Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(6):2406-2418. doi:10.7150/ijbs.70629 This issue Cite
Review
Immunologic Memory in Pregnancy: Focusing on Memory Regulatory T Cells
1. Institute of Reproductive Health, Center for Reproductive Medicine, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, P.R. China.
2. Department of Obstetrics and Gynecology, Liyuan Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, P.R. China.
3. Department of Obstetrics and Gynaecology, Faculty of Medicine, The Chinese University of Hong Kong, Prince of Wales Hospital, Shatin, Hong Kong, P.R. China.
4. C.S. Mott Center for Human Growth and Development, School of Medicine, Wayne State University, Detroit, MI, USA.
# These authors have contributed equally to this work.
Received 2021-12-31; Accepted 2022-2-17; Published 2022-3-6
Abstract
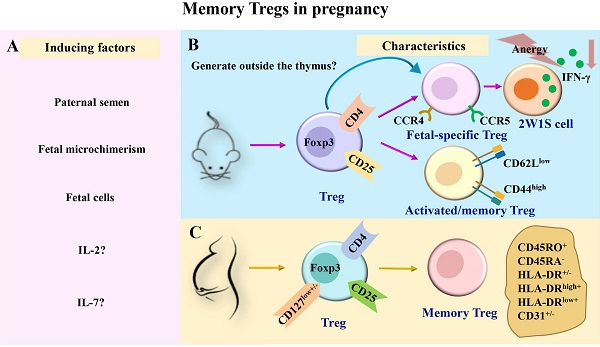
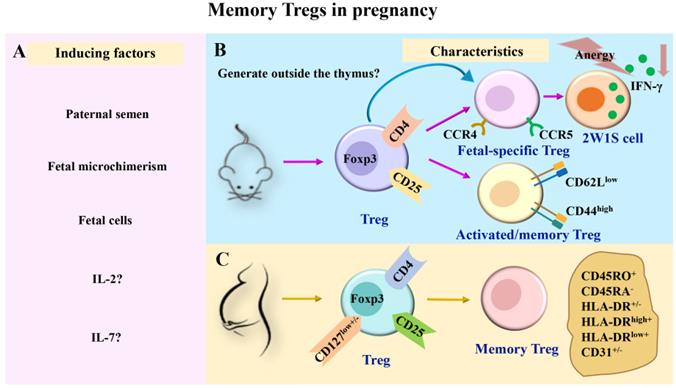
A successful pregnancy requires the maternal immune system to tolerate an allogeneic fetus. The incidence of preeclampsia and other complications related to impaired fetal tolerance is lower during the second pregnancy than during the first pregnancy. At the same time, compared with normal pregnant women in the previous pregnancy, patients with pregnancy complications in the previous pregnancy also have an increased risk of the disease when they become pregnant again. This difference may be related to the immunological memory of pregnancy. Regulatory T cells (Tregs) are immunosuppressive CD4+ T cells that play a predominant role in maintaining immune tolerance. In addition, Tregs possess immunological memory properties, including fetal or paternal-specific memory Tregs and Tregs expressing memory cell makers, forming an immunoregulatory memory against fetal antigens. In this review, we provide an overview of the characteristics of memory Tregs in pregnancy, evidence regarding the existence of memory Tregs in human pregnancy, as well as in mouse models. We also discuss the mechanism of memory Tregs induction, maintenance, and action. In addition, we described their changes during the first pregnancy, second pregnancy, postpartum, and pathological pregnancy in order to provide new targets for the diagnosis and treatment of pregnancy related diseases.
Keywords: regulatory T cells, memory, pregnancy, preeclampsia, gestational diabetes mellitus
Introduction
"Immune memory" implies that during the process of immunity, following reaction with a specific antigen, contact, and stimulation by the same antigen can quickly initiate secondary immunity to induce a stronger immune response. The pregnancy outcomes of secondary pregnancies are more favorable than that of primary pregnancies. The incidences of fetal growth restriction, fetal death, and low birth weight were lower in subsequent pregnancies [1, 2]. Simultaneously, the incidence of preeclampsia (PE) and other complications associated with impaired fetal tolerance in multiparas were lower than those in nulliparas [3, 4]. Multiparas are usually defined as women who have given birth at least twice to an infant, and nulliparas are defined as women who have never given birth. However, a second pregnancy with a different partner has an incidence similar to that of the first pregnancy [5, 6]. This might be due to immune memory during pregnancy. Thus, multiparous women may "remember" the antigen of the previous pregnancy and rapidly produce "protective" reactions such as tolerance when they are pregnant again to achieve a more favorable pregnancy outcome.
With the increase of the number of pregnancy losses, the incidence of re-abortion increases. Compared with normal pregnant women in the previous pregnancy, patients with pregnancy complications in the previous pregnancy also have an increased risk of the disease when they become pregnant again. This further shows that good pregnancy memory may lead to a good pregnancy outcome, while bad pregnancy memory may lead to adverse pregnancy outcomes. Recent studies have revealed that some immune memory cells are present in the decidua and peripheral blood during pregnancy, including B cells [7-9], T cells [1, 10-12], and natural killer (NK) cells [13, 14]. These cells expand rapidly after pregnancy, especially after the second pregnancy, and play different roles during pregnancy.
Regulatory T cells (Tregs) are immunosuppressive CD4+ T cells that play a pivotal role in maintaining self-tolerance by preventing immune responses against autoantigens [15, 16]. In mice, the number of Tregs selectively accumulates after pregnancy and remains at a high level for a long time after delivery, forming an immunomodulatory memory for fetal antigens. Moreover, the accelerated expansion of Tregs during the second pregnancy was almost entirely driven by the proliferation of fetal-specific forkhead box P3+ (Foxp3+) cells retained in the previous pregnancy. These fetal-specific Tregs can detect functional loss, which may be beneficial for maintaining pregnancy immune tolerance [17]. In humans, memory Tregs have also been found during early pregnancy [18]. Therefore, the importance of memory Tregs during pregnancy has been widely concerned and worthy of discussion.
The present review aimed to furnish novel research directions and possible therapeutic targets to further investigate memory Tregs in normal pregnancy and pregnancy-related complications. Herein, we summarized evidence of memory Tregs in humans and mouse models during pregnancy, the generation and maintenance of memory Tregs during pregnancy, and the role of memory Tregs in normal and pathological pregnancies.
Overview of Tregs in pregnancy
A successful pregnancy requires the maternal immune system to tolerate an allogeneic fetus [19, 20]. Disorders impacting immune tolerance may result in pregnancy loss, fetal growth restriction, premature birth, and PE [21-23]. Present in both the basal decidua and parietal decidua at the maternal-fetal interface [24, 25], Tregs cells are involved in the regulation of autoimmunity and tolerance toward the fetus by suppressing maternal immune responses [26-29]. The expansion of Tregs starts extremely early during pregnancy [30]. After this early increase, decidual Tregs remained elevated in the second trimester and then reduced before birth [31, 32].
In mice, the absence of Tregs in the first trimester of pregnancy can lead to pregnancy failure due to fetal immune rejection, either antibody-related Tregs depletion or CD25 depletion [33, 34]. Indeed, in the abortion-prone model of CBA/J × DBA/2 [35], it was observed that the embryo resorption rate increased, along with the simultaneous exhaustion of Tregs [10]. The adoptive transfer of Tregs from normal pregnant mice can prevent fetal rejection [36] and significantly reduce the fetal absorption rate [37, 38]. In addition to immune tolerance during the first trimester, depletion of functional Tregs in the third trimester can lead to premature delivery and adverse neonatal outcomes, which can be rescued by the adoptive transfer of Tregs [39].
In humans, the same phenomenon has been observed. A high proportion of Tregs cells is present in the peripheral blood and decidua of early pregnancy [40], expressing CD152 (cytotoxic T-lymphocyte-associated protein-4, CTLA-4). This increased Tregs cells number plays an important role in immune tolerance by effectively inhibiting the proliferation of autologous T cells [41]. In contrast, a lack of Tregs can result in pregnancy failure. The proportion of CD4+CD25bright T cells in the decidua of women with spontaneous abortion was significantly lower than that in women with induced abortion [41]. The frequency of Foxp3+CD4+ T cells in miscarriage with a normal embryo was significantly lower than those in miscarriage with an abnormal embryo [42]. In normal fertile non-pregnant women, peripheral Tregs expand in the late follicular phase, followed by a dramatic decrease in the luteal phase. However, the number of Tregs in women with recurrent spontaneous abortion was similarly low during follicular and luteal phases. In addition, their function was reportedly reduced [43]. Through the expression of protective factors including interleukin (IL) -10, transforming growth factor-β (TGF-β), heme oxygenase-1, indoleamine 2,3-dioxygenase, leukemia inhibitory factor, and negative costimulatory molecule (like CTLA-4, TIGIT) [44], Tregs at the maternal-fetal interface create a tolerant microenvironment and play an important role in avoiding fetal allograft rejection.
