10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(12):4768-4780. doi:10.7150/ijbs.75056 This issue Cite
Review
Long COVID and its Management
1. Centre of Reproduction, Development & Aging, Faculty of Health Sciences, University of Macau, Taipa, Macau, China
2. Institute of Translational Medicine, Faculty of Health Sciences, University of Macau, Taipa, Macau, China
3. Interventional Medical Centre, Zhuhai People's Hospital, Zhuhai Hospital Affiliated with Jinan University, Zhuhai 519000, China
4. Bioimaging and Stem Cell Core Facility, Faculty of Health Sciences, University of Macau, Taipa, Macau, China
Received 2022-5-12; Accepted 2022-6-22; Published 2022-7-11
Abstract
The pandemic of COVID-19 is the biggest public health crisis in 21st Century. Besides the acute symptoms after infection, patients and society are also being challenged by the long-term health complications associated with COVID-19, commonly known as long COVID. While health professionals work hard to find proper treatments, large amount of knowledge has been accumulated in recent years. In order to deal with long COVID efficiently, it is important for people to keep up with current progresses and take proactive actions on long COVID. For this purpose, this review will first introduce the general background of long COVID, and then discuss its risk factors, diagnostic indicators and management strategies. This review will serve as a useful resource for people to understand and prepare for long COVID that will be with us in the foreseeable future.
Keywords: COVID-19, SARS-CoV-2, SARS, long COVID, post-acute sequelae of SARS-CoV-2 (PASC), post-acute COVID syndrome (PACS), vaccine, management, risk factor, drug repurposing
Introduction
Coronavirus disease 2019 (COVID-19) has escalated into an unprecedented global pandemic since the first case was identified in December 2019 [1-3]. By June 20th 2022, more than 535 million individuals were infected with over 6.3 million deaths worldwide according to World Health Organization (WHO) [4]. Apart from the severe morbidity and mortality in the first few weeks after infection, up to 70% COVID-19 survivors may experience long-term medical complications [5-7]. The lingering symptoms after COVID-19 infection can last weeks to months, severely reducing the quality of life long after patients become virus-free. Such symptoms are generally reported as “long COVID” in common literature.
With so many affected patients, long COVID posts a global health challenge for the society [8]. In the US, by fall 2021, cumulative loss from direct economic losses in conjunction with mortality, morbidity and relative metal health complications of COVID-19 is estimated at $16 trillion [9]. The substantial burden of the pandemic should not be neglected. In just two years, a large volume of evidence has been accumulated on the pathological manifestation, treatments and preventative measures of long COVID [1]. It is necessary for health professionals and the general public to gain more knowledge about the disease. Therefore, this review will present our current understanding of long COVID and highlight the potential management strategies and therapeutics.
Acute COVID-19 and post-acute COVID-19 (long COVID)
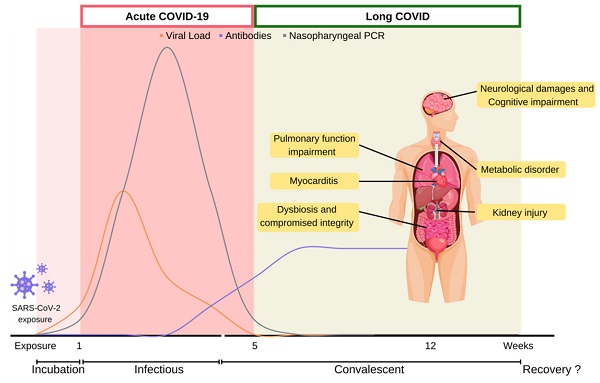
COVID-19 is caused by the infection of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), a singled-stranded RNA coronavirus [10]. After the initial infection, patient would experience distinct symptoms in acute and post-acute stages over the course of the disease (Figure 1).
Acute COVID-19 stage generally refers to the initial five weeks after SARS-CoV-2 infect patients. When SARS-CoV-2 first enters the host respiratory tract, it initiates infection through the binding of the spike protein with Angiotensin-converting enzyme II (ACE2) receptor, and then utilizes transmembrane protease serine 2 (TMPRSS2) to enter host cells. SARS-CoV-2 hijacks the host cellular machinery for viral RNA replication and protein production. SARS-CoV-2 load usually reaches its peak upon symptom onset within the initial weeks of infection, and first becomes detectable by reverse transcription polymerase chain reaction (RT-PCR) within the first week of infection. At the peak of infection, a patient could carry 109 to 1010 virions [11].
While the virion copy number is at its peak, the onset of infectious symptoms follows, which is referred to as acute COVID. Clinical manifestation of COVID-19 can range from asymptomatic, to severe fulminant illnesses leading to mortality. According to meta-analysis studies, mild flu-like symptoms such as fever and cough are commonly reported. In moderate to severe cases, onset acute respiratory distress syndrome (ARDS) may deteriorate rapidly and lead to the development of pneumonia and dyspnea. Severe pneumonia with ARDS progresses to pathological deteriorations including septic shock and multi-system organ failure, which account for high morbidity and mortality among severe infected patients [2, 5, 12-14]. Acute COVID stage usually lasts five weeks till the viral load gradually declines from its peak to undetectable. During this period, viral infection often disables host cellular machineries, leading to functional impairments, even cell death. Subsequent cytokines recruitment often contributes to systematic hyperinflammation and leads to progression of thrombosis even multi-organ failure and death [2, 13]. Majority of the confirmed cases (81-45%) were reported to be asymptomatic or mild disease at time of diagnosis. Approximately 25% of the initially asymptomatic individual would develop symptoms throughout COVID-19 acute stage [15]. The Chinese Center for Disease Control and Prevention reported 14% of the COVID-19 patients were hospitalized with severe illness including dyspnea and 5% were reported as critical cases with disease progression to respiratory failure, shock, or multi-organ impairment [16]. Additionally, critical or fatal risk of SARS-CoV-2 varied by age, pre-existing comorbidities and vaccination status. Pathophysiology of COVID-19 and its relevant biological disease course have been reviewed comprehensively elsewhere [12, 17].
Disease course of COVID-19. COVID-19 disease course is depicted into multiple stages depending on viral counts and symptoms presented over time. Infected host undergoes acute-COVID-19 which lasts five weeks from symptoms onset, until competent virions become undetectable. Persistent symptoms and long-term complications beyond five weeks from COVID-19 infection is defined as long COVID. COVID-19: Coronavirus Disease 2019; PCR: polymerase chain reaction; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
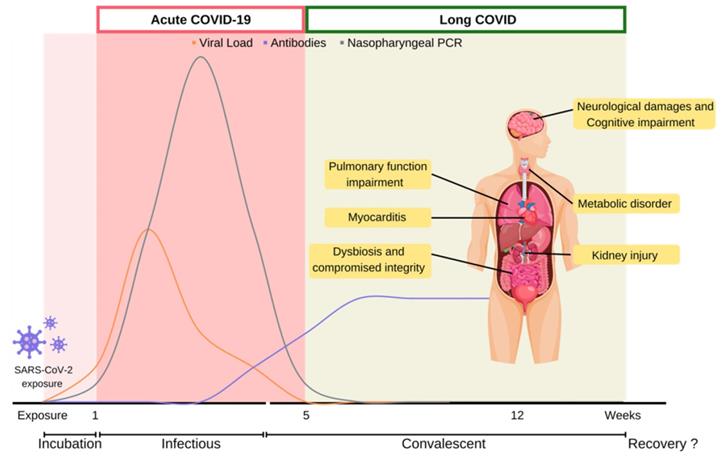
About three weeks after initial detection by RT-PCR, the viral load becomes undetectable. This marks the end of acute COVID, but also the start of post-acute stage. COVID-relevant health issues could linger around as long COVID for years even in patients who are asymptomatic during the acute stage. Survivors of virus infections are at risk of developing physiological complications and multi-organ impairment. The subacute and sequelae effects of SARS-CoV-2 infection are called various names, such as “long COVID”, “post-acute COVID syndrome”, “post-COVID-19 condition” and “long-haul COVID-19”. In response to the absence of a consensus definition for long COVID, WHO has made a clinical case definition to the public in delineating long COVID. “Post-COVID-19 condition occurs in individuals with a history of probable or confirmed SARS-CoV-2 infection, usually 3 months from the onset of COVID-19 with symptoms that last for at least 2 months and cannot be explained by an alternative diagnosis.” [18] The National Institute for Health and Care Excellence (NICE) defined post-COVID-19 syndrome as “signs and symptoms that develop during or after an infection consistent with COVID‑19, continue for more than 12 weeks and are not explained by an alternative diagnosis” [19]. Additionally, symptoms appearing during a timeframe of four-to-twelve-week post COVID-19 onset are regarded as ongoing symptomatic COVID-19. While the terminology for long COVID evolves as clinical evidence accumulates, the term “long COVID” will be used to discuss health issues during post-acute stage of COVID-19 in this review.
