Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(10):4088-4100. doi:10.7150/ijbs.69816 This issue Cite
Review
Extracellular Vesicles in Bone Homeostasis: Emerging Mediators of Osteoimmune Interactions and Promising Therapeutic Targets
Stomatology Hospital, School of Stomatology, Zhejiang University School of Medicine, Clinical Research Center for Oral Diseases of Zhejiang Province, Key Laboratory of Oral Biomedical Research of Zhejiang Province, Cancer Center of Zhejiang University, Hangzhou 310006, China.
#These authors contributed equally to the work and should be considered co-first authors.
Received 2021-12-7; Accepted 2022-5-27; Published 2022-6-21
Abstract
An imbalance in bone homeostasis results in bone loss and poor healing in bone diseases and trauma. Osteoimmune interactions, as a key contributor to bone homeostasis, depend on the crosstalk between mesenchymal stem cell-osteoblast (MSC-OB) and monocyte-macrophage (MC-Mφ) lineages. Currently, extracellular vesicles (EVs) are considered to be involved in cell-to-cell communication and represent a novel avenue to enhance our understanding of bone homeostasis and to develop novel diagnostic and therapeutic options. In this comprehensive review, we aim to present recent advances in the study of the effect of MC-Mφ-derived EVs on osteogenesis and the regulatory effects of MSC-OB-derived EVs on the differentiation, recruitment and efferocytosis of Mφ. Furthermore, we discuss the role of EVs as crucial mediators of the communication between these cell lineages involved in the development of common bone diseases, with a focus on osteoporosis, osteoarthritis, bone fracture, and periodontal disease. Together, this review focuses on the apparent discrepancies in current research findings and future directions for translating fundamental insights into clinically relevant EV-based therapies for improving bone health.
Keywords: Extracellular vesicle, Exosome, Osteoimmune interaction, Bone disease
Background
Osteoimmunology is an emerging field exploring immune processes and bone metabolism [1]. Bone cells (bone mesenchymal stem cells (BMSCs), osteoblasts (OBs), osteoclasts (OCs), and osteocytes), immune cells (T cells, B cells, neutrophils, and macrophages (Mφ)), hematopoietic stem cells (HSCs), and myeloid and lymphoid progenitor cells share the same micro-environment, this leads to them sharing a variety of molecules, functioning together as osteoimmunology. BMSCs, known as “universal cells”, have self-renewal capacity and multiple differentiation potency into OBs, chondrocytes, and adipocytes [2]. In pathological circumstances, significant unbalanced differentiation of BMSCs into adipocytes/chondrocytes occurs. Mφ as dynamic cells, derived from monocytes (MCs), participating in induction and resolution of metabolic/inflammation exhibit a significant degree of plasticity and heterogeneity through their function and biology. As important components of osteoimmunology, BMSCs and Mφ with same characteristic-remarkable genotypes, cellular phenotypes, and function plasticity sit at the center of a complex and tightly regulated system. Moreover, the intimate interactions between BMSC-OB and MC-Mφ are fundamental in maintaining bone homeostasis, as illustrated by the activation of tissue repair Mφ (M2) upon promoting BMSC osteogenic differentiation, a process that is impaired in most bone loss diseases (e.g. osteoporosis and periodontitis).
Brief description of mesenchymal stem cell-osteoblast (MSC-OB) lineage and monocyte-macrophage (MC-Mφ) lineage in bone tissues and their different modes of communication. (a) Conventional secretory pathways (including direct cell-cell contact and chemical receptor-mediated events) and (b) extracellular vesicles (EVs). Schematic picture was created with BioRender.
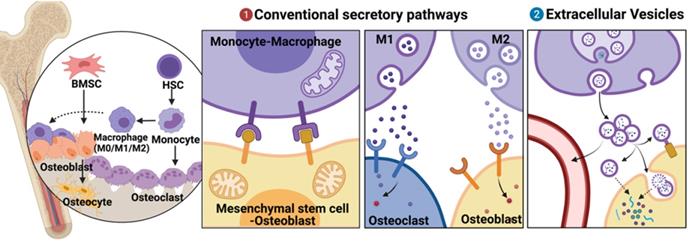
Mediators of osteoimmune interactions can be divided into two groups, namely, (i) conventional secretory pathways, including direct cell-cell contact and chemical receptor-mediated events (such as those involving cytokines and other chemical mediators) and (ii) extracellular vesicle (EV)-mediated events. The mechanisms of the former interactions have been thoroughly studied and elucidated [3, 4] (Fig. 1). However, EVs, which were considered only to be “garbage disposal” systems; are currently being increasingly recognized as playing important roles in short- and long-distance intercellular communications [5].
EVs are small vesicles of 30-150 nm in diameter with a lipid bilayer. Following the release of EVs by exocytosis from cells, they interact with the target cells and transport intracellular components including proteins, lipids, messenger RNAs (mRNAs), and microRNAs (miRNAs) to the cytosol of target cells via endocytosis [6]. EVs can also mediate signal cascades with secretory cells and the extracellular matrix (ECM) or can be released into the blood and lymphatic vessels to function in long-distance communication [7, 8]. As a cell-free therapeutic strategy for bone-related diseases and bone repair, EVs possess unique advantages such as nano size, non-toxicity, low immunogenicity, biocompatibility, and flexibility of use, thereby garnering attention [9]. There is now broad consensus that EVs are part of the intercellular signaling network that takes place in osteoimmunology. However, most studies were focused on two primary areas: intrinsic regulation in a single cell lineage (osteocyte, BMSCs, and osteoprogenitor) or cell-to-cell traffic in coupling of angiogenesis and osteogenesis. A comprehensive review of EVs-mediated regulation between MC-Mφ and MSC-OB is lacking.
Moreover, in terms of the mechanisms in communication between MC-Mφ and BMSC-OB via EVs, the most contentious aspect, at this time, are the functions of EVs derived from different types of Mφ on recipient MSC-OB cells. It should also be highlighted that regarding EVs derived from MSC-OB cells, impacting Mφ polarization should not always be seen as their main function; the other functions of these EVs (e.g., recruitment, efferocytosis, differentiation) are worth being known, despite being relatively understudied. The purpose of this article is to elucidate the crosstalk between MC-Mφ and MSC-OB in multiple ways, highlight cell-to-cell communication by EVs, cover advances in research on EVs derived from the MSC-OB lineage and MC-Mφ in bone disease. Finally, we discuss areas that are the most contentious and propose areas that are in particular need for further research in bone homeostasis.
MSC-OB regulation by MC-Mφ via EVs
MC-Mφ affect the proliferation, differentiation, recruitment, survival, function of MSC-OBs, and ultimately, bone homeostasis. MC migrates from the circulation to the local tissue and differentiates into Mφ, which plays specific roles. Mφ as highly heterogeneous and plastic cells can, depending on the stimulus and microenvironment, polarize into different types in vivo, including M0, M1, M2. Non-activated M0 can be classically activated by prostaglandin E2 (PGE2), tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6, which initiate the immune response and remove pathogens and tumor cells [10, 11].
Alternatively, the anti-inflammatory M2 phenotype is activated by IL-4, IL-13, or IL-10 and expresses high levels of arginase 1 (Arg1), IL-10, IL-1ra, and cluster of differentiation 206 (CD206), which plays a central role in tissue repair and neovascularization [4, 12]. In addition to Mφ, OC, which are also derived from the hematopoietic mononuclear precursor cells, can be stimulated by Mφ colony-stimulating factor (M-CSF) and receptor activator of NFκB ligand (RANKL) produced by OB to differentiate into bone resorptive cells [13]. These tissue-resident Mφ line the bone surface and contribute to osteoclastic bone resorption [14]. In this review, OC is included in the class of MC-Mφ according to their cell derivation.
Regulation of MSC-OB by MC-Mφ through conventional secretory pathways
Direct cell-to-cell contacts and chemical receptor-mediated events are conventional, effective regulatory mechanisms between the MSC-OB and MC-Mφ. It is reported that the osteal Mφ efferocytose apoptotic OB via αvβ3 or Mer linking proteins milk fat globule factor (MFG)-E8 or growth arrest specific 6 (Gas6) [15]. In addition, the direct contact between OB and OC involves interactions among ephrin B2(EFNB2)-ephrin type-B receptor 4 (EPHB4), FAS-Fas ligand (FASL), and neuropilin 1(NRP1)-semaphorin(SEMA3A), and it regulates cell proliferation, differentiation and survival [16].
In chemical receptor-mediated event regulation, M1 respond to the inflammatory environment, promoting inflammatory osteoclastogenesis via M-CSF, TNF-α, IL-1β, IL-6, IL-17, and IL-23 secretion [17]. M2 mediate the recruitment and differentiation of BMSCs by secreting CC motif chemokine ligand 2 (CCL2), CXC motif chemokine ligand 8 (CXCL8), stromal cell-derived factor 1 (SDF-1), and other chemokines.
Biomaterials can modulate Mφ polarization by inhibiting NFκB phosphorylation or by promoting the release of vascular endothelial growth factor (VEGF) and Arg1 [19,20]. These effects are mediated through the activation of bone morphogenic protein (BMP)-2/Smad and phosphoinositide 3-kinase (PI3K)/AKT signaling pathways, which facilitates bone tissue regeneration [18, 19]. Thus, converting the Mφ phenotype from pro-inflammatory to anti-inflammatory by modulating cytokines and signaling pathways, eventually alter its function and its capability to regulate the direction of BMSC differentiation and promote osteogenesis.
