10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(12):4629-4641. doi:10.7150/ijbs.73583 This issue Cite
Review
Humoral and Cellular Immune Responses of COVID-19 vaccines against SARS-Cov-2 Omicron variant: a systemic review
State Key Laboratory of Quality Research in Chinese Medicine, Institute of Chinese Medical Sciences, University of Macau, Macau SAR 999078, China
*These authors have contributed equally to this work.
Received 2022-4-3; Accepted 2022-5-21; Published 2022-7-11
Abstract
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has undergone multiple mutations since its emergence, and its latest variant, Omicron (B.1.1.529), is the most contagious variant of concern (VOC) which poses a major and imminent threat to public health. Since firstly reported by World Health Organization (WHO) in November 2021, Omicron variant has been spreading rapidly and has become the dominant variant in many countries worldwide. Omicron is the most mutated variant so far, containing 60 mutations in its genome, including 37 mutations in the S-protein. Since all current COVID-19 vaccines in use were developed based on ancestral SARS-CoV-2 strains, whether they are protective against Omicron is a critical question which has been the center of study currently. In this article, we systemically reviewed the studies regarding the effectiveness of 2- or 3-dose vaccines delivered in either homologous or heterologous manner. The humoral and cellular immune responses elicited by various vaccine regimens to protect against Omicron variant are discussed. Current understanding of the molecular basis underlying immune escape of Omicron was also analyzed. These studies indicate that two doses of vaccination are insufficient to elicit neutralizing antibody responses against Omicron variant. Nevertheless, Omicron-specific humoral immune responses can be enhanced by booster dose of almost all type vaccines in certain degree, and heterologous vaccination strategy may represent a better choice than homogenous regimens. Intriguingly, results of studies indicate that all current vaccines are still able to elicit robust T cell response against Omicron. Future focus should be the development of Omicron variant vaccine, which may induce potent humoral as well as cellular immune responses simultaneously against all known variants of the SARS-CoV-2 virus.
Keywords: SARS-COV-2, Omicron variant, vaccine, booster dose, humoral immune response, cellular immune response
1. Introduction
During the COVID-19 pandemic, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has evolved more than 1,500 different Pango lineages in more than 270 million cases reported [1]. The new coronavirus already had four major variant of concerns (VOCs), namely Alpha, Beta, Gamma, and Delta. On 24th November 2021, a new variant (B.1.1.529), named Omicron, was reported to WHO by South Africa. On 26th November, the WHO defined it as the fifth VOC. Omicron variant infection was found in 128 countries and territories by 4th January 2022 [2]. Studies have shown that Omicron has an even farther antigenic distance from the ancestor virus, as compared to that of Beta and Delta variants. As a result, the protective efficacy of previous vaccines against Omicron was markedly reduced [3].
As the most severely mutated strain, there are 18,261 mutations in various Omicron isolates, in which 97% of them are in coding regions [4]. The entry of the SARS-CoV-2 virus into the host cell is through the Spike-protein(S-protein) which acts on the angiotensin converting enzyme II (ACE2) receptor of the host cells [5]. There are 30 mutations in the S-protein of Omicron, most of which are in the receptor binding domain (RBD) region [6]. The RBD of Wuhan-Hu-1 has 1,273 amino acids, in contrast, the RBD of Delta and Omicron variants has 1,271 and 1,270 amino acids, respectively [7]. Several major previously appeared mutations and some novel mutations were found in Omicron variants, which increase the risk of infection and possibly resistance to existing vaccines [8].
Vaccination programs have been initiated in many countries, and in some countries, there is wider coverage. Vaccination elicits neutralizing antibodies against key viral proteins, such as S-protein, has been effectively slowing down the spread of the virus and reducing the rate of severe morbidity and mortality [9]. In spite of this, the reduced sensitivity of neutralizing antibodies to key viral proteins of the variants, including SARS-CoV-2 Omicron, resulted in an increase in the risk of immune escape and a reduction of the effectiveness of the vaccine [8, 10]. Currently, understanding the effectiveness of various vaccine regimens against Omicron variant has become the focus of research. Although remained incompletely understood, the humoral and cellular immune responses elicited by current vaccines to Omicron have been intensively studied. In this article, we systemically reviewed the latest progress in this fast-evolving field.
2. Major SARS-CoV-2 VOCs and the protective efficacy of vaccines
Alpha (B.1.1.7). At the end of December 2020 in the United Kingdom, a new SARS-CoV-2 virus was discovered. The Alpha strain, also known as the B.1.1.7 strain, contains mutations such as 69/70/144del, N501Y, A570D, P681H, T716I, S982A, and D1118H in the spike protein. Among them, the N501Y mutation can lead to enhanced viral adhesion, making it easier to enter host cells [11].
Beta (B.1.315). Beta strain is the main strain for secondary outbreaks of COVID-19. It was found in South Africa in October 2020 [12]. The spike protein of Beta strain contains 9 mutation sites, which are L18F, D80A, D215G, R246I, K417N, E484K, N501Y, D614G, and A701V [12]. Three mutations, e.g., K417N, E484K, and N501Y, are in the RBD which enhanced the binding affinity of B.1.315 to ACE2 receptor [13].
Gamma (P.1). Gamma strain was firstly spotted in Brazil in January 2021[14]. The spike protein of Gamma strain contains 11 mutations, including L18F, T20N, P26S, D138Y, R190S, H655Y, T1027I, D614G, K417T, E484K, and N501Y [15]. Among them, three mutations (L18F, K417T and E484K) are located in the RBD region which may improve the binding affinity of P.1 strain to ACE2 [15].
Delta (B.1.617.2). Delta strain is the fourth VOC which was initially found in India in December 2020. The spike protein of Delta strain contains 10 mutations (T19R, G142D, 156/157del, R158G, L452R, T478K, D614G, P681R and D950N) [16].
Omicron (B.1.1.529). On 26th November 2021, the fifth VOC was identified and named as Omicron. The variant has rapidly spread to many regions throughout Asia, Africa, Europe, and North America [17]. This strain is currently the most severely variant with many mutation sites in its spike protein species, including 69-70/142-144/211del A67V, T95I, Y145D, L212I, ins214EPE, T547K, D614G, H655Y, N679K, P681H, N764K, D796Y, N856K, Q954H, N969K, and L981F [18]. Omicron has further evolved into three subvariants, namely Omicron BA.1 (B.1.1.529.1), BA.2 (B.1.1.529.2), and BA.3 (B.1.1.529.3). Among them, BA.2 shares 32 same mutations with BA.1, while possessing distinct 28 mutations. BA.2 was found to be able to re-infect patients who were already infected with BA.1[19], suggesting that BA.2 may become the protagonist of the next wave of epidemic after the Omicron BA.1 pandemic [19].
Protective Effect of Vaccines against various VOCs of SARS-COV-2
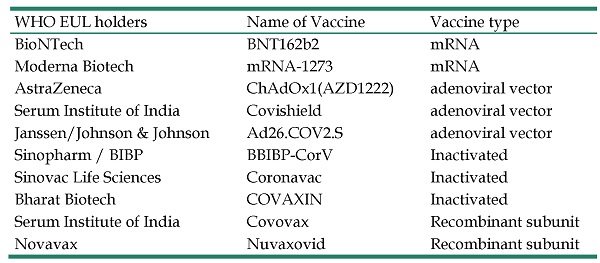
To date, four main categories of SARS-CoV-2 vaccines, e.g. mRNA, adenoviral vector, inactivated, and recombinant subunit vaccines, have been developed and approved for use. As of 15th March, 2022, a total of 216 vaccines are in development and 92 of them have entered the stage of human trials [20]. Due to the severity of the epidemic beyond the ability to control, WHO has included 10 vaccines on the emergency use listing (EUL), as summarized in Table 1. Studies showed that the most of vaccines are effective in protection against Alpha variant [21-24]. However, their efficacy against Beta variant was markedly decreased [22, 23]. Effect of vaccines against Gamma variant was further reduced [25], while the immune escape rate of Delta variant remains the same as that of Gamma variant [26].
SARS-CoV-2 Omicron is highly transmissible even among fully vaccinated individuals [27, 28], suggesting that this variant has the capacity to evade protective immune responses elicited by current vaccines. Compared to the original wild-type (WT) strain of SARS-CoV-2, Omicron variant has 60 amino acid mutations, 37 of which are in the S-protein, and these mutations in the S-protein RBD which binds to the ACE2 receptor will change binding epitopes to many current antibodies [29]. G446S is a key antibody escape site. By altering the local conformation of the binding interface, G664S provides greater resistance to a major class of antibodies binding to the right shoulder of the RBD [30]. Right shoulder represents a region of the RBD surface named by a conventional nomenclature compared with human torso [31]. The structural plasticity at the RBD-ACE2 interface promotes the accumulation of a large number of RBD mutations of Omicron variant which lead to the reduction of the efficacy of neutralizing antibody [32, 33].
WHO emergency use listing
| WHO EUL holders | Name of Vaccine | Vaccine type |
|---|---|---|
| BioNTech | BNT162b2 | mRNA |
| Moderna Biotech | mRNA-1273 | mRNA |
| AstraZeneca | ChAdOx1(AZD1222) | adenoviral vector |
| Serum Institute of India | Covishield | adenoviral vector |
| Janssen/Johnson & Johnson | Ad26.COV2.S | adenoviral vector |
| Sinopharm / BIBP | BBIBP-CorV | Inactivated |
| Sinovac Life Sciences | Coronavac | Inactivated |
| Bharat Biotech | COVAXIN | Inactivated |
| Serum Institute of India | Covovax | Recombinant subunit |
| Novavax | Nuvaxovid | Recombinant subunit |
The vaccine approved by WHO listed in EUL to expediting the availability of these products to people affected by a public health emergency.
