10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(12):4795-4808. doi:10.7150/ijbs.73485 This issue Cite
Review
Revealing the mystery of persistent smell loss in Long COVID patients
1. Center of Reproduction, Development & Aging, and Institute of Translational Medicine, Faculty of Health Sciences, University of Macau, Taipa, Macau, China.
2. Ministry of Education Frontiers Science Center for Precision Oncology, University of Macau, Taipa, Macau, China.
Received 2022-3-31; Accepted 2022-6-9; Published 2022-7-15
Abstract
COVID-19 is hopefully approaching its end in many countries as herd immunity develops and weaker strains of SARS-CoV-2 dominate. However, a new concern occurs over the long-term effects of COVID-19, collectively called “Long COVID”, as some symptoms of the nervous system last even after patients recover from COVID-19. This review focuses on studies of anosmia, i.e., impairment of smell, which is the most common sensory defect during the disease course and is caused by olfactory dysfunctions. It remains mysterious how the olfactory functions are affected since the virus can't invade olfactory receptor neurons. We describe several leading hypotheses about the mystery in hope to provide insights into the pathophysiology and treatment strategies for anosmia.
Keywords: COVID-19, long COVID, olfactory dysfunction
Introduction
Coronavirus disease-19 (COVID-19) has so far infected more than 400 million and killed nearly 6 million people worldwide since the first reported case in December 2019 [1]. It is caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), which infects target cells and hijacks their biological functions [2, 3]. The recently emerging variant of SARS-CoV-2, B.1.1.529 (Omicron), is much more transmissible than the previous variants and has spread rapidly in many areas of the world [4]. Early estimation indicates that Omicron is less severe than the previous variants, possibly due to its less efficient viral replication [5, 6]. Although the morbidity and mortality rates of COVID-19 have not yet slowed down, many countries are preparing to undertake COVID-19 as an endemic disease and slowly crawling back to normalcy through natural and vaccine-induced immunity [7].
However, what remains to be a concern regarding COVID-19 is the persistent symptoms, ranging from fatigue, headache, shortness of breath, smell and taste loss, and depression to psychiatric and cognition defects, which continue to affect millions in their daily life [8-11]. These symptoms, collectively called “Long COVID”, typically last more than 12 weeks and even longer than one year from the onset of the disease. A recent systematic review reported that more than 50% of the COVID-19 survivors suffer at least one of the post-acute sequelae of COVID-19 (PASC) or long COVID symptoms [12, 13]. One prevailing thought for long COVID is the viral persistence in COVID-19 patients, even after the recovery from the disease. Persistent SARS-CoV-2 infection found in certain cohorts with immune deficiency may be a major contributing factor to the persistent symptoms [14].
One of the most common symptoms associated with COVID-19 is the loss of smell, which was previously unrecognized in other CoV-related diseases. Multiple meta-analyses indicate that more than 50% of COVID-19 patients suffer from olfactory impairment during an early stage of the disease or even months after the recovery [15-17]. A study using a microencapsulation assay named University of Pennsylvania Smell Identification TEST reported higher prevalence of olfactory dysfunction (OD) than other studies did based on self-reports such as questionnaires and interviews [18]. The olfactory function is evolutionarily conserved as one of the oldest senses, given its importance in identifying food, mating partners, and escaping dangers [19]. Considering the physiological importance of olfaction and widespread OD prevalence among COVID-19 patients, here we summarize studies on the pathogenesis of OD manifested in COVID-19 and discuss the potential mechanisms of persistent OD as well as treatment options.
Olfactory dysfunctions associated with COVID-19
Intriguingly, many COVID-19 patients and survivors reported central nervous system (CNS) symptoms, including headache, olfactory and taste dysfunctions, seizure, stroke, and even long-term cognitive dysfunctions [11]. Several studies have detected both RNA and protein of SARS-CoV-2 in the brain tissues from COVID-19 autopsies and animal models. The manifested neuro-invasion by SARS-CoV-2 implicates that the virus enters through damaged endothelial cells of the blood-brain barrier (BBB), lymphatic systems associated with respiratory organs, infected immune cells, and neural-mucosal interface [20, 21]. Since the virus enters the brain via the neural-mucosal interface, OD is considered as an important diagnostic and prognostic indicator of the long-term neurological complications associated with COVID-19.
At least 40% of COVID-19 patients reported anosmia as an early sign of the disease, and nearly 70% of patients with OD claimed a reduced quality of life due to smell loss [13, 22]. More than 10% of the infected with COVID-19 may suffer prolonged anosmia, often more than one year from the onset of the disease [17, 23]. A similar but distinct symptom of the OD, called parosmia, described as a distorted or unmatched smell, was found to be less common than anosmia during the duration of COVID-19 but substantially higher in the follow-up surveys conducted months later [24]. This suggests that parosmia could be a valuable diagnostic marker for long COVID.
Epidemiological analysis of COVID-19 associated OD
Many meta-analyses have been carried out to study OD frequency concerning age, sex, potential risk factors such as smoking, and genetic associations. However, variable and even contradictory results were reported in associating OD with the aforementioned factors among COVID-19 patients [15]. This could be mainly due to the experimental variables of the study methods, design, and sample number. For example, most reports on olfactory defects due to COVID-19 are based on self-reports, questionnaires, and other subjective criteria that may hinder the objectivity of the studies.
Despite the inconclusive premises of the studies, several reports provided meaningful epidemiological observations associated with OD. First, smell loss was observed more commonly in asymptomatic COVID-19 cases than severe cases. A study demonstrates that patients with OD suffered less severely in terms of hospitalization and mortality rate [13]. Second, age is considered a major risk factor in COVID-19 related OD [25]. For instance, the expression of endogenous angiotensin-converting enzyme 2 (ACE2), the binding receptor of SARS-CoV-2 on target cells, increase with age in mice, suggestive of the age-dependent OD prevalence and severity [26]. One caveat of the studies is that there is a general decline of the olfaction in later life, which may lead to overestimation of the extent of smell loss. One large-cohort study involving more than 70,000 individuals who reported symptoms found an inverse correlation between age and the frequency of smell loss [27]. Third, several reports suggest no significant association between sex and OD regarding the prevalence and severity [18, 28]. It is interesting to note that a recent study examined the expression profiles of the obligate receptor ACE2 and the accessory protease transmembrane serine protease 2 (TMPRSS2) in thousands of cells from hundreds of biopsy samples from different individuals using single-cell RNA sequencing (scRNA-seq) [29]. The study indicates a strong correlation between sex, age, smoking, and expression of ACE2 and TMPRSS2, suggesting a higher prevalence of OD in males, elders, and smokers than in females, youngers, and non-smokers, respectively [29].
Structure and function of Olfactory system
Anatomy, organization, and physiology of the olfactory mucosa (OM)
The OM is in the roof of the nasal cavity and detects odorants as they enter the nasal cavity. It is a mucus-secreting membrane structure composed of the olfactory epithelium (OE) and lamina propria (LP) beneath. The OE houses multiple cell types, including olfactory receptor neurons (ORNs), also known as olfactory sensory neurons (OSNs), and many supporting/glial cells. ORNs are bipolar neurons with dendritic ends containing multiple protruding cilia which project into the mucosal cavity. The unmyelinated axon bundles extend through the cribriform plate to form a synapsis with mitral and tufted cells in the olfactory glomerulus within the olfactory bulb (OB). Subsequently, the synaptic signal is transmitted to the projection interneurons and ultimately to the primary olfactory cortex and anterior hippocampus for smell recognition and memory retrieval [30, 31].
Besides ORNs, the OE is composed of sustentacular cells (SCs), Bowman's gland ductal cells (BGDCs), horizontal and globose basal stem cells (HBCs and GBCs), and microvilli cells (MVCs) [32]. SCs are morphologically epithelial cells that enwrap the dendrites of the ORNs and mediate the functions of the ORNs by metabolic, secretory, and phagocytic activities [33, 34]. The primary function of BGDCs is secretion of fluids, including mucin, which forms the mucus layer covering the OE. HBC and GBC are multipotent stem cells located at the basal layer of the OE. They can differentiate into both neuronal and non-neuronal cell types to maintain the homeostasis of the OE [35]. MVCs are morphologically similar to SCs and send out microvillar toward the surface of the olfactory mucus, however, the precise function of MVCs is not yet known [36].
Chemosensory mechanism of the olfactory system
The chemosensory activation of the ORNs has been described in detail in the following reviews [37, 38]. Briefly, odorants reach the OE within the olfactory cavity; an odorant binds to a specific odorant receptor (OR), which belongs to the G protein-coupled receptor (GPCR) expressed in the cilia of the ORN. The OR bound by odorants activates a cAMP-based second messenger cascade and then the G protein, Golf, which further activates adenylyl cyclase III and increases cAMP level in the cilia. The subsequent opening of ORN channel triggers the Ca2+ influx and opens the chloride channel, leading to the depolarization of the ORN. The axons of the ORNs expressing a specific receptor and propagating an action potential migrate through the cribriform plate and join other ORNs to form fascicles, innervating glomeruli in the OB.
ORNs and smell recognition
ORN genes constitute the most extensive gene family in the mammalian genome [39]. The dimension and diversity of ORNs genes in the animal genome may be attributable to the need to detect and discriminate the distinct and diverse odorants [31]. The current hypothesis, known as the one neuron-one receptor hypothesis, describes that an ORN transmitting the signal for a unique and specific smell cue is facilitated by the monoallelic expression of a single OR in a mutually exclusive manner [40, 41]. Multiple mechanisms, including DNA recombination, gene conversion, RNA decay, and activation by the locus control region (LCR), have been implicated in achieving the single OR expression [41, 42]. Moreover, it has been demonstrated that the expression of an OR per ORN is critical to not only transmit a specific signal for a unique odorant detection but also to facilitate the axon guidance and correct targeting of the ORN to a specific glomerulus [43].
