10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(11):4400-4413. doi:10.7150/ijbs.72707 This issue Cite
Review
An Update on the Multifaceted Role of NF-kappaB in Endometriosis
1. Department of Gynecology, Women's Hospital, School of Medicine, Zhejiang University, Xueshi Road, Hangzhou 310006, China
2. Zhejiang Provincial Key Laboratory of Precision Diagnosis and Therapy for Major Gynecological Diseases, Women's Hospital, Zhejiang University School of Medicine, Hangzhou 310006, China
Received 2022-3-8; Accepted 2022-6-12; Published 2022-7-4
Abstract
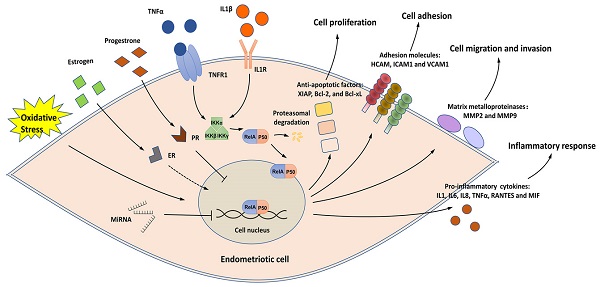
Endometriosis remains a common but challenging gynecological disease among reproductive-aged women with an unclear pathogenesis and limited therapeutic options. Numerous pieces of evidence suggest that NF-κB signaling, a major regulator of inflammatory responses, is overactive in endometriotic lesions and contributes to the onset, progression, and recurrence of endometriosis. Several factors, such as estrogen, progesterone, oxidative stress, and noncoding RNAs, can regulate NF-κB signaling in endometriosis. In the present review, we discuss the mechanisms by which these factors regulate NF-κB during endometriosis progression and provide an update on the role of NF-κB in affecting endometriotic cells, peritoneal macrophages (PMs) as well as endometriosis-related symptoms, such as pain and infertility. Furthermore, the preclinical drugs for blocking NF-κB signaling in endometriosis are summarized, including plant-derived medicines, NF-κB inhibitors, other known drugs, and the potential anti-NF-κB drugs predicted through the Drug-Gene Interaction Database. The present review discusses most of the studies concerning the multifaceted role of NF-κB signaling in endometriosis and provides a summary of NF-κB-targeted treatment in detail.
Keywords: NF-κB, endometriosis, peritoneal macrophage
Introduction
Endometriosis is a benign gynecological disease that affects 6%-10% of reproductive-aged women and 20%-50% of women with infertility [1, 2]. It is defined as the abnormal implantation of the endometrium outside the uterine cavity, particularly in the ovaries and pelvic peritoneum [3, 4]. The colonization and growth of ectopic endometrium can result in chronic pelvic pain, infertility, dysmenorrhea, and other clinical symptoms in endometriosis patients [3, 4]. Increased inflammatory responses in ectopic endometrial tissues are believed to be strongly associated with the pathogenesis of endometriosis, which is induced by the activation of proinflammatory factors and signaling pathways, as well as the increased infiltration of immune cells [5-7].
The activation of nuclear factor kappa B (NF-κB) in patients with endometriosis has been found to play a vital role in regulating disease progression through complex mechanisms [8]. As a superfamily of transcription factors, NF-κB has five members, including RelA (p65), RelB, c-Rel, NF-κB1 (p105/p50), and NF-κB2 (p100/p52) [9] (Figure 1A). All five members share a Rel homology domain (RHD), which is essential for homo or heterodimerization and binding to cognate DNA elements [9]. The resting state of NF-κB dimers is sequestered in the cytoplasm by a family of inhibitors of κB (IκB) proteins (such as IκBα, IκBβ, p105, and p100), which serve as inhibitors through their ankyrin repeats [10]. When NF-κB signaling is activated, IκB is phosphorylated by IκB kinases (IKKs) and degraded by the proteasome, which allows NF-κB dimers to enter the nucleus and elicit the transcriptional activity of downstream genes [8].
A simplified view of the structure and transduction process of NF-κB. (A) Structures of the members of the NF-κB signaling pathway. ① The NF-κB superfamily contains five members: RelA (p65), RelB, c-Rel, NF-κB1 (p105/p50), and NF-κB2 (p100/p52). p50 and p52 are shorter forms processed by p105 and p100, respectively. All members share a Rel homology domain (RHD), which contains two specific DNA-binding domains (DBDs) and a nuclear localization sequence. One of the DBDs is engaged in DNA recognition, and the other is involved in dimerization. ② The IκB family contains the three most important members: IKBα, IKBβ, and IKBγ. ③ The IKK complex contains the three most important members. GRR: glycine-rich region; ANK: ankyrin repeats; DD: death domain; TAD: transcription activation domain; LZ: leucine zipper domain; KD: kinase domain; HLH: helix-loop-helix domain; NBD: NEMO-binding domain; MOD/UBD: minimal oligomerization domain/ubiquitin-binding domain; ZF: zinc finger domain. (B) NF-κB signaling is activated in endometriotic cells. Stimuli, such as TNF-α and IL1, activate the IKK complex, triggering IKB phosphorylation and its subsequent proteasomal degradation. The RelA/p50 heterodimers then translocate to the nucleus and elicit transcriptional activity. Estrogen, progesterone, oxidative stress, and ncRNAs are the key regulators of NF-κB signaling, and the effects of estrogen on NF-κB signaling are controversial. Activation of the downstream genes of NF-κB signaling induces inflammatory responses and supports the survival, adhesion, migration, and invasion of endometriotic cells during endometriosis development.
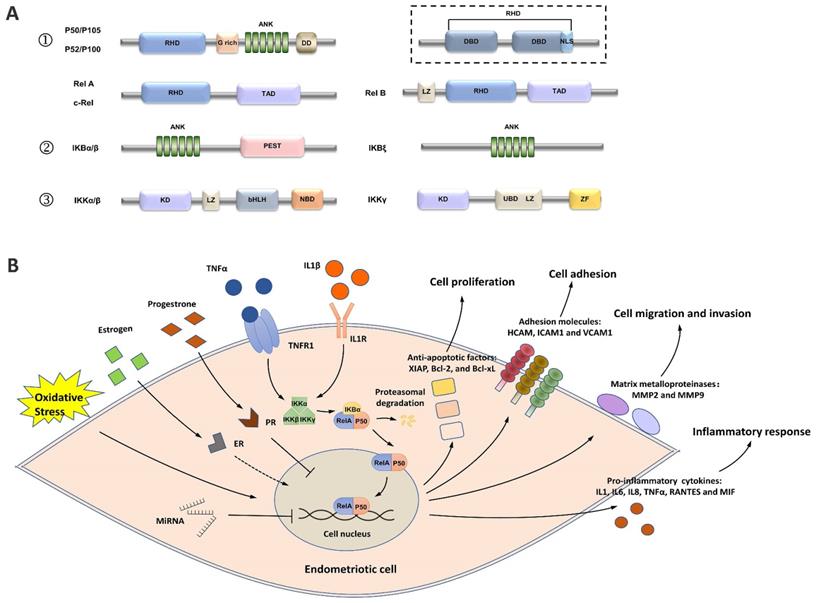
In endometriotic cells, NK-κB signaling is activated by stimuli, such as tumor necrosis factor α (TNF-α) and interleukin-1β (IL-1β) [11-19]. The IKK complex, which contains two catalytic subunits (IKK-1 [IKK-α] and IKK-2 [IKK-β]) and a noncatalytic accessory protein NF-κB essential modulator (NEMO [IKKγ]), is activated under stimulation (Figure 1B) [20]. The IKK complex then phosphorylates IκB proteins, allowing the cytoplasmic RelA/p50 heterodimer to be released and translocated to the nucleus [20]. The transcriptional activity of several proinflammatory cytokines/chemokines, such as IL1, IL6, IL8, TNF-α, RANTES, MIF, and ICAM1, is activated by NF-κB signaling, indicating the key role of NF-κB in the inflammatory responses in endometriosis [11, 13, 14, 18, 19, 21-27].
NK-κB signaling has a close relationship with key regulatory factors for the onset and progression of endometriosis, including estrogen, progesterone, oxidative stress, and noncoding RNAs (ncRNAs). In addition, NK-κB signaling can regulate the cellular behaviors of endometriotic cells and peritoneal macrophages (PMs) in the endometriotic milieu, as well as contribute to endometriosis-induced pain and infertility. In this review, we discuss the role of NF-κB in endometriosis pathogenesis and the relevant molecular mechanisms. We also summarize and predict known and potential anti-NF-κB drugs for endometriosis treatment.
Regulation of NF-κB signaling in endometriosis
Estrogen
The effects of estrogen signaling on NF-κB in endometriosis are controversial (Figure 2). An early study first discovered that estrogen and/or its receptors, estrogen receptor (ER) α and ERβ, can increase NF-κB activity in ectopic endometrial cells, contrary to their effect in normal endometrial stromal cells [28]. Repressor of estrogen receptor activity (REA), a key ER corepressor in the female reproductive tract, is downregulated in ectopic endometriotic lesions, contributing to the activation of estrogen signaling and enhanced NF-κB activity [29]. Mechanistically, estrogen signaling induces NF-κB activation in endometriotic cells by activating several proinflammatory pathways, such as CXCL12/CXCR4, PI3K/Akt, and thymic stromal lymphopoietin (TSLP) signaling [25, 30, 31].
Estrogen-stimulated NF-κB activation can promote the viability and proliferation of endometriotic cells. Mechanistically, NF-κB signaling can restrict autophagy-related cell death, inhibit the expression of PTEN and activate the PI3K/Akt and MAPK/ERK pathways [30, 31]. In addition, estrogen-induced NF-κB activity can also affect the polarization of PMs in the endometriotic milieu (see the “Macrophage polarization” section) [32].
The treatment of endometriotic cells with GnRHa, a hormone therapy that induces the hypoestrogenic state of ectopic endometrium, reduces NF-κB activation in endometriotic cells and further suppresses the expression of the proinflammatory cytokine IL8 [22]. This finding indicates that hormone therapies may have the potential to inhibit NF-κB signaling and reduce inflammation in ectopic endometrial lesions [22].