Effect of MC-Mφ-derived EVs on osteogenesis
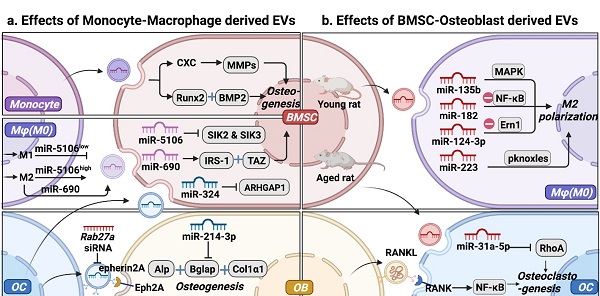
EVs, as an important medium of intercellular communication, carry molecular cargo and transfer bioactive components (Table 1 and Fig. 2a). Signals activated in MC promote the differentiation of BMSCs into bone lining cells. Karin et al. [20] demonstrated that human MC-EVs improve the secretion of RUNX2 (RUNX family transcription factor 2) and BMP-2 (bone morphogenic protein-2) in hBMSCs, promoting osteogenic differentiation. Arjen et al. [21] reported that MC-EVs upregulated the expression of genes coding matrix metalloproteinases (MMPs) and increased the secretion of CXC, thereby stimulating processes related to the reorganization of the ECM structure, directing differentiation towards bone lining cells, which could differentiate into bone-forming OB. Interestingly, exposure in the inflammatory context may not undermines MC-EVs function in osteogenesis. Ekström et al. demonstrated that LPS-stimulated human MCs releasing EVs promoted the gene expression of the osteogenic markers (e.g., RUNX2, BMP2) [22]. These findings indicate that the mechanisms by which MC-derived EVs promote osteogenesis are uncertain and require further investigation.
Mφ, as an essential component of the innate immune system, and bone marrow derived Mφ (BMM) is also closely associated with OB in the endosteal and periosteal surfaces. Furthermore, they present different phenotypes and functions in different microenvironments, and EVs derived from different Mφ phenotypes (M0-EVs, M1-EVs, and M2-EVs) play different roles in osteogenesis. Yu et al. [23] demonstrated that EVs secreted by different Mφ phenotypes could be internalized by BMSCs. Specifically, M1-EVs promoted the proliferation of BMSCs, with the highest expression levels of alkaline phosphatase (ALP), RUNX family transcription factor 2 (Runx2), osteocalcin (OCN), and BMP-2. In contrast, M2-EVs impaired the proliferation of BMSCs, whereas M0-EVs did not significantly influence the proliferation of BMSCs [23]. However, the unique role of different polarized Mφ in osteogenesis has not yet been elucidated. Yuan et al. [24] demonstrated that miR-5106 is highly enriched in M2-EVs and that it can be transferred to BMSCs where it targets SIK2 and SIK3, thereby accelerating bone remodeling. MiR-5106 expression reportedly decreased in M1-EVs, and M1-EV-treated cells exhibited similarly reduced mineral deposition and low levels of ALP compared with untreated cells [24]. These findings indicate that EVs from the same phenotype Mφ have different effects on the osteogenic differentiation of BMSCs. M2-EVs increased the expression of miR-690, IRS-1, and TAZ in BMSCs, inhibiting adipogenesis and promoting osteogenesis of BMSCs [25]. EVs with complex cargos have been suggested to play different roles in BMSC differentiation. Alternatively, different Mφ phenotypes regulate the osteogenesis of BMSCs through different signal pathways, depending on EVs and cytokines, separately or jointly [26].
Published Studies on Biogenesis of monocyte-macrophage (MC-Mφ) lineage derived EVs in osteogenesis
| Source and Kind | Methods: isolated and identified | Specific Cargo | Target cells and Genes | Function | Signaling Pathway | References |
|---|---|---|---|---|---|---|
| human monocytes-EVs | ·The miRCURY™ Exosome Isolation Kit OR ultracentrifugation: 120,000g for 2h, filtration through a 0.1-μm filter ·TEM, Western blot (CD90, TSG101, CD63 and Hsp70) | - | ·Human ATMSCs and Human BMSCs | Osteogenesis ↑ | MC‐EVs→MSC various cytokines by MSCs↑ (CXC chemokines and IL-1) → expression of MMPs↑ →Osteogenesis ↑ | Arjen Gebraad, 2018 |
| human monocytes-EVs | ·Centrifuged: 16,500g for 20 min, followed by filtration through a 0.22 µm filter ·Western blot (Hsp70, Tsg101 calnexin and Grp94), Flow cytometry (CD63, CD9, CD81) and Bioanalyzer analysis | - | ·Human BMSCs | Osteogenesis ↑ | Human monocytes-EVs improve the secretion of RUNX2 and BMP-2 in hBMSCs, promoting osteogenic differentiation | Karin Ekström, 2013 |
| Mouse M0 macrophages-EVs | ·Centrifuged: 2,000g for 30 min, and total exosome isolation reagent, then centrifuged at 10,000g for 60 min at 4 °C TEM, NTA, Western blot (CD81, CD63, CD9 and Alix) | - | ·Mouse BMSCs | No significant influence on the proliferation of BMSCs | - | Yu Xia, 2020 |
| Mouse M1 macrophages-EVs | ·Centrifuged: 2,000g for 30 min, and total exosome isolation reagent, then centrifuged at 10,000g for 60 min at 4 °C TEM, NTA, Western blot (CD81, CD63, CD9 and Alix) | - | ·Mouse BMSCs | Osteogenesis ↑ | M1-EVs promoted the proliferation of BMSCs (7-day time) | Yu Xia, 2020 |
| Mouse M2 macrophages-EVs | ·Centrifuged: 2,000g for 30 min, and total exosome isolation reagent, then centrifuged at 10,000g for 60 min at 4 °C ·TEM, NTA, Western blot (CD81, CD63, CD9 and Alix) | - | ·Mouse BMSCs | Osteogenesis ↓ | M2-EVs impaired the proliferation of BMSCs (7-day time) | Yu Xia, 2020 |
| M2 macrophages-EVs | ·Ultracentrifugation ·TEM, DLS, flow cytometry (CD63, CD81) | miR-5106 | ·Mouse BMSCs ·SIK2 and SIK3 | Osteogenesis ↑ | M2-EVs containing miR-5106 promote osteogenic differentiation of BMSC via suppressing the expression of SIK2 and SIK3 | Yuan Xiong, 2020 |
| Mouse M2 macrophages-EVs | ·MinuteTM efficient exosome precipitation reagent purchased from Inent Biotechnologies Company ·TEM, NTA, Western blot (CD81, CD63) | miR-690 | ·Mouse BMSCs ·IRS-1 and TAZ | Osteogenesis ↑ | M2-EVs delivered miR-690 into BMSCs and increased the expression of IRS-1 and TAZ | Ziyi Li, 2021 |
| Osteoclasts-EVs | ·Ultracentrifugation: 2000g for 20 min, 20,000g for 30 min, 120,000g for 70 min at 4 °C ORTotal Exosome Isolation Kit:10,000g for 1h at 2-8 °C ·Dynamic light scattering, Western blot (HSP70, TSG101, TFIIB and LaminA/C) and Flow cytometry (CD963) | mir-214 | ·Osteoblast ·EphrinA2/EphA2 | Osteogenesis ↓ | OC-EVs containing miR-214 though ephrinA2/EphA2 ligand induced osteoblast dysfunction, and the down-regulation can be rescue by Rab27a small interfering RNA | Weijian Sun, 2016 |
| Osteoclasts-EVs | ·Ultracentrifugation: 300g for 10 min, 820g for 15 min, 10,000g for 5 min at 4 °C and passage through a 0.8-μm syringe filter to remove cell debris, and final centrifugation at 100,000g for 2h at 4 °C | miR-214-3p | ·Osteoblast | Osteogenesis ↓ | Osteoclast-derived exosomal miR-214-3p could be transferred into osteoblasts to inhibit osteoblastic bone formation | Defang Li 2016 |
| Osteoclasts-EVs | ·Ultracentrifugation: 2000g for 15 min, 12,000g for 15min at 4 °C and 100,000g for 2h at 4 °C ·TEM, NTA, Western blot (Lamin A/C, histone 3 CD81 and TSG101) | ·miR-324 | ·Mouse BMSCs ·ARHGAP1 | Osteogenesis ↑ | Osteoclast-derived exosomal down-regulated ARHGAP1 in the RhoA/ROCK pathway to promote osteogenic differentiation | Mengmeng Li ang, 2021 |
OC functions as the only giant multinucleated cell, deriving from Mφ precursor and is mainly involved in bone resorption [27]. OC participates in normal bone accrual, growth, and modeling, thereby maintaining bone metabolism through calcium metabolism and its lifetime integrity [28]. OC-derived EVs (OC-EVs) contain multiple bone regulatory proteins that modulate OB formation [29]. Huynh et al. [29] found that RANK-rich OC-EVs inhibit the formation of OC-like multinucleated cells by suppressing the interaction of RANKL-RANK [30]. Interestingly, they found that OC-EVs, but not pre-OC-EVs, are rich in RANK, which promotes the differentiation of OC rather than the inhibition of OC-EVs in the formation of OC [30]. Sun et al. [31] found that OC-EVs bound to OB through an EFNA2/EphA2 interaction to impair OB function by releasing miR-214. Li et al. [32] reported that OC-derived exosomal miR-214-3p could be transferred into OB to suppress bone formation. Liang et al. [33] found that OC-EVs were rich in miR-324, whereas ARHGAP1, which inhibits osteogenic differentiation, was downregulated and the miR-324-enriched EV-modified scaffold promoted bone regeneration. These observations suggest that OC can activate OB on the other side of the bone through OC-EVs, similar to the coupling factors that mediate cell-cell coupling. The mechanism by which OC-EVs influence bone remodeling still needs to be investigated to improve our understanding of the interaction between OB and OC as a crucial mechanism of maintaining cell homeostasis [34]. In the bone microenvironment, EVs act as carriers of bioactive molecules that regulate osteogenesis through MC, modulate the Mφ polarization phenotype, and regulate OC, representing a new mode of intercellular communication. Subsequently, OC secretes EVs that carry different cargos, which differentially regulate osteogenesis.