However, unlike other sensory neurons, the olfactory system exhibits an extraordinarily complex organization to recognize a broad spectrum of odorant molecules and integrate the signals to transmit to the olfactory cortex. A recent study carefully analyzed the OR expressions through scRNA-seq of individual ORNs during olfactory development in Drosophila melanogaster, which revealed two critical principles [44]. First, the same set of receptor genes is expressed continuously in any given ORN during development. Second, the transcriptomic profiles are distinctively maintained in anatomically and functionally defined ORNs throughout the developmental stages. Thus, the OE is spatially organized and ORNs in any defined area may be associated with sensing of a specific group of odorant molecules.
The OM as key entry target for SARS-CoV-2
The mechanism by which SARS-CoV-2 enters the host cells has been discussed in recent reviews [45, 46]. Briefly, the trimeric form of the glycoprotein, Spike (S), encoded by the 3,822-bp S gene, binds to ACE2 and undergoes the cleavage catalyzed by TMPRSS2. Subsequently, the activated Spike protein fuses with the host cell membrane, followed by endocytosis of the viral particle. Given the location of the OM in the nasal cavity, the viral load in the OM is among the highest in the comparative analysis of the swab species from multiple organs, including bronchioles, pharynx, sputum, blood, and urine [47, 48]. More importantly, many cell types constituting the OE express both ACE2 and TMPRSS2 [49]. In fact, Hou et al., revealed the infection gradient based on the ACE2 expression level of various body tissues [50]. The viral titer was the highest in the nasal cavity, where ACE2 expression was the lowest in the alveoli of the respiratory tract [50]. Therefore, it is likely that the OM is the initial infection site for SARS-CoV-2 and manifests olfactory loss as one of the earliest symptoms of COVID-19. Finally, consistent with the notion that D614G mutation rapidly accelerated the viral SARS-CoV-2 transmission by increasing affinity between the receptor binding domain of S protein and ACE2, the G614 variant causes a statistically higher frequency (~6-fold) of anosmia than the D614 variant, further suggesting that the OD is a key diagnostic marker for COVID-19 pathogenesis [51].
Genetic factors as a determinant for COVID-19 associated OD
Whereas OD is affected by many factors in the nasal microenvironment including the viral titer, the pre-existing inflammatory condition or immune-deficiency [52], it may also be associated with the genotype of individuals, i.e., genetic factors may determine the prevalence and severity of COVID-19 associated OD.
First, East Asian populations displayed a 2-3-fold lower rate of olfactory and taste defects than the populations in western countries [53]. The higher susceptibility to the OD was not due to the disproportionate SARS-CoV-2 variant types or mutation rates found in different geographical locations. Instead, the differential expression of ACE2 due to polymorphism found in different populations were found to contribute to differential susceptibility [54, 55]. In particular, the allele frequency of an intronic variant SNP rs2285666 (G8790A) in Asians is nearly 2-fold higher than the other populations, and is associated with lower SARS-CoV-2 infection [56]. Another study indicated a higher concordance and correlation in anosmia among monozygotic twins than dizygotic twins, suggesting a correlation between genetics and the COVID-19-caused anosmia [57]. However, a study involving 1,700 variants from a genome database analyzed the polymorphisms in the coding region of ACE2 in different populations and found no direct correlation among the genetic polymorphism, susceptibility, and symptoms of COVID-19 [58].
Shelton et al., recently conducted a genome-wide association study among more than 69,000 self-reported COVID-19 patients [27], and identified the link of a locus at chr4q13.3 containing UGT2A1 and UGT2A2 to the smell loss [27]. This is the first study linking COVID-19-caused olfactory defects to genetic polymorphisms in large populations. Although the cellular mechanism related to this locus is unknown, drosophila homologs for UGT1A1 and UGT2A2 are known to play an important role in olfactory functions. Reduced detection of pheromone and other functional defects were observed with tissue-specific mutations and RNAi-mediated knockdown of UGT1A1 and UGT2A2 [59].
Discrepancy between the expression pattern of ACE2 and SARS-CoV-2 infection
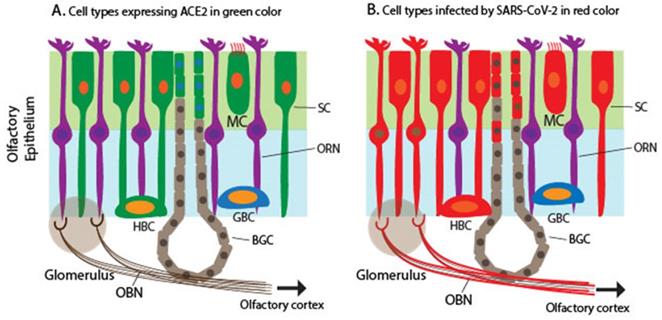
SARS-CoV-2 enters the host cells through its binding receptor ACE2 and protease TMPRSS2. Several scRNA-seq studies determined the expression pattern of ACE2 and TMPRSS2 in the nasal mucosa, particularly ciliated apical OE in humans, non-human primates, and mice [49, 60-63]. TMPRSS2 expression is ubiquitous in many cell types, including neuronal and non-neuronal cell types in the OM, although TMPRSS2 expression is higher in non-neuronal OE cells than in ORNs [49, 64]. A single cell meta-analysis of hundreds of tissue types from more than 200 human subjects demonstrates that the OM shares the expression programs, including ACE2/TMPRSS2 co-expression, with other SARS-CoV-2 target cells such as a subset of alveoli cells and airway secretory cells [29]. Together, ACE2 expression is considered the most significant susceptibility factor for viral entry. Multiple studies have shown that ACE2 and TMPRSS2 expression is restricted to SCs, MVCs, and BGDCs in the OE of humans and mice [60, 61, 65] (Figure 1A). As mentioned above, SCs are non-neuronal columnar epithelial cells and play a critical role in enwrapping ORNs and other supporting functions such as the nutrient exchange and removal of dead debris and neurons. SARS-CoV-2 infects mouse SCs that express human ACE2, leading to massive cell death of the OE, immune cell infiltration, and subsequent damage to the OE structure [65].
However, Meinhardt et al., recently documented the detection of S protein in the cytoplasm and perinuclear regions of two cell types: cells with epithelial morphology and dendrite-harboring cells in the OE [21]. This indicates that SARS-CoV-2 appears to infect both non-neuronal and neuronal cell types in the OE of the OM. The infection of ORNs is confirmed by the co-localization of S protein in a subset of TuJ1/NF200/OMP+ ORNs [21]. The finding was confirmed through detection of viral RNA in both ACE2-expressing non-neuronal cells and non-ACE2-expressing neuronal cells as well as in CNS tissues derived from COVID-19 autopsies [21], indicating that the virus invaded non-ACE2 expressing neurons including ORNs. Other studies have also shown various levels of SARS-CoV-2 proteins and RNA in human samples [60, 61, 66, 67](Figure 1B). Furthermore, animal studies, including mice, hamsters, and ferrets, produced similar results in that SARS-CoV-2 proteins or RNA were detected in non-ACE2 expressing cells [65, 68, 69]. The reason for the discrepancy between the ACE2 expression profile and SARS-CoV-2 infection pattern is unknown.
Most studies indicate that the infection of neuronal cell types not expressing ACE2 is a rare event, as indicated by the extremely low percentage of the co-localization between SARS-CoV-2 antigen and neural markers, particularly ORN markers. What are the possible routes of viral entry to ORNs? One possible mechanism by which the ORNs are infected is through the differentiation of the infected HBCs since HBCs have been shown to express low levels of ACE2 and TMPRSS2 [70]. Once the ORNs are infected, the virus could transverse along the ORN axons and infects the post-synaptic neurons through an uptake using the neurotransmitter system, further infecting olfactory bulb neurons (OBNs) and interneuron en route to the olfactory cortex. It has been shown that herpes simplex virus type I infects the CNS through this route by initially infecting OBNs [71]. Alternatively, ectopic ACE2 expression within a subset of neurons may result in the viral tropism of the neuronal cell types. Other cell-cell communication mechanisms such as the secretion and absorption of the extracellular vesicles (EV) and the transmission of the virus through the gap junction cannot be excluded [72, 73].
For neurons that don't express ACE2, they may be infected through induced expression of ACE2 following interferon (IFN) signaling. SARS-CoV-2 dsRNA induces the activation of type I IFN molecule in ACE2-expressing cells in the OE. Subsequent secretion of IFN-α activates type I IFN signaling and a series of IFN-stimulated genes (ISGs). The increased expression of ISGs provides anti-viral activities by stimulating innate immune cells [74]. Ziegler et al., demonstrated in vivo and in vitro that both type I and II IFN signaling could induce ACE2 expression in both nasal and respiratory epithelium [63]. This suggests that IFN-α and IFN-γ secreted from SCs and the infiltrating immune cells can induce ACE2 expression in the neighboring cells, including ORNs [63]. Interestingly, it was also shown that the non-structural proteins, i.e., nsp1 and nsp13 of SARS-CoV-2 could directly inhibit type I and II IFN signaling by blocking the phosphorylation of STAT1 and STAT2, indicating that there exist intimate and more complex cross-talks between the viral tropism and immune response [75, 76]. Activation of IFN signaling by SARS-CoV-2 infection, in turn, can induce ACE2 expression and exacerbate the infectivity of SARS-CoV-2. This gives the upper hand to the virus in infected cells that generally do not express ACE2.
