10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(6):2484-2496. doi:10.7150/ijbs.69802 This issue Cite
Research Paper
Acyl-CoA synthetase long-chain 3-mediated fatty acid oxidation is required for TGFβ1-induced epithelial-mesenchymal transition and metastasis of colorectal carcinoma
1. Key Laboratory of Carcinogenesis and Invasion, Chinese Ministry of Education, Department of Radiology, Xiangya Hospital, Central South University, Changsha, Hunan 410078, PR China
2. Cancer Research Institute, School of Basic Medicine, Central South University, Changsha, Hunan 410078, PR China
3. Hengyang Medical College, University of South China, Hengyang 421001, Hunan, PR China
4. Department of Neurosurgery, Xiangya Hospital, Central South University, Changsha, Hunan 410078, PR China
5. Hunan Key Laboratory of Oncotarget Gene, Hunan Cancer Hospital and The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, Hunan 410078, China
6. Key Laboratory of Biological Nanotechnology of National Health Commission, Central South University, Changsha, Hunan 410078, China
7. Molecular Imaging Research Center of Central South University, Changsha, Hunan 410078, China
8. National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, 410078, China
*These authors contribute equally to the paper
Abstract
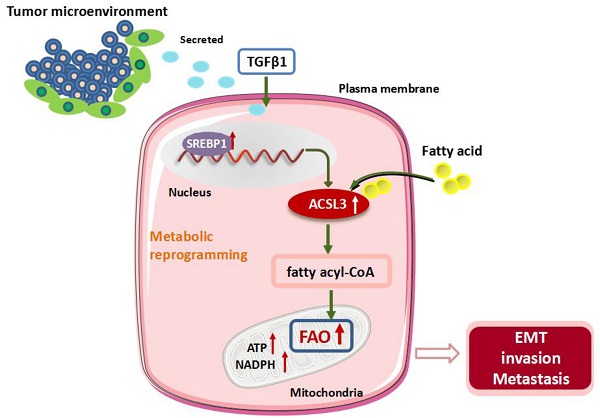
Cancer cells frequently undergo metabolic reprogramming to support tumorigenicity and malignancy, which is recognized as a hallmark of cancer. In addition to glycolysis and glutaminolysis, alterations in fatty acid (FA) metabolism have received increasing concerns in the past few years. Recently, accumulating evidence has shown that fatty acid β-oxidation (FAO) is abnormally activated in various tumors, which is associated with the machinery of proliferation, stemness, metastasis, and radiochemotherapeutic resistance of cancer cells. Acyl-CoA synthetases 3 (ACSL3) belongs to a family of enzymes responsible for converting free long-chain FAs into fatty acyl-CoA esters, which act as substrates both for lipid synthesis and FAO.
Here, we demonstrate that transforming growth factor beta 1 (TGFβ1) induces the up-regulation of ACSL3 through sterol regulatory element-binding protein 1 (SREBP1) signaling to promote energy metabolic reprogramming in colorectal carcinoma (CRC) cells. ACSL3 mediates the epithelial mesenchymal transition (EMT) and metastasis of CRC cells by activation of FAO pathway to produce ATP and reduced nicotinamide adenine dinucleotide phosphate (NADPH), which sustain redox homeostasis and fuel cancer cells for invasion and distal metastasis. Thus, targeting ACSL3 and FAO metabolic pathways might be exploited for therapeutic gain for CRC and other FAs- addicted cancers.
Keywords: Acyl-CoA synthetases 3, Fatty acid β-oxidation, Epithelial-to-mesenchymal transition, Metastasis, Colorectal carcinoma

 Global reach, higher impact
Global reach, higher impact