10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(13):5086-5102. doi:10.7150/ijbs.72770 This issue Cite
Review
From Chihuahua to Saint-Bernard: how did digestion and microbiota evolve with dog sizes
1. Université Clermont Auvergne, UMR 454 MEDIS UCA-INRAE, Clermont-Ferrand, France
2. Lallemand Animal Nutrition, Blagnac, France
3. Dômes Pharma, Pont-du-Château, France
4. Institute of Animal Nutrition, Freie Universität Berlin, Königin-Luise-Strasse 49, Berlin, Germany
5. Toxalim (Research Center in Food Toxicology), University of Toulouse, INRAE, ENVT, INP-Purpan, UPS, Toulouse, France
Received 2022-3-10; Accepted 2022-7-17; Published 2022-8-1
Abstract
Health and well-being of dogs are of paramount importance to their owners. Digestion plays a key role in dog health, involving physicochemical, mechanical and microbial actors. However, decades of breeding selection led to various dog sizes associated with different digestive physiology and disease sensitivity. Developing new products requires the consideration of all the multi-faceted aspects of canine digestion, the evaluation of food digestibility, drug release and absorption in the gut. This review paper provides an exhaustive literature survey on canine digestive physiology, focusing on size effect on anatomy and digestive parameters, with graphical representation of data classified as “small”, “medium” and “large” dogs. Despite the huge variability between protocols and animals, interesting size effects on gastrointestinal physiology were highlighted, mainly related to the colonic compartment. Colonic measurements, transit time permeability, fibre degradation, faecal short-chain fatty acid concentration and faecal water content increase while faecal bile acid concentration decreases with body size. A negative correlation between body weight and Proteobacteria relative abundance was observed suggesting an effect of dog body size on faecal microbiota. This paper gathers helpful in vivo data for academics and industrials and supports the development of new food and pharma products to move towards canine personalized nutrition and health.
Keywords: canine, digestive physiology, gut microbiota, petfood, veterinary products
Introduction
Canis lupus familiaris, also known as the domesticated dogs, belong to the Canidae family like the grey wolf (Canis lupus) and the dingo, a domestic dog returned to the wild. Descending from the grey wolf, dogs might have been the first animal domesticated by humans around 20.000 to 40.000 years ago [1]. Dogs were initially strict carnivores, but during the agricultural revolution they probably acquired the ability to digest starch and became facultative carnivores. Genes playing key roles in starch digestion (i.e. encoding for pancreatic amylase, membrane-bound intestinal maltase-glucoamylase and gene involved in glucose uptake) were selected during dog domestication [2]. Depending on their usefulness for humans, the Canis lupus familiaris subspecies have differentiated slowly, with the development of new canine species designated for specific tasks, such as herd protection (Mastiff), hunting (Pointer), cold hardiness (Siberian husky) or companion (Pekinese). Nowadays, the canine species includes approximatively 400 breeds with high size variability and weight ranging from 1 kg for a Chihuahua to 100 kg for a Saint-Bernard [3]. Dogs now occupy a full place in many families. Their health and well-being are therefore of paramount importance to their owners, to the extent that 7 % of French dogs have their own health insurance against 30 % of dogs homed in the United Kingdom and 80 % of dogs homed in Sweden (SantéVet/Ipsos, 2018). Digestion, a complex process involving many physicochemical, mechanical, and microbial mechanisms, is a key parameter in dog health. In particular, gut microbiota and its involvement in canine nutrition and health have increasingly been studied during the last decade. Developing new food or pharma products needs to consider all these multi-faceted aspects of canine digestion, to answer important questions such as food digestibility, micronutrient bioaccessibility, probiotic survival and activity, or drug release and absorption in the gut. Petfood manufacturers and veterinary companies aim to develop personalized ranges adapted to size (e.g. long-term growth of large breeds puppies, poor digestive tolerance and gastric dilatation volvulus for large dogs) or to address certain breed predispositions such as obesity in Labrador Retrievers or enteropathies in Terriers [4-7]. Nevertheless, the impact of dog size or breed on digestive parameters remains poorly described despite its full interest in canine nutrition and health.
This review paper provides for the first time an exhaustive survey of the literature on the impact of body size on dog's digestive physiology, in the entire gut from mouth to colon and feces, by gathering digestive anatomy, physicochemical parameters and gut microbiota variations. Relevant studies were identified, and information extracted regarding involved dogs (i.e. number of dogs, age, weight, breed, sex, reproduction state, living environment), nutrition (i.e. food type, feeding frequency, food's principal components) and analysis methods. Only in vivo studies on healthy adult dogs, fed with dry food or ingesting water were included. Here, canine body sizes were classified into three groups: “small” under 10 kg, “medium” between 10-30 kg and “large” up to 30 kg according to usual practice of main petfood suppliers. Then, the selected data were analyzed according to dog sizes and clarified through graphical representations to highlight a potential size effect on digestive parameters.
General observations of canine digestion and associated organs
External morphological differences observed between extreme dog sizes such as Chihuahua and Saint-Bernard obviously reveal internal anatomical modifications. The canine mature digestive tract length can represent 2.8 % to 7 % of the total body weight (BW), in a 60 kg and a 5 kg dog, respectively [8]. Since gastrointestinal tract (GIT) absolute length (in centimeters) is a reflect of dog height at the shoulder with a 6:1 ratio [9], it leads to the question: how does the size of dog impact digestive anatomy? Canine digestive anatomy is adapted to their facultative carnivorous diet (i.e. high-protein and high-fat diet) with a short and simple digestive tract. Digestion starts in the mouth with mastication process, helped by saliva. After swallowing, food boluses are transported through the esophagus into the stomach which is a J-shaped organ of glandular type, characterized by three anatomical compartments (i.e. fundus, corps and antrum) leading to the pylorus sphincter [10]. Canine gastric mucosal cells secrete hydrochloric acid (HCl), pepsin and lipase, which makes stomach essential in protein and lipid digestion. Canine stomach has a high dilatation capacity, varying from a maximal volume of 0.5 L for small dogs to 8 L in large dogs, which corresponds to the extreme quantity of food that a dog can ingest [10]. Digestion continues along the small intestine which is distributed as 10 % length for duodenum, 85 % for jejunum and 5 % for ileum [10,11]. Small intestine length measured post mortem is positively correlated (Pearson correlation of 0.672) to canine BW (from 240 cm for a 5 kg to 640 cm for a 33 kg dog), as well as small intestine width (weaker correlation, R2 = 0.36) [11]. Canine small intestine, together with peripheral organs such as pancreas and liver, have a key role in canine digestion process. Pancreas produces pancreatic juice delivered into duodenum and associated with protein, carbohydrate and lipid digestion. Liver, coupled with gallbladder, has a central role in lipid digestion through bile acid (BA) production and induction of increased intestinal peristalsis [12]. Small intestine is also a central player in nutrient absorption, allowed by the presence of microvilli at the surface of enterocytes. When measuring intestinal wall thickness at different levels of the GIT (descending duodenum, proximal and distal jejunum, proximal and distal ileum), higher values were observed for male dogs compared to female (except for distal ileum) but no correlation was found with dog sizes whatever the intestinal compartment [13]. Regarding small intestinal villus length, an old study from 1978 showed no correlation between dog weight and mucosal dimensions [14]. In adult dogs from various sizes, duodenal villus length was 722 ± 170 µm [15]. Jejunal villi were longer in small dogs like Pomeranian and Fox Terrier (900 μm) than in medium ones such as Newfoundland (500 µm) [16]. Lastly, ileal villus length was measured in medium size Greyhound female and values around 796-823 µm were found [17]. Canine large intestine measures around 20-80 cm with 2-3 cm diameter in medium dogs [10]. The three parts of the canine colon (i.e. ascending, transverse and descending) are not so well defined when compared to humans, with the particularity to be non-sacculated and devoid of sigmoid colon [10]. Ascending colon represents in medium size dog 20% of the colon length, while transverse and descending correspond to 30 % and 50 %, respectively. The two first parts are used for transport, electrolyte and water modification as well as for bacterial fermentation and storage areas, while descending colon mainly functions as conduit ending with rectum. Canine large intestine is involved in water and electrolyte absorption but also degradation of residual nutrients thanks to the fermentation activity of resident microorganisms called gut microbiota. Large intestine total length appears to vary according to dog's BW, from 32 cm for Miniature Poodles to 99 cm for Great Danes [18]. Volume and surface are also increased from Miniature Poodle to Great Dane (volume of 92 versus 2106 cm3, surface of 191 versus 1612 cm2). As the large intestine length increases with BW, the same positive relation is observed for absorption surface with a higher number of villi in large compared to small dogs [18]. Colonic crypts length was around 500-600 µm but without correlation with dog size [16]. To conclude, scarce anatomy data (only five publications) evidenced morphological differences depending on dog's BW (mainly related to the colonic compartment), even if important parameters have not been evaluated such as gastric wall thickness, intestinal microvilli characteristics (i.e. length or number) or peripheral organs anatomy and functions. Variations in digestive anatomy can obviously affect physicochemical parameters such as pH, digestive secretions and transit time, and consequently gut microbiota.
Methods of literature research
Our literature search was performed using PubMed (https://pubmed.ncbi.nlm.nih.gov) and Google Scholar (https://scholar.google.fr) with the key words “dog” OR “canine” AND “stomach”, “small intestine”, “intestine”, “duodenum”, “jejunum”, “ileum”, “ileal”, “colonic”, “large intestine”, “rectum”, “feces”, AND “anatomy”, “digestion”, “pH”, “enzyme”, “digestive secretion”, “digestibility”, “permeability”, “absorption”, “microbiota”, “bile acids”, “transit time”, “fatty acids”, “fermentation”, “gas”, “mucus” in all available years. The online database search was last performed in January 2022 on titles, abstracts and key words including original articles, reviews, thesis, and books. Relevant studies were identified after consultation of the main text, figures, and supplementary materials. Information regarding involved dogs (i.e. number of dogs, age, weight, breed, sex, reproduction state, living environment), diet (i.e. type of food, feeding frequency, composition of food), health (i.e. healthy dogs only) and analysis methods were collected. Only in vivo studies on adult dogs, fed with dry food or ingesting water were included in the literature survey.
We found a total of 163 studies, including 87 providing information on a single dog size, with only small dogs involved in 7 publications, only medium dogs in 71 and only large dogs in 9 (Figure S1). The three dog sizes (i.e. small, medium and large dogs) were compared together in 8 additional studies. In the remaining 68 studies, 45 integrated dogs without specifying their characteristics and other 22 included different sizes of dogs but didn't discriminate them in their analysis (both classed in the “unclassified” group). Concerning publication date, 40 studies were performed over 30 years, 76 studies have been done between 5 and 30 years ago and 47 were performed in the last 5 years. Only 10 studies were directly targeting the influence of dog size on canine digestive physiology.