However, some studies have reported the inhibitory effect of estrogen signaling on NF-κB in endometriosis [33-35]. Mechanistically, estrogen can reduce the expression of AGTR1, a gene that encodes the angiotensin II receptor, which is overexpressed and activates NF-κB signaling in endometriosis [34]. By applying a microarray-based technique, Han et al. found that TNFα/NF-κB signaling is downregulated by ERβ in the eutopic endometrium of a C57BL/6 mouse model [33]. In addition, NF-κB signaling can also be repressed by estrogen signaling in PMs [35]. Treating PMs with ERB-041, a selective ERβ agonist, inhibits NF-κB activation and its downstream inducible nitric oxide synthase (iNOS)/nitric oxide (NO) signaling during endometriosis progression [35].
SR-16234 is a selective estrogen receptor modulator that has potent antagonistic activity on ERα with weak partial agonist activity against the ERβ receptor [36]. In a BALB/c mouse model of endometriosis, treatment with SR-16234 substantially decreased NF-κB p65 expression and reduced the growth of endometriotic lesions [36]. In an open-label single-arm clinical trial, the oral administration of SR-16234 substantially relieved the pain symptoms of patients with endometriosis [37]. These findings indicate that selectively inhibiting estrogen/ERα signaling while activating estrogen/ERβ signaling may serve as a potential therapeutic strategy for endometriosis treatment.
NF-κB and estrogen in endometriosis. In endometriotic cells, estrogen can activate NF-κB signaling by activating CXCL12/CXCR4, PI3K/Akt, and TSLP signaling, and low REA expression also contributes to the activation of the estrogen/NF-κB axis. Estrogen-stimulated NF-κB activity further activates Akt and ERK signaling, represses PTEN expression, and induces production of the proinflammatory cytokines CCL2 and IL8. In addition, estrogen can inhibit NF-κB signaling in endometriotic cells by repressing AGTR1 expression. In peritoneal macrophages, estrogen/ERβ signaling can inhibit NF-κB activation and further inhibit NOS production.

Progesterone
The p65 subunit of NF-κB and progesterone receptor (PR) can repress each other through direct contact. The mutual repression of p65 and PR in the endometrium is involved in endometrial biologic alterations during the menstrual cycle and pathophysiologic processes, such as irregular uterine bleeding [38-40]. In a study of 109 patients with endometriosis, increased p65 expression and decreased PRB (a PR isoform) expression jointly served as biomarkers for the recurrence of ovarian endometrioma [41]. However, another study with 104 patients drew the opposite conclusion that the recurrence of ovarian endometriosis is associated with decreased p65 expression and increased PRB expression [42]. The contradictory conclusions of the two reports may have been partially caused by clinical, immunological, histochemical, inflammatory, and genetic-epigenetic heterogeneity of endometriotic tissues [43-45]. In addition, other factors, such as the low reproducibility of immunohistochemistry analysis, and the different patterns between recurrent and initial endometriosis, may have also affected the results [42]. These findings indicate that the relationship between p65/PR expression and endometriosis recurrence needs to be re-evaluated.
As a commonly used hormone drug for treating endometriosis, progesterone inhibits the NF-κB-induced production of proinflammatory factors in endometriotic cells [46, 47], and the combined use of progesterone and NF-κB inhibitors can remarkably increase the efficacy of alleviating endometriosis-related pain [48]. However, a recent study revealed that progesterone resistance, a central element during endometriosis progression, may weaken the inhibitory effect of progesterone on NF-κB by inducing aberrant endoplasmic reticulum stress in endometriotic tissues [49]. The upregulation of endoplasmic reticulum stress in endometriotic cells by its activator can remarkably inhibit NF-κB-induced inflammation by upregulating the NF-κB-negative regulators A20 and C/EBPβ, indicating the potential anti-NF-κB value of endoplasmic reticulum stress in endometriosis [49].
Oxidative stress
The relationship between NF-κB and oxidative stress in endometriosis is shown in Figure 3. The imbalance between pro-oxidants (free radical species, such as reactive oxygen species [ROS] and nitric oxide synthase [NOS] and antioxidants is implicated in the pathophysiology of endometriosis [50]. The overproduction of ROS in the pelvic cavity of patients with endometriosis is an important inducer of chronic NF-κB-mediated inflammatory responses [51-53]. Extracellular high mobility group box-1 (HMGB-1), a prototypical molecule of damage-associated molecular patterns, activates NF-κB in endometriotic cells by binding to its receptor, Toll-like receptor 4 (TLR4), and induces inflammatory responses in the environment of endometriosis with sustained oxidative stress [52, 53]. In addition, the HMGB-1/TLR4/NF-κB axis can also induce the proliferation and invasion of endometriotic cells and contribute to endometriosis-induced pain [52-54].
NF-κB and oxidative stress in endometriosis. Oxidative stress induces NF-κB activation in endometriotic cells through iron overload and by activating the HMGB-1/TLR4 and estrogen/ERα pathways. HNF1β, as a coactivator for NF-κB, can activate NF-κB to protect endometriotic cells against oxidative damage. NF-κB activation in endometriotic cells may also induce the production of pro-oxidants (iNOS and NO) and reduce antioxidant enzymes (SOD, GPx, HO, and CAT). In peritoneal macrophages, estrogen/ERβ signaling can inhibit the NF-κB/NOS/NO axis and may alleviate oxidative stress.
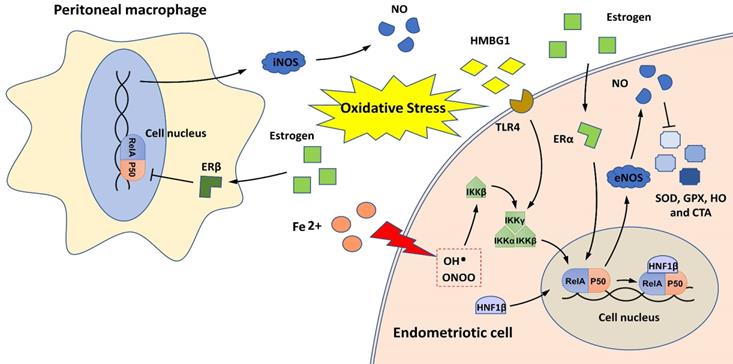
During retrograde menstruation, erythrocytes are carried into the pelvic cavity of patients with endometriosis, and the lysis of erythrocytes results in iron release, with free iron serving as a source of ROS [55]. Iron overload activates IKKβ and stimulates NF-κB signaling, conferring pro-endometriotic behaviors on endometrial stromal cells [27]. Hepatocyte nuclear factor-1 beta (HNF1β) is a homeobox transcription factor that is overexpressed in endometriotic cells [56]. It functions as a coactivator for NF-κB, and its activation can enhance the survivability of endometriotic cells in oxidative cellular environments [56].
NF-κB signaling may also contribute to oxidative stress overload in patients with endometriosis by promoting NOS production and decreasing the expression of antioxidant enzymes, such as superoxide dismutase (SOD), glutathione peroxidase (GPx), heme oxygenase (HO), and catalase (CAT) [35, 57, 58]. Notably, two estrogen receptors (ERα and ERβ) are engaged in the regulation of the NF-κB/NOS/NO axis; ERα activates this axis in endometrial stromal cells, and ERβ inhibits this axis in PMs [35, 57]. These findings indicate that estrogen signaling may have different effects on NF-κB-mediated oxidative stress in the ectopic endometrium and endometriotic milieu.
Noncoding RNAs
NcRNAs are a heterogeneous class of RNAs that do not encode proteins but participate in the pathophysiological processes of endometriosis by regulating gene expression [59]. MiRNAs are ncRNAs that are 19-25 nucleotides in length and negatively regulate gene expression by binding to the 3′-untranslated regions of target mRNAs [59]. Four types of miRNAs, namely, miR-16, miR-138, miR-182, and miR-199a, directly target the key NF-κB signaling-related genes IKKβ (targeted by miR-138 and miR-182) and p65 (targeted by miR-16 and miR-199a) to inhibit NF-κB signaling, and their expression is downregulated in ectopic endometrium [60-63]. In contrast, two miRNAs, namely, miR-9 and miR-22, indirectly activate NF-κB signaling by repressing the expression of sirtuin 1 (SIRT1), an NAD (+)-dependent deacetylase, to reduce inflammatory responses in the ectopic endometrium [64, 65]. The overexpression of miR-16, miR-138, miR-182, and miR-199a or the inhibition of miR-9 and miR-22 can block NF-κB signaling and further repress inflammatory responses and the survival, migration, and invasion abilities of endometriotic cells [60-65].
Long noncoding RNAs (lncRNAs) are another group of ncRNAs more than 200 nucleotides in length [59]. MALAT1, a lncRNA, is overexpressed in the ectopic endometrium and enhances the proliferation and invasion of endometriotic cells by activating the NF-κB/iNOS/MMP9 axis [58]. However, the mechanism by which MALAT1 activates NF-κB remains unclear.
Role of NF-κB in endometriosis pathogenesis
NF-κB regulates endometriotic cell behaviors
NF-κB signaling affects endometriosis progression by regulating the activities of endometriotic cells. The abnormal survivability of endometriotic cells is correlated with NF-κB-mediated activities, such as secretion of the proinflammatory cytokine IL8 and activation of the antiapoptotic molecules XIAP, Bcl-2, and Bcl-xL [18, 56, 66, 67]. In a Macaca fascicularis model of endometriosis, p65 knockdown by short hairpin RNA considerably reduced the expression of proliferating cell nuclear antigen (PCNA) and the microvessel density of ectopic lesions, indicating that NF-κB can be a therapeutic target for preventing the growth and angiogenesis of endometriotic lesions [68].
The aberrant adhesion of endometriotic cells is an initial step for the establishment of endometriosis [69]. Decoy receptor 3 (DcR3), a pleiotropic immunomodulator, can move retrograde to the ectopic endometrium with menstrual blood and subsequently induce upregulation of adhesion molecules in an NF-κB-dependent manner [70]. NF-κB signaling induces the high expression of key adhesion molecules in the ectopic endometrium, including homing cell adhesion molecule (CD44), ICAM-1, and vascular cell adhesion molecule-1 (VCAM-1). Inhibition of NF-κB can effectively reduce the adhesive ability of endometriotic cells [19, 70, 71].