Intercellular communication between mesenchymal stem cell-osteoblast (MSC-OB) lineage and monocyte-macrophage (MC-Mφ) lineage is mediated by extracellular vesicles (EVs). (a) Effect of MC-Mφ-derived EVs on osteogenesis. (b) MSC-OB-derived EVs regulate monocyte-macrophages recruitment, polarization, and function. Schematic picture was created with BioRender.
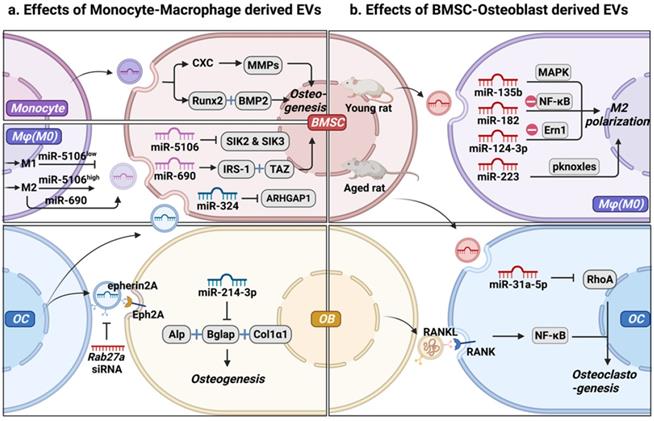
MC-Mφ regulated by MSC-OB via EVs
A dense spongy layer containing the commonly reported MSC-OB, including bone BMSC, OB, and osteocyte [35], serves as a barrier to protect the bone marrow. BMSCs have the potential for self-renewal and multidirectional differentiation [36]. OB is derived from mesenchymal precursor, which is transformed to committed pre-OB following the expression of RUNX2 and Osterix. These cell lineages in the bone surface continue to differentiate into matrix-producing OB [37]. Osteocytes are derived from OB embedded in the bone matrix and constitute over 90% of cells in the bone [38].
MC-Mφ are important immune cells in the bone marrow that are involved in inflammation and immune regulation, this suggests that in bone injuries or osteoarthritis, MSC-OB and MC-Mφ are tightly interrelated.
Regulation of MC-Mφ by MSC-OB through conventional secretory pathways
Studies on MSC-OB to MC-Mφ have focused on their secreted soluble factors such as M-CSF and transforming growth factor (TGF)-β [39]. A few studies have reported the direct cell-to-cell contact of MC-Mφ as target cells, and we found that most related studies have been based on BMSCs, and not on other types of MSC-OB [34]. BMSCs have been shown to reciprocally modulate Mφ polarization phenotypes and regeneration via multiple signaling pathways, such as the NFκB, signal transducer and activator of transcription 3 (STAT3), and Akt pathways [39-41]. BMSCs regulate Mφ recruitment and phenotypic polarization to promote tissue regeneration by secreting chemokines, cytokines, and other signaling factors [35]. In conclusion, BMSCs attenuate injury and promote healing by modifying the polarization status of Mφ and suppressing their inflammatory reaction.
MSC-OB-derived EVs regulate Mφ
BMSCs are multipotent stem cells found in the bone marrow and are attractive sources of regenerative medicine [42, 43]. They have strong paracrine, anti-inflammatory, immunoregulatory, and angiogenic capabilities [44]. In addition to the growth factors and cytokines produced by BMSCs, EVs have gained interest as intriguing signals that can shuttle payloads between cells. Recent studies have shown that the therapeutic benefits of BMSCs can be attributed to their EVs, and their use represents a potential cell-free strategy for osteogenesis [45]. Moreover, BMSC-EVs could be powerful tools for Mφ recruitment, polarization, and functionalization [46] (Table 2 and Fig. 2b). BMSC-EVs promote the regeneration of periodontal tissues, partially through their involvement in regulating Mφ polarization and TGF-β expression to modulate the inflammatory immune response [47]. Xu et al. [47] found that BMSC-EVs transformed Mφ subsets from the M1 to M2 phenotype in vitro under lipopolysaccharide (LPS) stimulation and reduced post-infarction inflammation by increasing M2 and degrading M1 polarization in the ischemic heart [48]. In tendon-to-bone healing, Shi et al. [48] demonstrated that BMSC-EVs promoted fibrocartilage formation by increasing M2 polarization [49]. In addition to identifying functional changes, studies have traced molecular mechanisms. For examples, Wang et al. showed that BMSC-EVs attenuated cartilage damage by carrying highly expressed miR-135b. This effect promoted M2 polarization of SMs by targeting mitogen-activated protein kinase (MAPK) [50]. Thus, BMSC-EVs are crucial candidates for the immunomodulation of Mφ polarization. Zhao et al. [51] proved that BMSC-EVs attenuated myocardial ischemia-reperfusion by shuttling miR-182, which modified Mφ polarization through the Toll-like receptor 4 (TLR4)/NFκB pathway. Li et al. [3] demonstrated that exosomal miR-124-3p derived from BMSCs attenuated nerve injury induced by spinal cord ischemia/reperfusion injury (SCIRI) by regulating endoplasmic reticulum to nucleus signaling 1 (Ern1) and M2 polarization. However, the expression of miR‐31a‐5p was notably higher in BMSC-EVs from aged rats than in those from young rats. This effect increased the osteoclastic number and function by blocking E2F2 activity, resulting in SAHF assembly, and the dysfunction was reversed by miR‐31a‐5p antagomir [52, 53]. Overall, we propose that MSC-EVs effectively modify Mφ polarization and promote cementogenic differentiation in the bone microenvironment. In turn, BMSC-EVs regulated M1 to M2 switching in Mφ and maintained the presence of M2 to promote tissue repair, thus forming a strong positive feedback effect.
