10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(12):4756-4767. doi:10.7150/ijbs.72461 This issue Cite
Review
COVID-19 Pandemic: Insights into Interactions between SARS-CoV-2 Infection and MAFLD
1. Cancer Center, Faculty of Health Sciences, University of Macau, Taipa, Macau SAR, China.
2. Centre for Precision Medicine Research and Training, Faculty of Health Sciences, University of Macau, Taipa, Macau SAR, China.
3. MOE Frontier Science Centre for Precision Oncology, University of Macau, Taipa, Macau SAR, China.
Received 2022-2-28; Accepted 2022-6-23; Published 2022-7-11
Abstract
COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has become an ongoing global health pandemic. Since 2019, the pandemic continues to cast a long shadow on all aspects of our lives, bringing huge health and economic burdens to all societies. With our in-depth understanding of COVID-19, from the initial respiratory tract to the later gastrointestinal tract and cardiovascular systems, the multiorgan involvement of this infectious disease has been discovered. Metabolic dysfunction-associated fatty liver disease (MAFLD), formerly named nonalcoholic fatty liver disease (NAFLD), is a major health issue closely related to metabolic dysfunctions, affecting a quarter of the world's adult population. The association of COVID-19 with MAFLD has received increasing attention, as MAFLD is a potential risk factor for SARS-CoV-2 infection and severe COVID-19 symptoms. In this review, we provide an update on the interactions between COVID-19 and MAFLD and its underlying mechanisms.
Keywords: COVID-19, SARS-CoV-2, MAFLD, NAFLD, Liver injury
Introduction
The coronavirus outbreak, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), led to the COVID-19 pandemic and has placed an unprecedented burden on our world, including health systems, social life, economics, and infrastructure [1, 2]. SARS-CoV-2, belonging to the Coronaviridae family with SARS-CoV and Middle East respiratory syndrome-related coronavirus (MERS-CoV), consists of a positive single-stranded RNA genome of approximately 30 kb [3-5]. SARS-CoV-2 shares 82% genome sequence similarity with SARS-CoV and 50% genome sequence homology with MERS-CoV [6]. As of the time of writing, there have been 528.8 million confirmed cases worldwide according to the WHO Coronavirus (COVID-19) Dashboard, and this number continues to increase. Statistically, approximately 119 people per 10,000 people die of COVID-19, which is possibly 10 times or more than the mortality rate of influenza. COVID-19 can cause multiple organ involvement in the body, and its symptoms vary from person to person, normally including fever, headache, and dyspnea [7, 8]. Symptoms of gastrointestinal involvement, such as nausea, vomiting, and diarrhea, and dysfunction of taste and smell have also been reported [9-11]. Approximately 5% of patients experience severe symptoms such as respiratory failure, shock and multiple organ dysfunction [12]. More than 50% of COVID-19 patients have reported underlying medical conditions, and approximately one-third have multiple comorbidities [13], which is associated with the high mortality of COVID-19.
Metabolic dysfunction-associated fatty liver disease (MAFLD), formerly known as nonalcoholic fatty liver disease (NAFLD), has been recently recognized by global experts [14, 15]. MAFLD is the most common cause of chronic liver disease and it affects approximately 25% of the adult population worldwide [16, 17]. The prevalence of MAFLD is expected to increase by 30% by 2030 as the prevalence of obesity, diabetes and metabolic syndrome continues to grow, as well as changes in people's lifestyle and eating habits continue [18]. MAFLD is characterized by hepatic steatosis with metabolic dysfunction. Without timely intervention, MAFLD may progress from nonalcoholic steatohepatitis (NASH) to liver cirrhosis and eventually hepatocellular carcinoma (HCC) [19, 20]. Liver injury from both SARS-CoV and MERS-CoV has been reported in past SARS and MERS pandemics [21-24]. Unlike the heart, lung, and gastrointestinal damage caused by SARS-CoV-2, the clinical significance of liver involvement has been controversial since the beginning of the COVID-19 pandemic [6, 25-30]. Increasing evidence has indicated that hepatic microvesicular steatosis and elevations in transaminase and bilirubin occur in COVID-19 patients. It is plausible to believe there is a link between SARS-CoV-2 infection and MAFLD, which has been proposed and discussed in previous studies [31-33]. In this review, we provide an update on the epidemiology of COVID-19 patients with liver dysfunction, its potential mechanisms, and the management approaches for MAFLD.
COVID-19 and liver dysfunction
Although the exact effect of COVID-19 on the liver is currently unclear, it can be noted that liver biochemistry abnormalities appear to be common in COVID-19 patients, affecting approximately 17-58% of individuals with COVID-19 [34-48] (Table 1). Liver biochemistry abnormalities manifest primarily as mild and moderate elevations of alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) in the early stages of the disease [25, 48-50]. A small number of patients may have increased serum bilirubin levels and decreased serum albumin levels, and rarely, increased levels of markers of bile duct damage, such as alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), and total bilirubin (TBIL) [51-53]. This suggests that liver injury caused by SARS-CoV-2 infection is mainly hepatocyte damage rather than cholestasis.
In addition to the liver function indices, histopathological examination of COVID-19 patients also suggests a link between COVID-19 and liver dysfunction. The first autopsy report of a patient who died of severe SARS-CoV-2 infection showed moderate microvesicular steatosis and mild lobular and portal venous activity in liver tissue [54]. Subsequently, other COVID-19 autopsies showed similar results, with Kupffer cell activation observed in addition to hepatic steatosis and vascular changes [35, 55-57]. In addition, liver biopsies from a cohort of 48 deceased COVID-19 patients revealed extensive luminal thrombosis at the portal and sinusoidal levels, as well as portal fibrosis with marked pericyte activation [58].
COVID-19 patients also exhibited more severe liver dysfunction [6, 48]. In an initial clinical investigation based on 99 cases, one COVID-19 patient developed severe hepatitis with ALT and AST levels of 7,590 U/L and 1,445 U/L, respectively [49]. Liver injury is much worse in severe COVID-19 patients than in patients with mild symptoms [25]. This suggests that comorbidity with liver disease in patients with SARS-CoV-2 infection could exacerbate COVID-19 disease and even lead to death. Singh et al. investigated the interaction of preexisting liver disease and COVID-19. Based on a large, diverse cohort of 2,780 COVID-19 patients in the United States, this study indicated that liver abnormalities were found in the vast majority of patients regardless of preexisting liver disease, but patients with liver disease were at higher risk for hospitalization and mortality [59]. Moreover, although abnormal liver function in most COVID-19 patients recovers after the course of the disease, permanent liver damage has been reported in severe COVID-19 cases [6, 49, 60]. As MAFLD is the most common cause of chronic liver disease, more attention needs to be paid to patients with MAFLD, not only to prevent these patients from being infected with SARS-CoV-2 but also to follow up the outcomes of infected patients.
Liver biochemistry abnormalities of COVID-19 patients
| Study | Region | Sample collection date | Sample size (n) | Elevated ALT | Elevated AST | Elevated ALP | Elevated GGT | Elevated TBIL |
|---|---|---|---|---|---|---|---|---|
| Guan[48] | Nationwide, China | 2019/12/11-2020/01/29 | 722-757 | 158/741 (21.3%) | 168/757 (22.2%) | NA | NA | 76/722 (10.5%) |
| Zhang[34] | Wuhan, China | 2019/12/29-2020/02/16 | 267 | 49 (18.4%) | 76 (28.5%) | NA | NA | NA |
| Cai[35] | Shenzhen, China | 2020/01/11-2020/02/21 | 417 | 54 (12.9%) | 76 (18.2%) | 101 (24.2%) | 68 (16.3%) | 99 (23.7%) |
| Xu[36] | Shanghai, China | 2020/01/20-2020/10/20 | 1003 | 295 (29.4%) | 176 (17.5%) | 26 (2.6%) | 134 (13.4%) | 40 (4.0%) |
| Ding[37] | Wuhan, China | 2020/01/28-2020/04/25 | 2073 | 501 (24.2%) | 545 (26.3%) | 165 (8.0%) | 443 (21.4%) | 71 (3.4%) |
| Fu[38] | Wuhan, China | 2020/02/01-2020/02/20 | 482 | 96 (19.9%) | 98 (20.3%) | NA | NA | 23 (4.8%) |
| Lv[39] | Wuhan, China | 2020/02/05-2020/03/23 | 2912 | 662 (22.7%) | 221 (7.5%) | 135 (4.6%) | 536 (18.4%) | 52 (1.8%) |
| Benedé-Ubieto[40] | Madrid, Spain | 2020/02/25-2020/04/23 | 799 | 204 (25.73%) | 446 (49.17%) | 186 (24.21%) | 270 (34.62%) | NA |
| Richardson[43] | New York, America | 2020/03/01-2020/04/04 | 5700 | 2176 (39.0%) | 3263 (58.4%) | NA | NA | NA |
| Weber[41] | Munich, Germany | 2020/03-2020/07 | 217 | 59 (27.2%) | 91 (41.9%) | 22 (10.1%) | 80 (36.9%) | 10 (4.6%) |
SARS-CoV-2 tropism of the liver
The extent to which organ-specific pathology correlates with direct viral replication or consequent immune and cardiovascular complications is clinically relevant. SARS-CoV-2 preferentially infects respiratory cells, but it can be detected in multiple other organs, including the liver [61-63], indicating a multiple-organ tropism of SARS-CoV-2. Notably, there are differences in the viral load and distribution across patients [7], which may require data from larger cohorts to illustrate the distribution of SARS-CoV-2 in different organs.
