10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(6):2609-2626. doi:10.7150/ijbs.70120 This issue Cite
Review
CAR-T Cell Therapy for Breast Cancer: From Basic Research to Clinical Application
1. Department of Pharmacy, Jiangsu Cancer Hospital, The Affiliated Cancer Hospital of Nanjing Medical University, Jiangsu Institute of Cancer Research, 210009, China.
2. Department of Clinical Pharmacy, School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, Nanjing, 211198, China.
3. Precision Medicine Center, First Affiliated Hospital of Gannan Medical University, Ganzhou, 341000, China.
4. Department of Clinical Pharmacy, School of Pharmacy, Nanjing Medical University, Nanjing, 211103, China.
Abstract
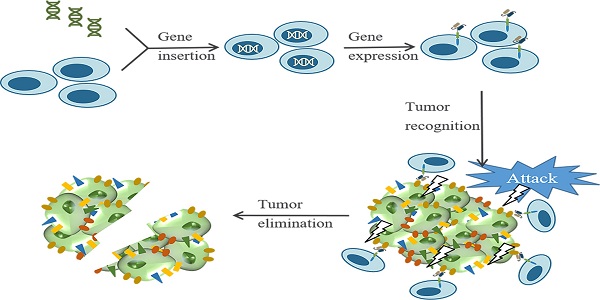
Breast cancer rises as the most commonly diagnosed cancer in 2020. Among women, breast cancer ranks first in both cancer incidence rate and mortality. Treatment resistance developed from the current clinical therapies limits the efficacy of therapeutic outcomes, thus new treatment approaches are urgently needed. Chimeric antigen receptor (CAR) T cell therapy is a type of immunotherapy developed from adoptive T cell transfer, which typically uses patients' own immune cells to combat cancer. CAR-T cells are armed with specific antibodies to recognize antigens in self-tumor cells thus eliciting cytotoxic effects. In recent years, CAR-T cell therapy has achieved remarkable successes in treating hematologic malignancies; however, the therapeutic effects in solid tumors are not up to expectations including breast cancer. This review aims to discuss the development of CAR-T cell therapy in breast cancer from preclinical studies to ongoing clinical trials. Specifically, we summarize tumor-associated antigens in breast cancer, ongoing clinical trials, obstacles interfering with the therapeutic effects of CAR-T cell therapy, and discuss potential strategies to improve treatment efficacy. Overall, we hope our review provides a landscape view of recent progress for CAR-T cell therapy in breast cancer and ignites interest for further research directions.
Keywords: CAR-T cell therapy, Breast cancer, Preclinical studies, Clinical trials, Tumor-associated antigens

 Global reach, higher impact
Global reach, higher impact