Published Studies on Biogenesis of mesenchymal stem cell-osteoblast (MSC-OB) lineage derived EVs in monocyte recruitment, polarization and function
| Source | Methods: isolated and identified | Specific Cargo | Target cells and Genes | Function | Signaling Pathway | References |
|---|---|---|---|---|---|---|
| Rat BMSC-EVs | ·Density‐gradient ultracentrifugation :2 subsequent centrifugations steps of 2500g for 15 minutes ·NTA, TEM and Western blot (CD63, CD81, HSP70 and Tsg101) | - | ·Raw264.7 and rat peritoneal macrophages | M2 polarization ↑ M1 polarization ↓ reduced the inflammation | BMSC-EVs + LPS+ Raw264.7→NF‐κB p65 ↓ → AKT1 /AKT2 → M2 ↑, M1 ↓ →IL‐6, TNF‐α, IL‐1β ↓ | Ruqin Xu, 2019 |
| Rat BMSC-EVs | · Utral centrifuged: 300g for 10 min, 2000g for 10 min. After centrifugation, 0.22 μm Steritop™ and Amicon ultra-15 spinning Filter Unit, the liquid was centrifuged at 100,000g for 60 min ·TEM, Western blot (CD9, CD63 and CD81) | - | ·HUVECs and macrophages | M1 polarization ↓ secretion of proinflammatory factors by M1 macrophages↓. | BMSC-EVs + HUVECs→ phosphorylation levels of VEGFR1 and VEGFR2 ↑, LATS1/2 and YAP1 ↓ BMSC-EVs→ M1 ↓ → TNF-α, IL-1β, IL-6, IL-8, and NOS-2 ↓ | Yao Huang, 2020 |
| Mouse BMSC-EVs | ·Ultracentrifugation: 2000g for 30 min, 10,000g for 30 min, 100 000g for 70 min; ·Zeta View system, TEM and Western blot (CD81, TSG101, and CD9) | - | ·Mouse Bone marrow-derived macrophages | M2 polarization ↑ M1 polarization ↓ | BMSC-EVs→ M2 polarization ↑ →fibrocartilage and biomechanical property ↑ | Youxing Shi, 2020 |
| Mouse BMSC-EVs | ·Filtration through a 0.22-μm filter and exosome isolation kit. ·TEM and flow cytometry (CD90) | miR-124-3p | ·Mouse Bone marrow-derived macrophages · Ern1 | M2 polarization ↑ | BMSCs containing miR-124-3p + macrophage →Ern1 expression ↓ → M2 ↑ | Ran Li, 2020 |
| Rat BMSC-EVs | ·Ultracentrifugation: 300g for 10 min, 2000g for 15 min, 10,000g for 30 min, and 100,000g for 70 min twice ·TEM, NTA and Western blot (CD9, CD63, TSG101, and Calnexin) | miR-31a-5p | · Osteoclasts · E2F2, SAHF | Osteoclasts numbers and function↑ | miR‐31a‐5p→ E2F2 ↓ → SAHF assembly induces cellular aging→ osteoclastic numbers and function↑ | Rongyao Xu, 2018 |
| Human JMSC and BMSC-EVs | ·Ultracentrifugation: 100,000g for 3 h, 3000g for 15 min, mixed with ExoQuick-TC, then centrifuged at 1500g for 30 min. Western blot (CD63 and CD81) and NTA | miR-223 | ·Human peripheral blood PBMC-derived macrophage pknox1 | M2 polarization ↑ Enhances cutaneous wound healing | miR-223 + BMSCs→ targeting pknox1→M2 polarization ↑ | Xiaoning He, 2019 |
| Mouse Osteoblast-EVs | ·Ultracentrifugation: 5000g for 10 min, 35,000g for 10 min, 100,000g for 70 min at 4 °C. ·Western blot (CD63) and TEM | - | · Osteoclasts | Osteoclasts numbers and function↑ | Osteoblasts-EVs via RANKL-RANK → osteoclast formation↑ | Alfredo Cappariello, 2018 |
| Osteocyte-EVs | - | - | - | - | - |
Furthermore, MCs could differentiate into bone resorbing OCs, which are independent of Mφ polarization, as OCs differentiate preferentially with pro-inflammatory MCs [54], whereas inducers that replace activated Mφ inhibit OC osteocyte differentiation [55]. Li et al. [56] demonstrated that EVs shed from OB containing the RANKL protein could be internalized by OC precursors via the stimulation of RANKL-RANK signaling to facilitate OC formation. A similar result was reported by Cappariello et al. [57], they found that OC-EVs interacted with OC precursors through the RANKL-RANK mechanism because RANKL-/- EVs did not preserve OC functionality.
Despite the scarcity of studies, the few that exist appear to demonstrate the efficacy of MSC-OB-derived EVs in Mφ recruitment. Huang et al. found that in contrast to young MSC-EVs, aging MSC-EVs failed to alter Mφ phenotypes and reduce Mφ recruitment [58]. After isolation of EVs of fibrodysplasia ossificans progressiva patients, analysis of the liquid chromatography/mass spectrometry results revealed that the heterotopic ossification occurs via the Ephrin B signaling pathway by aiding in Mφ chemotaxis and activation [59].
Apart from EVs secreted by viable BMSCs, apoptotic vesicles (apoVs) produced by apoptotic BMSCs have also been implicated in the regulation of Mφ functions. Zheng et al. [60] found that BMSC-derived apoVs could induce Mφ reprogramming in an exocytosis-dependent manner in the treatment of type 2 diabetes livers. Mechanistically, they demonstrated that proteins in BMSC-derived apoVs contribute to Mφ polarization towards the anti-inflammation phenotype. This phenomenon, however, has not yet been shown to occur in bone homeostasis. Given the increased fracture risk in adults with type 2 diabetes, future relative studies into the BMSC-derived apoVs applied in bone defect repair in diabetes mellitus will be of particular interest.
EVs are crucial mediators between MSC-OB and MC-Mφ in bone disease
Osteoporosis (OP)
Osteoporosis (OP) is the most common metabolic bone disorder characterized by low bone volume and microarchitectural destruction, which leads to susceptibility to bone fragility and fracture [61], especially in the aging population [62]. The dysfunctional activities of OB and OC are the primary causes of OP (Table 3 and Fig. 3a) [63]. OC, the only bone resorption cells, are also the current research priority. Li et al. [54] observed that miR-214-3p is rich in OC, and that its level in EVs increased. Furthermore, bone formation decreased in OC-specific miR-214-3p knock-in mice, and the OC-targeted RNA antagonist antagomir-214-3p reversed the inhibition in ovariectomized (OVX) mice [57]. Sun et al. [64] found that OC-EVs specifically recognized OB through the interaction between EFNA2 (carried by OC-EVs) and EphA2 (on OB). In addition, miR-27a has been found to be more highly expressed in normal mice than in OVX mice, and that it interacts with Dickkopf WNT signaling pathway inhibitor 2 (DKK2) to reduce the number of OCs, while reversing OP symptoms [65]. These results indicate that OC-EVs contain molecular cargo and regulate cell communication between OC and OB to inhibit bone formation.
Despite the imbalance between OB and OC, OP is also associated with a lack of sex steroid deficiency and chronic inflammation of aging [66]. There appears to be an important role for Mφ in the etiology of the bone disorder. These are accompanied by increased secretion of inflammatory cytokines, including TNFα, IL-1β, and IL-6, which increase the expression of RANKL, as a TNFα superfamily member in the bone marrow micro-environment, and is necessary for osteoclastogenesis [67]. Functionally, the osteogenic differentiation potential of BMSCs and the capacity for anti-inflammatory M2 polarization were notably decreases with increasing donor age [68, 69]. Mφ-derived EVs, as intercellular messengers, contain several hundred proteins such as annexins, heat shock protein (HSP)-60, HSP-70, and fibronectin, and mainly affect the differentiation of BMSC, OB, and OC. The accumulation of age-related molecules packaged by EVs in the bone microenvironment could cause OP [68]. Moreover, enhanced differentiation of BMSCs to adipocytes in the development of obesity, could also induces OP [70]. In the obese state, the balance is clearly tilted toward the proinflammatory macrophage phenotype, which induces BMSCs differentiate toward the adipogenic lineage.
However, many of the important questions about the extent to which MSCs are influenced by Mφ-derived EVs in pathological conditions of osteoporosis in obese people will require further research to answer. Comprehensive studies of EVs derived from Mφ in OP are far worse than those in cytokines, and more research on the cargo they carry is needed, which would provide a strategy to upregulate or downregulate intercellular messengers to interfere with the function of bone-resorbing OC (Fig. 3a). EVs are novel mediators in intercellular communication, and it is plausible that the modulation of EVs derived from Mφ rather than those from M2 could be a promising treatment strategy for individuals with OP.
Potential of extracellular vesicles (EVs) derived from bone-related cells and monocyte-macrophages in bone disease therapy. (a) Osteoporosis (OP), (b) bone fracture, (c) osteoarthritis (OA), and (d) periodontal disease. Schematic picture was created with BioRender.
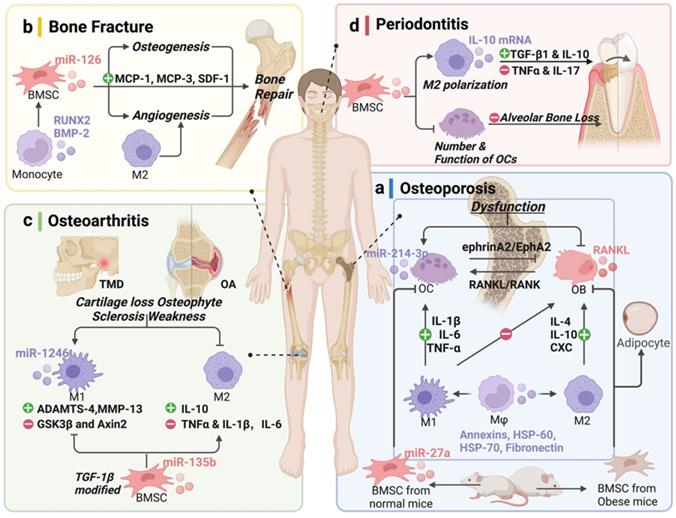
Function of bone relative or monocyte-macrophage lineage EVs in bone diseases (summarized above not be mentioned here)
| Disease | Source and Kind | Specific Cargo | Function | Regulatory details | References |
|---|---|---|---|---|---|
| Osteoporosis | Mice BMSC-EVs | miR-27a | Osteogenesis↑ bone density↑ levels of bone resorption markers↓ Osteoclasts number↓ | miR-27a inhibit DKK2 expression via Wnt/β-catenin pathway | Yan Wang, 2021 |
| Osteoarthritis | Mice BMSC-EVs | - | M2 polarization ↑ M1 polarization ↓ | Decrease the percentages of F4/80+ macrophages Down-regulate TNF-α Up-regulate IL-10 | Stella Cosenza, 2017 |
| Rat BMSC-EVs | M2 polarization ↑ M1 polarization ↓ | Decrease the expression of IL-1β, IL-6, and TNF-α, whereas IL-10 is released. | Jiyong Zhang, 2020 | ||
| Rat BMSC-EVs | miR-135b | M2 polarization ↑ M1 polarization ↓ | TGF-β1 modified BMSC-EVs via delivering miR-135b up-regulate the lower levels of serum inflammatory cytokines and induce the polarization of synovial macrophages to M2 in OA rats. | Rui Wang, 2021 | |
| TMJ inflammation | Mice M1-EVs | miR-1246 | Induce inflammation in condylar chondrocytes | miR-1246 inhibits GSK3β and Axin2 expression, causing activation of the Wnt/β-catenin pathway | Sisi Peng, 2021 |
| Bone fracture | Mice BMSC-EVs | miRNAs | Promote bone repair | Up-regulate expression of monocyte chemotactic protein-1 (MCP-1), MCP-3, and stromal cell-derived factor-1, maybe via miRNA | Koichi Murata, 2016 |
| Periodontitis | Rat BMSC-EVs | - | Inflammatory infiltration↓ Bone loss↓ | Decreased TNF-α and IL-17; increase IL-10 secretion, reduce osteoclast number | Yixin Zhang, 2018 |
| Mice BMSC-EVs | - | Alveolar bone loss↓ Inflammatory infiltration↓ Collagen destruction↓ | Regulate the function of osteoclasts and affect the macrophage polarization and TGF-β1 expression Regulate the OPG-RANKL-RANK pathway | Li Liu, 2021 | |
| Mice M2-EVs | IL-10 mRNA | osteogenesis↑ osteoclastogenesis ↓ | M2-EVs could activate the cellular IL-10/IL-10R pathway via delivering exosomal IL-10 mRNA to cells directly, regulating cell differentiation and bone metabolism. | Xutao Chen, 2022 |
Bone fracture
In bone fracture therapy, good blood transport and stable fixation are the basic necessary conditions [71]. Mφ and MSC-OB are the two most important cell lineages involved in fracture healing and bone remodeling [72]. Bone healing is characterized by a cascade of well-regulated complex biological processes involving different cell types [73]. During fracture healing, Mφ is found in the fracture site, and their depletion impairs effective healing [74]. Mφ has been reported to regulate inflammation through cytokine signaling and, more importantly, endocytosis and exocytosis in acute and chronic inflammation.