Symptoms of long COVID
It is estimated that 31%-69% of COVID-19 survivors will experience long COVID symptoms after initial recovery from SARS-CoV-2 infection [20, 21]. Generally, initial symptoms of long COVID symptoms include fatigue (29%), muscle pain, palpitations, cognitive impairment (28%), dyspnea (21%), anxiety (27%), chest pain, and arthralgia (18%) [22]. Among the UK population, fatigue is most prevalent among long COVID patients (51%), followed by dyspnea (35%), arthralgia (25%) and concentration difficulties (25%). Correspondingly, a recent meta-analysis of 36 studies identified fatigue, cognitive impairment, joint pain, anxiety, and depression as primary clinical symptoms of long COVID [5]. A massive international survey found fatigue, malaise and cognitive impairment as the most prevalence symptoms experienced among individuals with reported long COVID [23]. Furthermore, cognitive related symptoms were reported to develop at later long COVID stages. While many cases were initially reported in hospitalized COVID-19 patients who experienced severe symptoms during the acute stage, long COVID symptoms have also been documented in non-hospitalized or asymptomatic individuals [24]. Approximately 30% of non-hospitalized COVID-19 patients reported lingering symptoms 2 months after initial infections [25]. Similarly, less than 1% of COVID survivors achieved complete recovery at 80 days after infection [26]. The lingering symptoms of long COVID reflect chronical damages of multi-systemic organs. Such health conditions post a significant burden on the quality of life among COVID survivors (Figure 1) [27-29].
As a respiratory virus, SARS-CoV-2 infection leads to respiratory system dysfunction in long COVID. SARS-CoV-2 initially infects the alveolar epithelium, and induces chronic inflammation responses that trigger sustained production of inflammatory cytokines and reactive oxygen species (ROS) [17]. Additionally, disruption of cellular integrity activates fibroblasts to deposit collagen and fibronectin, leading to fibrotic changes to lung tissue. In the long-term, viral-induced complement activation and subsequent disruption of coagulant pathways favor the development of prolonged inflammation and hypercoagulable state, predisposing the patient to the risk of thrombosis [30]. National data from UK suggested that 36% of long COVID individuals experienced shortness of breath to a certain extent. 26% of long COVID individuals develop signs and symptoms of lung impairment [31]. Respiratory abnormalities involving changes in total lung capacity and airway function have also been reported following COVID-19 infection [32]. In addition, dyspnea is prevalent in COVID-19 survivors along with other respiratory symptoms, including chronic chough and reduction in exercise capacities [33].
Many long COVID patients also experience cardiovascular complications. The abundance of ACE2 receptors on cardiomyocytes provides a direct route for SARS-CoV-2 infection. Chronic inflammation of the cardiomyocytes can lead to myositis and cell death. Prolonged inflammation and cellular damage prompt fibrotic changes and reduce cell adhesion, all of which could lead to arrhythmia and the development of coagulopathy. In addition, inflammation of the autonomic nervous system might progress to postural orthostatic tachycardia syndrome (POTS) [34]. Persistent myocardial inflammation and increased cardiac troponin level are commonly present in COVID-19 patients 2 months after diagnosis. Evidence have suggested increased risk of cardiovascular compilations following SARS-CoV-2 infection, even among non-hospitalized patients. In particular, cardiovascular risk was associated with the severity of infection during acute COVID-19 [35]. Correspondingly, a prospective observational study found that 32% COVID-19 survivors have cardiac damages 3 months after the onset of infection [36]. 89% of long COVID patients reported cardiac associated symptoms, where 53% reported chest pain, 68% palpitations and 31% new onset of POTS [37].
Long COVID can also affect the central nervous system (CNS). Chronic neuro-inflammation activates glial cells which lead to neurodegenerative disorders. SARS-CoV-2 can permeate through the blood-brain barrier (BBB), and subsequent damage of the BBB permeability will further drive neuro-inflammation in the brain parenchyma. Pathological hyperinflammatory and hypercoagulable conditions may increase the risk of thrombotic events [38]. Furthermore, hyper-inflammation in the brainstem may lead to autonomic dysfunction. CNS dysfunction in the brain may contribute to long-term cognitive impairment. Multiple studies have proposed that CNS disturbance in response to neuro-inflammation could be responsible for the neuropsychiatric abnormalities, including chronic malaise, fatigue, sleeping disorder, ageusia and anosmia (loss of taste and smell), PTSD, conative impairment, and even stroke [7]. In a UK retrospective cohort study on 23,6379 confirmed COVID-19 cases, one in three subjects reported neuropsychiatric symptoms 6 months after SARS-CoV-2 infection [39]. Specifically, subjects with severe acute COVID-19 were at higher risk for neuropsychiatric morbidity. Imaging studies using structural magnetic resonance imaging (MRI) and positron emission tomography (PET) imaging showed brain alterations among patients with cognitive impairment during subacute COVID-19 stage when compared to healthy controls [40-42]. Combination of population and clinical data strongly suggest neurological involvement in long COVID.
SARS-CoV-2 induces both direct and indirect pathology which contribute to multi-organ dysfunction. Hyperinflammation of kidney tissues may activate the complement system, contributing to focal segmental glomerulosclerosis and glomerular involution [30]. Acute kidney injury has been reported among discharged COVID-19 patients, and 35% of the recovered patients have reduced kidney function [43]. SARS-CoV-2 triggers pancreatitis during acute-COVID. Pancreatic damage as observed in long COVID may be consequences from a combination of direct viral attack and indirect effect of systemic inflammation. The major SARS-CoV-2 receptor ACE2 is an endocrine regulator in the renin-angiotensin-aldosterone system, while the other SARS-CoV-2 receptor TMPRSS2 is found in pancreatic β cells. The viral infection directly impairs ACE2 and TMPRSS2-expressing cells, causing dysfunctions in renin-angiotensin-aldosterone system and disrupts metabolic homeostasis in the long term. Furthermore, systemic inflammation and corticosteroids administration increase the risk of bone demineralization and dermatologic complications [44].
Altered gastrointestinal system functions were reported in both acute- and subacute-COVID [22, 45]. Expression of ACE2 receptor made the esophagus and enterocytes prone to direct viral damage [46]. Upon viral clearance, prior hyperinflammatory state and microbiome imbalance may damage gastrointestinal integrity and function [47]. Current findings have emphasized changes in the gut microbiota in patients following COVID-19 infection [48-50].
Considering the diverse manifestations of the disease, the full clinical spectrum of symptoms experienced by these patients over time needs to be catalogued to develop a better understanding of their underlying pathology. This would also further aid in the characterization of the various phenotypes of long COVID and the risk factors associated with its development, which will allow effective design of clinical trials with improved treatment options.
Treatments and management of long COVID patients
Medical experts are using their best efforts to manage patients with long COVID. Although several guidelines on long COVID management have been released, there remains a large practical gap and specific treatments are not reviewed. While empirical evidence still awaits to inform clinical practice, a comprehensive review of clinical strategies adopted by clinicians will be valuable in guiding appropriate patient rehabilitation [51]. It is important to optimize clinical outcomes by considering patient safety and enhancing sophisticated diagnosis and holistic assessment. Furthermore, novel therapeutics for treating organ specific dysfunction and long COVID related symptoms will guide treatment development. WHO have empathized research priorities in refining clinical characterization and developing therapeutics for long COVID. At the same time, medical professionals are exploring clinical approaches in identifying and managing long COVID.