The high migration and invasion ability of endometriotic cells is considered the main cause of implantation and extension of the ectopic foci [72]. Studies have revealed that NF-κB signaling contributes to endometriotic cell migration and invasion through the transcriptional activation of matrix metalloproteinases (MMPs, especially MMP-2 and MMP-9), a family of zinc-dependent endopeptidases that are responsible for extracellular matrix degradation [71, 73-75]. Nasiri et al. exhibited potent inhibitory effects of the anti-NF-κB drugs aloe-emodin and aspirin on the invasion of endometriotic cells from patients with stage IV endometriosis [71]. In another study, IKKβ knockdown via short interfering RNA remarkably suppressed the migration and invasion of endometriotic cells, further indicating the critical role of NF-κB signaling in ectopic endometrial implantation [60].
NF-κB and macrophages in the endometriotic milieu
Activated PMs are involved in the pathological process of peritoneal endometriotic lesions [76]. In a study of 44 cases (22 with and 22 without endometriosis), a significantly higher proportion of NF-κB nuclear translocation was found in PMs from patients with endometriosis [77]. NF-κB activation contributes to the crosstalk between PMs and endometriotic cells and affects the polarization/differentiation of PMs in the ectopic milieu (Figure 4).
Crosstalk between PMs and endometriotic cells
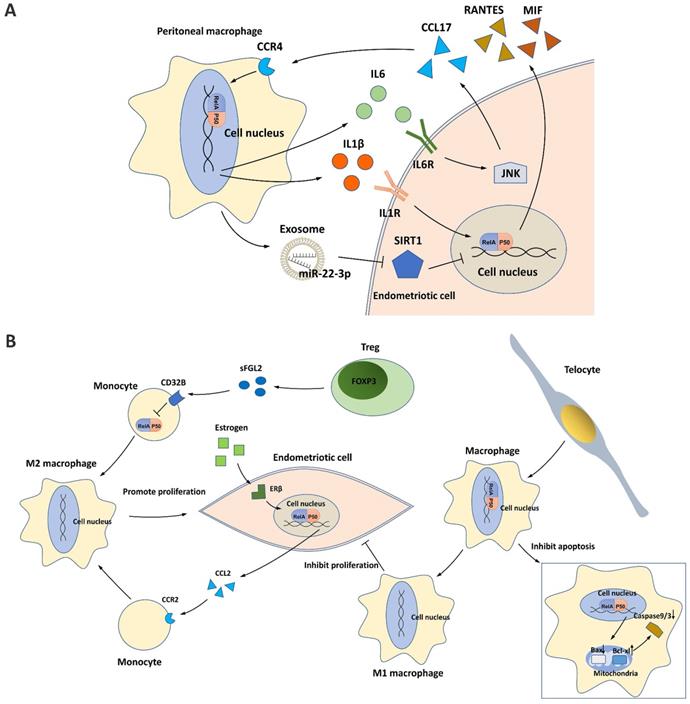
Endometriotic cells can activate NF-κB signaling in PMs by secreting CCL17, and this process is dependent on JNK signaling [78]. NF-κB activation in PMs subsequently induces the secretion of IL6, which in turn activates the JNK/CCL17 axis in endometriotic cells, forming crosstalk between PMs and endometriotic cells [78].
PMs can also activate NF-κB signaling in endometriotic cells through different mechanisms. IL1β, a potent macrophage cytokine produced from activated PMs in the ectopic milieu, activates NF-κB in endometriotic cells, resulting in the production of proinflammatory cytokines/chemokines, such as RANTES and MIF [13, 14, 21]. In addition, PMs can release exosomes that deliver miR-22-3p, a miRNA that activates NF-κB signaling in endometriotic cells by suppressing SIRT1 expression [65].
NF-κB and macrophages in endometriosis. (A) NF-κB signaling contributes to the crosstalk between peritoneal macrophages (PMs) and endometriotic cells. The IL6/JNK/CCL17/CCR4 axis induces NF-κB activation in PMs, which then promotes IL6 production and forms a positive loop. In addition, IL1β and exosome-derived miR-22-3p secreted by PMs induce NF-κB activation in endometriotic cells, which may promote secretion of the proinflammatory cytokines RANTES and MIF. (B) NF-κB signaling affects PM polarization in the endometriotic milieu. Tregs and estrogen/ERβ signaling induce M2 macrophage polarization and endometriotic cell proliferation by repressing NF-κB in monocytes and activating NF-κB in endometriotic cells. Telocytes promote M1 macrophage polarization while inhibiting endometriotic cell proliferation and mitochondria-mediated PM apoptosis by activating NF-κB in PMs.
Macrophage polarization
Traditionally, macrophages differentiate into classical proinflammatory M1 macrophages or alternative anti-inflammatory M2 macrophages in response to different environmental stimuli. In the endometriotic milieu, M1 macrophages secrete multiple cytokines/chemokines for inflammatory responses, inhibiting endometriotic cell proliferation and promoting tissue damage, whereas M2 macrophages possess an immunosuppressive ability that supports the survival and invasiveness of endometriotic cells, stimulates the growth and vascularization of ectopic endometrial lesions, and induces pain generation [78-82]. During the progression of endometriosis, NF-κB suppression in monocytes/macrophages enhances M2 macrophage polarization and inhibits M1 macrophage polarization, developing a pro-repair environment for neovascularization in ectopic lesions [80, 81]. One mechanism is the binding of soluble fibrinogen-like protein 2 secreted by the increased regulatory T cells (Tregs) in the endometriotic milieu to its receptor CD32B expressed on PMs [81]. Tregs activated by PMs further suppress NF-κB signaling and induce an immune tolerance environment for endometriosis progression [81]. Telocytes, a type of mesenchymal/stromal cell, were recently identified to enhance M1 macrophage polarization in the endometriotic milieu by activating NF-κB signaling, which helps suppress the onset of endometriosis [80]. In addition, NF-κB activated by telocytes can also promote macrophage proliferation by inhibiting mitochondrion-dependent apoptosis [80].
In endometriotic cells, NF-κB signaling is activated by estrogen/ERβ signaling and can enhance M2 macrophage polarization [32]. Mechanistically, estrogen-stimulated NF-κB signaling promotes CCL2 production, which recruits PMs and induces macrophage M2 polarization, thus promoting the pathogenesis of endometriosis [32].
NF-κB contributes to endometriosis-associated pain and infertility
NF-κB and pain
The presence of TRPA1/TRPV1-expressing nerve fibers in ectopic endometrium is one of the key factors for pain generation [83]. In a C57BL/6 mouse model of endometriosis, Fattori et al. observed that endometriosis-induced NF-κB activation contributes to increased calcium influx in TRPA1/TRPV1-expressing dorsal root ganglion (DRG) neurons [84]. This pattern of neuronal activation coincides with peripheral sensitization detected in the activation of NF-κB [85, 86].
In an SD rat model of endometriosis, NF-κB overexpression was found in the DRG and spinal dorsal horn (SDH), which was induced by the HMGB-1/TLR4/MyD88 pathway and contributed to mechanical hyperalgesia at the graft site of ectopic endometrium [54]. Inhibiting the expression of TLR4 or MyD88 could decrease NF-κB p65 phosphorylation in the DRG, alleviating chronic endometriosis-induced pain [54].
Nobiletin and andrographolide, which are plant-derived anti-NF-κB drugs, can remarkably improve response latency in animal models of endometriosis, confirming the potential of NF-κB as a target for reducing endometriosis-induced pain [87, 88].
NF-κB and infertility
In a study of 35 cases (15 infertile patients with endometriosis, 10 infertile patients with nonendometriotic ovarian cysts, and 10 healthy fertile women), the mean expression of NF-κB1 was remarkably higher in the ectopic endometrium of infertile patients with endometriosis than in the endometria of patients with nonendometriotic cysts and fertile patients [89]. After the surgical removal of endometrioma, the expression of NF-κB1 markedly decreased in endometriosis patients, suggesting that the overexpression of NF-κB in eutopic endometrium may contribute to endometriosis-associated infertility [89]. According to the results of another genetic association study of 438 cases (172 infertile patients with endometriosis, 77 cases of idiopathic infertility, and 189 healthy women), the 94 insertion/deletion ATTG polymorphism in the NFKB1 gene was positively correlated with endometriosis and idiopathic infertility [90]. More investigations should be performed to explore the relationship between NF-κB activation and the high infertility risk of patients with endometriosis.
Preclinical drugs for blocking NF-κB signaling in endometriosis
Preclinical in vivo and/or in vitro experiments have discovered a large number of drugs that inhibit NF-κB signaling and thus alleviate the development of endometriosis (Table 1) [13, 14, 16, 18, 19, 36, 46, 67, 75, 87, 88, 91-119]. In this section, we discuss the effects of some of these drugs on endometriotic cells/endometriosis-like lesions and the relevant mechanisms. We also predicted potential drugs that may affect NF-κB signaling in endometriosis through the Drug-Gene Interaction Database (DGIdb) (http://www.dgidb.org/) [120] to provide more potential drug treatment options for researchers.