MSC-OB and MC-Mφ participate in the inflammatory regulation of the fracture site (Table 3 and Fig. 3b). Bobby et al. [75] found that MC-derived EVs increased RUNX2 and BMP-2 levels, indicating the osteogenic differentiation of MSCs. BMSC-EVs regulate the expression of MC chemotactic protein-1 (MCP-1), MCP-3, and SDF-1 promote bone repair [76]. Moreover, because osteogenesis and angiogenesis are closely linked, EVs promote fracture healing by regulating the entire osteogenesis-angiogenesis process or Mφ polarization-angiogenesis coupling.
The role of M2 in promoting angiogenesis and wound healing has been clarified [77]. Mφ-EVs have angiogenetic potential in vitro and in vivo and could serve as a pro-angiogenic treatment for ischemic diseases [78]. These studies indicate that EVs not only participate in bone matrix generation and mineralization, but also show potential as a diagnostic tool, especially in some underlying diseases such as diabetes, autoimmune disease, and tumors [79].
Osteoarthritis (OA)
Osteoarthritis (OA) is a chronic joint disorder that results in joint pain and functional impairment [80]. It is mainly characterized by cartilage degradation, synovial inflammation, subchondral bone erosion, and osteophyte formation [81]. The most common risk factors for OA are age, sex, prior joint injury, obesity, genetic predisposition, and mechanical factors [82]. Currently, there are no effective curative therapies for OA, and because of their extensive proliferation and differentiation capacities, BMSC-EVs represent a promising approach to OA therapy.
In addition to the cartilage protective and regenerative effects of BMSC-EVs, their role in the immunomodulatory effect of Mφ has also been demonstrated, as Mφ and other innate immune cells release inflammatory cytokines, which promote cartilage damage [71, 83]. The increase in M1 is accompanied by hyperactivation of ADAMTS like 4 (ADAMTS-4) and MMP-13, and it aggravates cartilage loss, osteophyte formation, subchondral sclerosis, and periarticular weakness [84].
M2 polarization promotes articular homeostasis and regeneration in OA [9]. In temporomandibular joint (TMJ) inflammation, M1-EVs transfer miR-1246 to inhibit glycogen synthase kinase β (GSK3β) and Axin2 expression and, then, upregulate IL-6, IL-8, IL-1β, and MMPs, accelerating inflammation [85]. Interestingly, BMSC-EVs mediate the transformation of Mφ from the M1 to M2 phenotype to reduce joint tissue damage by inhibiting proinflammatory cytokines and releasing anti-inflammatory cytokines and transforming the percentage of CD4+CD8+ T cell subsets [86, 87]. BMSC-EVs inhibit M1 production and promote M2 generation. Synovial fluid expresses lower levels of the proinflammatory cytokines, IL-1β, IL-6, and TNF-α, whereas IL-10, an anti-inflammatory cytokine, is released. TGF-β1-modified BMSC-derived EVs via miR-135b corroborate this result [88]. Moreover, the 3D-printed ECM/gelatin methacrylate/exosome scaffold promoted the polarization of synovial Mφ to M2, exhibiting a potential therapy [89] (Table 3 and Fig. 3c). These studies revealed that EVs have the potential to effectively protect cartilage from degeneration and attenuate OA progression by modulating immunoreactivity, promoting the proliferation and migration of human OA chondrocytes, and regulating Mφ polarization.
Periodontitis
Periodontal diseases are initiated by dysbiosis of the commensal oral microbiota, which leads to tooth loss and could contribute to systemic inflammation [90]. Alveolar bone loss is a sign of periodontitis progression, and results from host immune and inflammatory responses to microbial challenges [91]. Therefore, modulating the host inflammatory and immune cell processes is a promising strategy to rescue bone resorption in periodontitis. Mφ play an important role in the innate immune system and interact with oral pathogens to influence the balance of the oral microbial community. Furthermore, balancing the M1/M2 ratio is a novel prospect.
BMSC-EVs promote the regeneration of periodontal tissues by inhibiting the function of OCs, affecting Mφ polarization, and regulating TGF-β1 expression, thereby modulating the inflammatory immune response [45]. Similarly, Zhang et al. [92] reported that BMSC-EVs decreased alveolar bone loss by regulating the expression of related cytokines and inhibiting osteoclastic bone resorption. M2-EVs could upregulate the IL-10 cytokine expression of BMSCs and BMMs via activating exosomal IL-10 mRNA to cells directly, which could promote osteogenesis while inhibiting osteoclastogenic differentiation and alveolar bone resorption [93]. Therefore, the application of BMSC/Mφ-derived EVs for the regeneration of periodontal tissue is a promising treatment strategy (Table 3 and Fig. 3d).
Controversy and future perspective
Although there are many areas of consensus regarding mechanisms of cell-cell communication via EVs in osteoimmunology, as with any rapidly growing field, there remain several challenges and areas of disagreement. It rapidly became clear that different Mφ phenotypes (M0, M1, and M2) have been proven to be involved in osteogenesis, and that M2 is the most beneficial for osteogenesis [94]. However, there is still no consensus on which EVs secreted by different Mφ phenotypes promote osteogenesis the most [94]. Yu et al. [84] found that the relative expression of osteogenesis genes was upregulated by M1-EVs but downregulated by M2-EVs. Conversely, Kang et al. [95] demonstrated that M0 and M2-EVs promoted repair/regeneration and M1-EVs inhibited bone repair. Mechanistically, they found similar miRNA cargo in M0 and M2 EVs and different miRNA cargo in M1 EVs. These controversial results could be partially explained by duration of action, culture conditions, and differences in techniques such as cell source, maturity of MC-Mφ, and co-culture conditions. Despite there is some debate, it is clear that EVs derived from MC and all Mφ phenotypes have the ability to regulate the osteogenesis of BMSCs, but the effectiveness of the process varies with physiological conditions and different phases through various signaling pathways that deliver different cargoes, resulting in different functions. In addition to conditioned media, intercellular communication can also be mediated through processes such as gap junctions, and autocrine and paracrine activities [32]. Overall, these conflicting results reflect the complexities of the regulation of EVs on MC-Mφ and MSC-OB, especially considering that the human body and the in vivo environment are extremely complex.
Recently, studies have reported novel discoveries and forms of EVs in bone metabolism regulation, and EVs secreted by bacteria and the damaged brain are two examples. All bacteria produce vesicles for the selective export of toxins and other virulence factors into host cells. Vesicles provide a self-preserving membrane remodeling mechanism despite their high energy cost [96, 97]. Vesicles from Aggregatibacter actinomycetemcomitans, the causative organism of periodontal and systemic diseases, can cross the blood-brain barrier to deliver small RNAs, which produce TNF-α [98]. This observation suggests that EVs from the bone microenvironment can be delivered to the entire body and be activated. Conversely, systematic EVs influence bone homeostasis by resisting the local microenvironment. Xia et al. [99] demonstrated that injured neurons in the damaged brain, mainly in the hippocampus, release EVs to accelerate bone formation through miR-328a-3p and miR-150-5p targeting forkhead box O4 (FOXO4) or Cbl proto-oncogene (CBL), respectively.
