10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(11):4532-4544. doi:10.7150/ijbs.70784 This issue Cite
Review
The Role of DACT Family Members in Tumorigenesis and Tumor Progression
1. Department of Neurosurgery, Guizhou Provincial People's Hospital, Guiyang, China.
2. Department of Anesthesiology, Guizhou Provincial People's Hospital, Guiyang, China.
3. Department of Nephrology, Guizhou Provincial People's Hospital, Guiyang, China.
4. Department of Neurosurgery, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China.
5. Department of Respiratory and Critical Care Medicine, Guizhou Provincial People's Hospital, Guiyang, China.
*These authors are co-first authors and contributed equally to this work.
Received 2022-1-6; Accepted 2022-4-21; Published 2022-7-11
Abstract
Disheveled-associated antagonist of β-catenin (DACT), which ubiquitously expressed in human tissue, is critical for regulating cell proliferation and several developmental processes in different cellular contexts. In addition, DACT is essential for some other cellular processes, such as cell apoptosis, migration and differentiation. Given the importance of DACT in these cellular processes, many scientists are gradually interested in studying the role of DACT in tumorigenesis and cancer progression. This review article focuses on the latest research regarding the essential functions and potential DACT mechanisms in the occurrence and progression of tumors. Our study indicates that DACT may act as a tumor biomarker for cancer diagnosis and prognosis, as well as a promising therapeutic target in cancers.
Keywords: DACT family members, Cancer, Proliferation, Apoptosis, Invasion, Prognosis
Introduction
It is well known that cancer remains the leading cause of human death, which results from accumulated genetic and epigenetic alterations of various cancer-related genes, including tumor suppressor genes (TSGs) and oncogenes [1-3]. TSGs are considered as crucial genes that are sufficient to control the growth of tumors and regulate many cellular biological processes, including induction of apoptosis and autophagy, inhibition of cell proliferation, suppression of invasion, and DNA damage repair [4-6]. Indeed, the inactivation of TSGs is an important mechanism contributing to tumorigenesis and the progression of cancer [7, 8]. Although more and more TSGs have been found and various therapies targeting these TSGs also have been developed over the past several decades, the prognosis of cancers remains poor. Therefore, it is urgent to identify new therapeutic targets and potential molecular mechanisms that regulate the development of cancer.
DACT family members (DACTs) were initially isolated from yeast-two hybrid screens with the Dishevelled (Dvl) PDZ domain as bait [9-11]. In humans, it has been reported that DACTs comprise three functional members: DACT1, DACT2, and DACT3, which share some conserved domains [12]. The DACTs encode a vital group of adaptor proteins that physically interact with different factors to maintain development and postnatal homeostasis [13]. As adaptors, DACTs exert cell biological function by regulating key signaling pathways, such as Wnt, TGF-β, YAP, and NF-κB signaling pathways (as summarized in Table 1) [14-19]. In recent years, accumulating evidence has revealed that DACTs are aberrantly expressed in many malignant tumors and associated with unfavorable survival. This review will discuss the possible function and underlying mechanisms of DACTs in tumor development and their values in cancer diagnosis and prognosis.
The expression and molecular mechanism of DACTs in tumors
| Molecules | Experiment subjects | Levels | Pathway | Properties | References |
|---|---|---|---|---|---|
| DACT1 | Gastric Cancer | Downregulate | N/A | Be associated with the poorer survival | [81] |
| DACT1 | Gastric Cancer | Downregulate | Nuclear Factor-κB signaling | Inhibits gastric cancer cell growth | [19] |
| DACT1 | Gastric Cancer | Downregulate | N/A | Plays a pivotal role as a potential tumor suppressor in migration and invasion of gastric cancer. | [98] |
| DACT1 | Gastric Cancer | N/A | Wnt/β-catenin signaling | Cyclin G2 suppresses Wnt/β-catenin signaling and inhibits gastric cancer cell growth and migration through DACT1 | [88] |
| DACT1 | Bladder urothelial carcinoma | Downregulate | N/A | Implicates in carcinogenesis and the progression | [36] |
| DACT1 | Cervical cancer | Downregulate | N/A | H1FX-AS1 inhibites cervical cancer cell proliferation, migration and invasion, while induces apoptosis by sponging miR-324-3p to up-regulate the DACT1 expression level | [37] |
| DACT1 | Cervical cancer | Downregulate | N/A | KDM1A can downregulate DACT1 expression through histone deacetylation and therefore suppress the proliferation and migration of cervical cancer cells | [38] |
| DACT1 | Breast cancer | Downregulate | Wnt/β-catenin signaling | Inhibits proliferation and migration | [25] |
| DACT1 | Non-small cell lung cancer tissues | Downregulate | N/A | Relates to the clinicopathological factors and is an independent risk factor for prognosis of the patients with NSCLC | [42] |
| DACT1 | Esophageal squamous carcinoma | Downregulate | N/A | Inhibits proliferation and migration | [50] |
| DACT1 | Hepatocellular Carcinoma | Downregulate | Wnt/β-catenin signaling | Related to the tumorigenesis and leads to cytoplasmic accumulation of β-catenin | [80] |
| DACT1 | Hepatocellular Carcinoma | N/A | Wnt/β-catenin signaling | miR-324-3p directly targets DACT1 and negatively regulates its expression in HCC cells | [66] |
| DACT1 | Type I ovarian cancer | Downregulate | Wnt/β-catenin signaling | Inhibits ovarian cancer growth, tumorigenesis, and induce autophagy | [39] |
| DACT1 | Leukemia | N/A | Wnt/β-catenin signaling | Inhibits Wnt/β-catenin signaling by reducing nuclear β-catenin levels and reduces P-glycoprotein expression in KG-1α cells | [89] |
| DACT1 | Bone metastasis of breast and prostate | N/A | Wnt/β-catenin signaling | TGF-β induces DACT1to form protein condensates in the cytoplasm to repress Wnt signaling | [73] |
| DACT2 | Colon cancer | Downregulate | Wnt/β-catenin signaling | Suppresses cell apoptosis and inhibiting cell proliferation both in vitro and in vivo | [15] |
| DACT2 | Glioma | Downregulate | Wnt/β-catenin and YAP signaling | Inhibits proliferation, cell cycle and enhance apoptosis, sensitivity to temozolomide, and suppress tumor growth | [16] |
| DACT2 | Breast cancer | Downregulate | Wnt/β-catenin signaling | Suppresses proliferation, invasion, tumor growth, migration, and invasion | [35] |
| DACT2 | Breast cancer | Downregulate | Wnt/β-catenin signaling | Inhibits the breast cancer cells proliferation and invasion | [35] |
| DACT2 | Breast cancer | NA | Wnt/β-catenin signaling | MiR-503-3p represses glycolysis and promotes mitochondrial oxidative phosphorylation in breast cancer by elevating DACT2 | [69] |
| DACT2 | Breast cancer | Downregulate | Wnt/β-catenin signaling | Suppresses breast cancer cell growth, induces G1/S phase arrest, inhibits Wnt/βcatenin signaling and suppresses breast cancer cell tumor growth in xenograft mice | [82] |
| DACT2 | Breast cancer | Downregulate | Wnt/β-catenin signaling | Induces breast cancer cell apoptosis in vitro, and further inhibites breast tumor cell proliferation, migration and EMT, through antagonizing Wnt/β-catenin and Akt/GSK-3 signaling. | [83] |
| DACT2 | Gastric cancer | Downregulate | Wnt/β-catenin signaling | MiR‑214 promotes the proliferation, migration and invasion of mixed gastric adenocarcinoma type gastric cancerMKN28 cells by suppressing the expression of DACT2 | [52] |
| DACT2 | Gastric cancer | Downregulate | Wnt/β-catenin signaling | Inhibits cell proliferation, migration and invasion in gastric cancer cells and suppresses gastric cancer xenografts in mice | [53] |
| DACT2 | Prostate Cancer | Downregulate | N/A | Suppresses prostate cancer cells migration and invasion | [55] |
| DACT2 | Hepatocellular Carcinoma | Downregulate | Wnt/β-catenin signaling | Suppresses cell proliferation and inhibit tumor growth | [47] |
| DACT2 | Hepatocellular Carcinoma | Downregulate | Wnt/β-catenin signaling | Suppresses liver cancer progression | [46] |
| DACT2 | Lung Cancer | Downregulate | Wnt/β-catenin signaling | Increases the anti-proliferation effect of gefitinib on NSCLC cells | [44] |
| DACT2 | Lung Cancer | Downregulate | Wnt/β-catenin signaling | Inhibits tumor growth | [45] |
| DACT2 | Esophageal squamous | Downregulate | TGF-βsignaling | Inhibits tumor growth, migration, invasion and reduce tumorigenicity | [49] |
| DACT2 | Esophageal squamous | Esophageal squamous | N/A | Inhibits proliferation and migration | [50] |
| DACT2 | Esophageal squamous | Esophageal squamous | Wnt/β-catenin signaling | Suppresses colony formation, cell migration, invasion in esophageal cancer cells, esophageal cancer cell xenograft growth, and Wnt signaling in human esophageal cancer cells | [51] |
| DACT2 | Papillary Thyroid cancer | Downregulate | Wnt/β-catenin signaling | Suppresses cell proliferation, invasion, and migration | [54] |
| DACT2 | Papillary Thyroid cancer | N/A | N/A | MiR-181a inhibits DACT2 by downregulating MLL3, leading to YAP-VEGF-mediated angiogenesis | [70] |
| DACT2 | Nasopharyngeal carcinoma | Downregulate | Wnt/β-catenin signaling | Inhibits nasopharyngeal carcinoma cell proliferation and metastasis | [85] |
| DACT3 | Colon cancer | Downregulate | Wnt/β-catenin signaling | Leads to inhibition of Wnt/β-catenin signaling and massive apoptosis of colorectal cancer cells | [14] |
| DACT3 | Non-small cell lung cancer | Downregulate | Wnt/β-catenin signaling | Inhibits the malignant phenotype of non-small cell lung cancer | [56] |
| DACT3 | Esophageal squamous | Downregulate | N/A | Unknown | [50] |
| DACT3 | Esophageal squamous | N/A | N/A | MiR-638 regulates expression of DACT3 and promotes autophagy as well as malignancy | [71] |
| DACT3 | Lung Cancer | N/A | N/A | MiR-31 diminishes Dkk-1 and DACT3 expression levels in normal respiratory epithelia and lung cancer cells | [72] |
N/A: Not Applicable.