The establishment of viral tropism depends on the susceptibility and permissiveness of a particular host cell. Studies have found that the entry of SARS-CoV-2 into cells is dependent on the expression of angiotensin-converting enzyme 2 (ACE2) [64, 65]. The receptor-binding domain (RBD) of the SARS-CoV-2 spike (S) protein has a high affinity for ACE2, and to achieve its function, SARS-CoV-2 binds to ACE2 to target cells [66]. Transmembrane serine protease 2 (TMPRSS2) in host cells further facilitates viral uptake by cleaving ACE2 and activating the SARS-CoV-2 S protein [67]. After attachment and cleavage of the S protein, SARS-CoV-2 is internalized through endocytosis, and the viral genome is released from the endosome [8, 68, 69]. Other host proteases, such as FURIN, are also thought to facilitate processing of the S protein [70]. Therefore, the expression of host cell proteins that contribute to SARS-CoV-2 infection provides early clues to speculate on the liver tropism of SARS-CoV-2.
To assess the expression of SARS-CoV-2 entry factors in liver tissue and their distribution across cell types, single-cell RNA sequencing (scRNA-seq) analysis was performed based on published single-cell datasets [71-74]. These studies indicate that ACE2 expression can be found on hepatocytes, but it is mainly expressed by liver cholangiocytes. This finding is consistent with the expression pattern of ACE2 in the liver and gallbladder in the Human Protein Atlas (https://www.proteinatlas.org/). In addition, TMPRSS2 and FURIN can also be detected in the liver and are broadly expressed rather than on specific cell types [72]. Notably, entry of SARS-CoV-2 into cells may be dependent on the expression of TMPRSS2. As long as TMPRSS2 is present, low or barely detectable levels of ACE2 still support SARS-CoV-2 entry into cells [75]. Therefore, one study analyzed the coexpression of ACE2 and TMPRSS2 based on three human liver single-cell transcriptomic datasets and found that very few hepatocytes (0.03-0.04%) coexpressed ACE2 and TMPRSS2 [76]. Moreover, another study showed that coexpression of ACE2 and TMPRSS2 only occurred in TROP2+ cholangiocyte-biased progenitor cells [77]. These scRNA-seq-based studies suggest that SARS-CoV-2 might preferentially infect cholangiocytes and cause damage to the bile ducts, but it is difficult to explain why the pattern of liver injury in COVID-19 patients is mainly hepatocellular rather than cholestatic [57]. Notably, transmission electron microscopy (TEM) revealed the presence of intact SARS-CoV-2 virus particles in the cytoplasm of hepatocytes from patients with COVID-19, which is direct evidence of SARS-CoV-2 infection of hepatocytes [55]. Therefore, analysis of the expression of these host genes may underestimate the hepatic tropism of SARS-CoV-2 due to technical limitations of scRNA-seq.
Experimental cell and organoid models are of great significance for exploring the tropism of SARS-CoV-2 to different types of cells in the liver and its mechanism. It has been reported that the complete SARS-CoV-2 life cycle is observed in both the liver cancer cell lines Huh-7 and HepG2, supporting the infection and replication of the virus in hepatocytes [78-80]. In addition, human primary hepatocytes and pluripotent stem cell-derived liver organoids also exhibit a permissive effect of hepatocytes for SARS-CoV-2 [81]. These studies provide favorable evidence for the hepatic tropism of SARS-CoV-2, suggesting that SARS-CoV-2 infection may directly lead to hepatocyte damage. Consistent with scRNA-seq data from liver tissue, human liver ductal organoids expressed ACE2 and TMPRSS2 and were infected by SARS-CoV-2, which impaired the function of cholangiocytes [82]. As current evidence has not found other resident cells in the liver, such as hepatic stellate cells and Kupffer cells, that express ACE2 [56, 76, 83], it is suggested that SARS-CoV-2 mainly enters and infects hepatocytes and cholangiocytes, causing liver dysfunction.
Interplay between MAFLD and COVID-19
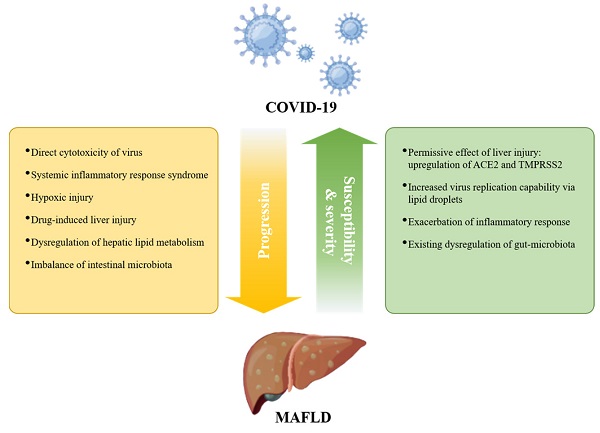
It has been shown that the presence of preexisting liver disease affects the prognosis of COVID-19 [59], but the impact of MAFLD on COVID-19 progression has been controversial [84-86]. Since MAFLD is a chronic disease affected by multiple factors and other metabolic dysfunctions, this inconsistency may be due to the extensive interaction of MAFLD with other metabolic comorbidities. Such confounding factors, as well as differences in diagnostic criteria, may lead to difficulties in analyzing risk factors for the COVID-19 pandemic [47, 87]. Nonetheless, obesity, hyperglycemia/diabetes, and cardiovascular disease have been identified as distinct risk factors for adverse clinical outcomes in COVID-19 [44, 62, 88]. The incidence of MAFLD in patients with COVID-19 is higher than that in the general population [89, 90], suggesting that MAFLD contributes to enhanced susceptibility to COVID-19. Furthermore, the organoid culture system provides direct evidence for NASH-enhanced permissive effects for SARS-CoV-2 [91]. ACE2 was upregulated upon experimental liver injury [92, 93], and hepatic ACE2 and TMPRSS2 expression were increased in MAFLD patients [94]. These findings possibly provide a mechanistic explanation for the increased susceptibility of MAFLD patients to SARS-CoV-2 (Figure 1).
Given the central role of the liver in the production of albumin, acute-phase reactants, and coagulation factors, liver dysfunction may affect multisystem manifestations of COVID-19, such as acute respiratory distress syndrome (ARDS), coagulation disorders, and multiorgan failure [6, 8, 24, 95-97], thereby exacerbating symptoms of COVID-19. MAFLD is an independent risk factor for severe COVID-19 in patients aged less than 60 years [57, 98-100]. MAFLD patients with coexisting obesity had a more than 6-fold increased risk of severe COVID-19 [101], and further studies indicated that the association of MAFLD with COVID-19 severity is independent of obesity or diabetes [102, 103]. In addition, one recent retrospective study with 359 COVID-19 patients found that the death rate and intubation rate were significantly higher in MAFLD patients than in the control group [104]. Liver function abnormalities and a longer viral shedding time caused by MAFLD contribute to COVID-19 progression [57], and the effect is more pronounced in MAFLD patients with advanced fibrosis [105-109]. Furthermore, an increased neutrophil-to-lymphocyte ratio (NLR) in MAFLD may exacerbate SARS-CoV-2-induced inflammatory storms, thereby aggravating COVID-19 symptoms [110].
