10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(5):1912-1932. doi:10.7150/ijbs.68484 This issue Cite
Research Paper
Metabolomics reveals that CAF-derived lipids promote colorectal cancer peritoneal metastasis by enhancing membrane fluidity
1. Department of Colon and Rectum Surgery, The Sixth Affiliated Hospital (Guangdong Gastrointestinal and Anal Hospital), Sun Yat-sen University, 26 Yuancun Erheng Road, Guangzhou, Guangdong, 510655, China.
2. Guangdong Provincial Key Laboratory of Colorectal and Pelvic Floor Disease, The Sixth Affiliated Hospital (Guangdong Gastrointestinal and Anal Hospital), Sun Yat-sen University, Guangzhou, Guangdong, 510655, China.
3. Department of Clinical Laboratory, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, 510655, China.
4. Sun Yat-sen University Metabolomics Center, Guangzhou, Guangdong, 510080, China.
#These authors contributed equally to this work.
Abstract
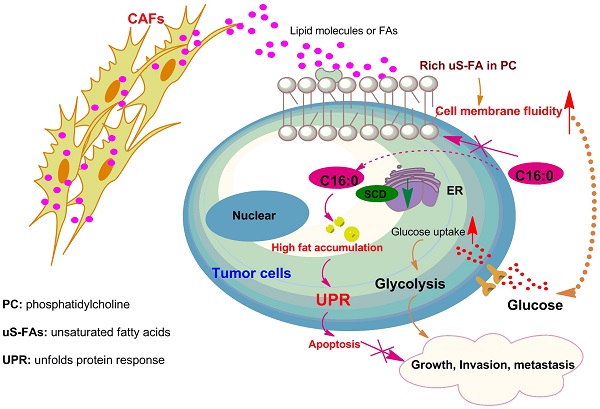
Patients with peritoneal metastasis (PM) of colorectal cancer (CRC) have poorer overall survival outcomes than those without PM. Cancer-associated fibroblasts (CAFs) are a major component of the tumor microenvironment and mediate CRC progression and PM. It is imperative to identify and develop novel therapeutic targets for PM-CRC driven by CAFs. Using lipidomics, we reveal that the abundance of phosphatidylcholine (PC) with unsaturated acyl chains was increased in clinical PM-CRC specimens. Additionally, we found that CAFs were present at a higher relative abundance in primary PM-CRC tumors and that membrane fluidity in CRC cells was increased after incubation with CAF-conditioned medium (CM) through three independent methods: lipidomics, fluorescence recovery after photobleaching (FRAP), and generalized polarization. Then, we found that increased membrane fluidity can enhance glucose uptake and metabolism, as supported by real-time bioenergetics analysis and U-13C glucose labeling. Interestingly, stearoyl-CoA desaturase 1 (SCD), the rate-limiting enzyme in the biosynthesis of unsaturated fatty acids (uS-FAs), was expressed at low levels in PM and associated with poor prognosis in CRC patients. Importantly, by untargeted metabolomics analysis and fatty acid ([U-13C]-stearic acid) tracing analyses, we found that CRC cells take up lipids and lipid-like metabolites secreted from CAFs, which may compensate for low SCD expression. Both in vitro and in vivo experiments demonstrated that sodium palmitate (C16:0) treatment could decrease the CAF-induced change in cell membrane fluidity, limit glucose metabolism, suppress cell invasiveness, and impair tumor growth and intraperitoneal dissemination. An increased C16:0 concentration was shown to induce apoptosis linked to lipotoxicity. Furthermore, C16:0 effectively enhanced the antitumor activity of 5-fluorouracil (5-FU) in vitro and was well tolerated in vivo. Taken together, these findings suggest that adding the saturated fatty acid (S-FA) C16:0 to neoadjuvant chemotherapy may open new opportunities for treating PM-CRC in the future.
Keywords: Lipidomics, colorectal cancer peritoneal metastasis, cancer-associated fibroblasts (CAFs), glucose metabolism, C16:0, metabolomics

 Global reach, higher impact
Global reach, higher impact