10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2022; 18(16):6084-6101. doi:10.7150/ijbs.76083 This issue Cite
Research Paper
HNRNPK/CLCN3 axis facilitates the progression of LUAD through CAF-tumor interaction
1. Department of Thoracic Surgery, The First Affiliated Hospital of Zhengzhou University, Zhengzhou University, Zhengzhou 450052, China.
2. Department of Clinical Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou University, Zhengzhou 450052, China.
3. Post-doctoral Station of Clinical Medicine, The First Affiliated Hospital of Zhengzhou University, Zhengzhou University, Zhengzhou 450052, China.
#These authors have contributed equally to this work and share first authorship.
Abstract
Background: Chloride channel 3 (CLCN3) is regulated by transcription-coactivator, however, it is unclear which core transcription factor regulates CLCN3. The role of CLCN3 in lung adenocarcinoma (LUAD) is unexplored and the relationship between CLCN3 and tumor microenvironment is unknown.
Methods: A 5′-biotin-labeled promoter probe of CLCN3 was used to pull down the promoter-binding transcription factor. Further study was investigated using LUAD samples, cell lines, and xenograft mice models, and the mechanism was explored.
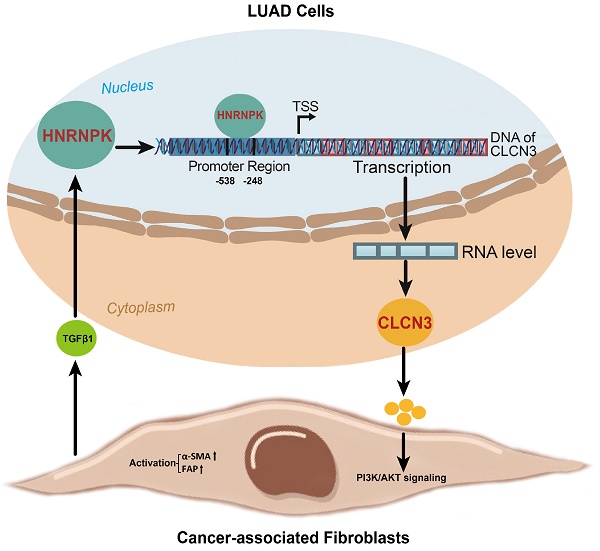
Results: CLCN3 was upregulated in human LUAD, and CLCN3 knockdown inhibited tumor proliferation and migration in vitro. Next, heterogeneous nuclear ribonucleoprotein K (HNRNPK) was first validated as a CLCN3 promoter-binding transcription factor. Mechanistically, HNRNPK knockdown suppressed the promoter activity of CLCN3, thus regulating CLCN3 expression at the transcriptional level, and the binding motif 'GCGAGG' and binding site '-538/-248 bp' were identified. Subsequently, the RNA-seq data illustrated that the primary functions of HNRNPK were similar to those of CLCN3. The results from in vitro and in vivo trials indicated that the expression and function of CLCN3 were regulated by HNRNPK. By isolating primary cancer-associated fibroblasts (CAFs) from human LUAD, we confirmed that decreased extracellular CLCN3 secretion induced by HNRNPK knockdown inhibited CAFs activation and TGF-β1 production, thus suppressing nuclear HNRNPK expression and LUAD progression in a feedback way. Furthermore, this phenomenon was rescued after the addition of TGF-β1, revealing that the HNRNPK/CLCN3 axis facilitated LUAD progression through intercellular interactions. Finally, we identified that CLCN3 and HNRNPK were upregulated and correlated with poor prognosis in LUAD patients.
Conclusions: HNRNPK/CLCN3 axis facilitates the progression of LUAD through CAF-tumor interaction.
Keywords: LUAD, CLCN3, HNRNPK, Transcription, CAFs

 Global reach, higher impact
Global reach, higher impact