Long COVID symptoms are presented heterogeneously, so patients need to be closely monitored. In order to develop effective treatment strategies, holistic assessment is necessary to consider pre-existing conditions and to identify specific symptoms. NICE outlines a set of evidence-based assessment and management approaches for treating patients with long COVID [52]. The NICE guideline recommends clinical investigation of long COVID as early as 4 weeks after initial acute symptoms. In addition, the National Institute of Health and Care Research (NIHR) also issued recommendations on the evaluation of long COVID symptoms and prioritizing care for certain populations [53].
In general, current clinical practice adopted a symptom-based approach in managing long COVID. Comprehensive assessment through medical history and examination is essential. It is recommended to obtain a complete assessment including full blood count, renal function test, C-reactive protein, liver function test, thyroid function, hemoglobin A1c (HbA1c), vitamin D, magnesium, B12, folate and ferritin levels [52]. The International Consensus Conference in Critical Care recommends adopting screening tests for prediction and identification of physical and mental impairment. A robust clinical care also requires additional assessments for appropriate referrals to specialists. Importantly, while long COVID is diagnosed, other non-COVID-19 related diagnosis should also be considered unless they could be excluded. Appropriate treatments are provided according to clinical symptoms [51]. For patients presenting with cardiopulmonary symptoms, chest imaging, electrocardiography and pulmonary function tests should be considered. Oxygen supplementation is commonly provided for patients with dyspnea and during pulmonary rehabilitation. In particular, corticosteroid treatments have been showed to resolve pneumonia and improve clinical functions. Sufficient patient support and rapport building are essential for disease recovery [51].
Mast cell activation syndrome (MCAS) and anti-histamine treatment
Previous findings have suggested the prevalence of mast cells activation syndrome in long COVID patients [54]. It is suggested that immune disturbance from SARS-CoV-2 infection may lead to aberrant mast cell activation and further initiate cascades of inflammatory responses contributing to allergic flare-up [54, 55]. Histamine antagonists have been used to relieve long COVID associated symptoms [55, 56]. However, the interaction of antihistamines with viral-altered ACE2 pathways remains to be elucidated. One concern is that antihistamines can be easily assessed over the counter and abusive usage may lead to problems. Misuse of antihistamines will cause abnormal levels of circulating dopamine and may be associated with dementia in the long-term. Future studies and clinical trials are required to investigate antihistamines as therapeutic treatment for long COVID.
Dietary supplements
In long COVID, chronic inflammation provokes multi-organ damage and exacerbates pre-existing conditions. Dietary supplements, such as vitamins and minerals, contain anti-inflammatory and anti-oxidative components, so they have become potential treatments for long COVID. A pilot study demonstrates that multivitamin supplements improve clinical symptoms among long COVID patients [57]. In addition, a commercial plant extract supplement from Panax ginseng and Eleutherococcus senticosus effectively relieved post-COVID fatigue and improved health status in 201 long COVID patients [58]. Nicotinamide ribose, a form of vitamin B3, is being examined for its effects of ameliorating cognitive dysfunctions and chronic fatigue in two clinical trials (NCT04809974, NCT04604704). Essential fatty acids, such as omega-3 (Eicosatetraenoic acid - EPA + docosahexaenoic acid - DHA), are also being examined for their functions in long COVID symptoms (NCT05121766).
Long COVID patients often have dysregulated lipid oxidation and lactate accumulation during physically active state, indicating compromised mitochondrial function [59]. The mitochondrial dysfunction in long COVID shares similar symptoms as the ones observed in Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Supplementation with Coenzyme Q10 (CoQ10) is found to reduce fatigue frequency and relieve oxidative stress among ME/CFS patients [60]. Currently, high-dose CoQ10 treatment is being investigated in a Phase II clinical trial in long COVID patients (NCT04960215).
Dietary supplements may also have beneficial effect in modulating systemic inflammation and immunity. Natural flavonoids such as luteolin and quercetin are promising immunomodulatory agents which have showed inhibitory effects on mast cells [61]. The influence of microbiota on immunity is well known, and long COVID leads to significant changes in gut flora [5, 49]. Dietary pro-biotics and pre-biotics are being evaluated on their impacts on clinical symptoms, immune function and biomarkers in long COVID patients (NCT04813718).
Other potential therapeutics
Because long COVID leads to systemic dysfunctions, various therapeutic strategies have been explored. Viral infection often compromises the immune system, which may increase the risk for opportunistic infections. Antibiotic and anti-viral compounds such as Azithromycin, Remdesivir and Favipiravir are being explored for their effectiveness in controlling long COVID (NCT04699097, NCT04978259, NCT04448119). In order to combat the extensive inflammatory state from COVID-19 infection, antibody treatments have also been studied, including Infliximab, Tocilizumab, Siltuximab, Anakinra and Leronlimab (NCT05220280, NCT04330638, NCT04330638, NCT04330638, NCT04678830). Meanwhile, antidepressants have been proposed to lessen long COVID symptoms by reducing peripheral inflammatory markers, thereby restoring immune function. Selective serotonin reuptake inhibitor and serotonin receptor modulator Vortioxetine is being examined in a clinical trial to treat long COVID (NCT05047952).
Clinical indicators for long COVID
In order to effectively treat long COVID, it is crucial to have accurate diagnosis. Early diagnosis is essential for effective management and improved prognosis. Several clinical indicators have been developed to guide the clinical diagnosis of long COVID.
Serum biochemistry
Acute COVID-19 infection disturbs the immune system response and predisposes a low-grade inflammation state which may continue over subacute phase. Systemic inflammatory markers were proposed as biomarkers for long COVID. For instance, D-dimer, c-reactive protein (CRP), interleukin-6 (IL-6), procalcitonin and neutrophil count found to be associated with persistence symptoms of long-COVID [33, 62-66]. Cardiac, hepatic and renal abnormalities are also observed among patients with abnormal CRP, procalcitonin and neutrophil count levels [67]. Analysis of endothelial function in 30 patients with long COVID reported distance ET-1 and RHI profile in long COVID patients [68]. In addition, neurodegenerative indicators including amyloid beta, neurofilament light, neurogranin, total tau, and p-T181-tau were elevated among long COVID patients when compared to healthy controls [38].
The heterogenic nature of long COVID may justify the inconsistence of evidence in some studies [69-71]. Furthermore, symptoms relapse and viral-shredding add challenges in diagnosing long COVID [72].
To this end, it would be very helpful for long COVID diagnosis if a set of biostable biomarkers are available independent of symptoms. A recent study found that the immunoglobin profile of IgM and IgG3 is linked with increasing risk for developing long COVID. In fact, IgM and IgG3 are secreted by B cells in response to interferon induction and IL-4 signaling. Impairment in interferon synthesis characterized by increased interleukin signaling may contribute to inefficient IgG isotype conversion, debilitating immunity regulation [73]. As the pandemic evolves, research emerges toward a predictive model for post-acute sequelae of COVID-19 [74].
Gut microbiota
Immunomodulatory functions of gut microbiome are well documented in various disease pathogenesis [75]. SARS-CoV-2 RNA can be detected from fecal samples during acute stage, indicating gastrointestinal (GI) involvement in COVID-19 pathology [46, 76]. Recent studies reveal that gut microbiome diversity and composition are changed post COVID-19 infection [48-50]. Increase in viral-induced cytokines may compromise intestinal integrity, facilitating the entry of bacteria and metabolites into circulation. Such dysbiosis triggers the innate immune responses, causing pulmonary dysfunction and secondary infections [77]. Furthermore, integrative multi-omics profiling reveals that subjects with GI complications exhibit unique T cell signature during COVID-19 recovery [20]. Current findings open opportunities for gut microbiota to be used as a therapeutical approach by priming inflammation responses. A prospective study of 106 subjects identified specific patterns of intestinal microbiome profile predicted long COVID symptoms. For examples, elevated levels of Ruminococcus gnavus, Bacteroides vulgatus and reduced levels of Faecalibacterium prausnitzi and butyrate-producing bacteria correlated with long COVID [49]. Such intricate association may serve as a detection tool for the occurrence of long COVID.
Proactive Management of long COVID
Considering that more and more people will likely be infected by emerging SARS-CoV-2 variants, long COVID will continue to affect the society immensely in the foreseeable future. It is critical for people to take proactive actions to relieve its potential impacts. In doing so, we need to understand the risk factors of long COVID, control the risk factors and avoid questionable practices.
