10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(13):5070-5085. doi:10.7150/ijbs.72706 This issue Cite
Review
Development and application of ribonucleic acid therapy strategies against COVID-19
1. School of Healthcare Technology, Chengdu Neusoft University, Sichuan, China
2. School of Life Science and Technology, University of Electronic Science and Technology of China, Sichuan, China
3. Ineye Hospital of Chengdu University of TCM, Sichuan, China
4. Medical College, Guizhou University, Guizhou, China
Received 2022-3-8; Accepted 2022-7-16; Published 2022-8-1
Abstract
The Coronavirus disease 2019 (COVID-19) pandemic is caused by the severe acute respiratory syndrome 2 coronavirus (SARS-CoV-2), remaining a global health crisis since its outbreak until now. Advanced biotechnology and research findings have revealed many suitable viral and host targets for a wide range of therapeutic strategies. The emerging ribonucleic acid therapy can modulate gene expression by post-transcriptional gene silencing (PTGS) based on Watson-Crick base pairing. RNA therapies, including antisense oligonucleotides (ASO), ribozymes, RNA interference (RNAi), aptamers, etc., were used to treat SARS-CoV whose genome is similar to SARV-CoV-2, and the past experience also applies for the treatment of COVID-19. Several studies against SARS-CoV-2 based on RNA therapeutic strategy have been reported, and a dozen of relevant preclinical or clinical trials are in process globally. RNA therapy has been a very active and important part of COVID-19 treatment. In this review, we focus on the progress of ribonucleic acid therapeutic strategies development and application, discuss corresponding problems and challenges, and suggest new strategies and solutions.
Keywords: ribonucleic acid therapy, COVID-19, SARS-CoV-2, antisense oligonucleotides, ribozymes, RNA interference
Introduction
The Coronavirus disease 2019 (COVID-19) is an emerging infectious disease caused by a new form of severe acute respiratory syndrome coronavirus (SARS-CoV), namely SARS-CoV-2 [1, 2]. As known so far, the SARS-CoV-2 spreads faster but is less pathogenic than SARS-CoV, which affected more than 8000 individuals all over the world with a fatality rate of 11% in 2002-2004 [3, 4]. Among those affected patients, the failure of lung or related cardiovascular system contributed to a large share of the death. An excessive pro-inflammatory response mediated by elevated inflammatory cytokines and chemokines can lead to lung injury and acute respiratory distress syndrome (ARDS). In addition, viral infection can cause a series of clinical symptoms, such as neurological complications, coagulation disorders, liver and kidney failures as well [5, 6]. Since the World Health Organization (WHO) declared the COVID-19 outbreak a global pandemic, as of January 31, 2022, 373,229,380 confirmed cases, including 5,658,702 deaths, have been reported [7, 8]. Another disturbing concern is the variability of SARS-CoV-2. From the Alpha variant to the latest Omicron variant, the pathogenicity and transmissibility of the virus are qualitatively changed compared with the original strain, adding to the complexity of and difficulty in the pandemic prevention and treatment [9, 10]. To keep COVID-19 at bay, scientists have developed various vaccines to enhance immunity, and accelerated the development of therapeutic drugs, including small molecule and antibody drugs [11-14]. Among them, ribonucleic acid therapeutic strategies, which saw rapidly development in the past few years, seem to be most potential in treating COVID-19.
The approved RNA therapeutic drugs and vaccines
| Categories | Drugs (Trade name) | Indications | Years | Locations | Developers | Reference |
|---|---|---|---|---|---|---|
| ASO | Fomivirsen(Vitravene) | CMV | 1998 | US | lonis/Novartis | [24] |
| ASO | Mipomersen(Kynamro) | HoFH | 2013 | US | lonis/Sanofi | [29] |
| ASO | Eteplirsen(Exondys 51) | DMD | 2016 | US | Sarepta Therapeutics | [30] |
| ASO | Nusinersen(Spinraza) | SAM | 2016 | US | lonis/Biogen | [31] |
| ASO | Inotersen(Tegsedi) | hATTR | 2018 | US | lonis | [32] |
| ASO | Volanesorsen(Waylivra) | FCS | 2019 | EU | lonis | [33] |
| ASO | Golodirsen(Vyondy 53) | DMD | 2019 | US | Sarepta Therapeutics | [34] |
| ASO | Viltolarsen(Viltepso) | DMD | 2020 | US/Japan | Nippon Shinyaku | [35] |
| ASO | Casimersen(AMONDYS 45) | DMD | 2021 | US | Sarepta Therapeutics | [36] |
| siRNA | Patisiran(Onpattro) | FAP | 2018 | US/EU | Alynlam | [25] |
| siRNA | Givosiran(Givlaari) | AHP | 2019 | US | Alynlam | [37] |
| siRNA | Lumasiran(OXLUMO) | PH1 | 2020 | US/EU | Alynlam | [38] |
| siRNA | Leqvio(Inclisiran) | Primary hypercholesterolaemia/mixed dyslipidaemia | 2020 | EU | Alynlam/Novartis | [39] |
| Aptamer | Pegaptanib(Macugen) | AMD | 2004 | EU | Eyetech/Pfizer | [40] |
| Vaccine | BNT162b2(Comirnaty) | COVID-19 | 2021 | US | BioNTech/Pfizer | [26] |
| Vaccine | mRNA-1273(Spikevax) | COVID-19 | 2021 | US | Moderna | [27] |
AHP, acute hepatic porphyria; AMD, age-related macular degeneration; CMV, cytomegalovirus; COVID-19, coronavirus disease 2019; DMD, duchenne muscular dystrophy; FAP, familial amyloidotic polyneuropathy; FCS, familial chylomicronemia syndrome; hereditary transthyretin amyloidosis; HoFH, homozygous familial hypercholesterolemia; PH1, primary hyperoxaluria type 1; SMA, Spinal muscular atrophy.
RNA Therapy
The classic central dogma of molecular biology believes that RNA is the transmitter of genetic information between DNA and proteins [15], and the discovery of catalytic RNA in the 1980s expanded the functions of RNA [16]. The Human Genome Project (HGP) and the progress in high-throughput sequencing technology dug up further information on the human genome, especially the gradual identification of the functions of a range of non-coding RNAs, completely changing researchers' perception of the RNA world [17]. It is now believed that RNA regulates the genes' functions in all living cells, and is closely related to many diseases [18]. In the 1990s, after the discovery of RNA interference (RNAi) [19], RNA-based biotherapeutics came into being. Researchers tried to control gene expression permanently or temporarily by transporting nucleic acids into cells so as to treat the diseases. Thus, the era of RNA therapy has begun.
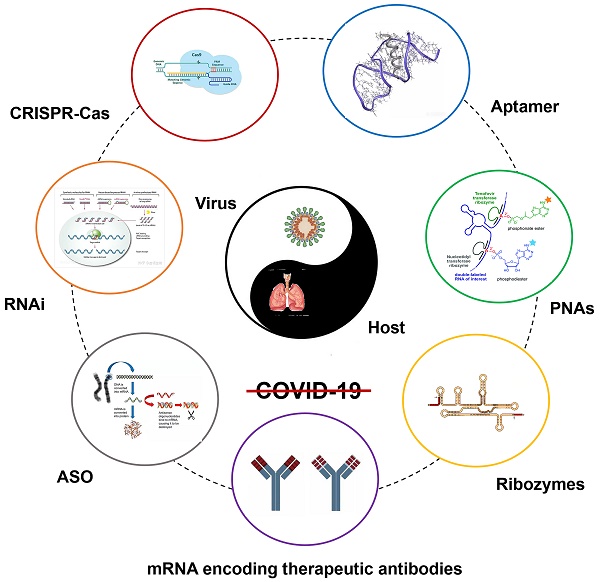
Under most circumstances, RNA therapies can be classified into several categories, including antisense oligonucleotides (ASO) which inhibit the translation of mRNA, RNAi, aptamers that could bind proteins and other molecular ligands, small activating RNAs (saRNAs) with the ability to activate genes, catalytic RNAs or called ribozymes, RNA vaccines and gene editing approaches such as CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats)-Cas (CRISPR-associated) systems [20]. RNA based biotherapeutics have natural advantages over the traditional treatments. Since most RNA therapeutic drugs depend on the principles of Watson-Crick base pairing, it can specifically and closely complement and bind to the target to serve the purpose of treatment [21, 22]. Besides, the therapeutic RNAs can be designed to suppress any genes, regardless of whether they are noncoding or highly expressed, and thus have the potential to treat a range of diseases theoretically. What's more, RNA can be synthesized through chemical methods, which is more cost-effective and convenient than other biologics. Therefore, RNA therapeutic drugs have significant potential in application because of their high efficacy, specificity and wide range of application [20].
Over the past two decades, a dozen of RNA therapeutic drugs have been approved by the US Food and Drug Administration (FDA). The specific list can be found in Table 1. Fomivirsen (trade name: Vitravene) is the first antisense oligonucleotide drug approved for the treatment of cytomegalovirus (CMV) retinitis complicated by AIDS patients [23]. Antiviral effects are exerted by antisense inhibition of CMV mRNA [24]. On August 10, 2018, the world's first small interfering RNA (siRNA) drug Patisiran (trade name: Onpattro) was approved by FDA. It is used to treat hereditary transthyretin-mediated amyloidosis and acute hepatic porphyria (AHP), as the first RNAi based drug in the world [25]. In 2021, faced with the growing threat from the COVID-19 pandemic, FDA approved two mRNA vaccines developed respectively by the companies of Pfizer/BioNtech and Moderna [26, 27]. So far, over 400 RNA-targeting drug development projects have been conducted worldwide, two-thirds of which are in the pre-investigational new drug (pre-IND) stage, one-third in early clinical trials (phase I or II), about 3% in phase III, and some waiting regulatory approval. As most of the RNA drugs deliver a good performance, such as Patisiran with sales over $150 million in 2019, the world's major pharmaceutical companies have made heavy investment in RNA therapeutic drugs to accelerate their development [28].
Viral infection is a common clinical disease [41], and can be a serious threat to human health as some viruses spread rapidly and have a high fatality rate. Today, there are two major interventions for viral infection: one is vaccines, the other is antiviral drugs to control clinical symptoms and reduce viral activity [42, 43]. However, the slow development of vaccines and the lack of specificity for most antiviral drugs make it more than urgent to find new antiviral strategies. The RNA therapeutic drugs may provide us with the answer [44]. Taking RNAi as an example, it can silence or suppress the expression of genes related to viral replication or some other important biological functions to fight against viral infection. Many researches on RNA therapy against viral infection are going on, including hepatitis B (HBV) [45], AIDS (HIV) [46] and hand-foot-and-mouth disease related virus [47], etc. In the detection and treatment of coronavirus infection, including SARS-CoV, Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and SARS-CoV-2 raging around the world over the past two years, several studies on RNA therapy have been reported [48, 49]. Those in vitro and clinical trials show that RNA therapeutic strategies can successfully inhibit the replication of the viruses, thus become the most potential useful tool in controlling COVID-19 [50].