Impact of body size on digestive physicochemical parameters
Gastrointestinal pH
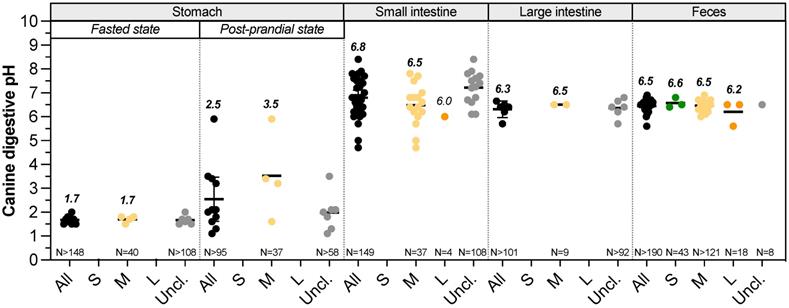
Gastrointestinal pH changes along the dog digestive tract (Fig. 1, Table S1). Mean salivary pH of medium dogs is around 7.3-7.8 and quickly decreases by 0.5 point with a stimulation using a piece of solid sugar on the tongue [19-21]. In the stomach, the arrival of food bolus stimulates HCl production. This compartment shows the lowest pH value along the GIT, allowing dogs to partially digest bones [22] and putrescent meat and largely depends on feed status. However, due to the paucity of data, it remains difficult to know how BW affects gastric, small intestinal and colonic pH (Fig. 1, Table S1). To date, gastric pH has not been assessed in small and large dogs [23-26]. Regarding medium dogs under fasted conditions, mean gastric pH of Beagles is around 1.5 (range 0.9-2.5), punctuated by occasional pH spikes with high frequency changes due to inter-individual variability [27]. Those values measured in laboratory animals are in accordance with pH found in mixed-breed owner dogs [28]. Small intestinal pH increases to value close to the neutrality because of the buffering capacity of pancreatic juice enriched in bicarbonate ions and bile [10]. It also increases from the proximal to the distal parts, from 6.5 to 8 in medium size dogs [29]. To date, there is no available study that investigates the influence of the dog size on duodenal and ileal pH [30]. The few studies investigating the canine jejunal pH measured a mean pH of 6.8 and 6.0 for medium and large dogs, respectively [31,32]. Only few studies investigated colonic pH using colonic cannula or wireless capsules, and once new, most of them do not discriminate dogs in terms of BW. Colonic pH is more acidic than the small intestine one, with mean values of 5-6.5 and 6.2, respectively for medium and large dogs, whereas there is no data concerning small dogs [29,33-35]. Most of the time, colonic pH is estimated using faeces and there is no information on how pH varies depending on colonic sections. The average canine faecal pH values are in accordance with colonic pH, mainly around 6.4-6.6, as observed in Fig. 1. For small dog group, three studies used faeces of 43 dogs and pH values vary weakly from 6.4 to 6.8 [36-38]. There are also plenty of data on the faecal pH of medium (more than 121 dogs) and large (18 dogs) size dogs, with a pH range of 6-6.9 and 5.6-6.5, respectively [39-42]. This is an accordance with some studies reporting that colonic and faecal pH of large dogs are more acidic than that of other size dogs fed with the same diet [18,43].
Digestive secretion
Enzymes. First digestion step occurs in the oral cavity with salivary enzymes (Table S2). Numerous recent studies measured amylase activity in saliva of healthy dogs [44-49]. Mean amylase activity varies from 26.5 to 37.3 UI/L of saliva in medium dogs according to literature (Table S2). One study involves 75 dogs from 8 to 42 kg (52 mixed breeds and 23 pure breeds) and measured 35.9 ± 41 UI/L amylase in saliva but results weren't discussed regarding dog sizes [48]. Lactate dehydrogenase and adenosine deaminase activities were also quantified in saliva, without classification with canine BW [45,48,50]. Gastric mucosa secretes gastric juice containing proteolytic (pepsin, chymosin) and lipolytic (lipase) enzymes [20,51]. In laboratory Beagles, gastric juice volume output increases with meal viscosity, from a total of 37.2 mL secreted for a low viscosity to 190 mL for a high viscosity meal [52]. Pancreatic juice, discharged in canine duodenum, has an alkaline pH (7.4-8.3). It contains amylase (2013 U/kg BW), lipase (9.8-33.3 mL 0.05 N NaOH/mL -no longer used unit of measure), phospholipases, cholesterases, proteases (old value of 407.5-2440 mg tyrosin/mL -no longer used unit of measure) and nucleases, without further detailed information [12,53]. Digestive secretions were mainly studied before 2000s, but values were not discriminated depending on dog sizes, and no study focuses on small and large dogs. However, enzymatic activities may vary according to the different diet composition (i.e. protein, lipid, fiber contents) adapted to each dog size.
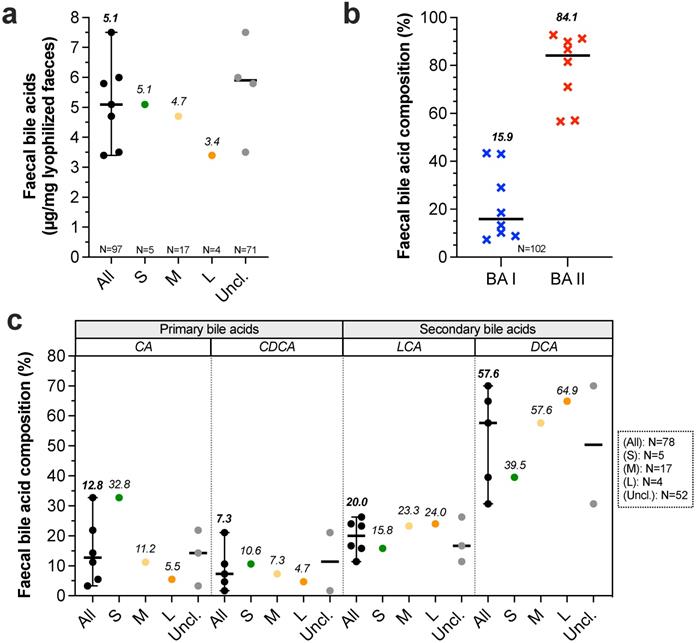
Bile salts. Bile is produced by liver, partially stored in gallbladder then discharged to duodenum during postprandial phase, allowing stimulation of intestinal motility, intestinal lipids saponification and vitamins A, D, E and K absorption. In liver, primary BA such as cholic acid (CA) and chenodeoxycholic acid (CDCA) are synthesized from cholesterol and conjugated to taurine or glycine [54]. Studies evaluating bile production in healthy dogs never discriminate dog sizes. Bile production was only evaluated in medium dogs and was found to be 29 mL/kg/24 h [55]. Once reached gallbladder, bile is up to 10 fold more concentrated than in liver with a total concentration around 50 (40-90) mmol/L [10,54,56,57]. Here, it contains up to 15 different BA but the three majors count for 99% of total pool, with 72.8% taurocholic acid, 20.3% taurodeoxycholic acid and 6.2% taurochenodeoxycholic acid [58]. In the small intestine, BA are deconjugated by gut microbiota and converted into secondary BA. 95% of BA are reabsorbed in ileum, return into liver and the 5% remaining fraction crosses colon [56]. Faecal BA concentrations were measured in three recent studies involving all dog sizes but curiously without BW distinction (Fig. 2A, Table S2). Authors found coherent results with concentrations ranged from 5.8 to 7.5 µg of total BA per mg of dry faeces [59-61]. Another recent study evaluated faecal BA concentrations in 24 healthy dogs [62]. After data retreatment (classification in size groups), small, medium and large dogs present respectively 5.1, 4.7 and 3.4 µg/mg of total BA per mg of dry faeces. This suggests a decrease in faecal BA concentrations with BW increase. Further analysis from 8 studies (Fig. 2B) indicates that relative percentages of faecal secondary BA (BA II: 84.9%) are higher than primary BA (BA I: 15.5%). Moreover, proportions of primary BA such as CA and CDCA seem to be inversely correlated to canine BW whereas the contrary is observed for secondary BA (only one study) (Fig. 2C). These results suggest that the microbiota activity, and notably the BA recycling, differs from small to large breed sizes.
Mucins. Mucins are produced by goblet cells all along the dog GIT [10]. Mucus thickness has been evaluated only in gastric compartment and stomach presents a mucosa covering mucin-layer of 576 μm and 425 μm, respectively in the antrum and fundus [10,63,64]. This mucin-layer allows protection of the epithelium against acidic pH of stomach and withstands bone fragments [65]. Influence of dog size on mucin secretion and mucin-layer thickness whatever the digestive compartment has never been assessed.