Known drugs that target NF-κB signaling in endometriosis.
| Drug | Function | Experimental model/condition | Reference |
|---|---|---|---|
| Plant/herbal extract | |||
| Baicalein | Inhibits endometriotic cell viability via suppressing NF-κB signaling. | hESCs isolated from patients with endometriosis | [92] |
| Imperatorin | Inhibits the growth and the histopathological features of endometriosis-like lesions via suppressing PI3K/Akt/NF-κB pathway. | SD rat model | [94] |
| 6-Shogaol | Inhibits inflammation of endometriosis-like lesions via suppressing NF-κB signaling. | SD rat model | [95] |
| Octyl Gallate | Inhibits inflammation of endometriosis-like lesions via suppressing NF-κB signaling. | Wistar rat model | [96] |
| HEABG; Ginsenoside Rg3 | Inhibits endometriotic cell proliferation via suppressing TNFα/NF-κB pathway. | hESCs isolated from patients with endometriosis | [16, 91] |
| Parthenolide | Reduces lesion size and growth of endometriosis-like lesions via suppressing TNFα/NF-κB pathway. | BALB/c mouse model | [97] |
| Curcumin | Inhibits inflammation of endometriosis-like lesions partly via suppressing NF-κB signaling. | hESCs isolated from patients with endometriosis | [98] |
| Inhibits the secretion of MIF in hESCs via suppressing IL-1β/NF-κB pathway. | hESCs isolated from patients with endometriosis | [13, 14] | |
| Inhibits the expression of IL-6, IL-8, MCP-1, ICAM-1 and VCAM-1 in hESCs via suppressing TNFα/NF-κB pathway. | hESCs isolated from patients with endometriosis | [19] | |
| Ameliorates decreased apoptotic responses during early endometriosis caused partly via suppressing NF-κB/MMP-3/FasL pathway. | BALB/c mouse model | [99] | |
| Nobiletin | Reduces lesion sizes, pain and inflammation of endometriosis-like lesions via suppressing NF-κB signaling. | hESCs isolated from patients with endometriosis; C57BL/6 mouse model | [87] |
| Andrographolide | Reduces lesion size and growth of endometriosis-like lesions and improves hyperalgesia via suppressing NF-κB signaling. | hESCs isolated from patients with endometriosis; SD rat model | [88] |
| Reduces the recurrence of endometriosis partly via suppressing NF-κB signaling. | BALB/c mouse model | [100] | |
| Glycyrrhizin | Inhibits the production of LPS-induced inflammatory mediators via suppressing NF-κB signaling. | Primary mouse endometrial epithelial cells | [117] |
| Costunolide | Promotes endometriotic cell apoptosis via suppressing NF-κB signaling. | Human endometriotic epithelial cell line 11Z | [93] |
| Artemisia princeps extract | Promotes endometriotic cell apoptosis via suppressing NF-κB signaling. | Human endometriotic epithelial cell line 11Z and 12Z | [67] |
| Cyperi rhizoma extract | Inhibits endometriotic cell adhesion and neurotrophin expression via suppressing Akt/NF-kB pathway. | Human endometriotic epithelial cell line 11Z and 12Z | [118] |
| NF-κB inhibitor | |||
| BAY 11-7085 | Inhibits endometriotic cell proliferation via suppressing NF-κB signaling. | hESCs isolated from patients with endometriosis | [101] |
| BAY 11-7085 and SN-50 | Promotes the apoptosis of endometriosis-like lesions via suppressing NF-κB signaling. | Nude mouse model of endometriosis | [102] |
| PDTC | Inhibits proliferation, angiogenesis, adhesion, migration and invasion of endometriotic cells via suppressing NF-κB signaling. | hESCs isolated from patients with endometriosis | [75, 103-105] |
| Other known drugs | |||
| hCG; Daidzein-rich isoflavone aglycones | Suppresses TNFα/NF-κB pathway in endometriotic cells. | hESCs isolated from patients with endometriosis | [106, 107] |
| Progesterone, dienogest, or danazol; GnRHa; Thalidomide | Suppresses TNFα/NF-κB/IL8 pathway in endometriotic cells. | hESCs isolated from patients with endometriosis | [46, 108, 109] |
| Pioglitazone | Inhibits endometriotic cell proliferation via suppressing TNFα/NF-κB/IL-8 pathway. | hESCs isolated from patients with endometriosis | [18] |
| BV6 | Inhibits endometriotic cell viability via suppressing TNFα/NF-κB/cIAPs pathway. | hESCs isolated from patients with endometriosis | [110] |
| Reduces endometriotic lesion size and represses the inflammatory and angiogenic activity of the endometriosis-like lesions via suppressing NF-κB/cIAPs pathway. | BALB/c mouse model | [111] | |
| CDDO-Me | Inhibits inflammation in endometriosis-like lesions via suppressing NF-κB signaling. | SD rat model | [112] |
| Disulfiram | Prevents endometriotic implant growing via suppressing NF-κB signaling. | Wistar rat model | [113] |
| SR-16234 | Inhibits the growth of endometriosis-like lesions partly via suppressing NF-κB signaling. | BALB/c mouse model | [36] |
| INT-777 | Suppresses TGR5/TNFα/NF-κB pathway in endometriotic cells. | hESCs isolated from patients with endometriosis | [116] |
| Niclosamide | Inhibits macrophage-induced inflammation and endometriotic cell viability partly via suppressing NF-κB signaling. | Human endometriotic epithelial cell line 12Z | [114] |
| hESCs isolated from patients with endometriosis | [115] | ||
| Trichostatin A | Suppresses TNFα/NF-κB pathway in endometriotic cells. | Human endometriotic stromal cell line YHES, and 22B; Human endometriotic epithelial cell line 11Z | [119] |
hESC: human endometrial stromal cell; PI3K: phosphatidylinositol 3 kinase; Akt: protein kinase B; SD: Sprague Dawley; HEABG: hexane extract of aged black garlic; TNFα: tumor necrosis factor alpha; IL: interleukin; MCP-1: monocyte chemoattractant protein 1; ICAM-1: intercellular adhesion molecule 1; VCAM-1: vascular cell adhesion molecule 1; MMP: matrix metalloproteinase; PDAC: pyrrolidine dithiocarbamate; LPS: lipopolysaccharide; TGR5: takeda-G-protein-receptor-5
Plant-derived medicines
Numerous studies have shown that plant extracts are a source of novel therapeutic methods for endometriosis [121], and NF-κB signaling was identified as the target of some of these plant-derived medicines [13, 14, 16, 19, 67, 87, 88, 91-100, 117, 118]. Curcumin derived from the rhizomes of Curcuma plants can repress TNFα/IL1β-induced NF-κB activation in human endometriotic cells, resulting in the reduced secretion of proinflammatory cytokines, such as IL6, IL8, and MIF, and the reduced expression of chemokines, such as MCP-1, and cell adhesion molecules, such as ICAM-1 and VCAM-1 [13, 14, 19]. In addition, in vivo experiments revealed that curcumin can also inhibit MMP3-dependent FasL-induced local immune cell death in the endometriotic milieu partly by suppressing NF-κB activation, which prevents the formation of the immune-tolerant environment in initial endometriotic development [99].
Andrographolide, an active ingredient extracted from Andrographis paniculate, is a potent NF-κB inhibitor in endometriosis. Mechanistically, andrographolide attenuates the DNA-binding activity of NF-κB and the expression of its downstream genes COX-2, TF, and NGF, suppressing the proliferation of endometriotic cells and reducing the size of ectopic lesions [88]. A recent study also found that the perioperative use of β-blockers and/or andrographolide can effectively inhibit the growth of residual lesions in a BALB/c mouse model of endometriosis, indicating the potential value of andrographolide in reducing the recurrence risk of endometriosis [100].
In addition to curcumin and andrographolide, other plant-derived medicines, such as ginsenoside Rg3, baicalein, costunolide, imperatorin, 6-shogaol, octyl gallate, parthenolide, HEABG, nobiletin, Artemisia princeps extract, glycyrrhizin, and Cypri rhizoma extract, also inhibit inflammation and endometriotic cell proliferation by suppressing NF-κB signaling [16, 67, 87, 91-97, 117, 118]. However, the exact mechanisms need further exploration.
NF-κB inhibitors
BAY 11-7085 is a synthetic compound that suppresses IκBα phosphorylation and prevents the release and nuclear translocation of NF-κB [122]. BAY 11-7085 treatment can remarkably inhibit DNA synthesis and the proliferation of endometriotic cells and induce cell apoptosis by activating caspase-mediated apoptosis [101]. SN50, a cell-permeable NF-κB inhibitory peptide, consists of a membrane-translocating motif and a nuclear localization sequence derived from the NF-κB p50 subunit, which specifically inhibits the nuclear translocation of NF-κB [123]. NF-κB inhibition through BAY 11-7085 or SN50 treatment reduced endometriotic lesions and diminished the initial development of endometriosis in a nude mouse model of endometriosis [102].
Pyrrolidine dithiocarbamate (PDTC), a diethyl derivative of dithiocarbamates, is another potent NF-κB inhibitor. It inhibits NF-κB signaling by repressing IκBα phosphorylation, nuclear p65 protein expression, and the DNA-binding activity of NF-κB subunits in endometriotic cells [105]. The expression of NF-κB target genes/molecules in endometriotic cells is also inhibited by PDTC treatment, which may suppress the proliferation (PCNA, CD31, CD34, Ki67, and survivin), angiogenesis (VEGF), adhesion (CD44), and migration/invasion (MMP2, MMP9) of endometriotic cells and reduce inflammatory responses (COX-2, PGE2) [75, 103-105].
Other known drugs
Pioglitazone is a peroxisome proliferator-activated receptor γ ligand that can inhibit TNFα-induced IL-8 expression and endometriotic cell proliferation by suppressing NF-κB signaling [18]. Notably, activation of the TNFα/NF-κB/IL8 pathway in endometriotic cells can also be inhibited by hormone or thalidomide treatment, providing other choices for blocking NF-κB signaling in endometriosis [46, 108, 109]. BV6 is a small-molecule antagonist of inhibitors of apoptosis proteins (IAPs) that are activated by NF-κB signaling in endometriosis. BV6 causes the proteasomal degradation of IAPs and suppresses their expression; thus, it inhibits endometriotic cell proliferation in vitro and the growth and inflammation of murine endometriosis-like lesions in vivo [110, 111].
Niclosamide is an antihelminthic drug used to treat parasitic infections. Recent studies have demonstrated that niclosamide can suppress macrophage-dependent endometriotic cell viability and cytokine/chemokine secretion through STAT3 and NF-κB signaling, but its therapeutic effect needs to be verified in animal models of endometriosis [114, 115].
Potential drugs
The druggability of NF-κB signaling-related genes was described using DGIdb, and potential drug-gene interactions were visualized using Cytoscape [124]. The results showed that RELA and NFKB1, two key NF-κB signaling-related genes, were theoretically regulated by 25 and 16 drugs, respectively (Figure 5). Among these drugs, four NF-κB inhibitors, namely, isoliquiritigenin, isorhamnetin, parthenolide, and rutin, prevented inflammatory responses and inhibited the development of endometriosis in preclinical studies [97, 125-127]. Other drugs, including dehydroxymethylepoxyquinomicin, edasalonexent, acacetin, artesunate, chrysoeriol, cudraflavone B, cynaropicrin, N-(3-oxododecanoyl) homoserine lactone, quercetagetin, sorbinil, tamarixetin, diosmetin, laquinimod, and triptolide, also have inhibitory effects on the NF-κB signaling-induced inflammatory response; thus, their effects on endometriosis need further exploration [128-136].
Discussion and Conclusion
Stimulated by proinflammatory factors, such as IL1β and TNFα, NF-κB signaling is overactive in ectopic endometrial tissues and enhances the proliferation, adhesion, migration, and invasion abilities of endometriotic cells. In addition, NF-κB signaling is involved in the crosstalk between endometriotic cells and PMs, as well as the regulation of PM polarization to affect endometriosis progression.