However, a recent study demonstrated that while MAFLD may be associated with severe COVID-19 at the population level, MAFLD is not a causal risk factor for severe COVID-19 based on two-sample Mendelian randomization (TSMR) analysis [111]. The authors mentioned that their results may be limited by the small sample size and other unknown clinical covariates [111]. In addition, TSMR studies consider the lifetime effects of genetic variation rather than short-term measurements of specific parameters, and in some cases Mendelian randomization and its biological plausibility may not hold. These may be the reasons for the inconsistent results. Nevertheless, we need to draw more attention to the association of MAFLD and its comorbidities with COVID-19 progression (Figure 1).
Interplay between COVID-19 and MAFLD. MAFLD may increase the susceptibility and severity of severe COVID-19; in turn, COVID-19 may promote the progression of preexisting MAFLD.
In turn, SARS-CoV-2 infection also aggravates MAFLD progression (Figure 1). Studies have shown that SARS-CoV-2 promotes metabolic complications at the systemic and organ levels, including hyperglycemia, hypertension, and low high-density lipoprotein cholesterol (HDL-C), in patients without preexisting metabolic disease [112, 113]. A study describing the clinical characteristics of patients with combined MAFLD and COVID-19 found more severe liver injury in MAFLD patients with COVID-19 than in uninfected MAFLD patients [114]. Moreover, another follow-up of 235 discharged patients with COVID-19 found that the prevalence of MAFLD at follow-up was 55.3%, which was greater than the prevalence of MAFLD at admission (37.3%) [115]. Therefore, special attention should be given to the management of patients with MAFLD during the COVID-19 pandemic. Next, we will focus on summarizing the possible molecular mechanisms by which COVID-19 affects MAFLD.
Potential mechanism for COVID-19 promoting MAFLD progression
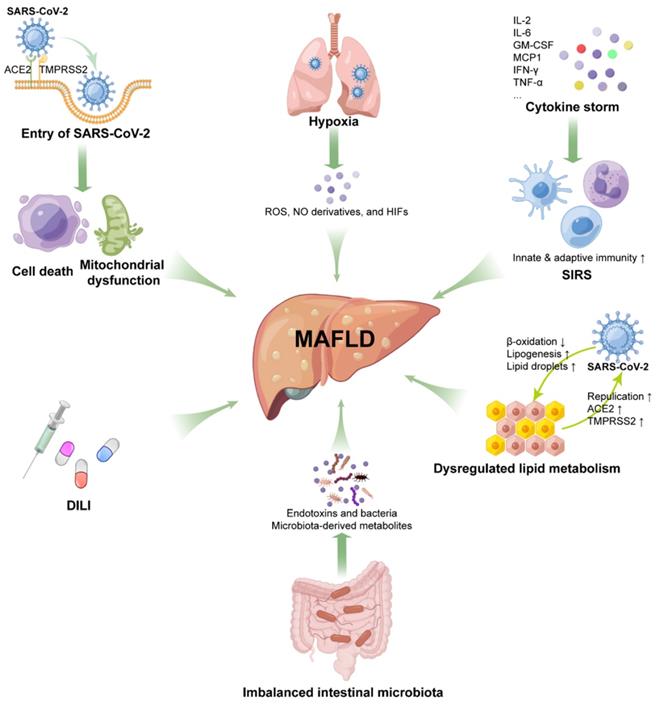
Direct cytotoxicity of virus
SARS-CoV-2 in the intestinal lumen can be transferred to the liver through portal blood flow, enter cells through the ACE2 receptor and actively replicate to induce direct damage to the liver [33]. Studies have shown that SARS-CoV-2 can induce apoptosis of infected cells [116, 117]. In situ hybridization analysis has revealed SARS-CoV-2 virions in the vascular lumen and portal endothelial cells of COVID-19 liver specimens [58], and TEM also found the presence of intact virions in the cytoplasm of hepatocytes [55]. Liver injury in COVID-19 patients is associated with the SARS-CoV-2 viral load, suggesting that the persistence of the virus may also cause direct damage to the liver [118]. It is known that impaired mitochondrial activity is associated with the pathogenesis of NAFLD/NASH [119]. Based on transcriptomic analysis and ultrastructural examination, SARS-CoV-2 infection suppressed hepatic mitochondrial activity and caused obvious mitochondrial swelling of hepatocytes [55, 120], strongly suggesting that SARS-CoV-2 directly causes cytopathic effects and contributes to MAFLD progression.
Systemic inflammatory response syndrome (SIRS)
There is increasing evidence that patients with severe COVID-19 have cytokine storm syndrome [121]. Elevated inflammatory biomarkers, such as C-reactive protein (CRP), ferritin, lactate dehydrogenase (LDH), D-dimer, IFN-γ, TNF-α, interleukin (IL)-2, IL-6, and monocyte chemoattractant protein-1 (MCP1), have been reported in severe COVID-19 patients [25, 122, 123]. MAFLD is thought to be related to innate immune-mediated inflammation. According to recent knowledge about cytokine storm syndrome in COVID-19 patients, we propose that MAFLD may be exacerbated by the inflammatory response of COVID-19. First, IL-6 levels are elevated in MAFLD patients and associated with hepatic steatosis [124, 125]. After SARS-CoV-2 infection, granulocyte-macrophage colony-stimulating factor (GM-CSF), produced by pathogenic T-cell activation, can activate CD14+CD16+ inflammatory monocytes to produce large amounts of IL-6 and other proinflammatory factors, which may contribute to MAFLD progression [126]. Second, MCP1 is also involved in aggravating steatohepatitis [127]. A recent study identified specific CD16+ T cells with enhanced cytotoxicity in severe COVID-19, which promote microvascular endothelial cell injury and the release of chemokines, including MCP1 [128]. Third, the liver contains the largest population of all tissue-resident macrophages (Kupffer cells), which contribute to the development of liver disease. In response to SARS-CoV-2 infection, lung resident macrophages can activate inflammasomes, secrete IL-1 and IL-18, and undergo pyroptosis, thereby leading to a hyperinflammatory state [129], indicating that the inflammasome-activated inflammatory response in Kupffer cells may also promote the progression of MAFLD. Fourth, cGAS-STING signaling-induced IFN has been shown to play an essential role in the development and progression of MAFLD [130]. SARS-CoV-2 infection induces cGAS-STING activation in endothelial cells through mitochondrial DNA release, resulting in cell death and type I IFN production [131]. As noted above, SARS-CoV-2 infection caused mitochondrial swelling in hepatocytes, suggesting that activation of cGAS-STING signaling may exacerbate MAFLD in COVID-19 patients.
Notably, autoimmune hepatitis symptoms have been described following SARS-CoV-2 vaccination [132-134]. SARS-CoV-2 infection elicits not only innate but also adaptive immune responses. It has been reported that commercial monoclonal antibodies against SARS-CoV-2 spike protein or nucleoprotein can cross-react with human tissue antigens [135, 136], which suggests that immune cross-reactivity may cause SARS-CoV-2 infection or vaccine-induced autoimmunity. A recent study also found that SARS-CoV-2-specific activated CD8 T cells were enriched in the liver after SARS-CoV-2 vaccination, thereby leading to hepatitis [137]. These studies suggest that in addition to the innate immune response caused by SARS-CoV-2 infection, the adaptive immune response may also contribute to the severity of MAFLD.
Hypoxic injury
In addition to SIRS, severe COVID-19 patients often experience other severe complications, including ARDS and multiple organ failure, which can cause hypoxia and shock, leading to liver ischemia and hypoxia [138]. Hepatic hypoxia in COVID-19 patients may lead to increased levels of ROS, NO derivatives, and hypoxia-inducible factors (HIFs) [33, 139, 140]. Increased HIFs in COVID-19 patients can promote obesity and insulin resistance [141], which are important risk factors for MAFLD. HIF-1α has been reported to have the potential to promote MAFLD development [142]. Moreover, hypoxia-induced HIF-2α overexpression aggravated MAFLD progression by inhibiting fatty acid β-oxidation and inducing lipogenesis in the liver through PPARα [143]. Therefore, such changes in severe COVID-19 patients may further exacerbate the progression of MAFLD.