Pathogenesis of OD caused by SARS-CoV-2
The temporary smell loss can be attributable to three primary sources of dysfunction in the olfactory system. First, the blockage of the nasal passage and mucosal tissue due to local inflammation can prevent odorant molecules from reaching the OE in the roof of the nasal cavity. The level of pro-inflammatory cytokines produced by the infected cells in nasal mucosa has been considered a strong indicator of the disease severity [77]. Second, the temporary olfactory dysfunction can be caused by an altered level or function of ORNs. Third, temporary functional defects in the signal processing parts of the olfactory system, i.e., olfactory bulbs and the olfactory cortex, can contribute to transient smell impairment.
Schematic to illustrate the expression of ACE2 encoding a receptor for SARS-CoV-2 and detection of SARS-CoV-2 in the olfactory mucosa. A. Cells expressing ACE2 in the olfactory mucosa, which are labeled in green, including SCs, MCs, a subset of BGDCs, and HBCs. B. Cells infected by SARS-CoV-2 in the olfactory mucosa, which are labeled in red, including SCs, MCs, HBCs, BGDCs, a subset of ORNs, and cells in the outer layers of OB (the mitral and glomerular neurons), based on the detection of the viral RNA and antigens.
In a hamster model, nasally applied SARS-CoV-2 massively damaged the OE as early as 2 days post-infection (dpi), demonstrated by the reduced thickness of the septum OE and shedding of the ORN cilia into the lumen [68]. The severity of the damage exacerbates 4 dpi and gradually decreases by 14 dpi. However, both the OE thickness and damage levels do not fully return to the normal levels [68]. Significantly, the cilia of the ORN lose as much as 90% of Golf protein detection in the ORN. The most severely affected cell type appears to be SC, as the majority of SCs are positive per immunostaining for SC-specific marker Keratin-18 (K18) and the SARS-CoV-2 N protein. Finally, increased macrophage and monocyte infiltration levels were observed in the OE and the lamina propria of the infected animals. Together, both the severely damaged SCs and infiltration of immune cells affect the integrity and functions of ORNs.
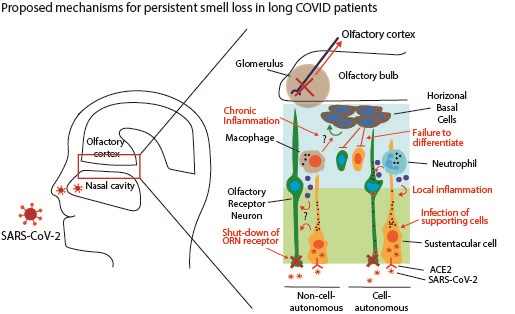
Most COVID-19 survivors with OD symptoms recover in a few weeks, whereas others continue to suffer anosmia for months after its onset [78]. For COVID-19 patients with transient anosmia, damaged cells in the OE are replenished by activation of HBCs. In contrast, chronic or persistent anosmia may be caused by the persistent presence of the virus in the OM of the patients, leading to chronic inflammation in many cell types, including ORNs [66]. In addition to prolonged inflammation, other mechanisms, i.e., cell-autonomous and non-cell-autonomous mechanisms may cause chronic or permanent dysfunction in the olfactory system and will be described below in detail.
Role of the olfactory pathway in SARS-CoV-2 entry to the CNS
Findings on autopsy samples from COVID-19 victims have revealed that SARS-CoV-2 virions invade the defined neural regions, including OB, trigeminal ganglions, and medulla oblongata, as determined per RT-PCR, in situ hybridization, and histocytochemistry [21]. Consistent with the viral penetration of the virus to the CNS, the infiltration of macrophages and CD8+ T lymphocytes in perivascular regions and widespread microglial activation throughout the brain were observed, indicating virus-induced neuroinflammation [21]. Others have also demonstrated the infection of brain tissues in both humans and mice by SARS-CoV-2, whereas the studies didn't propose a possible entry route to the brain [79, 80].
Recent studies have implicated multiple routes and mechanisms by which SARS-CoV-2 invades the CNS and brain. It has been suggested that the virus may directly enter the CNS through the brain capillary endothelial-like cells (BCECs) within the blood-brain-barrier (BBB) structure [81]. Analysis of cerebral-spinal fluid shows up-regulation in the expression of interferon-regulated genes in dendritic cells, along with activated T cells and natural killer (NK) cells. This is accompanied by increased interleukin-1 (IL-1) and IL-12, not seen in the blood plasma [82]. Another suggested mechanism involves neuropilin-1 (NRP1), a transmembrane glycoprotein with a capacity of binding to the furin-cleaved substrates. Cleaved S protein by furin or TMPRSS2 can activate NRP1 for the viral entry into NRP1-expressing endothelial and epithelial cells [83]. Meinhardt et al., proposed that the virus invades the CNS through the axons in the ORNs, which project to specific neuronal areas [21]. The presence of intact SARS-CoV-2 particles in ORNs, neuroanatomical regions, and olfactory track projections suggests neural invasion through axonal transportation [21]. More studies are needed to further define the mechanisms whereby SARS-CoV-2 invades the CNS.
Pathologic mechanism for COVID-19-associated persistent smell loss
Although the temporary smell loss caused by COVID-19 often returns to normal within a few weeks after the recovery from COVID-19, persistent olfactory loss can be a long complication affecting the life quality, for which little is known about the mechanisms. Based on the current literature, we discuss two leading and contrasting views on the cause of persistent anosmia.
Cell-autonomous mechanism
Analyses of olfactory tissues from COVID-19 autopsies indicated viral infection in the olfactory bulb (OB), based on the detection of the viral RNA and protein [21, 84, 85]. In addition, histological and neuroimaging analyses showed a high level of OB damage and both CD148 (an inflammatory marker) and viral antigen were detected in the outer layers of the OB isolated from COVID-19 autopsies [33, 80, 86]. As ACE2 expression is widely distributed in the glomerular and mitral layers within the OB, it is possible that SARS-CoV-2 infects the outer layers of the OB and triggers TLR4-mediated inflammatory response [49, 87].
It is estimated that OBNs undergo less than 1% turnover rate over 100 years based on 14C levels in genomic DNA in the human olfactory system [88]. OBNs are formed by the subsequent migration and maturation of the neuroblasts, differentiated from multipotent neural stem cells that originate from the subventricular zone in the cerebral cortex [89]. Thus, it is challenging to replenish OBNs following their damage or death due to the low neurogenesis potential in adult humans (Figure 2A).
Several animal models showed that SARS-CoV-2 infects, and causes massive damage to, the apical ciliated OE structure, and neutrophils and macrophages recruited to the OE further damage the OE [65, 66, 90]. Regardless of the ACE2 expression pattern, the primary targets for SARS-CoV-2 are SCs, MVCs, and BGDCs, but not ORNS located in the OE [60, 91]. Extensive damage to SCs in the OE leads to functional and structural loss of the OE despite the regenerative potential of stem cells residing in the basal layer of the OE after the injury. It is generally thought that both ORNs and supporting cells are continuously regenerated from stem cells within the OE after the injury. However, the persistent infection by virus and inflammatory microenvironment within the OE may undermine the rate of the regeneration potential and delay restoration of the OD even weeks after the recovery (Figure 2B). This may occur in conjunction with the death of OBNs, resulting in permanent smell loss.
Schematic to illustrate mechanisms for persistent OD associated with COVID-19. A. Infection of the mitral and glomerular cells by SARS-CoV-2 through ACE2-mediated cell entry and low neurogenesis potential of OBNs manifested in permanent smell loss. B. Massive damage and cell death of SCs and ciliated apical side of the OE by SARS-CoV-2 invading through ACE2, illustrated as “Y” shape. The extensive damage to the OE due to the SC cell death affects the ORN structures and functions, leading to permanent smell loss. C. Infiltrated IBA+ neutrophils and macrophages produce inflammatory cytokines, including CXCL10, IL-6, IL-1β, IFNβ, and IFNγ, and affects ORN functions. The persistent presence of the virus results in the prolonged inhibitory activity against the ORNs. D. SARS-CoV-2 infection of the HBCs disrupts ORN differentiation and maturation as well as the restoration of the ORN after the OE damage. Ultimately, the ORN functions are inhibited or damaged for an extended period. E. The exposure to TNFα triggers differential NFκB signaling in the HBCs. The early or chronic inflammation in the OE promotes differentiation and proliferation of HBCs by upregulating the expression of differentiation genes and p63, respectively. imORN and mORN stand for immature and mature ORNs, respectively. F. SARS-CoV-2 infection of SC causes chromatin re-organization such that the OR cluster no longer interacts with the enhancer sequence, thereby downregulating the expressions of OR and ORN signaling genes and disrupting the functions of ORN in the long term. The red dots, black dots, and the half-moon or crescent-moon-shaped objects inside the cells represent SARS-CoV-2, apoptotic granules, and dying nuclei, respectively.