Risk factors of long COVID
Ever since long COVID was first reported, much attention has been paid to identifying its risk factors. The understanding of risk factors allows people to correlate long COVID with various determinants such as pre-existing conditions, age, medical treatments, genetics and lifestyle. The specific correlations will guide clinical diagnosis and management of long COVID. Symptoms in long COVID arise from multisystem damage, a holistic approach should be considered when identifying at-risk individuals at early phase of the disease [53].
1. The severity of Acute COVID infection
Long COVID was first reported in hospitalized patients requiring mechanical ventilation during acute stage, suggesting the symptoms at this stage correlates with the prevalence and severity of long COVID [69, 78, 79] (Figure 2). It is reported that viral load during acute COVID correlates with the severity of long COVID manifestation [20]. Early viral clearance seems to be protective from long COVID responses. Low viral load or early viral clearance probably leads to lower grade inflammatory reactions during acute phase, which results in less damage to the host in long COVID. The COVID Symptom Study shows that symptom quantity during the initial infectious week is predictive of long COVID duration [74]. Multiple population-level survey studies also demonstrate the positive correlation between acute disease symptoms with long COVID [24, 80]. Among hospitalized population, persistent symptoms are more frequently reported in patients with history of intensive care unit (ICU) admission or mechanical ventilation [81, 82].
In the past three years, the continuous viral evolution leads to the emergence of numerous SARS-CoV-2 variants in global populations, which challenges the effectiveness of public health in diagnosis, vaccination, and treatments. Five variants of concern (VOC) have been widely reported, and they are Alpha, Beta, Gamma, Delta and Omicron variants [83]. Because of their specific mutations in viral genome, different variants have various transmissibility and virulence, which affects both Acute COVID-19 and long COVID in patients. Currently, Omicron variant is the dominant variant with enhanced transmissibility since February 2022, replacing Delta variant that was most prominent in 2021. A recent study showed that Omicron variant caused lower risk in developing long COVID in comparison to Delta variant among infected patients [84]. The finding is probably associated with the fact that Omicron variant generally caused less severe symptoms than Delta variant at Acute stage. However, because of its high transmissibility, more people will likely be infected by the Omicron variant. In this sense, Omicron variant associated long COVID will remain a major challenge for people in foreseeable future.
Proposed risk factors and relevant pathophysiological consequences of long COVID development. Old age, pre-existing comorbidities, and incomplete COVID-19 vaccination are well-established risk factors of acute-COVID-19 severity, which subsequently increase the risk for long COVID. Altered clinical parameters such as immunoglobins, inflammation cytokines, and microbiome profile could reflect the progression of long COVID. Such predisposition may also have an impact on recovery. COVID-19: Coronavirus Disease 2019; CRP: c-reactive protein; IgG: immunoglobulin G; IgM: immunoglobulin M; IL-6: interleukin 6; RAS: renin-angiotensin system; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
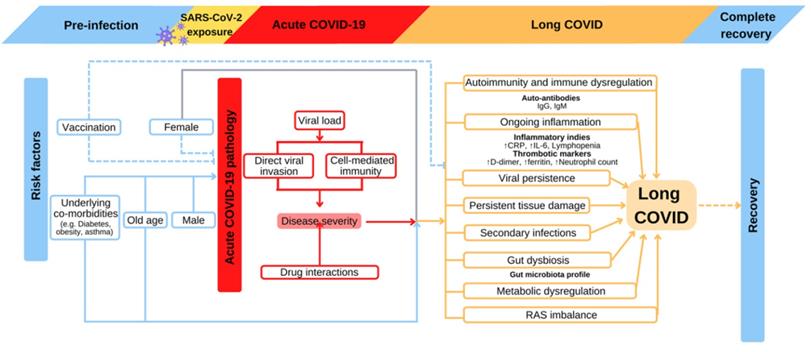
2. Pre-existing conditions
Pre-existing conditions correlate with the severity at acute stage, and are risk factors for developing long COVID. In a recent study, a combined analysis of UK primary care electronic health records and 10 population-based longitudinal studies revealed multiple risk factors for long COVID, including age, female gender, poor general health, asthma, and being overweight or obese [85]. In a prospective cohort study of 215 participants, long COVID developed in 94% of participants with a history of asthma bronchial, compared to 59% in those without [62]. The complex interactions of asthma severity, underlying conditions, as well as corticosteroid administration should be considered when managing long COVID. In addition to asthma, other pre-existing conditions were also reported to be long COVID risk factors. Pre-existing type 2 diabetes increase the risk of long COVID, as chronic inflammation associated with insulin resistance leads to a more profound immune responses during acute SARS-CoV-2 infection and subsequent COVID sequala [20]. Poor mental health is associated with 50% increased risk of developing long COVID and an increase in its relative severity [85, 86]. Interestingly, history of Epstein-Barr virus viremia might increase the risk for long COVID as suggested from single-cell sequencing of long COVID subjects [20]. With ongoing research, additional medical conditions may be identified as risk factors for long COVID.
3. Age
Advanced age is a main risk factor for the severity of acute infection, as well as the risk of long COVID. The COVID Symptom Study identified advanced age as a risk factor for long COVID [7]. Approximately one in five COVID survivor of over 70 years of age reported having lasting symptoms [87]. In contrast, self-reporting data from the UK's Office for National Statistic suggested subjects aged from 35 to 69 have the highest prevalence in experiencing long COVID [31]. Discrepancy of such results warrants further research to elucidate underlying mechanisms and identify long COVID patterns in different age groups. Considering that older patients are more likely to have pre-existing conditions and develop more severe acute responses, the increased risk of long COVID with advanced age may be a secondary effect.
4. Biological sex and sex hormones
Clinical data from epidemiological studies have drawn the attention toward the sex-discrepancy in long COVID [88]. In general, females under age 50 are five times more likely to develop long COVID symptoms post-discharge when comparing to male COVID-19 patients [89]. Multiple studies have observed higher prevalence of long COVID in female subjects than male [43, 81, 86, 90, 91].
Ovarian abnormality following HIV and Hepatitis B/C infections has been previously reported [92]. The abundance of ACE2 in ovarian granulosa cells and the hormonal disruption from SARS-CoV-2 may both contribute to ovarian dysfunction [25]. Specifically, perimenopausal and menopausal women are more prone to long COVID. Virus-mediated ovarian hormone disturbance interferes with systematic homeostasis reflects the inflammatory disorder over disease course in anticipating long COVID. However, overlapping symptoms of long COVID and menopause-related conditions pose additional challenges in the diagnosis and management of long COVID in this population.
Paradoxically, male sex is a risk factor for acute infection, but fewer long COVID cases are reported in male patients. It is noticed that long COVID studies are largely derived from self-reported data. Selection and reporting biases may arise from such methodological limitations. It is possible that part of the sex-associated long COVID prevalence may be linked to sex-dependent self-reporting. Meanwhile, sex-dependent discrepancy in medical care already exist in pre-COVID-19 era, so more complicated social issues could also lead to the increased number of female long COVID patients.
Proactive measures to relieve the potential impacts by Long COVID
Considering the enormous impact of SARS-CoV-2, the health burden of long COVID should not be neglected. The general public should consider proactive approaches to prevent long COVID. Summarized proactive measures are described in Figure 3.
1. Vaccination
Considering the impact of long covid to individual's wellbeing and society, it is important to take preventive measures besides trying to avoid infection. In the past two years, various vaccines have been developed for SARS-CoV-2. Although vaccines do not prevent infection, they significantly suppress morbidity and fatality. Two recent studies compared the long covid symptoms between unvaccinated patients and vaccinated patients in Israel [93] and the UK [94]. Both demonstrated that vaccination is strongly associated with the decrease of long COVID related symptoms. In fact, one of the studies showed that vaccinated people are no more likely to report long COVID symptoms than individuals without prior viral infection. In Switzerland, about 40% of death in connection with SARS-CoV-2 infection had not received COVID-19 vaccination in contrast to ~ 10% of death shared by individuals who had received at least single dose of vaccination [95]. These data suggest that vaccination is beneficial in controlling both acute and long COVID-19 symptoms.