3. Vaccine-induced humoral immune responses against Omicron variant
S-protein, a homotrimeric protein, is located on the surface of the virus membrane [34]. Each of its monomers is composed of two domains, S1 and S2. S1 is responsible for receptor binding and S2 is responsible for membrane binding. S1 consists of an RBD and an N-terminal domain (NTD), RBD directly binds to ACE2 and NTD is involved in the regulation of S-protein activation and influences the RBD binding process [34]. The traditional neutralizing antibodies are to block the binding site between RBD and ACE2, many vaccines are developed for this, such as BNT162b2 and ZF2001 [35]. However, recent studies found that anti-NTD also can inhibit the RBD binding processes [34]. Among 86 potent neutralizing antibodies identified in peripheral blood mononuclear cells from vaccinees, the diversity of anti-RBD antibodies is much higher than anti-NTD antibodies [36]. RBD and NTD have accumulated a high number of mutations in SARS-CoV-2 variants. These mutations have direct implications on virus infection rates through the higher affinity of RBD for the ACE2 receptor and escape of neutralizing antibodies [37]. Therefore, variants of SARS-CoV-2 can escape neutralization by maintaining these mutations, which results in the failure of vaccination. It is worth mentioning that SARS-CoV-2 RBD-specific memory B cell clones accumulated over time, and the pool of memory B cells may provide long-term protection [38]. Clonal relationships indicated that at least some of these somatic mutations lead to part of cross-reactivity of memory B cells [39]. Memory B cells play the central role in humoral immune response; they are generated in prolonged germinal center reactions [40]. These memory B cells actually increased between 1 month and 8 months after infection, accompanied with affinity maturation of antibodies. The kinetics of memory B cells in humans after an initial acute viral infection is poorly understood, and further study is needed [41]. A study on vaccinated individuals revealed that mRNA vaccines induced variant-specific memory B cells maintained for at least 6 months and such B cells could cross-bind with Alpha, Beta and Delta variants [39]. To date, the response of memory B cells to Omicron remains unclear.
In the following sections, we will discuss the humoral immune responses elicited by two or three doses of different types of vaccines against SARS-CoV-2 Omicron variants, followed by the analysis of evidence showing the enhanced efficacy of booster dose. The humoral immune responses elicited by heterologous versus homologous vaccination strategies are also compared.
Diminished humoral immune responses against Omicron by 2-dose of vaccines
mRNA Vaccines (BNT162b2 and mRNA-1273). There is compelling evidence that the efficacy of two doses of mRNA vaccines in neutralization of Omicron variant was markedly reduced. In the individuals vaccinated with two-dose of BNT162b or mRNA-1273, the titers of neutralizing antibodies against Omicron were 23~122 folds lower than antibodies against WT, ancestral strain or mutant strains (such as USA-WA1/2020, Victoria and D614G strains) [42-52]. However, the binding capacity of antibodies elicited by 2-does mRNA vaccines was preserved to some degree, as the difference in binding affinity of antibodies to RBD of Omicron and that of WT strain was minor, with 2.9-fold reduction in individuals vaccinated with 2-dose mRNA-1273 and 1.5-fold reduction after boosted with BNT162b2 as compared to WT [44]. Due to the differences in the design of study, experimental condition and sample sources, the results between the above studies may not be comparable.
Adenoviral vector vaccines (ChAdOx1 or AZD1222, Covishield and Ad26.COV2.S). It was shown that the effectiveness of two doses of ChAdOx1was reduced over time, with a limited protection against symptomatic disease caused by the Omicron variant for 20 to 24 weeks after the second vaccination [53]. A study shows that, in 21 out of 22 individuals vaccinated with two doses of ChAdOx1, the serum neutralizing antibody titers against Omicron dropped below detectable thresholds [50]. Similarly, another study found that none of the individuals received 2-dose of ChAdOx1 had cross-neutralization of the Omicron variant [54]. As for Covishield, a study found that the neutralizing antibody titers to Omicron variant were reduced by 23.15-fold in individuals vaccinated with two doses [55]. As for Ad26.COV2.S, Omicron neutralizing antibody titers were reduced by 17-fold to WT, with low level GMNT in individuals either vaccinated recently (42 IU/mL) or vaccinated six months later (33 IU/mL) was found in individuals received 1 dose vaccine [42]. Therefore, the humoral immune responses against Omicron elicited by adenoviral vector vaccines appear to be minimal.
Inactivated vaccines (BBIBP-CorV, CoronaVac and COVAXIN). It was shown that none of the individuals fully vaccinated with CoronaVac had detectable neutralizing antibodies against Omicron variant [56]. Another study found about 6.4-fold decrease of GMT against Omicron variant from recipients who received 2-dose of CoronaVac as compared to GMT against WT strain. Again, none of them was above 50% threshold of protection [57]. Plasma collected on day 14 after a second dose of CoronaVac showed a 12.5-fold reduction in average in neutralizing Omicron variants as compared to WT strain [58]. Another study showed that after 30 days of second dose, CoronaVac immunization generated detectable neutralizing antibodies against Omicron in only 17% of individuals, which dropped to 10% after 60 days [59]. As for BBIBP-CorV, it was shown that GMT against Omicron was still above the low limit after 4~8 months [60]. As compared with WT strain, a 1.52-fold reduction in levels of neutralizing antibody against the Omicron variant was observed in recipients who received 2-dose of BBIBP-CorV [61]. As for COVAXIN, one study found that the levels of neutralizing antibody against Omicron in individuals vaccinated with 2 doses were reduced 8.67~12.49-fold, as compared to B.1 strain. However, even 6 months after initial vaccination, the anti-S1-RBD and nucleocapsid IgG neutralizing antibodies could still be detected [62, 63].
Taken together, the above studies indicate that the titers of neutralizing antibodies against Omicron elicited by 2-dose of all current vaccines are markedly reduced, as compared to those against ancestral strain of SARS-CoV-2 (summarized in Table 2). These data indicate that 2-dose of vaccination are insufficient to generate satisfactory levels of protection against infection with Omicron and mild disease. In fact, such reduction appears to be more profound in individuals who received adenovirus vaccine, as compared to those who received mRNA vaccines [64]. Neutralizing antibodies against Omicron elicited by standard two inoculations of inactivated vaccines is often low, indicative of the limited capacity of this type of vaccine in the induction of humoral immune responses against Omicron [57, 61]. Nevertheless, although the full vaccination frequently fails to show a strong neutralizing antibody activity against Omicron variant, they still have clear public health benefit such as reduction of infectious viral load and transmission risk [65].
Humoral immune response against Omicron was enhanced by booster dose of vaccines
Since humoral immune responses against Omicron variant elicited by all current vaccines were markedly reduced, many countries and regions are calling for an additional booster vaccination six months after the second dose. It was shown that the booster dose resulted in a significant improvement in protection against mild diseases [53]. A real-world study that analyzed about 62,000 vaccinated individuals in UK revealed that participants who had received 2 or 3 doses of vaccines had a lower risk of hospitalization during Omicron infection. And individuals who had received 3 doses of the vaccine have a marked reduction on the duration of Omicron symptoms (2-dose: 8.3 days, 3-dose: 4.4 days ) [66]. The importance of booster dose is evident, since it can elicit higher level of neutralizing antibody against the Omicron [47].
It was shown that over 90% of individuals retained neutralizing activity against the Omicron after booster dose of mRNA vaccines [52]. As compared to 2-dose of mRNA vaccines, booster dose could enhance the titers of neutralizing antibodies against Omicron variant about 20~30 times [51, 67]. For example, individuals vaccinated with 3-dose of BNT162b2 demonstrated a minimal reduction of neutralizing antibody against Omicron (4-fold decrease) [48]. Another study also showed that the booster dose of BNT162b2 resulted in a potent neutralizing activity against Omicron (ED50= 722) in individuals with a low neutralizing activity after two doses of BNT162b2 (ED50 < 30) [68]. Similarly, after booster vaccination, the effective serum neutralizing activity in individuals received BNT162b2 was increased from 37% to 100% [49]. Other studies based on pseudo virus neutralization experiments also reported the similar result, with 16~31.8-fold increase in the neutralizing activity [42, 45, 46, 57]. The neutralizing activity against Omicron remained robust even 4 months after booster dose vaccination with BNT162b2 [69].