Memory Tregs in mice models
Appropriate animal models help explore the mechanisms of Tregs during pregnancy memory. Rosenblum et al. [45] first found Treg memory in transgenic mice. They crossed transgenic mice expressing a membrane-bound form of Ovalbumin (Ova) under the control of a tetracycline response element to transgenic mice expressing the tetracycline transactivator protein under the control of the keratin 5 promoters. Then the model antigen, Ova, could be inducibely expressed within the skin. It was further found that exposure to tissue autoantigen could lead to the activation of autoreactive Treg cells produced by autoantigen expression in the thymus. Activated Treg cells persist in target tissues and inhibit autoimmune response when repeatedly or chronically exposed to tissue autoantigens. In mice, the key challenge to examine Tregs in pregnancy memory is distinguishing between fetal and paternal-specific Tregs. I-Ab2W1S55-68 peptide and β-actin were co-expressed in transgenic male mice and then conceived with non-2W1S-expressing B6 females. The I-Ab2W1S55-68 peptide can replace fetal antigens. Endogenous maternal Tregs that replaced fetal antigens could be identified by major histocompatibility complex (MHC) class II tetramer enrichment. 2W1S+ Tregs are considered fetal-specific Tregs [17].
Another method to identify paternal antigen-specific Tregs is to use the MLS-1a superantigen for Treg expression on DBA/2 cells and recognition by the T cell receptor Vβ6. Tregs expressing Vβ6 are considered MLS-1a -specific Tregs [46]. These are called PA-specific Tregs (CD4+Foxp3+Vβ6+), which are considered paternal antigen-specific Tregs [47]. Although the second method has not been used to evaluate pregnancy memory, using this method, the authors put forward unique views regarding the effect of semen exposure on paternal alloantigen reactivity.
In diphtheria toxin receptor (DTR) mice, the target cells express DTR through gene editing and are conditionally eliminated by injecting diphtheria toxin (DT). In 2012, Rowe et al. [17] first applied this model to evaluate fetal-specific memory Tregs during pregnancy in mice. Foxp3DTR/DTR transgenic mice were first constructed, and then Foxp3+-specific ablation mice were obtained by DT injection. The authors also adoptively transferred CD45.1CD4 cells from Foxp3DTR/DTR mice donors to CD45.2CD4 receptor mice. Next, CD45.1Foxp3+CD4 cells in mice were specifically eliminated postpartum to assess the critical role of fetal-specific Tregs produced during pregnancy. This mouse model was also used in a subsequent study of Tregs in idiopathic preterm birth. Interestingly, the authors established a new mouse model by intraperitoneally injecting Foxp3DTR at 14.5 days postcoital (dpc) with 25 or 50 μg/kg DT, followed by 5 or 50 μg/kg/day DT 24 h later until delivery. The objective was to create a partial or total Foxp3 depletion model during pregnancy, where partial Foxp3 depletion during the second trimester approximated premature delivery in mice [39]. This provides critical insight. As DT depletion is transient, a mouse model with partial or total Foxp3 depletion, induced by injecting DT at different doses, may be more conducive for assessing the effects of Tregs at different pregnancy stages, as well as for constructing a more appropriate animal model. Furthermore, it may help investigate the role of memory Tregs during different stages of pregnancy.
Given ethical restrictions during human pregnancy assessments, current research remains focused on elucidating changes and functions of memory Tregs in peripheral blood during different pregnancy periods. Specific markers for fetal-specific Tregs are scarce. In addition, reports on altered Treg levels in the decidua during different gestational periods are lacking.
Characteristics of memory Tregs in pregnancy
Based on the literature, memory Tregs in pregnancy are mainly classified by two strategies. One is to classify memory Tregs by adding special memory markers to Tregs. This classification is predominantly based on the experience of memory T cells. The second strategy involves the inclusion of fetal or paternal-specific Tregs, which reportedly play an extremely important role in immune tolerance and memory of pregnancy.
Naïve T cells express CD45RA+, and memory T cells express CD45RO+ [48]. Memory T cells can rapidly differentiate into effector cells and new memory T cells after antigenic stimulation [49]. According to their locations and functions, they can be divided into central memory T cells (TCM), effector memory T cells (TEM), and tissue-resident memory T cells (TRM). TCM expresses chemokine receptor (CCR) 7+ in secondary lymphoid organs, but not in non-lymphoid tissues [50] such as lymph nodes and spleen. The other two memory T cells are CCR7- and are expressed in non-lymphoid tissues such as the skin, lung, or intestine [51]. After TCM activation, CD69 (a marker of activated T cells) is expressed on the cell surface, and TCM cells irreversibly lose CCR7 expression and proliferate to TEM [48]. Therefore, CCR7 is typically employed to distinguish between central and effector subtypes of memory T cells.
Recent studies have confirmed the presence of TregEM (CD25+CD45RA-CD62L-) and TregCM (CD25+CD45RA-CD62L+) in peripheral blood [52, 53], with significant differences in functions. The frequency and function of TregEM are reportedly suppressed in patients with delayed fracture union when compared with those of TregEM in normal patients. TregEM expressed more TGF-β and IL-10 when stimulated, which may be beneficial for the occurrence of anti-inflammatory effects [52]. However, evidence indicating the presence of TregEM and TregCM in human pregnancy is lacking, further studies are needed to show the existence, changes, and function of TregEM and TregCM in human pregnancy.
In mice, when the immune system recognizes tumor cells, CD44hi memory Tregs rapidly establish immune tolerance. The relative speed of these Tregs and effector T cells (Teffs) reaction determines the result of the antitumor immune response: tolerance or rejection [54]. Chen et al. [10] defined CD4+Foxp3+CD44highCD62Llow as activated/memory Tregs (amTregs) in the draining lymph node (dLN) during pregnancy. AmTregs were found to be present in both lymph nodes draining deep tissues and peripheral blood in non-pregnant mice [55, 56]. These cells can recognize autoantigens and avoid the development of autoimmune diseases [57]. Fetal and tumor microenvironments have similar immune tolerance mechanisms. Through the overall comparison of fetal and tumor microenvironments by mouse transcriptome, it is revealed strikingly similar in the expression of numerous immune-related pathways, including dynamic upregulation of Treg-related pathways in the pregnant uterus[58]. In addition, amTregs have the advantage of regulating the immune response of antitumor and anti-fetal effects, which is beneficial for embryo implantation and inducing immune tolerance during pregnancy[10, 54].
In human pregnancies, studies included memory Tregs (CD45RA-CD45RO+) and memory Treg subsets differentiated by human leukocyte antigen (HLA)-DR+/- and CD31+/- [18, 59-61]. HLA-DR is a type of HLA class II molecule, the others being HLA-DP and HLA-DQ and they are expressed on many antigens presenting cells, including monocytes, dendritic cells, and B cells. HLA-DR plays a role in antigen presentation [62]. And HLA-DR+ is typically employed as an activation marker for T cells and Tregs [63]. HLA-DR+ Tregs were found to express higher levels of Foxp3 and induce stronger inhibition than Tregs with HLA-DR- [64]. Schober et al. [59] divided the total CD4+CD127low+/-CD25+ Foxp3+ Tregs pool into four different Treg subgroups, including naïve Tregs (nTregs, CD45RA+ Tregs), DR-Tregs (HLA-DR-CD45RA- memory Tregs), DRlow+ Tregs (HLA-DRlow +CD45RA- memory Tregs), and DRhigh+ Tregs (HLA-DRhigh+CD45RA- memory Tregs).
CD31 was also used during human pregnancy to define memory Treg subsets, including CD31+ and CD31- memory Tregs [60]. CD31 is a platelet endothelial cell adhesion molecule-1, expressed on the surface of human granulocytes, monocytes, and platelets. Moreover, this molecule is enriched at the junction of the endothelial cells [65]. CD31 may be involved in leukocyte migration, angiogenesis, and integrin activation. In addition, its interaction with a heterophilic counter receptor on T cells can interfere with T cell activation and inhibit the response of T cells [66].
During pregnancy, some fetal or paternal-specific Tregs have been shown to possess memory. As early as 2008, Tilburgs et al. [67] reported that compared with peripheral blood, CD4+CD25bright T cells in full-term basal decidua and parietal decidua significantly enhanced the inhibition of fetal-specific umbilical cord blood cells. However, no difference was observed in the inhibition of third-party umbilical cord blood cells. The authors suggested that CD4+CD25bright T cells in maternal peripheral blood had specific "memory" to fetal-specific umbilical cord blood cells. In 2012, Rowe et al. [17] reported, for the first time, that repeated pregnancy initiated the accelerated accumulation of maternal Foxp3+ cells in pregnant mice. These cells were derived from pre-existing fetal-specific maternal Tregs retained from previous pregnancies, persisting post-delivery and maintaining the protective and regulatory memory of fetal antigens. Moreover, CD44 (expressed on activated and memory T cells) was increased in fetal-specific CD4+ T cells and maintained a 10-fold increase when compared with the non-pregnant control for 100 days after delivery. Subsequently, Gomez-Lopez et al. [39] determined the role of Tregs in preterm birth and suggested that complete depletion of Tregs in the second pregnancy had a more deleterious effect on neonatal survival than depletion during the first pregnancy. The authors concluded that pregnancy could imprint protective and regulatory memory in Tregs.