The fifth wave of COVID-19 outbreak in Hong Kong has alarmed the COVID-19 vaccine hesitancy and associated factors in the community [96, 97]. Vaccination coverage in Hong Kong declined with age, with 49% of individuals aged above 60 reported receiving at least two doses of COVID-19 vaccine [98]. The COVID-19-associated mortality was prevalent among unvaccinated individuals aged above 60 years. The increased risk of COVID-19 severity among older population groups suggested relative risk for long COVID. Data from Hong Kong highlighted the important to implement strategies to boost vaccine coverage, especially among older adults.
Proactive measures and treatment strategies invovled in relieving long COVID. Management of long COVID should be prioritized throughout the course of disease. Sufficient nutrients from a well-balanced diet, physical activity, appropriate PPE and COVID-19 vaccination are protective measures against SARS-CoV-2 infection and the progression of long COVID. Early viral clearance by preproperate clinical practice during acute-COVID-19 may alleviate viral infection and prevent poor clinical outcomes. Integrated management plan should be provided to individuals with long COVID, depending on assessments and targeted management. CoQ10: coenzyme Q10; COVID-19: Coronavirus Disease 2019; CRP: c-reactive protein; MCAS: mast cell activation syndrome; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
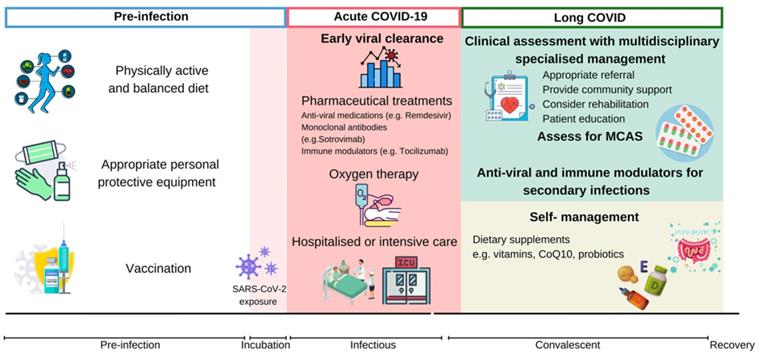
In addition to vaccines specific to SARS-CoV-2, people reported that other unrelated vaccine might also provide protective effects. A study on health care workers showed that recent influenza vaccination reduced the risk of SARS-CoV-2 infection and COVID-19 severity [99]. A retrospective study also showed that influenza vaccination significantly decreased the risks of sepsis, stroke and deep vein thrombosis associated with long COVID, and reduced the admission to Emergency Department and Intensive Care Unit weeks after initial infection [100]. Besides from the novel COVID vaccination, the bacillus Calmette-Guérin (BCG) vaccine against tuberculosis holds general protection from wide range of infectious diseases [101, 102]. In the era of COVID, the BCG vaccine has been proposed against SARS-CoV-2 severity where BCG vaccination is shown to have negatively associated with COVID-19 mortality [103, 104]. One study showed that BCG vaccine against COVID-19 was significant in young population, but was not as effective in older population [105]. However, another study from South Africa reported no protective effect by BCG vaccination against COVID-19 infection, severity and related mortality [106]. Above contradictory reports may contain analytic complications such as confounding mortality with tuberculosis and differences in demographics and diagnosis, so more human and animal studies are necessary to reveal whether influenza and BCG vaccines could stimulate the basal immune defense against SARS-CoV-2[107, 108]. In the absence of solid evidence, influenza and BCG vaccines are not recommended as a preventative vaccination for COVID-19[109].
2. Anti-inflammation treatment
Immunological aberrations owning to molecular mimicry induce autoantibody production which stimulates T cells and leads to tissue damage during acute COVID-19 infection [110]. Therefore, combating inflammation responses is critical in managing viral manifestation as well as clinical sequelae of SARS-CoV2. Dexamethasone is commonly used to treat inflammation in acute COVID-19 patients. Dexamethasone-treated COVID-19 patients were less likely to experience long COVID symptoms at 8-month follow-up in an observational study [111]. This study suggests that disease treatment approach for acute COVID could have profound impact on patients' wellbeing in the long run.
3. Nutritional control and lifestyle modifications
Nutritional management plays an important role in the management of chronic illness, and appropriate nutrition may mitigate the manifestations of viral infections [112-114]. Moreover, disparities in SARS-CoV-2 incidences across population indicate the plausibility of nutrition-related epigenetic variations among different populations [115]. For instance, high fat intake is found to downregulate the expression of ACE2 in animal models [116]. In addition to dietary patterns, lifestyle interventions and sleeping habits also have primordial function in shaping innate immune responses to external stimuli [117].
A meta-analysis of 361,934 participants reported a significant association between vitamin D insufficiency and increased risk of SARS-CoV-2 infection (OR = 1.43, 95% CI 1.00-2.05) [49]. This suggested the protective roles of vitamin D against SARS-CoV-2 [118]. Multiple observational studies and clinical trials have established the beneficial effect of vitamin D supplementation in preventing respiratory infections [119-121]. For instance, numerous ongoing clinical trials are evaluating the clinical outcomes of vitamin D supplementation in the context of COVID. generally, vitamin D is derived primarily from UVB radiation exposure and dietary intake [122]. Both UV-synthesized and ingested vitamin D then undergo two hydroxylation reactions to become activated as calcitriol. Activated vitamin D is involved in multiple immune functions, including the maintenance of barriers, antigen presentation, innate immunity and adaptive responses. Furthermore, the regulatory roles of vitamin D in the renin-angiotensin-aldosterone system (RAAS) may contribute to its therapeutic potential in mitigating SARS-CoV-2 pathogenesis. Despite the association between vitamin D deficiency and SARS-CoV-2 infection, evidence to support vitamin D supplementation for long COVID management is still lacking. Although recent study did not find an association between vitamin D levels and persistent long COVID symptoms, vitamin D deficiency is known to be associated with fatigue and muscle weakness. Additional research is warranted to explore the relationship between vitamin D and the pathology of long COVID. Optimal vitamin D intake from sunlight exposure and vitamin D rich food (fish, mushroom, and vitamin d-fortified foods) may have proactive effects in preventing COVID-19 infection and relative long COVID risk.
Probiotics are well known to reinforce immunity and counteract inflammation by restoring the gut microbiota. A growing body of research supports the beneficial role of probiotics in lung and mental health by modulating the gut-lung and gut-brain axes [45]. Recently, Lactobacillus plantarum is found to exhibit antiviral effects in SARS-CoV-2 infected intestinal-epithelial cells [123]. Although the beneficial role of probiotics is extensively reported, the interactions of probiotics supplementation for COVID-19 management are not fully elucidated [124, 125].
On the other hand, antibiotic administration has been reported to disrupt gut microflora which resulted in declined prognosis in mouse models of lung infection [47]. Antibiotic use can have several negative effects on the gut microbiota, including reduced species diversity, altered metabolic activity, and the selection of antibiotic-resistant organism. The risks associated with the use of antibiotics for managing long COVID requires continued research.
4. Controversial practices
Repurposed therapeutics, such as ivermectin and hydroxychloroquine are widely available in many countries, which may interfere with proper treatments such as vaccine and dexamethasone. Such situation may lead to increased risk for the development of long COVID.
Anti-parasitic drug, Ivermectin, is a candidate drug during the COVID-19 pandemic. Given the availability and affordability of Ivermectin, abusive usage of ivermectin was reported in some nations as a control effort for COVID-19. However, evidence supporting the utilization of these drugs relied on laboratory data. Clinical data supporting the administration of ivermectin had significant methodical limitations [126]. Meanwhile, Chloroquine/hydroxychloroquine, an anti-malarial treatment [127], has been wildly utilized and studied since the initial outbreak of COVID-19. Preliminary data reported enhanced viral clearance among COVID-19 infected patients with hydroxychloroquine compared with standard treatment [128]. However, as research evolved, the use of hydroxychloroquine became controversial due to the lack of well-designed studies and potential bias from media propaganda [129]. The adverse outcomes from the administration of such therapeutics in the context of long COVID have not been comprehensively reviewed. Furthermore, NIH recommended against the use of the aforementioned drugs for the treatment and prevention of COVID-19.