Structural and biological functions of DACT family members
DACTs are intracellular proteins that can bind to many molecules in the cytoplasm and nucleus [20]. All members are characterized by a PDZ-binding motif at the C terminus, a leucine zipper motif near the N terminus (leucine zipper, LZ), and two serine-rich domains (one in the C-terminal region and the other right after the leucine-zipper domain) [21, 22]. Studies have shown that structures and functional levels of DACTs are highly conserved [20]. Based on research at the amino acid sequence level, there are 799-aminoacids for DACT1 protein, 774-aminoacids for DACT2 protein, and 610-aminoacids for DACT3 protein [23, 24]. DACT1 and DACT2 share 28.8% total amino-acid identity. DACT3 is approximately 27% similar to DACT1 and 24% similar to DACT2 [12]. However, DACT1 are more similar to DACT3 than to DACT2 at the C-terminus [21]. At the cellular level, DACT1 is found throughout the cytoplasm as punctate spots and diffusely in the nucleus, while DACT2 protein locates mainly in the cytoplasm, and DACT3 is predominantly in the nucleus [14, 15, 25]. It has been previously shown that DACT1 and DACT2 are the nucleocytoplasmic shuttling proteins, containing both nuclear localization signal (NLS) and putative nuclear export signal (NES) [15, 26]. Immunofluorescent staining revealed that the N-terminal region of is totally localized in the cytoplasm and the C-terminal region settled in the nucleus, which suggested that NES and NLS are contained in N-terminal and C-terminal region, of the DACT1 or DACT2, respectively [15, 26]. It is worth noticing that the bioinformatic analysis found that there is a typical NES in N-terminal regions and at least one NLS in C-terminal regions of DACT3, meaning DACT3 may also be nucleocytoplasmic shuttling proteins like DACT1 and DACT2[15]. Thus, similarities and differences among the three DACTs suggest that each DACT paralog not only maintains conserved roles, but also has divergent functions in signal transduction.
DACTs play a critical role in the regulation of cellular function and vertebrate development. As reported previously, DACTs expression has been associated with the development of most organs and tissues, including the teeth, eye, heart, brain, and kidney [9, 27-30]. Additionally, DACT1 was reported to mediate TGF-β1-induced apoptosis of mesangial cells [31]. It has been also demonstrated that DACT1 downregulation facilitates proliferation and neuronal differentiation of neural stem cells [32]. Moreover, DACT1 was proved to be a novel atrial fibrillation-related gene by regulating connexin43 via cytoskeletal organization induced by β-catenin accumulation in cardiomyocytes [33]. Another study showed that the deletion of DACT2 significantly increases the proliferation rates of mouse embryonic fibroblasts cells during tooth development [20]. Besides, DACT3 has been reported to inhibit Wnt-induced epithelial-to-mesenchymal transition (EMT) in renal tubular cells [34]. Considering the fact that DACT2 is involved in so many cellular activities, it is natural to imagine that changes of their expression may lead to the occurrence of pathological conditions Indeed, Human DACT1, 2, and 3 genes are located on chromosomes 14q22.3, 6q27, and 19q13.32 region, respectively. Notably, the abnormalities in these regions are often tightly related to the occurrence of tumors [12, 14, 23, 24, 35].
DACTs in cancer development
DACT1 and cancer
Among three members in the DACT family, DACT1 is the best investigated in various types of cancer. Wang et al. reported that DACT1 was down-expressed in primary gastric cancers [19]. Meanwhile, low expression of DACT1 was proved in primary gastric cancer tissues compared with the adjacent nontumor tissues [19]. In bladder urothelial carcinoma, DACT1 protein was decreased or absent in bladder cancer tissues [36]. Conversely, high DACT1 expression was observed in almost all normal bladder samples [36]. A similar result was found in breast cancer, that DACT1 expression was significantly silenced or downregulated in cancerous tissues compared with paired surgical-margin tissues and normal breast tissues [25]. Further analysis based on the gene expression-based outcome for breast cancer online database showed that decreased expression of DACT1 was associated with estrogen receptor negative and higher histologic grade [25], indicating that DACT1 could be considered as a potential indicator for different histological subtypes of breast cancer. DACT1 was also significantly downregulated in cervical cancer tissues compared with its expression in normal tissues [37, 38]. Consistent with previous studies, DACT1 expression showed an obviously significant difference between either normal ovarian tissues or benign lesions and malignant tissues [39]. However, DACT1 was reported to be overexpressed in colon cancer and squamous cell carcinoma [40, 41], which indicates that the biological function of DACT1 varies among malignancies.
In addition, researchers reported that the expression of DACT1 was significantly down-regulated in non-small cell lung cancer tissues (NSCLC) compared with that in normal lung tissues [42]. Patients with low expression of DACT1 were significantly associated with poor prognosis [42]. Moreover, decreased expression was positively correlated with poor differentiation, high pathologic TNM stage, and lymph node metastasis of lung cancer [42]. However, in esophageal squamous cell carcinoma (ESCC), it demonstrated that DACT1 expression was increased compared with that in normal squamous cell epithelia [41]. Higher DACT1 expression was also shown to be significantly correlated with regional lymph node metastasis and P-stage [41]. All of these evidences strongly indicate that DACT1 may serve as a predictive factor for the prognosis of cancer, and the function of DACT1 may be dependent on cellular context.
DACT2 and cancer
Previous studies have reported that DACT2 expression was significantly depressed in primary breast cancer samples compared with adjacent normal breast tissues [35, 43]. Significant down-regulation of DACT2 expression was also observed in NSCLC tissues, colorectal cancer and hepatocellular carcinoma (HCC) tissues compared to normal tissues [44-48]. Additionally, the expression level of DACT2 was significantly reduced in primary esophageal cancer samples compared with adjacent normal esophageal mucosa, and further statistical analysis showed that the frequency of DACT2 silencing significantly correlated with differentiation and prognosis of ESCC [49-51]. Moreover, the decreased expression of DACT2 is closely correlated with the invasion, metastasis, occurrence, and development of gastric cancer, prostate, and papillary thyroid cancer [52-55]. Similarly, based on our findings, DACT2 was significantly down-expressed in glioma tissues compared with normal brain tissues and the paired adjacent tissues [16]. Expression levels of DACT2 in glioma tissues significantly correlated with the WHO grade, Karnofsky Performance Score and age[16]. Lower DACT2 expression had a significantly poorer overall survival compared to patients with higher DACT2 expression in gliomas with different grades [16].
DACT3 and cancer
Compared with DACT1 and 2, DACT3 has been less well characterized, and its function in tumorigenic signaling and development remains unclear. Recent research revealed that DACT3 expression was reduced in NSCLC tissue, which was correlated with lymph node metastasis and poor prognosis of NSCLC [56]. It also demonstrated that the expression of DACT3 is also consistently reduced in colorectal cancer [14]. Further analysis showed DACT3 expression was related to prognosis and validated to be associated with the pathological stage of colon cancer [57]. Interestingly, Luo et al. performed the weighted gene co-expression network analysis to identify the key modules and hub genes in bladder cancer, and they found that three hub genes (DACT3, TNS1, and MSRB3) were related to lymph node metastasis and prognostic of bladder cancer, which might provide new insights into the therapeutic target of bladder cancer [58].
Together, all these studies demonstrated that the association between DACTs expression and outcome in most types of cancers strengthened the importance of the role of DACTs in tumor progression and validated their potential value as a robust prognostic biomarker. The expressions of DACTs in cancers are shown in Table 1.