Impact of dog sizes on pH values in all digestive compartments. Results from studies measuring in dog's pH values in the stomach (under fasted or fed conditions), small intestine, large intestine and faeces are presented. Small dogs are plotted in green, medium dogs in yellow, large dogs in orange and unclassified dogs in grey. Raw data were pooled in “all” group (in black). Calculated median values are in italic bold, values for a single point in italic. Black bars represent 95% confidence intervals. The number of dogs involved in studies is indicated as “N=”
Impact of dog sizes on faecal bile acids. Results from studies in dog faeces quantifying total bile acids (BA) are represented in (a), further separated into primary (blue crosses) and secondary BA (red crosses) in (b). Detailed composition in cholic acid (CA), chenodeoxycholic acid (CDCA), lithocholic acid (LCA) and deoxycholic acid (DCA) is shown in (c). The same caption as used in Fig. 1 was applied
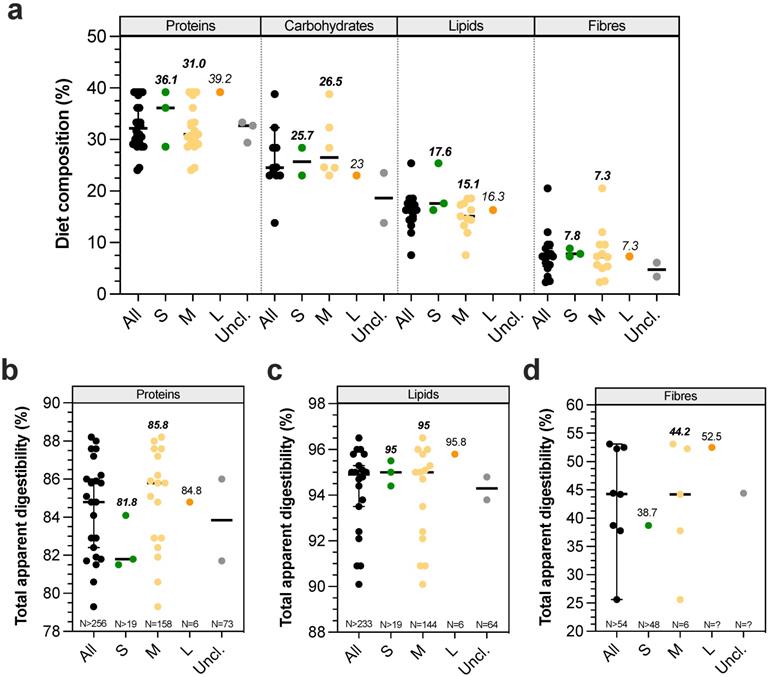
Diet composition and impact of dog sizes on total apparent digestibility. Nutritional composition of dry food diet used in canine studies is represented in (a). Results from studies investigating in dogs' total digestibility of proteins, lipids and fibres are presented in (b), (c) and (d), respectively. The same caption as used in Fig. 1 was applied
Nutrient digestibility
Digestibility defines the degree to which organic matter is digested by an animal. Its measure provides a qualitative and quantitative indicator of food's quality, i.e. the more digestible a food is, the higher the proportion of absorbed nutrients will be. Figure 3A gives an overview of canine dry food composition in dogs according to BW. Digestibility performances can be evaluated in dogs by measuring ileal or total (in faeces) apparent digestibility of a tested diet, and standardized digestibility could be obtained by deducing endogen products such as enzymes or metabolites delivered from intestinal cell desquamation. As previously observed for physicochemical parameters, digestibility studies are mainly focused on medium dogs and there are only two publications on small [66,67] and one on large dogs [66] (Table S3). Due to their invasive nature, only 4 studies have been performed with ileal cannula (to measure ileal digestibility), including 3 on medium dogs [68-71]. Lipid digestibility seems to be almost complete at the ileum level (i.e. 89.3-96.5%), with only around 3-5% increased digestibility when evaluating total digestibility in faecal samples. Ileal protein digestibility appears to be lower (51.3-76.2%), with higher variations certainly related to protein quality which largely influences this parameter [67,72-74]. Surprisingly, the only study investigating total dietary fibre digestibility found an ileal digestibility of 17.8%, while according to their definition fibres are only degraded in the large intestine [68]. Given the lack of data, it is impossible to conclude on a possible effect of dog BW on ileal nutrient digestibility. Total apparent protein (82-88%) and lipid (95-95.8%) digestibilities appear to be equal between different dog sizes, whatever the initial proportion of dietary proteins or lipids (Fig. 3B-C). In contrast, total apparent dietary fibre digestibility (Fig. 3D) appears to be higher in large than in small and medium dogs (52.5 ± 4% for Great Dane versus 39 ± 7.4% for Miniature poodle, and 26-38% for medium dogs) [42,75,76]. Indeed, it seems that fibre digestibility would be quite similar between small and medium dogs, while it would be improved in large dogs. In addition, faecal apparent digestibility of dry matter, organic matter and gross energy appears to be significantly higher for large compared to small dogs [66]. All in all, those results mean that the colonic fermentation seems to be more important in large than in medium and small breed size dogs.
Intestinal absorption
Permeability. During digestion process, food is broken down into small soluble compounds (amino acids, fatty acids, monosaccharides, minerals and vitamins), able to be absorbed mainly through the villi-covered wall of the small intestine. Nutrient passage through the epithelial wall is modulated by intestinal permeability, which is the property of epithelium to allow some molecules to be absorbed passively or actively through mucosa while avoiding the passage of microorganisms and macromolecules. Lactulose to L-rhamnose or lactulose to sucralose urinary ratios could be used to monitor changes in canine small and large intestine permeability, respectively [77]. A higher lactulose to L-rhamnose ratio is associated with a leakier small intestine, while a lower lactulose to L-rhamnose ratio indicates a higher colonic permeability. Using these methods, Weber et al. [78] observed an increased intestinal permeability in Giant Schnauzer and Great Danes (large dogs; lactulose to L-rhamnose ratio: 0.31) compared to small dogs (0.16), and Hernot et al. [77] found a higher colonic permeability in large dogs (lactulose to sucralose ratio: 0.35) than in small ones (0.51). Those differences could be related to modifications associated with dog size in intestinal area, pore size, frequency of tight junctions, differences in tightness of tight junctions or accessibility of luminal content to intestinal crypts [79]. Of note, breed differences were particularly noticed with a higher colonic permeability in Great Danes, as previously described [80,81]. An increased permeability could affect both nutrient, metabolite and electrolyte absorption but also microorganism's translocation. This may explain the weaker digestive tolerance of resistant-starch and higher digestive sensibility of large dogs compared to small ones, as discussed by Goudez et al. [82].
Passive absorption. Water, electrolytes and vitamins are absorbed through passive mechanisms in the small and large intestinal lumen. In healthy dogs, around 90% fluids crossing the colon are reabsorbed by mucosa [20,83]. Meyer et al. [81] demonstrated that total faecal water increases with dog BW, but the percentage of free faecal water decreases. This is of high interest because an increase in free faecal water content is associated with a higher colonic water content that can in turn influence in vivo drug dissolution, in the case of poorly soluble drugs for which dissolution continues in the large intestine [43]. Whereas small dogs tend to have drier stools, a tendency of poorer faecal consistency and higher water content is observed in larger dogs. Potassium and bicarbonate ions are secreted into the colonic lumen, whereas sodium and chloride ions are passively absorbed from luminal contents [83]. Uptake of sodium ions creates an hypertonic environment next to crypts, generating an electrochemical gradient across colonic mucosa which drives water uptake from luminal contents by osmosis [83]. Based on observation that large digestibility variations are observed within the same breed and between different breeds, Zentek and Meyer [80] compared mineral digestibility of four food types in Great Danes and Beagles. There was no breed difference for calcium, magnesium and phosphorous absorption (Table S3), while net colonic sodium absorption tended to be 9-23% lower in Great Danes compared to Beagles. These data were further supported by Weber et al. [36] describing an increase in sodium faecal content with an increase in BW (2.1 ± 0.7 g/kg DM in Miniature Poodle versus 6.1 ± 1.3 g/kg DM in Great Dane), traducing a lower sodium absorption by large dogs. Moreover, a reduction of colonic absorption of sodium has been particularly observed in Beagle, Labrador Retriever, Springer Spaniel and Münsterländer, suggesting a breed sensitivity [18]. Besides, Neri et al. [84] reported a significantly greater faecal potassium concentration in large compared to smaller dogs. Independently of dog sizes, 90% vitamin D, 80-90% vitamin A, 40-90% vitamin K and 35-50% vitamin E are absorbed by passive absorption in the proximal small intestine [20].
Active absorption. Active absorption processes in the small intestine implicate co-transporters (e.g. glucose or sodium-dependent transports) and concerns monosaccharides from carbohydrate degradation and peptides from protein degradation. Thus, 95% of monosaccharides are absorbed in the duodenum and proximal jejunum [20], and 30% of amino acids and 70% of tripeptides are absorbed and assimilated in the proximal jejunum [12]. Regarding lipids, 80% of fatty acids and monoglycerides are absorbed in form of micelles in the small intestine and resulting in chylomicrons that passed into the intestinal lymphatic capillary of villus by endocytosis. There is no available information on the influence of dog size on nutrient absorption. Moreover, the overall active transport capacity of small intestine has been assessed by examining urinary excretion ratio of D-xylose to 3-O-methyl-D-glucose [78]. Non-significantly different ratios of 0.57, 0.58 and 0.59 for small, medium and large dogs respectively have been reported, suggesting that small intestinal active transport is relatively consistent between sizes.
Mechanical digestion and gastrointestinal transit time
Motility. Canine gut motility was firstly evaluated using radiopaque markers, plastic beads or breath test. Recently, wireless motility capsule was developed to measure pressure, forces and gut contractions frequency. Using this method, Boscan et al. [85] observed in fed medium dogs a lower maximal amplitude contraction in the stomach compared to small intestine (52 mmHg versus 75 mmHg, respectively), coupled with higher gastric contraction frequency, with 3.7 contractions/min in the stomach versus 0.5 contraction/min in the small intestine. Another study involving dogs from different sizes observed similar tendency on maximal amplitude contraction (lower in stomach than in small intestine, with 0.2 versus 4.1 mmHg), but opposite results on frequency (0.8 in stomach versus 10.9 contractions/min in small intestine) [86]. Moreover, in this study, large intestinal contraction frequency seems to be similar to the gastric ones (0.6 contraction/min). Authors also calculated a motility index defined as the area under the pressure curve and the higher motility index was observed in small intestine (306.2 compared to 20 in stomach and 76.1 in colon). Using wireless motility capsule, Farmer et al. [87] found that motility indexes were higher in large intestine (199 mmHg*second/min) compared to small intestine (134 mmHg*second/min) and stomach (55 mmHg*second/min) with a similar maximum of 3.7 contractions/min in gastric compartment [88]. Lastly, no study has investigated how dog BW or size influences gut peristalsis.