The protective efficacy of 2 doses various vaccines against Omicron.
| Vaccine type | Vaccines | GMT (NT50) | Effective(fold) | Compared to | Reference | |
|---|---|---|---|---|---|---|
| mRNA vaccines | BNT162b2/mRNA-1273 | BNT162b2/mRNA-1273 | 121 | -22.9x | D614G | [51] |
| BNT162b2/mRNA-1273 | BNT162b2/mRNA-1273 | 17 | -30.6x | USA-WA1/2020 | [52] | |
| BNT162b2 | BNT162b2 | 1.71(OD) | -1.4x | WT | [74] | |
| BNT162b2 | BNT162b2 | -23x | USA-WA1/2020 | [44] | ||
| BNT162b2 | BNT162b2 | 22 | -25.5x | D614G | [45] | |
| BNT162b2 | BNT162b2 | 54 | -29.8x | Victoria | [50] | |
| BNT162b2 | BNT162b2 | 5.43-6.32 | -35.7∼39.9x | WT | [43] | |
| BNT162b2 | BNT162b2 | 7 | -22.9x | WT | [46] | |
| BNT162b2 | BNT162b2 | -122x | WT | [42] | ||
| BNT162b2 | BNT162b2 | 1.11 | -14.91x | WT | [47] | |
| BNT162b2 | BNT162b2 | 89 | -22x | WT | [123] | |
| BNT162b2 | BNT162b2 | -16x | D614G | [48] | ||
| BNT162b2 | BNT162b2 | 7 | -30.9x | WT | [57] | |
| BNT162b2 | BNT162b2 | 9 (ID50) | -14.3x | WT | [49] | |
| BNT162b2 | BNT162b2 | 6%(EC50>30) | -16.6x | D614G | [68] | |
| BNT162b2 | BNT162b2 | 9/20(IC50>16) | -2.2x | Alpha | [54] | |
| mRNA-1273 | mRNA-1273 | -43x | WT | [42] | ||
| mRNA-1273 | mRNA-1273 | -42x | USA-WA1/2020 | [44] | ||
| mRNA-1273 | mRNA-1273 | 27 | -84x | D614G | [70] | |
| mRNA-1273 | mRNA-1273 | 62 | -49x | |||
| mRNA-1273 | mRNA-1273 | 1/10(IC50>16) | -9x | Alpha | [54] | |
| Adenovirus vector vaccines | Ad26.COV2.S | -17x | WT | [42] | ||
| ChAdOx1 | ChAdOx1 | 1.17(OD) | -2.0x | WT | [74] | |
| ChAdOx1 | ChAdOx1 | 10 | -13.3x | Victoria | [50] | |
| ChAdOx1 | ChAdOx1 | 0/10(IC50>16) | -10x | Alpha | [54] | |
| ChAdOx1 | BNT162b2 | 14/20(IC50>16) | -1.4x | |||
| Covishield | Covishield | -23.15x | B.1 | [55] | ||
| ChAdOx1 | ChAdOx1 | 11%(EC50>30) | -8.1x | D615G | [68] | |
| Inactivated vaccines | BBIBP-CorV | BBIBP-CorV | 9.63 | -7x | WT | [60] |
| BBIBP-CorV | BBIBP-CorV | 55 | -1.52x | WT | [61] | |
| CoronaVac | CoronaVac | Inactive | USA-WA1/2020 | [56] | ||
| CoronaVac | CoronaVac | 5 | -6.4x | WT | [57] | |
| CoronaVac | CoronaVac | 20.9 | [59] | |||
| CoronaVac | CoronaVac | 53 | -12.5x | WT | [58] | |
| COVAXIN | COVAXIN | 4.9 | -12.49x | B.1 | [63] | |
| COVAXIN | COVAXIN | 4.11 | -8.67x | B.1 | [62] | |
If not specifically marked, the GMT(NT50) represented geometric mean of 50% neutralizing antibodies titers. EC50 represented 50% effective concentration. IC50 represented 50% inhibitor concentration. ID50 represented inhibitory serum dilution. Due to different studies use some different standard. OD represented optical density. OR represented odds ratio (positive/negative). USA-WA1/2020 represented the strain isolated from the first patient in the United States. We make the results more uniform by using multiples of the data of Omicron and others (WT virus or other variants).
The effect of booster dose of mRNA-1273 was also studied. For example, it has been reported that boosting with another dose of mRNA-1273 resulted in a 19~32.3-fold increase in cross-neutralization of the Omicron variant [42] [70]. One study estimated that the effectiveness of 3-dose of BNT162b2 and mRNA-1273 were 66% and 69%, respectively [71]. Another study showed that the reduction of neutralizing activities against Omicron in individuals received booster dose of BNT162b2 or mRNA-1273 was 7.5-fold and 16.7-fold respectively, as compared to their effect on USA-WA1/2020 [44]. Further investigation is needed to determine if such differences between two major mRNA vaccines could be indicator of better booster dose of vaccine.
The booster dose of inactivated vaccines could also enhance the production of neutralizing antibodies against Omicron variant. For example, it was reported that BBIBP-CorV vaccine booster resulted in a 1.5~5-fold increase in the neutralizing antibodies against Omicron, as compared to that elicited by 2-dose of the same vaccine [60] [61] [72]. Similarly, in recipients vaccinated with COVAXIN, the booster dose of same vaccine increased the levels of neutralizing antibodies against Omicron variant by 18.53-fold [62]. However, one study failed to observe the increase of neutralizing activity against Omicron by booster dose of CoronaVac [57].
Taken together, there is strong evidence that the booster dose of the most of current vaccines can markedly enhance the humoral immune responses against Omicron variant, in terms of GMT of neutralizing antibody in the serum (summarized in table 3). There is evidence that mRNA vaccines as booster dose can elicit markedly higher levels of neutralizing antibodies than inactivated vaccines (GMT for mRNA vaccines: 93- 3179, GMT for inactivated vaccines:7.6- 84) (summarized in table 3). It was shown that antibody diversity can be increased through repeated antigen exposure which may help neutralizing SARS-CoV-2 variants [73]. Indeed, the booster dose is able to improve the breadth of humoral response and cross-reactivity to mutated SARS-CoV-2 variants such as Omicron, in addition to increasing the neutralizing antibody titers [42]. The difference in cross-reactivity between two doses and third dose may be due to emerging neutralizing antibodies that can target new conserved epitopes on S-protein or increasing the affinity of existing neutralizing antibodies, rendering them less sensitive to mutations in their target epitopes, or both [42]. Thus, the booster dose of the most of current vaccines is necessary and can provide a stronger protection against Omicron variant, as compared to 2-dose vaccines. Nevertheless, although booster dose substantially increases the protection against Omicron, the immune responses triggered by it can wane over time [53]. To date, the effect of adenoviral vector vaccines as primary and booster vaccination on the titers of neutralizing antibody against Omicron has not been reported yet, since U.S. FDA just approved Ad26.COV2.S as booster dose on 21st October 2021.
Heterologous vaccination regimens improve humoral immune response against Omicron variant
In addition to the aforementioned booster dose with the same vaccine (homologous vaccination regimens), vaccines of different technical platforms, e.g. heterologous vaccination regimens, have been used in the priming dose and booster dose, in the hope of enhancing the production of neutralizing antibodies against Omicron variant. In the current vaccination program, almost all reported adenovirus vector vaccines were used as a priming vaccination and boosted with mRNA vaccines [42, 74]. It was found that GMT of neutralizing antibody against Omicron variant in individuals received 2-dose ChAdOx1 and a booster dose of BNT162b2 was comparable with GMT in individuals vaccinated with 3 doses of BNT162b2 [74]. It is worth noting that antibody binding doesn't represent the whole function of antibody, since neutralization is more important. A pseudo virus neutralization assay-based study indicated that Ad26.COV2.S vaccines boosted with mRNA-1273 also showed a substantially enhanced neutralizing activity against Omicron variant [42]. One study using VISION Network analysis found that one dose of Ad26.COV2.S vaccine plus one dose of mRNA vaccine yielded better protection than two doses of Ad26.COV2.S, and the efficacy of heterologous regimen was comparable to that of 3 doses of mRNA vaccines [75]. Further investigation is needed to verify the observation that booster dose of mRNA vaccine can improve the efficacy of adenovirus vaccine. In India, COVAXIN was used as booster dose in individuals received Covishield as prime dose and found that IgG titer increased 1.65- and 1.18-fold to those vaccinated with Covishield or COVAXIN only, respectively [55], suggesting that inactivated vaccine may also enhance humoral immune responses induced by adenovirus vaccine to certain degree.
Homologous booster strategy with inactivated vaccinees can also enhance the neutralizing effect against Omicron variant, but less potent than mRNA vaccines [57]. Thus, mRNA vaccine was proposed as booster dose for individuals received priming dose of inactivated vaccine. For example, it was shown that the individuals primarily vaccinated with 2-dose CoronaVac and received booster dose of BNT162b2 exhibited significantly elevated levels of Omicron-specific antibodies which was 1.4-fold higher than in individuals who received 2-dose of mRNA vaccines [56]. Another study using a viral microneutralization assay with SARS-CoV-2 Omicron live virus found that the mean titers of recipients vaccinated with 2-dose of CoronaVac and 1-dose BNT162b2 increased 27.5-fold, as compared to 2-dose of CoronaVac [59]. This improvement may be caused by the fact that beta-coronaviruses including SARS-CoV-2 variants share a conserved site in S-protein, and BNT162b2 used S-protein mRNA from the original Wuhan SARS-CoV-2 isolate [59]. In addition, ZF2001, a recombinant subunit vaccine which was developed with recombinant DNA technology using the RBD of the S-protein as antigen, as booster dose brought about 10-fold increase in GMT of neutralizing antibody against Omicron in the individuals received BBIBP-CorV as priming dose [60]. Another study that used the same vaccination regimens also found that booster dose elicited the neutralizing antibodies that targeted some conserved sites in RBD or NTD [61]. Moreover, a large-scale real-world data from Brazil showed that the effectiveness of 2-dose of CoronaVac was 55.0% against infection and 82.1% against severe outcomes during the period of 14~30 days after vaccination, and such effect was decreased to 34.7% and 72.5% after 180 days post vaccination, respectively. However, administration of booster dose of BNT162b2 after 6 months could markedly enhance the effectiveness to 92.7% against infection and 97.3% against severe outcomes in the following 14~30 days, although the elderly was less responsive to the booster dose [76].