With great progress in RNA therapy, it still has some limitations in practice. Due to its poor stability, RNA can be easily degraded in organisms by ribonuclease (RNase), making the biological half-life too short. The low efficiency of cellular uptake makes it difficult for the drugs to get into the target cells [20]. Besides, RNA itself is potentially immunogenic and likely to have off-target effects [51, 52]. Efforts to improve the features of natural nucleic acids and develop them into useful drugs are diminished by these factors. To overcome these shortages, various chemical modifications and novel delivery systems have been designed to make RNA drugs more accurate. RNA therapy is a rapidly developing field and has significant potential in fighting against SARS-CoV-2. This review, with a focus on the development and application of ribonucleic acid therapeutic strategies for COVID-19, addresses its problems and challenges along its progress, and puts forward some feasible strategies and solutions.
Potential Targets for RNA Therapy against COVID-19
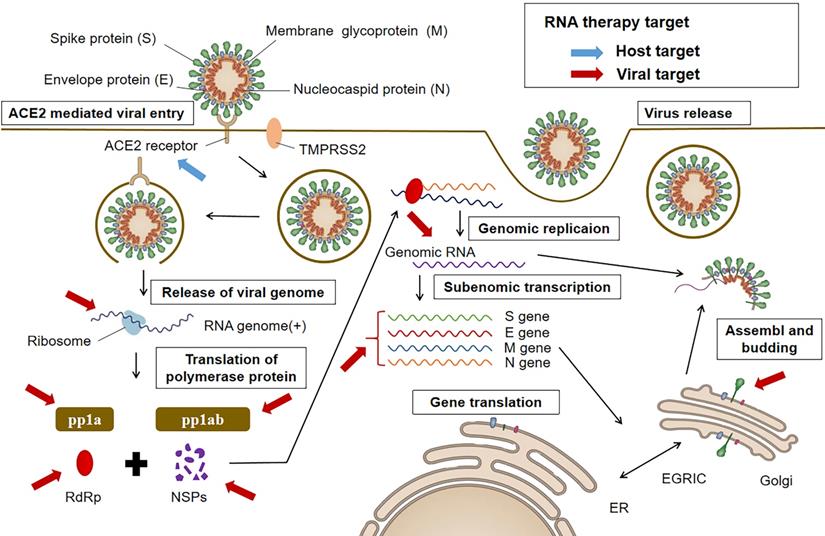
Though being theoretically feasible for RNA therapy to silence or suppress any protein, it is essential to select effective targets for the efficacy of the treatment. In general, there are two antiviral targeting strategies: (i) viral proteins essential in survival and replication, and (ii) related host factors including those involved in cellular entry and trafficking of the virus [51]. As shown in Figure 1, RNA therapeutic drugs against COVID-19 can also use the two potential different categories of targets.
Viral targets
Targeting viral proteins is a direct and effective approach. SARS-CoV-2 has a positive-sense, single-stranded RNA genome, which contains three different categories of genes: structural, nonstructural and accessory [2]. There are 4 structural genes including S, E, M, and N, encoding Spike, Envelope, Membrane, and Nucleocapsid protein respectively [53]. Among them, S-glycoprotein plays a major role in cell entry via angiotensin-converting enzyme 2 (ACE2) receptor, and so receives the most attention. S protein, with two subunits S1 and S2, determines the viral entry. S1 could directly contact with the host receptor ACE2 with its receptor-binding domain (RBD), while the fusion and entrance of the following membrane to the host cytoplasm of the viruses were mediated by S2 subunit [54-58]. Now S protein is the target mostly concerned about for various therapeutic approaches including RNA therapy [12]. Other structure proteins of SARS-CoV-2, the M protein plays a central role in viral morphogenesis, assembly and egress [12]. Further studies show that the domains formed by the N- and C-terminal of M protein may be the potential RNAi targets [59, 60]. The E protein, critical for viral envelope curvature, maturation, and budding, is also involved in ion channel activity that is required for pathogenesis of SARS-CoV and possibly SARS-CoV-2 [4]. In a study on mutations among 68 samples of SARS-CoV-2, 42 missense mutations in a majority of structural and nonstructural proteins were identified, but the mutations in the E protein were not[61], indicating that the conserved E protein may serve as a great viral target for RNA therapy. N protein, another viral structural protein, is incorporated into nucleocapsid and responsible for viral genome packing. As its domains can bind to RNA via its phosphorylated residues, the N protein may also be a suitable target [62].
The nonstructural gene ORF1ab encodes polypeptides pp1a and pp1ab (-1 ribosomal frameshift) which occupies about 2/3 of the genome at the 5′ end. Hydrolytic cleavage of pp1a and pp1ab produces a series of nonstructural protein (i.e., nsp1-16) that are essential for viral transcription and replication [53]. The productions of accessory genes, although not essential for viral structure or replication, could modulate host innate or adaptive immune response and play a crucial role in viral pathogenicity [63]. In theory, all proteins encoded by the SARS-CoV-2 genome are potential anti-virus targets for developing ribonucleic acid therapy strategies against COVID-19.
The untranslated region (UTR) is another important part on the genome of SARS-CoV-2. Several studies show that the 5'UTR and the 3'UTR are also of great significance in the RNA replication and transcription of virus [64]. The researcher found in an early study that the small interfering RNA (siRNA) which targeted to the 3`UTR of SARS-CoV could suppress the viral infection and replication in Vero E6 cells [65]. Another study showed that the therapeutic siRNA which directly targeted the leader sequence of SARS could also inhibit the viral replication in Vero E6 cells [66], suggesting that the UTR of SARS may serve as a potential target of RNAi. Since the secondary structures of the 5`UTR of SARS-CoV and SARS-CoV-2 are remarkably alike [64], these specific region sequences can also be the target for the treatment of COVID-19 using RNA therapeutics.
Host targets
As various interactions between the virus and the host proteins can be identified, host targeting is another strategy in antiviral therapy [67-70]. The viral infection and replication can be suppressed by blocking or inhibiting the key host factors. The ACE2, serving as the receptor of viral S protein, can facilitate the internalization of SARS-CoV and SARS-CoV-2 [71]. It has been shown that soluble ACE2 has a protective role in many organs including lungs, making the recombinant ACE2 seem as a suitable therapeutic strategy [72]. Subsequent studies pointed out that ACE2 had a paradoxical role in protecting organs and facilitating the viral internalization [73]. Thus, further research and discussions on the benefits and potential risks of targeting ACE2 as a therapeutic strategy in COVID-19 are needed [73].
The components of the endocytic pathway have been proposed as crucial targets for developing the therapeutic strategies for all CoV species [74], for the endocytic pathway is thought to be important in viral entry and replication. Chloroquine, with its ability to neutralize lysosomal pH and inhibit protease activity, was extensively used in SARS-CoV [75, 76]. It is also reported that Chloroquine and hydroxychloroquine could suppress the viral entry into cells by interfering with the interaction of SARS-CoV-2 with cell surface gangliosides [77]. However, the following study manifested that no significant difference was found in the outcome of patients receiving hydroxychloroquine, making this idea remain controversial [78]. A recent study showed that AP2-associated protein kinase 1 (AAK1) seemed to be a suitable target to interrupt the viral entry into host cells and its intracellular assembly [79, 80]. There are dozens of medically approved AAK1 inhibitors. Among these drugs, Baricitinib, a Janus kinase (JAK) inhibitor, which can bind to cyclin-G-associated kinase along with AAK1 to prevent the entrance of virus into cells and will not cause severe side effects even with higher doses, may be the most potential treatment against COVID-19 [81]. Another study found that AP2M1 as a crucial host factor in the infection of SARS-CoV and SARS-CoV-2. The specific siRNA, namely siAP2M, was used to knock down the AP2M1 protein involved in endocytosis and significantly suppressed the viral infection [82]. These studies demonstrate that the therapeutic strategy of inhibiting the key host factors in endocytosis is feasible.
Antisense Oligonucleotides (ASO)
The antisense therapeutics was proposed in the 1970s with the theoretical basis that gene expression could be modulated by nucleic acids [83]. The chemically synthesized 15-50 nucleotides in length are designed to bind to RNA targets via complementary base pairing for the prevention of mRNA transcription and translation. Transported into cells, ASO combines with the target mRNA, and RNA-DNA complex becomes a substrate for RNase H, leading to endonuclease-mediated transcript knockdown [84]. ASO can also directly target mRNA, inhibiting mRNA from 5′ capping, 3′ polyadenylation, and splicing, ultimately terminating protein production [84]. ASO therapy is considered to be one of the most effective nucleic acid therapeutic strategies for many diseases, including myopathies, neurodegenerative diseases, oncology, etc.[85-87]. Given the advantage of targeting any viral RNA of interest, ASO has already been applied in some highly pathogenic viral infections of Ribovirus, such as Ebola, influenza, Hepatite-C, Japanese Encephalitis, achieving certain efficacy [88-91]. It is also an early nucleic acid therapy with successful clinical application for its more potential action mechanisms, easier intracellular delivery, and stable clinical suitability. Fomivirsen (trade name: Vitravene), the first ASO drug, was approved by FDA in 1998 [24]. After that, 9 ASO drugs were approved (Table 1), and 50 more ASO candidates are in clinical trials [28, 92].
Studies on ASO antiviral therapy against SARS-CoV boomed following the SARS outbreak in 2003. ASO technologies have been advanced to reduce the severity of viral infection, and to diagnose and treat SARS-associated disease. Three ASO-related patents were published. Patent US20030224353 published by Stein David described ASO ORF1 which targeted region 217-245bp of SARS-CoV, to treat the ssRNA viral infection [93]. Patent WO2005013905 was filed by AVI BioPharma, Inc., which targeted 3′ terminal end of negative strand using the modified oligonucleotide compounds to treat ssRNA viral infection, including flavivirus, coronavirus, picornavirus, togavirus, calicivirus, and hepatitis E virus [94]. Ionis Pharmaceuticals designed hybrid DNA/RNA ASO for the disruption of the pseudoknot in the frameshift site of SARS-CoV to reduce the viral activity. Based on this ASO, Ionis Pharmaceuticals published the patent application WO2005023083 [95]. In addition to the patents, another study on ASO to target the conserved RNA elements that were required in the synthesis and translation of viral RNA, demonstrated that ASO could effectively inhibit the replication of SARS-CoV [96]. However, there appeared some unexpected results that SARS-CoV developed ASO drug resistance and escaped. Therefore, more rationality and caution are needed in designing ASO drugs for coronavirus.
Since the SARS-CoV-2 and SARS-CoV share 79.5% similarity in genome [80], ASO should also be capable of treating COVID-19 on a theoretical level. ASO drug can be designed to bind to the viral mRNA transcripts that encode the crucial proteins associated with the replication and transcription to disturb the viral expansion. Based on this hypothesis, in a recent study, nine ASO candidates were designed to target the genes of viral structure protein N and ORF1a, ORF1b and 5′-UTR of viral genome, of which were very important for the transcription and replication of SARS-CoV-2. The results showed that viral infection could be inhibited by blocking these key targets effectively, which needs further verifications [97]. Beyond targeting the important viral protein synthesis, another strategy is to directly target the viral genome itself, especially RNA viruses (other than retroviruses). ASO can be designed to target the conserved sequences in viral genome to disturb or completely degrade the genomic RNA for the elimination of SARS-CoV-2 [52]. However, more studies are needed due to the unexpected variability of RNA viruses, and the difficulty to find suitable conserved sequences of viral genome for ASO targeting. All in all, with its simplicity to design, low toxicity effect, high specificity, and low production cost, ASO therapy is still a very potential treatment for COVID-19 [98].