At present, in mice and humans, the reported memory Tregs during pregnancy include fetal-specific Tregs, amTregs, DR-Tregs, DR+Tregs, DRlow+ Tregs, DRhigh+ Tregs, CD31+ memory Tregs, CD31- memory Tregs. Table 1 summarizes the research on memory Tregs during pregnancy. It includes the grouping methods of memory Tregs, surface markers, material location, the proportion of different memory Tregs in healthy women, and the main conclusions of the study.
Memory Tregs in normal pregnancy
The changes in immune memory cells at different stages of pregnancy might help elucidate the function of immune memory cells during different stages of pregnancy. In mice, Rowe et al. [17] revealed that in spleen and lymph node cells, the fetal-specific Tregs increased with progressing pregnancy and peaked at 48 h postpartum, attaining levels 100 times higher than those observed before pregnancy; this was followed by a gradual decrease slowly that could be detected until day 100 post-delivery. Moreover, 2W1S+Foxp3+ cells (considered to be fetal-specific Tregs) upregulated Ki67, a proliferation marker during pregnancy. Although Ki67 expression decreased post-delivery, the proportion of 2W1S+Foxp3+ cells on day 100 post-delivery remained twice that observed before pregnancy. This finding may be related to the mechanism via which fetal-specific maternal Tregs proliferate during pregnancy and persist after delivery [17]. In the uterus of allogenic female BALB/c, the number and frequency of amTregs from day post-implantation (dpi) 6 to dpi 10 continued to increase in dLN, but not in non-dLN, when compared with the non-pregnant virgin controls [10].
In humans, HLA-DR+ memory Tregs exhibited significantly decreased inhibitory activity in the peripheral blood of normal pregnant women when compared with that in non-pregnant women. However, no significant differences were observed in HLA-DR- memory Tregs [18]. Treg subsets in the peripheral blood of five women with successful in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) pregnancy were analyzed from the beginning of pregnancy, revealing that nTregs transformed into DR+ and DR- memory Tregs. The transformation peaked at approximately seven weeks of pregnancy and then reversed until term [18]. During the early stages of pregnancy, trophoblast invasion and blood vessel formation mainly occur at the maternal-fetal interface, while Tregs inhibit inflammation and support maternal vascular adaptation to promote trophoblast invasion and placenta into the maternal blood supply [32]. Thus, the increase in memory Tregs during early pregnancy may play a crucial role in early embryo implantation and development. The study by Schlossberger et al. [18] also discovered an interesting phenomenon, in which the total Tregs were not altered with age, but subsets of DR+ and DR- memory Tregs increased with age. As these changes occurred simultaneously with age-related fertility decline, it appears that in addition to ovarian aging, age-related changes in the Treg pool composition might be related to female fertility loss. In addition, compared with non-pregnant women, there was a decrease in the percentage of DRhigh+ CD45RA- and DRlow+CD45RA- Tregs and an increase in the percentage of naïve DR-CD45RA+ Tregs during normal pregnancy from 10 to 20 weeks. These Treg subgroups remained stable until the term. Among them, DRhigh+CD45RA- Tregs showed stronger inhibitory activity [68]. In addition, this phenomenon is reversed at the initiation of natural-term delivery [69].
In a study assessing CD31+ memory Tregs, at the beginning of pregnancy, the proportion of recent thymic emigrant-regulatory T cells (RTE-Tregs, CD45RA+CD31+ Tregs) and CD31+ memory Tregs (CD45RA-CD31+ Tregs) decreased significantly, while the proportion of mature nTregs (CD45RA+CD31- Tregs) remained unaltered. Furthermore, the complementarity of CD45RA-CD31-memory Tregs increased [60]. CD31+ is mainly expressed in CD45RA+ T cells, which interferes with T cell activation. This may underlie the decreased proportion of CD31+ memory Tregs during pregnancy. Compared with non-pregnant women, CD4+CD45RA-Foxp3++ Tregs and CD4+CD45RA+Foxp3+ Tregs decreased during pregnancy (20w and 32w) and increased on day 4 post-delivery; however, the level remained lower than that in non-pregnant women [61].
In conclusion, during the early pregnancy, CD31+ memory Tregs decreased, but the DR+ Tregs, DR- Tregs, CD31- memory Tregs in humans and amTregs, fetal-specific Tregs in mice increased. During the second and third trimesters of pregnancy, fetal-specific Tregs in mice increased, and DR+ Tregs decreased in humans. The changes in memory Tregs during and after pregnancy in mice and humans are shown in Table 2.
Generation and maintenance of memory Tregs during pregnancy
All lymphocytes, especially those in the T cell lineage, have a sequence from naïve to effector to memory [70]. Different T cell subsets are activated through distinct pathways. CD8+ T cells can persist as self-renewal and numerically stable cell populations, meeting the most stringent definition of "memory." In contrast, the maintenance of CD4+ T cells is considered unstable and usually requires sustained low-level antigen stimulation [71, 72].
Thornton et al. [73] demonstrated that Helios is expressed in all thymocytes in the double negative stage 2 of thymic development, and may provide a basis for the source of Tregs. It has been reported that the expression of Helios (also known as IKZF2) in maternal Tregs with 2W1S fetal-specificity was gradually downregulated, decreased to as low as 40% during the third trimester of pregnancy, and then slowly increased post-delivery [17]. Moreover, it provides potential perspectives for the source of fetal-specific Tregs [17].
Summary of the studies on memory Tregs in pregnancy
| Grouping | Markers of memory Tregs | Sampling times and sources for memory Tregs | Proportion of memory Tregs in normal pregnancy | Main conclusions | References |
|---|---|---|---|---|---|
| ♀B6 ×♂Balb/c-2W1S; ♀B6 ×♂B6-2W1S; ♀B6 ×♂Balb/c. | CD4+2W1S+ Foxp3+ | Virgin; E 11.5; E 18.5; PP 2; PP 14; PP 30; PP 100 (in spleen and lymph nodes) | (Percentage of Foxp3+ among 2W1S+CD4+ cells) Virgin: 7%; E11.5: 21.1%; E18.5: 45.1%; PP2: 60.1%; PP14: 20.1%; PP30: 19.6%; PP100: 18.4%. | Pregnancy imprints Foxp3+CD4 cells to maintain the protective regulatory memory to the fetal antigen. | [17] |
| ♀Balb/c ×♂B6 | amTregs: CD4+Foxp3+CD44highCD62Llow | dpi 1; dpi 4; dpi 6; dpi 7; dpi 10; dpi 12 (in dLN and ndLN) | In dLN, the frequency of amTregs from dpi 6 to dpi 10 increased. | Early recruitment of amTregs in uterine dLNs was triggered by embryo implantation. | [10] |
| ♀Foxp3DTR ×♂BALB/C; Tregs depletion with DT. | Tregs: CD45+CD3+CD4+CD25+Foxp3+ | Delivery; 1w, 2w, and 3 w of postpartum (in decidua, myometrium, peripheral blood, placenta) | None. | The enhanced Tregs expansion during the second pregnancy was related to maternal-fetal tolerance, as well as the health of the newborn. | [39] |
| Patients undergoing IVF / ICSI: Pregnancy group (n = 36); Nonpregnant group (n = 160). | DR+ Tregs: D45RA-HLA-DR+ Tregs; DR- Tregs: CD45RA-HLA-DR- Tregs | 1 hour before embryo transfer; 7w; 14w; 21w; 28w (in peripheral blood) | 1 hour before embryo transfer in pregnancy group (Percentage in total Tregs); DR+ Tregs: 27.0%; DR- Tregs: 30.1%. | Compared with that in women with successful pregnancy, the percentage of DR-Tregs was increased in non-pregnant women. | [18] |
| Healthy pregnant women (n = 64); Dietary-adjusted GDM ( n = 21); Insulin-dependent GDM (n = 40). | DR- Tregs: CD45RA-HLA-DR-Tregs; DRlow+ Tregs: CD45RA-HLA-DRlow+ Tregs; DRhigh+ Tregs: CD45RA-HLA-DRhigh+Tregs. | 24 - 41 w (in peripheral blood) | Healthy pregnant group (Percentage in total Tregs): DR+ Treg: 22.8%; DRlow+ Treg: 20.3%; DRhigh+ Treg: 2.5%; DR-Treg: 29.9% . | ① The percentage of DR-Tregs was significantly higher in patients with dietary-adjusted GDM than that in healthy pregnancies; ② The percentages of DRlow+ Tregs and DRhigh+ Tregs were significantly higher in patients with insulin-dependent GDM than those in healthy pregnancies. | [59] |
| Non-pregnant women (n=31); Healthy pregnant women (n=169); PE ( n = 37). | CD31+ memory Tregs: CD45RA-CD31+ Tregs; CD31- memory Tregs: CD45RA-CD31- Tregs (Tregs: CD4+CD127low+/-Foxp3+). | 1st trimester; 2nd trimester; 3rd trimester; term (in peripheral blood) | 3rd trimester (Percentage in total Tregs): CD31+memory Tregs: 4%; CD31-memory Tregs: 66%. | ① At the beginning of pregnancy, RTE-Tregs differentiated into CD31-memory Tregs and were maintained until term delivery. ② In PE group, CD45RA-CD31+ memory Tregs were significantly increased. | [60] |
| Non-pregnant fertile women (n = 31); Healthy pregnant women (n = 135) ; PE (n = 27); HELLP (n = 15) ; CI (n = 30); PL (n = 24). | CD45RA-HLA-DR- Tregs; CD45RA-HLA-DRlow+ Tregs; CD45RA-HLA-DRhigh+ Tregs. | 24-42 weeks' gestation (in peripheral blood) | Healthy pregnant group (Percentage in Tregs): CD45RA-HLA-DR-Treg: 30.4%; CD45RA-HLA-DRlow+Treg: 23.6%; CD45RA-HLA-DRhigh+Treg: 4.8%. | PE and PL were characterized by distinct Treg subsets accompanied by a significant decrease in their suppressive activity. | [68] |
Note: Tregs not specifically defined in the table are CD4+CD127low+/-CD25+Foxp3+. amTregs: activated/memory Tregs; CI: cervical insufficiency; dLN: draining lymph node; dpi: day postimplantation; DT: diphtheria toxin; DTR: diphtheria toxin receptor; DR+ Tregs: HLA-DR+ memory Tregs; DR- Tregs: HLA-DR- memory Tregs; DRhigh+ Tregs: HLA-DRhigh+ memory Tregs; DRlow+ Tregs: HLA-DRlow+ memory Tregs; E: embryonic day; Foxp3+: forkhead box P3+; GDM: gestational diabetes mellitus; HELLP: haemolysis-elevated liver enzyme levels-low platelet count syndrome; IVF/ICSI: in vitro fertilization/intracytoplasmic sperm injection; ndLN: non-dLN; PE: preeclampsia; PL: preterm labour necessitating preterm delivery; PP: post-partum day; RTE-Tregs: recent thymic emigrant-regulatory T-cells; Tregs: Regulatory T cells; w: weeks of gestation; 1st trimester: the first trimester; 2nd trimester: the second trimester; 3rd trimester: the third trimester.