The protective efficacy of booster doses various vaccines against Omicron.
| Vaccine regimen | Prime vaccination | Booster dose | GMT | Effective (fold) | compared to | Reference | |
|---|---|---|---|---|---|---|---|
| Homologous vaccination | BNT162b2/mRNA-1273 | BNT162b2/mRNA-1273 | BNT162b2/mRNA-1273 | 3179 | +26.3 | 2-dose | [51] |
| BNT162b2/mRNA-1273 | BNT162b2/mRNA-1273 | BNT162b2/mRNA-1273 | 93 | +5.4x | 2-dose | [52] | |
| BNT162b2/mRNA-1273 | BNT162b2/mRNA-1273 | BNT162b2/mRNA-1273 | +38x | 2-dose | [67] | ||
| BNT162b2 | BNT162b2 | BNT162b2 | 3.37 (OD) | +1.9 | 2-dose | [74] | |
| BNT162b2 | BNT162b2 | BNT162b2 | -4x | D614G | [48] | ||
| BNT162b2 | BNT162b2 | BNT162b2 | 700 | +31.8 | 2-dose | [124] | |
| BNT162b2 | BNT162b2 | BNT162b2 | 164 | +23.4 | 2-dose | [46] | |
| BNT162b2 | BNT162b2 | BNT162b2 | +27x | 2-dose | [42] | ||
| BNT162b2 | BNT162b2 | BNT162b2 | 114.9 | +16x | 2-dose | [57] | |
| BNT162b2 | BNT162b2 | BNT162b2 | -7.5 | USA-WA1/2020 | [44] | ||
| BNT162b2 | BNT162b2 | BNT162b2 | 107.6 | +96.9x | 2-dose | [47] | |
| BNT162b2 | BNT162b2 | BNT162b2 | 100%(EC50>30) | +16.7x | 2-dose | [68] | |
| BNT162b2 | BNT162b2 | BNT162b2 | 1195 (ID50) | +132.8x | 2-dose | [49] | |
| mRNA-1273 | mRNA-1273 | mRNA-1273 | +19x | 2-dose USA-WA1/2020 | [42] [44] | ||
| mRNA-1273 | mRNA-1273 | mRNA-1273 | -16.7 | ||||
| mRNA-1273 | mRNA-1273 | mRNA-1273 | 620 | +24.1x | 2-dose | [70] | |
| mRNA-1273 | mRNA-1273 | mRNA-1273 | 2002 | +32.3x | |||
| BBIBP-CorV | BBIBP-CorV | BBIBP-CorV | 48.73 | +5.1x | 2-dose | [60] | |
| BBIBP-CorV | BBIBP-CorV | BBIBP-CorV | 84 | +1.5x | 2-dose | [61] | |
| BBIBP-CorV | BBIBP-CorV | BBIBP-CorV | 22.96 | -16.07x | WT | [72] | |
| CoronaVac | CoronaVac | CoronaVac | 7.6 | +1.5x | 2-dose | [57] | |
| COVAXIN | COVAXIN | COVAXIN | 76.14 | +18.53x | 2-dose | [62] | |
| Heterologous vaccination | Ad26.COV2.S | mRNA-1273 | +4x | 1-dose Ad26.COV2.S | [42] | ||
| ChAdOx1 | ChAdOx1 | BNT162b2 | 3.46 (OD) | +2.9x | 2-dose ChAdOx1 | [74] | |
| Covishield | COVAXIN | +1.65x | 2-dose Covishield | [55] | |||
| BBIBP-CorV | BBIBP-CorV | ZF2001 | 95.86 | +10x | 2-dose BBIBP-CorV | [60] | |
| BBIBP-CorV | BBIBP-CorV | ZF2001 | 172 | +3.1x | 2-dose BBIBP-CorV | [61] | |
| CoronaVac | CoronaVac | BNT162b2 | +10.1x | 2-dose CoronaVac | [56] | ||
| CoronaVac | CoronaVac | BNT162b2 | 52.8 | +10.6x | 2-dose CoronaVac | [57] | |
| CoronaVac | CoronaVac | BNT162b2 | 575.8 | +27.5x | 2-dose CoronaVac | [59] | |
If not specifically marked, the GMT(NT50) represented geometric mean of 50% neutralizing antibodies titers. EC50 represented 50% effective concentration. IC50 represented 50% inhibitor concentration. ID50 represented inhibitory serum dilution. Due to different studies use some different standard. OD represented optical density. OR represented odds ratio (positive/negative). USA-WA1/2020 represented the strain isolated from the first patient in the United States. We make the results more uniform by using multiples of the data of Omicron and others (WT virus or other variants) and the result of booster dose was compared with 2-dose.
In summary, there are strong evidence that the humoral immune responses against Omicron variant could be markedly increased by heterologous vaccination regimens (summarized in Table 3). More specifically, booster dose of mRNA vaccines can elicit high levels of neutralizing antibodies against Omicron variant in individuals received adenovirus vaccines or inactivated vaccines as priming dose. Using recombinant subunit vaccine to boost inactivated vaccines can also enhance the neutralizing activity against Omicron variant. Adenovirus vaccines may also be useful as booster dose to enhance the efficacy of inactivated vaccines. The results from recent studies strongly suggest that the heterologous regimens including different types of vaccines developed with distinct technology platform can induce stronger humoral immune responses to emerging variants including Omicron. Importantly, heterologous regimens containing at least one dose of mRNA vaccine markedly enhance the binding and neutralizing activities of elicited antibodies against Omicron variant, while its safety is comparable to that of homologous regimens, as shown by a UK study [77].
A recent UK study indicated that the effectiveness of heterologous vaccine regimens against hospitalization dropped from 92% to 83% just 10 weeks after a third dose [78], suggesting that the protection provided by a booster dose could also wane over time. Therefore, the efficacy of the fourth dose of vaccine was investigated. For example, two studies from China and Israel showed that the relatively high levels of cross-neutralizing antibodies against Omicron induced by the third dose of inactivated or mRNA vaccines could not be further enhanced by the fourth dose of vaccine. However, these studies found that the fourth dose was able to bring neutralizing antibody levels back to the peak after the third dose [79, 80], a phenomenon known as 'ceiling of immunity' [81]. These results favor the idea of continuing routine vaccination as long as SARS-CoV-2 persistence.
4. Vaccine-induced T cell immune responses against Omicron variant
T cell-mediated immune responses constitute the other essential arm of immune defense in the control of intracellular pathogens. For example, it had been shown that T cell responses play a critical role in virus clearance in the infection of influenza viruses, SARS-CoV and MERS-CoV [82-84]. In SARS-CoV-2 infection, lower frequencies of CD8 and CD4 T cells was associated with more severe disease [85, 86]. A recent study indicated that the coordinated SARS-CoV-2-specific adaptive immune responses are mediated by CD4 and CD8 T cells, and both subsets of T cells are protective and associated with milder disease [87]. CD4 and CD8 T cells are highly proliferative in patients infected with SARS-CoV-2 [88], and both these replicating CD8 and CD4 T cells expressed the elevated levels of cytotoxicity markers [89]. However, the effector function of T cells appears to fail in the severe patients [90]. In patients with COVID-19, T cells underwent an extensive remodeling in terms of abundance and phenotype [91]. The peak levels of SARS-CoV-2-specific memory CD4 and CD8 T cells was found to be in the first month of infection, and then slowly declined in the following 6~7 months. Central memory Th1 (T helper 1 cells) CD4 T cells dominate throughout the early infection and recovery period [92]. By comparison with health individuals, Th1 cells present in COVID-19 patients characteristically expressed CXCR3 and CCR6, as well as transcripts encoding anti-viral cytokines and chemokines, including CD154, IFN-γ, IL-2 and TNF [92], while their expression of IFN-γ, IL-2 was lower than influenza-reactive Th1 cells [93]. CD4 cells with cytotoxic activity, namely CD4 CTLs, can directly induce the death of major histocompatibility complex class II-expressing infected cells and recruit innate immune cells to enhance CD8 CTL responses [93]. The proportion of SARS-CoV-2-reactive regulatory T cells (Treg) also significantly is reduced in hospitalized patients and patients with an impaired Treg response to SARS-CoV-2 have a more robust CD4 CTL responses [93] (reviewed by us recently [94]). The levels of SARS-CoV-2-specific memory CD4 T cells are constant from 3 to 6 months following mRNA vaccines vaccination [39]. The most of vaccinees are able to elicit a robust CD4 T cell response up to 6 months after vaccination and the early CD4 T cell responses and humoral immune responses are correlated [39]. Robust CD8 T cells are crucial for the clearance of infected cells in the early phase of infection [90]. A preclinical study on rhesus macaques showed that CD8 T cells could contribute to the protection against SARS-CoV-2 when humoral immunity is insufficient [95]. During and after infection, GZMB (Granzyme B)-expressing CD8 T cells were clonally expanded, resulting in the generation of protective memory T cell responses against SARS-CoV-2 [91]. It was shown that memory CD4 T cells recognize SARS-CoV-2 virus structural and accessory proteins, while memory CD8 T cells prefer to recognize nucleocapsid protein [92]. Also, these T cell responses last for at least 6 months, as revealed by an 8-month-long serological measurement at a population scale [41]. All these evidence indicate that the robust T cell responses with the proper specificity, phenotype, and function induced by vaccines against SARS-CoV-2 can control the infection and time course of the disease, as well as can promote the optimization of the humoral immunity, although the major goal of most current vaccines is to elicit neutralizing antibodies [96, 97]. It is proposed that an ideal COVID-19 vaccine should stimulate the production of neutralizing antibodies and induce robust T cell-mediated immune responses simultaneously. Previously, S-protein as antigen was focused on the identification of the most of vaccine candidates. Non-spike proteins may need to be included in the development of future vaccines, in order to induce a broad epitope coverage, a process akin to natural infection of virus [97].
