatol. 2016;64:852-9
132. Ding E, Lu D, Wei L. et al. Predicting tumor recurrence using metabolic indices of (18)F-FDG PET/CT prior to orthotopic liver transplantationfor hepatocellular carcinoma. Oncol Lett. 2020;20:1245-1255
133. Lu D, Yang F, Lin Z. et al. A prognostic fingerprint in liver transplantation for hepatocellular carcinoma based on plasma metabolomics profiling. Eur J Surg Oncol. 2019;45:2347-2352
134. Grąt M, Kornasiewicz O, Lewandowski Z. et al. Combination of morphologic criteria and α-fetoprotein in selection of patients with hepatocellular carcinoma for liver transplantation minimizes the problem of posttransplant tumor recurrence. World J Surg. 2014;38:2698-707
135. Sternby Eilard M, Holmberg E, Naredi P. et al. Addition of alfa fetoprotein to traditional criteria for hepatocellular carcinoma improves selection accuracy in liver transplantation. Scand J Gastroenterol. 2018;53:976-983
136. Grąt M, Wronka KM, Stypułkowski J. et al. The Warsaw Proposal for the Use of Extended Selection Criteria in Liver Transplantation for Hepatocellular Cancer. Ann Surg Oncol. 2017;24:526-534
137. Xu X, Ke QH, Shao ZX. et al. The value of serum alpha-fetoprotein in predicting tumor recurrence after liver transplantation for hepatocellular carcinoma. Dig Dis Sci. 2009;54:385-8
138. Giard JM, Mehta N, Dodge JL. et al. Alpha-Fetoprotein Slope >7.5 ng/mL per Month Predicts Microvascular Invasion and Tumor Recurrence After Liver Transplantation for Hepatocellular Carcinoma. Transplantation. 2018;102:816-822
139. Piñero F, Costa P, Boteon YL. et al. Results of Liver Transplantation for Hepatocellular Carcinoma in a Multicenter Latin American Cohort Study. Ann Hepatol. 2018;17:256-267
140. Ekpanyapong S, Philips N, Loza BL. et al. Predictors, Presentation, and Treatment Outcomes of Recurrent Hepatocellular Carcinoma After Liver Transplantation: A Large Single Center Experience. J Clin Exp Hepatol. 2020;10:304-315
141. de Miguel M, Calvo E. Clinical Challenges of Immune Checkpoint Inhibitors. Cancer Cell. 2020;38:326-333
142. Spahn S, Roessler D, Pompilia R. et al. Clinical and Genetic Tumor Characteristics of Responding and Non-Responding Patients to PD-1 Inhibition in Hepatocellular Carcinoma. Cancers (Basel). 2020 12
143. Hsu WF, Chuang PH, Chen CK. et al. Predictors of response and survival in patients with unresectable hepatocellular carcinoma treated with nivolumab: real-world experience. Am J Cancer Res. 2020;10:4547-4560
144. Shao YY, Liu TH, Hsu C. et al. Early alpha-foetoprotein response associated with treatment efficacy of immune checkpoint inhibitors for advanced hepatocellular carcinoma. Liver Int. 2019;39:2184-2189
145. Kim HI, Lim J, Shim JH. Role of the alpha-fetoprotein response in immune checkpoint inhibitor-based treatment of patients with hepatocellular carcinoma. J Cancer Res Clin Oncol. 2021
146. Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017;17:559-572
147. Kurebayashi Y, Ojima H, Tsujikawa H. et al. Landscape of immune microenvironment in hepatocellular carcinoma and its additional impact on histological and molecular classification. Hepatology. 2018;68:1025-1041
148. Yarchoan M, Xing D, Luan L. et al. Characterization of the Immune Microenvironment in Hepatocellular Carcinoma. Clin Cancer Res. 2017;23:7333-7339
149. Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63:844-55
150. Li MS, Li PF, He SP. et al. The promoting molecular mechanism of alpha-fetoprotein on the growth of human hepatoma Bel7402 cell line. World J Gastroenterol. 2002;8:469-75
151. Lin B, Zhu M, Wang W. et al. Structural basis for alpha fetoprotein-mediated inhibition of caspase-3 activity in hepatocellular carcinoma cells. Int J Cancer. 2017;141:1413-1421