Expression regulation of DACT family members in cancers
Transcriptional and Posttranscriptional regulation of DACTs
Both transcriptional and posttranscriptional regulation play crucial roles in DACTs expression in cancers. MicroRNAs (miRNAs) are small non-coding RNAs that have been identified to play pivotal roles in various biological contexts by mediating the regulation of target gene expression at the posttranscriptional level [59-67]. In HCC, Gan et al. revealed that DACT1 might act as a target gene of miR-1269 by bioinformatics analysis [68]. Besides, miR-324-3p was demonstrated that it cloud directly target DACT1 and negatively regulate its expression in HCC cells [66]. Furthermore, rescue experiments revealed that DACT1 could reverse the effects of miR-324-3p in HCC cells [66]. Recently, evidence showed that miR-324-3p targeted the DACT1 transcript directly at its 3′UTR region [37]. However, lncRNA H1FX-AS1 modulates miR-324-3p-mediated inhibition of DACT1 in cervical cancer [37]. MiRNAs are also involved in the regulation of DACT2 expression. Zhao et al. showed that expression of miR-214 is elevated, but the expression of DACT2 mRNA is decreased in gastric cancer tissues, being closely correlated with the invasion, metastasis, occurrence, and development of gastric cancer [52]. Furthermore, miR‑214 can directly bind with the 3'‑UTR seeding region of DACT2 mRNA to regulate its expression [52]. Similarly, it was indicated that the expression of DACT2 increased in breast cancer tissues accompanied by decreased expression of miR-503-3p [69]. Meanwhile, DACT2 was further proved to be a direct target of miR-503-3p [69]. Recently, Wang et al. reported that miR-181a inhibited DACT2 by downregulating mixed-lineage leukemia 3(MLL3) expression in human umbilical vein endothelial cells, resulting in papillary thyroid cancer progression [70]. As a member of DACTs, the expression of DACT3 is also modulated by miRNAs in cancers. Ren et al. found a significantly negative correlation between DACT3 expression and miR-638 expression in ESCC and breast cancer tissues [71]. Importantly, it was further demonstrated that miR-638 might negatively regulate the expression of DACT3 by targeting the 3′UTR region of DACT3 to promote malignancy [71]. Interestingly, cigarette smoke cloud induces expression of miR-31, which directly interacts with DACT-3 to decrease the expression of DACT3 in lung cancer cells [72] (as summarized in Table 2).
miRNAs involved in the regulation of DACTs expression
| Molecules | MicroRNAs | Properties | References |
|---|---|---|---|
| DACT1 | miR-324-3p | MiR-324-3p negatively regulates DACT1 expression in HCC cells, and lncRNA H1FX-AS1 can act as a competing endogenous RNA of miR-324-3p to inhibit DACT1 cervical cancer progression | [37, 66] |
| DACT2 | miR-214 | MiR-214 inhabits DACT2 expression in gastric cancer tissues to promote the occurrence and development of gastric cancer | [52] |
| miR-503-3p | DACT2 increases accompanied by decreased expression of miR-503-3p in breast cancer tissues | [69] | |
| miR-181a | MiR-181a inhibits DACT2 by downregulating MLL3 expression, resulting papillary thyroid cancer progression | [70] | |
| DACT3 | miR-638 | MiR-638 negatively regulates expression of DACT3 to promote cancer malignancy | [71] |
| miR-31 | MiR-31 directly interacts with DACT-3 to decrease the expression of DACT3 in lung cancer cells | [72] |
Transcriptional level regulation is another primary method involved in DACTs expression regulation. Esposito et al. demonstrated that DACT1 was transcriptionally regulated by TGF-β to modulate Wnt signaling [73]. A recent study demonstrated that the rs9364433 single nucleotide polymorphism in gene promoter has an allele-specific effect on DACT2 expression modulated by transcription factor TFAP2A [44]. The G allele is associated with diminished TFAP2A binding leading to the transcriptional suppression of the DACT2 gene in NSCLC cell lines and tissues [44].
Epigenetic regulation of DACTs
Genetic and epigenetic variation work in concert to influence human health and disease [74]. Distinct from genetic mutation, epigenetic influences modify gene expression without permanent changes in the genomic sequence [74-76]. Indeed, recent studies demonstrated that epigenetic alterations are considered the main regulation mechanisms during carcinogenesis and cancer progression. The epigenetic modifications can be generally categorized into DNA methylation, histone proteins modifications, and mutations in chromatin remodeling complexes [76]. Notably, DNA methylation is the most extensively studied epigenetic mechanism that predominantly occurs in CpG islands (CGIs), preferentially located at the 5′ promoter region of more than 50% of human genes [75, 77, 78]. Hypermethylation of TSGs in CGI of the promoter regions is an alternative mechanism for TSG silencing and could occur early in tumorigenesis, thus serving as a promising tumor marker for diagnosis of multiple cancers [25].
Silencing of DACT family members can also be occurred by methylation of CpG islands in the promoter region, which plays an important role in tumorigenesis and development of tumors. For example, Yang et al. reported that the DACT1 gene was inactivated by hyper-methylation in the promoter region in nasopharyngeal carcinoma with 5-aza-deoxycytidine treatment, which can be reactivated by demethylation [79]. Promoter CpG methylation of DACT1 was also detected in breast cancer tissues, which was correlated with its downregulation [25]. Demethylation treatment of breast cancer cell lines restored expression of DACT1 along with promoter demethylation, as well as in hepatoma cell lines [80], suggesting that promoter methylation is a major mechanism for DACT1 silencing in breast cancer cells [25]. In addition, methylation of the DACT1 CpG island is common in bladder cancer, repressing DACT1 expression [36]. DACT1 gene hypermethylation was closely related to tumor size, grade, and stage, which indicates hypermethylation of DACT1 may be a potential prognostic factor progression of bladder urothelial carcinoma [36]. Based on a large-scale gene sequencing analysis of gastric cancer patients, it was found that different methylated levels of DACT1 promoter were identified in the gastric cancer tissues while unmethylated in normal gastric mucosal tissues [81]. Notably, three methylated CpG sites (CpG-515, CpG-435, and CpG-430) of DACT1 promoter were significantly associated with the poor survival of gastric cancer patients [81]. Similarly, Deng et al. demonstrated that gene silence of DACT1 was mediated by promoter methylation in gastric cancer cells, and DACT1 methylation was significantly associated with tumor metastasis, invasion, and advanced tumor stage [19].
According to previous reports, DNA methylation played a crucial role in the silencing of DACT2. Guo et al. found that DACT2 was frequently methylated in human ESCC, which may be one of the main mechanisms for DACT2 inactivation [50]. It also demonstrated that DACT2 was silenced by promoter hypermethylation in HCC, contributing to cancer progression [46]. Additionally, DACT2 was reported to be frequently methylated in human lung cancer, and methylation of DACT2 was associated with poor differentiation of lung cancer [45]. The similar results were reported in papillary thyroid cancer [54], breast cancer [35, 82, 83], esophageal cancer [50, 51], gastric cancer [53], colorectal cancer [84] and nasopharyngeal cancer [85]. However, it was shown that promoter methylation of DACT1 and DACT2 may not be a common event in oral squamous cell carcinoma [86], suggesting promoter methylation of DACTs has the tumor cell-specific, and the precise mechanism of this gene family inactivation need to be further studied.
DACT3 expression is also frequently reduced in cancers. However, promoter methylation may not be the primary mechanism that mediates DACT3 silencing. According to the research, histone modification may be the main regulated mechanism for the inactivation of DACT3 in colon cancer [14]. These results reinforce the importance of epigenetic regulation as a major mechanism of DACTs silencing in tumor progression. As shown in Table 3, the methylation status of DACTs is summarized.