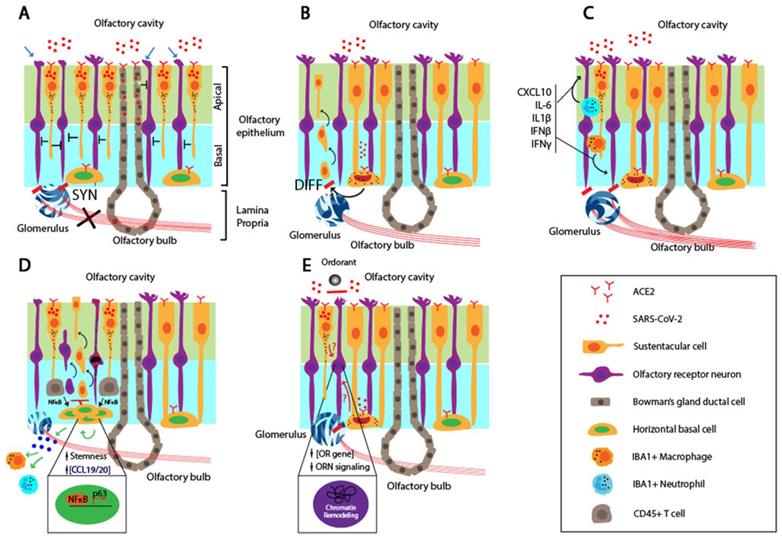
SARS-CoV-2 infection of the OE results in an inflammatory environment. A recent report by Ho et al. provided evidence for the degeneration of olfactory axon injury microvasculopathy in the post-mortem samples, and this indicates a local inflammation in the micro-vessels [92]. It is accompanied by the infiltration of the innate immune cells, as evidenced by co-staining for IBA, a myeloid marker, and the N protein of SARS-CoV-2 [66]. Myeloid-derived macrophages and neutrophils recruited to the infected OE secret pro-inflammatory cytokines. Moreover, biopsy samples from COVID-19 patients with persistent smell loss showed high levels of IBA+ immune cells and up-regulated expression of IL6 in the olfactory mucosa [66]. It is interesting to note that a high level of the viral genomic RNA, but not subgenomic RNA, was present in the olfactory mucosa, indicating no active viral replication in these cells [66]. Together, these findings suggest that the persistent inflammation in the OE affects the ORN and OBN functions (Figure 2C). The persistent presence of the genomic RNA of SARS-CoV-2 may induce IFN expression in an RNA-dependent and TLR-mediated manner, trigger ACE2 expression in neighboring cells, and cause their reinfection by the virus and infection of cells that don't express ACE2 [93, 94].
Brann et al., showed that most of the HBCs express the required receptors, ACE2 and TMPRSS2, which favors the entry of the SARS-CoV-2 [60]. KRT5-positive HBCs derived from biopsy samples of a COVID-19 patient were co-stained positive for ACE2 in the cell body [60]. Furthermore, the level of ACE2 is elevated upon the activation of HBCs, compared to the resting HBCs. In a murine model, the nucleocapsid (N) protein of the SARS-CoV-2 was detected in CK8+ SCs, CK8+/Sox9+ BGCs, and CK5+ HBCs. The SARS-CoV-2 infection of HBCs might cause persistent OD due to reduced regeneration capacity of the OE in a long term (Figure 2D). In cell-autonomous models, the OD directly involves the infection, followed by cell death or functional loss of the neuronal components, i.e., ORNs and OBNs, coupled with delayed or reduced regenerative capacity of the OE stem cells.
Non-cell-autonomous mechanism
Chronic rhinosinusitis (CRS) is a common chronic inflammatory disease of the airway caused by various conditions such as infection and dysfunctions of the sinus. Although its exact etiology is unknown, it has been shown that the infiltration of local immune cells and production of inflammatory cytokines cause the loss of ORNs and reduced olfactory function [95]. Anosmia associated with COVID-19 can be described in the context of CRS. While studying the cause of the smell impairment in CRS, Chen et al., identified a novel mechanism in which the prolonged inflammation locks the HBCs in an undifferentiated state, in part, by upregulating p63 [96]. The inflammation at the early stage of COVD-19 results in production of pro-inflammatory cytokines such as TNFα. It induces HBC to differentiate to ORNs in an NFκB-dependent manner. However, the persistent NFκB signaling lowers the differentiation potential of HBC by upregulating transcription factors involved in maintaining the stemness of HBC (Figure 2E). In this model, the infection or dysfunction of ORNs is not a prerequisite for persistent smell loss.
A recent study by Zazhytska et al., raised an intriguing idea in which SARS-CoV-2 suppresses the ORN functions without infecting the ORNs [67]. Right after the SARS-CoV-2 infection of golden hamsters, the authors detected the viral infection and massive death of the SCs. However, the ORN and OBN infection level was extremely scarce, and these cells remained intact. After transcriptomic analysis of all cells derived from the OE via scRNA-seq, the authors discovered the striking downregulation of ORs and ORN signaling genes in the ORNs. Furthermore, the nuclear chromatin structure was massively reorganized in the ORNs of the infected animals compared to mock controls. Using in situ HiC to measure and quantify the pairwise interactions between two chromosome regions, the authors revealed that physical interactions between OR gene clusters in cis- and trans-contacts were drastically reduced in the infected animals.
Interestingly, the serum depleted of cells by UV from infected hamsters induced a global disruption of the nuclear chromatin structure in ORNs in a non-cell-autonomous manner, which indicates that the soluble factors in the serum of the infected animals may induce the reorganization of the chromatin. The identity and origin of the soluble factors are not known. Nonetheless, the viral infection may induce secretion of the soluble factors, remodel the chromatin within the OR gene clusters, and downregulate the expression of ORs and corresponding signaling genes in the ORNs [67] (Figure 2F).
It has been recently suggested that the SARS-CoV-2 genome can integrate into the genome of host cells in a LINE-1 dependent manner [97]. The genomic RNA of SARS-CoV-2 is integrated into the genome of the cultured lung cells or organoids after the viral infection. The integration was evidenced by the chimeric reads spanning human-negative-strand RNA of SARS-CoV-2 were found in cells derived from deceased COVID-19 patients [97]. It is interesting to note that the detected RNA in the infected ORNs was genomic, but not subgenomic, RNA, indicating that the integrated viral RNAs are non-replicating in nature [66]. Nevertheless, the hypothesis or conclusion that the genomic RNA of SARS-CoV-2 integrates into the human genome met abundant critiques as currently, there is no plausible mechanism for the negative-strand RNA of SARS-CoV-2 to integrate into the human genome. Nonetheless, this phenomenon could be in line with the activation of IFN and ISGs by the viral RNA, leading to persistent infection and damage to the OE, hence permanent OD. Together, non-cell-autonomous models depict the transcriptomic changes in HBCs and chromatin remodeling in ORNs without SARS-CoV-2 infection as the primary causes of persistent OD.
Potential stem cell therapy for persistent OD
While most COVID-19 patients with the symptoms of OD recover from the smell loss, as many as 1.6 million people in the U.S. alone may suffer from permanent smell loss [98].
The current mainstream therapy for chronic or permanent olfactory dysfunctions include smell training, oral or topical steroids, and nonsteroidal oral medications [99-102]. Based on the available evidence, smell training is a recommendation with minimal harm effect and highest benefit in improving olfactory function. The only inconvenience is the need for a sustained daily training for months. The use of short-term oral and/or topical steroids is an option, especially for their anti-inflammatory activity. Considering the potential side effects relating to systemic corticosteroids, the patients should be carefully selected. Numerous nonsteroidal oral medications also show their values in relieving the symptoms of OD, especially when they benefit from wide accessibility and high tolerance. However, more rigorous evaluations are required when contradictory and inconsistent results are produced [103]. Recently, Platelet-rich plasma (PRP) therapy has been applied to treat anosmia patients in the US with a potential promise [104]. PRP therapy has been utilizing the injection of one's own plasma containing platelets to promote the healing of injured ligaments, joints, and muscles. For long term or permanent smell loss, the function of the olfactory neurons is often affected, with neurogenic exhaustion as the common feature. Stem cell-based therapy may be broadly effective in replacing the damaged or senescent ORNs. Given the current understanding of the olfactory and neural stem cells, they are potential sources for cell therapy of permanent OD to restore olfactory functions.
Stem cells in the olfactory mucosa
The OM is directly exposed to the external environment, thus vulnerable to insults including chemicals, viruses, and bacteria, which can cause neuronal cell damage and death. The presence of stem cells in OM maintains the homeostasis of the olfactory cells and functions. The pseudostratified OM consists of mature ORNs, supporting cells, and two distinct populations of basal cells. The two basal cell types are HBCs in flat morphology, positive for Keratin-5 (K5), and the most basally located cells in the OE and GBCs, which lie above the HBC layer. Unlike other parts of the nervous system, the OE retains the capacity for neurogenesis and maintains the olfactory sense during a lifetime [105]. It was thought that HBCs remain quiescent during a normal homeostatic state, and GBCs are sufficient to replenish the whole OE [106]. HBCs become active, morphologically changing from a flattened to pyramidal shape, and regenerate the entire OE when there is substantial damage to the OE [106, 107]. However, a fate-mapping study by Carter et al., demonstrated that HBCs are quiescent and multipotent and can differentiate into both neuronal, i.e., ORNs, and non-neuronal lineages, including SCs and BGDCs, even during the normal turnover of the OE [35]. Moreover, GBCs were derived from HBCs, indicating that HBCs give rise to all cell types, including GBCs in the OE [106]. It is important to note that p63, a transcription factor, is a master regulator of the fate determination, self-renewal, and differentiation of the dormant HBCs [108, 109].
GBCs are heterogeneous in morphology and express Lgr5 exclusively through Wnt-signaling [110]. The neural differentiation potential of GBCs is coordinated by sequential and distinct but overlapping expression of neural transcription factors, including Ascl1, Ngn1, and NeuroD1 [111]. GBCs and HBCs may represent the active and reserve stem cell populations, respectively, during the homeostasis of the OE [112].
Hauser et al., have identified a new type of resident stem cells called olfactory ecto-mesenchymal stem cells (OE-MSCs) in the lamina propria (LP)[113]. These cells were derived from the neural crest and showed a proliferation profile and multiple differentiation potential like bone marrow mesenchymal stem cells (BM-MSCs) [114]. OE-MSCs possess immunomodulatory activity in vitro as MSCs from other sources [114]. Finally, although the vomeronasal organ (VNO) is not a part of the OE, VNO is the secondary olfactory organ located in the lower part of the nasal cavity. It is primarily known to detect sexually and socially relevant chemicals and is thought to contain neural stem cells (NSCs) [115]. In the VNO, there are two types of stem cells: one mainly maintaining the VNO and the other replacing the lost neural cells [115].