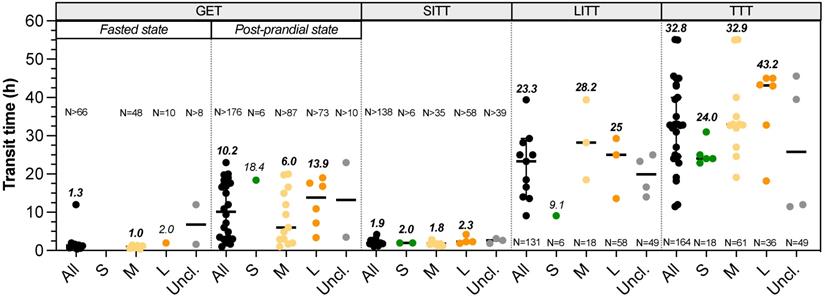
Transit time. There is no available data on the duration of oral phase in dogs, but they are well known to quickly swallow their whole food. Data on gastric emptying time (GET), small intestinal transit time (SITT), orocaecal transit time (OCTT), large intestinal transit time (LITT) and total transit time (TTT) can be found in the literature with homogeneous definition between studies (Fig. 4, Table S4). Three different studies evaluate the impact of dog size on GET fed animals. Weber et al. [75] showed no significant difference in half-gastric emptying time between four breeds dogs (i.e. Miniature Poodle, Standard Schnauzer, Giant Schnauzer and Great Danes) using radiopaque markers ingested with food (T50 = 6.4-7.8 h). Without specifying any values, Bourreau et al. [89] concluded on a longer GET in large compared to small breeds after ingestion of a dry food meal using breath test method. Contrarily, Boillat et al. [4] described a shorter GET in large compared to medium breeds (range 6.8-15 h), using wireless motility capsule immediately administered after a dry food meal. Thus, there is apparently no relationship between BW and GET not only in fed, but also in fasted animals (Fig. 4). Besides, a liquid meal conduced to a shorter GET compared to meat, with 90% emptying in 0.4 h and 50% in 1-3 h, respectively (unknown dog size and method) [90]. This suggests that canine gastric emptying is influenced by food consistency [91]. There is also no consensus on the effect of dog size on SITT. Oswald et al. [43] and Weber et al. [8] found no influence of breed or BW, while Boillat et al. [4] measured a shorter SITT in largest dogs, ranging 1.6-3.7 h without linking transit time and dog size [4,8,43]. OCTT was evaluated in dogs using very different methods. Some studies used sulfasalazine (converted into sulfapyridine in plasma) but do not employ the same threshold to define OCTT, i.e. either 50% conversion or first appearance in plasma [75,92], whereas more recent studies used wireless motility capsule. As a consequence, extremely variable results of OCTT are provided, from 2.2-2.8 h with sulfapyridine [75,92] to 20.7 h with capsule [93]. Whatever the method used, these authors conclude to an absence of correlation between OCTT and BW. Studies of Boillat et al. [4] and Warrit et al. [94] assessed LITT in dogs from several breeds and various BW using wireless motility capsule. Both works conclude on the absence of correlation between LITT and BW (Fig. 4), with T1/2 ranged 7.1-42.9 h [4] and 25.0 h (1.1-49.1 h) [86]. However, using plastic beads, researches revealed a longer LITT in large dogs (29.3 h for great Dane) than in small dogs (9.1 h for Miniature Poodle) and a significant positive correlation between LITT and BW, but also between LITT and shoulder height was demonstrated [92]. In this study, LITT accounts for 39% of mean TTT for small breed dogs and 70% for large ones, which means that longer transit times observed in large dogs could be related to a longer LITT. Lastly, TTT showed a clear positive correlation with BW, as highlighted in Fig. 4 [18]. When gathering the data obtained in all the available studies, TTT ranged from 22.9-31 h (calculated median 24 h) in small dogs, 19.1-55 h (median 32.9 h) in medium dogs and 18.2-45 h (median 43.2 h) in large dogs. Especially, using plastic beads in small and large breeds, TTT observed was 22.9 h in Miniature Poodle and 43.3 h in Great Dane, whilst giant Schnauzer showed an even higher TTT of 55 h [95]. This result was explained by the authors through a high stress sensitivity of giant Schnauzer that could influence their transit time in refraining their defecation, emphasizing a breed effect in addition to body size influence.
Impact of dog sizes on gastrointestinal transit time. Results from studies in dogs evaluating gastric emptying time (GET) under fasted or fed status, small intestinal transit time (SITT), large intestinal transit time (LITT) and total transit time (TTT) are represented. The same caption as used in Fig. 1 was applied
Impact of body size on microbial parameters
Gut microbiota composition
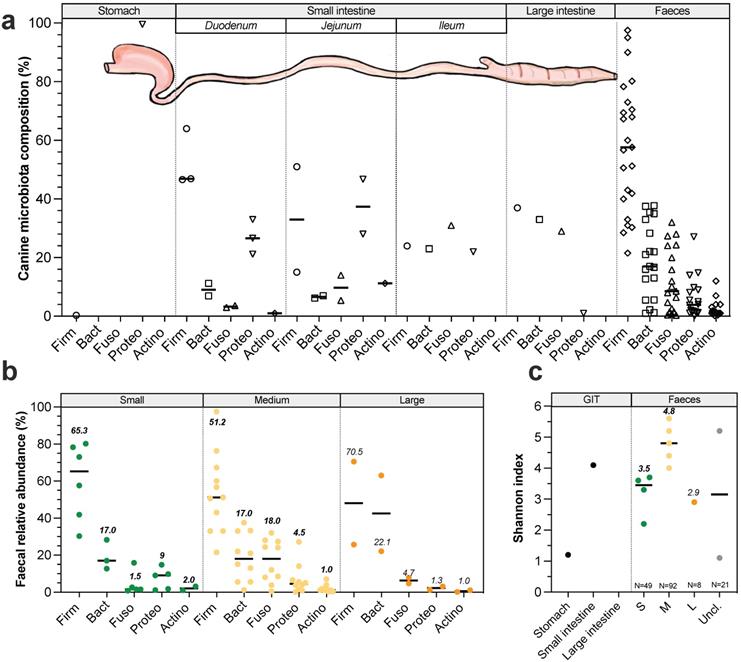
Longitudinal variations. In dogs like in other mammals, microorganisms colonize the entire GIT from mouth to rectum. All along GIT, there are longitudinal variations in gut microbiota composition due to changes in pH, substrate concentrations (including oxygen and nutrient availability) and transit time [64,96,97]. Gut microbiota has been weakly described in dogs (compared to humans) and most of available studies have been performed since 2003 (for detailed information see Table S5). Canine oral microbiota present similar number (around 350 bacterial taxa from 148 genera) but significantly different populations compared to the human ones [98] and is mainly colonized by Proteobacteria (45%), Bacteroidetes (25%) and Firmicutes (19%). Most abundant species are Porphyromonas cangingivalis and Porphyromonas gulae [99,100]. Regarding the other digestive compartments, studies have been mostly performed on faeces to avoid invasive procedures. Stomach is the less colonized compartment with 104 to 105 colony forming units (CFU) per gram of content in medium dogs, mainly composed by Proteobacteria (Fig. 5A) including Helicobacter spp. that are potential pathogenic strains [31,96,101]. Small intestine contains 105 to 107 CFU/g of content [31,101]. Duodenum (Fig. 5A) is colonized by Firmicutes (calculated median 47%), Proteobacteria (27%), Bacteroidetes (9%), Fusobacteria (3%) and Actinobacteria (1%), whereas jejunum is characterized by a higher abundance in Proteobacteria (37%), Actinobacteria (11%) and Fusobacteria (10%), together with lower percentages of Firmicutes (33%) and Bacteroidetes (7%) [28,102,103]. Ileum (Fig. 5A) is dominated by 31% Fusobacteria, 24% Firmicutes, 23% Bacteroidetes and 22% Proteobacteria [104]. These abundances should be considered with caution as they have been found in a single study performed in 6 medium dogs. As for other mammals, large intestine is the most colonized part of the GIT, with up to 109 to 1011 CFU/g of content [96]. According to a unique publication using 16S Illumina sequencing to investigate microbiota composition from 6 healthy Hound dogs [104], colonic digesta is dominated by 37% Firmicutes, 33% Bacteroidetes, 29% Fusobacteria and 1% Proteobacteria including E. coli-like organisms (Fig. 5A). It's interesting to highlight that majority of taxa colonizing the colon are also found in canine faeces [105] which seems to be rather different from the human situation where a significant number of mucus-adherent bacteria from the colon are not found in faeces [105]. No study has investigated dog size effect on gut microbiota composition elsewhere than in stools, and the main variations in faeces are presented in Figure 5B and 5C. Whatever dog sizes, faecal microbiota of healthy dogs is dominated by three main bacterial phyla: Firmicutes, Bacteroidetes and Fusobacteria [105]. Bacteria from Actinobacteria and Proteobacteria phyla are also found in canine faeces but in a lesser proportion. Of interest, a variable relative abundance of Bacteroidetes was reported and was inversely correlated to Fusobacteria relative abundance indicating they might occupy the same ecological niches [106]. Fusobacteria and Proteobacteria seem to be more abundant in dogs than in other omnivorous, probably related to diet changes [107]. Unlike in human where Fusobacterium is frequently associated with diseases, in dogs this genus is related to non-stressful conditions and is therefore probably a marker of an healthy state, especially because its abundance increases when dogs have access to the outside [43]. In small dogs faeces (Fig. 5B), average Firmicutes proportions vary widely from 30 to 80%, followed by 13-28% Bacteroidetes, while a lower abundance of Proteobacteria (1-15%), Fusobacteria (1-16%) and Actinobacteria (1-3%) was detected [108-110]. Medium dogs display similar value ranges of Firmicutes (15-98%), Bacteroidetes (0.1-34%), Proteobacteria (0.1-27%) and Actinobacteria (1%), but a larger proportion of Fusobacteria (0.1-40%) compared to small dogs [108,111]. Only one study investigated faecal microbiota composition in 8 large dogs and quantified 71% Firmicutes, 22% Bacteroidetes, 5% Fusobacteria, and 1% Actinobacteria, with interestingly a much lower abundance of Proteobacteria (1%) than in small and medium dogs [40]. In few studies, canine faecal diversity was followed with Shannon index and calculated medians seem to be higher in medium dogs (4.8, four studies) compared to small (3.5, five studies) and large dogs (2.9, a single study) (Fig. 5C). In addition to Bacteria (representing 98%), canine faecal microbiota also contains 1.1% Archaea, 0.4% Fungi and 0.4% viruses, mainly bacteriophages [112,113]. Fungal part of the faecal microbiota is composed by 97.9% Ascomycota and 1% Basidiomycota [114]. Even if methanogen Archaea have been detected in healthy dogs faeces, there is no information on their methanogen potential [114].