DACT family commonly methylated in cancers
| Tissue | Gene | Assay | Methylation prevalence (%) | References | ||
|---|---|---|---|---|---|---|
| Normal | Para-cancer | cancer | ||||
| Breast cancer | DACT1 | MSP | 0% (0/15) | 0% (0/11) | 29.9% (40/134) | [25] |
| Breast cancer | DACT2 | MSP | N/A | 0% (0/15) | 32.9% (26/79) | [43] |
| Breast cancer | DACT2 | MSP | N/A | N/A | 83% (10/12) | [35] |
| Breast cancer | DACT2 | MSP | N/A | 66.7% (16/24) | 49.7% (76/153) | [82] |
| Breast cancer | DACT2 | MSP/BGS | 0% (0/14) | 20% (1/5) | 73% (107/147) | [83] |
| Bladder urothelial carcinoma | DACT1 | MSP | 25% (7/29) | N/A | 58.62% (17/29) | [36] |
| Prostate cancer | DACT2 | MSP/BGS | N/A | N/A | 68.1% (32/47) | [55] |
| Gastric cancer | DACT1 | MSP | N/A | N/A | 29.3% (60/205) | [19] |
| Gastric cancer | DACT1 | MSP | 0% (0/25) | N/A | 28.3% (25/459) | [81] |
| Gastric cancer | DACT1 | MSP | 0% (0/20) | N/A | 29.3% (60/205) | [98] |
| Gastric cancer | DACT2 | MSP | 0% (0/8) | N/A | 55.7% (93/167) | [53] |
| Nasopharyngeal cancer | DACT1 | MSP | N/A | N/A | 70.9% (44/62) | [86] |
| Nasopharyngeal cancer | DACT2 | MSP | 0% (0/8) | N/A | 91% (29/32) | [85] |
| Colon cancer | DACT2 | BGS | 0% (0/12) | N/A | 43.3% (29/67) | [15] |
| DACT2 | MSP | N/A | N/A | 46% (23/50) | [48] | |
| Esophageal squamous | DACT1 | MSP/BGS | N/A | 16.4%-47.8% (26/159-76/159) | 43.4%-54.1% (69/159-86/159) | [50] |
| Esophageal squamous | DACT2 | MSP/BGS | N/A | 21.4% (34/159) | 52.3% (83/159) | [50] |
| Esophageal squamous | DACT2 | MSP/BGS | 0% (0/27) | N/A | 69% (87/126) | [51] |
| Esophageal squamous | DACT3 | MSP/BGS | N/A | 3.8% | 5.7% | [50] |
| Hepatocellular carcinoma | DACT1 | MSP | 0% (0/3) | 18% (9/43) | 51% (22/43) | [80] |
| Hepatocellular carcinoma | DACT2 | MSP | N/A | N/A | 54.84% (34/62) | [47] |
| Lung cancer | DACT2 | MSP | 0% (0/4) | N/A | 41% (43/106) | [45] |
| Papillary thyroid cancer | DACT2 | MSP | 0% (0/10) | N/A | 64.6% | [54] |
| Oral squamous carcinoma | DACT2 | MSP | N/A | N/A | 70.2% (33/47) | [86] |
| Bladder urothelial carcinoma | DACT1 | MSP | N/A | 25% (7/29) | 58.62% (17/29) | [36] |
| Breast cancer | DACT1 | MSP | 0% (0/15) | 0% (0/11) | 29.9%(40/134) | [25] |
| DACT2 | MSP | N/A | 0% (0/15) | 32.9% (26/79) | [43] | |
| MSP | N/A | N/A | 83% (10/12) | [35] | ||
| MSP | N/A | 66.7% (16/24) | 49.7%(76/153) | [82] | ||
| MSP/BGS | 0% (0/14) | 20% (1/5) | 73% (107/147) | [83] | ||
| Bladder urothelial carcinoma | DACT1 | MSP | 25% (7/29) | N/A | 58.62% (17/29) | [36] |
| Prostate cancer | DACT2 | MSP/BGS | N/A | N/A | 68.1% (32/47) | [55] |
| Gastric cancer | DACT1 | MSP | N/A | N/A | 29.3% (60/205) | [19] |
| MSP | 0% (0/25) | N/A | 28.3% (25/459) | [81] | ||
| MSP | 0% (0/20) | N/A | 29.3% (60/205) | [98] | ||
| DACT2 | MSP | 0% (0/8) | N/A | 55.7% (93/167) | [53] | |
| Nasopharyngeal cancer | DACT1 | MSP | N/A | N/A | 70.9% (44/62) | [86] |
| DACT2 | MSP | 0% (0/8) | N/A | 91% (29/32) | [85] | |
| Colon cancer | DACT2 | BGS | 0% (0/12) | N/A | 43.3% (29/67) | [15] |
| DACT2 | MSP | N/A | N/A | 46% (23/50) | [48] | |
| Esophageal squamous | DACT1 | MSP/BGS | N/A | 16.4%-47.8% (26/159-76/159) | 43.4%-54.1% (69/159-86/159) | [50] |
| DACT2 | MSP/BGS | N/A | 21.4% (34/159) | 52.3% (83/159) | [50] | |
| MSP/BGS | 0% (0/27) | N/A | 69% (87/126) | [51] | ||
| DACT3 | MSP/BGS | N/A | 3.8% | 5.7% | [50] | |
| Hepatocellular carcinoma | DACT1 | MSP | 0% (0/3) | 18% (9/43) | 51% (22/43) | [80] |
| DACT2 | MSP | N/A | N/A | 54.84% (34/62) | [47] | |
| Lung cancer | DACT2 | MSP | 0% (0/4) | N/A | 41% (43/106) | [45] |
| Papillary thyroid cancer | DACT2 | MSP | 0% (0/10) | N/A | 64.6% | [54] |
| Oral squamous carcinoma | DACT2 | MSP | N/A | N/A | 70.2% (33/47) | [86] |
| Bladder urothelial carcinoma | DACT1 | MSP | N/A | 25% (7/29) | 58.62% (17/29) | [36] |
DACT family members regulate cancer cell proliferation and cell cycle
Uncontrolled proliferation is characteristic of cancer cells and represents one of the hallmarks of neoplastic growth [87]. The cell proliferation inhibition capacity of DACTs is discovered in various cancers including gastric cancer [19, 52, 53, 88], leukemia [89], breast cancer [25, 35, 69, 82, 83], cervical cancer, HCC [46, 66], lung cancer [45, 56], papillary thyroid cancer [54, 70], esophageal cancer [50, 51, 71, 90], glioma [16], nasopharyngeal carcinoma [85, 91]. Mechanically, DACTs exert their proliferation‐inhibitory effects through changes in signaling pathways. Zhu et al. demonstrated that DACT1 overexpression led to a strong cell cycle arrest at the G0/G1 phase through inhibiting Wnt/β-catenin signaling by reducing nuclear β-catenin levels, which resulted in KG-1α cell proliferation inhibition [89]. Yin et al. found that DACT1 reduced the expression of active β-catenin and its downstream target gene c-MYC in breast cancer cells, thus inhibiting breast cancer cell proliferation [25]. In addition, Wang et al. reported that DACT1 suppressed gastric cancer cell proliferation via decreasing the expression of NF-κB signaling downstream factors, including oncogenic interleukin-8 (IL-8) and tumor necrosis factor-α (TNF-α) [19]. DACT1 was also proved that interact with proteins to regulate cell proliferation. Gao et al. found that DACT1 was a cyclin G2-interacting protein that was required for the cyclin G2-mediated inhibition of β-catenin expression [88]. Cyclin G2 inhibited the activity of CKI to phosphorylate DACT1, causing growth arrest in gastric cancer cells [88]. In cervical cancer, DACT1 was identified as a target gene of the lysine-specific histone demethylase 1A (KDM1A) [38]. KDM1A could downregulate DACT1 expression through histone deacetylation to enhance the proliferation of cervical cancer cells [38]. Moreover, Shi et al. demonstrated that H1FX-AS1 served as a ceRNA of miR-324-3p to upregulate the DACT1 expression, which induced inhibition of the proliferation of cervical cancer [37]. Similarly, DACT1 was also negatively regulated by miR-324-3p, and DACT1 inhibited HCC growth by decreasing the accumulation of both cytoplasmic and nuclear β-catenin and expression of c-Myc and cyclin D1 [66].
DACT2 has also been reported to be frequently down-expressed in multiple human cancers, which suppressed the proliferation of cancer cells. Restoration of DACT2 expression cloud suppresses human breast cancer cells growth through inducing G1/S checkpoint arrest [82]. The expression of cyclinD1 and cyclinE1, which were critical proteins for G1‐S progression, were decreased through inhibiting the Wnt/β-catenin signaling pathway after DACT2 overexpression [35, 82, 83]. Similarly, DACT2 knockdown in HCC cells and papillary thyroid cancer induced G1/S arrest of cell cycle and significant suppression of cell growth, as well as in lung cancer [45, 46]. Our previous study proved that proliferation was inhibited, and G1/S arrest was enhanced by overexpression of DACT2 in glioma cells [16]. Other mechanism research found the expression of PCNA and cyclinD1 were decreased after overexpression of DACT2, and restoration of DACT2 can suppress upregulation of p-YAP and prevent YAP translocating into the nucleus and sequestering in the cytoplasm to degrade through inactivation of Wnt/β-catenin signaling pathway [16]. According to previous researches, DACT2 was regulated by various miRNAs to participate in the regulation of the proliferation of tumors. MiR-503-3p derived from macrophage directly targeted on DACT2 [69]. Reduction of miR-503-3p repressed glycolysis and promoted mitochondrial oxidative phosphorylation in breast cancer cells and decreased tumor growth by overexpressing DACT2 and inactivating the Wnt/β-catenin signaling pathway [69]. Hypoxia-induced exosomal miR-181a from papillary thyroid cancer targeted and inhibited MLL3, leading to the downregulation of DACT2, which contributed to tumor growth [70]. However, it was demonstrated that re-expression of DACT2 inhibited the expression of cyclinB1 and the cyclin B1-Cdk1 (Cyclin-Dependent Kinase 1, also known as cell division control protein kinase 2, CDC2) complex CDC2, and increased the levels of p-CDC2 (Y15) in esophageal and gastric cancers by inhibiting Wnt signaling pathway [51, 53]. Additionally, Zhang et al. also reported that DACT2-restored expression reduced p-Smad2/3, an index of TGF-β activity, via both proteasome and lysosomal pathways, which cloud induce G2/M phase arrest in esophageal cancer [90].
As a member of the DACT family, DACT3 is also involved in tumor growth. DACT3 transfection inhibited c-Myb expression of NSCLC cells, as well as c-Myc expression and β-catenin nuclear translocation to inhibit cell proliferation [56]. Over-expression of miR-31 significantly enhanced proliferation by direct interaction with Dickkopf-1 and DACT-3 in lung cancer [72].