Differentiation of olfactory stem cells in vitro and in vivo
HBCs are quiescent in general and don't proliferate in vivo and vitro. However, Peterson et al., could culture HBCs in a medium mimicking the respiratory epithelium (RE) culture system, including dual-SMAD inhibitors and TGFα [116]. Following excision of p63 and retinoic acid treatment, HBCs can be activated and differentiated into neuronal and non-neuronal lineage cells (including GBCs), respectively [116]. Moreover, engrafted HBCs fully restore the methyl bromide-lesioned OE by differentiating to all the OE cell types, including ORNs [116]. Attempts have also been made to purify and expand GBCs in vitro. GBCs were isolated using immunoselection with antibodies against GBC-specific markers and expanded to large quantities in the presence of TGFβR1 inhibitors [117, 118].
Stem cell therapy for COVID-19-associated persistent OD
While the transient OE loss could be restored naturally from activation and differentiation of basal stem cells, the permanent olfactory loss may incur more than 10% of COVID-19 survivors. While nasal steroid and anti-inflammatory sprays are widely used to accelerate the restoration of transient damages, stem cell-based approaches could provide a lasting solution in curing permanent OD.
First, the persistent inflammation in the OE after SARS-CoV-2 infection may attribute to persistent and permanent smell loss. MSCs have been implicated as an excellent cell type to alleviate dysregulated immunity through the secretion of anti-inflammatory cytokines and the expression of immunomodulatory surface proteins [119, 120]. MSCs are easy to culture, scale up, and characterize in vitro and can be easily tested in animal models [121-124]. Furthermore, MSCs are low in immunogenicity in the allogeneic engraftment [125, 126]. Pre-conditioning of MSCs via hypoxic culture or treatment with an inflammatory stimulus such as lipopolysaccharide enhances their immunomodulatory effects due to increased secretion of anti-inflammatory cytokines, including NO, IL-10, and TGFβ, and decreased secretion of inflammatory cytokines, including IL-1, IL-6, and TNFα [127]. Finally, MSCs can directly modulate T cell activity by suppressing effector T cell proliferation and increasing regulatory T cell expansion through elevated expression of immune-modulatory ligands such as programmed cell death ligand-1 [123, 128]. Given the presence and potential immunomodulatory effects of OE-MSCs in vivo, the effective engraftment of the exogenous MSCs into the nasal mucosa or OE could diminish the OE damage and functional loss of ORNs. However, the retention of locally injected MSCs into the OE may gradually go down due to wash-out, cell death, and immune rejection. Antibody-assisted or genetically modified targeting can efficiently deliver and retain the cells on-site [115]. For example, SCs are the only cell types that express K18 in the OE. By labeling MSCs with a peptide or an antibody that binds to K18, local injection of the modified MSCs could maximize the retention of the cells, thus reducing the inflammatory environment and accelerating the restoration of the olfactory functions.
Second, the engraftment of HBCs and GBCs may hold a good promise for OD patients whose HBCs and GBCs are depleted due to viral infection or chronic NF-κB mediated inflammation. In multiple animal models, in which OE legion is induced via olfactory bulbectomy or toxic chemicals such as methyl bromide, engraftment of HBCs and GBCs has fully restored the OE [116, 117]. Kurtenbach et al., developed an inducible hyposmia mouse model via conditional deletion of Ift88, a ciliopathy-related gene in ORNs [117]. In this model, without ciliation, ORNs are incapable of odor transduction, whereas intranasal delivery of purified GBCs can produce odor-responsive ORNs, reinnervate olfactory bulbs, and recover the olfactory behavior of injured mice [117]. It has also been reported that in vitro cultured HBCs, following engraftment into methyl bromide-lesioned OE, can restore the OE by regenerating both neuronal and non-neuronal cell types in the OE [116].
A summary of stem cell therapies of OD used in humans and animal models.
| Stem cell | Source | Species | Disease model | Administration | Function | Effect | Refs. |
|---|---|---|---|---|---|---|---|
| Mouse GBC | Olfactory epithelium | Mouse | Inducible hyposmia mouse model by conditional deletion of the IFT88 gene | Intranasally delivering the cell droplets | Grafted GBC can engraft into the OE, produce odor-responsive OSN, and reinnervate the OB | Cell treated mice show recovered olfactory behavior | [117] |
| Mouse HBC | Olfactory epithelium | Mouse | Olfactotoxic gas methyl bromide lesion | Intranasally grafting | RA pretreated HBC can engraft into lesioned OE, and generate all OE cell types, including OSN | In vitro cultured and expanded HBC can contribute to the regeneration of lesioned OE after transplantation | [116] |
| Mouse Neural stem cells | Olfactory epithelium | Mouse | None | Heterotopic grafting through Stereotaxic injection | OE-NSC can integrate the SVZ and proliferate, further migrate towards OB, and differentiate into neuron in the OB | OE-NSC dierived neurons exhibit electrophysilogical properties similar to endogenous neurons | [129] |
| Rat BMMSC | Cell line | Rat | Triton X-100 irrigation to injure the OM | Cell suspension locally injected into OE | Transplanted BMSC can engraft to the damaged OM and elevat the expression of nerve growth factor and brain-derived neurotrophic factor | Cell treated OM have restored cellular composition | [132] |
| Human ADSC | Aspirated adipose tissue | Mouse | Dichlobenil inoculation to damage the olfactory region | Cell suspension injected through tail vein | Transplanted ADSC can engraft in the lesioned OE and induce neuroregenerative process | Cell treated mice can respond to odorant stimulation activity through electroolfactogram test | [133] |
Third, it is thought that neural stem cells (NSCs) residing in the VNO can replenish damaged olfactory bulb neurons. For such NSC-based cell therapy, it is essential to understand the differentiation potential of olfactory NSCs in vivo. Li et al., recently showed that VNO-derived NSCs migrated to the olfactory bulb and differentiated into olfactory interneurons after engraftment into the subventricular zone [129]. In another study, strips of OE were directly grafted to the olfactory bulb with long-term survival. However, it was not addressed whether the damaged OE functions were restored [130]. The stem cell therapies of OD in humans and animal models are summarized in Table 1.
In addition to the great therapeutic potential, some potential risk of stem cell therapy must be cautiously considered, including immune reactivity, side effects and safety. Ethical consideration or immune rejection can be solved by using autologous derived stem cells. In the pilot study discussed above, no serious adverse effect or death directly related to the implantation of stem cells. Concerns about the biosafety of stem cell transplantation have been reported in many other studies, and the behavior of transplanted stem cells can be guaranteed in many ways [131]. Nonetheless, many factors still challenge the establishment of stem cell based therapy in treating OD, such as the source, density and quality of stem cells, the administration route, dosage and frequency.
Conclusion and perspectives
Olfactory dysfunction, the most detected symptoms in long COVID, severely affects the quality of life. While most of the smell impairments are minor in their nature and fully restored within 4 weeks, thanks to the regenerative capability of stem cells residing in the basal layer of the OE. However, more than 10% of the affected, which could be extrapolated to affect more than 40 million people worldwide, may have compromised quality of life and even face life-threatening risks due to their inability to detect toxic compounds, fire, or gas. As outlined in the review, SARS-CoV-2 may infect ACE2-expressing cells, including SCs, MCs, and BGDCs, in the OE and hijack the essential cellular functions, such as the maintenance of the integrity of the ORN organization and the transmission of odorant signaling through the GPCRs and neural synapses. Surprisingly, ORNs and even OBNs were infected according to animal studies and analysis of postmortem samples, although these cells do not express the receptors for the viral entry.
Since stem cells in the OE express ACE2, they can also be targeted by SARS-CoV-2. The findings thus far have strongly suggested compromised regenerative potential of stem cells in the OE contributing to permanent OD. The persistent or permanent OD is attributable to both cell-autonomous and non-cell-autonomous mechanisms in which the regenerative potential of the OE is compromised. Further underpinning the cellular and molecular mechanisms may provide a new basis for immediate and long-term translational research on various olfactory stem cells and testing their therapeutic potential.
Abbreviations
COVID-19: Coronavirus disease-19
SARS-CoV-2: Severe acute respiratory syndrome coronavirus-2
OD: Olfactory dysfunction
CNS: Central nervous system
BBB: Blood-brain barrier
ACE2: Angiotensin-converting enzyme 2
TMPRSS2: Transmembrane serine protease 2
OM: Olfactory mucosa
OE: Olfactory epithelium
RE: Respiratory epithelium
LP: Lamina propria
ORN: Olfactory receptor neuron
OR: Olfactory receptor
OB: Olfactory bulb
SC: Sustentacular cell
MVC: Microvilli cell
BGDC: Bowman's gland ductal cell
HBC: Horizontal basal cell
GBC: Globose basal cell
GPCR: G protein-coupled receptor
IFN: Interferon
ISG: IFN-stimulated genes
DPI: Days post-infection
Scrams: Single-cell RNA-sequencing
BCEC: Brain capillary endothelial-like cell
OMP: Olfactory marker protein
OE-MSC: Olfactory ecto-mesenchymal stem cells
VNO: Vomeronasal Organ
OBX: Olfactory bulbectomy
NSC: Neural stem cell
IF: Immunofluorescence
ISH: In situ hybridization
HCC: Histocytochemistry
Acknowledgements
This work was supported with the University of Macau Research Committee funds MYRG #2017-00124-FHS and #2020-00140-FHS, Macau Science and Technology Development Fund (FDCT) #0112-2018-A3 and #0002-2020-AKP, and FDCT-National Natural Science Foundation of China joint grant #0008-2019-AFJ to R.X. The authors thank the council members of the Macau Society for Stem Cell Research for inspiring discussion.