It is interesting to ask if the mutation of SARS-CoV-2 would result in the evasion of T cell-mediated immune responses. Indeed, the single point mutation may eliminate the response of individual T cell clone. However, such mutation is unlike to abolish the overall cell-mediated immunity, for the reason that T cell receptors can recognize over 1,400 different SARS-CoV-2 epitopes (382 for CD4 cells and 1,052 for CD8 cells) at the population level [98]. A study showed that 93% of CD4 and 97% of CD8 T cell epitopes in COVID-19 patients infected with different variants are completely conserved [99]. And the SARS-COV-2-specific CD4 and CD8 T cell responses are less dominated by spike protein epitopes, and the mutation on spike protein will not influence the T cell responses [100]. All these results suggest that SARS-CoV-2-specific T cell responses may remain robust cross-reactive to Omicron variant and protect vaccinees from severity and death, even though all vaccines were developed based on an ancestral virus.
Indeed, it was shown that SARS-CoV-2-specific T cell responses induced by current vaccines retain robust reactivity to Omicron variant, albeit such crucial role of T cell immunity is relatively underestimated [101]. In fact, the response of polyclonal memory T cells is not affected by single amino acid substitutions or deletions across large peptidomes [99]. In a study found that the individuals with primary series vaccination with mRNA1273, or BNT162b2, or Ad26.COV2.S have similar median effector T cell reactivity (in terms of IFN-γ ELISpot spot forming units of SFU/106 PBMCs) against Omicron S-protein, as compared with that against WT SARS-CoV-2 (WT: 43, Omicron: 42), while prior infection could bring better performance (WT:311, Omicron:315 ). Even in individuals with undetectable Omicron neutralizing antibody, effector T cell responses were still measurable [102]. Based on samples obtained at 28 days after vaccination with ChAdOx-1, or Ad26.COV2.S, or mRNA-1273, or BNT162b2, a study showed that 94% individuals (46/49 ) had S-specific CD4 T cells and there were no significant differences between the responses of these CD4 T cells to WT, Omicron and other VOCs [103]. In contrast, the responses of CD8 T cells to Omicron could only be detected in 63% of recipients (31/49). These T cells responses were maintained at least for 6 months after booster dose [103]. Also, mRNA booster vaccine recipients had significantly stronger T cells responses to Omicron S-protein at the time of breakthrough infection [104]. In another study, it was found that about 15% vaccinated individuals (5 out of 32) exhibited a loss of CD8 T cell recognition of Omicron [105]. This study also showed that the vaccination with 2-dose of BNT162b2 or 2-dose of Ad26.COV2.S could trigger CD4 T cell responses to the Omicron [105], while Ad26.COV2.S induced stronger spike-specific CD8 T cell responses [106]. At 8 months after vaccination, the median CD8 T cell responses were 0.016% with the BNT162b2, 0.017% with the mRNA-1273, and Ad26.COV2.S were highest among them which was 0.12% [107]. It was shown that the vaccination with BNT162b2 or Ad26.COV2.S could elicit broadly CD4 and CD8 T cell responses against Omicron variant, including in the subsets of central and effector memory cells, and such T cell responses were durable and could retain 8 months after vaccination [108]. In the macaques vaccinated with BNT162b2 and Ad26.COV2.S, 4 of 30 failed to control viral replication with negligible Omicron-specific CD8 T cell response even they had moderate neutralizing antibodies titers, other 26 macaques demonstrated rapid control of virus [109]. It is worth mentioning that, similar to the humoral responses of the heterologous vaccination regimens, the advantage of a diverse immune response has been demonstrated in cell-mediated immunity. For example, one study found that individuals vaccinated with BNT162b2 largely preserved CD8 T cell recognition of Omicron S-protein epitopes because about 85.3% (n = 208) of the HLA class I epitopes were not affected on the amino acid sequence level [46]. Another study reported that SARS-CoV-2 spike-specific CD4 and CD8 T cells in individuals vaccinated with BNT162b2 provided extensive immune protection against Omicron, with median frequencies of antigen specific CD4 T cells and CD8 T cells that cross-recognized Omicron were 91% and 92%, respectively [110]. The peripheral blood mononuclear cells from 8 donors vaccinated with BNT162b2 demonstrated equivalent levels of responses to both ancestral and Omicron S-protein [111]. Tracking individual clones of SARS-CoV-2-specific CD8 T cells showed that an IFN signature marked long-lived, circulating memory CD8 T cells persisting even 1-year, and such cells expressed CD45RA, IL-7 receptor-α and T cell factor 1 [112]. Thus, T cell responses elicited by vaccines against Omicron can maintain for a considerable long time.
The performance of inactivated vaccine CoronaVac is poorer than mRNA vaccines in eliciting the generation of neutralizing antibody. However, inactivated vaccines appeared to have the capacity to induce even stronger CD4 and CD8 T cell responses to the structural protein, such as highly abundant, conserved, and immunogenic N protein, as compared with BNT162b2 [113]. This result is in line with the results of two clinical studies in Turkey and Chile which showed that inactivated vaccines effectively prevented COVID-19, including severe disease and death [114, 115]. CoronaVac could induce more effector memory T cell response to S, N, Envelope and Matrix protein of SARS-CoV-2, yielding long-term cross-reactive cellular memory during infection with SARS-CoV-2 variants and the average magnitude of structural and S-specific T-cell responses were higher in CoronaVac-vaccinated subjects than BNT162b2-vaccinated subjects [113]. Theoretically, more broad cellular immune responses can be triggered by whole viral protein contained in inactivated vaccine. In recipients of inactivated vaccine, T cell epitope recognition is abundant and the most of them are conserved [98], while Omicron variant have not evolved extensive mutation which can escape T cell responses because of the variety of HLA haplotypes between individuals and virtually all individuals with existing SARS-CoV-2-specific CD8 T cells recognize Omicron variant [116]. This may explain why current inactivated vaccine (CoronaVac) rendered same effectiveness in protection against severe outcome of patients with Omicron variant, as compared to BNT162b2 [117], although inactivated vaccines is known to elicit weaker humoral immune responses against Omicron. Moreover, it was shown that CoronaVac recipients had higher levels of FcγRIIIa-binding antibodies that could stimulate antibody-dependent cell cytotoxicity of NK cells, as compared with that in BNT162b2 recipients [113].
T cell responses against non-spike structural and accessory proteins of WT and Omicron variants are preserved in all individuals with prior infection, while individuals only received S-protein-targeting vaccine without prior infection lack of such responses [102]. A study showed that effector T cells increase more in individuals boosted with BNT162b2, as compared to individuals boosted with CoronaVac, in those received CoronaVac as prime vaccination [118]. These support the notion that vaccination with vaccines using different technical platforms which target various epitopes can elicit a wider range of T cell responses, and repeated exposure to antigen may enhance cross-reactive T cell responses. Moreover, using different vaccine regimens in prime and booster vaccinations may represent a better solution for emerging variants. In particular, the use of inactivated vaccines containing whole virus proteins which is rich in highly conserved and immunogenic proteins to stimulate T cell responses, may provide better protection. To date, the understanding of responses of T cells to Omicron remains incomplete. Therefore, in-depth understandings of the molecular basis underlying T cell responses which play a decisive role in the resistance of transmission and infection of Omicron variant need further investigation.
5. Closing remarks
To date, all the current vaccines are designed based on the ancestral SARS-CoV-2 virus, thus there is an urgent need for the new generation of vaccines specifically targeting Omicron variant or its subvariants (such as BA.2, BA.3, Deltacron and XE). In fact, it is the current efforts of pharmaceutical companies including Pfizer and BioNTech[119]. However, there are some potential challenges for the development of Omicron vaccines. For example, antigenic and virological characterization of the various Omicron sub-variant BA.1, BA.2, BA.3, Deltacron and XE remain to be thoroughly studied, while the new mutant strains may emerge before Omicron vaccines can be produced and delivered on a large scale. Since the performance of any newly developed vaccine may be affected by acquired immunity triggered by previous vaccines, further research is also needed to address questions such as whether different vaccines interact with each other, and if epitope similarities or differences between sub-variants affect the performance of Omicron vaccines. Nevertheless, further understanding of humoral and cellular immune responses, as well as their underlying molecular mechanism, elicited by current vaccine regimens against Omicron variant would be crucial for containing current surge of COVID-19 with existing tools. Moreover, in-depth study in this line could also be helpful to device future vaccines against emerging mutant strains of SARS-CoV-2. To develop vaccines constantly against new SARS-CoV-2 variants does not seem to be a long-term solution, especially for a rapidly mutated virus like SARS-CoV-2. More innovative technologies should be developed. For example, it was proposed that a cocktail vaccine that combines the structural and non-structural viral antigen for a broader range immunity [120], or contains the protein that is highly conservative from virus such as nucleocapsid structural protein [121, 122] may effective in protection against emerging variants.
Taken together, recent studies have been focusing on the understanding of humoral as well as cellular immune responses against Omicron variants elicited by current vaccine regimens, which underlies the effectiveness of vaccines on the highly mutated variants of SARS-CoV-2. These studies clearly indicate that the levels of vaccine-elicited neutralizing antibody against Omicron variants are reduced to various degrees depending on the route of technology, while robust T cell mediated immune responses are largely preserved in the individual who has received any approved vaccines. This at least partially explains why the infectivity and transmissibility of SARS-CoV-2 Omicron variant is extremely high, while the clinical severity and death are markedly reduced. There is compelling evidence that the booster dose as well as heterologous vaccination represent a strategy to enhance the overall protective efficacy of current vaccines. Further understanding of cellular and molecular basis underlying the Omicron-specific humoral as well as cellular immune responses elicited by current vaccines would be helpful for the design of more effective vaccines to prevent the emerging variants of SARS-CoV-2.