Changes of memory Tregs in healthy and pathological pregnancies
| Memory Tregs | Mouse or human | During normal pregnancy | PE | Dietary GDM | Insulin GDM | HELLP | PL | Failure of ART | References | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st trimester | 2nd trimester | 3rd trimester | |||||||||
| amTregs | mouse | ↑ | [10] | ||||||||
| fetal-specific Tregs | mouse | ↑ | ↑ | ↑ | [17] | ||||||
| DR+ Tregs | human | ↑ | ↓ | ↓ | [18] | ||||||
| DR- Tregs | human | ↑ | ↑ | ↑ | ↑ | [18] [68] | |||||
| DRlow+ Tregs | human | ↓ | ↑ | ↑ | ↑ | [59] [68] | |||||
| DRhigh+ Tregs | human | ↓ | ↑ | ↑ | ↑ | [59] [68] | |||||
| CD31+ memory Tregs | human | ↓ | ↑ | [60] | |||||||
| CD31- memory Tregs | human | ↑ | [60] | ||||||||
Note: amTregs: activated/memory Tregs (CD4+Foxp3+CD44highCD62Llow); ART: Assisted Reproductive Technology; CD31+ memory Tregs: (CD45RA-CD31+ Tregs); CD31- memory Tregs: (CD45RA-CD31- Tregs); DR+ Tregs: HLA-DR+ memory Tregs (CD45RA-HLA-DR+ Tregs); DR- Tregs: HLA-DR- memory Tregs (CD45RA-HLA-DR- Tregs); DRlow+ Tregs: HLA-DRlow+ memory Tregs (CD45RA-HLA-DRlow+ Tregs); DRhigh+ Tregs: HLA-DRhigh+ memory Tregs (CD45RA-HLA-DRhigh+ Tregs); GDM: gestational diabetes mellitus; HELLP: haemolysis-elevated liver enzyme levels-low platelet count syndrome; PE: preeclampsia; PL: irresistible preterm delivery; Tregs: Regulatory T cells; 1st trimester: the first trimester; 2nd trimester: the second trimester; 3rd trimester: the third trimester.
A recent mouse study has shown that Foxp3+ Tregs specific for paternal antigens were produced outside the thymus and accumulated in the placenta. The study reported that female mice with impaired extrathymic Treg induction exhibited increased fetal absorption [74]. Peripheral-derived Tregs (pTregs) are observed by Samstein et al. [74] from day E12 of gestation in mice. They proved that the lack of pTreg cells will lead to the increase of spontaneous abortion. Tilburgs et al. [67] suggested that fetal-specific Tregs were preferentially recruited from maternal peripheral blood to the maternal-fetal interface, where they may contribute to the local regulation of fetal-specific responses and further contribute to pregnancy outcomes. Therefore, fetal-specific Tregs in mice might be generated outside the thymus, but more conclusive evidence still needs to be accumulated.
To date, factors known to induce memory Tregs during pregnancy include paternal semen induction, fetal cell induction, fetal microchimerism induction, and cytokine production.
Paternal semen
Before pregnancy, female reproductive tissues, especially immune cells, first come into contact with male semen. Paternal antigens may be recognized by female immune cells during mating and can further produce memory.
Using a mouse tumor inoculation model and delayed-type hypersensitivity in mice, Robertson et al. [75] reported that sperm in seminal plasma and semen played an important role in inducing paternal antigen-specific tolerance. Accordingly, exposure to semen during mating can promote functional tolerance to paternal alloantigen. This effect may be mediated by the expansion of the Treg pool. The size of peptides presents in DBA/2 < 10 kD boosted the abortion rate. These discuss the role of seminal plasma peptides on the establishment of immune tolerance to the fetus [76]. Another study showed that, after mating with 2W1S+ male mice, the number of 2W1S+ Tregs was increased in infertile female mice (infertile female mice were constructed by irradiation with 100 rads); however, these levels were lower than those observed in pregnant mice [17]. This provides a new concept for the formation of semen-induced Treg immune memory before pregnancy. In addition, postpartum memory Tregs may be exposed to paternal antigens following exposure to seminal fluid, which may benefit the maintenance of memory Tregs. This conjecture remains to be confirmed.
Fetal cells and fetal microchimerism
The embryo carries antigens that differ from those of the mother. Numerous fetal cells (and fragments) flow into the maternal circulation during normal pregnancy [77], providing abundant fetal and placental antigens for the maternal immune system. Reportedly, the presence of fetal cells in postpartum maternal circulation [78, 79] may cause antigenic activation of memory T cell populations [80, 81]. This may lead to the persistence of postpartum immune memory cells. After mating in mice 12-21 days, proliferation fetal cells were unequivocally demonstrated in maternal spleen and bone marrow [82]. Bianchi et al. [80] revealed that male fetal progenitor cells could survive in maternal blood for up to 27 years after delivery. Fetal cells and acellular substances are transferred to the maternal circulation during early pregnancy, and fetal cells may survive in the maternal circulation and tissues for life. This substance is called "fetal microchimerism" [83-86].
Recent findings suggested that these microchimeric cells expressing antigenic traits were purposefully retained within mothers and their offspring to promote genetic fitness by improving the outcome of future pregnancies [87]. Microchimerism may persist in the maternal circulation post-pregnancy. Reportedly, the rapid increase in Tregs in uterine dLN begins 3 or 4 days after embryo implantation. Embryo implantation triggers early recruitment of amTregs in mouse uterine dLN [10]. The proliferation of memory Tregs in early pregnancy is driven by fetal antigens that exhibit Treg specificity. Subsequent studies assessing surface markers of Tregs in dLN failed to detect CD103, CTLA-4, inducible co-stimulator (ICOS), programmed cell death protein-1 (PD-1), CD25, and glucocorticoid-induced changes in tumor necrosis factor receptor-related proteins [10].
Cytokines
It has been reported that IL-2 can induce the production of memory Tregs [88]. Moreover, Low-dose IL-2 induced Tregs expansion can improve recurrent spontaneous abortion in a model of mouse miscarriage. In contrast, a recent study found that IL-2 was non-essential to maintaining CD44hiCD62LlowCCR7low Tregs [89]. The role of IL-2 in pregnant memory Tregs warrants further investigations.
The total frequency of memory marker CD27 positive B cells increased during pregnancy, persisted during pregnancy, produced IL-10, and clustered with Foxp3 post cells [7]. IL-10 secreted by B cells contributes to the induction and maintenance of placental Tregs and plays a pivotal role in healthy pregnancies. Notably, memory Tregs are primarily maintained by IL-7 in the skin [88, 90]. However, the effects of IL-10 and IL-7 on memory Tregs during pregnancy have not been reported.