Author contributions
J.W.P., X.W., and R.X. conceived of and designed the study and wrote the manuscript. R.X. gave the final approval of the manuscript.
Competing Interests
R.X. is a founder of ImStem Biotechnology, Inc., a stem cell company. The other authors declare no competing financial interests.
References
1. COVID-19 dashboard. 2022. Available from:https://coronavirus.jhu.edu/map.html
2. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020;324:782-93
3. Yi Y, Lagniton PNP, Ye S, Li EQ, Xu RH. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int J Biol Sci. 2020;16:1753-66
4. Mostafavi E, Dubey AK, Teodori L, Ramakrishna S, Kaushik A. SARS-CoV-2 Omicron variant: A next phase of the COVID-19 pandemic and a call to arms for system sciences and precision medicine. Medcomm. 2022;3(1e):119
5. Wolter N, Jassat W, Walaza S, Welch R, Moultrie H, Groome M. et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet. 2022;399:437-46
6. Zhao HJ, Lu L, Peng Z, Chen LL, Meng XJ, Zhang CY. et al. SARS-CoV-2 Omicron variant shows less efficient replication and fusion activity when compared with Delta variant in TMPRSS2-expressed cells. Emerg Microbes Infec. 2022;11:277-83
7. Park JW, Lagniton PNP, Liu Y, Xu RH. mRNA vaccines for COVID-19: what, why and how. Int J Biol Sci. 2021;17:1446-60
8. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A. et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep-Uk. 2021;11:16144
9. Michelen M, Manoharan L, Elkheir N, Cheng V, Dagens A, Hastie C. et al. Characterising long COVID: a living systematic review. BMJ Glob Health. 2021;6(9e):005427
10. Rogers JP, Chesney E, Oliver D, Pollak TA, McGuire P, Fusar-Poli P. et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7:611-27
11. Fotuhi M, Mian A, Meysami S, Raji CA. Neurobiology of COVID-19. J Alzheimers Dis. 2020;76:3-19
12. Groff D, Sun A, Ssentongo AE, Ba DM, Parsons N, Poudel GR. et al. Short-term and Long-term Rates of Postacute Sequelae of SARS-CoV-2 Infection: A Systematic Review. JAMA Netw Open. 2021;4:e2128568
13. Wei G, Gu J, Gu Z, Du C, Huang X, Xing H. et al. Olfactory Dysfunction in Patients With Coronavirus Disease 2019: A Review. Front Neurol. 2021;12:783249
14. Moran E, Cook T, Goodman AL, Gupta RK, Jolles S, Menon DK. et al. Persistent SARS-CoV-2 infection: the urgent need for access to treatment and trials. Lancet Infect Dis. 2021;21:1345-7
15. Saniasiaya J, Islam MA, Abdullah B. Prevalence of Olfactory Dysfunction in Coronavirus Disease 2019 (COVID-19): A Meta-analysis of 27,492 Patients. Laryngoscope. 2021;131:865-78
16. Tong JY, Wong A, Zhu D, Fastenberg JH, Tham T. The Prevalence of Olfactory and Gustatory Dysfunction in COVID-19 Patients: A Systematic Review and Meta-analysis. Otolaryng Head Neck. 2020;163:3-11
17. Renaud M, Thibault C, Le Normand F, McDonald EG, Gallix B, Debry C. et al. Clinical Outcomes for Patients With Anosmia 1 Year After COVID-19 Diagnosis. JAMA Netw Open. 2021;4:e2115352
18. Agyeman AA, Chin KL, Landersdorfer CB, Liew D, Ofori-Asenso R. Smell and Taste Dysfunction in Patients With COVID-19: A Systematic Review and Meta-analysis. Mayo Clin Proc. 2020;95:1621-31
19. Jacobs LF. From chemotaxis to the cognitive map: the function of olfaction. Proc Natl Acad Sci U S A. 2012;109(Suppl 1):10693-700
20. Kalra RS, Dhanjal JK, Meena AS, Kalel VC, Dahiya S, Singh B. et al. COVID-19, Neuropathology, and Aging: SARS-CoV-2 Neurological Infection, Mechanism, and Associated Complications. Front Aging Neurosci. 2021;13:662786
21. Meinhardt J, Radke J, Dittmayer C, Franz J, Thomas C, Mothes R. et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 2021;24:168-175
22. Saniasiaya J, Prepageran N. Impact of olfactory dysfunction on quality of life in coronavirus disease 2019 patients: a systematic review. J Laryngol Otol. 2021;135:947-52
23. Boscolo-Rizzo P, Hummel T, Hopkins C, Dibattista M, Menini A, Spinato G. et al. High prevalence of long-term olfactory, gustatory, and chemesthesis dysfunction in post-COVID-19 patients: a matched case-control study with one-year follow-up using a comprehensive psychophysical evaluation. Rhinology. 2021;59:517-27
24. Ohla K, Veldhuizen MG, Green T, Hannum ME, Bakke AJ, Moein S. et al. Increasing incidence of parosmia and phantosmia in patients recovering from COVID-19 smell loss. medRxiv. 2021. 2021 08.28.21262763
25. Cristillo V, Pilotto A, Cotti Piccinelli S, Zoppi N, Bonzi G, Gipponi S. et al. Age and subtle cognitive impairment are associated with long-term olfactory dysfunction after COVID-19 infection. J Am Geriatr Soc. 2021;69:2778-80
26. Brechbuhl J, Lopes AC, Wood D, Bouteiller S, de Valliere A, Verdumo C. et al. Age-dependent appearance of SARS-CoV-2 entry sites in mouse chemosensory systems reflects COVID-19 anosmia-ageusia symptoms. Commun Biol. 2021;4:880
27. Shelton JF, Shastri AJ, Fletez-Brant K, Auton A, Chubb A, Fitch A. et al. The UGT2A1/UGT2A2 locus is associated with COVID-19-related loss of smell or taste. Nature Genetics. 2022
28. Printza A, Katotomichelakis M, Valsamidis K, Metallidis S, Panagopoulos P, Panopoulou M. et al. Smell and Taste Loss Recovery Time in COVID-19 Patients and Disease Severity. J Clin Med. 2021 10
29. Muus C, Luecken MD, Eraslan G, Sikkema L, Waghray A, Heimberg G. et al. Single-cell meta-analysis of SARS-CoV-2 entry genes across tissues and demographics. Nat Med. 2021;27:546-59
30. Gottfried JA, Smith APR, Rugg MD, Dolan RJ. Remembrance of odors past: Human olfactory cortex in cross-modal recognition memory. Neuron. 2004;42:687-95
31. Su CY, Menuz K, Carlson JR. Olfactory Perception: Receptors, Cells, and Circuits. Cell. 2009;139:45-59
32. Chen CR, Kachramanoglou C, Li D, Andrews P, Choi D. Anatomy and cellular constituents of the human olfactory mucosa: a review. J Neurol Surg B Skull Base. 2014;75:293-300
33. Laurendon T, Radulesco T, Mugnier J, Gerault M, Chagnaud C, El Ahmadi AA. et al. Bilateral transient olfactory bulb edema during COVID-19-related anosmia. Neurology. 2020;95:224-5
34. Choi R, Goldstein BJ. Olfactory Epithelium: Cells, Clinical Disorders, and Insights from an Adult Stem Cell Niche. Laryngoscope Invest. 2018;3:35-42
35. Iwai N, Zhou Z, Roop DR, Behringer RR. Horizontal basal cells are multipotent progenitors in normal and injured adult olfactory epithelium. Stem Cells. 2008;26:1298-306
36. Genovese F, Tizzano M. Microvillous cells in the olfactory epithelium express elements of the solitary chemosensory cell transduction signaling cascade. Plos One. 2018 13
37. Lledo PM, Gheusi G, Vincent JD. Information processing in the mammalian olfactory system. Physiol Rev. 2005;85:281-317
38. Uchida N, Poo C, Haddad R. Coding and Transformations in the Olfactory System. Annu Rev Neurosci. 2014;37:363-85
39. Zhang XM, Firestein S. The olfactory receptor gene superfamily of the mouse. Nat Neurosci. 2002;5:124-33
40. Chess A, Simon I, Cedar H, Axel R. Allelic Inactivation Regulates Olfactory Receptor Gene-Expression. Cell. 1994;78:823-34
41. Serizawa S, Miyamichi K, Sakano H. One neuron-one receptor rule in the mouse olfactory system. Trends Genet. 2004;20:648-53
42. Tan L, Li Q, Xie XS. Olfactory sensory neurons transiently express multiple olfactory receptors during development. Mol Syst Biol. 2015;11:844
43. Feinstein P, Bozza T, Rodriguez I, Vassalli A, Mombaerts P. Axon guidance of mouse olfactory sensory neurons by odorant receptors and the beta2 adrenergic receptor. Cell. 2004;117:833-46
44. McLaughlin CN, Brbic M, Xie Q, Li T, Horns F, Kolluru SS. et al. Single-cell transcriptomes of developing and adult olfactory receptor neurons in Drosophila. Elife. 2021 10
45. Jackson CB, Farzan M, Chen B, Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Bio. 2022;23:3-20
46. V'Kovski P, Kratzel A, Steiner S, Stalder H, Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. 2021;19:155-70
47. Bwire GM, Majigo MV, Njiro BJ, Mawazo A. Detection profile of SARS-CoV-2 using RT-PCR in different types of clinical specimens: A systematic review and meta-analysis. J Med Virol. 2021;93:719-25
48. Wang W, Xu Y, Gao R, Lu R, Han K, Wu G. et al. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020;323:1843-4