Drug-induced liver injury (DILI)
At the beginning of the COVID-19 outbreak, there was no evidence-based drug treatment. Various drugs are used clinically to combat COVID-19, such as antiviral drugs (remdesivir, lopinavir/ritonavir), antibiotics (macrolides), antimalarial/antirheumatic drugs (hydroxychloroquine), immunomodulatory drugs (corticosteroids, tocilizumab) and anti-fever medications (acetaminophen), but many of these drugs have been considered to be hepatotoxic [144, 145]. The use of lopinavir and ritonavir has been reported to be independently associated with elevated ALT/AST in patients with COVID-19 [146]. The presence of underlying metabolic abnormalities and MAFLD can contribute to DILI [30, 57]; on the other hand, MAFLD also aggravates the hepatotoxicity of drugs such as acetaminophen, promoting the progression of MAFLD to NASH and even cirrhosis [144]. Corticosteroid, the recommended drug for severe COVID-19, is also clearly associated with steatosis or glycogen deposition [145]. Therefore, for patients with chronic liver disease, the risk of liver injury should be considered when choosing medications to treat COVID. The use of drugs with high hepatotoxicity may promote the progression of MAFLD.
Dysregulation of hepatic lipid metabolism
In the infectious state, activated innate immunity not only directly triggers and amplifies liver inflammation but also interferes with the regulation of lipid metabolism, thereby promoting the development of liver fibrosis in MAFLD/NASH patients [147]. Proteomic and metabolomic analyses revealed dyslipidemia in COVID-19 patients, such as lipid accumulation and downregulation of apolipoproteins [148, 149]. In turn, it was found that SARS-CoV-2 infection can modulate pathways of lipid synthesis and uptake, thereby increasing lipid droplet (LD) accumulation in human cells. Meanwhile, SARS-CoV-2 can highjack LDs to enhance its replication capacity [150]. Mechanistically, recent studies have indicated that ACE2 plays an important role in maintaining metabolic homeostasis. SARS-CoV-2 infection impairs ACE2 expression, which in turn induces metabolic abnormalities [151]. This metabolic imbalance caused by ACE2 impairment may promote MAFLD progression in COVID-19 patients.
Imbalance of intestinal microbiota
The gastrointestinal tract is not only the primary habitat for human microbiota but is also a target for SARS-CoV-2 infection, as it expresses high levels of ACE2 and TMPRSS2 [152]. There is increasing evidence that disruption of the microbiota balance during COVID-19 is associated with disease severity and mortality [153]. The intestine has an interaction with the liver through the liver-gut axis, and the intestinal microbiota plays an important role in MAFLD progression [19, 154], suggesting that intestinal dysbiosis may contribute to the severity of MAFLD during COVID-19.
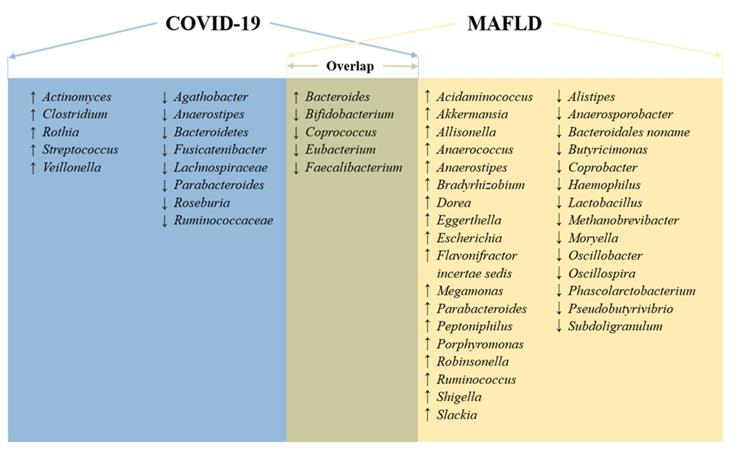
Based on a pilot study of 15 patients with COVID-19, alterations in intestinal microbiota and their association with susceptibility to severe disease have been reported [155]. Compared to the microbiota of the healthy group, anti-inflammatory bacteria such as Eubacterium ventriosum, Faecalibacterium prausnitzii, Roseburia, and Lachnospiraceae decreased, but opportunistic pathogens Clostridium hathewayi, Actinomyces viscosus, and Bacteroides nordii increased in patients with COVID-19. A study of 30 COVID-19 patients revealed that COVID-19 patients have a higher abundance of opportunistic pathogens, such as Streptococcus, Rothia, Veillonella and Actinomyces, and a lower abundance of beneficial symbionts [156]. Another cohort (62 COVID-19 patients) also found that Roseburia and Faecalibacterium decreased, while Clostridium and Streptococcus increased in COVID-19 patients [157]. Furthermore, a larger cohort (100 COVID-19 patients) from two hospitals showed that several gut commensals with known immunomodulatory potential, such as Faecalibacterium prausnitzii, Eubacterium rectale and several bifidobacterial species, were depleted in patients, and this perturbed composition in patients with COVID-19 is concordant with disease severity [158]. These studies indicate enrichment of opportunistic pathogens and depletion of commensals in the intestinal microbiota of COVID-19 patients.
According to a recent review about the relationship between intestinal microbiota and NAFLD in humans, we found that both diseases shared common altered bacteria, such as Bacteroides, Eubacterium, Faecalibacterium, Coprococcus, and Bifidobacterium (Figure 2) [159]. Therefore, intestinal dysbiosis can cause intestinal and liver inflammation through translocation of endotoxins and bacteria due to increased intestinal permeability. Then, intestinal dysbiosis-induced hepatic inflammation, together with the SIRS mentioned above, further exacerbates MAFLD.
Moreover, intestinal commensal-derived metabolites are involved in the development and progression of MAFLD, fibrosis and cirrhosis [159]. For instance, lactate, ethanol, lipopolysaccharide (LPS), and trimethyl N-oxide (TMAO) can accelerate MAFLD progression, while short-chain fatty acids (SCFAs), such as acetate, proprionate, and butyrate, can have anti-inflammatory properties, thus preventing the progression of MAFLD [159]. According to a hamster model, the abundance of bacteria known to produce SCFAs, such as Ruminococcaceae and Lachnospiraceae, was reduced following SARS-CoV-2 infection, as was the amount of systemic SCFAs [160]. Although this was obtained from animal experiments, it reflects that alterations in microbial metabolites caused by disturbances in the intestinal microbiota can affect the risk of COVID-19. On the other hand, intestinal dysbiosis is also present in MAFLD, which leads to changes in the levels of bacterial metabolites such as butyrate and bile acids through the liver-gut axis [161]. Consequently, there is an increased risk of local and systemic low-grade inflammation and decreased anti-inflammatory capacity in the intestine, thereby increasing the severity of COVID-19.
In short, SARS-CoV-2 infection can promote the occurrence and development of MAFLD through multiple pathways (Figure 3). As the research moves along, more underlying mechanisms will be discovered.
Overlapping microbiota and general signatures in COVID-19 and MAFLD. Microbiota with an up arrow was found to be more abundant in patients with MAFLD or COVID-19 than in healthy individuals, and vice versa.
Potential mechanisms for MAFLD progression under SARS-CoV-2 infection. Direct cytotoxicity of SARS-CoV-2, hypoxia-mediated liver injury, drug-induced liver injury, systemic inflammatory response syndrome (SIRS), dysregulated lipid metabolism, and imbalanced intestinal microbiota are involved in MAFLD initiation and progression.