The maintenance of memory Tregs during pregnancy remains poorly understood. The underlying rationale could be related to the prolonged existence of fetal cells in maternal circulation. It is similar to antigen stimulation at the later stages of persistent infection. Notably, the incidence of PE in the second pregnancy was lower than that in the first pregnancy [3]. However, when the interval between two pregnancies is prolonged, the risk of PE continues to increase in women who have repeated pregnancies. Functional changes and maintenance mechanisms of activated memory Tregs need to be comprehensively investigated in future investigations. The induction and characteristics of memory Tregs during pregnancy are shown in Fig. 1.
The induction and characteristics of memory Tregs in pregnancy. (A) During pregnancy, Tregs in maternal peripheral blood may be induced to generate memory Tregs by paternal semen, fetal microchimerism, fetal cells, and cytokines such as IL-2 and IL-7. (B-C) Previous studies have divided memory Tregs into different subsets in mice and humans. In pregnant mice, memory Tregs were divided into fetal-specific Tregs and activated/memory Tregs (CD4+Foxp3+CD44highCD62Llow). Among them, fetal-specific Tregs may be generated outside the thymus, and they secreted IFN-γ reduced and exhibited a state of anergy (B). In pregnant women, memory Tregs were divided into HLA-DR+ memory Tregs (CD45RA-HLA-DR+ Tregs), HLA-DR- memory Tregs (CD45RA-HLA-DR- Tregs), HLA-DRlow+ memory Tregs (CD45RA-HLA-DRlow+ Tregs), HLA-DRhigh+ memory Tregs (CD45RA-HLA-DRhigh+ Tregs), CD31+ memory Tregs (CD45RA-CD31+ Tregs) and CD31- memory Tregs (CD45RA-CD31- Tregs) (C). IL, interleukin; TGF-β, transforming growth factor-β; Tregs, regulatory T cells; Foxp3, forkhead box P3; CCR, chemokine receptor; IFN-γ, interferon-γ.
Memory Tregs in the second pregnancy
Accumulated evidence indicates that the incidence of pregnancy-related complications such as PE in multiparas were lower than that in nulliparas [3]. The memory characteristics of immune cells play a key role in repeated pregnancies. A previous study revealed a population of pregnancy-trained decidual NK cells (PTdNKs) was amplified in the decidua of women with repeated pregnancy when compared with that in the decidua of women during the first pregnancy. PTdNKs were characterized by high expression of NKG2C and leukocyte immunoglobulin-like receptor B1 transcripts. In addition, these cells secrete high levels of vascular endothelial growth factor-α (VEGF-α), which may be beneficial for angiogenesis and pregnancy [13].
Using their established adoptive transfer mouse model, Barton et al. [91] identified fetal-specific T cells. They found that these cells did not increase after the second mating pregnancy with the same partner. However, fetal-specific T cells in the second pregnancy highly expressed CD43 and PD-1, with a lower expression of CD127, which may contribute to the success of subsequent pregnancies to a certain extent [91]. The same phenomenon was observed in mouse CD8+T cells in another study by Kinder and colleagues [1]. Compared with the first allogeneic pregnancy, the levels of PD-1 and LAG-3, expressed by maternal CD8+ T cells stimulated by the fetus, gradually increased during the second pregnancy. Simultaneously, the memory of fetal expressed antigen during the second pregnancy weakened the cytolysis function of maternal CD8+ T cells initiated by the previous pregnancy, thus affording protection against fetal loss [1].
Unlike CD8+T cells, which weakened the cytolysis function in the second pregnancy, fetal-specific Tregs maintained at a high level after parturition in mice and expanded rapidly in the second pregnancy, showing the characteristics of immune tolerance. But they are conducive to the maintenance of pregnancy and against fetal loss. Rowe et al. [17] revealed that during the second pregnancy, the accelerated expansion of Tregs was almost entirely driven by proliferating fetal-specific Foxp3+ cells retained from the previous pregnancy, which was further conducive to pregnancy. The amplification rate was considerably faster in fetal-specific Foxp3+ cells than in nTregs. They also found an interesting phenomenon that 2W1S+ CD4 fetal-specific T cells maintained reduced interferon-γ (IFN-γ) secretion after pregnancy, that is, they showed anergy. And the anergy between maternal CD4 cells was not inherent in cells but maintained by the postpartum environment [17]. Another study found that compared with the first pregnancy, neonatal mortality was higher when Tregs were exhausted during the third trimester of the second pregnancy. The mortality rate associated with complete depletion was higher than that observed following partial depletion [39]. In addition, during repeated pregnancy, the newborns who survived in the group with partial or complete lack of Tregs were thinner and presented worse outcomes than their control group counterparts. These results support the concept that pregnancy imprints protective regulatory memories [39]. Furthermore, the amplification of Tregs during the second pregnancy was related to maternal-fetal tolerance, as well as neonatal health.
Few studies have been documented in the function of memory Tregs during the second pregnancy in humans. These studies focused on the expansion of immune memory cells in the second pregnancy to produce immune tolerance, which is further conducive to the maintenance of pregnancy. Clarifying the function and changes of memory Tregs during the second pregnancy is pivotal to reducing pregnancy-related complications during repeated pregnancies and elucidating the disease mechanism.
Memory Tregs in pathological pregnancy
An imbalance in immune tolerance can induce pregnancy complications. A study assessing 763,795 patients has reported that the risk of PE was 4.1% during the first pregnancy and 1.7% during subsequent pregnancies. However, a PE risk of 14.7% was observed during the second pregnancy in women who had experienced PE during the first pregnancy and was approximately 1% in multiparous women without a history of PE [92]. Moreover, a large meta-analysis of data from more than 1.5 million patients found that women who experienced stillbirth, preterm birth, and fetal growth restriction before pregnancy exhibited a significantly increased risk for each condition during subsequent pregnancies [93]. These results indicate that good pregnancy memory may lead to a good pregnancy outcome, while poor pregnancy memory may be associated with the subsequent increase in the incidence of pregnancy-complicated diseases.
Recently, changes in memory Tregs have been detected in association with pregnancy complications, which could be employed as a new target for diagnosing and treating pregnancy complications. Previous studies assessed changes in memory Tregs during pregnancy complications in PE, gestational diabetes mellitus (GDM), preterm delivery, and the success of assisted reproductive technology (ART).
PE
PE affects 5%-8% of pregnancies and is the main cause of fetal and maternal mortality and morbidity [94]. In women with PE, there is a negative correlation between Tregs and memory B cells, which is characterized by a systematic decrease of Tregs and an increase of memory B cells [9]. But in memory Tregs, Clonal expansion of the CD4+CD25HICD127-CD45RA- Treg population has been observed in the decidua of healthy term pregnancies. The failure of clonal expansion may be related to the occurrence of PE [95].
In patients with PE, RTE-Tregs differentiate into CD31+ memory Tregs rather than CD31- memory Tregs. CD31+ memory Tregs reportedly decrease during normal pregnancy [60]. However, the underlying mechanism remains unclear. A study by Steinborn et al. [68] revealed that the percentages of DRlow+ CD45RA- Tregs and DRhigh+CD45RA- Tregs were significantly increased in pregnant women with PE. Moreover, the percentage of DRhigh+CD45RA- Tregs was significantly upregulated in patients with hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome. Nevertheless, this study did not define DRlow+CD45RA- Tregs and DRhigh+CD45RA- Tregs as memory Tregs, although CD45RA- is typically defined as a memory marker.
GDM
GDM refers to diabetes mellitus with normal glucose metabolism or potentially impaired glucose tolerance before pregnancy, which appears or is diagnosed during pregnancy. The reported incidence among pregnant women ranges between 2%-9% [96] and appears to be growing. Recent studies have revealed that GDM is characterized by chronic systemic inflammation and increased humoral immune responses [97]. Tregs can reportedly modulate the excessive enhancement of immune responses. It has been shown that although the inhibitory activity of Tregs was significantly decreased in patients with GDM, no difference in the total percentage of Tregs was detected.
Interestingly, in the subgroup analysis of Tregs, a considerably higher number of DR-memory Tregs was observed in dietary-adjusted pregnant patients with GDM than in healthy pregnancies. However, insulin-dependent patients with GDM exhibited considerably higher levels of DRlow+ Tregs and DRhigh+ Tregs [59]. It has been suggested that the cellular function of pregnancy memory may be related not only to immunity but also to metabolism. Indeed, previous studies have suggested that proliferation depends on glycolysis, and memory depends on fatty acid oxidation [98]. However, whether the increase in memory Tregs is related to metabolism remains to be determined.
Taken together, these studies suggest that DRlow+ memory Tregs and DRhigh+ memory Tregs may be unfavorable for pregnancy. This finding is similar to the increase in DRlow+ memory Tregs and DRhigh+ memory Tregs observed in PE. However, in normal pregnancy, these two cell types are decreased [68]. DR+ Tregs and DR- Tregs increased in the early stage of normal pregnancy, as shown in Table 2. However, the mechanism is not clear, especially in the second pregnancy.