Estrogen plays a key role in regulating NF-κB signaling in endometriosis and has promoting and inhibitory effects. Progesterone has a clear inhibitory effect on NF-κB activity, and hormone therapies, such as GnRHa and progesterone treatment, can remarkably inhibit NF-κB-related inflammatory responses in endometriotic cells.
Oxidative stress is a key inducer of NF-κB signaling in endometriotic cells by activating the HMGB-1/TLR4/NF-κB axis and inducing iron overload. NF-κB signaling can in turn contribute to oxidative stress by activating NOS/NO signaling and decreasing the expression of antioxidant enzymes. NcRNAs, such as miRNAs and lncRNAs, are abundantly found in the human endometrium and were recently identified to regulate the expression of NF-κB-related genes in endometriotic cells.
In patients with endometriosis, the activation of NF-κB signaling is associated with endometriosis-related pain and infertility. In preclinical studies of endometriosis, plant-derived drugs, NF-κB inhibitors, and other known drugs have been widely evaluated and have shown potent anti-NF-κB effects that alleviate disease progression. Other drugs that have the potential to target NF-κB-related molecules are predicted in this review using DGIdb.
Notably, most NF-κB-related endometriosis studies chose normal/ectopic endometrial stromal cells for subsequent experiments. However, several studies also revealed that the role of NF-κB in endometrial epithelial cells shares several similarities with its role in endometrial stromal cells, such as the regulation by TNFα, estrogen signaling, and ncRNAs [24, 31, 61]; the promotion of cell proliferation, migration, invasion, and adhesion [56, 73, 74]; and the contribution to the resistance of ROS stress-induced cell apoptosis [56]. In addition, several drugs exert anti-endometriotic effects by downregulating NF-κB expression in endometrial epithelial cells [67, 93, 117-119].
Potential interactions between drugs and key NF-κB signal-related genes, RELA and NFKB1. Drugs with an interaction score ≥ 0.2 were screened out. Triangles with sizes from small to large and colors from light to dark represent interaction scores from low to high.
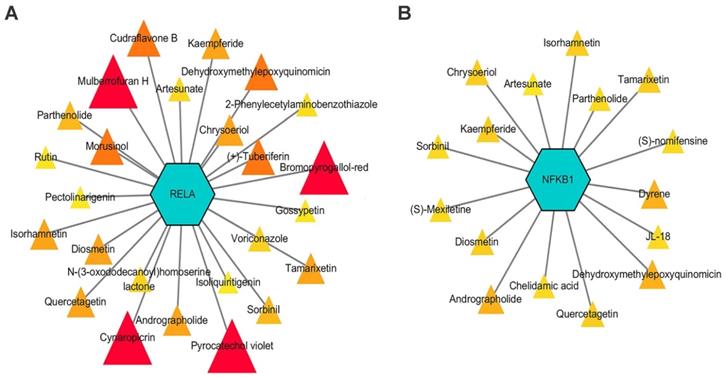
In conclusion, aberrant NF-κB signaling is involved in several aspects of endometriosis pathogenesis. Future studies should discover and develop more drugs that inhibit NF-κB signaling and further test their efficacy in clinical trials.
Abbreviations
NF-κB: nuclear factor-kappa B; IL1: interleukin1; IL6: interleukin 6; IL8: interleukin 8; RANTES: regulated on activation normal T cell expressed and secreted; MIF: macrophage migration inhibitory factor; ER: estrogen receptor; CXCL12: C-X-C motif chemokine 12; CXCR4: C-X-C chemokine receptor type 4; PI3K/Akt: phosphoinositide 3-kinase/ protein kinase B; PTEN: phosphatase and tensin homolog; MAPK/ERK: mitogen-activated protein kinases/extracellular signal-regulated kinase; GnRHa: gonadotrophin-releasing hormone analogues; NAD: nicotinamide adenine dinucleotide; XIAP: X-linked inhibitor of apoptosis; Bcl-2: B-cell lymphoma 2; Bcl-xL: B-cell lymphoma-extra-large; JNK: jun N-terminal kinase; CCL17: C-C motif chemokine 17; CCL 2: C-C motif chemokine 2; TRPA1: transient receptor potential ankyrin 1; TRPV1: transient receptor potential vanilloid-1; MYD88: myeloid differentiation factor-88 adaptor protein; MCP1: monocyte chemoattractant protein 1; TF: tissue factor; NGF: nerve growth factor; COX2: cyclooxygenase-2.
Acknowledgements
Funding
This work was supported by the National Key R&D Program of China (grant number 2017YFC1001202) and the National Natural Science Foundation of China (grant numbers 81974225 and 82171636).
Author contributions
YML took part in the conception of this review, drafted the manuscript and prepared the figures. JZW and XMZ revised the article and performed the final approval of the version to be submitted.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Giudice LC. Clinical practice. Endometriosis. The New England journal of medicine. 2010;362:2389-98
2. Gao X, Outley J, Botteman M, Spalding J, Simon JA, Pashos CL. Economic burden of endometriosis. Fertil Steril. 2006;86:1561-72
3. Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nature reviews Endocrinology. 2014;10:261-75
4. Kapoor R, Stratopoulou CA, Dolmans MM. Pathogenesis of Endometriosis: New Insights into Prospective Therapies. Int J Mol Sci. 2021 22
5. Jiang L, Yan Y, Liu Z, Wang Y. Inflammation and endometriosis. Frontiers in bioscience (Landmark edition). 2016;21:941-8
6. Vallvé-Juanico J, Houshdaran S, Giudice LC. The endometrial immune environment of women with endometriosis. Human reproduction update. 2019;25:564-91
7. Symons LK, Miller JE, Kay VR, Marks RM, Liblik K, Koti M. et al. The Immunopathophysiology of Endometriosis. Trends in molecular medicine. 2018;24:748-62
8. Kaponis A, Iwabe T, Taniguchi F, Ito M, Deura I, Decavalas G. et al. The role of NF-kappaB in endometriosis. Frontiers in bioscience (Scholar edition). 2012;4:1213-34
9. Oeckinghaus A, Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harbor perspectives in biology. 2009;1:a000034
10. Hayden MS, Ghosh S. NF-κB, the first quarter-century: remarkable progress and outstanding questions. Genes & development. 2012;26:203-34
11. Xiu-li W, Su-ping H, Hui-hua D, Zhi-xue Y, Shi-long F, Pin-hong L. NF-kappaB decoy oligonucleotides suppress RANTES expression and monocyte chemotactic activity via NF-kappaB inactivation in stromal cells of ectopic endometrium. Journal of clinical immunology. 2009;29:387-95
12. Yu J, Francisco AMC, Patel BG, Cline JM, Zou E, Berga SL. et al. IL-1β Stimulates Brain-Derived Neurotrophic Factor Production in Eutopic Endometriosis Stromal Cell Cultures: A Model for Cytokine Regulation of Neuroangiogenesis. The American journal of pathology. 2018;188:2281-92
13. Veillat V, Lavoie CH, Metz CN, Roger T, Labelle Y, Akoum A. Involvement of nuclear factor-kappaB in macrophage migration inhibitory factor gene transcription up-regulation induced by interleukin- 1 beta in ectopic endometrial cells. Fertility and sterility. 2009;91:2148-56
14. Cao WG, Morin M, Metz C, Maheux R, Akoum A. Stimulation of macrophage migration inhibitory factor expression in endometrial stromal cells by interleukin 1, beta involving the nuclear transcription factor NFkappaB. Biology of reproduction. 2005;73:565-70
15. Miyamoto A, Taniguchi F, Tagashira Y, Watanabe A, Harada T, Terakawa N. TNFalpha gene silencing reduced lipopolysaccharide-promoted proliferation of endometriotic stromal cells. Am J Reprod Immunol. 2009;61:277-85
16. Kim KH, Park JK, Choi YW, Kim YH, Lee EN, Lee JR. et al. Hexane extract of aged black garlic reduces cell proliferation and attenuates the expression of ICAM-1 and VCAM-1 in TNF-α-activated human endometrial stromal cells. International journal of molecular medicine. 2013;32:67-78
17. Taniguchi F, Harada T, Miyakoda H, Iwabe T, Deura I, Tagashira Y. et al. TAK1 activation for cytokine synthesis and proliferation of endometriotic cells. Mol Cell Endocrinol. 2009;307:196-204
18. Ohama Y, Harada T, Iwabe T, Taniguchi F, Takenaka Y, Terakawa N. Peroxisome proliferator-activated receptor-gamma ligand reduced tumor necrosis factor-alpha-induced interleukin-8 production and growth in endometriotic stromal cells. Fertil Steril. 2008;89:311-7
19. Kim KH, Lee EN, Park JK, Lee JR, Kim JH, Choi HJ. et al. Curcumin attenuates TNF-α-induced expression of intercellular adhesion molecule-1, vascular cell adhesion molecule-1 and proinflammatory cytokines in human endometriotic stromal cells. Phytotherapy research: PTR. 2012;26:1037-47
20. Napetschnig J, Wu H. Molecular basis of NF-κB signaling. Annual review of biophysics. 2013;42:443-68
21. Lebovic DI, Chao VA, Martini JF, Taylor RN. IL-1beta induction of RANTES (regulated upon activation, normal T cell expressed and secreted) chemokine gene expression in endometriotic stromal cells depends on a nuclear factor-kappaB site in the proximal promoter. The Journal of clinical endocrinology and metabolism. 2001;86:4759-64
22. Sakamoto Y, Harada T, Horie S, Iba Y, Taniguchi F, Yoshida S. et al. Tumor necrosis factor-alpha-induced interleukin-8 (IL-8) expression in endometriotic stromal cells, probably through nuclear factor-kappa P activation: Gonadotropin-releasing hormone agonist treatment reduced IL-8 expression. JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM. 2003;88:730-5
23. Wieser F, Vigne JL, Ryan I, Hornung D, Djalali S, Taylor RN. Sulindac suppresses nuclear factor-kappaB activation and RANTES gene and protein expression in endometrial stromal cells from women with endometriosis. J Clin Endocrinol Metab. 2005;90:6441-7