Currently, bone metabolism is drawing considerable attention. HucMSC-EVs enhanced the shift from adipogenic to osteogenic differentiation of BMSCs and inhibited OC formation by transferring C-type lectin domain containing 11A (CLEC11A). HucMSC-EVs also improved bone formation, reduced marrow fat accumulation, and downregulated bone resorption in OVX and tail suspension-induced hindlimb disuse osteoporotic mice [100]. Studies have focused on lipid metabolism and the function of glucose and amino acid metabolism in the bone microenvironment. Furthermore, the mechanism by which the metabolites are delivered is worth future investigation. More interestingly, Islam et al. found that mitochondrial DNA and mitochondrial cargo of BMSCs could be delivered to Mφ via EVs [101]. These “useless” damaged mitochondria in BMSCs are “treasures” for Mφ, which can enable Mφ to express greater bioenergy [102].
The emphasis in this Review has highlight major concepts of how EVs functioning in intriguing biological links between BMSCs osteogenesis and M2 activation in middle and advanced stage of bone reconstruction/remodeling. Such interaction between Mφ and BMSCs based on EVs can summarize as a positive feedback or a vicious circle. M2 polarization promotes the osteogenic differentiation of BMSCs, increasing the number of OBs and leading to the secretion of a large number of “anti-inflammatory EVs” in benefiting to switching inflammation bone microenvironment. Conversely, BMSCs switching to adipogenesis will promote M1 differentiation, producing a large amount of “inflammatory EVs”, and that the inflammatory events may lead to fat accumulation, in turn, promoting M1 differentiation, causing inflammation. In various disease models, we must consider that BMSCs and Mφ have the potential for plasticity and multiple differentiation, in which EVs plays a significant role. Thus, as many anti-inflammatory EVs as possible are need to promote osteogenesis.
Conclusions
Evidence of the crosstalk between MSCs and Mφ through EVs is accumulating. However, the exact role of pre-MC regulation in MSC-OB in bone regeneration and the regulation of EVs derived from OB, OC, and osteocytes on MC-Mφ warrant further investigation. Osteoimmunology plays an important role in autoimmune diseases through the crosstalk between MSC and Mφ by EVs in hematologic malignancies, osteopetrosis, inflammation, and other pathological conditions.
In this review, we focus on Mφ and BMSCs largely because of their strong differentiation ability, flexible regulatory ability, and easy accessibility. An understanding of these mechanisms would enhance the appreciation of skeletal biology and facilitate the establishment of targeted approaches to modify bone mass and develop new concepts for applying osteoimmune interaction mechanisms.
Abbreviations
EV: extracellular vesicle; MSC: mesenchymal stem cell; OB: osteoblast; MC: monocyte; Mφ: macrophage; ECM: extracellular matrix; BMSC: bone mesenchymal stem cell; PGE2: prostaglandin E2; TNF-α: tumor necrosis factor-α; IL: Interleukin; Arg1: arginase 1; CD206: cluster of differentiation 206; OC: osteoclast; M-CSF: macropahge colony-stimulating factor; RANKL: receptor activator of nuclear factor kappa-Β ligand; MFG-E: milk fat globule factor-E; EPHB4: ephrin type-B receptor 4; FASL: FAS-Fas ligand; NRP1: neuropilin 1; SEMA3A: semaphorin 3A; CCL2: CC motif chemokine ligand 2; CXCL8: CXC motif chemokine ligand 8; SDF-1: stromal cell-derived factor 1; VEGF: vascular endothelial growth factor; RUNX2: RUNX family transcription factor 2; BMP-2: bone morphogenic protein-2; PI3K: phosphoinositide 3-kinase; MMP: matrix metalloproteinase; BMM: bone marrow-derived macrophage; ALP: alkaline phosphatase; OCN: osteocalcin; TGF-β: transforming growth factor β; STAT3: signal transducer and activator of transcription 3; LPS: lipopolysaccharide; MAPK: mitogen-activated protein kinase; TLR4: Toll-like receptor 4; SCIRI: spinal cord ischemia/reperfusion injury; Ern1: endoplasmic reticulum to nucleus signaling 1; OSM: Oncostatin M; OVX: ovariectomized; DKK2: Dickkopf WNT signaling pathway inhibitor 2; HSP-60: heat shock protein; MCP-1: MC chemotactic protein-1; TMJ: temporomandibular joint.
Acknowledgements
This work was supported by the National Natural Science Foundation of China [grant number 81771118], Zhejiang Provincial Key Research and Development project [grant number 2021C03059].
Competing Interests
The authors have declared that no competing interest exists.
References
1. Murthy MB. Osteoimmunology - Unleashing the concepts. J Indian Soc Periodontol. 2011;15:190-8
2. Yun WS, Choi JS, Ju HM, Kim MH, Choi SJ, Oh ES. et al. Enhanced Homing Technique of Mesenchymal Stem Cells Using Iron Oxide Nanoparticles by Magnetic Attraction in Olfactory-Injured Mouse Models. Int J Mol Sci. 2018 19
3. Li R, Zhao KC, Ruan Q, Meng CY, Yin F. Bone marrow mesenchymal stem cell-derived exosomal microRNA-124-3p attenuates neurological damage in spinal cord ischemia-reperfusion injury by downregulating Ern1 and promoting M2 macrophage polarization. Arthritis Res Ther. 2020 22
4. Guihard P, Danger Y, Brounais B, David E, Brion R, Delecrin J. et al. Induction of osteogenesis in mesenchymal stem cells by activated monocytes/macrophages depends on Oncostatin M signaling. Bone. 2012;50:S83-S
5. Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654-U72
6. Pitt JM, Kroemer G, Zitvogel L. Extracellular vesicles: masters of intercellular communication and potential clinical interventions. J Clin Invest. 2016;126:1139-43
7. Cocozza F, Grisard E, Martin-Jaular L, Mathieu M, Thery C. SnapShot: Extracellular Vesicles. Cell. 2020;182:262 -+
8. Colombo M, Raposo G, Thery C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu Rev Cell Dev Bi. 2014;30:255-89
9. Ailuno G, Baldassari S, Lai F, Florio T, Caviglioli G. Exosomes and Extracellular Vesicles as Emerging Theranostic Platforms in Cancer Research. Cells-Basel. 2020 9
10. Kong XY, Gao J. Macrophage polarization: a key event in the secondary phase of acute spinal cord injury. J Cell Mol Med. 2017;21:941-54
11. Fukui S, Iwamoto N, Takatani A, Igawa T, Shimizu T, Umeda M. et al. M1 and M2 Monocytes in Rheumatoid Arthritis: A Contribution of Imbalance of M1/M2 Monocytes to Osteoclastogenesis. Front Immunol. 2018 8
12. Zhang YH, He M, Wang Y, Liao AH. Modulators of the Balance between M1 and M2 Macrophages during Pregnancy. Front Immunol. 2017 8
13. Yang MH, Mailhot G, Birnbaum MJ, MacKay CA, Mason-Savas A, Odgren PR. Expression of and role for ovarian cancer G-protein-coupled receptor 1 (OGR1) during osteoclastogenesis. J Biol Chem. 2006;281:23598-605
14. Kim JM, Lin C, Stavre Z, Greenblatt MB, Shim JH. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells-Basel. 2020 9
15. Quan JJ, Hou YL, Long WL, Ye S, Wang ZY. Characterization of different osteoclast phenotypes in the progression of bone invasion by oral squamous cell carcinoma. Oncol Rep. 2018;39:1043-51
16. Sinder BP, Pettit AR, McCauley LK. Macrophages: Their Emerging Roles in Bone. J Bone Miner Res. 2015;30:2140-9
17. Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S. et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines (vol 41, pg 14, 2014). Immunity. 2014;41:339-40
18. Ge YW, Liu XL, Yu DG, Zhu ZA, Ke QF, Mao YQ. et al. Graphene-modified CePO4 nanorods effectively treat breast cancer-induced bone metastases and regulate macrophage polarization to improve osteo-inductive ability (vol 19, 11, 2021). J Nanobiotechnol. 2021 19
19. Zhao SJ, Kong FQ, Jie J, Li Q, Liu H, Xu AD. et al. Macrophage MSR1 promotes BMSC osteogenic differentiation and M2-like polarization by activating PI3K/AKT/GSK3 beta/beta-catenin pathway. Theranostics. 2020;10:17-35
20. Ekstrom K, Omar O, Graneli C, Wang X, Vazirisani F, Thomsen P. Monocyte exosomes stimulate the osteogenic gene expression of mesenchymal stem cells. PLoS One. 2013;8:e75227