Interestingly, DACTs exerted different roles in the regulation of colorectal cancer cell progression. It was found that DACT1 increased the nuclear and cytoplasmic fractions of β-catenin via phosphorylated GSK-3β at Ser9 to promote cell proliferation in colon cancer [40]. However, reactivating DACT2 transcription significantly inhibited nuclear β-catenin expression to inactivate the Wnt/β-catenin pathway, which consequently restricted colorectal cancer cells proliferation [15, 84].
DACTs family members regulate cancer cell apoptosis
Resistance to apoptosis is a remarkable hallmark of malignancies. Apoptosis is a programmed cell death, which is critical for removing unessential cells like tumor cells. Specific mechanisms of apoptosis in anti-carcinogenic action include activating poly (ADP-ribose) polymerase (PARP), subsequently fragments of effector caspases, and the Bcl-2 (B-cell lymphoma 2) family of proteins [92, 93]. Zhu et al. demonstrated that DACT1-transfected KG-1α leukemia cells had increased expression of pro-apoptotic proteins Bax and decreased expression of anti-apoptotic proteins Bcl-2, which revealed that DACT1 might activate intrinsic apoptotic pathways [89]. It is reported that re‐expression of DACT1 in breast and gastric cells appears to induce apoptosis through a caspase-dependent pathway, including activation of caspase-9, followed by cleavage of downstream caspase effectors caspase-3 and caspase-7, ultimately stimulating the activation of PARP and cellular disassembly and apoptosis [19, 25].
Moreover, DACT1 downregulated anti-apoptotic genes Bcl2 and Bcl-XL, which contributed to the prevention of mitochondrial apoptosis, leading to activation of the downstream apoptotic protease cascade [19]. Similar to DACT1, ectopic expression of DACT2 led to a significant increase of apoptotic cells [15, 16, 91], induction on the expression of Bax in glioma cells [16], and the enhanced cleavage of PARP in colon cancer cells [15]. In addition, it is suggested that overexpression of DACT3 resulted in a dramatic activation of caspase 3 in colorectal cancer cells and induced a sharp drop in the mitochondrial transmembrane potential that is characteristic of apoptosis [15].
Regarding the signaling pathways implicated in the regulation of cell apoptosis, DACTs have been proved to promote cell apoptosis through blocking Wnt/β-catenin [14, 15, 25, 89, 91], NF-κB [19], YAP [16] signaling pathways.
DACT family members regulate cancer cell migration and invasion
Cell migration and invasion are central to morphogenesis and to multiple aspects of tumor metastasis [94, 95]. Matrix metalloproteinases (MMPs) are a family of zinc‐containing endopeptidases collectively capable of degrading extracellular matrix components. Among the members of the MMP family, MMP-2 and MMP-9 have been widely studied and linked to increased invasion ability in various cancers [96, 97]. It was reported that DACTs could inhibit the invasion and metastasis through decreasing expression and activity of MMP-2 and MMP-9 in gastric cancer [53, 98], colorectal cancer [84], esophageal cancer [51]. EMT is characterized by the loss of cell-cell adhesions and gain of migratory and invasive traits, which governs tumor cell metastasis [99]. The molecular characteristics of EMT contain the suppression of epithelial markers (e.g., E-cadherin) and the concomitant promotion of mesenchymal markers such as N-cadherin, Fibronectin DACT2 directly interacts with β-catenin, and Vimentin [100]. Wang et al. revealed that DACT2 upregulated the expression of E-cadherin, which was enriched in the cellular membrane, especially at cell-cell contact region, indicating that DACT2 restored E-cadherin junction stability. Moreover, on E-cadherin knockdown, cell invasive ability was significantly increased in DACT2-expressing colon cancer cells, indicating that E-cadherin blockade could partially relieve the anti-metastatic potential of DACT2 [15]. In addition, forced transfection of DACT2 effectively reversed EMT to mesenchymal-to-epithelial transition in breast cancer cells, resulting in the upregulation of E-cadherin and downregulation of Vimentin [83].
Mechanically, DACTs exert its migration- and invasion-inhibitory effects through Wnt/β-catenin [15, 25, 35, 51, 53, 54, 73, 83, 88, 91], planar cell polarity (PCP) [98], TGF-β/Smad2/3 [49] and Akt/GSK-3 signaling pathway [83]. It is suggested that cyclin G2 could interact with DACT1 and inhibit the ability of CKI to phosphorylate DACT1, thereby stimulating β-catenin degradation in a GSK-3β-dependent in gastric cancer [88]. Increasing evidence has revealed critical contributions of the PCP pathway to tumor metastasis [101-103]. Liu et al. reported that DACT1 regulated the PCP pathway by promoting Dvl-2 degradation and suppressing the active form of JNK in gastric cancer cells [98]. Another study indicated that restored expression of DACT2, the activity of TGF-β/Smad2/3 was suppressed via both proteasome and lysosomal degradation pathways, leading to F-actin rearrangement that might depend on the involvement of cofilin and ezrin-redixin-moesin protein in ESCC [49]. Moreover, the data from breast cancer research indicated that expression of p-AKT and p-GSK-3β dramatically decreased in breast cancer cells upon DACT2 re-expression, which indicated DACT2 might regulate Akt/GSK-3 signaling pathway to involve in migration and invasion [83]. Up to now, the Wnt/β-catenin signaling pathway is the most well-studied pathway regulated by DACTs. Enormous studies provided evidence that DACTs antagonize Wnt/β-catenin signaling by decreasing active β-catenin levels in cancers. In colon cancer, DACT2 binding to nuclear β-catenin, preventing it from forming a complex with its partner lymphoid enhancer-binding factor 1, was an important mechanism for DACT2-mediated Wnt/β-catenin signaling inhibition [15]. Consistently, DACT3 interacted with and down-regulated Dvl2 protein and attenuated the Wnt-responsive Top flash reporter expression, which agrees with the inhibitory effect of DACT3 on Wnt signaling in colorectal cancer. As shown in Table 1 and Figure 1, different mechanisms of DACTs in regulating progression of cancers are summarized.
Molecular mechanism of DACTs in tumors.
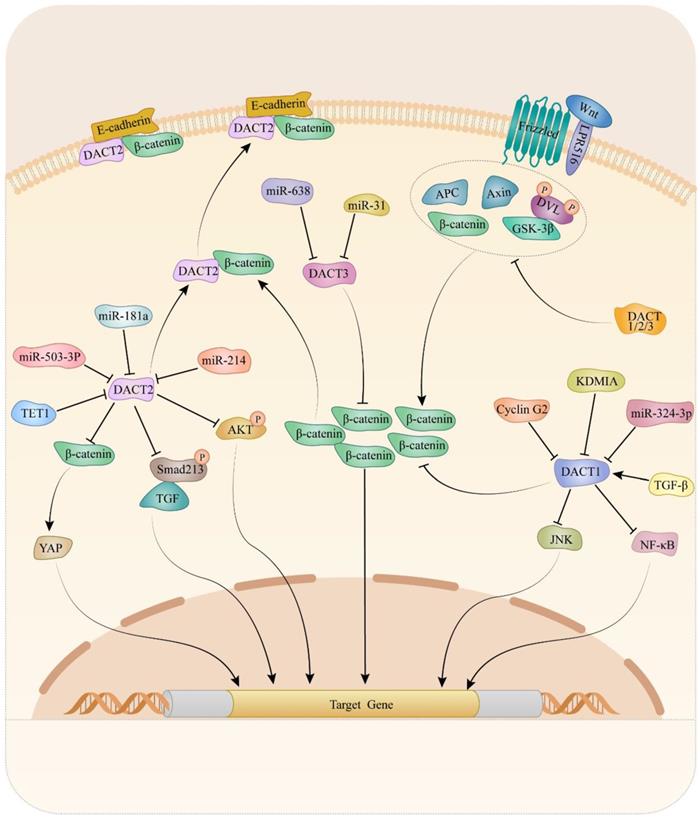
DACT family members act as the positive regulator to enhance autophagy
Autophagy is a regulated self-eating process that eliminates the cellular materials, such as aggregated cytoplasmic proteins or aged and damaged organelles through lysosomal degradation [104]. It has been shown autophagy is tightly involved in the Wnt/β-catenin pathway, and DACT1 and DACT3 are positive regulators to enhance autophagy. Ruo-nan Li et al. found DACT1 enhanced autophagy which enhances LC3 lipidation and p62/SQSTM1 degradation [105]. Mechanistic studies suggest that DACT1 enhanced the Atg14L-Beclin1-Vps34 complex formation to drive autophagy [106]. DACT1 acts as an adaptor to increase the ubiquitination of Dvl2 mediated by the von Hippel-Lindau tumor suppressor and mediates the Vps34-Beclin1 complex formation induced by protein aggregates under starvation in turn [107]. In addition, literature reports that miR-638 regulates autophagy of ESCC and BC cells. Yanli Ren et al. elucidated that miRNA-638 promotes autophagy and malignant phenotypes of cancer cells via directly suppressing DACT3 [104]. Unfortunately, there is no more research on the mechanism of DACT3 in autophagy. It needs to further investigate the molecular mechanism of DACT3 in cancer cell autophagy and its potential therapeutic implications.