The life cycle of SARS-CoV-2 and potential targets for RNA therapy. All targets can be grouped into two categories. One is the anti-virus (denoted in red), such as the structure genes of viral genome. Another is the anti-host category (denoted in blue), such as ACE2 receptor.
Ribozymes
The discovery of catalytic RNA has dramatically complemented and improved the understanding of RNA in the 1980s. Catalytic RNA, also known as Ribozymes, can bind and cleave phosphodiester in nucleic acid to induce specific degradation of target mRNA for the inhibition of gene expression [99]. Being natural but also able to chemically synthesized, ribozymes are believed to treat viral infectious diseases effectively for its gene editing activities. Meanwhile, more efforts are needed in the design of ribozymes. The target region is required to be quite conserved and critical for the survival or replication of the virus, and accessible so that the ribozymes can bind to it easily and smoothly [99]. With its weak RNase resistance, Ribozymes often perform a short-term effect in vivo [100, 101]. Therefore, chemical modification and appropriate delivery system are always added to increase therapy robustness. Another concern is that the cleavage effects in vivo are often different from those in vitro [102]. Despite these disadvantages, ribozymes are still regarded as an important and effective antiviral therapy. Compared with some other RNA drugs, ribozymes have high specificity and low immunogenicity [103]. Ribozyme-based studies have already been carried out in a series of RNA viruses including HCV, HIV, and influenza [104-107], but few reports about the ribozyme therapy on coronavirus-induced diseases. This may be caused by the fact that ribozymes require for the conserved regions of the genome and the coronavirus families are prone to new mutations, making the design relatively difficult. In addition, in the last two decades, except for SARS-CoV and MERS-CoV, which caused small-scale viral infections in certain areas, coronaviruses did not cause large-scale epidemics until the outbreak of COVID-19 in 2020. In an early study, a therapeutic RNA/DNA chimeric ribozyme was designed to recognize and cleave the conserved common regions and regions with loop structures in genome of coronavirus, including SARS, and a patent application was applied in this research (JP2007043942)[108]. As the SARS-CoV-2 and SARS-CoV share highly similar genome [80], this designed chimeric ribozyme may also be potentially useful for COVID-19's treatment and related studies.
In recent years, gene-editing technology represented by CRISPR/Cas system is on a rise. Some studies have tried to use gene therapy technologies in combination with ribozymes to modulate gene expression so as to treat some intractable diseases. Ribozymes in conjunction with Cas9, or even Cpf1 nuclease are believed to improve the efficiency of treatment strategies [109]. CRISPR-RGP, a gene-editing system based on Streptococcus pyogenes (Sp) Cas9, was designed against Plasmodium. A ribozyme-guide-ribozyme (RGR) single guide RNA (sgRNA) expression strategy with RNA polymerase II promoters were utilized in the system, and a position-dependent but strand-independent reduction in gene expression was induced by the catalytically dead SpCas9 (dSpCas9) binding to the upstream region of target genes. This robust gene-editing system can be used in the treatment of malaria by generating gene disruptions and insertions of coding sequences in Plasmodium [110]. Similar studies with other gene therapy technologies on HIV have been carried out [111]. Therefore, gene editing or gene therapy combined with ribozymes provides a possibility to treat SARS-CoV-2 infection.
RNA Interference (RNAi)
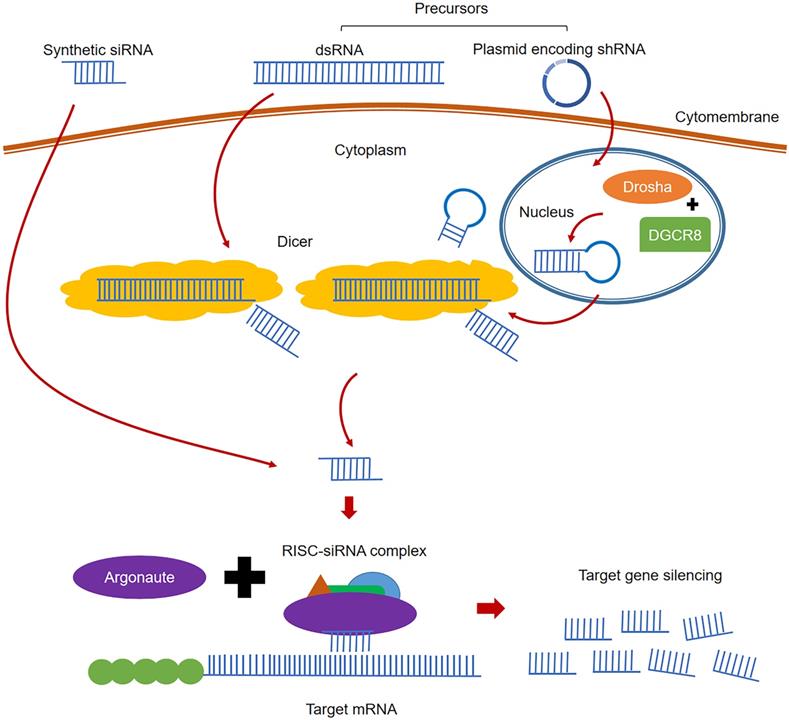
RNA interference (RNAi), the post-transcriptional gene silencing (PTGS) phenomenon, is induced by double strand RNA (dsRNA) which could efficiently and specifically degrade the homologous mRNAs [19]. RNAi is an inherent habitant of living organisms, which can be triggered by small interfering RNA (siRNA) or dsRNA. The classical process of RNAi is shown in Figure 2. In the cytoplasm, the long pieces of dsRNA are firstly cleaved into siRNA (21-23 nt in length) fragments by the enzyme Dicer [112]. Unlike ribozymes or ASOs that directly bind to target RNA, siRNA is combined with the protein Argonaute (Ago) and forms a complex called RNA-induced silencing complex (RISC) [113]. With siRNA being unwound, the activated RISC complex containing the antisense strand (or guide strand) of siRNA will specifically target and degrade the complementary mRNA [114]. Two methods are used to artificially induce RNAi using siRNA strategy: directly delivering the designed siRNA into target cells via appropriate transporters, or using designed plasmids encoding short hairpin RNA (shRNA). When the plasmid is sent into target cells, shRNA is produced and cleaved by Drosha and DGCR8 in the nucleus. After transferring into the cytoplasm by exportin 5, shRNA can be cleaved by enzyme Dicer and turn into siRNAs to induce RNAi [115]. Since siRNA can suppress gene expression by the RISC mechanism, target genes will see continuous degradation, resulting in extremely high potency and sustained activities compared with other RNA therapies. RNAi requires extremely strict recognition to degrade specific sequences, making its specificity highly unparalleled. In addition, small doses of siRNA could trigger sustained gene inhibition, and the effect increase with its concentration level [115]. Based on these advantages of gene modulation, RNAi technology has been widely used to explore gene function and in the treatment of infectious diseases and malignant tumors [116-120]. Currently, there are four siRNA-based drugs approved (Table 1) [25, 37, 39, 121], and more than 20 candidates in clinical trials [28].
Past experience of RNAi against SARS-CoV
RNAi technology was applied in the previous treatment research for the SARS epidemic. SARS-CoV could enter human cells through the S protein binding to the host receptor ACE2, which is similar to SARS-CoV-2. Therefore, S protein has become an important target selection in RNAi therapy. Several studies demonstrated that the viral load can be effectively reduced by silencing the S protein using siRNA strategy [65, 122, 123]. N protein incorporated into nucleocapsid and responsible for viral genome packing is also considered as a viral suppressor of RNAi in host cells [124]. The studies of targeting gene encoding N protein using shRNA approach were also carried out, with effective reduction in viral loads [125-127]. An interesting finding in the studies is that inhibition of N-gene could lead to an increase in the secretion of INF-β, which also can help cells to fight viral infection. Apart from S and N protein, another structure protein M of SARS-CoV is also explored. A former study concluded that targeting the 3` portion of gene encoding M protein could effectively cut the target mRNA levels, but left the inhibition of SARS-CoV uninvestigated [128].
The classical processes of RNAi.
In addition to structural genes, genes encoding nonstructural proteins and accessory proteins and the UTR regions are also important targets of RNAi therapy. The RNA-dependent RNA polymerase (RdRp) gene was highly conserved in different coronaviruses and considered to be an effective target [129]. Studies using siRNA and shRNA showed effective silencing of gene encoding RdRp[130-132]. However, the RdRp gene appeared to have different sensitivity to different siRNA sequences, which required more caution and rationality in the design of siRNA. In a separate study, shRNA was designed to target a leader sequence thought to be conserved in all coronaviruses, and a reduction in the viral load was observed [133]. Another study focused on nsp1, a non-structural protein of SARS-CoV which was derived from the 5′ leader end of the genome. The cells transfected with the designed shRNA can resist the viral invasion and enjoy adequate protection [134]. The subgenomic RNAs (sgRNAs), which are generated from discontinuous transcription during the synthesis of negative-strand genomic RNA of coronavirus, are also of great importance. The translation of most viral genes (the structural and accessory proteins) occurs via sgRNAs as the intermediates [135]. In a past study, several accessory proteins derived from the sgRNAs of SARS-CoV were considered to have no significant sequence homology compared with other coronaviruses. The previous experiments demonstrated that these proteins were suitable and effective targets as well as the S protein [136].
RNAi therapy against SARS-CoV-2
Since the SARS-CoV-2 is 79% similar to the SARS-CoV in genome, theoretically, RNAi strategies for SARS should be effective in treating COVID-19 as well [137]. siRNA can be designed to target the structural genes including S, E, M and N proteins to inhibit the entrance of virus into host cells and disturb the viral replication [138]. Besides the structural genes, the leader sequence seems to be another suitable choice [133]. The experience of SARS-related research can assist the development of therapeutic siRNA targeting leader sequences to inhibit the replication of SARS-CoV-2. In a recent study, eight siRNAs targeting the highly conserved 5`-UTR of SARS-CoV-2 were designed. siCOV6, the most promising candidate, could reduce the viral replication and prevent cytopathic effect from developing by targeting the leader sequence that was present in all sgRNAs [139]. Targeting Nsps is another important strategy for the effective inhibition of viral replication in reducing the viral symptoms [134]. There are totally 15 nsps, nsp1 to nsp10 and nsp12 to nsp16, which are encoded by genes on ORF1ab and ORF1a in the genome of SARS-CoV-2. Nsp12 is the RdRp of the virus and can co-regulate the viral replication with nsp5 (3C-like protease) [138]. Several previous studies showed the effective suppression of SARS-CoV by using siRNA to target RdRp gene, and this strategy may also be feasible for SARS-CoV-2. In a recent study, nsp9 and nsp10 were found to interact with NF-κB-repressing factor (NKFR) to facilitate interleukin-8 (IL-8) induction in lung epithelial A549 cells. The designed siRNAs were used to target these two nsps, and the introduction of IL-8 was observed to decrease effectively, which could help to reduce the level of inflammation and minimize damages to the lung [69].