Variations in gut microbiota composition along the canine digestive tract and impact of dog sizes. Main bacteria populations found in the different compartments of the dog gastrointestinal tract are represented in (a). Bacteria counts are expressed in colony forming units (CFU) per gram of digestive content. Results from studies exploring by 16S rRNA Illumina sequencing canine microbiota composition (regardless of dog size) in the different digestive compartments are presented in (b). Influence of dog sizes on faecal microbiota composition at the phylum level is shown in (c) and corresponding Shannon index in (d). Canine main phyla are Firmicutes (Firm), Bacteroidetes (Bact), Fusobacteria (Fuso), Proteobacteria (Proteo) and Actinobacteria (Actino). The same caption as used in Fig. 1 was applied
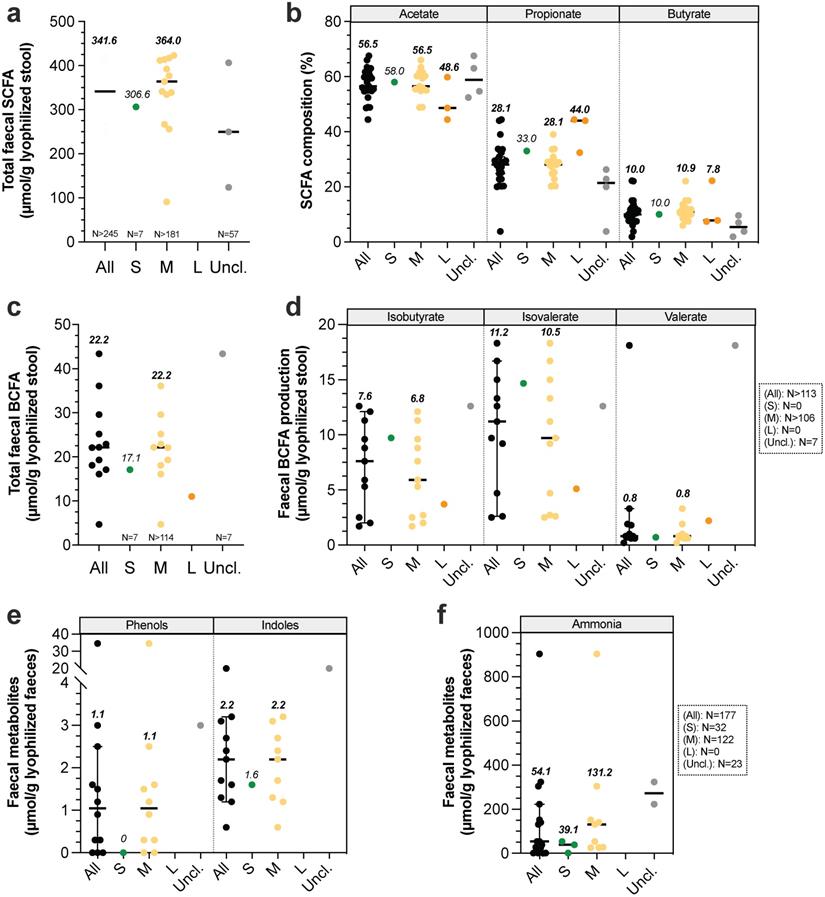
Impact of dog sizes on faecal microbial products production. Results from studies in dogs measuring total faecal major short-chain fatty acids (SCFA, i.e. acetate, propionate and butyrate) are presented in (a) and detailed in (b). Similarly, influence of dog size on major branched-chain fatty acids production (BCFA, i.e. isobutyrate, isovalerate and valerate) is shown in (c) and detailed in (d). Effect of dog size on other microbial metabolites are presented in (e) for phenols and indoles and (f) for ammonia. The same caption as used in Fig. 1 was applied
Radial variations. In addition to longitudinal variations, there are also radial changes in gut microbial composition that starts to be described in human [115] but are still in infancy in dogs. Indeed, the entire gut epithelium is covered by a mucus layer that offers an alternative source of host-derived nutrients. This mucus is colonized by a specific mucus-adherent microbiota (namely mucosal microbiota) and seems to play a key role in host homeostasis [116]. Of note, there is a lack of studies on the canine mucosal microbiota. Only two studies investigated the mucosa-associated bacteria on the outer mucus layer in the colon of healthy dogs, using targeted FISH approach [117,118]. Analysis of colonic biopsy samples from healthy Boxers revealed that bacteria appear to be restricted to the outer mucus layer, as no bacteria was detected within the mucosa [117]. In addition, Cassmann et al. [118] demonstrated that free ileal and colonic mucus of healthy young dogs (< 2 years old) was mainly colonized by Bacteroidetes spp. and Eubacteria, while Eubacteria represented the major bacteria attached to adherent mucus. Authors reported that there were almost no bacteria attached to surface epithelium or contained within mucosa. Of interest, Akkermansia muciniphila, a well-known mucin-degrading bacteria in humans, inversely correlated to obesity, was not yet identified in canine faeces [119].
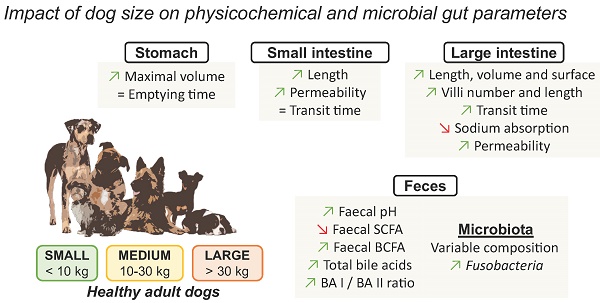
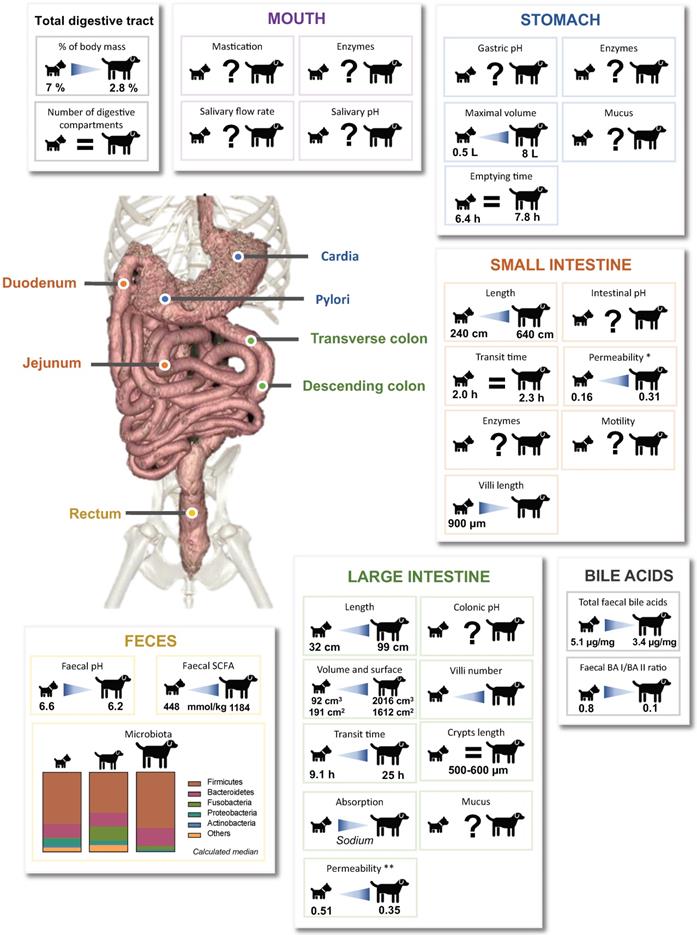
Overview of the impact of dog sizes on digestive physiology and faecal microbiota composition and activity. Key parameters of the oral, gastric, intestinal and colonic compartments from the canine digestive tract are summarized. Specified values were obtained from reports comparing in a same study the results obtained for small and large dogs. Lack of data are represented by “?”, BA: bile acid, SCFA: short chain fatty acids. *: Lactulose/L-rhamnose ratio, **: Lactulose/sucralose ratio
Gut microbiota metabolic activities and functions
Gut microbiota is known to play a key role in host homeostasis and health maintenance, as it is implicated in many nutritional (e.g. vitamin synthesis, fibre degradation), immunological (immune system maturation) and physiological processes (e.g. vascularization, epithelium integrity, “barrier” effect against pathogens and lipid digestion via the metabolism of primary BA into secondary BA) [20,120]. At a functional level, whatever the type of food, identified gene content of microbiome from medium dogs was not modified and was associated with the metabolism of carbohydrates (12.5-13%), proteins (8.1-9.1%), DNA (7.1-7.4%), cell wall and capsule (7-7.6%), amino acids and derivatives (6.8-6.9%), cofactors, vitamins, prosthetic groups and pigments (5.7-6%) and bacterial virulence (6.2-7.2%) [112]. These results underline that all microbiota functions are far to be already discovered, as proved by the remaining 42.8% non-affiliated genes. Guard and Suchodolski [121] have studied faeces from 8 healthy dogs (2.7 to 31.8 kg) and observed high inter-variability microbiota composition between animals, while bacteria's functions were very consistent. Thus, even if gut microbiota composition highly vary between dogs, the functional potential seems to be unchanged whatever dog sizes [20]. Gut microbiota metabolic activity leads to gas and short-chain fatty acid (SCFA) production from soluble fibres. SCFAs stimulate intestinal motility and can be further used as an energy source for colonocytes, liver and brain. The three main SCFAs are acetate, propionate and butyrate, with faecal relative percentages of 60:25:15 [122]. Non-digested protein from diet and endogen proteins are also metabolized by gut microbiota, leading to the production of branched chain fatty-acids (BCFA), ammonia, indoles and phenols [18]. Canine faecal protein degradation products are associated with deleterious effects, such as poor faecal quality, inflammation and kidney diseases in dogs and colorectal cancer in humans [123,124]. Canine SCFA production was only evaluated in faecal samples (Fig. 6A-B, Table S6). Values were mainly obtained in medium dogs (especially Beagles) and are widely variable due to many differences in design study (e.g. type of food, food composition in carbohydrates, methods, type of units). However, in a study performed by Weber et al. [36] , the authors compared SCFA production between small, medium and large dogs and demonstrated that total SCFA concentration in stool significantly increased with BW, with 448 ± 67, 894 ± 80 and 1184 ± 259 mmol/kg of lyophilized faeces for small, medium and large dogs respectively. This is consistent with a longer LITT in large breed dogs that may promote microbial fermentation. Large quantity of organic acids produced could thus exceeds colonic mucosa absorption capacity, thereby leading to an accumulation in lumen, a decrease of colonic pH and an increased faecal excretion [18]. Similarly, total BCFAs were measured only in faecal samples, and mainly in medium dogs (Fig. 6C, Table S6). BCFA concentrations seem to be lower in small dogs (a unique value of 17.1 µmol/g) compared to medium ones (calculated median of 22.2 µmol/g). Moreover, BCFA composition was only studied in medium dogs, with a calculated median concentration of 6.8 µmol/g isobutyrate, 10.5 µmol/g isovalerate and 0.8 µmol/g valerate (Fig. 6D). Phenols, indoles and ammonia concentrations were also poorly studied in small and medium dogs and to our knowledge never measured in large dogs (Fig. 6E-F). Based on our calculated medians, it appears that these products are found in higher concentrations in medium than in small dogs. Lastly, to our knowledge there is no data on gas production in dogs and the two studies on gas composition focused on malodorous compounds such as hydrogen sulphide [125,126].