49. Bilinska K, Jakubowska P, Von Bartheld CS, Butowt R. Expression of the SARS-CoV-2 Entry Proteins, ACE2 and TMPRSS2, in Cells of the Olfactory Epithelium: Identification of Cell Types and Trends with Age. Acs Chem Neurosci. 2020;11:1555-62
50. Hou YXJ, Okuda K, Edwards CE, Martinez DR, Asakura T, Dinnon KH. et al. SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell. 2020;182(2):429-446
51. von Bartheld CS, Hagen MM, Butowt R. The D614G Virus Mutation Enhances Anosmia in COVID-19 Patients: Evidence from a Systematic Review and Meta-analysis of Studies from South Asia. Acs Chem Neurosci. 2021;12:3535-49
52. Gallo O, Locatello LG, Mazzoni A, Novelli L, Annunziato F. The central role of the nasal microenvironment in the transmission, modulation, and clinical progression of SARS-CoV-2 infection. Mucosal Immunol. 2021;14:305-16
53. Butowt R, von Bartheld CS. Anosmia in COVID-19: Underlying Mechanisms and Assessment of an Olfactory Route to Brain Infection. Neuroscientist. 2021;27:582-603
54. Asselta R, Paraboschi EM, Mantovani A, Duga S. ACE2 and TMPRSS2 variants and expression as candidates to sex and country differences in COVID-19 severity in Italy. Aging (Albany NY). 2020;12:10087-98
55. Cao Y, Li L, Feng Z, Wan S, Huang P, Sun X. et al. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020;6:11
56. Alimoradi N, Sharqi M, Firouzabadi D, Sadeghi MM, Moezzi MI, Firouzabadi N. SNPs of ACE1 (rs4343) and ACE2 (rs2285666) genes are linked to SARS-CoV-2 infection but not with the severity of disease. Virology Journal. 2022;19:48
57. Williams FMK, Freidin MB, Mangino M, Couvreur S, Visconti A, Bowyer RCE. et al. Self-Reported Symptoms of COVID-19, Including Symptoms Most Predictive of SARS-CoV-2 Infection, Are Heritable. Twin Res Hum Genet. 2020;23:316-21
58. Cao YN, Li L, Feng ZM, Wan SQ, Huang PD, Sun XH. et al. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020;6:11
59. Fraichard S, Legendre A, Lucas P, Chauvel I, Faure P, Neiers F. et al. Modulation of Sex Pheromone Discrimination by a UDP-Glycosyltransferase in Drosophila melanogaster. Genes-Basel. 2020;11(3):237
60. Brann DH, Tsukahara T, Weinreb C, Lipovsek M, Van den Berge K, Gong BY. et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci Adv. 2020 6(31)
61. Fodoulian L, Tuberosa J, Rossier D, Boillat M, Kan C, Pauli V. et al. SARS-CoV-2 Receptors and Entry Genes Are Expressed in the Human Olfactory Neuroepithelium and Brain. Iscience. 2020;23(12):101839
62. Sungnak W, Huang N, Becavin C, Berg M, Queen R, Litvinukova M. et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nature Medicine. 2020;26:681-687
63. Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN. et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell. 2020;181:1016-1035
64. Saraiva LR, Ibarra-Soria X, Khan M, Omura M, Scialdone A, Mombaerts P. et al. Hierarchical deconstruction of mouse olfactory sensory neurons: from whole mucosa to single-cell RNA-seq. Sci Rep. 2015;5:18178
65. Ye Q, Zhou J, He Q, Li RT, Yang G, Zhang Y. et al. SARS-CoV-2 infection in the mouse olfactory system. Cell Discov. 2021;7(1):49
66. de Melo GD, Lazarini F, Levallois S, Hautefort C, Michel V, Larrous F. et al. COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci Transl Med. 2021;13(596):eabf8396
67. Zazhytska M, Kodra A, Hoagland DA, Frere J, Fullard JF, Shayya H. et al. Non-cell autonomous disruption of nuclear architecture as a potential cause of COVID-19 induced anosmia. Cell. 2022;185(6):1052-1064
68. Bryche B, St Albin A, Murri S, Lacote S, Pulido C, Ar Gouilh M. et al. Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters. Brain Behav Immun. 2020;89:579-86
69. Sia SF, Yan LM, Chin AWH, Fung K, Choy KT, Wong AYL. et al. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature. 2020;583:834-8
70. Zhang AJ, Lee AC-Y, Chu H, Chan JF-W, Fan Z, Li C. et al. Severe Acute Respiratory Syndrome Coronavirus 2 Infects and Damages the Mature and Immature Olfactory Sensory Neurons of Hamsters. Clin Infect Dis. 2021;73:e503-e12
71. Barnett EM, Cassell MD, Perlman S. Two neurotropic viruses, herpes simplex virus type 1 and mouse hepatitis virus, spread along different neural pathways from the main olfactory bulb. Neuroscience. 1993;57:1007-25
72. Basu R, Banerjee K, Bose A, Das Sarma J. Mouse Hepatitis Virus Infection Remodels Connexin43-Mediated Gap Junction Intercellular Communication In vitro and In vivo. J Virol. 2015;90:2586-99
73. Bello-Morales R, Ripa I, Lopez-Guerrero JA. Extracellular Vesicles in Viral Spread and Antiviral Response. Viruses. 2020;12(6):623643
74. Su S, Jiang S. A suspicious role of interferon in the pathogenesis of SARS-CoV-2 by enhancing expression of ACE2. Signal Transduct Target Ther. 2020;5:71
75. Xia HJ, Cao ZG, Xie XP, Zhang XW, Chen JYC, Wang HL. et al. Evasion of Type I Interferon by SARS-CoV-2. Cell Rep. 2020 33
76. Lei XB, Dong XJ, Ma RY, Wang WJ, Xiao X, Tian ZQ. et al. Activation and evasion of type I interferon responses by SARS-CoV-2. Nat Commun. 2020 11
77. Gulluev M, Yucel A, Kahraman ME, Bor MA. Measurement of some serum cytokines in nasal polyp and evaluation of its correlation with disease severity. Eur Arch Oto-Rhino-L. 2021;278:3345-9
78. Tognetti A, Thunell E, Olsson MJ, Greilert N, Havervall S, Thålin C. et al. High prevalence of olfactory disorders 18 months after contracting COVID-19. medRxiv. 2022. 2022 01.20.22269490
79. Song E, Zhang C, Israelow B, Lu-Culligan A, Prado AV, Skriabine S. et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J Exp Med. 2021 218
80. Solomon IH, Normandin E, Bhattacharyya S, Mukerji SS, Keller K, Ali AS. et al. Neuropathological Features of Covid-19. N Engl J Med. 2020;383:989-92
81. Krasemann S, Haferkamp U, Pfefferle S, Woo MS, Heinrich F, Schweizer M. et al. The blood-brain barrier is dysregulated in COVID-19 and serves as a CNS route for SARS-CoV-2. Stem Cell Rep. 2022;17:307-20
82. Song E, Bartley CM, Chow RD, Ngo TT, Jiang R, Zamecnik CR. et al. Divergent and self-reactive immune responses in the CNS of COVID-19 patients with neurological symptoms. Cell Rep Med. 2021;2:100288
83. Cantuti-Castelvetri L, Ojha R, Pedro LD, Djannatian M, Franz J, Kuivanen S. et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370:856-60
84. Xydakis MS, Albers MW, Holbrook EH, Lyon DM, Shih RY, Frasnelli JA. et al. Post-viral effects of COVID-19 in the olfactory system and their implications. Lancet Neurol. 2021;20:753-61
85. Fabbri VP, Foschini MP, Lazzarotto T, Gabrielli L, Cenacchi G, Gallo C. et al. Brain ischemic injury in COVID-19-infected patients: a series of 10 post-mortem cases. Brain Pathol. 2021;31:205-10
86. Matschke J, Lutgehetmann M, Hagel C, Sperhake JP, Schroder AS, Edler C. et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19:919-29
87. Klingenstein M, Klingenstein S, Neckel PH, Mack AF, Wagner AP, Kleger A. et al. Evidence of SARS-CoV2 Entry Protein ACE2 in the Human Nose and Olfactory Bulb. Cells Tissues Organs. 2020;209:155-64
88. Bergmann O, Liebl J, Bernard S, Alkass K, Yeung MS, Steier P. et al. The age of olfactory bulb neurons in humans. Neuron. 2012;74:634-9
89. Bond AM, Ming GL, Song H. Adult Mammalian Neural Stem Cells and Neurogenesis: Five Decades Later. Cell Stem Cell. 2015;17:385-95
90. Khan M, Yoo SJ, Clijsters M, Backaert W, Vanstapel A, Speleman K. et al. Article Visualizing in deceased COVID-19 patients how SARS-CoV-2 attacks the respiratory and olfactory mucosae but spares the olfactory bulb. Cell. 2021;184:5932 -+
91. Nakashima N, Nakashima K, Takaku-Nakashima A, Takano M. Olfactory receptor neurons express olfactory marker protein but not calpain 5 from the same genomic locus. Mol Brain. 2019 12
92. Ho CY, Salimian M, Hegert J, O'Brien J, Choi SG, Ames H. et al. Postmortem Assessment of Olfactory Tissue Degeneration and Microvasculopathy in Patients With COVID-19. JAMA Neurol. 2022
93. Aboudounya MM, Heads RJ. COVID-19 and Toll-Like Receptor 4 (TLR4): SARS-CoV-2 May Bind and Activate TLR4 to Increase ACE2 Expression, Facilitating Entry and Causing Hyperinflammation. Mediat Inflamm. 2021. 2021