21. Gebraad A, Kornilov R, Kaur S, Miettinen S, Haimi S, Peltoniemi H. et al. Monocyte-derived extracellular vesicles stimulate cytokine secretion and gene expression of matrix metalloproteinases by mesenchymal stem/stromal cells. FEBS J. 2018;285:2337-59
22. Ekstrom K, Omar O, Graneli C, Wang XQ, Vazirisani F, Thomsen P. Monocyte Exosomes Stimulate the Osteogenic Gene Expression of Mesenchymal Stem Cells. Plos One. 2013 8
23. Xia Y, He XT, Xu XY, Tian BM, An Y, Chen FM. Exosomes derived from M0, M1 and M2 macrophages exert distinct influences on the proliferation and differentiation of mesenchymal stem cells. PeerJ. 2020;8:e8970
24. Xiong Y, Chen L, Yan C, Zhou W, Yu T, Sun Y. et al. Correction to: M2 Macrophagy-derived exosomal miRNA-5106 induces bone mesenchymal stem cells towards osteoblastic fate by targeting salt-inducible kinase 2 and 3. J Nanobiotechnology. 2021;19:88
25. Li ZY, Wang YF, Li SL, Li YK. Exosomes Derived From M2 Macrophages Facilitate Osteogenesis and Reduce Adipogenesis of BMSCs. Front Endocrinol. 2021 12
26. Crippa GE, Beloti MM, Cardoso CR, Silva JS, Rosa AL. Effect of growth hormone on in vitro osteogenesis and gene expression of human osteoblastic cells is donor-age-dependent. J Cell Biochem. 2008;104:369-76
27. Meng J, Zhou C, Zhang W, Wang W, He B, Hu B. et al. Stachydrine prevents LPS-induced bone loss by inhibiting osteoclastogenesis via NF-kappaB and Akt signalling. J Cell Mol Med. 2019;23:6730-43
28. Italiani P, Boraschi D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front Immunol. 2014;5:514
29. Yuan FL, Wu QY, Miao ZN, Xu MH, Xu RS, Jiang DL. et al. Osteoclast-Derived Extracellular Vesicles: Novel Regulators of Osteoclastogenesis and Osteoclast-Osteoblasts Communication in Bone Remodeling. Front Physiol. 2018;9:628
30. Huynh N, VonMoss L, Smith D, Rahman I, Felemban MF, Zuo J. et al. Characterization of Regulatory Extracellular Vesicles from Osteoclasts. J Dent Res. 2016;95:673-9
31. Sun W, Zhao C, Li Y, Wang L, Nie G, Peng J. et al. Osteoclast-derived microRNA-containing exosomes selectively inhibit osteoblast activity. Cell Discov. 2016;2:16015
32. Li D, Liu J, Guo B, Liang C, Dang L, Lu C. et al. Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation. Nat Commun. 2016;7:10872
33. Liang M, Yin X, Zhang S, Ai H, Luo F, Xu J. et al. Osteoclast-derived small extracellular vesicles induce osteogenic differentiation via inhibiting ARHGAP1. Mol Ther Nucleic Acids. 2021;23:1191-203
34. Gon Y, Shimizu T, Mizumura K, Maruoka S, Hikichi M. Molecular techniques for respiratory diseases: MicroRNA and extracellular vesicles. Respirology. 2020;25:149-60
35. Chen KX, Jiao YR, Liu L, Huang M, He C, He WZ. et al. Communications Between Bone Marrow Macrophages and Bone Cells in Bone Remodeling. Front Cell Dev Biol. 2020 8
36. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD. et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-7
37. Baniwal SK, Shah PK, Shi Y, Haduong JH, DeClerck YA, Gabet Y. et al. Runx2 promotes both osteoblastogenesis and novel osteoclastogenic signals in ST2 mesenchymal progenitor cells. Osteoporosis Int. 2012;23:1399-413
38. Sims NA, Martin TJ. Osteoclasts Provide Coupling Signals to Osteoblast Lineage Cells Through Multiple Mechanisms. Annu Rev Physiol. 2020;82:507-29
39. Gao S, Mao F, Zhang B, Zhang L, Zhang X, Wang M. et al. Mouse bone marrow-derived mesenchymal stem cells induce macrophage M2 polarization through the nuclear factor-kappa B and signal transducer and activator of transcription 3 pathways. Exp Biol Med. 2014;239:366-75
40. Zhao SJ, Kong FQ, Jie J, Li Q, Liu H, Xu AD. et al. Macrophage MSR1 promotes BMSC osteogenic differentiation and M2-like polarization by activating PI3K/AKT/GSK3beta/beta-catenin pathway. Theranostics. 2020;10:17-35
41. Liu F, Qiu HB, Xue M, Zhang S, Zhang XW, Xu JY. et al. MSC-secreted TGF-beta regulates lipopolysaccharide-stimulated macrophage M2-like polarization via the Akt/FoxO1 pathway. Stem Cell Res Ther. 2019 10
42. Zhang WL, Chi CT, Meng XH, Liang SD. miRNA-15a-5p facilitates the bone marrow stem cell apoptosis of femoral head necrosis through the Wnt/beta-catenin/PPAR gamma signaling pathway. Mol Med Rep. 2019;19:4779-87
43. Jia ZW, Yang PS, Wu YH, Tang Y, Zhao YC, Wu JH. et al. Comparison of biological characteristics of nucleus pulposus mesenchymal stem cells derived from non-degenerative and degenerative human nucleus pulposus. Exp Ther Med. 2017;13:3574-80
44. Kishore R, Khan M. More Than Tiny Sacks Stem Cell Exosomes as Cell-Free Modality for Cardiac Repair. Circ Res. 2016;118:330-43
45. Liu L, Guo SJ, Shi WW, Liu Q, Huo FJ, Wu YF. et al. Bone Marrow Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Promote Periodontal Regeneration. Tissue Eng Pt A. 2021;27:962-76
46. Huang Y, He B, Wang L, Yuan B, Shu H, Zhang FC. et al. Bone marrow mesenchymal stem cell-derived exosomes promote rotator cuff tendon-bone healing by promoting angiogenesis and regulating M1 macrophages in rats. Stem Cell Res Ther. 2020 11
47. Zhang F, Wang HS, Wang XF, Jiang GM, Liu H, Zhang G. et al. TGF-beta induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget. 2016;7:52294-306
48. Xu RQ, Zhang FC, Chai RJ, Zhou WY, Hu M, Liu B. et al. Exosomes derived from pro-inflammatory bone marrow-derived mesenchymal stem cells reduce inflammation and myocardial injury via mediating macrophage polarization. J Cell Mol Med. 2019;23:7617-31
49. Shi YX, Kang X, Wang YJ, Bian XT, He G, Zhou M. et al. Exosomes Derived from Bone Marrow Stromal Cells (BMSCs) Enhance Tendon-Bone Healing by Regulating Macrophage Polarization. Med Sci Monitor. 2020 26
50. Wang R, Xu B. TGF-beta1-modified MSC-derived exosomal miR-135b attenuates cartilage injury via promoting M2 synovial macrophage polarization by targeting MAPK6. Cell Tissue Res. 2021;384:113-27
51. Zhao JX, Li XL, Hu JX, Chen F, Qiao SH, Sun X. et al. Mesenchymal stromal cell-derived exosomes attenuatemyocardial ischaemia-reperfusion injury throughmiR-182-regulated macrophage polarization. Cardiovasc Res. 2019;115:1205-16
52. Huynh N, VonMoss L, Smith D, Rahman I, Felemban MF, Zuo J. et al. Characterization of Regulatory Extracellular Vesicles from Osteoclasts. Journal of Dental Research. 2016;95:673-9
53. Tao SC, Guo SC. Extracellular vesicles in bone: "dogrobbers" in the "eternal battle field". Cell Commun Signal. 2019 17
54. Seeling M, Hillenhoff U, David JP, Schett G, Tuckermann J, Lux A. et al. Inflammatory monocytes and Fcgamma receptor IV on osteoclasts are critical for bone destruction during inflammatory arthritis in mice. Proc Natl Acad Sci U S A. 2013;110:10729-34
55. Zaiss MM, Kurowska-Stolarska M, Bohm C, Gary R, Scholtysek C, Stolarski B. et al. IL-33 shifts the balance from osteoclast to alternatively activated macrophage differentiation and protects from TNF-alpha-mediated bone loss. J Immunol. 2011;186:6097-105
56. Li DF, Liu J, Guo BS, Liang C, Dang L, Lu C. et al. Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation. Nature Communications. 2016 7
57. Xu RY, Shen X, Si YM, Fu Y, Zhu WW, Xiao T. et al. MicroRNA-31a-5p from aging BMSCs links bone formation and resorption in the aged bone marrow microenvironment. Aging Cell. 2018 17
58. Huang R, Qin C, Wang J, Hu Y, Zheng G, Qiu G. et al. Differential effects of extracellular vesicles from aging and young mesenchymal stem cells in acute lung injury. Aging (Albany NY). 2019;11:7996-8014
59. Hrkac S, Novak R, Salai G, Grazio S, Vlahovic T, Grgurevic L. Heterotopic ossification vs. fracture healing: Extracellular vesicle cargo proteins shed new light on bone formation. Bone Rep. 2022;16:101177
60. Zheng CX, Sui BD, Zhang X, Hu JC, Chen J, Liu J. et al. Apoptotic vesicles restore liver macrophage homeostasis to counteract type 2 diabetes. J Extracell Vesicles. 2021 10
61. Xing H, Wang X, Xiao SS. Osseointegration of Layer-by-Layer Polyelectrolyte Multilayers Loaded with IGF1 and Coated on Titanium Implant Under Osteoporotic Condition (Retraction of Vol 12, Pg 7709, 2017). Int J Nanomed. 2021;16:7231 -