Discussion and general conclusion
In an original way, this review gives an overview of available literature concerning the effect of dog sizes (i.e. “small”, “medium” and “large” sizes) on digestive anatomy and associated physicochemical and microbial parameters, illustrating data with both synthetic graphs (Fig. 1 to Fig. 6) and exhaustive tabs (Table S1 to S6). Even if our conclusions may be hampered by the paucity and old age of many data, as well as the huge variability between experimental protocols (diet composition, measurement methods and data analysis processes) and animals (live or dead, anesthetized or not, companion or laboratory animals, environment), we evidenced clear effects of dog's BW on gastrointestinal physiology, mainly in relation with the colonic compartment (Fig. 7). Large intestine length, area and volume clearly increase with dog size. This seems to be associated with a higher colonic transit time that can affect nutrient and water absorption, gut microbiota composition and activity, as well as faecal moisture. Thus, sodium and potassium absorption are lower in larger dogs resulting in a higher concentration in faecal samples. Large dogs are also characterized by a higher intestinal permeability that can induce a backflow of absorbed electrolytes into the colonic lumen, translated into a luminal retention of electrolytes and water [18]. Besides, a longer colonic residence time in large dogs should promote microbial fermentations and especially a higher fibre degradation by resident bacteria. This higher fermentation capacity results in a stronger production of SCFAs leading to a diminution in faecal pH, and to a potential disturbance of water absorption due to the high osmotic power of SCFAs [36]. Together with an increased colonic permeability, excessive SCFAs production would induce water retention in the colon, associated with higher faecal water content and loose watery stools frequently observed in large dogs [43,127]. In addition, faecal concentrations of microbial degradation products from proteins (phenol, indole, ammonium and BCFAs) seem to be positively associated with dog BW, which again may be explained by a longer transit time. Moreover, our data analysis suggests an increase in Fusobacteria according to BW (observed between small and medium dogs), which can be related to an increase in protein metabolites [65,124]. As certain bacteria are fully involved in BA deconjugation, changes in microbiota composition depending on dog's BW can also be linked to modifications in BA concentrations, inversely correlated with BW.
Further studies would be necessary to enhance available data on physicochemical parameters, especially in the upper GIT, but also on gut microbiota that remains very poorly described in each digestive compartment and not described at all in the mucus layer. Lastly, our bibliographic review revealed the large predominance of some breeds (i.e. Miniature Poodle, Beagle, Standard and Giant Schnauzer and Great Dane) and breeds showing well-known specific digestive particularities (like German Shepherd) or specific energy needs (like Husky, Great Danes or Terriers) [80]. It would be therefore of high interest to further analyze current data by considering not only the effect of body size but also that of breeds. Taken together, all the specificities raised in large dog digestive physiology may be correlated to their high sensitivity to diet and digestive diseases [18]. Finally, all these data concerning the effect of dog size on their digestive physiology can be helpful for the development of new food or veterinary products at the individual level, in accordance with a personalization step intended by petfood and pharma companies. In full accordance with the 3R rules (aiming to reduce animal experiments), such in vivo data also provide key information necessary to develop and validate in vitro gut models adapted to each dog sizes for in-depth mechanistic studies on dog digestive physiology [128].
Abbreviations
BA: bile acids; BCFA: branched-chain fatty acids; BW: body weight; CA: cholic acid; CDCA: chenodeoxycholic acid; CFU: colony forming units; DCA: deoxycholic acid; GET: gastric emptying time; HCl: hydrochloric acid; LCA: lithocholic acid; LITT: large intestine transit time; OCTT: orocaecal transit time; SCFA: short-chain fatty acids; SITT: small intestine transit time; TTT: total transit time.
Supplementary Material
Supplementary tables.
Author contributions
SBD, EA and DH had the idea to make a literature review on this topic and designed the review. CD performed the literature survey, data analysis and figures design. SBD and CD wrote the first draft of the manuscript. All authors critically revised and approved the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Botigué LR, Song S, Scheu A. et al. Ancient European dog genomes reveal continuity since the Early Neolithic. Nat Commun. 2017;8:16082
2. Axelsson E, Ratnakumar A, Arendt M-L. et al. The genomic signature of dog domestication reveals adaptation to a starch-rich diet. Nature. 2013;495:360-4
3. Grandjean D, Haymann F. Encyclopédie du chien Royal Canin. 2010.
4. Boillat CS, Gaschen FP, Hosgood GL. Assessment of the relationship between body weight and gastrointestinal transit times measured by use of a wireless motility capsule system in dogs. Am J Vet Res. 2010;71:898-902
5. German AJ, Helps CR, Hall EJ, Day MJ. Cytokine mRNA expression in mucosal biopsies from German shepherd dogs with small intestinal enteropathies. Dig Dis Sci. 2000;45:7-17
6. Osto M, Lutz TA. Translational value of animal models of obesity-Focus on dogs and cats. Eur J Pharmacol. 2015;759:240-52
7. Raffan E, Dennis RJ, O'Donovan CJ. et al. A Deletion in the Canine POMC Gene Is Associated with Weight and Appetite in Obesity-Prone Labrador Retriever Dogs. Cell Metab. 2016;23:893-900
8. Weber M. Influence of size on the dog's digestive function. Bul de l'Ac Vét de France. 2006 327
9. Morris JG, Rogers GR. Comparative aspects of nutrition and metabolism of dogs and cats. In: Nutrition of the dog and cat. Cambridge University Press. Cambridge. 1989:35-66
10. Kararli TT. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos. 1995;16:351-80
11. López Albors O, Rojo D, Sarriá R, Soria F, Pérez Cuadrado E, Latorre R. Morphometry of the canine intestine with reference to the use of double balloon endoscopy. Vet J. 2011;190:113-8
12. Robin A. Les enterpathies excudatives chez le chien: actualités, diagnostiques et analyse retrospective de series de cas cliniques. [Lyon]: Université Claude Bernard. 2007
13. Sarriá R, Latorre R, Henroteaux M. et al. Morphometric study of the layers of the canine small intestine at five sampling sites. Vet J. 2012;192:498-502
14. Hart IR, Kidder DE. The quantitative assessment of normal canine small intestinal mucosa. Res Vet Sci. 1978;25:157-62
15. Washabau RJ, Day MJ, Willard MD. et al. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J Vet Intern Med. 2010;24:10-26
16. Baum B, Meneses F, Kleinschmidt S, Nolte I, Hewicker-Trautwein M. Age-related histomorphologic changes in the canine gastrointestinal tract: a histologic and immunohistologic study. World J Gastroenterol. 2007;13:152-7
17. Feldman EJ, Dowling RH, McNaughton J, Peters TJ. Effects of oral versus intravenous nutrition on intestinal adaptation after small bowel resection in the dog. Gastroenterology. 1976;70:712-9
18. Weber MP, Biourge VC, Nguyen PG. Digestive sensitivity varies according to size of dogs: a review. J Anim Physiol Anim Nutr (Berl). 2017;101:1-9
19. Smeets-Peeters M, Watson T, Minekus M, Havenaar R. A review of the physiology of the canine digestive tract related to the development of in vitro systems. Nutrition Research Reviews. 1998;11:45-69
20. Durand A. Entéropathies exsudatives chez le chien: proposition d'une stratégie thérapeutique diététique et médiacale. Université Claude Bernard. 2010
21. Larmas M, Scheinin A. Studies on dog saliva. I. Some physico-chemical characteristics. Acta Odontol Scand. 1971;29:205-14
22. de Godoy MRC, Vermillion R, Bauer LL. et al. In vitro disappearance characteristics of selected categories of commercially available dog treats. J Nutr Sci. 2014;3:e47
23. Lui CY, Amidon GL, Berardi RR, Fleisher D, Youngberg C, Dressman JB. Comparison of gastrointestinal pH in dogs and humans: implications on the use of the beagle dog as a model for oral absorption in humans. J Pharm Sci. 1986;75:271-4
24. Yamada I, Haga K. Measurement of gastric pH during digestion of a solid meal in dogs. Chem Pharm Bull. 1990;38:1755-6
25. Akimoto M, Nagahata N, Furuya A, Fukushima K, Higuchi S, Suwa T. Gastric pH profiles of beagle dogs and their use as an alternative to human testing. Eur J Pharm Biopharm. 2000;49:99-102
26. Sagawa K, Li F, Liese R, Sutton SC. Fed and fasted gastric pH and gastric residence time in conscious beagle dogs. Journal of Pharmaceutical Sciences. 2009;98:2494-500
27. Mahar KM, Portelli S, Coatney R, Chen EP. Gastric pH and Gastric Residence Time in Fasted and Fed Conscious Beagle Dogs using the Bravo® pH System. Journal of Pharmaceutical Sciences. 2012;101:2439-48
28. Garcia-Mazcorro JF, Dowd SE, Poulsen J, Steiner JM, Suchodolski JS. Abundance and short-term temporal variability of fecal microbiota in healthy dogs. Microbiologyopen. 2012;1:340-7
29. Koziolek M, Grimm M, Bollmann T. et al. Characterization of the GI transit conditions in Beagle dogs with a telemetric motility capsule. Eur J Pharm Biopharm. 2019;136:221-30
30. Martinez M. Applying the biopharmaceutics classification system to veterinary pharmaceutical products Part II. Physiological considerations. Advanced Drug Delivery Reviews. 2002;54:825-50