Abbreviations
SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2; RBD: receptor binding district; EUL: emergency use listing; VOC: variants of concern; NTD: N-terminal structural domain; GMT: geometry mean titers; Th1: T helper 1 cells; Tfh: follicular helper T cell; TNF: Tumor necrosis factor; IFN-γ: Interferon-γ; WT: Wild type; ACE2: angiotensin-converting enzyme II; S-protein: Spike-protein; GMNT: geometry mean neutralization titers.
Acknowledgements
This work was funded by grants from Macau Science and Technology Development Fund (0099/2021/A2 and 0056/2019/AFJ), University of Macau (CPG2022-00024-ICMS and MYRG2019-00169-ICMS), Guangdong-Hong Kong-Macau Joint Lab on Chinese Medicine and Immune Disease Research (EF006/ICMS-CX/2021/GDSTC), Applied Research Programs of Guangdong-Hong Kong-Macao Innovation Center sponsored by Guangzhou Development District (EF032/ICMS-CX/2021/RITH).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Garcia-Beltran WF, St Denis KJ, Hoelzemer A, Lam EC, Nitido AD, Sheehan ML. et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2022;185:457-66 e4
2. Ghebreyesus TA. WHO Director-General's opening remarks at the 150th session of the Executive Board — 24 January 2022. 2022.
3. Dejnirattisai W, Huo J, Zhou D, Zahradnik J, Supasa P, Liu C. et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell. 2022;185:467-84 e15
4. Bansal K, Kumar S. Mutational cascade of SARS-CoV-2 leading to evolution and emergence of omicron variant. 2021.
5. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W. et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-3
6. Daria S, Bhuiyan MA, Islam MR. Detection of highly muted coronavirus variant Omicron (B.1.1.529) is triggering the alarm for South Asian countries: Associated risk factors and preventive actions. J Med Virol. 2021
7. Kumar S, Thambiraja TS, Karuppanan K, Subramaniam G. Omicron and Delta variant of SARS-CoV-2: A comparative computational study of spike protein. J Med Virol. 2021
8. Araf Y, Akter F, Tang YD, Fatemi R, Parvez MSA, Zheng C. et al. Omicron variant of SARS-CoV-2: Genomics, transmissibility, and responses to current COVID-19 vaccines. J Med Virol. 2022
9. Kannan S, Shaik Syed Ali P, Sheeza A. Omicron (B.1.1.529) - variant of concern - molecular profile and epidemiology: a mini review. Eur Rev Med Pharmacol Sci. 2021;25:8019-22
10. Lazarevic I, Pravica V, Miljanovic D, Cupic M. Immune Evasion of SARS-CoV-2 Emerging Variants: What Have We Learnt So Far? Viruses. 2021 13
11. Luan B, Wang H, Huynh T. Enhanced binding of the N501Y-mutated SARS-CoV-2 spike protein to the human ACE2 receptor: insights from molecular dynamics simulations. FEBS Lett. 2021;595:1454-61
12. Wibmer CK, Ayres F, Hermanus T, Madzivhandila M, Kgagudi P, Oosthuysen B. et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Nat Med. 2021;27:622-5
13. Laffeber C, de Koning K, Kanaar R, Lebbink JHG. Experimental Evidence for Enhanced Receptor Binding by Rapidly Spreading SARS-CoV-2 Variants. J Mol Biol. 2021;433:167058
14. Chakraborty C, Bhattacharya M, Sharma AR. Present variants of concern and variants of interest of severe acute respiratory syndrome coronavirus 2: Their significant mutations in S-glycoprotein, infectivity, re-infectivity, immune escape and vaccines activity. Reviews in Medical Virology. 2021
15. Serpeloni JM, Lima Neto QA, Lucio LC, Ramao A, Carvalho de Oliveira J, Gradia DF. et al. Genome interaction of the virus and the host genes and non-coding RNAs in SARS-CoV-2 infection. Immunobiology. 2021;226:152130
16. Silva S, Pena L. Collapse of the public health system and the emergence of new variants during the second wave of the COVID-19 pandemic in Brazil. One Health. 2021;13:100287
17. Sun Y, Lin W, Dong W, Xu J. Origin and evolutionary analysis of the SARS-CoV-2 Omicron variant. J Biosaf Biosecur. 2022;4:33-7
18. Zinatizadeh MR, Zarandi PK, Zinatizadeh M, Yousefi MH, Amani J, Rezaei N. Efficacy of mRNA, adenoviral vector, and perfusion protein COVID-19 vaccines. Biomed Pharmacother. 2022;146:112527
19. Chen J, Wei GW. Omicron BA.2 (B.1.1.529.2): high potential to becoming the next dominating variant. ArXiv. 2022
20. COVID-19 Vaccine & Therapeutics Tracker
21. Muik A, Wallisch AK, Sanger B, Swanson KA, Muhl J, Chen W. et al. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine-elicited human sera. Science. 2021;371:1152-3
22. Abu-Raddad LJ, Chemaitelly H, Butt AA, National Study Group for C-V. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N Engl J Med. 2021;385:187-9
23. Wu K, Werner AP, Koch M, Choi A, Narayanan E, Stewart-Jones GBE. et al. Serum Neutralizing Activity Elicited by mRNA-1273 Vaccine. N Engl J Med. 2021;384:1468-70
24. Sadoff J, Gray G, Vandebosch A, Cardenas V, Shukarev G, Grinsztejn B. et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med. 2021;384:2187-201
25. Vignier N, Berot V, Bonnave N, Peugny S, Ballet M, Jacoud E. et al. Breakthrough Infections of SARS-CoV-2 Gamma Variant in Fully Vaccinated Gold Miners, French Guiana, 2021. Emerg Infect Dis. 2021;27:2673-6
26. Bruxvoort KJ, Sy LS, Qian L, Ackerson BK, Luo Y, Lee GS. et al. Effectiveness of mRNA-1273 against delta, mu, and other emerging variants of SARS-CoV-2: test negative case-control study. BMJ. 2021;375:e068848
27. Brandal LT, MacDonald E, Veneti L, Ravlo T, Lange H, Naseer U. et al. Outbreak caused by the SARS-CoV-2 Omicron variant in Norway, November to December 2021. Euro Surveill. 2021 26
28. Espenhain L, Funk T, Overvad M, Edslev SM, Fonager J, Ingham AC. et al. Epidemiological characterisation of the first 785 SARS-CoV-2 Omicron variant cases in Denmark, December 2021. Euro Surveill. 2021 26
29. Yin W, Xu Y, Xu P, Cao X, Wu C, Gu C. et al. Structures of the Omicron Spike trimer with ACE2 and an anti-Omicron antibody. Science. 2022: eabn8863.
30. Wang K, Jia Z, Bao L, Wang L, Cao L, Chi H. et al. Memory B cell repertoire from triple vaccinees against diverse SARS-CoV-2 variants. Nature. 2022
31. Dejnirattisai W, Zhou D, Ginn HM, Duyvesteyn HME, Supasa P, Case JB. et al. The antigenic anatomy of SARS-CoV-2 receptor binding domain. Cell. 2021;184:2183-200 e22
32. Wu LY, Zhou LP, Mo MX, Liu TT, Wu CK, Gong CY. et al. SARS-CoV-2 Omicron RBD shows weaker binding affinity than the currently dominant Delta variant to human ACE2. Signal Transduct Tar. 2022 7
33. McCallum M, Czudnochowski N, Rosen LE, Zepeda SK, Bowen JE, Walls AC. et al. Structural basis of SARS-CoV-2 Omicron immune evasion and receptor engagement. Science. 2022: eabn8652.
34. Li Y, Wang T, Zhang JR, Shao B, Gong HP, Wang YS. et al. Exploring the Regulatory Function of the N-terminal Domain of SARS-CoV-2 Spike Protein through Molecular Dynamics Simulation. Advanced Theory and Simulations. 2021 4
35. Min L, Sun Q. Antibodies and Vaccines Target RBD of SARS-CoV-2. Front Mol Biosci. 2021;8:671633
36. Cao Y, Yisimayi A, Bai Y, Huang W, Li X, Zhang Z. et al. Humoral immune response to circulating SARS-CoV-2 variants elicited by inactivated and RBD-subunit vaccines. Cell Res. 2021;31:732-41
37. Gomez CE, Perdiguero B, Esteban M. Emerging SARS-CoV-2 Variants and Impact in Global Vaccination Programs against SARS-CoV-2/COVID-19. Vaccines (Basel). 2021 9
38. Sokal A, Chappert P, Barba-Spaeth G, Roeser A, Fourati S, Azzaoui I. et al. Maturation and persistence of the anti-SARS-CoV-2 memory B cell response. Cell. 2021;184:1201-13 e14
39. Goel RR, Painter MM, Apostolidis SA, Mathew D, Meng W, Rosenfeld AM. et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science. 2021;374:abm0829
40. Turner JS, O'Halloran JA, Kalaidina E, Kim W, Schmitz AJ, Zhou JQ. et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature. 2021;596:109-13
41. Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE. et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021 371
42. Garcia-Beltran WF, St Denis KJ, Hoelzemer A, Lam EC, Nitido AD, Sheehan ML. et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. medRxiv. 2021
43. Lu L, Mok BW, Chen LL, Chan JM, Tsang OT, Lam BH. et al. Neutralization of SARS-CoV-2 Omicron variant by sera from BNT162b2 or Coronavac vaccine recipients. Clin Infect Dis. 2021
44. Carreno JM, Alshammary H, Tcheou J, Singh G, Raskin A, Kawabata H. et al. Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron. Nature. 2021
45. Lusvarghi S, Pollett SD, Neerukonda SN, Wang W, Wang R, Vassell R. et al. SARS-CoV-2 Omicron neutralization by therapeutic antibodies, convalescent sera, and post-mRNA vaccine booster. bioRxiv. 2021
46. Muik A, Lui BG, Wallisch AK, Bacher M, Muhl J, Reinholz J. et al. Neutralization of SARS-CoV-2 Omicron by BNT162b2 mRNA vaccine-elicited human sera. Science. 2022: eabn7591.