Conclusion
SARS-CoV-2 has been evolving quickly in the past two years, and multiple variants have gained increased abilities to infect patients or evade the protection by vaccination. Human species will likely co-exist with the virus for many years to come, so long COVID will remain a global challenge to health care system and economy. In order to effectively deal with long COVID, it is important to raise public awareness of its risk factors and take proper management options. With increasing knowledge of COVID-19, the society will be able to protect people's interests if everyone acts together.
Acknowledgements
This work was funded by the University of Macau (File No. MYRG2018-00135-FHS and MYRG2019-00147-FHS), by the Science and Technology Development Fund, Macau SAR (File No. 0059/2019/A1, 0123/2019/A3, and FDCT/0011/2019/AKP) and by Zhuhai City Health Bureau (File No. Zhuhai HLHPTP201702).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Ag. 2020 55
2. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19) A Review. Jama-J Am Med Assoc. 2020;324:782-93
3. Zhou F, Yu T, Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study (vol 395, pg 1054, 2020). Lancet. 2020;395:1038 -
4. WHO. WHO COVID-19 Dashboard. Geneva: World Health Organization. 2020
5. Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. Global Prevalence of Post-Acute Sequelae of COVID-19 (PASC) or Long COVID: A Meta-Analysis and Systematic Review. medRxiv. 2021. 2021 11.15.21266377
6. Huang C, Huang L, Wang Y, Li X, Ren L, Gu X. et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220-32
7. Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re'em Y. et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019
8. Briggs A, Vassall A. Count the cost of disability caused by COVID-19. Nature. 2021;593:502-5
9. Cutler DM, Summers LH. The COVID-19 Pandemic and the Trillion Virus. JAMA. 2020;324:1495-6
10. Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S. et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271 -+
11. Sender R, Bar-On YM, Gleizer S, Bernshtein B, Flamholz A, Phillips R. et al. The total number and mass of SARS-CoV-2 virions. Proceedings of the National Academy of Sciences. 2021;118:e2024815118
12. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet. 2020;395:497-506
13. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-62
14. Li JZ, Gong XB, Wang ZG, Chen RZ, Li TY, Zeng DY. et al. Clinical features of familial clustering in patients infected with 2019 novel coronavirus in Wuhan, China. Virus Res. 2020 286
15. Oran DP, Topol EJ. The Proportion of SARS-CoV-2 Infections That Are Asymptomatic: A Systematic Review. Ann Intern Med. 2021;174:655-62
16. Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. Jama. 2020;323:1239-42
17. Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: A review. Clinical immunology. 2020;215:108427
18. Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV, Condition WHOCCDWGoP-C-. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2021
19. NICE. COVID-19 rapid guideline: managing the long-term effects of COVID-19. Manchester: National Institute for Health and Care Excellence. 2020
20. Su Y, Yuan D, Chen DG, Ng RH, Wang K, Choi J. et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185:881-95.e20
21. Tenforde MW, Kim SS, Lindsell CJ, Rose EB, Shapiro NI, Files DC. et al. Symptom Duration and Risk Factors for Delayed Return to Usual Health Among Outpatients with COVID-19 in a Multistate Health Care Systems Network - United States, March-June 2020. Mmwr-Morbid Mortal W. 2020;69:993-8
22. Carfi A, Bernabei R, Landi F, Aute GAC-P. Persistent Symptoms in Patients After Acute COVID-19. Jama-J Am Med Assoc. 2020;324:603-5
23. Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re'em Y. et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. eClinicalMedicine. 2021 38
24. Stavem K, Ghanima W, Olsen MK, Gilboe HM, Einvik G. Persistent symptoms 1.5-6 months after COVID-19 in non-hospitalised subjects: a population-based cohort study. Thorax. 2021;76:405-7
25. Huang Y, Pinto MD, Borelli JL, Mehrabadi MA, Abrihim H, Dutt N. et al. COVID Symptoms, Symptom Clusters, and Predictors for Becoming a Long-Hauler: Looking for Clarity in the Haze of the Pandemic. medRxiv. 2021
26. Goërtz YMJ, Van Herck M, Delbressine JM, Vaes AW, Meys R, Machado FVC. et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res. 2020;6:00542-2020
27. Szekanecz Z, Valyi-Nagy I. Post-acute COVID-19 syndrome. Orv Hetil. 2021;162:1067-78
28. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-64
29. Zarei M, Bose D, Nouri-Vaskeh M, Tajiknia V, Zand R, Ghasemi M. Long-term side effects and lingering symptoms post COVID-19 recovery. Rev Med Virol. 2021: e2289.
30. Crook H, Raza S, Nowell J, Young M, Edison P. Long covid—mechanisms, risk factors, and management. BMJ. 2021;374:n1648
31. ONS. Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK. In: Statistic OfN, editor. Newport. 2022
32. Mo X, Jian W, Su Z, Chen M, Peng H, Peng P. et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. European Respiratory Journal. 2020;55:2001217
33. Mandal S, Barnett J, Brill SE, Brown JS, Denneny EK, Hare SS. et al. 'Long-COVID': a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. 2021;76:396-8
34. Goldstein DS. The possible association between COVID-19 and postural tachycardia syndrome. Heart Rhythm. 2021;18:508-9
35. Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-90
36. Dennis A, Wamil M, Alberts J, Oben J, Cuthbertson DJ, Wootton D. et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. 2021;11:e048391
37. Dani M, Dirksen A, Taraborrelli P, Torocastro M, Panagopoulos D, Sutton R. et al. Autonomic dysfunction in 'long COVID': rationale, physiology and management strategies. Clinical Medicine. 2021;21:e63-e7
38. Sun B, Tang N, Peluso MJ, Iyer NS, Torres L, Donatelli JL. et al. Characterization and Biomarker Analyses of Post-COVID-19 Complications and Neurological Manifestations. Cells. 2021;10:386
39. Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. The Lancet Psychiatry. 2021;8:416-27
40. Hosp JA, Dressing A, Blazhenets G, Bormann T, Rau A, Schwabenland M. et al. Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID-19. Brain. 2021;144:1263-76
41. Manca R, De Marco M, Ince PG, Venneri A. Heterogeneity in Regional Damage Detected by Neuroimaging and Neuropathological Studies in Older Adults With COVID-19: A Cognitive-Neuroscience Systematic Review to Inform the Long-Term Impact of the Virus on Neurocognitive Trajectories. Front Aging Neurosci. 2021;13:646908 -
42. Douaud G, Lee S, Alfaro-Almagro F, Arthofer C, Wang C, McCarthy P. et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. medRxiv. 2022. 2021 06.11.21258690
43. Huang C, Huang L, Wang Y, Li X, Ren L, Gu X. et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. The Lancet. 2021;397:220-32
44. Liu D, Ahmet A, Ward L, Krishnamoorthy P, Mandelcorn ED, Leigh R. et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy, Asthma & Clinical Immunology. 2013;9:30
45. de Oliveira GLV, Oliveira CNS, Pinzan CF, de Salis LVV, Cardoso CRdB. Microbiota Modulation of the Gut-Lung Axis in COVID-19. Frontiers in Immunology. 2021 12
46. Lamers MM, Beumer J, Van Der Vaart J, Knoops K, Puschhof J, Breugem TI. et al. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;369:50-4
47. Schuijt TJ, Lankelma JM, Scicluna BP, de Sousa e Melo F, Roelofs JJ, de Boer JD. et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut. 2016;65:575-83