Conclusions and perspectives
Numerous clinical studies have indicated that liver dysfunction is a common feature in COVID-19 patients and correlates with disease severity, suggesting an interaction between MAFLD and COVID-19, not only in that MAFLD contributes to the susceptibility and severity of COVID-19 but also in that COVID-19 may induce and accelerate the progression of MAFLD (Figure 1). Most existing studies are retrospective studies, which may be affected by confounding factors such as small sample size, limited survey area, and differences in diagnostic criteria. A multisample, multicenter long-term follow-up study is also required to explore the long-term consequences of the potential association between MAFLD and SARS-CoV-2 infection. Undeniably, multiple basic and clinical studies have demonstrated the tropism of SARS-CoV-2 to the liver. While human genome integration of SARS-CoV-2 is still under debate [162, 163], we cannot ignore the long-term effects of its infection on our bodies, especially the liver. This review summarizes multiple potential mechanisms by which COVID-19 drives the progression of MAFLD, namely, direct damage from viral infection, systemic inflammatory response, hypoxic injury, drug-induced liver injury, dysregulation of liver metabolism, and intestinal dysbiosis. As the epidemic continues, we should strengthen the monitoring and management of COVID-19 patients with MAFLD.
The generally recommended management for patients with MAFLD is similar to that of healthy individuals. Meanwhile, enhanced personal protection [164] along with good lifestyle habits [165] (including weight loss advice, nutritional guidance, and diabetes management) may reduce the chance and severity of COVID-19 infection and slow the progression of liver injury. Considering the possible increased risk of severe COVID-19, the European Association for the Study of the Liver (EASL) recommends that all MAFLD patients infected with SARS-CoV-2 should receive standardized and timely diagnosis and treatment as soon as possible [166]. In addition, MAFLD patients often have other metabolic comorbidities, such as hyperglycemia, obesity, and hypertension, which are risk factors affecting the prognosis of COVID-19. Monitoring and management of these metabolic disorders can minimize the risk of a poor prognosis in MAFLD patients with SARS-CoV-2 infection [167]. As new therapeutic drugs are being developed for COVID-19, potential hepatotoxic drug-drug interactions need to be assessed, and timely adjustment of medications for patients with MAFLD may also reduce the risk of adverse outcomes.
In conclusion, the COVID-19 pandemic continues, and new variants of SARS-CoV-2, such as Omicron BA.1 and BA.2, are spreading more rapidly and aggressively. Since MAFLD is considered a growing chronic pandemic affecting a quarter of the global adult population, we need to pay more attention to COVID-19 patients with MAFLD and develop new therapeutic strategies, such as imatinib and methazolamide treatment [151], to improve metabolic complications caused by COVID-19. Moreover, we need to establish a long-term follow-up program to monitor the prognosis and incidence of liver cancer in these patients.
Acknowledgements
This work is supported by National Natural Science Foundation of China (81672603 and 81401978), startup research grant from the University of Macau (SRG2021-00008-FHS), and Macao Science and Technology Development Fund grant (0011/2019/AKP). The figures were created with Figdraw and BioRender.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Nicola M, Alsafi Z, Sohrabi C, Kerwan A, Al-Jabir A, Iosifidis C. et al. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int J Surg. 2020;78:185-93
2. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J. et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727-33
3. Marra MA, Jones SJM, Astell CR, Holt RA, Brooks-Wilson A, Butterfield YSN. et al. The Genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399-404
4. Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nat Med. 2020;26:450-2
5. de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14:523-34
6. Zhang C, Shi L, Wang F-S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-30
7. Hanley B, Naresh KN, Roufosse C, Nicholson AG, Weir J, Cooke GS. et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020;1:e245-e53
8. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020;324:782-93
9. Lechien JR, Chiesa-Estomba CM, Place S, Van Laethem Y, Cabaraux P, Mat Q. et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020;288:335-44
10. Mao R, Liang J, Shen J, Ghosh S, Zhu L-R, Yang H. et al. Implications of COVID-19 for patients with pre-existing digestive diseases. Lancet Gastroenterol Hepatol. 2020;5:425-7
11. Jin X, Lian J-S, Hu J-H, Gao J, Zheng L, Zhang Y-M. et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002-9
12. Control CfD, Prevention. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). 2020.
13. Thakur B, Dubey P, Benitez J, Torres JP, Reddy S, Shokar N. et al. A systematic review and meta-analysis of geographic differences in comorbidities and associated severity and mortality among individuals with COVID-19. Sci Rep. 2021;11:8562
14. Méndez-Sánchez N, Bugianesi E, Gish RG, Lammert F, Tilg H, Nguyen MH. et al. Global multi-stakeholder endorsement of the MAFLD definition. Lancet Gastroenterol Hepatol. 2022;7:388-90
15. Eslam M, Sanyal AJ, George J. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020 158
16. Marjot T, Moolla A, Cobbold JF, Hodson L, Tomlinson JW. Nonalcoholic Fatty Liver Disease in Adults: Current Concepts in Etiology, Outcomes, and Management. Endocr Rev. 2020 41
17. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M. et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20
18. Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J. et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69:896-904
19. Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18:223-38
20. Rinella ME, Sanyal AJ. Management of NAFLD: a stage-based approach. Nat Rev Gastroenterol Hepatol. 2016;13:196-205
21. Chau T-N, Lee K-C, Yao H, Tsang T-Y, Chow T-C, Yeung Y-C. et al. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39:302-10
22. Alsaad KO, Hajeer AH, Al Balwi M, Al Moaiqel M, Al Oudah N, Al Ajlan A. et al. Histopathology of Middle East respiratory syndrome coronovirus (MERS-CoV) infection - clinicopathological and ultrastructural study. Histopathology. 2018;72:516-24
23. Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID-19 and the liver. J Hepatol. 2020;73:1231-40
24. Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020 40
25. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506
26. Oyelade T, Alqahtani J, Canciani G. Prognosis of COVID-19 in Patients with Liver and Kidney Diseases: An Early Systematic Review and Meta-Analysis. Trop Med Infect Dis. 2020 5
27. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-62
28. Wu C, Chen X, Cai Y, Xia Ja, Zhou X, Xu S. et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934-43
29. Bangash MN, Patel J, Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5:529-30
30. Boeckmans J, Rodrigues RM, Demuyser T, Piérard D, Vanhaecke T, Rogiers V. COVID-19 and drug-induced liver injury: a problem of plenty or a petty point? Arch Toxicol. 2020;94:1367-9
31. Xu Y, Yang X, Bian H, Xia M. Metabolic dysfunction associated fatty liver disease and coronavirus disease 2019: clinical relationship and current management. Lipids Health Dis. 2021;20:126
32. Steenblock C, Schwarz PEH, Ludwig B, Linkermann A, Zimmet P, Kulebyakin K. et al. COVID-19 and metabolic disease: mechanisms and clinical management. Lancet Diabetes Endocrinol. 2021;9:786-98