47. Nemet I, Kliker L, Lustig Y, Zuckerman N, Erster O, Cohen C. et al. Third BNT162b2 Vaccination Neutralization of SARS-CoV-2 Omicron Infection. N Engl J Med. 2022;386:492-4
48. Laurie MT, Liu J, Sunshine S, Peng J, Black D, Mitchell AM. et al. SARS-CoV-2 variant exposures elicit antibody responses with differential cross-neutralization of established and emerging strains including Delta and Omicron. medRxiv. 2021
49. Gruell H, Vanshylla K, Tober-Lau P, Hillus D, Schommers P, Lehmann C. et al. mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 Omicron variant. Nat Med. 2022
50. Dejnirattisai W, Shaw RH, Supasa P, Liu C, Stuart ASV, Pollard AJ. et al. Reduced neutralisation of SARS-CoV-2 omicron B.1.1.529 variant by post-immunisation serum. The Lancet. 2022;399:234-6
51. Zeng C, Evans JP, Qu P, Faraone J, Zheng YM, Carlin C. et al. Neutralization and Stability of SARS-CoV-2 Omicron Variant. bioRxiv. 2021
52. Edara VV, Manning KE, Ellis M, Lai L, Moore KM, Foster SL. et al. mRNA-1273 and BNT162b2 mRNA vaccines have reduced neutralizing activity against the SARS-CoV-2 Omicron variant. bioRxiv. 2021
53. Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N Engl J Med. 2022
54. Rossler A, Riepler L, Bante D, von Laer D, Kimpel J. SARS-CoV-2 Omicron Variant Neutralization in Serum from Vaccinated and Convalescent Persons. N Engl J Med. 2022
55. Sapkal G, Srivastava RK, Dwivedi G, Sahay RR, Yadav PD, Deshpande GR. et al. Immune responses against different variants of SARS-CoV-2 including omicron following six months of administration of heterologous prime-boost COVID-19 vaccine. J Travel Med. 2022
56. Perez-Then E, Lucas C, Monteiro VS, Miric M, Brache V, Cochon L. et al. Neutralizing antibodies against the SARS-CoV-2 Delta and Omicron variants following heterologous CoronaVac plus BNT162b2 booster vaccination. Nat Med. 2022
57. Peiris M, Cheng S, Mok CKP, Leung Y, Ng S, Chan K. et al. Neutralizing antibody titres to SARS-CoV-2 Omicron variant and wild-type virus in those with past infection or vaccinated or boosted with mRNA BNT162b2 or inactivated CoronaVac vaccines. Res Sq. 2022
58. Wang Y, Ma Y, Xu Y, Liu J, Li X, Chen Y. et al. Resistance of SARS-CoV-2 Omicron variant to convalescent and CoronaVac vaccine plasma. Emerg Microbes Infect. 2022;11:424-7
59. Campos GRF, Almeida NBF, Filgueiras PS, Corsini CA, Gomes SVC, de Miranda DAP. et al. Booster dose of BNT162b2 in a CoronaVac primary vaccination protocol improves neutralization of SARS-CoV-2 Omicron variant. medRxiv. 2022
60. Ai J, Zhang H, Zhang Y, Lin K, Zhang Y, Wu J. et al. Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost. Emerg Microbes Infect. 2022;11:337-43
61. Wang X, Zhao X, Song J, Wu J, Zhu Y, Li M. et al. Homologous or heterologous booster of inactivated vaccine reduces SARS-CoV-2 Omicron variant escape from neutralizing antibodies. Emerg Microbes Infect. 2022;11:477-81
62. Deshpande GR, Yadav PD, Abraham P, Nyayanit DA, Sapkal GN, Shete AM. et al. Booster dose of the inactivated COVID-19 vaccine BBV152 (Covaxin) enhances the neutralizing antibody response against alpha, Beta, Delta and omicron variants of concern. J Travel Med. 2022
63. Yadav PD, Sapkal GN, Sahay RR, Patil DY, Deshpande GR, Jain R. et al. Elevated neutralization of Omicron with sera of COVID-19 recovered and breakthrough cases vaccinated with Covaxin than two dose naive vaccinees. J Infect. 2022
64. Syed AM, Ciling A, Khalid MM, Sreekumar B, Chen PY, Kumar GR. et al. Omicron mutations enhance infectivity and reduce antibody neutralization of SARS-CoV-2 virus-like particles. medRxiv. 2022
65. Puhach O, Adea K, Hulo N, Sattonnet P, Genecand C, Iten A. et al. Infectious viral load in unvaccinated and vaccinated individuals infected with ancestral, Delta or Omicron SARS-CoV-2. Nat Med. 2022
66. Menni C, Valdes AM, Polidori L, Antonelli M, Penamakuri S, Nogal A. et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. The Lancet. 2022
67. Schmidt F, Muecksch F, Weisblum Y, Da Silva J, Bednarski E, Cho AL. et al. Plasma Neutralization of the SARS-CoV-2 Omicron Variant. New England Journal of Medicine. 2022;386:599-601
68. Planas D, Saunders N, Maes P, Guivel-Benhassine F, Planchais C, Buchrieser J. et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2021
69. Xia H, Zou J, Kurhade C, Cai H, Yang Q, Cutler M. et al. Neutralization and durability of 2 or 3 doses of the BNT162b2 vaccine against Omicron SARS-CoV-2. Cell Host Microbe. 2022
70. Doria-Rose NA, Shen X, Schmidt SD, O'Dell S, McDanal C, Feng W. et al. Booster of mRNA-1273 Strengthens SARS-CoV-2 Omicron Neutralization. medRxiv. 2021
71. Accorsi EK, Britton A, Fleming-Dutra KE, Smith ZR, Shang N, Derado G. et al. Association Between 3 Doses of mRNA COVID-19 Vaccine and Symptomatic Infection Caused by the SARS-CoV-2 Omicron and Delta Variants. JAMA. 2022
72. YangYang Gong X, Yang L Li J, Zhang J Wei L. et al. Regular and booster vaccination with inactivated vaccines enhance the neutralizing activity against Omicron variant both in the breakthrough infections and vaccinees. J Infect. 2022
73. Muecksch F, Weisblum Y, Barnes CO, Schmidt F, Schaefer-Babajew D, Wang Z. et al. Affinity maturation of SARS-CoV-2 neutralizing antibodies confers potency, breadth, and resilience to viral escape mutations. Immunity. 2021;54:1853-68 e7
74. Faustini S, Shields A, Banham G, Wall N, Al-Taei S, Tanner C. et al. Cross reactivity of spike glycoprotein induced antibody against Delta and Omicron variants before and after third SARS-CoV-2 vaccine dose in healthy and immunocompromised individuals. J Infect. 2022
75. Natarajan K PN, Dascomb K, et al. Effectiveness of Homologous and Heterologous COVID-19 Booster Doses Following 1 Ad.26.COV2.S (Janssen [Johnson & Johnson]) Vaccine Dose Against COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults — VISION Network, 10 States, December 2021-March 2022. MMWR Morb Mortal Wkly Rep. 2022; 71
76. Cerqueira-Silva T, Katikireddi SV, de Araujo Oliveira V, Flores-Ortiz R, Junior JB, Paixao ES. et al. Vaccine effectiveness of heterologous CoronaVac plus BNT162b2 in Brazil. Nat Med. 2022
77. Stuart ASV, Shaw RH, Liu X, Greenland M, Aley PK, Andrews NJ. et al. Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): a single-blind, randomised, phase 2, non-inferiority trial. The Lancet. 2022;399:36-49
78. COVID-19 variants identified in the UK
79. Regev-Yochay G, Gonen T, Gilboa M, Mandelboim M, Indenbaum V, Amit S. et al. Efficacy of a Fourth Dose of Covid-19 mRNA Vaccine against Omicron. N Engl J Med. 2022;386:1377-80
80. Wang J, Deng C, Liu M, Liu Y, Li L, Huang Z. et al. Four doses of the inactivated SARS-CoV-2 vaccine redistribute humoral immune responses away from the Receptor Binding Domain. 2022.