48. Lu Z, Zhou H, Wu W-K, Fu T, Yan M, He Z. et al. Alterations in the Composition of Intestinal DNA Virome in Patients With COVID-19. Frontiers in Cellular and Infection Microbiology. 2021 11
49. Liu Q, Mak JWY, Su Q, Yeoh YK, Lui GC-Y, Ng SSS. et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut. 2022;71:544
50. Vestad B, Ueland T, Lerum TrV, Dahl TBr, Holm K, Barratt-Due A. et al. Gut microbiota alterations in patients with persistent respiratory dysfunction three months after severe COVID-19. medRxiv. 2021
51. Nurek M, Rayner C, Freyer A, Taylor S, Järte L, MacDermott N. et al. Recommendations for the recognition, diagnosis, and management of long COVID: a Delphi study. British Journal of General Practice. 2021;71:e815-e25
52. NICE. COVID-19 rapid guideline: managing the long-term effects of COVID-19. London: National Institute for Health and Care Excellence. 2020
53. NIHR. LIVING WITH COVID19. London Natioanl Institute for Health Research. 2021
54. Weinstock LB, Brook JB, Walters AS, Goris A, Afrin LB, Molderings GJ. Mast cell activation symptoms are prevalent in Long-COVID. Int J Infect Dis. 2021;112:217-26
55. Glynne P, Tahmasebi N, Gant V, Gupta R. Long COVID following mild SARS-CoV-2 infection: characteristic T cell alterations and response to antihistamines. J Investig Med. 2022;70:61-7
56. Pinto MD, Lambert N, Downs CA, Abrahim H, Hughes TD, Rahmani AM. et al. Antihistamines for Postacute Sequelae of SARS-CoV-2 Infection. J Nurse Pract. 2022;18:335-8
57. Naureen Z, Dautaj A, Nodari S, Fioretti F, Dhuli K, Anpilogov K. et al. Proposal of a food supplement for the management of post-COVID syndrome. Eur Rev Med Pharmacol Sci. 2021;25:67-73
58. Rossato MS, Brilli E, Ferri N, Giordano G, Tarantino G. Observational study on the benefit of a nutritional supplement, supporting immune function and energy metabolism, on chronic fatigue associated with the SARS-CoV-2 post-infection progress. Clinical Nutrition ESPEN. 2021;46:510-8
59. Memme JM, Erlich AT, Phukan G, Hood DA. Exercise and mitochondrial health. The Journal of Physiology. 2021;599:803-17
60. Wood E, Hall KH, Tate W. Role of mitochondria, oxidative stress and the response to antioxidants in myalgic encephalomyelitis/chronic fatigue syndrome: A possible approach to SARS-CoV-2 'long-haulers'? Chronic Dis Transl Med. 2021;7:14-26
61. Hagenlocher Y, Lorentz A. Immunomodulation of mast cells by nutrients. Molecular Immunology. 2015;63:25-31
62. Cervia C, Zurbuchen Y, Taeschler P, Ballouz T, Menges D, Hasler S. et al. Immunoglobulin signature predicts risk of post-acute COVID-19 syndrome. Nat Commun. 2022 13
63. Zhao YM, Shang YM, Song WB, Li QQ, Xie H, Xu QF. et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25:100463
64. Liao B, Liu Z, Tang L, Li L, Gan Q, Shi H. et al. Longitudinal clinical and radiographic evaluation reveals interleukin-6 as an indicator of persistent pulmonary injury in COVID-19. Int J Med Sci. 2021;18:29-41
65. Marvisi M, Ferrozzi F, Balzarini L, Mancini C, Ramponi S, Uccelli M. First report on clinical and radiological features of COVID-19 pneumonitis in a Caucasian population: Factors predicting fibrotic evolution. Int J Infect Dis. 2020;99:485-8
66. Phetsouphanh C, Darley DR, Wilson DB, Howe A, Munier CML, Patel SK. et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol. 2022;23:210-6
67. Raman B, Cassar MP, Tunnicliffe EM, Filippini N, Griffanti L, Alfaro-Almagro F. et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine. 2021;31:100683
68. Haffke M, Freitag H, Rudolf G, Seifert M, Doehner W, Scherbakov N. et al. Endothelial dysfunction and altered endothelial biomarkers in patients with post-COVID-19 syndrome and chronic fatigue syndrome (ME/CFS). Journal of Translational Medicine. 2022;20:138
69. Townsend L, Dyer AH, Jones K, Dunne J, Mooney A, Gaffney F. et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. Plos One. 2020 15
70. Moreno-Perez O, Merino E, Leon-Ramirez JM, Andres M, Ramos JM, Arenas-Jimenez J. et al. Post-acute COVID-19 syndrome. Incidence and risk factors: A Mediterranean cohort study. J Infection. 2021;82:378-83
71. Fricke LM, Glockner S, Dreier M, Lange B. Impact of non-pharmaceutical interventions targeted at COVID-19 pandemic on influenza burden - a systematic review. J Infect. 2021;82:1-35
72. Fernandez-de-Las-Penas C, Palacios-Cena D, Gomez-Mayordomo V, Cuadrado ML, Florencio LL. Defining Post-COVID Symptoms (Post-Acute COVID, Long COVID, Persistent Post-COVID): An Integrative Classification. Int J Environ Res Public Health. 2021 18
73. Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N. et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science (New York, NY). 2020;369:718-24
74. Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC. et al. Attributes and predictors of long COVID. Nat Med. 2021 27
75. Belkaid Y, Hand Timothy W. Role of the Microbiota in Immunity and Inflammation. Cell. 2014;157:121-41
76. Xu Y, Li X, Zhu B, Liang H, Fang C, Gong Y. et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:502-5
77. Farsi Y, Tahvildari A, Arbabi M, Vazife F, Sechi L, Hashem A. et al. Diagnostic, Prognostic, and Therapeutic Roles of Gut Microbiota in COVID-19: A Comprehensive Systematic Review. Frontiers in Cellular and Infection Microbiology. 2022 12
78. Yong SJ. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis (Lond). 2021;53:737-54
79. van den Borst B, Peters JB, Brink M, Schoon Y, Bleeker-Rovers CP, Schers H. et al. Comprehensive Health Assessment 3 Months After Recovery From Acute Coronavirus Disease 2019 (COVID-19). Clin Infect Dis. 2021;73:E1089-E98
80. Cirulli ET, Schiabor Barrett KM, Riffle S, Bolze A, Neveux I, Dabe S. et al. Long-term COVID-19 symptoms in a large unselected population. medRxiv. 2020. 2020 10.07.20208702
81. Halpin SJ, McIvor C, Whyatt G, Adams A, Harvey O, McLean L. et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J Med Virol. 2021;93:1013-22
82. Taboada M, Carinena A, Moreno E, Rodriguez N, Dominguez MJ, Casal A. et al. Post-COVID-19 functional status six-months after hospitalization. J Infection. 2021;82:E31-E3
83. WorldHealthOrganization. Tracking SARS-CoV-2 variants. 2022.
84. Antonelli M, Pujol JC, Spector TD, Ourselin S, Steves CJ. Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. The Lancet. 2022;399:2263-4
85. Thompson EJ, Williams DM, Walker AJ, Mitchell RE, Niedzwiedz CL, Yang TC. et al. Risk factors for long COVID: analyses of 10 longitudinal studies and electronic health records in the UK. medRxiv. 2021. 2021 06.24.21259277
86. Poyraz BC, Poyraz CA, Olgun Y, Gurel O, Alkan S, Ozdemir YE. et al. Psychiatric morbidity and protracted symptoms after COVID-19. Psychiatry Res. 2021;295:113604
87. Sugiyama A, Miwata K, Kitahara Y, Okimoto M, Abe K, E B. et al. Long COVID occurrence in COVID-19 survivors. Sci Rep-Uk. 2022;12:6039
88. Stewart S, Newson L, Briggs TA, Grammatopoulos D, Young L, Gill P. Long COVID risk - a signal to address sex hormones and women's health. The Lancet Regional Health - Europe. 2021 11
89. Sigfrid L, Drake TM, Pauley E, Jesudason EC, Olliaro P, Lim WS. et al. Long Covid in adults discharged from UK hospitals after Covid-19: A prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg Health-Eu. 2021 8
90. Simani L, Ramezani M, Darazam IA, Sagharichi M, Aalipour MA, Ghorbani F. et al. Prevalence and correlates of chronic fatigue syndrome and post-traumatic stress disorder after the outbreak of the COVID-19. J Neurovirol. 2021;27:154-9
91. Bai F, Tomasoni D, Falcinella C, Barbanotti D, Castoldi R, Mulè G. et al. Female gender is associated with long COVID syndrome: a prospective cohort study. Clin Microbiol Infect. 2021: S1198-743X(21)00629-7.