Premature birth
In developed countries, premature birth remains the primary cause of morbidity and mortality, with a reported incidence rate of approximately 5%-9% [99]. Gomez-Lopez et al. [39] reported the immunomodulatory effects of Tregs during the third stage of pregnancy in mice. Treg deficiency may lead to idiopathic preterm births and poor perinatal outcomes. Interestingly, the authors revealed that complete depletion of Tregs during the third trimester of the second pregnancy minimally impacted the preterm birth rate while significantly influencing neonatal survival [39]. Another study reported that the percentages of DR-CD45RA- Tregs and DRlow+CD45RA- Tregs were significantly higher in human preterm births than in healthy pregnancies. However, no change in Treg subsets was detected in patients with simple cervical insufficiency and no preterm birth [68]. Accordingly, it can be suggested that distinct mechanisms possibly underlie preterm birth and cervical insufficiency.
ART outcome
Memory Tregs may be associated with ART outcome. In the study of Schlossberger et al. [18], they determined the association of total Treg pool and distinct Treg subsets (na ̈ıve CD45RA+ Tregs, HLA-DR- and HLA-DR+ memory Tregs) with the success of IVF/ICSI treatment. They found that the percentage of Tregs within the total CD4+ T cell pool was not different between successfully and non-successfully IVF/ICSI-treated women. However, there were a decreased percentage of na ̈ıve CD45RA+ Tregs and an increased percentage of HLA-DR- memory Tregs within the total Treg pool in non-successfully IVF/ICSI-treated women. They suggested that the ART success might be related to composition of the total Treg pool with na ̈ıve CD45RA+ Tregs and HLA-DR- memory Tregs.
RPL and miscarriage with normal fetal karyotype
Extensive studies have shown that systemic and local maldistribution and dysfunction of Tregs could be one of the etiologies of RPL and miscarriage with normal fetal karyotype [95, 100]. However, there are less studies targeting memory Tregs in RPL and miscarriage, which highlights this topic as future research direction. In a recent study, Tsuda et al. [95] found that the frequency of CD4+CD45RA-CD25+CD127low/- effector Tregs (also defined as one of memory Treg subsets) among CD4+CD25+CD127low/- total Tregs in decidua were significantly lower in miscarriage with normal chromosomal karyotyped embryo than 1st trimester normal pregnancy. They suggested that the decreased number of decidual effector Tregs might be related to the pathogenesis of miscarriage with normal fetal karyotype, for the effector Treg subset might contain fetal antigen specific populations in humans.
As is known that the number of miscarriages increases, the probability of miscarriage in the next pregnancy also increases. However, whether memory Tregs are correlated with the occurrence of repeated miscarriages with normal fetal karyotype is unknown and needs further investigations.
Table 2 summarizes the changes of memory Tregs in healthy and pathological pregnancies in humans. It seems that there are higher different memory Treg subsets in different pregnancy complications. However, the exact role of the different memory Treg subsets in the subsequent pregnancies of women who experienced pregnancy-related complications during their first pregnancy remains unclear and needs further investigations.
Conclusions
In conclusion, paternal semen, fetal cells, fetal microchimerism, and cytokines may induce the transformation of Tregs into memory Tregs during pregnancy. They include fetal-specific Tregs and Tregs expressing memory makers. During normal pregnancy, the good memory Tregs contribute to embryo implantation and play a role in immune tolerance. The increase in detrimental memory Tregs may be related to pregnancy complications. In human pregnancy, data regarding the changes and functions of memory Tregs in repeated pregnancies are limited. In mice, the role of memory Tregs in pathological pregnancy is lacking. An in-depth study on the role of memory Tregs in normal and pathological pregnancies, especially in the subsequent pregnancy, will help to uncover the mechanism underlying pregnancy-related diseases, which could further afford novel strategies for the clinical diagnosis and treatment of pathological pregnancies. Moreover, identifying more conclusive markers for memory Tregs' function is extremely important to fully elucidate the role of memory Tregs in human pregnancy.
Abbreviations
amTregs: activated/memory Tregs; ART: assisted reproductive technology; CCR: chemokine receptor; dLN: draining lymph node; dpc: days postcoital; dpi: day postimplantation; DT: diphtheria toxin; DTR: diphtheria toxin receptor; Foxp3+: forkhead box P3+; GDM: gestational diabetes mellitus; HELLP syndrome: patients with hemolysis, elevated liver enzymes, and low platelets syndrome; HLA: human leukocyte antigen; ICOS: inducible co-stimulator; IFN-γ: interferon-γ; IL: interleukin; IVF/ICSI: in vitro fertilization/intracytoplasmic sperm injection; MHC: major histocompatibility complex; NK: natural killer; Ova: Ovalbumin; PD-1: Programmed Cell Death Protein-1; PE: preeclampsia; PTdNKs: pregnancy-trained decidual NK cells; pTregs: Peripheral-derived Tregs; RPL: recurrent pregnancy loss; TCM: central memory T cells; Teffs: effector T cells; TEM: effector memory T cells; TGF-β: transforming growth factor-β; Tregs: Regulatory T cells; TRM: tissue-resident memory T cells; VEGF-α: vascular endothelial growth factor-α.
Acknowledgements
Funding
This work was supported by the National Natural Science Foundation of China (No. 81871186).
Author Contributions
Y.J.Z. and L.S. wrote the paper and made the figure; T.Z., K.P.M., and L.J. provided the insights for this manuscript, and edited the manuscript; G.M. revised the manuscript; A.H.L. conceptualized, supervised, revised and finally approved the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Kinder JM, Turner LH, Stelzer IA, Miller-Handley H, Burg A, Shao TY. et al. CD8(+) T Cell Functional Exhaustion Overrides Pregnancy-Induced Fetal Antigen Alloimmunization. Cell Rep. 2020;31:107784
2. Ahinkorah BO, Seidu AA, Ameyaw EK, Budu E, Bonsu F, Mwamba B. Beyond counting induced abortions, miscarriages and stillbirths to understanding their risk factors: analysis of the 2017 Ghana maternal health survey. BMC Pregnancy Childbirth. 2021;21:140
3. Trupin LS, Simon LP, Eskenazi B. Change in paternity: a risk factor for preeclampsia in multiparas. Epidemiology. 1996;7:240-4
4. Mikolajczyk RT, Zhang J, Ford J, Grewal J. Effects of interpregnancy interval on blood pressure in consecutive pregnancies. Am J Epidemiol. 2008;168:422-6
5. Fe Eney JG, Scott JS. Pre-eclampsia and changed paternity. Eur J Obstet Gynecol Reprod Biol. 1980;11:35-8
6. Tubbergen P, Lachmeijer A, Althuisius SM, Vlak M, Geijn H, Dekker GA. Change in paternity: a risk factor for preeclampsia in multiparous women? J Reprod Immunol. 1999;45:81-8
7. Benner M, Feyaerts D, García CC, Inci N, Molen RGVD. Clusters of Tolerogenic B Cells Feature in the Dynamic Immunological Landscape of the Pregnant Uterus. Cell Reports. 2020;32:108204
8. Wang L, Jiang P, Zhao S, Liu H, Liu L, Mor G. et al. The dynamic profile and potential function of B-cell subsets during pregnancy. Cell Mol Immunol. 2020;18:1082-4
9. Zeng B, Kwak-Kim J, Liu Y, Liao AH. Treg cells are negatively correlated with increased memory B cells in pre-eclampsia while maintaining suppressive function on autologous B-cell proliferation. Am J Reprod Immunol. 2013;70:454-63
10. Chen T, Darrasse-Jeze G, Bergot AS, Courau T, Churlaud G, Valdivia K. et al. Self-specific memory regulatory T cells protect embryos at implantation in mice. J Immunol. 2013;191:2273-81
11. Powell RM, Lissauer D, Tamblyn J, Beggs A, Cox P, Moss P. et al. Decidual T Cells Exhibit a Highly Differentiated Phenotype and Demonstrate Potential Fetal Specificity and a Strong Transcriptional Response to IFN. J Immunol. 2017;199:3406-17
12. Kieffer TE, Faas MM, Scherjon SA, Prins JR. Pregnancy persistently affects memory T cell populations. J Reprod Immunol. 2017;119:1-8
13. Gamliel M, Goldman-Wohl D, Isaacson B, Gur C, Stein N, Yamin R. et al. Trained Memory of Human Uterine NK Cells Enhances Their Function in Subsequent Pregnancies. Immunity. 2018;48:951-62
