ISSN: 1449-2288
Int J Biol Sci 2022; 18(8):3374-3389. doi:10.7150/ijbs.70742 This issue Cite
Research Paper
TBK1 Facilitates GLUT1-Dependent Glucose Consumption by suppressing mTORC1 Signaling in Colorectal Cancer Progression
1. Department of General Surgery, the First Affiliated Hospital of Soochow University, Suzhou, Jiangsu 215006, China.
2. Department of Gastrointestinal Surgery, Changzhi People's Hospital, The Affiliated Hospital of Changzhi Medical College, Changzhi, Shanxi 046000, China.
3. Department of Gastrointestinal Surgery, Changshu No. 2 Hospital, Suzhou, Jiangsu 215006, China.
4. Department of Biochemistry and Molecular Biology, Soochow University Medical College, Suzhou, Jiangsu 215123, China.
5. Department of General Surgery, the Affiliated Hospital of Jiangsu University, Zhenjiang, Jiangsu 212000, China.
6. Department of Medicine, Soochow University Medical College, Suzhou, Jiangsu 215006, China.
#These authors contributed equally to this work.
Abstract
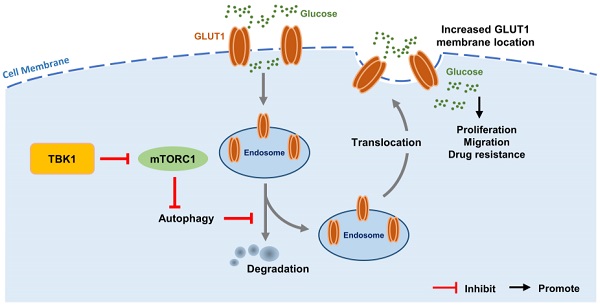
Intestinal inflammation is a vital precipitating factor of colorectal cancer (CRC), but the underlying mechanisms are still elusive. TANK-binding kinase 1 (TBK1) is a core enzyme downstream of several inflammatory signals. Recent studies brought the impacts of TBK1 in malignant disease to the forefront, we found aberrant TBK1 expression in CRC is correlated with CRC progression. TBK1 inhibition impaired CRC cell proliferation, migration, drug resistance and tumor growth. Bioinformatic analysis and experiments in vitro showed overexpressed TBK1 inhibited mTORC1 signaling activation in CRC along with elevated GLUT1 expression without inducing GLUT1 translation. TBK1 mediated mTORC1 inhibition induces intracellular autophagy, which in turn decreasing GLUT1 degradation. As a rescue, blocking of autophagosome and retromer respectively via autophagy-related gene 7 (ATG7) or TBC1 Domain Family Member 5 (TBC1D5) silence diminished the regulation of TBK1 to GLUT1. GLUT1 staining presented that TBK1 facilitated GLUT1 membrane translocation which subsequently enhanced glucose consumption. Inhibitor of TBK1 also decreased GLUT1 expression which potentiated drug-sensitivity of CRC cell. Collectively, TBK1 facilitates glucose consumption for supporting CRC progression via initiating mTORC1 inhibition induced autophagy which decreases GLUT1 degradation and increases GLUT1 membrane location. The adaptive signaling cascade between TBK1 and GLUT1 proposes a new strategy for CRC therapy.
Keywords: TBK1, GLUT1, Colorectal cancer, mTORC1, Autophagy

 Global reach, higher impact
Global reach, higher impact