24. Grund EM, Kagan D, Tran CA, Zeitvogel A, Starzinski-Powitz A, Nataraja S. et al. Tumor necrosis factor-alpha regulates inflammatory and mesenchymal responses via mitogen-activated protein kinase kinase, p38, and nuclear factor kappaB in human endometriotic epithelial cells. Molecular pharmacology. 2008;73:1394-404
25. Chang KK, Liu LB, Li H, Mei J, Shao J, Xie F. et al. TSLP induced by estrogen stimulates secretion of MCP-1 and IL-8 and growth of human endometrial stromal cells through JNK and NF-κB signal pathways. Int J Clin Exp Pathol. 2014;7:1889-99
26. Zhang Y, Huang O, Zhang W, Liu L, Xu C. Astragaloside IV exerts anti-inflammatory role in endometriosis by downregulating TLR4/NF-kappa B pathway. TROPICAL JOURNAL OF PHARMACEUTICAL RESEARCH. 2019;18:539-45
27. Alvarado-Díaz CP, Núñez MT, Devoto L, González-Ramos R. Iron overload-modulated nuclear factor kappa-B activation in human endometrial stromal cells as a mechanism postulated in endometriosis pathogenesis. Fertil Steril. 2015;103:439-47
28. Guzeloglu-Kayisli O, Halis G, Taskiran S, Kayisli UA, Arici A. DNA-binding ability of NF-kappaB is affected differently by ERalpha and ERbeta and its activation results in inhibition of estrogen responsiveness. Reproductive sciences (Thousand Oaks, Calif). 2008;15:493-505
29. Zhao Y, Chen Y, Kuang Y, Bagchi MK, Taylor RN, Katzenellenbogen JA. et al. Multiple Beneficial Roles of Repressor of Estrogen Receptor Activity (REA) in Suppressing the Progression of Endometriosis. Endocrinology. 2016;157:900-12
30. Mei J, Zhu XY, Jin LP, Duan ZL, Li DJ, Li MQ. Estrogen promotes the survival of human secretory phase endometrial stromal cells via CXCL12/CXCR4 up-regulation-mediated autophagy inhibition. Hum Reprod. 2015;30:1677-89
31. Zhang H, Zhao X, Liu S, Li J, Wen Z, Li M. 17betaE2 promotes cell proliferation in endometriosis by decreasing PTEN via NFkappaB-dependent pathway. Mol Cell Endocrinol. 2010;317:31-43
32. Gou Y, Li X, Li P, Zhang H, Xu T, Wang H. et al. Estrogen receptor β upregulates CCL2 via NF-κB signaling in endometriotic stromal cells and recruits macrophages to promote the pathogenesis of endometriosis. Human reproduction (Oxford, England). 2019;34:646-58
33. Han SJ, Lee JE, Cho YJ, Park MJ, O'Malley BW. Genomic Function of Estrogen Receptor β in Endometriosis. Endocrinology. 2019;160:2495-516
34. Zhang Z, Yuan Y, He L, Yao X, Chen J. Involvement of angiotensin II receptor type 1/NF-κB signaling in the development of endometriosis. Exp Ther Med. 2020;20:3269-77
35. Xiu-li W, Wen-jun C, Hui-hua D, Su-ping H, Shi-long F. ERB-041, a selective ER beta agonist, inhibits iNOS production in LPS-activated peritoneal macrophages of endometriosis via suppression of NF-kappaB activation. Molecular immunology. 2009;46:2413-8
36. Khine YM, Taniguchi F, Nagira K, Nakamura K, Ohbayashi T, Osaki M. et al. New insights into the efficacy of SR-16234, a selective estrogen receptor modulator, on the growth of murine endometriosis-like lesions. Am J Reprod Immunol. 2018;80:e13023
37. Harada T, Ohta I, Endo Y, Sunada H, Noma H, Taniguchi F. SR-16234, a Novel Selective Estrogen Receptor Modulator for Pain Symptoms with Endometriosis: An Open-label Clinical Trial. Yonago acta medica. 2017;60:227-33
38. Kalkhoven E, Wissink S, van der Saag PT, van der Burg B. Negative interaction between the RelA(p65) subunit of NF-kappaB and the progesterone receptor. The Journal of biological chemistry. 1996;271:6217-24
39. González-Ramos R, Rocco J, Rojas C, Sovino H, Poch A, Kohen P. et al. Physiologic activation of nuclear factor kappa-B in the endometrium during the menstrual cycle is altered in endometriosis patients. Fertil Steril. 2012;97:645-51
40. Zhang GH, Cui LJ, Li AY, Zhang JP, Liu Y, Zhao JS. et al. Endometrial breakdown with sustained progesterone release involves NF-κB-mediated functional progesterone withdrawal in a mouse implant model. Molecular reproduction and development. 2016;83:780-91
41. Shen F, Wang Y, Lu Y, Yuan L, Liu X, Guo SW. Immunoreactivity of progesterone receptor isoform B and nuclear factor kappa-B as biomarkers for recurrence of ovarian endometriomas. Am J Obstet Gynecol. 2008;199:486.e1-e10
42. Han AR, Lee TH, Kim S, Lee HY. Risk factors and biomarkers for the recurrence of ovarian endometrioma: about the immunoreactivity of progesterone receptor isoform B and nuclear factor kappa B. Gynecol Endocrinol. 2017;33:70-4
43. Koninckx PR, Ussia A, Adamyan L, Wattiez A, Gomel V, Martin DC. Heterogeneity of endometriosis lesions requires individualisation of diagnosis and treatment and a different approach to research and evidence based medicine. Facts, views & vision in ObGyn. 2019;11:57-61
44. Saare M, Krigul KL, Laisk-Podar T, Ponandai-Srinivasan S, Rahmioglu N, Lalit Kumar PG. et al. DNA methylation alterations-potential cause of endometriosis pathogenesis or a reflection of tissue heterogeneity? Biol Reprod. 2018;99:273-82
45. Saare M, Rekker K, Laisk-Podar T, Rahmioglu N, Zondervan K, Salumets A. et al. Challenges in endometriosis miRNA studies - From tissue heterogeneity to disease specific miRNAs. Biochimica et biophysica acta Molecular basis of disease. 2017;1863:2282-92
46. Horie S, Harada T, Mitsunari M, Taniguchi F, Iwabe T, Terakawa N. Progesterone and progestational compounds attenuate tumor necrosis factor alpha-induced interleukin-8 production via nuclear factor kappa B inactivation in endometriotic stromal cells. Fertility and sterility. 2005;83:1530-5
47. Zhao D, Lebovic DI, Taylor RN. Long-term progestin treatment inhibits RANTES (regulated on activation, normal T cell expressed and secreted) gene expression in human endometrial stromal cells. J Clin Endocrinol Metab. 2002;87:2514-9
48. Maia H Jr, Haddad C, Casoy J. Combining oral contraceptives with a natural nuclear factor-kappa B inhibitor for the treatment of endometriosis-related pain. International journal of women's health. 2013;6:35-9
49. Choi J, Jo M, Lee E, Lee DY, Choi D. Nuclear factor-kappa B signaling in endometriotic stromal cells is not inhibited by progesterone owing to an aberrant endoplasmic reticulum stress response: a possible role for an altered inflammatory process in endometriosis. Mol Hum Reprod. 2021 27
50. Scutiero G, Iannone P, Bernardi G, Bonaccorsi G, Spadaro S, Volta CA. et al. Oxidative Stress and Endometriosis: A Systematic Review of the Literature. Oxidative medicine and cellular longevity. 2017;2017:7265238
51. Nanda A, K T, Banerjee P, Dutta M, Wangdi T, Sharma P. et al. Cytokines, Angiogenesis, and Extracellular Matrix Degradation are Augmented by Oxidative Stress in Endometriosis. Annals of laboratory medicine. 2020;40:390-7
52. Yun BH, Chon SJ, Choi YS, Cho S, Lee BS, Seo SK. Pathophysiology of Endometriosis: Role of High Mobility Group Box-1 and Toll-Like Receptor 4 Developing Inflammation in Endometrium. PLoS One. 2016;11:e0148165
53. Yun BH, Kim S, Chon SJ, Kim GH, Choi YS, Cho S. et al. High mobility group box-1 promotes inflammation in endometriotic stromal cells through Toll-like receptor 4/nuclear factor-kappa B. American journal of translational research. 2021;13:1400-10
54. Su W, Cui H, Wu D, Yu J, Ma L, Zhang X. et al. Suppression of TLR4-MyD88 signaling pathway attenuated chronic mechanical pain in a rat model of endometriosis. Journal of neuroinflammation. 2021;18:65
55. Defrere S, Lousse JC, Gonzalez-Ramos R, Colette S, Donnez J, Van Langendonckt A. Potential involvement of iron in the pathogenesis of peritoneal endometriosis. Mol Hum Reprod. 2008;14:377-85
56. Preya UH, Woo JH, Choi YS, Choi JH. Hepatocyte nuclear factor-1 beta protects endometriotic cells against apoptotic cell death by up-regulating the expression of antiapoptotic genes†. Biol Reprod. 2019;101:686-94
57. Cho YJ, Park SB, Han M. Di-(2-ethylhexyl)-phthalate induces oxidative stress in human endometrial stromal cells in vitro. Mol Cell Endocrinol. 2015;407:9-17
58. Yu J, Chen LH, Zhang B, Zheng QM. The modulation of endometriosis by lncRNA MALAT1 via NF-κB/iNOS. European review for medical and pharmacological sciences. 2019;23:4073-80
59. Panir K, Schjenken JE, Robertson SA, Hull ML. Non-coding RNAs in endometriosis: a narrative review. Hum Reprod Update. 2018;24:497-515
60. Wang X, Ren R, Shao M, Lan J. MicroRNA-16 inhibits endometrial stromal cell migration and invasion through suppression of the inhibitor of nuclear factor-κB kinase subunit β/nuclear factor-κB pathway. Int J Mol Med. 2020;46:740-50
61. Zhang A, Wang G, Jia L, Su T, Zhang L. Exosome-mediated microRNA-138 and vascular endothelial growth factor in endometriosis through inflammation and apoptosis via the nuclear factor-κB signaling pathway. Int J Mol Med. 2019;43:358-70
62. Wu M, Zhang Y. MiR-182 inhibits proliferation, migration, invasion and inflammation of endometrial stromal cells through deactivation of NF-κB signaling pathway in endometriosis. Molecular and cellular biochemistry. 2021;476:1575-88