62. Wu SY, Yu Q, Sun Y, Tian J. Synergistic effect of a LPEMF and SPIONs on BMMSC proliferation, directional migration, and osteoblastogenesis. Am J Transl Res. 2018;10:1431-43
63. Cao X. Targeting osteoclast-osteoblast communication (vol 17, pg 1344, 2011). Nat Med. 2011;17:1693 -
64. Deng LL, Wang YP, Peng Y, Wu Y, Ding YD, Jiang YH. et al. Osteoblast-derived microvesicles: A novel mechanism for communication between osteoblasts and osteoclasts. Bone. 2015;79:37-42
65. Wang Y, Zhou XQ, Wang DL. Mesenchymal Stem Cell-Derived Extracellular Vesicles Inhibit Osteoporosis via MicroRNA-27a-Induced Inhibition of DKK2-Mediated Wnt/beta-Catenin Pathway. Inflammation. 2021
66. Yu B, Wang CY. Osteoporosis: The Result of an 'Aged' Bone Microenvironment. Trends Mol Med. 2016;22:641-4
67. Li J, Ayoub A, Xiu Y, Yin X, Sanders JO, Mesfin A. et al. TGFbeta-induced degradation of TRAF3 in mesenchymal progenitor cells causes age-related osteoporosis. Nat Commun. 2019;10:2795
68. Boulestreau J, Maumus M, Jorgensen C, Noel D. Extracellular vesicles from mesenchymal stromal cells: Therapeutic perspectives for targeting senescence in osteoarthritis. Adv Drug Deliver Rev. 2021 175
69. Boulestreau J, Maumus M, Rozier P, Jorgensen C, Noel D. Mesenchymal Stem Cell Derived Extracellular Vesicles in Aging. Front Cell Dev Biol. 2020 8
70. Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheum. 2006;2:35-43
71. Liu SY, Xu X, Liang SJ, Chen ZH, Zhang Y, Qian AR. et al. The Application of MSCs-Derived Extracellular Vesicles in Bone Disorders: Novel Cell-Free Therapeutic Strategy. Front Cell Dev Biol. 2020 8
72. Schmidt-Bleek K, Kwee BJ, Mooney DJ, Duda GN. Boon and Bane of Inflammation in Bone Tissue Regeneration and Its Link with Angiogenesis. Tissue Eng Part B-Re. 2015;21:354-64
73. Medhat D, Rodriguez CI, Infante A. Immunomodulatory Effects of MSCs in Bone Healing. Int J Mol Sci. 2019 20
74. Cappariello A, Loftus A, Muraca M, Maurizi A, Rucci N, Teti A. Osteoblast-Derived Extracellular Vesicles Are Biological Tools for the Delivery of Active Molecules to Bone. J Bone Miner Res. 2018;33:517-33
75. Johnson BL, III, Kuethe JW, Caldwell CC. Neutrophil derived microvesicles: emerging role of a key mediator to the immune response. Endocr Metab Immune Disord Drug Targets. 2014;14:210-7
76. Furuta T, Miyaki S, Ishitobi H, Ogura T, Kato Y, Kamei N. et al. Mesenchymal Stem Cell-Derived Exosomes Promote Fracture Healing in a Mouse Model. Stem Cell Transl Med. 2016;5:1620-30
77. Hsieh CC, Wang CH. Aspirin Disrupts the Crosstalk of Angiogenic and Inflammatory Cytokines between 4T1 Breast Cancer Cells and Macrophages. Mediat Inflamm. 2018. 2018
78. Gangadaran P, Rajendran RL, Oh JM, Hong CM, Jeong SY, Lee SW. et al. Extracellular vesicles derived from macrophage promote angiogenesis In vitro and accelerate new vasculature formation In vivo. Exp Cell Res. 2020 394
79. Qiao Z, Greven J, Horst K, Pfeifer R, Kobbe P, Pape HC. et al. Fracture Healing and the Underexposed Role of Extracellular Vesicle-Based Cross Talk. Shock. 2018;49:486-96
80. Szychlinska MA, Trovato FM, Di Rosa M, Malaguarnera L, Puzzo L, Leonardi R. et al. Co-Expression and Co-Localization of Cartilage Glycoproteins CHI3L1 and Lubricin in Osteoarthritic Cartilage: Morphological, Immunohistochemical and Gene Expression Profiles. Int J Mol Sci. 2016 17
81. Deroyer C, Charlier E, Neuville S, Malaise O, Malaise M, de Seny D. Cemip (Kiaa1199) Induces a Fibrosis_Like Process in Osteoarthritic Chondrocytes. Osteoarthr Cartilage. 2019;27:S156-S7
82. Morales-Ivorra I, Romera-Baures M, Roman-Vinas B, Serra-Majem L. Osteoarthritis and the Mediterranean Diet: A Systematic Review. Nutrients. 2018 10
83. Wu XX, Wang YW, Xiao Y, Crawford R, Mao XZ, Prasadam I. Extracellular vesicles: Potential role in osteoarthritis regenerative medicine. J Orthop Transl. 2020;21:73-80
84. Sun AJR, Panchal SK, Friis T, Sekar S, Crawford R, Brown L. et al. Obesity-associated metabolic syndrome spontaneously induces infiltration of pro-inflammatory macrophage in synovium and promotes osteoarthritis. Plos One. 2017 12
85. Peng S, Yan Y, Li R, Dai H, Xu J. Extracellular vesicles from M1-polarized macrophages promote inflammation in the temporomandibular joint via miR-1246 activation of the Wnt/beta-catenin pathway. Ann N Y Acad Sci. 2021
86. Cosenza S, Toupet K, Maumus M, Luz-Crawford P, Blanc-Brude O, Jorgensen C. et al. Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics. 2018;8:1399-410
87. Cosenza S, Ruiz M, Toupet K, Jorgensen C, Noel D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep-Uk. 2017 7
88. Zhang JY, Rong YL, Luo CY, Cui WD. Bone marrow mesenchymal stem cell-derived exosomes prevent osteoarthritis by regulating synovial macrophage polarization. Aging-Us. 2020;12:25138-52
89. Chen PF, Zheng L, Wang YY, Tao M, Xie Z, Xia C. et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics. 2019;9:2439-59
90. Shaddox LM, Morford LA, Nibali L. Periodontal health and disease: The contribution of genetics. Periodontol 2000. 2021;85:161-81
91. Hienz SA, Paliwal S, Ivanovski S. Mechanisms of Bone Resorption in Periodontitis. J Immunol Res. 2015. 2015
92. Zhang YX, Xiong Y, Chen XW, Chen CF, Zhu ZM, Li L. Therapeutic effect of bone marrow mesenchymal stem cells pretreated with acetylsalicylic acid on experimental periodontitis in rats. Int Immunopharmacol. 2018;54:320-8
93. Chen XT, Wan Z, Yang L, Song S, Fu ZY, Tang K. et al. Exosomes derived from reparative M2-like macrophages prevent bone loss in murine periodontitis models via IL-10 mRNA. J Nanobiotechnol. 2022 20
94. Chen Z, Wu C, Gu W, Klein T, Crawford R, Xiao Y. Osteogenic differentiation of bone marrow MSCs by beta-tricalcium phosphate stimulating macrophages via BMP2 signalling pathway. Biomaterials. 2014;35:1507-18
95. Kang MY, Huang CC, Lu Y, Shirazi S, Gajendrareddy P, Ravindran S. et al. Bone regeneration is mediated by macrophage extracellular vesicles. Bone. 2020 141
96. Schwechheimer C, Kuehn MJ. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat Rev Microbiol. 2015;13:605-19
97. Briaud P, Carroll RK. Extracellular Vesicle Biogenesis and Functions in Gram-Positive Bacteria. Infect Immun. 2020 88
98. McMillan HM, Kuehn MJ. The extracellular vesicle generation paradox: a bacterial point of view. EMBO J. 2021;40:e108174
99. Han EC, Choi SY, Lee Y, Park JW, Hong SH, Lee HJ. Extracellular RNAs in periodontopathogenic outer membrane vesicles promote TNF-alpha production in human macrophages and cross the blood-brain barrier in mice. Faseb J. 2019;33:13412-22
100. Xia W, Xie J, Cai ZQ, Liu XH, Wen J, Cui ZK. et al. Damaged brain accelerates bone healing by releasing small extracellular vesicles that target osteoprogenitors. Nature Communications. 2021 12
101. Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K. et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18:759-U153
102. Phinney DG, Di Giuseppe M, Njah J, Sala E, Shiva S, St Croix CM. et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nature Communications. 2015 6
Author contact
![]() Corresponding authors: Zhjian Xie, E-mail: xzj66edu.cn; Zhuo Chen, E-mail: zoechenedu.cn; Stomatology Hospital, School of Stomatology, Zhejiang University School of Medicine, Clinical Research Center for Oral Diseases of Zhejiang Province, Key Laboratory of Oral Biomedical Research of Zhejiang Province, Cancer Center of Zhejiang University, Hangzhou 310006, China.
Corresponding authors: Zhjian Xie, E-mail: xzj66edu.cn; Zhuo Chen, E-mail: zoechenedu.cn; Stomatology Hospital, School of Stomatology, Zhejiang University School of Medicine, Clinical Research Center for Oral Diseases of Zhejiang Province, Key Laboratory of Oral Biomedical Research of Zhejiang Province, Cancer Center of Zhejiang University, Hangzhou 310006, China.

 Global reach, higher impact
Global reach, higher impact