The design of RNAi using computational approaches
In the past decade, with the rapid development of artificial intelligence and the continuous accumulation of biological data, computer technology plays an increasingly important role in drug design. Compared with traditional method of small molecule drug screening, the computational algorithms represented by deep learning can remarkably shorten the development time and cut the cost. In research to fight SARS-CoV-2, due to the time-consuming experimental methods, computational approaches can focus on thousands of solutions simultaneously, and effectively narrow down the cases for experimental validation. Several studies on the RNAi therapy against COVID-19 through the computational methods were reported (Seen in Supplementary Table 1). In one research project, genes encoding nucleocapsid phosphoprotein and surface glycoprotein of SARS-CoV-2 were chosen as the targets, and 8 siRNA molecules selected from 78 candidates were predicted to effectively silence the target genes for the antiviral treatment [140]. Nucleocapsid is encoded by N gene and responsible for viral encapsidation and genome packing. In one attempt, several bioinformatic tools were applied for the design of a duplex siRNA molecule that did not fit any off-target sequences. The results demonstrated that it could effectively silence the N gene, with therapeutic potential for SARS-CoV-2 [141]. In another study, the online software siDirect version 2.0 was used to predict the highly effective siRNAs taking the leader sequence of viral genome as the target, and the HNADOCK online server was applied in molecular docking to validate siRNA affinity for the targets. Four potential siRNAs were revealed in the results. Among them, the siRNA with sequence 5'GUUUAGAGAACAGAUCUACAA3' possesses the greatest potential to inhibit the leader sequence of SARS-CoV-2 with least off-target effect [48]. ORF1ab, the largest gene in the genome of SARS-CoV-2, encodes polyprotein PP1ab and PP1a which are the key factors in viral transcription and replication. In a separate study, some bioinformatic approaches were used to predict the potential siRNA by screening siRNA library targeting the ORF1ab gene. In the results, a total of 10 siRNA candidates were sorted out as the potential therapeutic agents against COVID-19 [142]. With RdRp as another important potential target for RNAi therapy, a dozen of siRNAs were designed to silence RdRp gene to cure COVID-19 in a recent study. Out of the expectation, the target of designed siRNA revealed no significantly matches with the entire human genome, which excludes any possibility for off-target silencing of the siRNA [49]. In addition to these structural or non-structural genes, the viral genome can also be targeted by siRNA. In one attempt, siRNAs were designed to directly target the evolutionarily conserved regions in the SARS-CoV-2 genome to down-regulate or silence its RNA. These siRNA candidates which had high affinity and low side effects were considered to be the potential therapeutics [143].
Industry applications on RNAi therapy against coronavirus
Since the SARS epidemic, dozens of patents for RNA-based therapeutic strategies have been issued. Most of them are based on RNAi therapy, and some are treated with antisense RNA. The review of siRNA-based patents issued or under consideration for coronaviruses is shown in Table 2. Due to the limited number of infected patients and areas of SARS and MERS, and the fact that similar viral infection incidents did not occur after the end of the epidemic in a short run, these treatment patents for coronaviruses were rarely developed into drugs or therapeutic strategies [4]. As a result, after the outbreak of COVID-19, there is still no specific cure for SRAS-CoV-2. However, with the advantages of RNAi technology in treating viral diseases and the research experience of RNA therapy against SARS-CoV, some pharmaceutical companies have published their recent research achievements. Hundreds of siRNAs have been designed to target the highly conserved regions of SARS-CoV-2 or the key factors that are responsible for the viral transcription or replication together with the delivery systems. With the ongoing epidemic of COVID-19, these siRNAs could eventually be developed into specific drugs for clinical application.
Review of siRNA-based patents issued or under consideration for Coronaviruses
| Patent number | Target region | Virus | Year |
|---|---|---|---|
| CN1458281 | RdRP, S protein, M protein,packaging protein | SARS-CoV | 2003 |
| CN1465584 | various | SARS-CoV | 2003 |
| CN1548054 | RdRP (RNA polymerase) | SARS-CoV | 2003 |
| CN1569233 | RdRp, helicase, nucleoprotein N, proteolytic enzyme gene | SARS-CoV | 2003 |
| CN1569878 | target sequence in the patent | SARS-CoV | 2003 |
| CN1590545 | RdRp, S protein, M gene, UP4, UP5, N | SARS-CoV | 2003 |
| CN1609116 | replicase polymerase guide region | SARS-CoV | 2003 |
| CN1609117 | replicase polymerase guide region | SARS-CoV | 2003 |
| US20030224353 | ORF1 | SARS-CoV | 2003 |
| CN1648249 | M, N, E gene | SARS-CoV | 2004 |
| US20050020525 | various | SARS-CoV | 2005 |
| JP2006238724 | various | SARS-CoV | 2005 |
| WO2005019410 | Nsp-1, Nsp-9, S protein | SARS-CoV | 2005 |
| CN101085986 | ORF3a | SARS-CoV | 2006 |
| CN101113158 | RdRP (RNA polymerase) | SARS-CoV | 2006 |
| CN101173275 | M1 and M2 gene | SARS-CoV | 2006 |
| EP1482037 | replicase (Pol) region | SARS-CoV | 2006 |
| US20050004063 | replicase A1, S, N, M, E gene | SARS-CoV | 2006 |
| WO200409238 | NC_004718 | SARS-CoV | 2007 |
| US20070270360 | NC_004718 | SARS-CoV | 2007 |
| CN101182517 | S, Nsp-9, Nsp-10, Nsp-13, E, M, N proteins | SARS-CoV | 2007 |
| JP2008253188 | DNaseX | SARS-CoV | 2007 |
| CN102453712 | PI4KB, PI4KA | SARS-CoV | 2010 |
| US20040192626 | various | SARS-CoV | 2012 |
| US8653252 | Replicase (Pol) region;pS3Xs (pGL3 with SARS sense, antisense target) and one without SARS target | SARS-CoV | 2014 |
| CN107488660 | ORF3 25962 25983bp of GenBank DQ497008 | SARS-CoV | 2017 |
| WO2017044507 | P.L. pro, RdRp, S protein | MERS-CoV | 2019 |
| WO2005023083 | various | SARS-CoV-2 | 2020 |
Other RNA Therapy
Aptamer
Aptamers, single-stranded RNA or DNA molecules, can fold and form stable three-dimensional structures by complementary base pairing, electrostatic interactions, and hydrogen bonding, and bind to target proteins with high specificity to modulate the functions [144-146]. Although most of aptamers are based on DNA molecules, for the significant advantages of high affinity, facile chemical modification, low-cost, low-temperature sensitivity, rapid synthesis, and large-scale production, RNA aptamers have also been widely used, especially in some antiviral studies[147-149]. In this review, we focused on the RNA aptamers against coronavirus infection. Nowadays, aptamers are often combined with other therapeutic agents, such as siRNA and ribozymes [145]. In a recent study, siRNA technology combined with aptamers have been applied to suppress HIV-1 expression [150]. Researches on aptamers against SARS-CoV have also been reported. In two Korea patent applications (KR2009128837 and KR2012139512), aptamer technology was used to inhibit the SARS virus, which all provide a new RNA therapeutic strategy for the fight against SARS-CoV-2 [108].
Aptamers also have a wide application in diagnosis for their high affinity to bind to target proteins. During the SARS epidemic, aptamer technology was proved to be useful in the diagnosis of SARS-CoV. By targeting antigenic viral proteins, aptamers were also considered promising in the diagnosis of COVID-19 [151, 152]. On this subject, several studies have stated that the aptamer technology combined with the enzyme-linked oligonucleotide assay to diagnose COVID-19 disease are being developed by Aptamer Group [153, 154]. Pinpoint Science has proposed a new approach for rapid detection of SARS-CoV-2 by an aptamer-based nanosensor [155]. With the progress in aptamer technology, a future where COVID-19 can be diagnosed at speed and with accuracy is foreseeable.
Peptide nucleic acids (PNAs)
PNA is a specific kind of antisense therapy, in which N-(2-aminoethyl) glycine units has replaced the nucleic acid phosphodiester backbone via a methyl carbonyl binder [156]. Similar to ASO, PNAs can specifically bind to target DNA or RNA according to Watson-Crick base pairing to modulate gene expression [157]. PNAs can exist stably in vivo or in vitro as the result of the resistance to nucleases or proteases, and have low toxicity due to low or no binding affinity to serum proteins [158], making them important candidates for antisense therapeutics. In some earlier anti-HIV studies, PNAs molecules were designed to target certain regions, including gag gene, dimerization initiation site and att sequences of linear pro-viral DNA's U3 and U5 region. The results demonstrated that PNAs could effectively inhibit the reverse transcription and prevent the integration of the viral genome and the host genome, showing a great antiviral effect [159-161].
The studies on PNAs against coronaviruses is limited. -1 Ribosomal frameshifting (PRF) hardly occurs during translation naturally. However, it can be triggered by specific signals which increases the possibility of tRNA slippage up to 50% [162]. The RPF signal consists of two elements, a heptanucleotide slippery site and a downstream tertiary RNA structure in the form of an RNA pseudoknot. SARS-CoV initiates -1 RPF at the three-helix-containing RNA pseudoknot [163]. The control of -1 PRF efficiency has been shown to be critical for the maintenance of correct stoichiometric ratios of viral replicase proteins [164]. In a previous study, a fully compatible sequence-specific PNA was designed to block the -1 PRF signal which was in charge of the synthesis of viral RNA replicase polyproteins, and the viral replication was significantly inhibited [165]. Since the structure of the -1 PRF signal is extremely conserved and stable, the result is also helpful for the design of PNAs against COVID-19. Another problem affecting the PNAs strategy for the treatment is the slow cellular uptake of PNAs [157]. Therefore, necessary modifications or appropriate delivery system such as nanotechnological tools should have been applied in the design of PNAs.
mRNA encoding therapeutic antibodies
Neutralizing antibodies are important biological products against various infectious viral pathogen. Compared with the traditional production and purification processes of therapeutic antibodies, delivering the genetic information of the antibody including DNA or mRNA to produce biologicals in situ is a cost- and labor-effective manner and a new antiviral option [166]. In early attempts, naked plasmid DNA (pDNA) was injected into the quadriceps of mice and the local expression of the encoded proteins was observed [167]. However, considering the potential integration of the pDNA into the host genome and the fear of anti-DNA autoantibodies, there is no pDNA-based drug been marketed so far even if several pDNA-encoded antibodies were evaluated in phase II-III clinical trials. In recent years, with the progress of in vitro transcribed (IVT) mRNA technology, it has become possible to use engineered mRNA to generate therapeutic antibodies in vivo. IVT mRNA can efficiently induce protein expression, avoiding risks such as be integrated into the host genome. With the improvement of delivery systems, the stability and translatability of the engineered mRNA have also been greatly increased. mRNA therapeutics has been applied in several bio-medical fields including the therapeutic cancer vaccine [168, 169], prophylactic vaccine [170, 171] and different infectious diseases [172, 173].