31. Mentula S, Harmoinen J, Heikkilä M. et al. Comparison between cultured small-intestinal and fecal microbiotas in beagle dogs. Appl Environ Microbiol. 2005;71:4169-75
32. Kalantzi L, Persson E, Polentarutti B. et al. Canine Intestinal Contents vs. Simulated Media for the Assessment of Solubility of Two Weak Bases in the Human Small Intestinal Contents. Pharm Res. 2006;23:1373-81
33. Smith HW. Observations on the flora of the alimentary tract of animals and factors affecting its composition. J Pathol. 1965;89:95-122
34. Lidbury JA, Suchodolski JS, Ivanek R, Steiner JM. Assessment of the Variation Associated with Repeated Measurement of Gastrointestinal Transit Times and Assessment of the Effect of Oral Ranitidine on Gastrointestinal Transit Times Using a Wireless Motility Capsule System in Dogs. Veterinary Medicine International. 2012;2012:1-8
35. Warrit K, Boscan P, Ferguson LE. et al. Minimally invasive wireless motility capsule to study canine gastrointestinal motility and pH. The Veterinary Journal. 2017;227:36-41
36. Weber MP, Hernot D, Nguyen PG, Biourge VC, Dumon HJ. Effect of size on electrolyte apparent absorption rates and fermentative activity in dogs. J Anim Physiol Anim Nutr (Berl). 2004;88:356-65
37. Beloshapka AN, de Godoy MRC, Detweiler KB. et al. Apparent total tract macronutrient digestibility, fecal characteristics, and fecal fermentative end-product concentrations of healthy adult dogs fed bioprocessed soy protein. J Anim Sci. 2016;94:3826-34
38. Igarashi H, Ohno K, Matsuki N. et al. Analysis of fecal short chain fatty acid concentration in miniature dachshunds with inflammatory colorectal polyps. J Vet Med Sci. 2017;79:1727-34
39. Cutrignelli MI, Bovera F, Tudisco R. et al. In vitro fermentation characteristics of different carbohydrate sources in two dog breeds (German shepherd and Neapolitan mastiff). J Anim Physiol Anim Nutr. 2009;93:305-12
40. Sandri M, Dal Monego S, Conte G, Sgorlon S, Stefanon B. Raw meat-based diet influences faecal microbiome and end products of fermentation in healthy dogs. BMC Veterinary Research [Internet]. 2016 [cited 21 October 2019]; 13. Available at: http://bmcvetres.biomedcentral.com/articles/10.1186/s12917-017-0981-z
41. Eisenhauer L, Vahjen W, Dadi T, Kohn B, Zentek J. Effects of Brewer's spent grain and carrot pomace on digestibility, fecal microbiota, and fecal and urinary metabolites in dogs fed low- or high-protein diets1. J Anim Sci. 2019;97:4124-33
42. Nogueira JPDS, He F, Mangian HF, Oba PM, De Godoy MRC. Dietary supplementation of a fiber-prebiotic and saccharin-eugenol blend in extruded diets fed to dogs. J Anim Sci. 2019;97:4519-31
43. Oswald H, Sharkey M, Pade D, Martinez MN. Canine gastrointestinal physiology: Breeds variations that can influence drug absorption. European Journal of Pharmaceutics and Biopharmaceutics. 2015;97:192-203
44. Contreras-Aguilar MD, Tecles F, Martínez-Subiela S, Escribano D, Bernal LJ, Cerón JJ. Detection and measurement of alpha-amylase in canine saliva and changes after an experimentally induced sympathetic activation. BMC Vet Res. 2017;13:266
45. Iacopetti I, Perazzi A, Badon T, Bedin S, Contiero B, Ricci R. Salivary pH, calcium, phosphorus and selected enzymes in healthy dogs: a pilot study. BMC Vet Res. 2017;13:330
46. Sanguansermsri P, Jenkinson HF, Thanasak J. et al. Comparative proteomic study of dog and human saliva. PLoS ONE. 2018;13:e0208317
47. Tecles F, Escribano D, Contreras-Aguilar MD. et al. Evaluation of adenosine deaminase in saliva and serum, and salivary α-amylase, in canine pyometra at diagnosis and after ovariohysterectomy. The Veterinary Journal. 2018;236:102-10
48. Ricci R, Perazzi A, Badon T, Bedin S, Iacopetti I. Effect of storage on long-term stability of salivary α-amylase, lysozyme, lactate dehydrogenase, calcium and phosphorus in dogs. Vet J. 2018;242:44-7
49. Hong H-R, Oh Y-I, Kim YJ, Seo K-W. Salivary alpha-amylase as a stress biomarker in diseased dogs. J Vet Sci. 2019;20:e46
50. Lavy E, Goldberger D, Friedman M, Steinberg D. pH Values and Mineral Content of Saliva in Different Breeds of Dogs. Israel Journal of Veterinary Medicine. 2012;67:244-2448
51. Aspinall V. Anatomy and Physiology of the Dog and Cat 8. The Digestive System. Veterinary Nursing Journal. 2004;19:94-9
52. Ehrlein H-J, Pröve J. Effect of viscosity of test meals on gastric emptying in dogs. Exp Physiol. 1982;67:419-25
53. Kienzle E. Enzyme activity in pancreatic tissue, intestinal mucosa and chyme of dogs in relation to age and diet. Journal of Animal Physiology and Animal Nutrition. 1988:276-88
54. Kakimoto T, Kanemoto H, Fukushima K, Ohno K, Tsujimoto H. Effect of a high-fat-high-cholesterol diet on gallbladder bile acid composition and gallbladder motility in dogs. American Journal of Veterinary Research. 2017;78:1406-13
55. Madrid JA, Salido GM, Mañas M, Martinez de Victoria E, Mataix FJ. Use of a bidirectional cannula to study biliary secretion in conscious dogs. Lab Anim. 1983;17:307-10
56. Nagahara T, Ohno K, Kanemoto H. et al. Effect of prednisolone administration on gallbladder emptying rate and gallbladder bile composition in dogs. American Journal of Veterinary Research. 2018;79:1050-6
57. Larcheveque S. Etat des connaissances sur la mucocèle biliaire chez le chien: étude bibliographique. Faculté de médecine de Créteil. 2019
58. Washizu T, Ishida T, Washizu M, Tomoda I, Kaneko JJ. Changes in bile acid composition of serum and gallbladder bile in bile duct ligated dogs. J Vet Med Sci. 1994;56:299-303
59. Schmidt M, Unterer S, Suchodolski JS. et al. The fecal microbiome and metabolome differ between dogs fed Bones and Raw Food (BARF) diets and dogs fed commercial diets. Loor JJ, Ed. PLoS ONE. 2018;13:e0201279
60. Blake AB, Guard BC, Honneffer JB, Lidbury JA, Steiner JM, Suchodolski JS. Altered microbiota, fecal lactate, and fecal bile acids in dogs with gastrointestinal disease. Staley C, Ed. PLoS ONE. 2019;14:e0224454
61. Manchester AC, Webb CB, Blake AB. et al. Long-term impact of tylosin on fecal microbiota and fecal bile acids of healthy dogs. J Vet Intern Med. 2019;33:2605-17
62. Guard BC, Honneffer JB, Jergens AE. et al. Longitudinal assessment of microbial dysbiosis, fecal unconjugated bile acid concentrations, and disease activity in dogs with steroid-responsive chronic inflammatory enteropathy. Journal of Veterinary Internal Medicine. 2019;33:1295-305
63. Bickel M, Kauffman GL. Gastric gel mucus thickness: effect of distention, 16,16-dimethyl prostaglandin e2, and carbenoxolone. Gastroenterology. 1981;80:770-5
64. Etienne-Mesmin L, Chassaing B, Desvaux M. et al. Experimental models to study intestinal microbes-mucus interactions in health and disease. FEMS Microbiol Rev. 2019;43:457-89
65. Moon CD, Young W, Maclean PH, Cookson AL, Bermingham EN. Metagenomic insights into the roles of Proteobacteria in the gastrointestinal microbiomes of healthy dogs and cats. MicrobiologyOpen. 2018;7:e00677
66. Weber M, Martin L, Biourge V, Nguyen P, Dumon H. Influence of age and body size on the digestibility of a dry expanded diet in dogs. J Anim Physiol Anim Nutr (Berl). 2003;87:21-31
67. Nery J, Biourge V, Tournier C. et al. Influence of dietary protein content and source on fecal quality, electrolyte concentrations, and osmolarity, and digestibility in dogs differing in body size1. Journal of Animal Science. 2010;88:159-69
68. Bednar GE, Murray SM, Patil AR, Flickinger EA, Merchen NR, Fahey GC. Selected animal and plant protein sources affect nutrient digestibility and fecal characteristics of ileally cannulated dogs. Arch Tierernahr. 2000;53:127-40
69. Flickinger EA, Schreijen EMWC, Patil AR. et al. Nutrient digestibilities, microbial populations, and protein catabolites as affected by fructan supplementation of dog diets. Journal of Animal Science. 2003;81:2008-18
70. Propst EL, Flickinger EA, Bauer LL, Merchen NR, Fahey GC. A dose-response experiment evaluating the effects of oligofructose and inulin on nutrient digestibility, stool quality, and fecal protein catabolites in healthy adult dogs. J Anim Sci. 2003;81:3057-66
71. Hendriks WH, Thomas DG, Bosch G, Fahey GC. Comparison of ileal and total tract nutrient digestibility of dry dog foods. J Anim Sci. 2013;91:3807-14
72. Zentek J, Fricke S, Hewicker-Trautwein M, Ehinger B, Amtsberg G, Baums C. Dietary protein source and manufacturing processes affect macronutrient digestibility, fecal consistency, and presence of fecal Clostridium perfringens in adult dogs. J Nutr. 2004;134:2158S-2161S
73. Pinna C, Vecchiato CG, Bolduan C. et al. Influence of dietary protein and fructooligosaccharides on fecal fermentative end-products, fecal bacterial populations and apparent total tract digestibility in dogs. BMC Vet Res [Internet]. 2018 [cited 7 November 2019]; 14. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5859515/
74. Carrière F, Laugier R, Barrowman JA, Douchet I, Priymenko N, Verger R. Gastric and pancreatic lipase levels during a test meal in dogs. Scand J Gastroenterol. 1993;28:443-54
75. Weber MP, Stambouli F, Martin LJ, Dumon HJ, Biourge VC, Nguyen PG. Influence of age and body size on gastrointestinal transit time of radiopaque markers in healthy dogs. American Journal of Veterinary Research. 2002;63:677-82
76. Detweiler KB, He F, Mangian HF, Davenport GM, de Godoy MRC. Effects of high inclusion of soybean hulls on apparent total tract macronutrient digestibility, fecal quality, and fecal fermentative end-product concentrations in extruded diets of adult dogs. J Anim Sci. 2019;97:1027-35
77. Hernot DC, Nery J, Biourge VC, Martin LJ, Dumon HJ, Nguyen PG. Colonic permeability is higher in Great Danes compared with smaller breed-dogs. J Anim Physiol Anim Nutr (Berl). 2009;93:703-9
