10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(6):2392-2405. doi:10.7150/ijbs.70620 This issue Cite
Research Paper
GPR125 positively regulates osteoclastogenesis potentially through AKT-NF-κB and MAPK signaling pathways
1. Division in Cellular and Molecular Medicine, Department of Pathology and Laboratory Medicine, Tulane University School of Medicine, Tulane University, New Orleans, Louisiana, USA.
2. Department of Pathology, School of Medicine, University of Alabama at Birmingham, Birmingham, Alabama, USA.
3. Engineering Research Center of Molecular & Neuroimaging, Ministry of Education, School of Life Science and Technology, Xidian University, Xi'an, Shaanxi 710126, China.
*Contributed equally to this work
Abstract
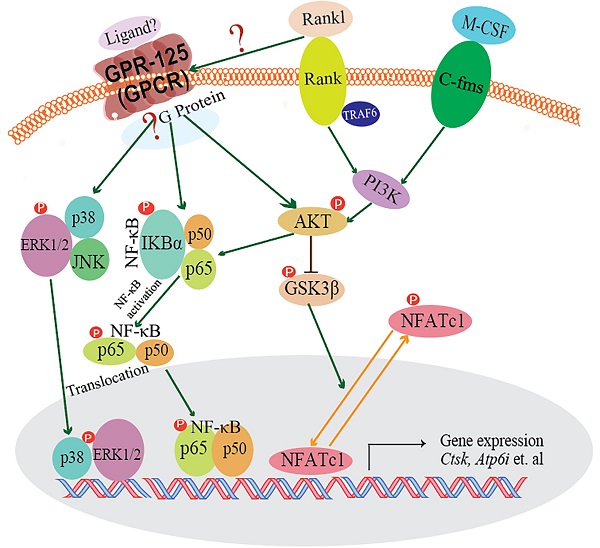
G-protein-coupled receptors (GPCRs) signaling is critical to cell differentiation and activation. However, the function of GPCRs in osteoclast differentiation and activation remains unclear. We found that the G-protein coupled receptor 125 (GPCR 125) gene (Gpr125) gene was highly expressed in osteoclasts through RNA-sequencing technology, qRT-PCR, and Western blot analysis. We characterized the role of GPCR125 in osteoclast differentiation and activation by loss-of-function and gain-of-function methods in osteoclasts. Osteoclasts with lentivirus-mediated GPR125 silencing demonstrated a dramatic reduction in differentiation and impaired bone resorption function. In contrast, overexpression of Gpr125 in osteoclasts increased NFATC1 expression and enhanced osteoclast differentiation and enhanced osteoclast-mediated bone resorption. These results indicated that GPCR125 positively regulates osteoclast formation and function. Following receptor activator of nuclear factor kappa-Β ligand (RANKL) stimulation, the expression levels of MAPK signaling pathway proteins phosphorylated-ERK (p-ERK) and phosphorylated-p38 (p-p38) were significantly decreased in the Gpr125 knockdown (sh-GPR125) group compared to its control group. We also found that phosphorylated AKT (p-AKT) expression was downregulated, as well as nuclear factor kappa-B (NF-κB) signaling pathway protein phosphorylated-IKB alpha (p-IKBα). Our results demonstrated that GPCR125 positively regulates osteoclasts via RANKL-stimulated MAPK and AKT-NF-κB signaling pathways, and GPCR125 could potentially be utilized as a novel therapeutic target in bone related diseases including osteoporosis.
Keywords: GPCR 125, Gpr125, osteoclasts, bone resorption, Akt signaling pathway, NFκB signaling pathway, MAPK signaling pathway

 Global reach, higher impact
Global reach, higher impact