In a study treating respiratory viral infections, the engineered mRNA encoding a bispecific single-domain antibody against the influenza A virus was constructed and delivered into lungs of mice before the viral infection. The reduced viral titers and morbidities in mice were observed [174]. In addition, mRNA therapeutic strategy has also been applied in the treatment against COVID-19. A safe and cost-effective platform to express neutralizing antibody in the lung with replicating mRNA using alphavirus replicon particle (VRP) delivery system is developed. The replicating mRNA encoding therapeutic antibody is intranasally delivered to target lung of mice. The experiment result shows that SARS-CoV-2 can efficiently be suppressed with reducing viral titer and less tissue damage [175]. Although there are still some concerns such as the intrinsic immunogenicity and transmission efficiency, a growing number of studies have shown that the local delivery of mRNA encoding therapeutic antibodies in the lungs could be a promising pulmonary antiviral prophylactic treatment [176].
CRISPR/Cas technology
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats)-Cas (CRISPR-associated) systems have greatly improved our ability to edit genes and modulate gene expression [177]. Except for its genome-editing ability, the diagnostic potential was also noticed by scientists. In 2017, Feng Zhang's group have reported SHERLOCK, the first CRISPR-based nucleic acid detection technique [178]. SHERLOCK allows multiplexed, portable, and ultra-sensitive detection of RNA and DNA from clinical samples. Recently, CRISPR/Cas technology has also been applied in the diagnosis of COVID-19. An assay design tool and a research method based on the CRISPR-Cas13 was designed. It was reported that the system could detect specifically SARS-CoV-2 and 66 related viruses in patient specimens [179].
In addition to the diagnosis, CRISPR/Cas technology has already been used in the treatment of SARS-CoV-2. PAC-MAN (prophylactic antiviral CRISPR in human cells), based on CRISPR-Cas13 technology, is found to effectively degrade the SARS-CoV-2 sequences in human lung epithelial cells. In this system, the CRISPR-derived RNAs (crRNAs) were designed and screened, and the crRNAs could target the conserved viral regions to cleave SARS-CoV-2 genome [180]. There are totally 22 crRNA pools analyzed by bioinformatics tools which can target all sequenced coronaviruses in PAC-MAN system in this study. Thus, PAC-MAN is a promising strategy to combat coronavirus by targeting different regions of a virus or different coronavirus strains simultaneously with the crRNA pool, preventing possible viral escapes [180].
Challenges and Perspectives
The right target selection is the first step in RNA therapy against COVID-19, whether ASOs or RNAi strategy. The targets should be conserved in the viral genome and very important for the life cycle of the virus. Similar to other treatments such as therapeutic antibodies, the structural genes of SARS-CoV-2 genome which encode S, E, M and N proteins, as well as crucial NSPs and accessory proteins are all potential therapeutic targets [12]. Among them, S protein with the function to help viruses enter cells by binding the host ACE2 receptor has attracted much more attention. Almost all RNA therapies have tried to target S protein to suppress the SARS-CoV-2 [55, 60, 123]. In addition, proteins responsible for the important viral physiological functions, such as transcription and replication, taking RdRp as an example, are often selected as therapeutic targets too [49]. One concern for the treatment is the variability of coronavirus. At present, dozens of new variants of SARS-CoV-2 have been discovered, posting serious challenges to its diagnosis and treatment [10]. Therefore, further in-depth research is needed to explore the conserved or specific functional regions suitable for therapeutic targeting in the viral genome by comparing the differences between variants. Another therapeutic strategy is the host targeting, such as targeting ACE2 receptors or some other key host factors in the process of viral transcription or replication [73]. However, the suppression of these host genes might cause different unexpected symptoms in humans. The design of RNA therapeutic strategy against host targets should be more cautious.
Another challenge is the stability of RNA-related drugs. It is well known that RNA has weak resistance to nucleases, and is rapidly metabolized in cells. These disadvantages have significantly limited the development of nucleic acid drugs. To solve these problems, several biological techniques, including chemical modifications to the RNA as well as drug delivery system, have been applied in drug design. A wide variety of chemical modifications, including modifications in nucleobase, backbone and sugar moiety, have been studied to improve the affinity and nuclease resistance of the RNA therapeutics [181-185]. The therapeutic efficacy of RNA drugs can be considerably enhanced by the chemical modifications through improving pharmacokinetic characteristics, making it a general and common approach for the design of nucleic acid strategy. In addition to the chemical modifications, the drug delivery system is another probable approach. Drug delivery system or the carrier technology can help drugs be taken up by cells more efficiently, reach the therapeutic targets more directly, enhance the targeting effect and biological activity, and prevent the drugs from being degraded by nucleases [186]. Liposomes, cationic polymer complexes, dendrimers, polymer microsomes, peptide protein delivery vehicles, and nanoparticles are all thought to be suitable choices of delivery systems [20]. Among them, nanostructure lipid carrier (NLC) is considered the most promising drug delivery system at present. Owing to the suitable volume, high drug loading efficiency and specific physical and chemical properties, the NLC system can improve the stability of nucleic acid drugs, complete drug delivery, release the drug effectively, and avoid the drug degradation in vivo. NLC technology has already been applied in Patisiran, the first approved siRNA-based drug [25]. Therapeutic siRNA is encapsulated in lipid-based nanoparticles to prevent the degradation by exonuclease while facilitating the transport process in vivo, which is also applicable to the development of nucleic acid drugs for coronaviruses. Because of the acute lung injury and inflammatory response caused by SARS-CoV-2, RNA therapeutic drugs cannot get through the barriers composed of the intense mucus layer, alveolar macrophages and pulmonary proteases [187]. Therefore, effective chemical modifications and drug delivery system such as NLC will boost the development of RNA therapeutic strategies against COVID-19.
How to deliver RNA therapeutic drugs to the lungs is another concern. Inhalational delivery, verified by accumulating evidence, is the optimal drug delivery approach compared to some conventional methods. Oral, nasal, and other inhalation delivery have several advantages, such as the ease of administration and the rapid onset of action, which is already used in some drugs and vaccines [188, 189]. In 2003, the live attenuated influenza vaccines in the form of nasal spray were approved in US for its better mucosal immune responses [190]. Inhalation delivery, or pulmonary delivery, is commonly applied in respiratory diseases. Drops, liquid sprays, powder sprays or gels are the main forms in the treatment. In recent years, nanoparticle formulations, with much garnered attention in the development of RNA therapy because of their ability to carry nucleic acid drugs, can also be used for inhalational delivery. In one RNAi study of lung cancer, therapeutic siRNA was encapsulated in liposomes and then delivered to lungs as spray freeze-dried nanoparticles for treatment [191]. Other studies on nanoparticle formulations for pulmonary delivery, such as dry powder chitosan nanoparticles and nanoparticles with the pulmonary surfactant Curosurf, showed that both can deliver therapeutic RNA molecules to lungs through inhalation [192, 193]. In a recent exploration, a novel lipid nanoparticle (LNP) delivery system is developed and therapeutic siRNAs which are encapsulated in the LNPs are transported to lungs directly. By using LNP system, SARS-CoV-2 can be greatly inhibited by screened siRNAs which can target the highly conserved regions of the viral genome [50]. Another interesting finding was that siRNA formulated with the traditional polymeric carrier polyethyleneimine (PEI) appeared to have the same therapeutic effect as the siRNA without a carrier. The result suggested that inhalation approach might be able to maintain the activity of "free" siRNA molecules [194]. Therefore, inhalational approaches serve as a reliable delivery strategy of great significance in winning the battle against SARS-CoV-2 using a nanoparticle delivery system or the “free” therapeutic RNA molecules.
Still there are some pharmacodynamic-related challenges on RNA therapy, including off-target effects and potential immunotoxicity. Some biological technologies such as chemical modifications can hold the key to solutions. However, a more directed effort is required by experts and funding agencies to develop simple, cost-effective, and easy-to-use formulations of RNA therapeutics, to accelerate the clinical application of RNA-based antiviral drugs.
Conclusion
The outbreak of COVID-19, a particularly contagious respiratory disease caused by the novel coronavirus SARS-CoV-2, has highlighted the urgent need for new therapeutic strategies that are rapid, precise, stable, and target-specific for treatment. In this review, we summarized the research experience of ribonucleic acid therapy strategies against SARS-CoV-2, including ASO, ribozymes, RNAi technology, aptamer, etc., and described different RNA therapeutics in terms of mechanisms, application methods, and shortages to improve. Besides, it paid special attention on the computational approaches in RNAi technology for COVID-19. RNA therapeutic strategies in this review aimed at viral therapy for SARS-CoV-2, provide sufficient evidence of their potential. In the end, from the perspectives of the existing literature, available technical tools, a large number of relevant scientific studies and persistent clinical trials, it is believed that more novel RNA therapeutic drugs will emerge for diagnosis, treatment and prevention of COVID-19 in the near future.
Supplementary Material
Supplementary table 1.
Acknowledgements
The authors would like to thank Miss Yuqing Gong for the proofreading of the manuscript and the support from the National Natural Science Foundation of China (grant number 62071099, 62172078 and 61901130), Science and Technology Department of Guizhou Province of China (grant number: [2020]1Y407).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W. et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-3
2. Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG. et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265-9
3. Kao RY, Tsui WH, Lee TS, Tanner JA, Watt RM, Huang JD. et al. Identification of novel small-molecule inhibitors of severe acute respiratory syndrome-associated coronavirus by chemical genetics. Chem Biol. 2004;11:1293-9
4. Rabaan AA, Al-Ahmed SH, Haque S, Sah R, Tiwari R, Malik YS. et al. SARS-CoV-2, SARS-CoV, and MERS-COV: A comparative overview. Infez Med. 2020;28:174-84
5. Harrison AG, Lin T, Wang P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020;41:1100-15
6. Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J. et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect. 2020;81:e16-e25
7. Bedford J, Enria D, Giesecke J, Heymann DL, Ihekweazu C, Kobinger G. et al. COVID-19: towards controlling of a pandemic. Lancet. 2020;395:1015-8
8. WHO. WHO Coronavirus Disease (COVID-19) Dashboard
9. Araf Y, Akter F, Tang YD, Fatemi R, Parvez MSA, Zheng C. et al. Omicron variant of SARS-CoV-2: Genomics, transmissibility, and responses to current COVID-19 vaccines. J Med Virol. 2022
10. Kumar S, Thambiraja TS, Karuppanan K, Subramaniam G. Omicron and Delta variant of SARS-CoV-2: A comparative computational study of spike protein. J Med Virol. 2021
11. Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce BF. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol. 2021;21:626-36
12. Ning L, Abagna HB, Jiang Q, Liu S, Huang J. Development and application of therapeutic antibodies against COVID-19. Int J Biol Sci. 2021;17:1486-96
13. Ita K. Coronavirus Disease (COVID-19): Current Status and Prospects for Drug and Vaccine Development. Arch Med Res. 2021;52:15-24
14. Aileni M, Rohela GK, Jogam P, Soujanya S, Zhang B. Biotechnological Perspectives to Combat the COVID-19 Pandemic: Precise Diagnostics and Inevitable Vaccine Paradigms. Cells. 2022 11
15. Crick F. Central dogma of molecular biology. Nature. 1970;227:561-3
16. Kruger K, Grabowski PJ, Zaug AJ, Sands J, Gottschling DE, Cech TR. Self-splicing RNA: autoexcision and autocyclization of the ribosomal RNA intervening sequence of Tetrahymena. Cell. 1982;31:147-57
17. Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J. et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860-921
18. Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152:1298-307
19. Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806-11
20. Zhao Y, Shu R, Liu J. The development and improvement of ribonucleic acid therapy strategies. Mol Ther Nucleic Acids. 2021;26:997-1013
21. Gaynor JW, Campbell BJ, Cosstick R. RNA interference: a chemist's perspective. Chem Soc Rev. 2010;39:4169-84
22. Dhuri K, Bechtold C, Quijano E, Pham H, Gupta A, Vikram A. et al. Antisense Oligonucleotides: An Emerging Area in Drug Discovery and Development. J Clin Med. 2020 9
23. de Smet MD, Meenken CJ, van den Horn GJ. Fomivirsen - a phosphorothioate oligonucleotide for the treatment of CMV retinitis. Ocul Immunol Inflamm. 1999;7:189-98
24. Highleyman L. Fomivirsen. BETA. 1998;29:31
25. Adams D, Gonzalez-Duarte A, O'Riordan WD, Yang CC, Ueda M, Kristen AV. et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N Engl J Med. 2018;379:11-21
