10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(16):6052-6067. doi:10.7150/ijbs.75188 This issue Cite
Research Paper
Intermittent Fasting Attenuates Hallmark Vascular and Neuronal Pathologies in a Mouse Model of Vascular Cognitive Impairment
1. Memory Aging and Cognition Centre, Department of Pharmacology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore
2. Department of Biochemistry, Yong Loo Lin School of Medicine, National University of Singapore, Singapore
3. Healthy Longevity Translational Research Program, Yong Loo Lin School of Medicine, National University of Singapore, Singapore
4. Centre for Healthy Longevity, National University Health System (NUHS), Singapore
5. Centre for Cardiovascular Biology and Disease Research, Department of Microbiology, Anatomy, Physiology and Pharmacology, School of Agriculture, Biomedicine and Environment, La Trobe University, Bundoora, VIC, Australia
6. School of Pharmacy, Sungkyunkwan University, Suwon, Republic of Korea
Abstract
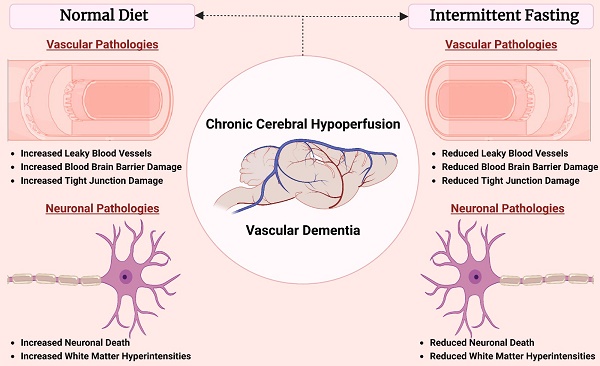
Background - Chronic cerebral hypoperfusion (CCH) is an important pathophysiological mechanism of vascular cognitive impairment (VCI). The heterogeneous effects of CCH complicate establishing single target therapies against VCI and its more severe form, vascular dementia (VaD). Intermittent fasting (IF) has multiple targets and is neuroprotective across a range of disease conditions including stroke, but its effects against CCH-induced neurovascular pathologies remain to be elucidated. We therefore assessed the effect of IF against CCH-associated neurovascular pathologies and investigated its underlying mechanisms.
Methods - Male C57BL/6NTac mice were subjected to either ad libitum feeding (AL) or IF (16 hours of fasting per day) for 4 months. In both groups, CCH was experimentally induced by the bilateral common carotid artery stenosis (BCAS) method. Sham operated groups were used as controls. Measures of leaky microvessels, blood-brain barrier (BBB) permeability, protein expression of tight junctions, extracellular matrix components and white matter changes were determined to investigate the effect of IF against CCH-induced neurovascular pathologies.
Results - IF alleviated CCH-induced neurovascular pathologies by reducing the number of leaky microvessels, BBB breakdown and loss of tight junctional proteins. In addition, IF mitigated the severity of white matter lesions, and maintained myelin basic protein levels, while concurrently reducing hippocampal neuronal cell death. Furthermore, IF reduced the CCH-induced increase in levels of matrix metalloproteinase (MMP)-2 and its upstream activator MT1-MMP, which are involved in the breakdown of the extracellular matrix that is a core component of the BBB. Additionally, we observed that IF reduced CCH-induced increase in the oxidative stress marker malondialdehyde, and increased antioxidant markers glutathione and superoxide dismutase. Overall, our data suggest that IF attenuates neurovascular damage, metalloproteinase and oxidative stress-associated pathways, and cell death in the brain following CCH in a mouse model of VCI.
Conclusion - Although IF has yet to be assessed in human patients with VaD, our data suggest that IF may be an effective means of preventing the onset or suppressing the development of neurovascular pathologies in VCI and VaD.
Keywords: Vascular dementia, Vascular cognitive impairment, Intermittent fasting, Neuronal death, White matter lesions, Blood brain barrier breakdown, Chronic cerebral hypoperfusion

 Global reach, higher impact
Global reach, higher impact