ISSN: 1449-2288
Int J Biol Sci 2022; 18(16):6102-6113. doi:10.7150/ijbs.73915 This issue Cite
Research Paper
Reversion of glucocorticoid-induced senescence and collagen synthesis decrease by LY294002 is mediated through p38 in skin
1. Department of Biological Science and Technology, College of Life Sciences, China Medical University, Taichung 406, Taiwan, ROC.
2. Ph.D. Program for Biotechnology Industry, China Medical University, Taichung 406, Taiwan, ROC.
3. Department of Dermatology, Taipei City Hospital, Renai Branch, Taipei, Taiwan, ROC.
4. Center for General Education, Mackay Junior College of Medicine, Nursing, and Management, Taipei, Taiwan, ROC.
5. Division of Breast Surgery, Department of Surgery, China Medical University Hospital, Taichung 40447, Taiwan, ROC.
6. Cardiovascular and Mitochondrial Related Disease Research Center, Hualien Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Hualien 970, Taiwan, ROC.
7. Center of General Education, Buddhist Tzu Chi Medical Foundation, Tzu Chi University of Science and Technology, Hualien 970, Taiwan, ROC.
8. Department of Medical Research, China Medical University Hospital, China Medical University, Taichung 404, Taiwan, ROC.
9. Graduate Institute of Biomedical Sciences, China Medical University, Taichung 404, Taiwan, ROC.
10. Department of Medical Laboratory Science and Biotechnology, Asia University, Taichung 413, Taiwan, ROC.
*Equal contributions.
Abstract
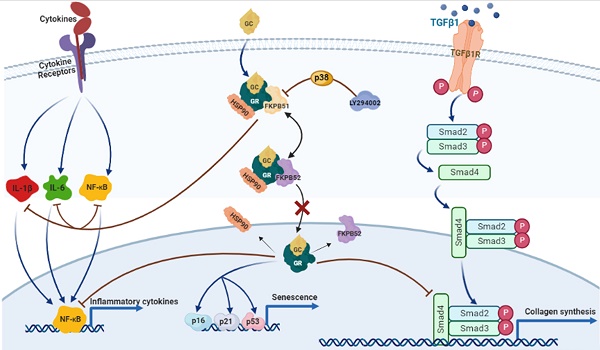
Glucocorticoids (GCs) are the most common treatment for inflammatory skin disorders; however, they show several adverse side effects, including atrophy and collagen decrease following chronic treatment. In particular, transcription factors and p38 signaling for collagen synthesis have been shown to be suppressed by the active glucocorticoid receptor (GR). LY294002 (LY), a phosphoinositide 3-kinase (PI3K) inhibitor, has been reported to protect keratinocytes in epidermis against GC-induced hypoplasia; however, its protective effect in dermis remains unclear. Furthermore, clobetasol propionate (CP) is the most used commercial synthetic GC, yet studies on how CP causes side effects in dermal fibroblasts are limited. In this study, dermal atrophy was modeled using CP in human dermal fibroblasts (HDFs) and C57BL/6 mice. CP treatment significantly upregulated FK506 binding protein 5 (FKBP51), an atrophy marker (2.4 ± 0.25 and 3.3 ± 0.3 fold in in vitro and in vivo, respectively), phosphorylated GR (1.96 ± 0.08 and 2.29 ± 0.25 fold in in vitro and in vivo, respectively), decreased fibroblast proliferation (82.71 ± 1.95% in in vitro), reduced collagen synthesis (0.36 ± 0.05 and 0.3 ± 0.1 fold in in vitro and in vivo, respectively), and induced aging, all of which were reversed by LY treatment (from 1.43 ± 0.08 to 2.8 ± 0.12 fold) without showing growth inhibition and exerting the anti-inflammation of CP. Interestingly, the protective effect of LY was dose-dependently reversed by treatment with a p38 inhibitor and reached 2.9 ± 0.15 fold at dose 20 µM. Taken together, our results demonstrate that LY reduced CP-induced upregulation of the atrophy marker FKBP51, GR phosphorylation, and GR nuclear translocation via the activation of p38, whilst maintaining the anti-inflammatory effect of glucocorticoids.
Keywords: glucocorticoid, clobetasol propionate, LY294002, skin atrophy, p38 MAPK, dermal fibroblasts

 Global reach, higher impact
Global reach, higher impact