26. Vergnes JN. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2021;384:1577
27. Teo SP. Review of COVID-19 mRNA Vaccines: BNT162b2 and mRNA-1273. J Pharm Pract. 2021 8971900211009650
28. Wang F, Zuroske T, Watts JK. RNA therapeutics on the rise. Nat Rev Drug Discov. 2020;19:441-2
29. Mipomersen. Am J Cardiovasc Drugs. 2010; 10: 271-9.
30. Syed YY. Eteplirsen: First Global Approval. Drugs. 2016;76:1699-704
31. Neil EE, Bisaccia EK. Nusinersen: A Novel Antisense Oligonucleotide for the Treatment of Spinal Muscular Atrophy. J Pediatr Pharmacol Ther. 2019;24:194-203
32. Keam SJ. Inotersen: First Global Approval. Drugs. 2018;78:1371-6
33. Paik J, Duggan S. Volanesorsen: First Global Approval. Drugs. 2019;79:1349-54
34. Heo YA. Golodirsen: First Approval. Drugs. 2020;80:329-33
35. Dhillon S. Viltolarsen: First Approval. Drugs. 2020;80:1027-31
36. Shirley M. Casimersen: First Approval. Drugs. 2021;81:875-9
37. Scott LJ. Givosiran: First Approval. Drugs. 2020;80:335-9
38. Scott LJ, Keam SJ. Lumasiran: First Approval. Drugs. 2021;81:277-82
39. Lamb YN. Inclisiran: First Approval. Drugs. 2021;81:389-95
40. Chapman JA, Beckey C. Pegaptanib: a novel approach to ocular neovascularization. Ann Pharmacother. 2006;40:1322-6
41. Chilamakuri R, Agarwal S. COVID-19: Characteristics and Therapeutics. Cells. 2021 10
42. Li M, Liu X, Zhou Y, Su SB. Interferon-lambdas: the modulators of antivirus, antitumor, and immune responses. J Leukoc Biol. 2009;86:23-32
43. Aliyu IA, Ling KH, Md Hashim N, Chee HY. Annexin A2 extracellular translocation and virus interaction: A potential target for antivirus-drug discovery. Rev Med Virol. 2019;29:e2038
44. Lundstrom K. Viral Vectors Applied for RNAi-Based Antiviral Therapy. Viruses. 2020 12
45. Yao J, Yu W, Chang Y, Ren J, Xu D, Han S. et al. Targeted screening of SiRNA directed HBV polymerase gene for effective inhibition of HBV expression. J Huazhong Univ Sci Technolog Med Sci. 2008;28:266-71
46. Katakowski JA, Palliser D. siRNA-based topical microbicides targeting sexually transmitted infections. Curr Opin Mol Ther. 2010;12:192-202
47. Tan EL, Tan TM, Tak Kwong Chow V, Poh CL. Inhibition of enterovirus 71 in virus-infected mice by RNA interference. Mol Ther. 2007;15:1931-8
48. Pandey AK, Verma S. An in silico analysis of effective siRNAs against COVID-19 by targeting the leader sequence of SARS-CoV-2. Adv Cell Gene Ther. 2021 e107
49. Shawan M, Sharma AR, Bhattacharya M, Mallik B, Akhter F, Shakil MS. et al. Designing an effective therapeutic siRNA to silence RdRp gene of SARS-CoV-2. Infect Genet Evol. 2021;93:104951
50. Idris A, Davis A, Supramaniam A, Acharya D, Kelly G, Tayyar Y. et al. A SARS-CoV-2 targeted siRNA-nanoparticle therapy for COVID-19. Mol Ther. 2021;29:2219-26
51. Uludag H, Parent K, Aliabadi HM, Haddadi A. Prospects for RNAi Therapy of COVID-19. Front Bioeng Biotechnol. 2020;8:916
52. Berber B, Aydin C, Kocabas F, Guney-Esken G, Yilancioglu K, Karadag-Alpaslan M. et al. Gene editing and RNAi approaches for COVID-19 diagnostics and therapeutics. Gene Ther. 2021;28:290-305
53. Clayton E, Rohaim MA, Bayoumi M, Munir M. The Molecular Virology of Coronaviruses with Special Reference to SARS-CoV-2. Adv Exp Med Biol. 2021;1352:15-31
54. Wang Q, Zhang Y, Wu L, Niu S, Song C, Zhang Z. et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell. 2020;181:894-904 e9
55. Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S. et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215-20
56. Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A. et al. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A. 2020;117:11727-34
57. Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H. et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221-4
58. Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444-8
59. Neuman BW, Kiss G, Kunding AH, Bhella D, Baksh MF, Connelly S. et al. A structural analysis of M protein in coronavirus assembly and morphology. J Struct Biol. 2011;174:11-22
60. Muhseen ZT, Hameed AR, Al-Hasani HMH, Tahir Ul Qamar M, Li G. Promising terpenes as SARS-CoV-2 spike receptor-binding domain (RBD) attachment inhibitors to the human ACE2 receptor: Integrated computational approach. J Mol Liq. 2020;320:114493
61. Phan T. Genetic diversity and evolution of SARS-CoV-2. Infect Genet Evol. 2020;81:104260
62. Hurst KR, Koetzner CA, Masters PS. Identification of in vivo-interacting domains of the murine coronavirus nucleocapsid protein. J Virol. 2009;83:7221-34
63. Michel CJ, Mayer C, Poch O, Thompson JD. Characterization of accessory genes in coronavirus genomes. Virol J. 2020;17:131
64. Baldassarre A, Paolini A, Bruno SP, Felli C, Tozzi AE, Masotti A. Potential use of noncoding RNAs and innovative therapeutic strategies to target the 5'UTR of SARS-CoV-2. Epigenomics. 2020;12:1349-61
65. Wu CJ, Huang HW, Liu CY, Hong CF, Chan YL. Inhibition of SARS-CoV replication by siRNA. Antiviral Res. 2005;65:45-8
66. Li BJ, Tang Q, Cheng D, Qin C, Xie FY, Wei Q. et al. Using siRNA in prophylactic and therapeutic regimens against SARS coronavirus in Rhesus macaque. Nature Medicine. 2005;11:944-951
67. Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM. et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459-68
68. Gordon DE, Hiatt J, Bouhaddou M, Rezelj VV, Ulferts S, Braberg H. et al. Comparative host-coronavirus protein interaction networks reveal pan-viral disease mechanisms. Science. 2020 370
69. Li J, Guo M, Tian X, Wang X, Yang X, Wu P. et al. Virus-Host Interactome and Proteomic Survey Reveal Potential Virulence Factors Influencing SARS-CoV-2 Pathogenesis. Med (N Y). 2021;2:99-112 e7
70. Zhou N, Bao J, Ning Y. H2V: a database of human genes and proteins that respond to SARS-CoV-2, SARS-CoV, and MERS-CoV infection. BMC Bioinformatics. 2021;22:18
71. Prabakaran P, Xiao X, Dimitrov DS. A model of the ACE2 structure and function as a SARS-CoV receptor. Biochem Biophys Res Commun. 2004;314:235-41
72. Rossi GP, Sanga V, Barton M. Potential harmful effects of discontinuing ACE-inhibitors and ARBs in COVID-19 patients. Elife. 2020 9
73. Xiao L, Sakagami H, Miwa N. ACE2: The key Molecule for Understanding the Pathophysiology of Severe and Critical Conditions of COVID-19: Demon or Angel? Viruses. 2020 12
74. Yang N, Shen HM. Targeting the Endocytic Pathway and Autophagy Process as a Novel Therapeutic Strategy in COVID-19. Int J Biol Sci. 2020;16:1724-31
75. Keyaerts E, Vijgen L, Maes P, Neyts J, Van Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun. 2004;323:264-8
76. Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG. et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69
77. Fantini J, Di Scala C, Chahinian H, Yahi N. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int J Antimicrob Agents. 2020;55:105960
78. Spinelli FR, Ceccarelli F, Di Franco M, Conti F. To consider or not antimalarials as a prophylactic intervention in the SARS-CoV-2 (Covid-19) pandemic. Ann Rheum Dis. 2020;79:666-7
79. Richardson P, Griffin I, Tucker C, Smith D, Oechsle O, Phelan A. et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395:e30-e1
80. Lu R, Zhao X, Li J, Niu P, Yang B, Wu H. et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565-74
81. Itani R, Tobaiqy M, Al Faraj A. Optimizing use of theranostic nanoparticles as a life-saving strategy for treating COVID-19 patients. Theranostics. 2020;10:5932-42
82. Wang PG, Tang DJ, Hua Z, Wang Z, An J. Sunitinib reduces the infection of SARS-CoV, MERS-CoV and SARS-CoV-2 partially by inhibiting AP2M1 phosphorylation. Cell Discov. 2020;6:71
83. Paterson BM, Roberts BE, Kuff EL. Structural gene identification and mapping by DNA-mRNA hybrid-arrested cell-free translation. Proc Natl Acad Sci U S A. 1977;74:4370-4
84. Schubert S, Kurreck J. Oligonucleotide-based antiviral strategies. Handb Exp Pharmacol. 2006;173:261-87
85. Sardone V, Zhou H, Muntoni F, Ferlini A, Falzarano MS. Antisense Oligonucleotide-Based Therapy for Neuromuscular Disease. Molecules. 2017 22
86. Leavitt BR, Tabrizi SJ. Antisense oligonucleotides for neurodegeneration. Science. 2020;367:1428-9
87. Muth CC. ASO Therapy: Hope for Genetic Neurological Diseases. JAMA. 2018;319:644-6
88. Chery J, Petri A, Wagschal A, Lim SY, Cunningham J, Vasudevan S. et al. Development of Locked Nucleic Acid Antisense Oligonucleotides Targeting Ebola Viral Proteins and Host Factor Niemann-Pick C1. Nucleic Acid Ther. 2018;28:273-84
89. de Jong YP, Jacobson IM. Antisense therapy for hepatitis C virus infection. J Hepatol. 2014;60:227-8
90. Lenartowicz E, Nogales A, Kierzek E, Kierzek R, Martinez-Sobrido L, Turner DH. Antisense Oligonucleotides Targeting Influenza A Segment 8 Genomic RNA Inhibit Viral Replication. Nucleic Acid Ther. 2016;26:277-85
91. Zhang L, Li Q, Ding X, Zhang B, Zhang Q, Qu X. et al. Antisense Oligonucleotides Targeting Raf-1 Block Japanese Encephalitis Virus In Vitro and In Vivo. Nucleic Acid Ther. 2017;27:78-86
92. Ma CC, Wang ZL, Xu T, He ZY, Wei YQ. The approved gene therapy drugs worldwide: from 1998 to 2019. Biotechnol Adv. 2020;40:107502
93. Smith Alvin W IPL, Stein David A, Skilling Douglas E. Antisense antiviral agent and method for treating ssRNA Viral Infection. In: INC AB, editor. USA. 2005
94. PL I. Sense Antiviral Compound And Method For Treating ssRNA Viral Infection. 2004.
95. Crooke ST ED, Sampath R, Freıer SM, Massıre C, Hofstadler SA. et al. Compositions and methods for the treatment of severe acute respiratory syndrome (SARS). 2004.
