10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(14):5575-5590. doi:10.7150/ijbs.70504 This issue Cite
Research Paper
A novel methylated cation channel TRPM4 inhibited colorectal cancer metastasis through Ca2+/Calpain-mediated proteolysis of FAK and suppression of PI3K/Akt/mTOR signaling pathway
1. Department of Pathology, Sir Run Run Shaw Hospital, Zhejiang University, Hangzhou 310016, Zhejiang, China.
2. Key Laboratory of Biotherapy of Zhejiang Province, Sir Run Run Shaw Hospital, Zhejiang University, Hangzhou 310016, China.
3. Department of Pathology, The First People's Hospital of Fuyang, Hangzhou 311400, China.
#The authors contribute equally.
Abstract
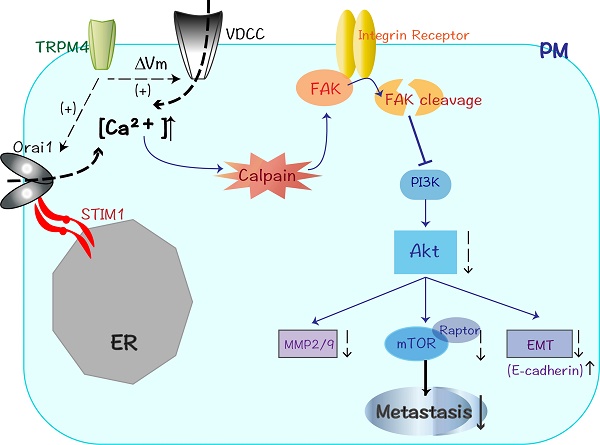
Colorectal cancer (CRC) is an aggressive malignancy with poor prognosis. It is imperative to elucidate the potential molecular mechanisms that regulate CRC cell aggressiveness. In present study, the transient receptor potential melastatin 4 (TRPM4), a calcium-activated nonselective cation channel, is downregulated in CRC as a novel methylated tumor suppressor gene (TSG). The reduced mRNA level of TRPM4 is due to the epigenetic methylation of its promoter CpG island (CGI). Moreover, ectopic expression of TRPM4 inhibited tumor growth and metastasis both in vitro and in vivo. Our experiments also demonstrate that TRPM4 restructures the CRC cytoskeleton and activates the Ca2+-mediated calpain pathway through enhancing calcium influx. The western blot analysis shows that the expression of focal adhesion kinase (FAK), a calpain-mediated proteolytic substrate, is markedly suppressed after ectopic overexpression of TRPM4, besides, Akt (also known as protein kinase B, PKB), phosphatidylinositol 3-kinase (PI3K) as well as its central target mTOR have significantly decreased expression accompanied by elevated E-cadherin and restrained matrix metalloproteinases (MMP2/MMP9) expression. The inhibition of protease calpain effectively relieves the retard of FAK/Akt signals and reverses the migration suppression of TRPM4. Taken together, TRPM4, identified as a novel methylated TSG, employs intracellular Ca2+ signals to activate calpain-mediated cleavage of FAK and impede CRC migration and invasion through modulating the PI3K/Akt/mTOR signaling cascade, providing the first evidence that TRPM4 is likely to be a significant biomarker and potential target for CRC therapy.
Keywords: TRPM4, tumor suppressor gene, methylation, metastasis

 Global reach, higher impact
Global reach, higher impact