33. Portincasa P, Krawczyk M, Smyk W, Lammert F, Di Ciaula A. COVID-19 and non-alcoholic fatty liver disease: Two intersecting pandemics. Eur J Clin Invest. 2020;50:e13338
34. Zhang J-J, Cao Y-Y, Tan G, Dong X, Wang B-C, Lin J. et al. Clinical, radiological, and laboratory characteristics and risk factors for severity and mortality of 289 hospitalized COVID-19 patients. Allergy. 2021;76:533-50
35. Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y. et al. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566-74
36. Xu W, Huang C, Fei L, Li Q, Chen L. Dynamic Changes in Liver Function Tests and Their Correlation with Illness Severity and Mortality in Patients with COVID-19: A Retrospective Cohort Study. Clin Interv Aging. 2021;16:675-85
37. Ding Z-Y, Li G-X, Chen L, Shu C, Song J, Wang W. et al. Association of liver abnormalities with in-hospital mortality in patients with COVID-19. J Hepatol. 2021;74:1295-302
38. Fu Y, Zhu R, Bai T, Han P, He Q, Jing M. et al. Clinical Features of Patients Infected With Coronavirus Disease 2019 With Elevated Liver Biochemistries: A Multicenter, Retrospective Study. Hepatology. 2021;73:1509-20
39. Lv Y, Zhao X, Wang Y, Zhu J, Ma C, Feng X. et al. Abnormal Liver Function Tests Were Associated With Adverse Clinical Outcomes: An Observational Cohort Study of 2,912 Patients With COVID-19. Front Med (Lausanne). 2021;8:639855 -
40. Benedé-Ubieto R, Estévez-Vázquez O, Flores-Perojo V, Macías-Rodríguez RU, Ruiz-Margáin A, Martínez-Naves E. et al. Abnormal Liver Function Test in Patients Infected with Coronavirus (SARS-CoV-2): A Retrospective Single-Center Study from Spain. J Clin Med. 2021;10:1039
41. Weber S, Hellmuth JC, Scherer C, Muenchhoff M, Mayerle J, Gerbes AL. Liver function test abnormalities at hospital admission are associated with severe course of SARS-CoV-2 infection: a prospective cohort study. Gut. 2021;70:1925-32
42. Sultan S, Altayar O, Siddique SM, Davitkov P, Feuerstein JD, Lim JK. et al. AGA Institute Rapid Review of the Gastrointestinal and Liver Manifestations of COVID-19, Meta-Analysis of International Data, and Recommendations for the Consultative Management of Patients with COVID-19. Gastroenterology. 2020 159
43. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW. et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052-9
44. Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A. et al. Clinical Characteristics of Covid-19 in New York City. N Engl J Med. 2020;382:2372-4
45. Youssef M, H Hussein M, Attia AS, M Elshazli R, Omar M, Zora G. et al. COVID-19 and liver dysfunction: A systematic review and meta-analysis of retrospective studies. J Med Virol. 2020;92:1825-33
46. Hundt MA, Deng Y, Ciarleglio MM, Nathanson MH, Lim JK. Abnormal Liver Tests in COVID-19: A Retrospective Observational Cohort Study of 1,827 Patients in a Major U.S. Hospital Network. Hepatology. 2020;72:1169-76
47. Marjot T, Webb GJ, Barritt AS, Moon AM, Stamataki Z, Wong VW. et al. COVID-19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol. 2021;18:348-64
48. Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X. et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-20
49. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y. et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-13
50. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J. et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-9
51. Elmunzer BJ, Spitzer RL, Foster LD, Merchant AA, Howard EF, Patel VA. et al. Digestive Manifestations in Patients Hospitalized With Coronavirus Disease 2019. Clin Gastroenterol Hepatol. 2021 19
52. Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX. et al. Acute Liver Injury in COVID-19: Prevalence and Association with Clinical Outcomes in a Large U.S. Cohort. Hepatology. 2020;72:807-17
53. Xie H, Zhao J, Lian N, Lin S, Xie Q, Zhuo H. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: A retrospective study. Liver Int. 2020;40:1321-6
54. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C. et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-2
55. Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L. et al. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807-16
56. Lax SF, Skok K, Zechner P, Kessler HH, Kaufmann N, Koelblinger C. et al. Pulmonary Arterial Thrombosis in COVID-19 With Fatal Outcome: Results From a Prospective, Single-Center, Clinicopathologic Case Series. Ann Intern Med. 2020;173:350-61
57. Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y. et al. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73:451-3
58. Sonzogni A, Previtali G, Seghezzi M, Grazia Alessio M, Gianatti A, Licini L. et al. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020;40:2110-6
59. Singh S, Khan A. Clinical Characteristics and Outcomes of Coronavirus Disease 2019 Among Patients With Preexisting Liver Disease in the United States: A Multicenter Research Network Study. Gastroenterology. 2020 159
60. An Y-W, Song S, Li W-X, Chen Y-X, Hu X-P, Zhao J. et al. Liver function recovery of COVID-19 patients after discharge, a follow-up study. Int J Med Sci. 2021;18:176-86
61. Adachi T, Chong J-M, Nakajima N, Sano M, Yamazaki J, Miyamoto I. et al. Clinicopathologic and Immunohistochemical Findings from Autopsy of Patient with COVID-19, Japan. Emerg Infect Dis. 2020 26
62. Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M. et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33:1007-14
63. Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L. et al. Multiorgan and Renal Tropism of SARS-CoV-2. N Engl J Med. 2020;383:590-2
64. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S. et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020 181
65. Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444-8
66. Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A. et al. Cell entry mechanisms of SARS-CoV-2. Proceedings of the National Academy of Sciences. 2020;117:11727
67. Glowacka I, Bertram S, Müller MA, Allen P, Soilleux E, Pfefferle S. et al. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol. 2011;85:4122-34
68. Perlman S, Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbiol. 2009;7:439-50
69. Wang H, Yang P, Liu K, Guo F, Zhang Y, Zhang G. et al. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008;18:290-301
70. Coutard B, Valle C, de Lamballerie X, Canard B, Seidah NG, Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Research. 2020;176:104742
71. Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185-92
72. Pirola CJ, Sookoian S. SARS-CoV-2 virus and liver expression of host receptors: Putative mechanisms of liver involvement in COVID-19. Liver Int. 2020;40:2038-40
73. Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A. et al. Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019-nCoV Infection. bioRxiv. 2020. 2020 02.03.931766
74. Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;526:135-40
75. Shulla A, Heald-Sargent T, Subramanya G, Zhao J, Perlman S, Gallagher T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J Virol. 2011;85:873-82
76. De Smet V, Verhulst S, van Grunsven LA. Single cell RNA sequencing analysis did not predict hepatocyte infection by SARS-CoV-2. J Hepatol. 2020;73:993-5
77. Seow JJW, Pai R, Mishra A, Shepherdson E, Lim TKH, Goh BKP. et al. Single-Cell RNA-seq Reveals Angiotensin-Converting Enzyme 2 and Transmembrane Serine Protease 2 Expression in TROP2(+) Liver Progenitor Cells: Implications in Coronavirus Disease 2019-Associated Liver Dysfunction. Front Med (Lausanne). 2021;8:603374
78. Chu H, Chan JF-W, Yuen TT-T, Shuai H, Yuan S, Wang Y. et al. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study. Lancet Microbe. 2020;1:e14-e23
