ISSN: 1449-2288
Int J Biol Sci 2023; 19(1):1-12. doi:10.7150/ijbs.77181 This issue Cite
Research Paper
Vimentin epigenetic deregulation in Bladder Cancer associates with acquisition of invasive and metastatic phenotype through epithelial-to-mesenchymal transition
1. Cancer Biology and Epigenetics Group, Research Center of IPO Porto (CI-IPOP) / RISE@CI-IPOP (Health Research Network), Portuguese Oncology Institute of Porto (IPO Porto) / Porto Comprehensive Cancer Centre (Porto.CCC), Rua Dr. António Bernardino de Almeida, 4200-072, Porto, Portugal.
2. INEGI-LAETA, Faculty of Engineering, University of Porto, Campus FEUP, Rua Dr. Roberto Frias 400, 4600-465, Porto, Portugal.
3. Department of Pathology and Molecular Immunology, School of Medicine and Biomedical Sciences-University of Porto (ICBAS-UP), Rua de Jorge Viterbo Ferreira 228, 4050-313, Porto, Portugal.
4. Department of Pathology, Portuguese Oncology Institute of Porto / Porto Comprehensive Cancer Center (Porto.CCC), Rua Dr. António Bernardino de Almeida, 4200-072, Porto, Portugal.
5. Institut Curie, UMR144, Centre de Recherche, 75005 Paris, France.
*These authors contributed equally to this work.
Abstract
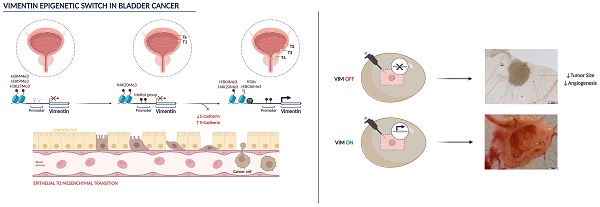
Bladder cancer (BlCa) is the ninth most common cancer worldwide, associated with significant morbidity and mortality. Thus, understand the biological mechanisms underlying tumour progression is of great clinical significance. Vimentin (VIM) is (over)expressed in several carcinomas, putatively in association with EMT. We have previously found that VIM promoter methylation accurately identified BlCa and VIM expression associated with unfavourable prognosis. Herein, we sought to investigate VIM expression regulation and its role in malignant transformation of BlCa.
Analysis of tissue samples disclosed higher VIM transcript, protein, and methylation levels in BlCa compared with normal urothelium. VIM protein and transcript levels significantly increased from non-muscle invasive (NMIBC) to muscle-invasive (MIBC) cases and to BlCa metastases. Inverse correlation between epithelial CDH1 and VIM, and a positive correlation between mesenchymal CDH2 and VIM were also observed. In BlCa cell lines, exposure to demethylating agent increased VIM protein, with concomitant decrease in VIM methylation. Moreover, exposure to histone deacetylases pan-inhibitor increased the deposit of active post-translational marks (PTMs) across VIM promoter. In primary normal urothelium cells, lower levels of active PTMs with concomitant higher levels of repressive marks deposit were observed. Finally, VIM knockdown in UMUC3 cell line increased epithelial-like features and decreased migration and invasion in vitro, decreasing tumour size and angiogenesis in vivo.
We demonstrated that VIM promoter is epigenetically regulated in normal and neoplastic urothelium, which determine a VIM switch associated with EMT and acquisition of invasive and metastatic properties. These findings might allow for development of new, epigenetic-based, therapeutic strategies for BlCa.
Keywords: Vimentin, Methylation, Histones posttranslational modifications, Bladder Cancer, EMT.

 Global reach, higher impact
Global reach, higher impact