14. Foley B, Cooley S, Verneris MR, Curtsinger J, Luo X, Waller EK. et al. Human Cytomegalovirus (CMV)-Induced Memory-like NKG2C+ NK Cells Are Transplantable and Expand In vivo in Response to Recipient CMV Antigen. J Immunol. 2012;189:5082-8
15. La Rocca C, Carbone F, Longobardi S, Matarese G. The immunology of pregnancy: Regulatory T cells control maternal immune tolerance toward the fetus. Immunol Lett. 2014;162:41-8
16. Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775-87
17. Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490:102-6
18. Schlossberger V, Schober L, Rehnitz J, Schaier M, Zeier M, Meuer S. et al. The success of assisted reproduction technologies in relation to composition of the total regulatory T cell (Treg) pool and different Treg subsets. Hum Reprod. 2013;28:3062-73
19. Zenclussen AC, Schumacher A, Zenclussen ML, Wafula P, Volk HD. Immunology of pregnancy: cellular mechanisms allowing fetal survival within the maternal uterus. Expert Rev Mol Med. 2007;9:1-14
20. Yang F, Zheng Q, Jin L. Dynamic Function and Composition Changes of Immune Cells During Normal and Pathological Pregnancy at the Maternal-Fetal Interface. Front Immunol. 2019;10:2317
21. Darmochwal-Kolarz D, Kludka-Sternik M, Tabarkiewicz J, Kolarz B, Rolinski J, Leszczynska-Gorzelak B. et al. The predominance of Th17 lymphocytes and decreased number and function of Treg cells in preeclampsia. J Reprod Immunol. 2012;93:75-81
22. Jin LP, Chen QY, Zhang T, Guo PF, Li DJ. The CD4+CD25 bright regulatory T cells and CTLA-4 expression in peripheral and decidual lymphocytes are down-regulated in human miscarriage. Clin Immunol. 2009;133:402-10
23. Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci. 2011;1221:80-7
24. Sindram-Trujillo AP, Scherjon SA, van Hulst-van Miert PP, Kanhai HH, Roelen DL, Claas FH. Comparison of decidual leukocytes following spontaneous vaginal delivery and elective cesarean section in uncomplicated human term pregnancy. J Reprod Immunol. 2004;62:125-37
25. Tilburgs T, Roelen DL, van der Mast BJ, van Schip JJ, Kleijburg C, de Groot-Swings GM. et al. Differential distribution of CD4(+)CD25(bright) and CD8(+)CD28(-) T-cells in decidua and maternal blood during human pregnancy. Placenta. 2006 27 Suppl A: S47-53
26. Mold JE, McCune JM. Immunological tolerance during fetal development: from mouse to man. Adv Immunol. 2012;115:73-111
27. Mohammadi S, Abdollahi E, Nezamnia M, Esmaeili SA, Tavasolian F, Sathyapalan T. et al. Adoptive transfer of Tregs: A novel strategy for cell-based immunotherapy in spontaneous abortion: Lessons from experimental models. Int Immunopharmacol. 2021;90:107195
28. Guerin LR, Prins JR, Robertson SA. Regulatory T-cells and immune tolerance in pregnancy: a new target for infertility treatment? Hum Reprod Update. 2009;15:517-35
29. Figueiredo AS, Schumacher A. The T helper type 17/regulatory T cell paradigm in pregnancy. Immunology. 2016;148:13-21
30. Zhang YH, Sun HX. Immune checkpoint molecules in pregnancy: Focus on regulatory T cells. Eur J Immunol. 2020;50:160-9
31. Dimova T, Nagaeva O, Stenqvist AC, Hedlund M, Kjellberg L, Strand M. et al. Maternal Foxp3 expressing CD4+ CD25+ and CD4+ CD25- regulatory T-cell populations are enriched in human early normal pregnancy decidua: a phenotypic study of paired decidual and peripheral blood samples. Am J Reprod Immunol. 2011;66(Suppl 1):44-56
32. Robertson SA, Care AS, Moldenhauer LM. Regulatory T cells in embryo implantation and the immune response to pregnancy. J Clin Invest. 2018;128:4224-35
33. Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266-71
34. Darrasse-Jeze G, Klatzmann D, Charlotte F, Salomon BL, Cohen JL. CD4+CD25+ regulatory/suppressor T cells prevent allogeneic fetus rejection in mice. Immunol Lett. 2006;102:106-9
35. Zhao A, Xiong M, Zhang Y, Bao S, Zhang J, Qiu L. et al. Adoptive transfer of mFas ligand into dendritic cells influences the spontaneous resorption rate in the CBA/J x DBA/2 mouse model. Fertil Steril. 2010;93:1700-5
36. Zenclussen AC, Gerlof K, Zenclussen ML, Sollwedel A, Bertoja AZ, Ritter T. et al. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am J Pathol. 2005;166:811-22
37. Yin Y, Han X, Shi Q, Zhao Y, He Y. Adoptive transfer of CD4+CD25+ regulatory T cells for prevention and treatment of spontaneous abortion. Eur J Obstet Gynecol Reprod Biol. 2012;161:177-81
38. Habicht A, Dada S, Jurewicz M, Fife BT, Yagita H, Azuma M. et al. A link between PDL1 and T regulatory cells in fetomaternal tolerance. J Immunol. 2007;179:5211-9
39. Gomez-Lopez N, Arenas-Hernandez M, Romero R, Miller D, Garcia-Flores V, Leng Y. et al. Regulatory T Cells Play a Role in a Subset of Idiopathic Preterm Labor/Birth and Adverse Neonatal Outcomes. Cell Rep. 2020;32:107874
40. Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004;112:38-43
41. Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod. 2004;10:347-53
42. Inada K, Shima T, Nakashima A, Aoki K, Ito M, Saito S. Characterization of regulatory T cells in decidua of miscarriage cases with abnormal or normal fetal chromosomal content. J Reprod Immunol. 2013;97:104-11
43. Arruvito L, Sanz M, Banham AH, Fainboim L. Expansion of CD4+CD25+and FOXP3+ regulatory T cells during the follicular phase of the menstrual cycle: implications for human reproduction. J Immunol. 2007;178:2572-8
44. Alijotas-Reig J, Llurba E, Gris JM. Potentiating maternal immune tolerance in pregnancy: a new challenging role for regulatory T cells. Placenta. 2014;35:241-8
45. Rosenblum MD, Gratz IK, Paw JS, Lee K, Marshak-Rothstein A, Abbas AK. Response to self antigen imprints regulatory memory in tissues. Nature. 2011;480:538-42
46. Nagahama K, Fehervari Z, Oida T, Yamaguchi T, Ogawa O, Sakaguchi S. Differential control of allo-antigen-specific regulatory T cells and effector T cells by anti-CD4 and other agents in establishing transplantation tolerance. Int Immunol. 2009;21:379-91
47. Shima T, Inada K, Nakashima A, Ushijima A, Ito M, Yoshino O. et al. Paternal antigen-specific proliferating regulatory T cells are increased in uterine-draining lymph nodes just before implantation and in pregnant uterus just after implantation by seminal plasma-priming in allogeneic mouse pregnancy. J Reprod Immunol. 2015;108:72-82
48. Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745-63
49. Lanzavecchia A, Sallusto F. Understanding the generation and function of memory T cell subsets. Curr Opin Immunol. 2005;17:326-32
50. Campbell JJ, Murphy KE, Kunkel EJ, Brightling CE, Soler D, Shen Z. et al. CCR7 expression and memory T cell diversity in humans. J Immunol. 2001;166:877-84
51. Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708-12
52. Wang J, Jiang H, Qiu Y, Wang Y, Sun G, Zhao J. Effector memory regulatory T cells were most effective at suppressing RANKL but their frequency was downregulated in tibial fracture patients with delayed union. Immunol Lett. 2019;209:21-7
53. Lei H, Kuchenbecker L, Streitz M, Sawitzki B, Vogt K, Landwehr-Kenzel S. et al. Human CD45RA(-) FoxP3(hi) Memory-Type Regulatory T Cells Show Distinct TCR Repertoires With Conventional T Cells and Play an Important Role in Controlling Early Immune Activation. Am J Transplant. 2015;15:2625-35
54. Darrasse-Jèze G, Bergot AS, Durgeau A, Billiard F, Klatzmann D. Tumor emergence is sensed by self-specific CD44hi memory Tregs that create a dominant tolerogenic environment for tumors in mice. J Clin Invest. 2009;119:2648-62
55. Mhanna V, Fourcade G, Barennes P, Quiniou V, Pham HP, Ritvo PG. et al. Impaired Activated/Memory Regulatory T Cell Clonal Expansion Instigates Diabetes in NOD Mice. Diabetes. 2021;70:976-85
56. Bergot AS, Chaara W, Ruggiero E, Mariotti-Ferrandiz E, Dulauroy S, Schmidt M. et al. TCR sequences and tissue distribution discriminate the subsets of naive and activated/memory Treg cells in mice. Eur J Immunol. 2015;45:1524-34
57. Fisson S, Darrasse-Jeze G, Litvinova E, Septier F, Klatzmann D, Liblau R. et al. Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state. J Exp Med. 2003;198:737-46
58. Nehar-Belaid D, Courau T, Derian N, Florez L, Ruocco MG, Klatzmann D. Regulatory T Cells Orchestrate Similar Immune Evasion of Fetuses and Tumors in Mice. J Immunol. 2016;196:678-90