63. Dai L, Gu L, Di W. MiR-199a attenuates endometrial stromal cell invasiveness through suppression of the IKKβ/NF-κB pathway and reduced interleukin-8 expression. Mol Hum Reprod. 2012;18:136-45
64. Zheng J, Shao S, Dai C, Guan S, Chen H. miR-9-5p promotes the invasion and migration of endometrial stromal cells in endometriosis patients through the SIRT1/NF-κB pathway. Int J Clin Exp Pathol. 2020;13:1859-66
65. Zhang L, Li HH, Yuan M, Li D, Wang GY. Exosomal miR-22-3p derived from peritoneal macrophages enhances proliferation, migration, and invasion of ectopic endometrial stromal cells through regulation of the SIRT1/NF-κB signaling pathway. European review for medical and pharmacological sciences. 2020;24:571-80
66. Iba Y, Harada T, Horie S, Deura I, Iwabe T, Terakawa N. Lipopolysaccharide-promoted proliferation of endometriotic stromal cells via induction of tumor necrosis factor alpha and interleukin-8 expression. Fertil Steril. 2004;82(Suppl 3):1036-42
67. Kim JH, Jung SH, Yang YI, Ahn JH, Cho JG, Lee KT. et al. Artemisia leaf extract induces apoptosis in human endometriotic cells through regulation of the p38 and NFκB pathways. J Ethnopharmacol. 2013;145:767-75
68. Zhu F, Liu M, Pan Y, Wang X, Chen Y. [Small hairpin RNA targeting inhibition of NF-κB gene in endometriosis therapy of Macaca fascicularis]. Zhonghua Fu Chan Ke Za Zhi. 2015;50:48-53
69. Witz CA. Cell adhesion molecules and endometriosis. Semin Reprod Med. 2003;21:173-82
70. Tsai HW, Huang MT, Wang PH, Huang BS, Chen YJ, Hsieh SL. Decoy receptor 3 promotes cell adhesion and enhances endometriosis development. J Pathol. 2018;244:189-202
71. Nasiri N, Babaei S, Moini A, Eftekhari-Yazdi P. Controlling Semi-Invasive Activity of Human Endometrial Stromal Cells by Inhibiting NF-kB Signaling Pathway Using Aloe-emodin and Aspirin. Journal of reproduction & infertility. 2021;22:227-40
72. Lu Q, Huang Y, Wu J, Guan Y, Du M, Wang F. et al. T-cadherin inhibits invasion and migration of endometrial stromal cells in endometriosis. Hum Reprod. 2020;35:145-56
73. Zhang H, Li M, Wang F, Liu S, Li J, Wen Z. et al. Endometriotic epithelial cells induce MMPs expression in endometrial stromal cells via an NFkappaB-dependent pathway. Gynecol Endocrinol. 2010;26:456-67
74. Li Y, Wang X, Wang X, Wan L, Liu Y, Shi Y. et al. PDCD4 suppresses proliferation, migration, and invasion of endometrial cells by inhibiting autophagy and NF-κB/MMP2/MMP9 signal pathway. Biol Reprod. 2018;99:360-72
75. Zhang JJ, Xu ZM, Zhang CM, Dai HY, Ji XQ, Wang XF. et al. Pyrrolidine dithiocarbamate inhibits nuclear factor-κB pathway activation, and regulates adhesion, migration, invasion and apoptosis of endometriotic stromal cells. Molecular human reproduction. 2011;17:175-81
76. Hogg C, Horne AW, Greaves E. Endometriosis-Associated Macrophages: Origin, Phenotype, and Function. Front Endocrinol (Lausanne). 2020;11:7
77. Lousse JC, Van Langendonckt A, González-Ramos R, Defrère S, Renkin E, Donnez J. Increased activation of nuclear factor-kappa B (NF-kappaB) in isolated peritoneal macrophages of patients with endometriosis. Fertility and sterility. 2008;90:217-20
78. Zhou WJ, Hou XX, Wang XQ, Li DJ. The CCL17-CCR4 axis between endometrial stromal cells and macrophages contributes to the high levels of IL-6 in ectopic milieu. American journal of reproductive immunology (New York, NY: 1989). 2017 78
79. Bacci M, Capobianco A, Monno A, Cottone L, Di Puppo F, Camisa B. et al. Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease. Am J Pathol. 2009;175:547-56
80. Huang YL, Zhang FL, Tang XL, Yang XJ. Telocytes Enhances M1 Differentiation and Phagocytosis While Inhibits Mitochondria-Mediated Apoptosis Via Activation of NF-κB in Macrophages. Cell transplantation. 2021;30:9636897211002762
81. Hou XX, Wang XQ, Zhou WJ, Li DJ. Regulatory T cells induce polarization of pro-repair macrophages by secreting sFGL2 into the endometriotic milieu. Communications biology. 2021;4:499
82. Wu J, Xie H, Yao S, Liang Y. Macrophage and nerve interaction in endometriosis. J Neuroinflammation. 2017;14:53
83. Bohonyi N, Pohóczky K, Szalontai B, Perkecz A, Kovács K, Kajtár B. et al. Local upregulation of transient receptor potential ankyrin 1 and transient receptor potential vanilloid 1 ion channels in rectosigmoid deep infiltrating endometriosis. Molecular pain. 2017;13:1744806917705564
84. Fattori V, Franklin NS, Gonzalez-Cano R, Peterse D, Ghalali A, Madrian E. et al. Nonsurgical mouse model of endometriosis-associated pain that responds to clinically active drugs. Pain. 2020;161:1321-31
85. Kanngiesser M, Häussler A, Myrczek T, Küsener N, Lim HY, Geisslinger G. et al. Inhibitor kappa B kinase beta dependent cytokine upregulation in nociceptive neurons contributes to nociceptive hypersensitivity after sciatic nerve injury. The journal of pain. 2012;13:485-97
86. Souza GR, Cunha TM, Silva RL, Lotufo CM, Verri WA Jr, Funez MI. et al. Involvement of nuclear factor kappa B in the maintenance of persistent inflammatory hypernociception. Pharmacology, biochemistry, and behavior. 2015;134:49-56
87. Wei X, Shao X. Nobiletin alleviates endometriosis via down-regulating NF-κB activity in endometriosis mouse model. Bioscience reports. 2018 38
88. Zheng Y, Liu X, Guo SW. Therapeutic potential of andrographolide for treating endometriosis. Hum Reprod. 2012;27:1300-13
89. Celik O, Celik E, Turkcuoglu I, Yilmaz E, Ulas M, Simsek Y. et al. Surgical removal of endometrioma decreases the NF-kB1 (p50/105) and NF-kB p65 (Rel A) expression in the eutopic endometrium during the implantation window. Reprod Sci. 2013;20:762-70
90. Bianco B, Lerner TG, Trevisan CM, Cavalcanti V, Christofolini DM, Barbosa CP. The nuclear factor-kB functional promoter polymorphism is associated with endometriosis and infertility. Hum Immunol. 2012;73:1190-3
91. Huang R, Chen S, Zhao M, Li Z, Zhu L. Ginsenoside Rg3 attenuates endometriosis by inhibiting the viability of human ectopic endometrial stromal cells through the nuclear factor-kappaB signaling pathway. Journal of gynecology obstetrics and human reproduction. 2020;49:101642
92. Jin Z, Huang J, Zhu Z. Baicalein reduces endometriosis by suppressing the viability of human endometrial stromal cells through the nuclear factor-κB pathway in vitro. Experimental and therapeutic medicine. 2017;14:2992-8
93. Kim JH, Yang YI, Lee KT, Park HJ, Choi JH. Costunolide induces apoptosis in human endometriotic cells through inhibition of the prosurvival Akt and nuclear factor kappa B signaling pathway. Biol Pharm Bull. 2011;34:580-5
94. Ma T, Liu P, Wei J, Zhao M, Yao X, Luo X. et al. Imperatorin alleviated endometriosis by inhibiting the activation of PI3K/Akt/NF-κB pathway in rats. Life sciences. 2021;274:119291
95. Wang D, Jiang Y, Yang X, Wei Q, Wang H. 6-Shogaol reduces progression of experimental endometriosis in vivo and in vitro via regulation of VGEF and inhibition of COX-2 and PGE2-mediated inflammatory responses. The Korean journal of physiology & pharmacology: official journal of the Korean Physiological Society and the Korean Society of Pharmacology. 2018;22:627-36
96. Bustami A, Lestari WP, Hayuningrum CF, Wuyung PE, Wibowo H, Natadisastra RM. The Anti-Inflammatory Effect of Octyl Gallate Through Inhibition of Nuclear Factor-κB (NF-κB) Pathway in Rat Endometriosis Model. Journal of reproduction & infertility. 2020;21:169-75
97. Takai E, Taniguchi F, Nakamura K, Uegaki T, Iwabe T, Harada T. Parthenolide reduces cell proliferation and prostaglandin E2 [corrected] in human endometriotic stromal cells and inhibits development of endometriosis in the murine model. Fertility and sterility. 2013;100:1170-8
98. Chowdhury I, Banerjee S, Driss A, Xu W, Mehrabi S, Nezhat C. et al. Curcumin attenuates proangiogenic and proinflammatory factors in human eutopic endometrial stromal cells through the NF-κB signaling pathway. Journal of cellular physiology. 2019;234:6298-312
99. Jana S, Paul S, Swarnakar S. Curcumin as anti-endometriotic agent: implication of MMP-3 and intrinsic apoptotic pathway. Biochemical pharmacology. 2012;83:797-804
100. Long Q, Zheng H, Liu X, Guo SW. Perioperative Intervention by β-Blockade and NF-κB Suppression Reduces the Recurrence Risk of Endometriosis in Mice Due to Incomplete Excision. Reproductive sciences (Thousand Oaks, Calif). 2019;26:697-708
101. Nasu K, Nishida M, Ueda T, Yuge A, Takai N, Narahara H. Application of the nuclear factor-kappaB inhibitor BAY 11-7085 for the treatment of endometriosis: an in vitro study. American journal of physiology Endocrinology and metabolism. 2007;293:E16-23
102. González-Ramos R, Van Langendonckt A, Defrère S, Lousse JC, Mettlen M, Guillet A. et al. Agents blocking the nuclear factor-kappaB pathway are effective inhibitors of endometriosis in an in vivo experimental model. Gynecologic and obstetric investigation. 2008;65:174-86