152. Dudich E, Semenkova L, Dudich I. et al. Alpha-fetoprotein antagonizes X-linked inhibitor of apoptosis protein anticaspase activity and disrupts XIAP-caspase interaction. Febs j. 2006;273:3837-49
153. Gao Q, Qiu SJ, Fan J. et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586-93
154. Pardee AD, Shi J, Butterfield LH. Tumor-derived α-fetoprotein impairs the differentiation and T cell stimulatory activity of human dendritic cells. J Immunol. 2014;193:5723-32
155. Guerra N, Tan YX, Joncker NT. et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28:571-80
156. Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480-9
157. Grimm CF, Ortmann D, Mohr L. et al. Mouse alpha-fetoprotein-specific DNA-based immunotherapy of hepatocellular carcinoma leads to tumor regression in mice. Gastroenterology. 2000;119:1104-12
158. Hirayama M, Nishimura Y. The present status and future prospects of peptide-based cancer vaccines. Int Immunol. 2016;28:319-28
159. Ghasemi F, Rostami S, Ghayour-Mobarhan M. et al. Current progress in the development of therapeutic vaccines for chronic hepatitis B virus infection. Iran J Basic Med Sci. 2016;19:692-704
160. Vollmer CM Jr, Eilber FC, Butterfield LH. et al. Alpha-fetoprotein-specific genetic immunotherapy for hepatocellular carcinoma. Cancer Res. 1999;59:3064-7
161. Lu Z, Zuo B, Jing R. et al. Dendritic cell-derived exosomes elicit tumor regression in autochthonous hepatocellular carcinoma mouse models. J Hepatol. 2017;67:739-748
162. Li J, Huang S, Zhou Z. et al. Exosomes derived from rAAV/AFP-transfected dendritic cells elicit specific T cell-mediated immune responses against hepatocellular carcinoma. Cancer Manag Res. 2018;10:4945-4957
163. Lee JH, Lee Y, Lee M. et al. A phase I/IIa study of adjuvant immunotherapy with tumour antigen-pulsed dendritic cells in patients with hepatocellular carcinoma. Br J Cancer. 2015;113:1666-76
164. Hanke P, Serwe M, Dombrowski F. et al. DNA vaccination with AFP-encoding plasmid DNA prevents growth of subcutaneous AFP-expressing tumors and does not interfere with liver regeneration in mice. Cancer Gene Ther. 2002;9:346-55
165. Wang XP, Wang QX, Lin HP. et al. Recombinant heat shock protein 70 functional peptide and alpha-fetoprotein epitope peptide vaccine elicits specific anti-tumor immunity. Oncotarget. 2016;7:71274-71284
166. Nakagawa H, Mizukoshi E, Kobayashi E. et al. Association Between High-Avidity T-Cell Receptors, Induced by α-Fetoprotein-Derived Peptides, and Anti-Tumor Effects in Patients With Hepatocellular Carcinoma. Gastroenterology. 2017;152:1395-1406.e10
167. Shen J, Wang LF, Zou ZY. et al. Phase I clinical study of personalized peptide vaccination combined with radiotherapy for advanced hepatocellular carcinoma. World J Gastroenterol. 2017;23:5395-5404
168. Butterfield LH, Ribas A, Dissette VB. et al. A phase I/II trial testing immunization of hepatocellular carcinoma patients with dendritic cells pulsed with four alpha-fetoprotein peptides. Clin Cancer Res. 2006;12:2817-25
169. Nakamoto Y, Mizukoshi E, Kitahara M. et al. Prolonged recurrence-free survival following OK432-stimulated dendritic cell transfer into hepatocellular carcinoma during transarterial embolization. Clin Exp Immunol. 2011;163:165-77
170. Taïeb J, Chaput N, Zitvogel L. Dendritic cell-derived exosomes as cell-free peptide-based vaccines. Crit Rev Immunol. 2005;25:215-23
171. Pitt JM, Charrier M, Viaud S. et al. Dendritic cell-derived exosomes as immunotherapies in the fight against cancer. J Immunol. 2014;193:1006-11