Conclusion and future vision
In this review, we have sought here to synthesize the advances in the DACTs and their roles in various cancers and highlighted the function and mechanisms of DACTs in cancer cell proliferation, apoptosis, migration, and invasion, and autophagy, which may contribute to forming an elementary framework for understanding the complex biologic changes induced by the DACT family, and help us to develop more targeted implementation strategies. Until now, research on inhibiting or promoting cancer progression by regulating DACTs has remained in the experimental stage. We hope continuing elucidation of cancer pathogenesis, including precise molecular mechanism, to make most utility of the DACT family members.
In clinical practice, DACTs expression is associated with clinicopathological parameters and survival of tumor patients. On this basis, DACT could act as a diagnostic and prognostic biomarker for cancer patients. Nevertheless, confirming the prognostic and diagnostic value of DACTs in cohort studies with well-designed and larger-size needs to be conducted. Further exploring the role of DACTs in more cancer and whether DACTs can serve as a drug target may have important research value. We hope that our review will attract the attention of the scientific research community and clinical practitioners and encourage them to carry out treatments targeting DACTs as soon as possible.
Abbreviations
DACT: Dishevelled-associated antagonist of β-catenin; TSGs: tumor suppressor genes; Dvl: Dishevelled; NES: nuclear export signal; NLS: nuclear localization signal; EMT: epithelial-mesenchymal transition; NSCLC: non-small cell lung cancer; ESCC: esophageal squamous cell carcinoma; HCC: hepatocellular carcinoma; miRNAs: MicroRNAs; KDM1A: histone demethylase 1A; MLL3: mixed-lineage leukemia 3; CGIs: CpG islands; MSP: Methylation-specific PCR; BGS: bisulfite genomic sequencing; PARP: poly (ADP-ribose) polymerase; Bcl-2: B-cell lymphoma 2; MMP: Matrix metalloproteinases; PCP: planar cell polarity.
Acknowledgements
Funding
This work was supported by the National Natural Science Foundation of China (81960454, 81960344), the Guizhou Provincial Science and Technology Projects ([2020]1Z066), Guizhou Provincial People's Hospital Doctor Foundation ([2018]06 and [2018]03) and Guizhou Provincial People's Hospital National Science Foundation (GPPH-NSFC-2019-13, 2019-17 and GPPH-NSFC-D-2019-17).
Author contributions
Jiqin Zhang and Yu Zeng wrote the manuscript. Ying Tan, Jian Liu and Yan Zha contributed to the critical revision/review of the manuscript. Jianhe Yue, Weijia Liu, Lin Liu, Xin Lin and Guoqiang Han edited the manuscript. All authors approved the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Jakoube P, Cutano V, Gonzà Lez-Morena JM, Keckesova Z. Mitochondrial tumor suppressors-the energetic enemies of tumor progression. Cancer Res. 2021
2. Meng Y, Wang L, Chen D, Chang Y, Zhang M, Xu JJ. et al. LAPTM4B: an oncogene in various solid tumors and its functions. Oncogene. 2016;35:6359-65
3. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-74
4. Sherr CJ. Principles of tumor suppression. Cell. 2004;116:235-46
5. Vurusaner B, Poli G, Basaga H. Tumor suppressor genes and ROS: complex networks of interactions. Free Radic Biol Med. 2012;52:7-18
6. Ma B, Cao W, Li W, Gao C, Qi Z, Zhao Y. et al. Dapper1 promotes autophagy by enhancing the Beclin1-Vps34-Atg14L complex formation. Cell Res. 2014;24:912-24
7. Tao Y, Liu S, Briones V, Geiman TM, Muegge K. Treatment of breast cancer cells with DNA demethylating agents leads to a releasee of Pol II stalling at genes with DNA-hypermethylated regions upstream of TSS. Nucleic Acids Res. 2011;39:9508-20
8. Lodygin D, Hermeking H. The role of epigenetic inactivation of 14-3-3sigma in human cancer. Cell Res. 2005;15:237-46
9. Cheyette BN, Waxman JS, Miller JR, Takemaru K, Sheldahl LC, Khlebtsova N. et al. Dapper, a Dishevelled-associated antagonist of beta-catenin and JNK signaling, is required for notochord formation. Dev Cell. 2002;2:449-61
10. Gloy J, Hikasa H, Sokol SY. Frodo interacts with Dishevelled to transduce Wnt signals. Nat Cell Biol. 2002;4:351-7
11. Mandal A, Waxman J. Retinoic acid negatively regulates dact3b expression in the hindbrain of zebrafish embryos. Gene expression patterns: GEP. 2014;16:122-9
12. Waxman J. S, Hocking A. M, Stoick C. L.Moon R. T. Zebrafish Dapper1 and Dapper2 play distinct roles in Wnt-mediated developmental processes. Development. 2004;131:5909-21
13. Sensiate LA, Sobreira DR, Da Veiga FC, Peterlini DJ, Pedrosa AV, Rirsch T. et al. Dact gene expression profiles suggest a role for this gene family in integrating Wnt and TGF-β signaling pathways during chicken limb development. Developmental dynamics: an official publication of the American Association of Anatomists. 2014;243:428-39
14. Jiang X, Tan J, Li J, Kivimäe S, Yang X, Zhuang L. et al. DACT3 is an epigenetic regulator of Wnt/beta-catenin signaling in colorectal cancer and is a therapeutic target of histone modifications. Cancer Cell. 2008;13:529-41
15. Wang S, Dong Y, Zhang Y, Wang X, Xu L, Yang S. et al. DACT2 is a functional tumor suppressor through inhibiting Wnt/β-catenin pathway and associated with poor survival in colon cancer. Oncogene. 2015;34:2575-85
16. Tan Y, Li QM, Huang N, Cheng S, Zhao GJ, Chen H. et al. Upregulation of DACT2 suppresses proliferation and enhances apoptosis of glioma cell via inactivation of YAP signaling pathway. Cell Death Dis. 2017;8:e2981
17. Zhang L, Zhou H, Su Y, Sun Z, Zhang H, Zhang L. et al. Zebrafish Dpr2 inhibits mesoderm induction by promoting degradation of nodal receptors. Science (New York, NY). 2004;306:114-7
18. Zhang J. A new trick to tune down TGF-beta signal. Cell Res. 2004;14:439-40
19. Wang S, Kang W, Go MY, Tong JH, Li L, Zhang N. et al. Dapper homolog 1 is a novel tumor suppressor in gastric cancer through inhibiting the nuclear factor-κB signaling pathway. Molecular medicine (Cambridge, Mass). 2012;18:1402-11
20. Li X, Florez S, Wang J, Cao H, Amendt BA. Dact2 represses PITX2 transcriptional activation and cell proliferation through Wnt/beta-catenin signaling during odontogenesis. PLoS One. 2013;8:e54868
21. Fisher DA, Kivimäe S, Hoshino J, Suriben R, Martin PM, Baxter N. et al. Three Dact gene family members are expressed during embryonic development and in the adult brains of mice. Developmental dynamics: an official publication of the American Association of Anatomists. 2006;235:2620-30
22. Kivimäe S, Yang XY, Cheyette BN. All Dact (Dapper/Frodo) scaffold proteins dimerize and exhibit conserved interactions with Vangl, Dvl, and serine/threonine kinases. BMC Biochem. 2011;12:33
23. Katoh M, Katoh M. Identification and characterization of rat Dact1 and Dact2 genes in silico. Int J Mol Med. 2005;15:1045-9
24. Katoh M, Katoh M. Identification and characterization of human DAPPER1 and DAPPER2 genes in silico. Int J Oncol. 2003;22:907-13
25. Yin X, Xiang T, Li L, Su X, Shu X, Luo X. et al. DACT1, an antagonist to Wnt/β-catenin signaling, suppresses tumor cell growth and is frequently silenced in breast cancer. Breast cancer research: BCR. 2013;15:R23
26. Gao X, Wen J, Zhang L, Li X, Ning Y, Meng A. et al. Dapper1 is a nucleocytoplasmic shuttling protein that negatively modulates Wnt signaling in the nucleus. The Journal of biological chemistry. 2008;283:35679-88
27. Hikasa H, Sokol SY. The involvement of Frodo in TCF-dependent signaling and neural tissue development. Development (Cambridge, England). 2004;131:4725-34
28. Kettunen P, Kivimäe S, Keshari P, Klein OD, Cheyette BN, Luukko K. Dact1-3 mRNAs exhibit distinct expression domains during tooth development. Gene expression patterns: GEP. 2010;10:140-3
29. Lee WC, Hough MT, Liu W, Ekiert R, Lindström NO, Hohenstein P. et al. Dact2 is expressed in the developing ureteric bud/collecting duct system of the kidney and controls morphogenetic behavior of collecting duct cells. American journal of physiology Renal physiology. 2010;299:F740-51
30. Brott BK, Sokol SY. A vertebrate homolog of the cell cycle regulator Dbf4 is an inhibitor of Wnt signaling required for heart development. Dev Cell. 2005;8:703-15
31. Jardim DP, Poço PCE, Campos AH Dact1, a Wnt-Pathway Inhibitor, Mediates Human Mesangial Cell TGF-β1-Induced Apoptosis. J Cell Physiol. 2017; 232: 2104-11.
