10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2023; 19(1):50-65. doi:10.7150/ijbs.73530 This issue Cite
Research Paper
PROX1-mediated epigenetic silencing of SIRT3 contributes to proliferation and glucose metabolism in colorectal cancer
1. Department of Gastrointestinal Medical Oncology, Fudan University Shanghai Cancer Center, Shanghai, 200032, China.
2. Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, 200032, China.
3. Department of Medical Oncology, Zhongshan Hospital, Fudan University, Shanghai, 200032, China.
4. Department of Colorectal Surgery, Fudan University Shanghai Cancer Center, Shanghai, 200032, China.
5. Department of Pulmonary Medicine, Shanghai Chest Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, 200032, China.
6. Department of Gastroenterology & Clinical Nutrition, The 452nd Hospital of PLA, Chengdu 610000, Sichuan, China.
7. Department of Surgery, United Health Services Southern California Medical Education Consortium, Temecula Valley Hospital, Temecula, CA 92592, USA.
8. Department of Pathology, Fudan University Shanghai Cancer Center, Shanghai, 200032, China.
9. Institute of Pathology, Fudan University, Shanghai, 200032, China.
*These authors contributed equally to this work.
Abstract
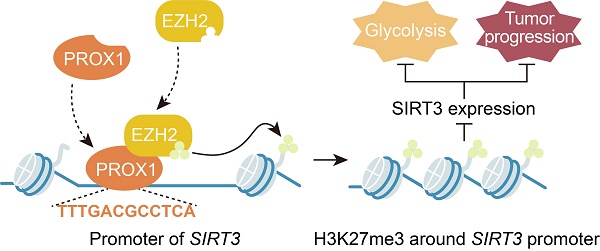
Prospero-related homeobox 1 (PROX1) is a homeobox transcription factor known to promote malignant transformation and stemness in human colorectal cancer (CRC). However, the biological function of PROX1 in metabolic rearrangement in CRC remains unclear. Here, we aimed to uncover the relationship between the expression profile and role of PROX1 and CRC cell glucose metabolism and to elucidate the underlying molecular mechanism. PROX1 expression was significantly upregulated in human CRC tissues and positively associated with the maximum standardized uptake value (SUVmax), a measure of tissue 18-fluoro-2-deoxy-D-glucose uptake and an indicator of glycolysis and tumor cell activity, in patients with CRC. Knockdown of PROX1 suppressed CRC cell proliferation and glucose metabolism in vitro and in vivo. Mechanistically, through a physical interaction, PROX1 recruited EZH2 to the SIRT3 promoter and inhibited SIRT3 promoter activity. Moreover, PROX1 or EZH2 knockdown decreased cell glycolysis by targeting SIRT3. Clinically, high PROX1 expression combined with low SIRT3 expression predicted poor prognosis in patients with CRC. Thus, our study suggests that the PROX1-EZH2 complex positively regulates cell proliferation and glucose metabolism by engaging SIRT3 in CRC, which may serve as a promising therapeutic strategy for CRC.
Keywords: PROX1, SIRT3, EZH2, colorectal cancer, aerobic glycolysis, prognosis

 Global reach, higher impact
Global reach, higher impact