59. Schober L, Radnai D, Spratte J, Kisielewicz A, Steinborn A. The role of regulatory T cell (Treg) subsets in gestational diabetes mellitus. Clin Exp Immunol. 2014 177
60. Wagner MI, Mai C, Schmitt E, Mahnke K, Meuer S, Eckstein V. et al. The role of recent thymic emigrant-regulatory T-cell (RTE-Treg) differentiation during pregnancy. Immunol Cell Biol. 2015;93:858-67
61. Forsberg A, Abrahamsson TR, Nilsson L, Ernerudh J, Duchen K, Jenmalm MC. Changes in peripheral immune populations during pregnancy and modulation by probiotics and omega-3 fatty acids. Sci Rep. 2020;10:18723
62. Palojarvi A, Petaja J, Siitonen S, Janer C, Andersson S. Low monocyte HLA-DR expression as an indicator of immunodepression in very low birth weight infants. Pediatr Res. 2013;73:469-75
63. Loewendorf AI, Nguyen TA, Yesayan MN, Kahn DA, Derya U. Normal Human Pregnancy Results in Maternal Immune Activation in the Periphery and at the Uteroplacental Interface. PLoS One. 2014;9:e96723
64. Baecher-Allan C, Wolf E, Hafler DA. MHC class II expression identifies functionally distinct human regulatory T cells. J Immunol. 2006;176:4622-31
65. Lertkiatmongkol P, Liao D, Mei H, Hu Y, Newman PJ. Endothelial functions of platelet/endothelial cell adhesion molecule-1 (CD31). Curr Opin Hematol. 2016;23:253-9
66. Prager E, Sunder-Plassmann R, Hansmann C, Koch C, Holter W, Knapp W. et al. Interaction of CD31 with a heterophilic counterreceptor involved in downregulation of human T cell responses. J Exp Med. 1996;184:41-50
67. Tilburgs T, Roelen DL, van der Mast BJ, de Groot-Swings GM, Kleijburg C, Scherjon SA. et al. Evidence for a selective migration of fetus-specific CD4+CD25bright regulatory T cells from the peripheral blood to the decidua in human pregnancy. J Immunol. 2008;180:5737-45
68. Steinborn A, Schmitt E, Kisielewicz A, Rechenberg S, Seissler N, Mahnke K. et al. Pregnancy-associated diseases are characterized by the composition of the systemic regulatory T cell (Treg) pool with distinct subsets of Tregs. Clin Exp Immunol. 2012;167:84-98
69. Schober L, Radnai D, Schmitt E, Mahnke K, Sohn C, Steinborn A. Term and preterm labor: decreased suppressive activity and changes in composition of the regulatory T-cell pool. Immunol Cell Biol. 2012;90:935-44
70. Gratz IK, Rosenblum MD, Abbas AK. The life of regulatory T cells. Ann N Y Acad Sci. 2013;1283:8-12
71. Nelson RW, McLachlan JB, Kurtz JR, Jenkins MK. CD4+ T cell persistence and function after infection are maintained by low-level peptide:MHC class II presentation. J Immunol. 2013;190:2828-34
72. Kinder JM, Jiang TT, Clark DR, Chaturvedi V, Xin L, Ertelt JM. et al. Pregnancy-induced maternal regulatory T cells, bona fide memory or maintenance by antigenic reminder from fetal cell microchimerism? Chimerism. 2014;5:16-9
73. Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y. et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433-41
74. Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012;150:29-38
75. Robertson SA, Guerin LR, Bromfield JJ, Branson KM, Ahlstrom AC, Care AS. Seminal fluid drives expansion of the CD4+CD25+ T regulatory cell pool and induces tolerance to paternal alloantigens in mice. Biol Reprod. 2009;80:1036-45
76. Clark DA, Rahmati M, Gohner C, Bensussan A, Markert UR, Chaouat G. Seminal plasma peptides may determine maternal immune response that alters success or failure of pregnancy in the abortion-prone CBAxDBA/2 model. J Reprod Immunol. 2013;99:46-53
77. Herzenberg LA, Bianchi DW, Schroder J, Cann HM, Iverson GM. Fetal cells in the blood of pregnant women: detection and enrichment by fluorescence-activated cell sorting. Proc Natl Acad Sci U S A. 1979;76:1453-5
78. Rijnink EC, Penning ME, Wolterbeek R, Wilhelmus S, Zandbergen M, Duinen SV. et al. Tissue microchimerism is increased during pregnancy: a human autopsy study. Mol Hum Reprod. 2015:857-64
79. Deshmukh H, Way SS. Immunological Basis for Recurrent Fetal Loss and Pregnancy Complications. Annu Rev Pathol. 2018;14:185-210
80. Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, Demaria MA. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proceedings of the National Academy of Sciences. 1996;93:705-8
81. David M, Lissauer Karen, P Piper. et al. Fetal microchimerism: the cellular and immunological legacy of pregnancy. Expert Rev Mol Med. 2009;11:e33
82. Philip PJ, Ayraud N, Masseyeff R. Transfer, tissue localization and proliferation of fetal cells in pregnant mice. Immunol Lett. 1982;4:175-8
83. Gammill HS, Guthrie KA, Aydelotte TM, Waldorf KMA, Nelson JL. Effect of parity on fetal and maternal microchimerism: interaction of grafts within a host? Blood. 2010;116:2706-12
84. O'Donoghue K, Chan J, de la Fuente J, Kennea N, Sandison A, Anderson JR. et al. Microchimerism in female bone marrow and bone decades after fetal mesenchymal stem-cell trafficking in pregnancy. Lancet. 2004;364:179-82
85. Olivia J, Holland and, Caitlin Linscheid. et al. Minor Histocompatibility Antigens Are Expressed in Syncytiotrophoblast and Trophoblast Debris: Implications for Maternal Alloreactivity to the Fetus. Am J Pathol. 2012;180:256-66
86. Liegeois A, Gaillard MC, Ouvre E, Lewin D. Microchimerism in pregnant mice. Transplant Proc. 1981;13:1250-2
87. Kinder JM, Stelzer IA, Arck PC, Way SS. Immunological implications of pregnancy-induced microchimerism. Nat Rev Immunol. 2017;17:483-94
88. Gratz IK, Truong HA, Yang SH, Maurano MM, Lee K, Abbas AK. et al. Cutting Edge: memory regulatory t cells require IL-7 and not IL-2 for their maintenance in peripheral tissues. J Immunol. 2013;190:4483-7
89. Smigiel KS, Richards E, Srivastava S, Thomas KR, Dudda JC, Klonowski KD. et al. CCR7 provides localized access to IL-2 and defines homeostatically distinct regulatory T cell subsets. J Exp Med. 2014;211:121-36
90. Sanchez Rodriguez R, Pauli ML, Neuhaus IM, Yu SS, Arron ST, Harris HW. et al. Memory regulatory T cells reside in human skin. J Clin Invest. 2014;124:1027-36
91. Barton BM, Xu R, Wherry EJ, Porrett PM. Pregnancy promotes tolerance to future offspring by programming selective dysfunction in long-lived maternal T cells. J Leukoc Biol. 2017;101:975-87
92. Hernandez-Diaz S, Toh S, Cnattingius S. Risk of pre-eclampsia in first and subsequent pregnancies: prospective cohort study. BMJ. 2009;338:b2255
93. Malacova E, Regan A, Nassar N, Raynes-Greenow C, Leonard H, Srinivasjois R. et al. Risk of stillbirth, preterm delivery, and fetal growth restriction following exposure in a previous birth: systematic review and meta-analysis. BJOG. 2018;125:183-92
94. Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. European Journal of Obstetrics Gynecology & Reproductive Biology. 2013;170:1-7
95. Tsuda S, Zhang X, Hamana H, Shima T, Ushijima A, Tsuda K. et al. Clonally Expanded Decidual Effector Regulatory T Cells Increase in Late Gestation of Normal Pregnancy, but Not in Preeclampsia, in Humans. Front Immunol. 2018;9:1934
96. Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352:2477-86
97. Ategbo JM, Grissa O, Yessoufou A, Hichami A, Dramane KL, Moutairou K. et al. Modulation of adipokines and cytokines in gestational diabetes and macrosomia. J Clin Endocrinol Metab. 2006;91:4137-43
98. Pearce EL, Poffenberger MC, Chang CH, Jones RG. Fueling immunity: insights into metabolism and lymphocyte function. Science. 2013;342:1242454
99. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75-84
100. Hu X, Zhu Q, Wang Y, Wang L, Li Z, Mor G. et al. Newly characterized decidual Tim-3+ Treg cells are abundant during early pregnancy and driven by IL-27 coordinately with Gal-9 from trophoblasts. Hum Reprod. 2020;35:2454-66
Author contact
![]() Corresponding author: Ai-Hua Liao, MD, PhD. Institute of Reproductive Health, Center for Reproductive Medicine, Tongji Medical College, Huazhong University of Science and Technology, No. 13 Hangkong Road, 430030 Wuhan, P.R. China. Tel: +86-27-8369-3513 Fax: +86-27-8369-3513 E-mail: aihua_liaoedu.cn
Corresponding author: Ai-Hua Liao, MD, PhD. Institute of Reproductive Health, Center for Reproductive Medicine, Tongji Medical College, Huazhong University of Science and Technology, No. 13 Hangkong Road, 430030 Wuhan, P.R. China. Tel: +86-27-8369-3513 Fax: +86-27-8369-3513 E-mail: aihua_liaoedu.cn

 Global reach, higher impact
Global reach, higher impact