32. Jiao S, Liu Y, Yao Y, Teng J. miR-124 promotes proliferation and neural differentiation of neural stem cells through targeting DACT1 and activating Wnt/β-catenin pathways. Mol Cell Biochem. 2018;449:305-14
33. Hou J, Yue Y, Hu B, Xu G, Su R, Lv L. et al. DACT1 Involvement in the Cytoskeletal Arrangement of Cardiomyocytes in Atrial Fibrillation by Regulating Cx43. Brazilian journal of cardiovascular surgery. 2019;34:711-22
34. Xue H, Xiao Z, Zhang J, Wen J, Wang Y, Chang Z. et al. Disruption of the Dapper3 gene aggravates ureteral obstruction-mediated renal fibrosis by amplifying Wnt/β-catenin signaling. The Journal of biological chemistry. 2013;288:15006-14
35. Guo L, Wang X, Yang Y, Xu H, Zhang Z, Yin L. et al. Methylation of DACT2 contributes to the progression of breast cancer through activating WNT signaling pathway. Oncol Lett. 2018;15:3287-94
36. Cheng H, Deng Z, Wang Z, Zhang W, Su J. The role of aberrant promoter hypermethylation of DACT1 in bladder urothelial carcinoma. J Biomed Res. 2012;26:319-24
37. Shi X, Huo J, Gao X, Cai H, Zhu W. A newly identified lncRNA H1FX-AS1 targets DACT1 to inhibit cervical cancer via sponging miR-324-3p. Cancer Cell Int. 2020;20:358
38. Zeng L, Chen C, Yao C. Histone Deacetylation Regulated by KDM1A to Suppress DACT1 in Proliferation and Migration of Cervical Cancer. Anal Cell Pathol (Amst). 2021;2021:5555452
39. Li RN, Liu B, Li XM, Hou LS, Mu XL, Wang H. et al. DACT1 Overexpression in type I ovarian cancer inhibits malignant expansion and cis-platinum resistance by modulating canonical Wnt signalling and autophagy. Sci Rep. 2017;7:9285
40. Yuan G, Wang C, Ma C, Chen N, Tian Q, Zhang T. et al. Oncogenic function of DACT1 in colon cancer through the regulation of β-catenin. PLoS One. 2012;7:e34004
41. Hou J, Li EM, Shen JH, Qing-Zhao, Wu ZY, Xu XE. et al. Cytoplasmic HDPR1 is involved in regional lymph node metastasis and tumor development via beta-catenin accumulation in esophageal squamous cell carcinoma. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 2011;59:711-8
42. Yang ZQ, Zhao Y, Liu Y, Zhang JY, Zhang S, Jiang GY. et al. Downregulation of HDPR1 is associated with poor prognosis and affects expression levels of p120-catenin and beta-catenin in nonsmall cell lung cancer. Mol Carcinog. 2010;49:508-19
43. Marusa Borgonio-Cuadra V, Miranda-Duarte A, Rojas-Toledo X, Garcia-Hernandez N, Alfredo Sierra-Ramirez J, Cardenas-Garcia M. et al. Association between promoter hypermethylation of the DACT2 gene and tumor stages in breast cancer. Journal of BUON: official journal of the Balkan Union of Oncology. 2018;23:361-5
44. Zhang N, Li Y, Xie M, Song Y, Liu J, Lei T. et al. DACT2 modulated by TFAP2A-mediated allelic transcription promotes EGFR-TKIs efficiency in advanced lung adenocarcinoma. Biochem Pharmacol. 2020;172:113772
45. Jia Y, Yang Y, Brock MV, Zhan Q, Herman JG, Guo M. Epigenetic regulation of DACT2, a key component of the Wnt signalling pathway in human lung cancer. The Journal of pathology. 2013;230:194-204
46. Gao S, Yang Z, Zheng ZY, Yao J, Zhang F, Wu LM. et al. Reduced expression of DACT2 promotes hepatocellular carcinoma progression: involvement of methylation-mediated gene silencing. World J Surg Oncol. 2013;11:57
47. Zhang X, Yang Y, Liu X, Herman JG, Brock MV, Licchesi JD. et al. Epigenetic regulation of the Wnt signaling inhibitor DACT2 in human hepatocellular carcinoma. Epigenetics. 2013;8:373-82
48. Jalilvand A, Soltanpour M. S. Investigating the methylation status of DACT2 gene and its association with MTHFR C677T polymorphism in patients with colorectal cancer. Mol Biol Res Commun. 2019;8:53-8
49. Hou J, Liao LD, Xie YM, Zeng FM, Ji X, Chen B. et al. DACT2 is a candidate tumor suppressor and prognostic marker in esophageal squamous cell carcinoma. Cancer Prev Res (Phila). 2013;6:791-800
50. Guo YL, Shan BE, Guo W, Dong ZM, Zhou Z, Shen SP. et al. Aberrant methylation of DACT1 and DACT2 are associated with tumor progression and poor prognosis in esophageal squamous cell carcinoma. J Biomed Sci. 2017;24:6
51. Zhang M, Linghu E, Zhan Q, He T, Cao B, Brock MV. et al. Methylation of DACT2 accelerates esophageal cancer development by activating Wnt signaling. Oncotarget. 2016;7:17957-69
52. Zhao L, Fan W, Fan Y, Gao S. MicroRNA-214 promotes the proliferation, migration and invasion of gastric cancer MKN28 cells by suppressing the expression of Dact2. Exp Ther Med. 2018;16:4909-17
53. Yu Y, Yan W, Liu X, Jia Y, Cao B, Yu Y. et al. DACT2 is frequently methylated in human gastric cancer and methylation of DACT2 activated Wnt signaling. Am J Cancer Res. 2014;4:710-24
54. Zhao Z, Herman J. G, Brock M. V, Sheng J, Zhang M, Liu B, et al. Methylation of DACT2 promotes papillary thyroid cancer metastasis by activating Wnt signaling. PLoS One. 2014;9:e112336
55. Li S, Yin L, Huang K, Zhao Y, Zhang H, Cai C. et al. Downregulation of DACT-2 by Promoter Methylation and its Clinicopathological Significance in Prostate Cancer. J Cancer. 2019;10:1755-63
56. Zhao H, Yang L, Han Y, Li H, Ling Z, Wang Y. et al. Dact3 inhibits the malignant phenotype of non-small cell lung cancer through downregulation of c-Myb. Int J Clin Exp Pathol. 2017;10:11580-7
57. Zhou XG, Huang XL, Liang SY, Tang SM, Wu SK, Huang TT. et al. Identifying miRNA and gene modules of colon cancer associated with pathological stage by weighted gene co-expression network analysis. Onco Targets Ther. 2018;11:2815-30
58. Luo C, Huang B, Wu Y, Xu Y, Ou W, Chen J. et al. Identification of Lymph Node Metastasis-Related Key Genes and Prognostic Risk Model in Bladder Cancer by Co-Expression Analysis. Frontiers in molecular biosciences. 2021;8:633299
59. Agbu P, Carthew RW. MicroRNA-mediated regulation of glucose and lipid metabolism. Nature reviews Molecular cell biology. 2021;22:425-38
60. Maul J, Alterauge D, Baumjohann D. MicroRNA-mediated regulation of T follicular helper and T follicular regulatory cell identity. Immunol Rev. 2019;288:97-111
61. Kiltschewskij D, Cairns MJ. Temporospatial guidance of activity-dependent gene expression by microRNA: mechanisms and functional implications for neural plasticity. Nucleic Acids Res. 2019;47:533-45
62. Gebert LFR, MacRae IJ. Regulation of microRNA function in animals. Nature reviews Molecular cell biology. 2019;20:21-37
63. Jonas S, Izaurralde E. Towards a molecular understanding of microRNA-mediated gene silencing. Nature reviews Genetics. 2015;16:421-33
64. Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nature reviews Drug discovery. 2017;16:203-22
65. Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nature reviews Cancer. 2015;15:321-33
66. Tuo H, Wang Y, Wang L, Yao B, Li Q, Wang C. et al. MiR-324-3p promotes tumor growth through targeting DACT1 and activation of Wnt/β-catenin pathway in hepatocellular carcinoma. Oncotarget. 2017;8:65687-98
67. Zhang H, Fu L. The role of ALDH2 in tumorigenesis and tumor progression: Targeting ALDH2 as a potential cancer treatment. Acta pharmaceutica Sinica B. 2021;11:1400-11
68. Gan TQ, Tang RX, He RQ, Dang YW, Xie Y, Chen G. Upregulated MiR-1269 in hepatocellular carcinoma and its clinical significance. Int J Clin Exp Med. 2015;8:714-21
69. Huang S, Fan P, Zhang C, Xie J, Gu X, Lei S. et al. Exosomal microRNA-503-3p derived from macrophages represses glycolysis and promotes mitochondrial oxidative phosphorylation in breast cancer cells by elevating DACT2. Cell death discovery. 2021;7:119