92. Kurmanova AM, Kurmanova GM, Lokshin VN. Reproductive dysfunctions in viral hepatitis. Gynecol Endocrinol. 2016;32:37-40
93. Kuodi P, Gorelik Y, Zayyad H, Wertheim O, Wiegler KB, Jabal KA. et al. Association between vaccination status and reported incidence of post-acute COVID-19 symptoms in Israel: a cross-sectional study of patients tested between March 2020 and November 2021. medRxiv. 2022. 2022 01.05.22268800
94. Antonelli M, Penfold RS, Merino J, Sudre CH, Molteni E, Berry S. et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. The Lancet Infectious Diseases. 2022;22:43-55
95. FOPH. COVID-19 Switzerland. Switzerland: Federal Office of Public Health. 2020
96. Ma A, Parry J. When Hong Kong's “dynamic zero” covid-19 strategy met omicron, low vaccination rates sent deaths soaring. BMJ. 2022;377:o980
97. Xiao J, Cheung JK, Wu P, Ni MY, Cowling BJ, Liao Q. Temporal changes in factors associated with COVID-19 vaccine hesitancy and uptake among adults in Hong Kong: Serial cross-sectional surveys. The Lancet Regional Health - Western Pacific. 2022 23
98. Smith DJ, Hakim AJ, Leung GM, Xu W, Schluter WW, Novak RT. et al. COVID-19 Mortality and Vaccine Coverage - Hong Kong Special Administrative Region, China, January 6, 2022-March 21, 2022. China CDC Wkly. 2022;4:288-92
99. Tayar E, Abdeen S, Alah MA, Chemaitelly H, Bougmiza I, Ayoub HH. et al. Effectiveness of influenza vaccination against SARS-CoV-2 infection among healthcare workers in Qatar. medRxiv. 2022. 2022 05.09.22274802
100. Taghioff SM, Slavin BR, Holton T, Singh D. Examining the potential benefits of the influenza vaccine against SARS-CoV-2: A retrospective cohort analysis of 74,754 patients. Plos One. 2021;16:e0255541-e
101. Junqueira-Kipnis AP, dos Anjos LRB, Barbosa LCdS, da Costa AC, Borges KCM, Cardoso AdRO. et al. BCG revaccination of health workers in Brazil to improve innate immune responses against COVID-19: A structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:881
102. Charoenlap S, Piromsopa K, Charoenlap C. Potential role of Bacillus Calmette-Guérin (BCG) vaccination in COVID-19 pandemic mortality: Epidemiological and Immunological aspects. Asian Pac J Allergy Immunol. 2020;38:150-61
103. Escobar LE, Molina-Cruz A, Barillas-Mury C. BCG vaccine protection from severe coronavirus disease 2019 (COVID-19). Proc Natl Acad Sci U S A. 2020;117:17720-6
104. Borges KCM, da Costa AC, de Souza Barbosa LC, Ribeiro KM, Dos Anjos LRB, Kipnis A. et al. Tuberculosis, BCG Vaccination and COVID-19: Are They Connected? Mini Rev Med Chem. 2022
105. Fu W, Ho P-C, Liu C-L, Tzeng K-T, Nayeem N, Moore JS. et al. Reconcile the debate over protective effects of BCG vaccine against COVID-19. Sci Rep-Uk. 2021;11:8356
106. Upton CM, van Wijk RC, Mockeliunas L, Simonsson USH, McHarry K, van den Hoogen G. et al. Safety and efficacy of BCG re-vaccination in relation to COVID-19 morbidity in healthcare workers: A double-blind, randomised, controlled, phase 3 trial. EClinicalMedicine. 2022;48:101414
107. de Bree LCJ, Marijnissen RJ, Kel JM, Rosendahl Huber SK, Aaby P, Benn CS. et al. Bacillus Calmette-Guérin-Induced Trained Immunity Is Not Protective for Experimental Influenza A/Anhui/1/2013 (H7N9) Infection in Mice. Front Immunol. 2018;9:869
108. Arts RJW, Moorlag S, Novakovic B, Li Y, Wang SY, Oosting M. et al. BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity. Cell Host Microbe. 2018;23:89-100.e5
109. Organization WH. Bacille Calmette-Guérin (BCG) vaccination and COVID-19. 2020.
110. Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann HH, Zhang Y. et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370:423 -+
111. Milne A, Maskell S, Sharp C, Hamilton F, Arnold D. Impact of dexamethasone on persistent symptoms of COVID-19: an observational study. medRxiv. 2021. 2021 11.17.21266392
112. Gallagher-Allred CR, Voss AC, Finn SC, McCamish MA. Malnutrition and clinical outcomes: the case for medical nutrition therapy. J Am Diet Assoc. 1996;96:361-6 9; quiz 7-8
113. Katona P, Katona-Apte J. The interaction between nutrition and infection. Clin Infect Dis. 2008;46:1582-8
114. Morais AHD, Aquino JD, da Silva-Maia JK, Vale SHD, Maciel BLL, Passos TS. Nutritional status, diet and viral respiratory infections: perspectives for severe acute respiratory syndrome coronavirus 2. Brit J Nutr. 2021;125:851-62
115. Cao W, Li T. COVID-19: towards understanding of pathogenesis. Cell Res. 2020;30:367-9
116. Yu HR, Tain YL, Tiao MM, Chen CC, Sheen JM, Lin IC. et al. Prenatal dexamethasone and postnatal high-fat diet have a synergistic effect of elevating blood pressure through a distinct programming mechanism of systemic and adipose renin-angiotensin systems. Lipids Health Dis. 2018 17
117. Netea MG, Dominguez-Andres J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E. et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20:375-88
118. Chiodini I, Gatti D, Soranna D, Merlotti D, Mingiano C, Fassio A. et al. Vitamin D Status and SARS-CoV-2 Infection and COVID-19 Clinical Outcomes. Frontiers in Public Health. 2021 9
119. Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P. et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583
120. Jolliffe DA, Camargo CA Jr, Sluyter JD, Aglipay M, Aloia JF, Ganmaa D. et al. Vitamin D supplementation to prevent acute respiratory infections: a systematic review and meta-analysis of aggregate data from randomised controlled trials. The Lancet Diabetes & Endocrinology. 2021;9:276-92
121. Rastogi A, Bhansali A, Khare N, Suri V, Yaddanapudi N, Sachdeva N. et al. Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study). Postgraduate Medical Journal. 2022;98:87-90
122. Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol Rev. 2016;96:365-408
123. Liu Y, Liu Q, Jiang Y, Yang W, Huang H, Shi C. et al. Surface-displayed porcine IFN-λ3 in Lactobacillus plantarum inhibits porcine enteric coronavirus infection of porcine intestinal epithelial cells. 2020.
124. Baud D, Dimopoulou Agri V, Gibson GR, Reid G, Giannoni E. Using probiotics to flatten the curve of coronavirus disease COVID-2019 pandemic. Frontiers in public health. 2020;8:186
125. Khailova L, Baird CH, Rush AA, Barnes C, Wischmeyer PE. Lactobacillus rhamnosus GG treatment improves intestinal permeability and modulates inflammatory response and homeostasis of spleen and colon in experimental model of Pseudomonas aeruginosa pneumonia. Clinical Nutrition. 2017;36:1549-57
126. Popp M, Stegemann M, Metzendorf MI, Gould S, Kranke P, Meybohm P. et al. Ivermectin for preventing and treating COVID-19. Cochrane Database of Systematic Reviews. 2021
127. Ben-Zvi I, Kivity S, Langevitz P, Shoenfeld Y. Hydroxychloroquine: from malaria to autoimmunity. Clin Rev Allergy Immunol. 2012;42:145-53
128. Gautret P, Lagier J-C, Parola P, Hoang VT, Meddeb L, Mailhe M. et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Ag. 2020;56:105949
129. Singh B, Ryan H, Kredo T, Chaplin M, Fletcher T. Chloroquine or hydroxychloroquine for prevention and treatment of COVID-19. Cochrane Database of Systematic Reviews. 2021
Author contact
![]() Corresponding author: Guokai Chen, Centre of Reproduction, Development & Aging, Faculty of Health Sciences, University of Macau, Taipa, Macau, China. +853 8822 4985/ GuokaiChenedu.mo
Corresponding author: Guokai Chen, Centre of Reproduction, Development & Aging, Faculty of Health Sciences, University of Macau, Taipa, Macau, China. +853 8822 4985/ GuokaiChenedu.mo

 Global reach, higher impact
Global reach, higher impact