172. Wang Y, Yang X, Yu Y. et al. Immunotherapy of patient with hepatocellular carcinoma using cytotoxic T lymphocytes ex vivo activated with tumor antigen-pulsed dendritic cells. J Cancer. 2018;9:275-287
173. Tam JP. Synthetic peptide vaccine design: synthesis and properties of a high-density multiple antigenic peptide system. Proc Natl Acad Sci U S A. 1988;85:5409-13
174. Li Z, Wang XP, Lin HP. et al. Anti-tumor immunity elicited by cross-linking vaccine heat shock protein 72 and alpha-fetoprotein epitope peptide. Neoplasma. 2015;62:713-21
175. Lan YH, Li YG, Liang ZW. et al. A DNA vaccine against chimeric AFP enhanced by HSP70 suppresses growth of hepatocellular carcinoma. Cancer Immunol Immunother. 2007;56:1009-16
176. Wang XP, Wang QX, Lin HP. et al. Glycoprotein 96 and α-fetoprotein cross-linking complexes elicited specific antitumor immunity. Cancer Biother Radiopharm. 2013;28:406-14
177. Heyman B, Yang Y. New developments in immunotherapy for lymphoma. Cancer Biol Med. 2018;15:189-209
178. Vairy S, Garcia JL, Teira P. et al. CTL019 (tisagenlecleucel): CAR-T therapy for relapsed and refractory B-cell acute lymphoblastic leukemia. Drug Des Devel Ther. 2018;12:3885-3898
179. Liu H, Xu Y, Xiang J. et al. Targeting Alpha-Fetoprotein (AFP)-MHC Complex with CAR T-Cell Therapy for Liver Cancer. Clin Cancer Res. 2017;23:478-488
180. Wang Z, Chen W, Zhang X. et al. A long way to the battlefront: CAR T cell therapy against solid cancers. J Cancer. 2019;10:3112-3123
181. Newick K, O'Brien S, Moon E. et al. CAR T Cell Therapy for Solid Tumors. Annu Rev Med. 2017;68:139-152
182. Zhu W, Peng Y, Wang L. et al. Identification of α-fetoprotein-specific T-cell receptors for hepatocellular carcinoma immunotherapy. Hepatology. 2018;68:574-589
183. Sun L, Guo H, Jiang R. et al. Engineered cytotoxic T lymphocytes with AFP-specific TCR gene for adoptive immunotherapy in hepatocellular carcinoma. Tumour Biol. 2016;37:799-806
184. Docta RY, Ferronha T, Sanderson JP. et al. Tuning T-Cell Receptor Affinity to Optimize Clinical Risk-Benefit When Targeting Alpha-Fetoprotein-Positive Liver Cancer. Hepatology. 2019;69:2061-2075
185. Luo X, Cui H, Cai L. et al. Selection of a Clinical Lead TCR Targeting Alpha-Fetoprotein-Positive Liver Cancer Based on a Balance of Risk and Benefit. Front Immunol. 2020;11:623
186. Li Z, Gong H, Liu Q. et al. Identification of an HLA-A*24:02-restricted α-fetoprotein signal peptide-derived antigen and its specific T-cell receptor for T-cell immunotherapy. Immunology. 2020;159:384-392
187. Cai L, Caraballo Galva LD, Peng Y. et al. Preclinical Studies of the Off-Target Reactivity of AFP(158)-Specific TCR Engineered T Cells. Front Immunol. 2020;11:607
188. Kloss CC, Condomines M, Cartellieri M. et al. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat Biotechnol. 2013;31:71-5
189. Barrett DM, Grupp SA, June CH. Chimeric Antigen Receptor- and TCR-Modified T Cells Enter Main Street and Wall Street. J Immunol. 2015;195:755-61
Author contact
![]() Corresponding author: Xiao Xu, Department of Hepatobiliary and Pancreatic Surgery, The Center for Integrated Oncology and Precision Medicine, Affiliated Hangzhou First People's Hospital, Zhejiang University School of Medicine, Hangzhou 310006, China. Tel: 13588191177. E-mail: zjxuedu.cn.
Corresponding author: Xiao Xu, Department of Hepatobiliary and Pancreatic Surgery, The Center for Integrated Oncology and Precision Medicine, Affiliated Hangzhou First People's Hospital, Zhejiang University School of Medicine, Hangzhou 310006, China. Tel: 13588191177. E-mail: zjxuedu.cn.

 Global reach, higher impact
Global reach, higher impact