70. Wang Y, Cen A, Yang Y, Ye H, Li J, Liu S. et al. miR-181a, delivered by hypoxic PTC-secreted exosomes, inhibits DACT2 by downregulating MLL3, leading to YAP-VEGF-mediated angiogenesis. Molecular therapy Nucleic acids. 2021;24:610-21
71. Ren Y, Chen Y, Liang X, Lu Y, Pan W, Yang M. MiRNA-638 promotes autophagy and malignant phenotypes of cancer cells via directly suppressing DACT3. Cancer Lett. 2017;390:126-36
72. Xi S, Yang M, Tao Y, Xu H, Shan J, Inchauste S. et al. Cigarette smoke induces C/EBP-β-mediated activation of miR-31 in normal human respiratory epithelia and lung cancer cells. PLoS One. 2010;5:e13764
73. Esposito M, Fang C, Cook KC, Park N, Wei Y, Spadazzi C. et al. TGF-β-induced DACT1 biomolecular condensates repress Wnt signalling to promote bone metastasis. Nat Cell Biol. 2021;23:257-67
74. Pfister SX, Ashworth A. Marked for death: targeting epigenetic changes in cancer. Nature reviews Drug discovery. 2017;16:241-63
75. Lu Y, Chan YT, Tan HY, Li S, Wang N, Feng Y. Epigenetic regulation in human cancer: the potential role of epi-drug in cancer therapy. Mol Cancer. 2020;19:79
76. Agrawal K, Das V, Vyas P, Hajdúch M. Nucleosidic DNA demethylating epigenetic drugs - A comprehensive review from discovery to clinic. Pharmacol Ther. 2018;188:45-79
77. Lister R, Pelizzola M, Dowen RH, Hawkins RD, Hon G, Tonti-Filippini J. et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462:315-22
78. Bird AP. CpG-rich islands and the function of DNA methylation. Nature. 1986;321:209-13
79. Yang JH, Lin LK, Zhang S. Effects of DACT1 methylation status on invasion and metastasis of nasopharyngeal carcinoma. Biol Res. 2019;52:31
80. Yau TO, Chan CY, Chan KL, Lee MF, Wong CM, Fan ST. et al. HDPR1, a novel inhibitor of the WNT/beta-catenin signaling, is frequently downregulated in hepatocellular carcinoma: involvement of methylation-mediated gene silencing. Oncogene. 2005;24:1607-14
81. Deng J, Liang H, Zhang R, Ying G, Xie X, Yu J. et al. Methylated CpG site count of dapper homolog 1 (DACT1) promoter prediction the poor survival of gastric cancer. Am J Cancer Res. 2014;4:518-27
82. Li J, Zhang M, He T, Li H, Cao T, Zheng L. et al. Methylation of DACT2 promotes breast cancer development by activating Wnt signaling. Sci Rep. 2017;7:3325
83. Xiang T, Fan Y, Li C, Li L, Ying Y, Mu J. et al. DACT2 silencing by promoter CpG methylation disrupts its regulation of epithelial-to-mesenchymal transition and cytoskeleton reorganization in breast cancer cells. Oncotarget. 2016;7:70924-35
84. Lu L, Wang Y, Ou R, Feng Q, Ji L, Zheng H. et al. DACT2 Epigenetic Stimulator Exerts Dual Efficacy for Colorectal Cancer Prevention and Treatment. Pharmacol Res. 2018;129:318-28
85. Zhang Y, Fan J, Fan Y, Li L, He X, Xiang Q. et al. The new 6q27 tumor suppressor DACT2, frequently silenced by CpG methylation, sensitizes nasopharyngeal cancer cells to paclitaxel and 5-FU toxicity via β-catenin/Cdc25c signaling and G2/M arrest. Clin Epigenetics. 2018;10:26
86. Schussel J. L, Kalinke L. P, Sassi L. M, de Oliveira B. V, Pedruzzi P. A, Olandoski M, et al. Expression and epigenetic regulation of DACT1 and DACT2 in oral squamous cell carcinoma. Cancer Biomark. 2015;15:11-7
87. Marcu LG. Imaging Biomarkers of Tumour Proliferation and Invasion for Personalised Lung Cancer Therapy. Journal of personalized medicine. 2020 10
88. Gao J, Zhao C, Liu Q, Hou X, Li S, Xing X. et al. Cyclin G2 suppresses Wnt/β-catenin signaling and inhibits gastric cancer cell growth and migration through Dapper1. Journal of experimental & clinical cancer research: CR. 2018;37:317
89. Zhu K, Jiang B, Yang Y, Hu R, Liu Z. DACT1 overexpression inhibits proliferation, enhances apoptosis, and increases daunorubicin chemosensitivity in KG-1α cells. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2017;39:1010428317711089
90. Qu Y, Dang S, Hou P. Gene methylation in gastric cancer. Clin Chim Acta. 2013;424:53-65
91. Fan J, Zhang Y, Mu J, He X, Shao B, Zhou D. et al. TET1 exerts its anti-tumor functions via demethylating DACT2 and SFRP2 to antagonize Wnt/β-catenin signaling pathway in nasopharyngeal carcinoma cells. Clin Epigenetics. 2018;10:103
92. Noh S, Choi E, Hwang CH, Jung JH, Kim SH, Kim B. Dietary Compounds for Targeting Prostate Cancer. Nutrients. 2019 11
93. Grilo AL, Mantalaris A. Apoptosis: A mammalian cell bioprocessing perspective. Biotechnol Adv. 2019;37:459-75
94. Stuelten CH, Parent CA, Montell DJ. Cell motility in cancer invasion and metastasis: insights from simple model organisms. Nature reviews Cancer. 2018;18:296-312
95. Vilchez Mercedes SA, Bocci F, Levine H, Onuchic JN, Jolly MK, Wong PK Decoding leader cells in collective cancer invasion. Nature reviews Cancer. 2021.
96. Chan CY, Lin TY, Sheu JJ, Wu WC, Huang CY. Matrix metalloproteinase-13 is a target gene of high-mobility group box-containing protein 1 in modulating oral cancer cell invasion. J Cell Physiol. 2019;234:4375-84
97. Nagumo Y, Kandori S, Tanuma K, Nitta S, Chihara I, Shiga M. et al. PLD1 promotes tumor invasion by regulation of MMP-13 expression via NF-κB signaling in bladder cancer. Cancer Lett. 2021;511:15-25
98. Liu Y, Zhang J, Yu W, Zhang X, Wang G, Zhao Z. Dapper homolog 1 alpha suppresses metastasis ability of gastric cancer through inhibiting planar cell polarity pathway. Oncotarget. 2016;7:81423-34
99. Liang H, Zhao X, Wang C, Sun J, Chen Y, Wang G. et al. Systematic analyses reveal long non-coding RNA (PTAF)-mediated promotion of EMT and invasion-metastasis in serous ovarian cancer. Mol Cancer. 2018;17:96
100. Li Y, Tian M, Liu W, Wang D, Zhou Z, Pei Q. et al. Follistatin-Like 3 Enhances Invasion and Metastasis via β-Catenin-Mediated EMT and Aerobic Glycolysis in Colorectal Cancer. Frontiers in cell and developmental biology. 2021;9:660159
101. VanderVorst K, Hatakeyama J, Berg A, Lee HCarraway KL. Cellular and molecular mechanisms underlying planar cell polarity pathway contributions to cancer malignancy. Semin Cell Dev Biol. 2018;81:78-87
102. Daulat AM, Borg JP. Wnt/Planar Cell Polarity Signaling: New Opportunities for Cancer Treatment. Trends in cancer. 2017;3:113-25
103. Daulat AM, Bertucci F, Audebert S, Sergé A, Finetti P, Josselin E. et al. PRICKLE1 Contributes to Cancer Cell Dissemination through Its Interaction with mTORC2. Dev Cell. 2016;37:311-25
104. Ren Y, Chen Y, Liang X, Lu Y, Pan W.Yang M. MiRNA-638 promotes autophagy and malignant phenotypes of cancer cells via directly suppressing DACT3. Cancer Lett. 2017;390:126-36
105. Li R. N, Liu B, Li X. M, Hou L. S, Mu X. L, Wang H, et al. DACT1 Overexpression in type I ovarian cancer inhibits malignant expansion and cis-platinum resistance by modulating canonical Wnt signalling and autophagy. Sci Rep. 2017;7:9285
106. Ma B, Cao W, Li W, Gao C, Qi Z, Zhao Y. et al. Dapper1 promotes autophagy by enhancing the Beclin1-Vps34-Atg14L complex formation. Cell Res. 2014;24:912-24
107. Ma B, Liu B, Cao W, Gao C, Qi Z, Ning Y. et al. The Wnt Signaling Antagonist Dapper1 Accelerates Dishevelled2 Degradation via Promoting Its Ubiquitination and Aggregate-induced Autophagy. J Biol Chem. 2015;290:12346-54
Author contact
![]() Corresponding authors: Ying Tan, E-mail: tanyingcom; Jian Liu, E-mail: liujiancom; Yan Zha, E-mail: chayancom.
Corresponding authors: Ying Tan, E-mail: tanyingcom; Jian Liu, E-mail: liujiancom; Yan Zha, E-mail: chayancom.

 Global reach, higher impact
Global reach, higher impact