78. Weber MP, Martin LJ, Dumon HJ, Biourge VC, Nguyen PG. Influence of age and body size on intestinal permeability and absorption in healthy dogs. Am J Vet Res. 2002;63:1323-8
79. Bjarnason I, MacPherson A, Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995;108:1566-81
80. Zentek J, Meyer H. Normal handling of diets-are all dogs created equal? J Small Anim Pract. 1995;36:354-9
81. Meyer H, Zentek J, Habernoll H, Maskell I. Digestibility and compatibility of mixed diets and faecal consistency in different breeds of dog. Zentralbl Veterinarmed A. 1999;46:155-65
82. Goudez R, Weber M, Biourge V, Nguyen P. Influence of different levels and sources of resistant starch on faecal quality of dogs of various body sizes. Br J Nutr. 2011;106(Suppl 1):S211-215
83. Rolfe V. Colonic fluid and electrolyte transport in health and disease. Vet Clin North Am Small Anim Pract. 1999;29:577-88 viii
84. Neri M, Phillips SF, Fich A, Haddad AC. Canine ileocolonic sphincter: flow, transit, and motility before and after sphincterotomy. Am J Physiol. 1991;260:G284-289
85. Boscan P, Cochran S, Monnet E, Webb C, Twedt D. Effect of prolonged general anesthesia with sevoflurane and laparoscopic surgery on gastric and small bowel propulsive motility and pH in dogs. Vet Anaesth Analg. 2013;41:73-81
86. Warrit K, Boscan P, Ferguson LE, Bradley AM, Dowers KL, Twedt DC. Effect of hospitalization on gastrointestinal motility and pH in dogs. Journal of the American Veterinary Medical Association. 2017;251:65-70
87. Farmer AD, Wegeberg A-ML, Brock B. et al. Regional gastrointestinal contractility parameters using the wireless motility capsule: inter-observer reproducibility and influence of age, gender and study country. Aliment Pharmacol Ther. 2018;47:391-400
88. Farmer AD, Ruffle JK, Hobson AR. Linaclotide increases cecal pH, accelerates colonic transit, and increases colonic motility in irritable bowel syndrome with constipation. Neurogastroenterol Motil. 2019;31:e13492
89. Bourreau J, Hernot D, Bailhache E. et al. Gastric emptying rate is inversely related to body weight in dog breeds of different sizes. J Nutr. 2004;134:2039S-2041S
90. Dressman JB. Comparison of canine and human gastrointestinal physiology. Pharmaceutical Research. 1986;3:123-31
91. Ménard O, Famelart M-H, Deglaire A. et al. Gastric Emptying and Dynamic In Vitro Digestion of Drinkable Yogurts: Effect of Viscosity and Composition. Nutrients. 2018 10
92. Hernot DC, Dumon HJ, Biourge VC, Martin LJ, Nguyen PG. Evaluation of association between body size and large intestinal transit time in healthy dogs. Am J Vet Res. 2006;67:342-7
93. Balsa IM, Culp WTN, Drobatz KJ, Johnson EG, Mayhew PD, Marks SL. Effect of Laparoscopic-assisted Gastropexy on Gastrointestinal Transit Time in Dogs. J Vet Intern Med. 2017;31:1680-5
94. Warrit K, Boscan P, Ferguson LE. et al. Minimally invasive wireless motility capsule to study canine gastrointestinal motility and pH. The Veterinary Journal. 2017;227:36-41
95. Hernot DC, Biourge VC, Martin LJ, Dumon HJ, Nguyen PG. Relationship between total transit time and faecal quality in adult dogs differing in body size. J Anim Physiol Anim Nutr. 2005;89:189-93
96. Hooda S, Minamoto Y, Suchodolski JS, Swanson KS. Current state of knowledge: the canine gastrointestinal microbiome. Animal Health Research Reviews. 2012;13:78-88
97. Friedman ES, Bittinger K, Esipova TV. et al. Microbes vs. chemistry in the origin of the anaerobic gut lumen. Proc Natl Acad Sci U S A. 2018;115:4170-5
98. Dewhirst FE, Klein EA, Thompson EC. et al. Correction: The Canine Oral Microbiome. Ravel J, Ed. PLoS ONE [Internet]. 2012 [cited 23 May 2022]; 7. Available at: https://dx.plos.org/10.1371/annotation/c2287fc7-c976-4d78-a28f-1d4e024d568f
99. Bell JA, Kopper JJ, Turnbull JA, Barbu NI, Murphy AJ, Mansfield LS. Ecological Characterization of the Colonic Microbiota of Normal and Diarrheic Dogs. Interdisciplinary Perspectives on Infectious Diseases. 2008;2008:1-17
100. Niemiec BA, Gawor J, Tang S, Prem A, Krumbeck JA. The bacteriome of the oral cavity in healthy dogs and dogs with periodontal disease. Am J Vet Res. 2021;83:50-8
101. Benno Y, Nakao H, Uchida K, Mitsuoka T. Impact of the advances in age on the gastrointestinal microflora of beagle dogs. J Vet Med Sci. 1992;54:703-6
102. Xenoulis PG, Palculict B, Allenspach K, Steiner JM, Van House AM, Suchodolski JS. Molecular-phylogenetic characterization of microbial communities imbalances in the small intestine of dogs with inflammatory bowel disease. FEMS Microbiol Ecol. 2008;66:579-89
103. Suchodolski JS, Dowd SE, Westermarck E. et al. The effect of the macrolide antibiotic tylosin on microbial diversity in the canine small intestine as demonstrated by massive parallel 16S rRNA gene sequencing. BMC Microbiol. 2009;9:210
104. Suchodolski JS, Camacho J, Steiner JM. Analysis of bacterial diversity in the canine duodenum, jejunum, ileum, and colon by comparative 16S rRNA gene analysis. FEMS Microbiol Ecol. 2008;66:567-78
105. Pilla R, Suchodolski JS. The Role of the Canine Gut Microbiome and Metabolome in Health and Gastrointestinal Disease. Front Vet Sci. 2020;6:498
106. Vázquez-Baeza Y, Hyde ER, Suchodolski JS, Knight R. Dog and human inflammatory bowel disease rely on overlapping yet distinct dysbiosis networks. Nat Microbiol. 2016;1:16177
107. Simon M. L'anxiété chez le chien, les répercussions sur le microbiote intestinal: intérêt de l'utilisation des probiotiques dans la prise en charge thérapeutique. [Internet]. [Toulouse]: Ecole Nationale Vétérinaire de Toulouse. 2019 Available at: https://oatao.univ-toulouse.fr/25833/
108. Kim H, Rather IA, Kim H. et al. A Double-Blind, Placebo Controlled-Trial of a Probiotic Strain Lactobacillus sakei Probio-65 for the Prevention of Canine Atopic Dermatitis. J Microbiol Biotechnol. 2015;25:1966-9
109. Omatsu T, Omura M, Katayama Y. et al. Molecular diversity of the faecal microbiota of Toy Poodles in Japan. J Vet Med Sci. 2018;80:749-54
110. Reddy KE, Kim H-R, Jeong JY. et al. Impact of Breed on the Fecal Microbiome of Dogs under the Same Dietary Condition. Journal of Microbiology and Biotechnology. 2019;29:1947-56
111. Algya KM, Cross T-WL, Leuck KN. et al. Apparent total-tract macronutrient digestibility, serum chemistry, urinalysis, and fecal characteristics, metabolites and microbiota of adult dogs fed extruded, mildly cooked, and raw diets1. Journal of Animal Science. 2018;96:3670-83
112. Swanson KS, Dowd SE, Suchodolski JS. et al. Phylogenetic and gene-centric metagenomics of the canine intestinal microbiome reveals similarities with humans and mice. ISME J. 2011;5:639-49
113. Suchodolski JS. Companion animals symposium: microbes and gastrointestinal health of dogs and cats. J Anim Sci. 2011;89:1520-30
114. Deng P, Swanson KS. Gut microbiota of humans, dogs and cats: current knowledge and future opportunities and challenges. Br J Nutr. 2015;113:S6-17
115. Albenberg L, Esipova TV, Judge CP. et al. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology. 2014;147:1055-1063.e8
116. Etienne-Mesmin L, Chassaing B, Desvaux M. et al. Experimental models to study intestinal microbes-mucus interactions in health and disease. FEMS Microbiol Rev. 2019;43:457-89
117. Simpson KW, Dogan B, Rishniw M. et al. Adherent and invasive Escherichia coli is associated with granulomatous colitis in boxer dogs. Infect Immun. 2006;74:4778-92
118. Cassmann E, White R, Atherly T. et al. Alterations of the Ileal and Colonic Mucosal Microbiota in Canine Chronic Enteropathies. Isaacson RE, Ed. PLoS ONE. 2016;11:e0147321
119. Garcia-Mazcorro JF, Minamoto Y, Kawas JR, Suchodolski JS, de Vos WM. Akkermansia and Microbial Degradation of Mucus in Cats and Dogs: Implications to the Growing Worldwide Epidemic of Pet Obesity. Vet Sci. 2020 7
120. Andoh A. Physiological Role of Gut Microbiota for Maintaining Human Health. Digestion. 2016;93:176-81
121. Guard BC, Suchodolski JS. HORSE SPECIES SYMPOSIUM: Canine intestinal microbiology and metagenomics: From phylogeny to function1. Journal of Animal Science. 2016;94:2247-61
122. Mondo E, Marliani G, Accorsi PA, Cocchi M, Di Leone A. Role of gut microbiota in dog and cat's health and diseases. Open Vet J. 2019;9:253-8
123. Hughes R, Magee EA, Bingham S. Protein degradation in the large intestine: relevance to colorectal cancer. Curr Issues Intest Microbiol. 2000;1:51-8
124. Ephraim E, Cochrane C-Y, Jewell DE. Varying Protein Levels Influence Metabolomics and the Gut Microbiome in Healthy Adult Dogs. Toxins (Basel). 2020 12
125. Giffard CJ, Collins SB, Stoodley NC, Butterwick RF, Batt RM. Administration of charcoal, Yucca schidigera, and zinc acetate to reduce malodorous flatulence in dogs. J Am Vet Med Assoc. 2001;218:892-6
126. Collins SB, Perez-Camargo G, Gettinby G, Butterwick RF, Batt RM, Giffard CJ. Development of a technique for the in vivo assessment of flatulence in dogs. Am J Vet Res. 2001;62:1014-9
127. Zentek J, Hall EJ, German A. et al. Morphology and immunopathology of the small and large intestine in dogs with nonspecific dietary sensitivity. J Nutr. 2002;132:1652S-4S
128. Deschamps C, Denis S, Humbert D. et al. In vitro models of the canine digestive tract as an alternative to in vivo assays: Advances and current challenges. ALTEX. 2022
Author contact
![]() Corresponding author: Stéphanie Blanquet-Diot, UMR 454 MEDIS, University Clermont Auvergne, INRAe, 28 place Henri Dunant, 63000 Clermont-Ferrand, France. (+33473178390; stephanie.blanquetfr)
Corresponding author: Stéphanie Blanquet-Diot, UMR 454 MEDIS, University Clermont Auvergne, INRAe, 28 place Henri Dunant, 63000 Clermont-Ferrand, France. (+33473178390; stephanie.blanquetfr)

 Global reach, higher impact
Global reach, higher impact