79. Ou X, Liu Y, Lei X, Li P, Mi D, Ren L. et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11:1620
80. Harcourt J, Tamin A, Lu X, Kamili S, Sakthivel SK, Murray J. et al. Severe Acute Respiratory Syndrome Coronavirus 2 from Patient with Coronavirus Disease, United States. Emerg Infect Dis. 2020;26:1266-73
81. Yang L, Han Y, Nilsson-Payant BE, Gupta V, Wang P, Duan X. et al. A Human Pluripotent Stem Cell-based Platform to Study SARS-CoV-2 Tropism and Model Virus Infection in Human Cells and Organoids. Cell Stem Cell. 2020 27
82. Zhao B, Ni C, Gao R, Wang Y, Yang L, Wei J. et al. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020;11:771-5
83. Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-7
84. Valenti L, Jamialahmadi O, Romeo S. Lack of genetic evidence that fatty liver disease predisposes to COVID-19. J Hepatol. 2020;73:709-11
85. Ji D, Cheng G, Lau G. Reply to: "NAFLD is a predictor of liver injury in COVID-19 hospitalized patients but not of mortality, disease severity on the presentation or progression - The debate continues". J Hepatol. 2021;74:484-5
86. Mushtaq K, Khan MU, Iqbal F, Alsoub DH, Chaudhry HS, Ata F. et al. NAFLD is a predictor of liver injury in COVID-19 hospitalized patients but not of mortality, disease severity on the presentation or progression - The debate continues. J Hepatol. 2021;74:482-4
87. Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M. et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202-9
88. Cai Q, Chen F, Wang T, Luo F, Liu X, Wu Q. et al. Obesity and COVID-19 Severity in a Designated Hospital in Shenzhen, China. Diabetes Care. 2020;43:1392-8
89. Medeiros AK, Barbisan CC, Cruz IR, de Araújo EM, Libânio BB, Albuquerque KS. et al. Higher frequency of hepatic steatosis at CT among COVID-19-positive patients. Abdom Radiol (NY). 2020;45:2748-54
90. Pan L, Huang P, Xie X, Xu J, Guo D, Jiang Y. Metabolic associated fatty liver disease increases the severity of COVID-19: A meta-analysis. Dig Liver Dis. 2021;53:153-7
91. McCarron S, Bathon B, Conlon DM, Abbey D, Rader DJ, Gawronski K. et al. Functional Characterization of Organoids Derived From Irreversibly Damaged Liver of Patients With NASH. Hepatology. 2021;74:1825-44
92. Paizis G, Tikellis C, Cooper ME, Schembri JM, Lew RA, Smith AI. et al. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 2005;54:1790-6
93. Huang Q, Xie Q, Shi C-C, Xiang X-G, Lin L-Y, Gong B-D. et al. Expression of angiotensin-converting enzyme 2 in CCL4-induced rat liver fibrosis. Int J Mol Med. 2009;23:717-23
94. Meijnikman AS, Bruin S, Groen AK, Nieuwdorp M, Herrema H. Increased expression of key SARS-CoV-2 entry points in multiple tissues in individuals with NAFLD. J Hepatol. 2021;74:748-9
95. Xie M, Chen Q. Insight into 2019 novel coronavirus - An updated interim review and lessons from SARS-CoV and MERS-CoV. Int J Infect Dis. 2020;94:119-24
96. Sun J, Aghemo A, Forner A, Valenti L. COVID-19 and liver disease. Liver Int. 2020;40:1278-81
97. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS. et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417-8
98. Zhou Y-J, Zheng KI, Wang X-B, Yan H-D, Sun Q-F, Pan K-H. et al. Younger patients with MAFLD are at increased risk of severe COVID-19 illness: A multicenter preliminary analysis. J Hepatol. 2020;73:719-21
99. Zhou Y-J, Zheng KI, Wang X-B, Sun Q-F, Pan K-H, Wang T-Y. et al. Metabolic-associated fatty liver disease is associated with severity of COVID-19. Liver Int. 2020;40:2160-3
100. Ji D, Qin E, Lau G. Reply to: 'Younger patients with MAFLD are at increased risk of severe COVID-19 illness: A multicenter preliminary analysis'. J Hepatol. 2020;73:722
101. Zheng KI, Gao F, Wang X-B, Sun Q-F, Pan K-H, Wang T-Y. et al. Letter to the Editor: Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;108:154244
102. Wang G, Wu S, Wu C, Zhang Q, Wu F, Yu B. et al. Association between non-alcoholic fatty liver disease with the susceptibility and outcome of COVID-19: A retrospective study. J Cell Mol Med. 2021;25:11212-20
103. Gao F, Zheng KI, Wang X-B, Yan H-D, Sun Q-F, Pan K-H. et al. Metabolic associated fatty liver disease increases coronavirus disease 2019 disease severity in nondiabetic patients. J Gastroenterol Hepatol. 2021;36:204-7
104. Vazquez-Medina MU, Cerda-Reyes E, Galeana-Pavon A, Lopez-Luna CE, Ramirez-Portillo PM, Ibanez-Cervantes G. et al. Interaction of metabolic dysfunction-associated fatty liver disease and nonalcoholic fatty liver disease with advanced fibrosis in the death and intubation of patients hospitalized with coronavirus disease 2019. Hepatol Commun. 2022
105. Yoo HW, Jin HY, Yon DK, Effenberger M, Shin YH, Kim SY. et al. Non-alcoholic Fatty Liver Disease and COVID-19 Susceptibility and Outcomes: a Korean Nationwide Cohort. J Korean Med Sci. 2021
106. Gimeno-Miguel A, Bliek-Bueno K, Poblador-Plou B, Carmona-Pírez J, Poncel-Falcó A, González-Rubio F. et al. Chronic diseases associated with increased likelihood of hospitalization and mortality in 68,913 COVID-19 confirmed cases in Spain: A population-based cohort study. PLoS One. 2021;16:e0259822
107. Younossi ZM, Stepanova M, Lam B, Cable R, Felix S, Jeffers T. et al. Independent Predictors of Mortality Among Patients With NAFLD Hospitalized With COVID-19 Infection. Hepatol Commun. 2021
108. Roca-Fernández A, Dennis A, Nicholls R, McGonigle J, Kelly M, Banerjee R. et al. Hepatic Steatosis, Rather Than Underlying Obesity, Increases the Risk of Infection and Hospitalization for COVID-19. Front Med (Lausanne). 2021;8:636637
109. Targher G, Mantovani A, Byrne CD, Wang X-B, Yan H-D, Sun Q-F. et al. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut. 2020;69:1545-7
110. Targher G, Mantovani A, Byrne CD, Wang XB, Yan HD, Sun QF. et al. Detrimental effects of metabolic dysfunction-associated fatty liver disease and increased neutrophil-to-lymphocyte ratio on severity of COVID-19. Diabetes Metab. 2020;46:505-7
111. Li J, Tian A, Zhu H, Chen L, Wen J, Liu W. et al. Mendelian Randomization Analysis Reveals No Causal Relationship Between Nonalcoholic Fatty Liver Disease and Severe COVID-19. Clin Gastroenterol Hepatol. 2022
112. Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS. et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017-32
113. Wei X, Zeng W, Su J, Wan H, Yu X, Cao X. et al. Hypolipidemia is associated with the severity of COVID-19. J Clin Lipidol. 2020;14:297-304
114. Huang R, Zhu L, Wang J, Xue L, Liu L, Yan X. et al. Clinical features of COVID-19 patients with non-alcoholic fatty liver disease. Hepatol Commun. 2020
115. Milic J, Barbieri S, Gozzi L, Brigo A, Beghé B, Verduri A. et al. Metabolic-Associated Fatty Liver Disease Is Highly Prevalent in the Postacute COVID Syndrome. Open Forum Infect Dis. 2022;9:ofac003
116. Ren Y, Shu T, Wu D, Mu J, Wang C, Huang M. et al. The ORF3a protein of SARS-CoV-2 induces apoptosis in cells. Cell Mol Immunol. 2020;17:881-3
117. Li F, Li J, Wang P-H, Yang N, Huang J, Ou J. et al. SARS-CoV-2 spike promotes inflammation and apoptosis through autophagy by ROS-suppressed PI3K/AKT/mTOR signaling. Biochim Biophys Acta Mol Basis Dis. 2021;1867:166260
118. Wong GL-H, Yip TC-F, Wong VW-S, Tse Y-K, Hui DS-C, Lee S-S. et al. SARS-CoV-2 Viral Persistence Based on Cycle Threshold Value and Liver Injury in Patients With COVID-19. Open Forum Infect Dis. 2021;8:ofab205
119. Koliaki C, Szendroedi J, Kaul K, Jelenik T, Nowotny P, Jankowiak F. et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015;21:739-46
120. Miller B, Silverstein A, Flores M, Xiang W, Cao K, Kumagai H. et al. SARS-CoV-2 induces a unique mitochondrial transcriptome signature. 2020.
121. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033-4
122. Liu J, Li S, Liu J, Liang B, Wang X, Wang H. et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763
123. Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62-75
124. Fricker ZP, Pedley A, Massaro JM, Vasan RS, Hoffmann U, Benjamin EJ. et al. Liver Fat Is Associated With Markers of Inflammation and Oxidative Stress in Analysis of Data From the Framingham Heart Study. Clin Gastroenterol Hepatol. 2019 17
125. Simon TG, Trejo MEP, McClelland R, Bradley R, Blaha MJ, Zeb I. et al. Circulating Interleukin-6 is a biomarker for coronary atherosclerosis in nonalcoholic fatty liver disease: Results from the Multi-Ethnic Study of Atherosclerosis. Int J Cardiol. 2018;259:198-204
126. Miele L, Napodano C, Cesario A, De Magistris A, Pocino K, Basile U. et al. COVID-19, adaptative immune response and metabolic-associated liver disease. Liver International. 2021;41:2560-77
127. Gao B, Tsukamoto H. Inflammation in Alcoholic and Nonalcoholic Fatty Liver Disease: Friend or Foe? Gastroenterology. 2016;150:1704-9
128. Georg P, Astaburuaga-García R, Bonaguro L, Brumhard S, Michalick L, Lippert LJ. et al. Complement activation induces excessive T cell cytotoxicity in severe COVID-19. Cell. 2022 185
129. Sefik E, Qu R, Junqueira C, Kaffe E, Mirza H, Zhao J. et al. Inflammasome activation in infected macrophages drives COVID-19 pathology. Nature. 2022
130. Chen R, Du J, Zhu H, Ling Q. The role of cGAS-STING signalling in liver diseases. JHEP Rep. 2021;3:100324
131. Di Domizio J, Gulen MF, Saidoune F, Thacker VV, Yatim A, Sharma K. et al. The cGAS-STING pathway drives type I IFN immunopathology in COVID-19. Nature. 2022
132. Chow KW, Pham NV, Ibrahim BM, Hong K, Saab S. Autoimmune Hepatitis-Like Syndrome Following COVID-19 Vaccination: A Systematic Review of the Literature. Dig Dis Sci. 2022
133. Cao Z, Gui H, Sheng Z, Xin H, Xie Q. Letter to the editor: Exacerbation of autoimmune hepatitis after COVID-19 vaccination. Hepatology. 2022;75:757-9
134. Zin Tun GS, Gleeson D, Al-Joudeh A, Dube A. Immune-mediated hepatitis with the Moderna vaccine, no longer a coincidence but confirmed. J Hepatol. 2022;76:747-9
135. Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clinical Immunology. 2020;217:108480
136. Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nature Reviews Immunology. 2021;21:195-7
137. Boettler T, Csernalabics B, Salié H, Luxenburger H, Wischer L, Salimi Alizei E. et al. SARS-CoV-2 vaccination can elicit a CD8 T-cell dominant hepatitis. J Hepatol. 2022
138. Feng G, Zheng KI, Yan Q-Q, Rios RS, Targher G, Byrne CD. et al. COVID-19 and Liver Dysfunction: Current Insights and Emergent Therapeutic Strategies. J Clin Transl Hepatol. 2020;8:18-24
139. Dar WA, Sullivan E, Bynon JS, Eltzschig H, Ju C. Ischaemia reperfusion injury in liver transplantation: Cellular and molecular mechanisms. Liver Int. 2019;39:788-801
140. Rosser BG, Gores GJ. Liver cell necrosis: cellular mechanisms and clinical implications. Gastroenterology. 1995;108:252-75
141. Gonzalez FJ, Xie C, Jiang C. The role of hypoxia-inducible factors in metabolic diseases. Nat Rev Endocrinol. 2018;15:21-32
142. Gorden DL, Myers DS, Ivanova PT, Fahy E, Maurya MR, Gupta S. et al. Biomarkers of NAFLD progression: a lipidomics approach to an epidemic. J Lipid Res. 2015;56:722-36
143. Chen J, Chen J, Fu H, Li Y, Wang L, Luo S. et al. Hypoxia exacerbates nonalcoholic fatty liver disease via the HIF-2α/PPARα pathway. Am J Physiol Endocrinol Metab. 2019;317:E710-E22
144. Ferron P-J, Gicquel T, Mégarbane B, Clément B, Fromenty B. Treatments in Covid-19 patients with pre-existing metabolic dysfunction-associated fatty liver disease: A potential threat for drug-induced liver injury? Biochimie. 2020;179:266-74
145. Nardo AD, Schneeweiss-Gleixner M, Bakail M, Dixon ED, Lax SF, Trauner M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021;41:20-32
146. Yip TC-F, Lui GC-Y, Wong VW-S, Chow VC-Y, Ho TH-Y, Li TC-M. et al. Liver injury is independently associated with adverse clinical outcomes in patients with COVID-19. Gut. 2021;70:733-42
147. Bai L, Li H. Innate immune regulatory networks in hepatic lipid metabolism. J Mol Med (Berl). 2019;97:593-604
148. Bruzzone C, Bizkarguenaga M, Gil-Redondo R, Diercks T, Arana E, García de Vicuña A. et al. SARS-CoV-2 Infection Dysregulates the Metabolomic and Lipidomic Profiles of Serum. iScience. 2020;23:101645
149. Shen B, Yi X, Sun Y, Bi X, Du J, Zhang C. et al. Proteomic and Metabolomic Characterization of COVID-19 Patient Sera. Cell. 2020;182:59-72.e15
150. Dias SSG, Soares VC, Ferreira AC, Sacramento CQ, Fintelman-Rodrigues N, Temerozo JR. et al. Lipid droplets fuel SARS-CoV-2 replication and production of inflammatory mediators. PLoS Pathog. 2020;16:e1009127-e
151. Li Z, Peng M, Chen P, Liu C, Hu A, Zhang Y. et al. Imatinib and methazolamide ameliorate COVID-19-induced metabolic complications via elevating ACE2 enzymatic activity and inhibiting viral entry. Cell Metab. 2022
152. Nowak JK, Lindstrom JC, Kalla R, Ricanek P, Halfvarson J, Satsangi J. Age, Inflammation, and Disease Location Are Critical Determinants of Intestinal Expression of SARS-CoV-2 Receptor ACE2 and TMPRSS2 in Inflammatory Bowel Disease. Gastroenterology. 2020;159:1151-4 e2
153. Wang B, Zhang L, Wang Y, Dai T, Qin Z, Zhou F. et al. Alterations in microbiota of patients with COVID-19: potential mechanisms and therapeutic interventions. Signal Transduct Target Ther. 2022;7:143
154. Iruzubieta P, Medina JM, Fernández-López R, Crespo J, de la Cruz F. A Role for Gut Microbiome Fermentative Pathways in Fatty Liver Disease Progression. J Clin Med. 2020;9:1369
155. Zuo T, Zhang F, Lui GCY, Yeoh YK, Li AYL, Zhan H. et al. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology. 2020;159:944-55 e8
156. Gu S, Chen Y, Wu Z, Chen Y, Gao H, Lv L. et al. Alterations of the Gut Microbiota in Patients With Coronavirus Disease 2019 or H1N1 Influenza. Clin Infect Dis. 2020;71:2669-78
157. Tao W, Zhang G, Wang X, Guo M, Zeng W, Xu Z. et al. Analysis of the intestinal microbiota in COVID-19 patients and its correlation with the inflammatory factor IL-18. Medicine in Microecology. 2020;5:100023
158. Yeoh YK, Zuo T, Lui GC, Zhang F, Liu Q, Li AY. et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70:698-706
159. Aron-Wisnewsky J, Vigliotti C, Witjes J, Le P, Holleboom AG, Verheij J. et al. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat Rev Gastroenterol Hepatol. 2020;17:279-97
160. Sencio V, Machelart A, Robil C, Benech N, Hoffmann E, Galbert C. et al. Alteration of the gut microbiota following SARS-CoV-2 infection correlates with disease severity in hamsters. Gut Microbes. 2022;14:2018900
161. Brandl K, Schnabl B. Intestinal microbiota and nonalcoholic steatohepatitis. Curr Opin Gastroenterol. 2017;33:128-33
162. Zhang L, Richards A, Barrasa MI, Hughes SH, Young RA, Jaenisch R. Reverse-transcribed SARS-CoV-2 RNA can integrate into the genome of cultured human cells and can be expressed in patient-derived tissues. Proc Natl Acad Sci U S A. 2021 118
163. Smits N, Rasmussen J, Bodea GO, Amarilla AA, Gerdes P, Sanchez-Luque FJ. et al. No evidence of human genome integration of SARS-CoV-2 found by long-read DNA sequencing. Cell Rep. 2021;36:109530
164. Rezasoltani S, Hatami B, Yadegar A, Asadzadeh Aghdaei H, Zali MR. How Patients With Chronic Liver Diseases Succeed to Deal With COVID-19? Front Med (Lausanne). 2020;7:398
165. Zhong P, Xu J, Yang D, Shen Y, Wang L, Feng Y. et al. COVID-19-associated gastrointestinal and liver injury: clinical features and potential mechanisms. Signal Transduct Target Ther. 2020;5:256
166. Boettler T, Marjot T, Newsome PN, Mondelli MU, Maticic M, Cordero E. et al. Impact of COVID-19 on the care of patients with liver disease: EASL-ESCMID position paper after 6 months of the pandemic. JHEP Rep. 2020;2:100169
167. Wong GL-H, Wong VW-S, Thompson A, Jia J, Hou J, Lesmana CRA. et al. Management of patients with liver derangement during the COVID-19 pandemic: an Asia-Pacific position statement. Lancet Gastroenterol Hepatol. 2020;5:776-87
Author contact
![]() Corresponding author: Qiang Chen, qiangchedu.mo; Tel: (853) 8822-4965; Fax: (853) 8822-2341
Corresponding author: Qiang Chen, qiangchedu.mo; Tel: (853) 8822-4965; Fax: (853) 8822-2341

 Global reach, higher impact
Global reach, higher impact