ISSN: 1449-2288
Int J Biol Sci 2022; 18(4):1434-1450. doi:10.7150/ijbs.57178 This issue Cite
Research Paper
CRL4-DCAF8L1 Regulates BRCA1 and BARD1 Protein Stability
1. Department of Cell Biology, School of Basic Medical Sciences, Peking University Health Science Center, Beijing, 100191, China
2. Department of Pathology, School of Basic Medical Sciences, Peking University Health Science Center, Beijing, 100191, China
3. Department of Biochemsitry; School of Basic Medical Sciences, Peking University Health Science Center, Beijing, 100191, China
4. Medical and Health Analysis Center, Peking University, Beijing, 100191, China
5. Department of Pathology, Weifang Medical University, Shandong, 261041, China
6. The Irma H. Russo, MD Breast Cancer Research Laboratory, Fox Chase Cancer Center-Temple University Health System, Philadelphia, PA, 19111, USA
# These authors contribute equally to the article
Abstract
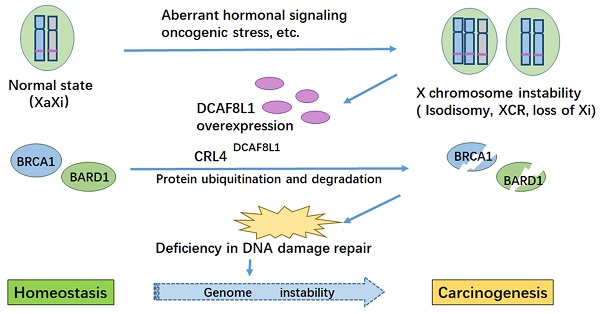
BRCA1 is frequently down-regulated in breast cancer, the underlying mechanism is unclear. Here we identified DCAF8L1, an X-linked gene product, as a DDB1-Cullin associated Factor (DCAF) for CUL4 E3 ligases to target BRCA1 and BARD1 for proteasomal degradation. Forced expression of DCAF8L1 caused reduction of BRCA1 and BARD1, and impaired DNA damage repair function, conferring increased sensitivity to irradiation and DNA damaging agents, as well as Olaparib, a PARPi anticancer drug; while depletion of DCAF8L1 restored BRCA1 and suppressed the growth of its xenograft tumors. Furthermore, the expression of DCAF8L1 was induced in human H9 ES cells during transition from primed to naïve state when Xi chromosome was reactivated. Aberrant expression of DCAF8L1 was observed in human breast fibroadenoma and breast cancer. These findings suggest that CRL4DCAF8L1 is an important E3 ligase that may participate in the development of breast cancer, probably through regulating the stability of BRCA1 and BARD1 tumor suppressor, linking BRCA1 and X chromosome inactivation to breast carcinogenesis.
Keywords: BRCA1, BARD1, DCAF8L1, ubiquitination, breast cancer, X chromosome inactivation

 Global reach, higher impact
Global reach, higher impact