103. Celik O, Hascalik S, Elter K, Tagluk ME, Gurates B, Aydin NE. Combating endometriosis by blocking proteasome and nuclear factor-kappaB pathways. Human reproduction (Oxford, England). 2008;23:2458-65
104. Zhang JJ, Xu ZM, Dai HY, Ji XQ, Duan YY, Zhang CM. et al. Application of the nuclear factor-κB inhibitor pyrrolidine dithiocarbamate for the treatment of endometriosis: an in vitro study. Fertility and sterility. 2010;94:2942-4
105. Zhang JJ, Xu ZM, Chang H, Zhang CM, Dai HY, Ji XQ. et al. Pyrrolidine dithiocarbamate attenuates nuclear factor-ĸB activation, cyclooxygenase-2 expression and prostaglandin E2 production in human endometriotic epithelial cells. Gynecologic and obstetric investigation. 2011;72:163-8
106. Huber AV, Saleh L, Prast J, Haslinger P, Knöfler M. Human chorionic gonadotrophin attenuates NF-kappaB activation and cytokine expression of endometriotic stromal cells. Molecular human reproduction. 2007;13:595-604
107. Takaoka O, Mori T, Ito F, Okimura H, Kataoka H, Tanaka Y. et al. Daidzein-rich isoflavone aglycones inhibit cell growth and inflammation in endometriosis. The Journal of steroid biochemistry and molecular biology. 2018;181:125-32
108. Yagyu T, Kobayashi H, Matsuzaki H, Wakahara K, Kondo T, Kurita N. et al. Thalidomide inhibits tumor necrosis factor-alpha-induced interleukin-8 expression in endometriotic stromal cells, possibly through suppression of nuclear factor-kappaB activation. The Journal of clinical endocrinology and metabolism. 2005;90:3017-21
109. Sakamoto Y, Harada T, Horie S, Iba Y, Taniguchi F, Yoshida S. et al. Tumor necrosis factor-alpha-induced interleukin-8 (IL-8) expression in endometriotic stromal cells, probably through nuclear factor-kappa B activation: gonadotropin-releasing hormone agonist treatment reduced IL-8 expression. The Journal of clinical endocrinology and metabolism. 2003;88:730-5
110. Taniguchi F, Higaki H, Izawa M, Azuma Y, Hirakawa E, Deura I. et al. The cellular inhibitor of apoptosis protein-2 is a possible target of novel treatment for endometriosis. American journal of reproductive immunology (New York, NY: 1989). 2014;71:278-85
111. Taniguchi F, Uegaki T, Nakamura K, Mon KY, Harada T, Ohbayashi T. et al. Inhibition of IAP (inhibitor of apoptosis) proteins represses inflammatory status via nuclear factor-kappa B pathway in murine endometriosis lesions. American journal of reproductive immunology (New York, NY: 1989). 2018 79
112. Siracusa R, D'Amico R, Cordaro M, Peritore AF, Genovese T, Gugliandolo E. et al. The Methyl Ester of 2-Cyano-3,12-Dioxooleana-1,9-Dien-28-Oic Acid Reduces Endometrial Lesions Development by Modulating the NFkB and Nrf2 Pathways. International journal of molecular sciences. 2021 22
113. Celik O, Ersahin A, Acet M, Celik N, Baykus Y, Deniz R. et al. Disulfiram, as a candidate NF-κB and proteasome inhibitor, prevents endometriotic implant growing in a rat model of endometriosis. European review for medical and pharmacological sciences. 2016;20:4380-9
114. Sekulovski N, Whorton AE, Shi M, MacLean JA II, Hayashi K. Endometriotic inflammatory microenvironment induced by macrophages can be targeted by niclosamide†. Biology of reproduction. 2019;100:398-408
115. Sekulovski N, Whorton AE, Tanaka T, Hirota Y, Shi M, MacLean JA. et al. Niclosamide suppresses macrophage-induced inflammation in endometriosis†. Biology of reproduction. 2020;102:1011-9
116. Lyu D, Tang N, Wang J, Zhang Y, Chang J, Liu Z. et al. TGR5 agonist INT-777 mitigates inflammatory response in human endometriotic stromal cells: A therapeutic implication for endometriosis. International immunopharmacology. 2019;71:93-9
117. Wang XR, Hao HG, Chu L. Glycyrrhizin inhibits LPS-induced inflammatory mediator production in endometrial epithelial cells. Microbial pathogenesis. 2017;109:110-3
118. Ahn JH, Choi JM, Kang ES, Yoo JH, Cho YJ, Jang DS. et al. The Anti-Endometriotic Effect of Cyperi Rhizoma Extract, Inhibiting Cell Adhesion and the Expression of Pain-Related Factors through Akt and NF-kB Pathways. Medicina (Kaunas, Lithuania). 2022 58
119. Wu Y, Starzinski-Powitz A, Guo SW. Constitutive and tumor necrosis factor-alpha-stimulated activation of nuclear factor-kappaB in immortalized endometriotic cells and their suppression by trichostatin A. Gynecol Obstet Invest. 2010;70:23-33
120. Wagner AH, Coffman AC, Ainscough BJ, Spies NC, Skidmore ZL, Campbell KM. et al. DGIdb 2.0: mining clinically relevant drug-gene interactions. Nucleic acids research. 2016;44:D1036-44
121. Meresman GF, Götte M, Laschke MW. Plants as source of new therapies for endometriosis: a review of preclinical and clinical studies. Hum Reprod Update. 2021;27:367-92
122. Pierce JW, Schoenleber R, Jesmok G, Best J, Moore SA, Collins T. et al. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. The Journal of biological chemistry. 1997;272:21096-103
123. Lin YZ, Yao SY, Veach RA, Torgerson TR, Hawiger J. Inhibition of nuclear translocation of transcription factor NF-kappa B by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. The Journal of biological chemistry. 1995;270:14255-8
124. Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome research. 2003;13:2498-504
125. Hsu YW, Chen HY, Chiang YF, Chang LC, Lin PH, Hsia SM. The effects of isoliquiritigenin on endometriosis in vivo and in vitro study. Phytomedicine: international journal of phytotherapy and phytopharmacology. 2020;77:153214
126. Ilhan M, Ali Z, Khan IA, Taştan H, Küpeli Akkol E. Bioactivity-guided isolation of flavonoids from Urtica dioica L. and their effect on endometriosis rat model. Journal of ethnopharmacology. 2019;243:112100
127. Talebi H, Farahpour MR, Hamishehkar H. The effectiveness of Rutin for prevention of surgical induced endometriosis development in a rat model. Scientific reports. 2021;11:7180
128. Funakoshi T, Yamashita K, Ichikawa N, Fukai M, Suzuki T, Goto R. et al. A novel NF-κB inhibitor, dehydroxymethylepoxyquinomicin, ameliorates inflammatory colonic injury in mice. Journal of Crohn's & colitis. 2012;6:215-25
129. Wu JY, Chen YJ, Bai L, Liu YX, Fu XQ, Zhu PL. et al. Chrysoeriol ameliorates TPA-induced acute skin inflammation in mice and inhibits NF-κB and STAT3 pathways. Phytomedicine: international journal of phytotherapy and phytopharmacology. 2020;68:153173
130. Ko W, Kim KW, Quang TH, Yoon CS, Kim N, Lee H. et al. Cudraflavanone B Isolated from the Root Bark of Cudrania tricuspidata Alleviates Lipopolysaccharide-Induced Inflammatory Responses by Downregulating NF-κB and ERK MAPK Signaling Pathways in RAW264.7 Macrophages and BV2 Microglia. Inflammation. 2021;44:104-15
131. Hayata M, Watanabe N, Kamio N, Tamura M, Nodomi K, Tanaka K. et al. Cynaropicrin from Cynara scolymus L. suppresses Porphyromonas gingivalis LPS-induced production of inflammatory cytokines in human gingival fibroblasts and RANKL-induced osteoclast differentiation in RAW264.7 cells. Journal of natural medicines. 2019;73:114-23
132. Kravchenko VV, Kaufmann GF, Mathison JC, Scott DA, Katz AZ, Grauer DC. et al. Modulation of gene expression via disruption of NF-kappaB signaling by a bacterial small molecule. Science (New York, NY). 2008;321:259-63
133. Baek S, Kang NJ, Popowicz GM, Arciniega M, Jung SK, Byun S. et al. Structural and functional analysis of the natural JNK1 inhibitor quercetagetin. Journal of molecular biology. 2013;425:411-23
134. Song XM, Yu Q, Dong X, Yang HO, Zeng KW, Li J. et al. Aldose reductase inhibitors attenuate β-amyloid-induced TNF-α production in microlgia via ROS-PKC-mediated NF-κB and MAPK pathways. Int Immunopharmacol. 2017;50:30-7
135. Shaji SK, G D, Sunilkumar D, Pandurangan N, Kumar GB, Nair BG. Nuclear factor-κB plays an important role in Tamarixetin-mediated inhibition of matrix metalloproteinase-9 expression. Eur J Pharmacol. 2021;893:173808
136. Chen Y, Wang Y, Liu M, Zhou B, Yang G. Diosmetin exhibits anti-proliferative and anti-inflammatory effects on TNF-α-stimulated human rheumatoid arthritis fibroblast-like synoviocytes through regulating the Akt and NF-κB signaling pathways. Phytotherapy research: PTR. 2020;34:1310-9
Author contact
![]() Corresponding author: Department of Gynecology, Women's Hospital, School of Medicine, Zhejiang University, Xueshi Road, Hangzhou 310006, China. Zhejiang Provincial Key Laboratory of Precision Diagnosis and Therapy for Major Gynecological Diseases, Women's Hospital, Zhejiang University School of Medicine, Hangzhou 310006, China. Tel: 86-571-87061501-2131; E-mail: zhangxinmedu.cn
Corresponding author: Department of Gynecology, Women's Hospital, School of Medicine, Zhejiang University, Xueshi Road, Hangzhou 310006, China. Zhejiang Provincial Key Laboratory of Precision Diagnosis and Therapy for Major Gynecological Diseases, Women's Hospital, Zhejiang University School of Medicine, Hangzhou 310006, China. Tel: 86-571-87061501-2131; E-mail: zhangxinmedu.cn

 Global reach, higher impact
Global reach, higher impact