ISSN: 1449-2288
Int J Biol Sci 2022; 18(10):4203-4218. doi:10.7150/ijbs.70583 This issue Cite
Research Paper
TOP1 inhibition induces bifurcated JNK/MYC signaling that dictates cancer cell sensitivity
1. Department of Surgery, Samuel Oschin Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA 90048, USA.
2. Key Laboratory for Breast Cancer Diagnosis and Treatment, Shantou University Medical College Cancer Hospital, Shantou 515041, China.
3. Loma Linda University, Department of Basic Sciences, 11085 Campus Street Mortensen Hall 219, Loma Linda, CA 92354, USA.
* Qizhi Liu and Stacey Chung contributed equally to this work.
Abstract
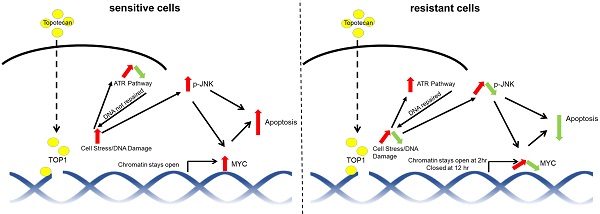
Rationale: Triple-negative breast cancer (TNBC) does not respond to anti-estrogen and anti-HER2 therapies and is commonly treated by chemotherapy. TNBC has a high recurrence rate, particularly within the first 3 years. Thus, there is an urgent clinical need to develop more effective therapies for TNBC. Topoisomerase I (TOP1) inhibitors cause DNA damage, making these drugs desirable for TNBC treatment since DNA repair machinery is defective in this subtype of breast cancer. Among the main molecular subtypes of breast cancer, the TNBC cell lines exhibited the highest TOP1 inhibition sensitivity. However, clinically used TOP1 inhibitors, such as topotecan and irinotecan, have shown limited clinical applications and the reasons remain unclear. Understanding the mechanism of differential responses to TOP1 blockade and identifying the predictive markers for cancer cell sensitivity will help further TOP1-targeted therapy for TNBC treatment and improve the clinical use of TOP1 inhibitors.
Methods: Viability assays were used to evaluate breast cancer cell sensitivity to topotecan and other TOP1 inhibitors as well as TOP2 inhibitors. An in vitro-derived topotecan-resistant TNBC cell model and TNBC xenograft models were employed to confirm cancer cell response to TOP1 blockade. RNA-seq was used to identify potential predictive markers for TNBC cell response to TOP1 blockade. Western blotting and qRT-PCR were performed to measure the protein levels and RNA expression. ATAC-seq and luciferase reporter assays were used to examine MYC transcriptional regulations. The effects of MYC and JNK in cancer cell response to TOP1 inhibition were validated via loss-of-function and gain-of-function experiments.
Results: We observed two distinct and diverging cancer cell responses - sensitive versus resistant to TOP1 inhibition, which was confirmed by TNBC xenograft mouse models treated by topotecan. TNBC cells exhibited bifurcated temporal patterns of ATR pathway activation upon TOP1 inhibitor treatment. The sensitive TNBC cells showed an “up then down” dynamic pattern of ATR/Chk1 signaling, while the resistant TNBC cells exhibited a “persistently up” profile. On the contrary, opposite temporal patterns of induced expression of MYC, a key regulator and effector of DNA damage, were found in TNBC cells treated by TOP1 inhibitors. Mechanistically, we showed that TOP1-induced JNK signaling upregulated MYC expression. Furthermore, pharmacological inhibition of ATR reversed TNBC cell resistance to topotecan, whereas MYC knockdown and JNK inhibition reduced cancer cell sensitivity.
Conclusions: Dynamic temporal profiles of induced ATR/Chk1 and JNK activation as well as MYC expression, may predict cancer cell response to TOP1 inhibitors. JNK activation-mediated constitutive elevation of MYC expression may represent a novel mechanism governing cancer cell sensitivity to TOP1-targeting therapy. Our results may provide implications for identifying TNBC patients who might benefit from the treatment with TOP1 inhibitors.
Keywords: ATR, MYC, JNK, TOP1 inhibitor, Triple-negative Breast Cancer

 Global reach, higher impact
Global reach, higher impact