81. Mallapaty S. Fourth dose of COVID vaccine offers only slight boost against Omicron infection. Nature. 2022
82. Zhong W, Roberts AD, Woodland DL. Antibody-independent antiviral function of memory CD4+ T cells in vivo requires regulatory signals from CD8+ effector T cells. J Immunol. 2001;167:1379-86
83. Zhao J, Zhao J, Perlman S. T cell responses are required for protection from clinical disease and for virus clearance in severe acute respiratory syndrome coronavirus-infected mice. J Virol. 2010;84:9318-25
84. Zhao J, Alshukairi AN, Baharoon SA, Ahmed WA, Bokhari AA, Nehdi AM. et al. Recovery from the Middle East respiratory syndrome is associated with antibody and T-cell responses. Sci Immunol. 2017 2
85. Mathew D, Giles JR, Baxter AE, Oldridge DA, Greenplate AR, Wu JE. et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science. 2020;369:1209 -+
86. Wang J, Jiang M, Chen X, Montaner LJ. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J Leukoc Biol. 2020;108:17-41
87. Rydyznski Moderbacher C, Ramirez SI, Dan JM, Grifoni A, Hastie KM, Weiskopf D. et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell. 2020;183:996-1012 e19
88. Neidleman J, Luo X, Frouard J, Xie G, Gill G, Stein ES. et al. SARS-CoV-2-Specific T Cells Exhibit Phenotypic Features of Helper Function, Lack of Terminal Differentiation, and High Proliferation Potential. Cell Rep Med. 2020;1:100081
89. Bieberich F, Vazquez-Lombardi R, Yermanos A, Ehling RA, Mason DM, Wagner B. et al. A Single-Cell Atlas of Lymphocyte Adaptive Immune Repertoires and Transcriptomes Reveals Age-Related Differences in Convalescent COVID-19 Patients. Front Immunol. 2021;12:701085
90. Bergamaschi L, Mescia F, Turner L, Hanson AL, Kotagiri P, Dunmore BJ. et al. Longitudinal analysis reveals that delayed bystander CD8(+) T cell activation and early immune pathology distinguish severe COVID-19 from mild disease. Immunity. 2021;54:1257 -+
91. Notarbartolo S, Ranzani V, Bandera A, Gruarin P, Bevilacqua V, Putignano AR. et al. Integrated longitudinal immunophenotypic, transcriptional and repertoire analyses delineate immune responses in COVID-19 patients. Sci Immunol. 2021 6
92. Cohen KW, Linderman SL, Moodie Z, Czartoski J, Lai LL, Mantus G. et al. Longitudinal analysis shows durable and broad immune memory after SARS-CoV-2 infection with persisting antibody responses and memory B and T cells. Cell Reports Medicine. 2021 2
93. Meckiff BJ, Ramirez-Suastegui C, Fajardo V, Chee SJ, Kusnadi A, Simon H. et al. Imbalance of Regulatory and Cytotoxic SARS-CoV-2-Reactive CD4(+) T Cells in COVID-19. Cell. 2020;183:1340-53 e16
94. Wang Y, Zheng J, Islam MS, Yang Y, Hu Y, Chen X. The role of CD4(+)FoxP3(+) regulatory T cells in the immunopathogenesis of COVID-19: implications for treatment. Int J Biol Sci. 2021;17:1507-20
95. McMahan K, Yu J, Mercado NB, Loos C, Tostanoski LH, Chandrashekar A. et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590:630-4
96. DiPiazza AT, Graham BS, Ruckwardt TJ. T cell immunity to SARS-CoV-2 following natural infection and vaccination. Biochem Biophys Res Commun. 2021;538:211-7
97. Hellerstein M. What are the roles of antibodies versus a durable, high quality T-cell response in protective immunity against SARS-CoV-2? Vaccine X. 2020;6:100076
98. Grifoni A, Sidney J, Vita R, Peters B, Crotty S, Weiskopf D. et al. SARS-CoV-2 human T cell epitopes: Adaptive immune response against COVID-19. Cell Host Microbe. 2021;29:1076-92
99. Tarke A, Sidney J, Methot N, Yu ED, Zhang Y, Dan JM. et al. Impact of SARS-CoV-2 variants on the total CD4(+) and CD8(+) T cell reactivity in infected or vaccinated individuals. Cell Rep Med. 2021;2:100355
100. Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR. et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell. 2020;181:1489-501 e15
101. Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol. 2022;23:186-93
102. Naranbhai V, Nathan A, Kaseke C, Berrios C, Khatri A, Choi S. et al. T cell reactivity to the SARS-CoV-2 Omicron variant is preserved in most but not all prior infected and vaccinated individuals. medRxiv. 2022
103. GeurtsvanKessel CH, Geers D, Schmitz KS, Mykytyn AZ, Lamers MM, Bogers S. et al. Divergent SARS CoV-2 Omicron-reactive T- and B cell responses in COVID-19 vaccine recipients. Sci Immunol. 2022: eabo2202.
104. Woldemeskel BA, Garliss CC, Aytenfisu TY, Johnston TS, Beck EJ, Dykema AG. et al. SARS-CoV-2 -specific immune responses in boosted vaccine recipients with breakthrough infections during the Omicron variant surge. JCI Insight. 2022
105. Keeton R, Tincho MB, Ngomti A, Baguma R, Benede N, Suzuki A. et al. T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature. 2022
106. Atmar RL, Lyke KE, Deming ME, Jackson LA, Branche AR, El Sahly HM. et al. Homologous and Heterologous Covid-19 Booster Vaccinations. N Engl J Med. 2022;386:1046-57
107. Collier AY, Yu J, McMahan K, Liu J, Chandrashekar A, Maron JS. et al. Differential Kinetics of Immune Responses Elicited by Covid-19 Vaccines. N Engl J Med. 2021;385:2010-2
108. Liu J, Chandrashekar A, Sellers D, Barrett J, Jacob-Dolan C, Lifton M. et al. Vaccines Elicit Highly Conserved Cellular Immunity to SARS-CoV-2 Omicron. Nature. 2022
109. Chandrashekar A, Yu J, McMahan K, Jacob-Dolan C, Liu J, He X. et al. Vaccine protection against the SARS-CoV-2 Omicron variant in macaques. Cell. 2022
110. Gao Y, Cai C, Grifoni A, Muller TR, Niessl J, Olofsson A. et al. Ancestral SARS-CoV-2-specific T cells cross-recognize the Omicron variant. Nat Med. 2022;28:472-6
111. Cohen H, Rotem S, Elia U, Bilinsky G, Levy I, Chitlaru T, et al. T Cell Response following Anti-COVID-19 BNT162b2 Vaccination Is Maintained against the SARS-CoV-2 Omicron B.1.1.529 Variant of Concern. Viruses. 2022; 14
112. Adamo S, Michler J, Zurbuchen Y, Cervia C, Taeschler P, Raeber ME. et al. Signature of long-lived memory CD8(+) T cells in acute SARS-CoV-2 infection. Nature. 2022;602:148-55
113. Mok CKP, Cohen CA, Cheng SMS, Chen C, Kwok KO, Yiu K. et al. Comparison of the immunogenicity of BNT162b2 and CoronaVac COVID-19 vaccines in Hong Kong. Respirology. 2021
114. Jara A, Undurraga EA, Gonzalez C, Paredes F, Fontecilla T, Jara G. et al. Effectiveness of an Inactivated SARS-CoV-2 Vaccine in Chile. N Engl J Med. 2021;385:875-84
115. Tanriover MD, Doganay HL, Akova M, Guner HR, Azap A, Akhan S. et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398:213-22
116. Redd AD, Nardin A, Kared H, Bloch EM, Abel B, Pekosz A. et al. Minimal Crossover between Mutations Associated with Omicron Variant of SARS-CoV-2 and CD8(+) T-Cell Epitopes Identified in COVID-19 Convalescent Individuals. mBio. 2022: e0361721.
117. McMenamin ME, Nealon J, Lin Y, Wong JY, Cheung JK, Lau EHY. et al. Vaccine effectiveness of two and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong. medRxiv. 2022. 2022 03.22.22272769
118. Kuloğlu ZE, El R, Guney-Esken G, Tok Y, Talay ZG, Barlas T. et al. Effect of BTN162b2 and Coronavac boosters on humoral and cellular immunity of individuals previously fully vaccinated with Coronavac against SARS-CoV-2: A longitudinal study. Allergy. 2022
119. Jiang M, Liu J, Yang D, Tross D, Li P, Chen F. et al. A TNFR2 antibody by countering immunosuppression cooperates with HMGN1 and R848 immune stimulants to inhibit murine colon cancer. Int Immunopharmacol. 2021;101:108345
120. Ong E, Wong MU, Huffman A, He YQ. COVID-19 Coronavirus Vaccine Design Using Reverse Vaccinology and Machine Learning. Front Immunol. 2020 11
121. Dutta NK, Mazumdar K, Gordy JT. The Nucleocapsid Protein of SARS-CoV-2: a Target for Vaccine Development. J Virol. 2020 94
122. Oliveira SC, de Magalhaes MTQ, Homan EJ. Immunoinformatic Analysis of SARS-CoV-2 Nucleocapsid Protein and Identification of COVID-19 Vaccine Targets. Front Immunol. 2020;11:587615
123. Cele S, Jackson L, Khoury DS, Khan K, Moyo-Gwete T, Tegally H. et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. 2021
124. Kotaki R, Adachi Y, Moriyama S, Onodera T, Fukushi S, Nagakura T. et al. SARS-CoV-2 Omicron-neutralizing memory B-cells are elicited by two doses of BNT162b2 mRNA vaccine. Sci Immunol. 2022: eabn8590.
Author contact
![]() Corresponding author: Prof. Xin Chen, Institute of Chinese Medical Sciences, University of Macau, Avenida da Universidade, Taipa, Macau SAR 999078, China. Tel.: (853) 8822 4513; Fax: (853) 2884 1358. E-mail address: xchenedu.mo; ORCID: http://orcid.org/0000-0002-2628-4027
Corresponding author: Prof. Xin Chen, Institute of Chinese Medical Sciences, University of Macau, Avenida da Universidade, Taipa, Macau SAR 999078, China. Tel.: (853) 8822 4513; Fax: (853) 2884 1358. E-mail address: xchenedu.mo; ORCID: http://orcid.org/0000-0002-2628-4027

 Global reach, higher impact
Global reach, higher impact