96. Neuman BW, Stein DA, Kroeker AD, Churchill MJ, Kim AM, Kuhn P. et al. Inhibition, escape, and attenuated growth of severe acute respiratory syndrome coronavirus treated with antisense morpholino oligomers. J Virol. 2005;79:9665-76
97. Barrey E, Burzio V, Dhorne-Pollet S, Eléout J, Delmas B. Think Different with RNA Therapy: Can Antisense Oligonucleotides Be Used to Inhibit Replication and Transcription of SARS-Cov-2? 2020.
98. Mansoor M, Melendez AJ. Advances in antisense oligonucleotide development for target identification, validation, and as novel therapeutics. Gene Regul Syst Bio. 2008;2:275-95
99. Puerta-Fernandez E, Romero-Lopez C, Barroso-delJesus A, Berzal-Herranz A. Ribozymes: recent advances in the development of RNA tools. FEMS Microbiol Rev. 2003;27:75-97
100. Koseki S, Tanabe T, Tani K, Asano S, Shioda T, Nagai Y. et al. Factors governing the activity in vivo of ribozymes transcribed by RNA polymerase III. J Virol. 1999;73:1868-77
101. Trang P, Hsu A, Zhou T, Lee J, Kilani AF, Nepomuceno E. et al. Engineered RNase P ribozymes inhibit gene expression and growth of cytomegalovirus by increasing rate of cleavage and substrate binding. J Mol Biol. 2002;315:573-86
102. Li W, Liu Y, Wang Y, Li R, Trang P, Tang W. et al. Engineered RNase P Ribozymes Effectively Inhibit the Infection of Murine Cytomegalovirus in Animals. Theranostics. 2018;8:5634-44
103. Lilley DM. Structure, folding and mechanisms of ribozymes. Curr Opin Struct Biol. 2005;15:313-23
104. Feng Y, Leavitt M, Tritz R, Duarte E, Kang D, Mamounas M. et al. Inhibition of CCR5-dependent HIV-1 infection by hairpin ribozyme gene therapy against CC-chemokine receptor 5. Virology. 2000;276:271-8
105. Lieber A, He CY, Polyak SJ, Gretch DR, Barr D, Kay MA. Elimination of hepatitis C virus RNA in infected human hepatocytes by adenovirus-mediated expression of ribozymes. J Virol. 1996;70:8782-91
106. Mitsuyasu RT, Merigan TC, Carr A, Zack JA, Winters MA, Workman C. et al. Phase 2 gene therapy trial of an anti-HIV ribozyme in autologous CD34+ cells. Nat Med. 2009;15:285-92
107. Tang XB, Hobom G, Luo D. Ribozyme mediated destruction of influenza A virus in vitro and in vivo. J Med Virol. 1994;42:385-95
108. Liu C, Zhou Q, Li Y, Garner LV, Watkins SP, Carter LJ. et al. Research and Development on Therapeutic Agents and Vaccines for COVID-19 and Related Human Coronavirus Diseases. ACS Cent Sci. 2020;6:315-31
109. He Y, Zhang T, Yang N, Xu M, Yan L, Wang L. et al. Self-cleaving ribozymes enable the production of guide RNAs from unlimited choices of promoters for CRISPR/Cas9 mediated genome editing. J Genet Genomics. 2017;44:469-72
110. Walker MP, Lindner SE. Ribozyme-mediated, multiplex CRISPR gene editing and CRISPR interference (CRISPRi) in rodent-infectious Plasmodium yoelii. J Biol Chem. 2019;294:9555-66
111. Dash PK, Kaminski R, Bella R, Su H, Mathews S, Ahooyi TM. et al. Sequential LASER ART and CRISPR Treatments Eliminate HIV-1 in a Subset of Infected Humanized Mice. Nat Commun. 2019;10:2753
112. Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363-6
113. Rand TA, Ginalski K, Grishin NV, Wang X. Biochemical identification of Argonaute 2 as the sole protein required for RNA-induced silencing complex activity. Proc Natl Acad Sci U S A. 2004;101:14385-9
114. Matranga C, Tomari Y, Shin C, Bartel DP, Zamore PD. Passenger-strand cleavage facilitates assembly of siRNA into Ago2-containing RNAi enzyme complexes. Cell. 2005;123:607-20
115. Kim D, Rossi J. RNAi mechanisms and applications. Biotechniques. 2008;44:613-6
116. Carthew RW, Sontheimer EJ. Origins and Mechanisms of miRNAs and siRNAs. Cell. 2009;136:642-55
117. Setten RL, Rossi JJ, Han SP. The current state and future directions of RNAi-based therapeutics. Nat Rev Drug Discov. 2019;18:421-46
118. van den Berg F, Limani SW, Mnyandu N, Maepa MB, Ely A, Arbuthnot P. Advances with RNAi-Based Therapy for Hepatitis B Virus Infection. Viruses. 2020 12
119. Kelleher AD, Cortez-Jugo C, Cavalieri F, Qu Y, Glanville AR, Caruso F. et al. RNAi therapeutics: an antiviral strategy for human infections. Curr Opin Pharmacol. 2020;54:121-9
120. Magalhaes M, Alvarez-Lorenzo C, Concheiro A, Figueiras A, Santos AC, Veiga F. RNAi-based therapeutics for lung cancer: biomarkers, microRNAs, and nanocarriers. Expert Opin Drug Deliv. 2018;15:965-82
121. Shah VN, Pyle L. Lumasiran, an RNAi Therapeutic for Primary Hyperoxaluria Type 1. N Engl J Med. 2021;385:e69
122. Qin ZL, Zhao P, Zhang XL, Yu JG, Cao MM, Zhao LJ. et al. Silencing of SARS-CoV spike gene by small interfering RNA in HEK 293T cells. Biochem Biophys Res Commun. 2004;324:1186-93
123. Zhang Y, Li T, Fu L, Yu C, Li Y, Xu X. et al. Silencing SARS-CoV Spike protein expression in cultured cells by RNA interference. FEBS Lett. 2004;560:141-6
124. Cui L, Wang H, Ji Y, Yang J, Xu S, Huang X. et al. The Nucleocapsid Protein of Coronaviruses Acts as a Viral Suppressor of RNA Silencing in Mammalian Cells. J Virol. 2015;89:9029-43
125. Tao P, Zhang J, Tang N, Zhang BQ, He TC, Huang AL. Potent and specific inhibition of SARS-CoV antigen expression by RNA interference. Chin Med J (Engl). 2005;118:714-9
126. Zhao P, Qin ZL, Ke JS, Lu Y, Liu M, Pan W. et al. Small interfering RNA inhibits SARS-CoV nucleocapsid gene expression in cultured cells and mouse muscles. FEBS Lett. 2005;579:2404-10
127. Cao YL, Wang Y, Guo R, Yang F, Zhang Y, Wang SH. et al. Identification and characterization of three novel small interference RNAs that effectively down-regulate the isolated nucleocapsid gene expression of SARS coronavirus. Molecules. 2011;16:1544-58
128. Qin ZL, Zhao P, Cao MM, Qi ZT. siRNAs targeting terminal sequences of the SARS-associated coronavirus membrane gene inhibit M protein expression through degradation of M mRNA. J Virol Methods. 2007;145:146-54
129. Vicenti I, Zazzi M, Saladini F. SARS-CoV-2 RNA-dependent RNA polymerase as a therapeutic target for COVID-19. Expert Opin Ther Pat. 2021;31:325-37
130. Lu A, Zhang H, Zhang X, Wang H, Hu Q, Shen L. et al. Attenuation of SARS coronavirus by a short hairpin RNA expression plasmid targeting RNA-dependent RNA polymerase. Virology. 2004;324:84-9
131. Meng B, Lui YW, Meng S, Cao C, Hu Y. Identification of effective siRNA blocking the expression of SARS viral envelope E and RDRP genes. Mol Biotechnol. 2006;33:141-8
132. He ML, Zheng B, Peng Y, Peiris JS, Poon LL, Yuen KY. et al. Inhibition of SARS-associated coronavirus infection and replication by RNA interference. JAMA. 2003;290:2665-6
133. Li T, Zhang Y, Fu L, Yu C, Li X, Li Y. et al. siRNA targeting the leader sequence of SARS-CoV inhibits virus replication. Gene Ther. 2005;12:751-61
134. Ni B, Shi X, Li Y, Gao W, Wang X, Wu Y. Inhibition of replication and infection of severe acute respiratory syndrome-associated coronavirus with plasmid-mediated interference RNA. Antivir Ther. 2005;10:527-33
135. Masters PS. The molecular biology of coronaviruses. Adv Virus Res. 2006;66:193-292
136. Akerstrom S, Mirazimi A, Tan YJ. Inhibition of SARS-CoV replication cycle by small interference RNAs silencing specific SARS proteins, 7a/7b, 3a/3b and S. Antiviral Res. 2007;73:219-27
137. Donia A, Bokhari H. RNA interference as a promising treatment against SARS-CoV-2. Int Microbiol. 2021;24:123-4
138. Wang S, Zhang Y, Liu S, Peng H, Sun L. Coronaviruses and the Associated Potential Therapeutics for the Viral Infections. 2020.