94. Torabi A, Mohammadbagheri E, Akbari Dilmaghani N, Bayat AH, Fathi M, Vakili K. et al. Proinflammatory Cytokines in the Olfactory Mucosa Result in COVID-19 Induced Anosmia. Acs Chem Neurosci. 2020;11:1909-13
95. Kern RC. Chronic sinusitis and anosmia: pathologic changes in the olfactory mucosa. Laryngoscope. 2000;110:1071-7
96. Chen MF, Reed RR, Lane AP. Chronic Inflammation Directs an Olfactory Stem Cell Functional Switch from Neuroregeneration to Immune Defense. Cell Stem Cell. 2019;25:501 -+
97. Zhang LG, Richards A, Barrasa MI, Hughes SH, Young RA, Jaenisch R. Reverse-transcribed SARS-CoV-2 RNA can integrate into the genome of cultured human cells and can be expressed in patient-derived tissues. P Natl Acad Sci USA. 2021 118
98. Khan AM, Kallogjeri D, Piccirillo JF. Growing Public Health Concern of COVID-19 Chronic Olfactory Dysfunction. JAMA Otolaryngol Head Neck Surg. 2022;148:81-2
99. Gupta S, Kallogjeri D, Farrell NF, Lee JJ, Smith HJ, Khan AM. et al. Development and Validation of a Novel At-home Smell Assessment. Jama Otolaryngol. 2022
100. Hummel T, Rissom K, Reden J, Hahner A, Weidenbecher M, Huttenbrink KB. Effects of olfactory training in patients with olfactory loss. Laryngoscope. 2009;119:496-9
101. Konstantinidis I, Tsakiropoulou E, Constantinidis J. Long term effects of olfactory training in patients with post-infectious olfactory loss. Rhinology. 2016;54:170-5
102. Neta FI, Fernandes ACL, Vale AJM, Pinheiro FI, Cobucci RN, Azevedo EP. et al. Pathophysiology and possible treatments for olfactory-gustatory disorders in patients affected by COVID-19. Curr Res Pharmacol Drug Discov. 2021;2:100035
103. Hura N, Xie DX, Choby GW, Schlosser RJ, Orlov CP, Seal SM. et al. Treatment of post-viral olfactory dysfunction: an evidence-based review with recommendations. Int Forum Allergy Rhinol. 2020;10:1065-86
104. Klug TR, D.; Chaskes, M.; Souza, G. D.; Pribitkin, E. Treatment of refractory anosmia with topical platelet rich plasma. Otolaryngology - Head and Neck Surgery. 2021;165(1 SUPPL):P344-P5
105. Carter LA, MacDonald JL, Roskams AJ. Olfactory horizontal basal cells demonstrate a conserved multipotent progenitor phenotype. J Neurosci. 2004;24:5670-83
106. Leung CT, Coulombe PA, Reed RR. Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nat Neurosci. 2007;10:720-6
107. Brann JH, Ellis DP, Ku BS, Spinazzi EF, Firestein S. Injury in aged animals robustly activates quiescent olfactory neural stem cells. Front Neurosci-Switz. 2015 9
108. Fletcher RB, Prasol MS, Estrada J, Baudhuin A, Vranizan K, Choi YG. et al. p63 Regulates Olfactory Stem Cell Self-Renewal and Differentiation. Neuron. 2011;72:748-59
109. Schnittke N, Herrick DB, Lin B, Peterson J, Coleman JH, Packard AI. et al. Transcription factor p63 controls the reserve status but not the stemness of horizontal basal cells in the olfactory epithelium. Proc Natl Acad Sci U S A. 2015;112:E5068-77
110. Chen MF, Tian SH, Yang XL, Lane AP, Reed RR, Liu HJ. Wnt-Responsive Lgr5(+) Globose Basal Cells Function as Multipotent Olfactory Epithelium Progenitor Cells. Journal of Neuroscience. 2014;34:8268-76
111. Cau E, Casarosa S, Guillemot F. Mash1 and Ngn1 control distinct steps of determination and differentiation in the olfactory sensory neuron lineage. Development. 2002;129:1871-80
112. Schwob JE, Jang W, Holbrook EH, Lin B, Herrick DB, Peterson JN. et al. Stem and progenitor cells of the mammalian olfactory epithelium: Taking poietic license. J Comp Neurol. 2017;525:1034-54
113. Hauser S, Widera D, Qunneis F, Muller J, Zander C, Greiner J. et al. Isolation of Novel Multipotent Neural Crest-Derived Stem Cells from Adult Human Inferior Turbinate. Stem Cells Dev. 2012;21:742-56
114. Rui K, Zhang ZJ, Tian J, Lin X, Wang XH, Ma J. et al. Olfactory ecto-mesenchymal stem cells possess immunoregulatory function and suppress autoimmune arthritis. Cell Mol Immunol. 2016;13:401-8
115. Brann JH, Firestein S. Regeneration of New Neurons Is Preserved in Aged Vomeronasal Epithelia. Journal of Neuroscience. 2010;30:15686-94
116. Peterson J, Lin B, Barrios-Camacho CM, Herrick DB, Holbrook EH, Jang W. et al. Activating a Reserve Neural Stem Cell Population In vitro Enables Engraftment and Multipotency after Transplantation. Stem Cell Rep. 2019;12:680-95
117. Kurtenbach S, Goss GM, Goncalves S, Choi R, Hare JM, Chaudhari N. et al. Cell-Based Therapy Restores Olfactory Function in an Inducible Model of Hyposmia. Stem Cell Rep. 2019;12:1354-65
118. Chen X, Fang H, Schwob JE. Multipotency of purified, transplanted globose basal cells in olfactory epithelium. J Comp Neurol. 2004;469:457-74
119. Weiss ARR, Dahlke MH. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs. Front Immunol. 2019 10
120. Jiang B, Yan L, Wang X, Li E, Murphy K, Vaccaro K. et al. Concise Review: Mesenchymal Stem Cells Derived from Human Pluripotent Cells, an Unlimited and Quality-Controllable Source for Therapeutic Applications. Stem Cells. 2019;37:572-81
121. Wang XY, Jiang B, Sun HY, Zheng DJ, Zhang ZW, Yan L. et al. Noninvasive application of mesenchymal stem cell spheres derived from hESC accelerates wound healing in a CXCL12-CXCR4 axis-dependent manner. Theranostics. 2019;9:6112-28
122. Yan L, Jiang B, Li EQ, Wang XY, Ling QJ, Zheng DJ. et al. Scalable Generation of Mesenchymal Stem Cells from Human Embryonic Stem Cells in 3D. Int J Biol Sci. 2018;14:1196-210
123. Wang X, Lazorchak AS, Song L, Li E, Zhang Z, Jiang B. et al. Immune modulatory mesenchymal stem cells derived from human embryonic stem cells through a trophoblast-like stage. Stem Cells. 2016;34:380-91
124. Liao H, Wang H, Rong X, Li E, Xu RH, Peng Y. Mesenchymal Stem Cells Attenuate Radiation-Induced Brain Injury by Inhibiting Microglia Pyroptosis. Biomed Res Int. 2017;2017:1948985
125. Schu S, Nosov M, O'Flynn L, Shaw G, Treacy O, Barry F. et al. Immunogenicity of allogeneic mesenchymal stem cells. J Cell Mol Med. 2012;16:2094-103
126. Borkar R, Wang X, Zheng D, Miao Z, Zhang Z, Li E. et al. Human ESC-derived MSCs enhance fat engraftment by promoting adipocyte reaggregation, secreting CCL2 and mobilizing macrophages. Biomaterials. 2021;272:120756
127. Saparov A, Ogay V, Nurgozhin T, Jumabay M, Chen WCW. Preconditioning of Human Mesenchymal Stem Cells to Enhance Their Regulation of the Immune Response. Stem Cells Int. 2016. 2016
128. Davies LC, Heldring N, Kadri N, Le Blanc K. Mesenchymal Stromal Cell Secretion of Programmed Death-1 Ligands Regulates T Cell Mediated Immunosuppression. Stem Cells. 2017;35:766-76
129. Li Q, Siri T, Bressan C, de Koninck Y, Saghatelyan A. Developmental Potential and Plasticity of Olfactory Epithelium Stem Cells Revealed by Heterotopic Grafting in the Adult Brain. Stem Cell Rep. 2020;14:692-702
130. Yagi S, Costanzo RM. Grafting the olfactory epithelium to the olfactory bulb. Am J Rhinol Allergy. 2009;23:239-43
131. Wang X, Jiang B, Sun H, Zheng D, Zhang Z, Yan L. et al. Noninvasive application of mesenchymal stem cell spheres derived from hESC accelerates wound healing in a CXCL12-CXCR4 axis-dependent manner. Theranostics. 2019;9:6112-28
132. Kwon JW, Jo HG, Park SM, Ku CH, Park DJ. Engraftment and regenerative effects of bone marrow stromal cell transplantation on damaged rat olfactory mucosa. Eur Arch Otorhinolaryngol. 2016;273:2585-90
133. Franceschini V, Bettini S, Pifferi S, Menini A, Siciliano G, Ognio E. et al. Transplanted human adipose tissue-derived stem cells engraft and induce regeneration in mice olfactory neuroepithelium in response to dichlobenil subministration. Chem Senses. 2014;39:617-29
Author contact
![]() Corresponding author: Jung Woo Park, Faculty of Health Sciences, University of Macau, Taipa, Macau, China. Email: jungwparkedu.mo. Ren-He Xu, Faculty of Health Sciences, University of Macau, Taipa, Macau, China. Email: renhexuedu.mo.
Corresponding author: Jung Woo Park, Faculty of Health Sciences, University of Macau, Taipa, Macau, China. Email: jungwparkedu.mo. Ren-He Xu, Faculty of Health Sciences, University of Macau, Taipa, Macau, China. Email: renhexuedu.mo.

 Global reach, higher impact
Global reach, higher impact