10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(10):4171-4186. doi:10.7150/ijbs.69332 This issue Cite
Research Paper
CCDC65, a Gene Knockout that leads to Early Death of Mice, acts as a potentially Novel Tumor Suppressor in Lung Adenocarcinoma
1. Cancer Center, Integrated Hospital of Traditional Chinese Medicine, Southern Medical University, 510315 Guangzhou, China.
2. Cancer Research Institute, Southern Medical University, Guangzhou, Guangdong, 510515, China.
3. Respiratory Department, Peking University Shenzhen Hospital, Shenzhen, 518034, China.
4. Department of Oncology, Dali Bai Autonomous Prefecture People's Hospital, Dali, Yunnan, 671000, China.
5. Department of Pulmonary and Critical Care Medicine, The Third Xiangya Hospital of Central South University, Changsha, 410000, China.
6. Department of Oncology, Nanfang Hospital, Southern Medical University, 510515 Guangzhou, China.
7. Department of Hepatobiliary Surgery, The First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, 710061, China.
8. School of Pharmacy, Guangdong Medical University, Dongguan, Guangdong 523808, China.
9. Affiliated Cancer Hospital & Institute of Guangzhou Medical University, Guangzhou Municipal and Guangdong Provincial Key Laboratory of Protein Modification and Degradation, State Key Laboratory of Respiratory Disease, School of Basic Medical Sciences, Guangzhou Medical University Guangzhou 510095, Guangdong, China.
#These authors contributed equally to this work.
Abstract
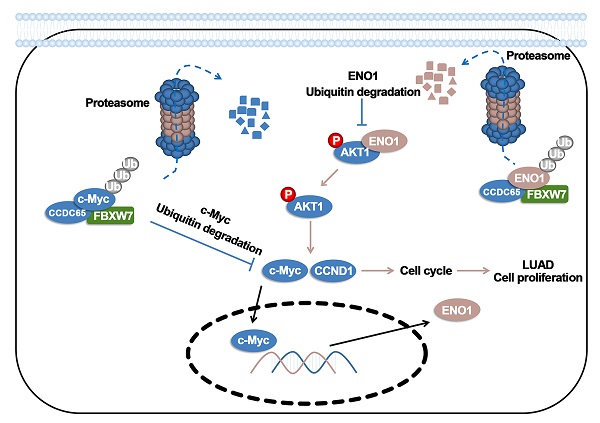
CCDC65 is a member of the coiled-coil domain-containing protein family and was only reported in gastric cancer by our group. We first observed that it is downregulated in lung adenocarcinoma based on the TCGA database. Reduced CCDC65 protein was shown as an unfavorable factor promoting the clinical progression in lung adenocarcinoma. Subsequently, CCDC65-/- mice were found possibly dead of hydrocephalus. Compared with the CCDC65+/+ mice, the downregulation of CCDC65 in CCDC65+/- mice significantly increased the formation ability of lung cancer induced by urethane. In the subsequent investigation, we observed that CCDC65 functions as a tumor suppressor repressing cell proliferation in vitro and in vivo. Molecular mechanism showed that CCDC65 recruited E3 ubiquitin ligase FBXW7 to induce the ubiquitination degradation of c-Myc, an oncogenic transcription factor in tumors, and reduced c-Myc binding to ENO1 promoter, which suppressed the transcription of ENO1. In addition, CCDC65 also recruited FBXW7 to degrade ENO1 protein by ubiquitinated modulation. The downregulated ENO1 further reduced the phosphorylation activation of AKT1, which thus inactivated the cell cycle signal. Our data demonstrated that CCDC65 is a potential tumor suppressor by recruiting FBWX7 to suppress c-Myc/ENO1-induced cell cycle signal in lung adenocarcinoma.
Keywords: CCDC65, c-Myc, ENO1, Knockdown mice, Ubiquitin degradation, Cell cycle

 Global reach, higher impact
Global reach, higher impact