139. Tolksdorf B, Nie C, Niemeyer D, Rohrs V, Berg J, Lauster D. et al. Inhibition of SARS-CoV-2 Replication by a Small Interfering RNA Targeting the Leader Sequence. Viruses. 2021 13
140. Chowdhury UF, Sharif Shohan MU, Hoque KI, Beg MA, Sharif Siam MK, Moni MA. A computational approach to design potential siRNA molecules as a prospective tool for silencing nucleocapsid phosphoprotein and surface glycoprotein gene of SARS-CoV-2. Genomics. 2021;113:331-43
141. Bappy SS, Shibly AZ, Sultana S, Mohiuddin AKM, Kabir Y. Designing potential siRNA molecule for the nucleocapsid(N) gene silencing of different SARS-CoV-2 strains of Bangladesh: Computational approach. Comput Biol Chem. 2021;92:107486
142. Hasan M, Ashik AI, Chowdhury MB, Tasnim AT, Nishat ZS, Hossain T. et al. Computational prediction of potential siRNA and human miRNA sequences to silence orf1ab associated genes for future therapeutics against SARS-CoV-2. Inform Med Unlocked. 2021;24:100569
143. Rohani N, Ahmadi Moughari F, Eslahchi C. DisCoVering potential candidates of RNAi-based therapy for COVID-19 using computational methods. PeerJ. 2021;9:e10505
144. Burnett JC, Rossi JJ. RNA-based therapeutics: current progress and future prospects. Chem Biol. 2012;19:60-71
145. Thevendran R, Sarah S, Tang TH, Citartan M. Strategies to bioengineer aptamer-driven nanovehicles as exceptional molecular tools for targeted therapeutics: A review. J Control Release. 2020;323:530-48
146. Jayasena SD. Aptamers: an emerging class of molecules that rival antibodies in diagnostics. Clin Chem. 1999;45:1628-50
147. Valencia-Resendiz DG, Palomino-Vizcaino G, Tapia-Vieyra JV, Benitez-Hess ML, Leija-Montoya AG, Alvarez-Salas LM. Inhibition of Human Papillomavirus Type 16 Infection Using an RNA Aptamer. Nucleic Acid Ther. 2018;28:97-105
148. Yu X, Gao Y, Xue B, Wang X, Yang D, Qin Y. et al. Inhibition of hepatitis C virus infection by NS5A-specific aptamer. Antiviral Res. 2014;106:116-24
149. Kwon HM, Lee KH, Han BW, Han MR, Kim DH, Kim DE. An RNA aptamer that specifically binds to the glycosylated hemagglutinin of avian influenza virus and suppresses viral infection in cells. PLoS One. 2014;9:e97574
150. Zhou J, Lazar D, Li H, Xia X, Satheesan S, Charlins P. et al. Receptor-targeted aptamer-siRNA conjugate-directed transcriptional regulation of HIV-1. Theranostics. 2018;8:1575-90
151. Ahn DG, Jeon IJ, Kim JD, Song MS, Han SR, Lee SW. et al. RNA aptamer-based sensitive detection of SARS coronavirus nucleocapsid protein. Analyst. 2009;134:1896-901
152. Jang KJ, Lee NR, Yeo WS, Jeong YJ, Kim DE. Isolation of inhibitory RNA aptamers against severe acute respiratory syndrome (SARS) coronavirus NTPase/Helicase. Biochem Biophys Res Commun. 2008;366:738-44
153. Kang J, Yeom G, Ha SJ, Kim MG. Development of a DNA aptamer selection method based on the heterogeneous sandwich form and its application in a colorimetric assay for influenza A virus detection. New Journal of Chemistry. 2019
154. Lee KH, Zeng H. Aptamer-Based ELISA Assay for Highly Specific and Sensitive Detection of Zika NS1 Protein. Anal Chem. 2017;89:12743-8
155. Liebich S. Solubilized oligonucleotide modified RNA (UPDATED) aptamers designed against the RBD motif of Spike protein delivered in dose-dependent manner through inhalation. 2020.
156. Nielsen PE, Egholm M, Berg RH, Buchardt O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science. 1991;254:1497-500
157. Pellestor F, Paulasova P. The peptide nucleic acids (PNAs), powerful tools for molecular genetics and cytogenetics. Eur J Hum Genet. 2004;12:694-700
158. Shakeel S, Karim S, Ali A. Peptide nucleic acid (PNA) — a review. Journal of Chemical Technology and Biotechnology. 2006
159. Koppelhus U, Zachar V, Nielsen PE, Liu X, Eugen-Olsen J, Ebbesen P. Efficient in vitro inhibition of HIV-1 gag reverse transcription by peptide nucleic acid (PNA) at minimal ratios of PNA/RNA. Nucleic Acids Res. 1997;25:2167-73
160. Kaushik N, Basu A, Pandey VN. Inhibition of HIV-1 replication by anti-trans-activation responsive polyamide nucleotide analog. Antiviral Res. 2002;56:13-27
161. Tripathi S, Chaubey B, Barton BE, Pandey VN. Anti HIV-1 virucidal activity of polyamide nucleic acid-membrane transducing peptide conjugates targeted to primer binding site of HIV-1 genome. Virology. 2007;363:91-103
162. Namy O, Rousset JP, Napthine S, Brierley I. Reprogrammed genetic decoding in cellular gene expression. Mol Cell. 2004;13:157-68
163. Baranov PV, Henderson CM, Anderson CB, Gesteland RF, Atkins JF, Howard MT. Programmed ribosomal frameshifting in decoding the SARS-CoV genome. Virology. 2005;332:498-510
164. Plant EP, Rakauskaite R, Taylor DR, Dinman JD. Achieving a golden mean: mechanisms by which coronaviruses ensure synthesis of the correct stoichiometric ratios of viral proteins. J Virol. 2010;84:4330-40
165. Ahn DG, Lee W, Choi JK, Kim SJ, Plant EP, Almazan F. et al. Interference of ribosomal frameshifting by antisense peptide nucleic acids suppresses SARS coronavirus replication. Antiviral Res. 2011;91:1-10
166. Van Hoecke L, Roose K. How mRNA therapeutics are entering the monoclonal antibody field. J Transl Med. 2019;17:54
167. Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A. et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465-8
168. Van Lint S, Goyvaerts C, Maenhout S, Goethals L, Disy A, Benteyn D. et al. Preclinical evaluation of TriMix and antigen mRNA-based antitumor therapy. Cancer Res. 2012;72:1661-71
169. Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC. et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396-401
170. Bahl K, Senn JJ, Yuzhakov O, Bulychev A, Brito LA, Hassett KJ. et al. Preclinical and Clinical Demonstration of Immunogenicity by mRNA Vaccines against H10N8 and H7N9 Influenza Viruses. Mol Ther. 2017;25:1316-27
171. Richner JM, Jagger BW, Shan C, Fontes CR, Dowd KA, Cao B. et al. Vaccine Mediated Protection Against Zika Virus-Induced Congenital Disease. Cell. 2017;170:273-83 e12
172. Kose N, Fox JM, Sapparapu G, Bombardi R, Tennekoon RN, de Silva AD. et al. A lipid-encapsulated mRNA encoding a potently neutralizing human monoclonal antibody protects against chikungunya infection. Sci Immunol. 2019 4
173. Tiwari PM, Vanover D, Lindsay KE, Bawage SS, Kirschman JL, Bhosle S. et al. Engineered mRNA-expressed antibodies prevent respiratory syncytial virus infection. Nat Commun. 2018;9:3999
174. Van Hoecke L, Verbeke R, De Vlieger D, Dewitte H, Roose K, Van Nevel S. et al. mRNA Encoding a Bispecific Single Domain Antibody Construct Protects against Influenza A Virus Infection in Mice. Mol Ther Nucleic Acids. 2020;20:777-87
175. Li JQ, Zhang ZR, Zhang HQ, Zhang YN, Zeng XY, Zhang QY. et al. Intranasal delivery of replicating mRNA encoding neutralizing antibody against SARS-CoV-2 infection in mice. Signal Transduct Target Ther. 2021;6:369
176. Sanz L, Alvarez-Vallina L. Engineered mRNA and the Rise of Next-Generation Antibodies. Antibodies (Basel). 2021 10
177. Wright AV, Nunez JK, Doudna JA. Biology and Applications of CRISPR Systems: Harnessing Nature's Toolbox for Genome Engineering. Cell. 2016;164:29-44
178. Gronowski AM. Who or What is SHERLOCK? EJIFCC. 2018;29:201-4
179. Metsky HC, Freije CA, Kosoko-Thoroddsen T-SF, Sabeti PC, Myhrvold C. CRISPR-based surveillance for COVID-19 using genomically-comprehensive machine learning design. bioRxiv. 2020. 2020 02.26.967026
180. Abbott TR, Dhamdhere G, Liu Y, Lin X, Goudy L, Zeng L. et al. Development of CRISPR as a prophylactic strategy to combat novel coronavirus and influenza. bioRxiv. 2020. 2020 03.13.991307
181. Peacock H, Kannan A, Beal PA, Burrows CJ. Chemical modification of siRNA bases to probe and enhance RNA interference. J Org Chem. 2011;76:7295-300
182. Lennox KA, Behlke MA. Chemical modification and design of anti-miRNA oligonucleotides. Gene Ther. 2011;18:1111-20
183. Fattal E, Barratt G. Nanotechnologies and controlled release systems for the delivery of antisense oligonucleotides and small interfering RNA. Br J Pharmacol. 2009;157:179-94
184. Manoharan M. 2'-carbohydrate modifications in antisense oligonucleotide therapy: importance of conformation, configuration and conjugation. Biochim Biophys Acta. 1999;1489:117-30
185. Snead NM, Escamilla-Powers JR, Rossi JJ, McCaffrey AP. 5' Unlocked Nucleic Acid Modification Improves siRNA Targeting. Mol Ther Nucleic Acids. 2013;2:e103
186. Sun Y, Zhao Y, Zhao X, Lee RJ, Teng L, Zhou C. Enhancing the Therapeutic Delivery of Oligonucleotides by Chemical Modification and Nanoparticle Encapsulation. Molecules. 2017 22
187. Youngren-Ortiz SR, Gandhi NS, Espana-Serrano L, Chougule MB. Aerosol Delivery of siRNA to the Lungs. Part 1: Rationale for Gene Delivery Systems. Kona. 2016;33:63-85
188. Bhavane R, Karathanasis E, Annapragada AV. Agglomerated vesicle technology: a new class of particles for controlled and modulated pulmonary drug delivery. J Control Release. 2003;93:15-28
189. Velasquez LS, Shira S, Berta AN, Kilbourne J, Medi BM, Tizard I. et al. Intranasal delivery of Norwalk virus-like particles formulated in an in situ gelling, dry powder vaccine. Vaccine. 2011;29:5221-31
190. Fiore AE, Bridges CB, Cox NJ. Seasonal influenza vaccines. Curr Top Microbiol Immunol. 2009;333:43-82
191. Fukushige K, Tagami T, Naito M, Goto E, Hirai S, Hatayama N. et al. Developing spray-freeze-dried particles containing a hyaluronic acid-coated liposome-protamine-DNA complex for pulmonary inhalation. Int J Pharm. 2020;583:119338
192. Muralidharan P, Malapit M, Mallory E, Hayes D Jr, Mansour HM. Inhalable nanoparticulate powders for respiratory delivery. Nanomedicine. 2015;11:1189-99
193. Merckx P, De Backer L, Van Hoecke L, Guagliardo R, Echaide M, Baatsen P. et al. Surfactant protein B (SP-B) enhances the cellular siRNA delivery of proteolipid coated nanogels for inhalation therapy. Acta Biomater. 2018;78:236-46
194. Ito T, Okuda T, Takayama R, Okamoto H. Establishment of an Evaluation Method for Gene Silencing by Serial Pulmonary Administration of siRNA and pDNA Powders: Naked siRNA Inhalation Powder Suppresses Luciferase Gene Expression in the Lung. J Pharm Sci. 2019;108:2661-7
Author contact
![]() Corresponding authors: Prof Bifang He. Email: bfheedu.cn and Prof. Jian Huang. Email: hjedu.cn
Corresponding authors: Prof Bifang He. Email: bfheedu.cn and Prof. Jian Huang. Email: hjedu.cn

 Global reach, higher impact
Global reach, higher impact