10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(8):3470-3483. doi:10.7150/ijbs.73398 This issue Cite
Research Paper
A Positive Feedback Loop between Inactive VHL-Triggered Histone Lactylation and PDGFRβ Signaling Drives Clear Cell Renal Cell Carcinoma Progression
1. State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, Guangdong, China
2. Department of Colorectal Surgery and Guangdong Provincial Key Laboratory of Colorectal and Pelvic Floor Diseases, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China
3. Department of Urology, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China
4. Department of Colorectal Surgery, Sun Yat-sen University Cancer Center, Guangzhou, Guangdong, China
5. Department of Hepatobiliary Oncology, Sun Yat-sen University Cancer Center, Guangzhou, Guangdong, China
6. Department of Pancreatobiliary Surgery, Sun Yat-sen University Cancer Center, Guangzhou, Guangdong, China
7. Department of Head and Neck Surgery, Sun Yat-sen University Cancer Center, Guangzhou, Guangdong, China
8. Department of Ultrasound, Second Clinical College of Jinan University, Shenzhen People's Hospital, Shenzhen, Guangdong, China
9. Department of Pharmacy, The Third Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
*The authors (JFY, LL, CYZ, and XYL) were co-first authors.
#The authors (HSL, CZ, and SL) contributed equally as co-corresponding authors.
Abstract
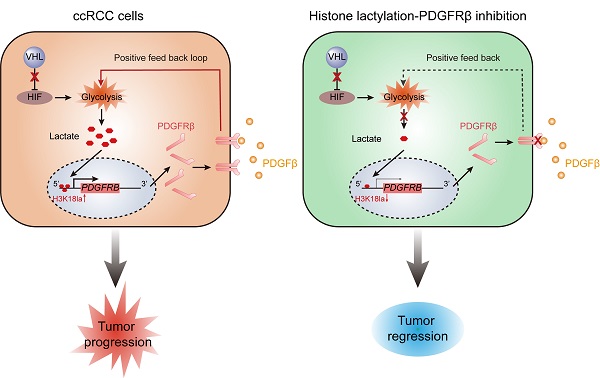
Inactive von Hippel-Lindau (VHL) is linked to metabolic reprogramming and plays pivotal roles in the pathogenesis of clear cell renal cell carcinoma (ccRCC). Here, we identify a previously unknown oncogenic role for inactive VHL in actively triggering histone lactylation to promote ccRCC progression. In patients with ccRCC, inactive VHL positively correlates with the presence of histone lactylation, and high levels of histone lactylation indicates poor patient prognosis. Inactive VHL-triggered histone lactylation contributes to ccRCC progression by activating the transcription of platelet-derived growth factor receptor β (PDGFRβ). In turn, PDGFRβ signaling is shown to stimulate histone lactylation, thereby forming an oncogenic positive feedback loop in ccRCC. Target correction of aberrant histone lactylation represses the growth and metastasis of ccRCC in vivo. More importantly, the combined inhibition of histone lactylation and PDGFRβ significantly reinforces the therapeutic efficacy. This work underscores the importance of histone lactylation in facilitating ccRCC progression and suggests targeting the positive feedback loop between histone lactylation and PDGFRβ signaling might provide a promising therapeutic strategy for ccRCC patients.
Keywords: Histone lactylation, inactive VHL, PDGFRβ, ccRCC

 Global reach, higher impact
Global reach, higher impact