10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2023; 19(1):66-88. doi:10.7150/ijbs.73936 This issue Cite
Review
Targeting FGF21 in cardiovascular and metabolic diseases: from mechanism to medicine
1. Department of Endocrinology, Institute of Endocrine and Metabolic Diseases, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, Clinical Research Hospital of Chinese Academy of Sciences (Hefei), University of Science and Technology of China, Hefei, Anhui, 230001, China.
2. Changshu Hospital Affiliated to Soochow University, Changshu No.1 People's Hospital, Changshu 215500, Jiangsu Province, China.
3. Changshu Hospital Affiliated to Nanjing University of Chinese Medicine, Changshu, China.
Received 2022-4-14; Accepted 2022-9-18; Published 2023-1-1
Abstract
Cardiovascular and metabolic disease (CVMD) is becoming increasingly prevalent in developed and developing countries with high morbidity and mortality. In recent years, fibroblast growth factor 21 (FGF21) has attracted intensive research interest due to its purported role as a potential biomarker and critical player in CVMDs, including atherosclerosis, coronary artery disease, myocardial infarction, hypoxia/reoxygenation injury, heart failure, type 2 diabetes, obesity, and nonalcoholic steatohepatitis. This review summarizes the recent developments in investigating the role of FGF21 in CVMDs and explores the mechanism whereby FGF21 regulates the development of CVMDs. Novel molecular targets and related pathways of FGF21 (adenosine 5'-monophosphate-activated protein kinase, silent information regulator 1, autophagy-related molecules, and gut microbiota-related molecules) are highlighted in this review. Considering the poor pharmacokinetics and biophysical properties of native FGF21, the development of new generations of FGF21-based drugs has tremendous therapeutic potential. Related preclinical and clinical studies are also summarized in this review to foster clinical translation. Thus, our review provides a timely and insightful overview of the physiology, biomarker potential, molecular targets, and therapeutic potential of FGF21 in CVMDs.
Keywords: fibroblast growth factor 21, cardiovascular diseases, metabolic diseases, targets, therapeutics
1. Introduction
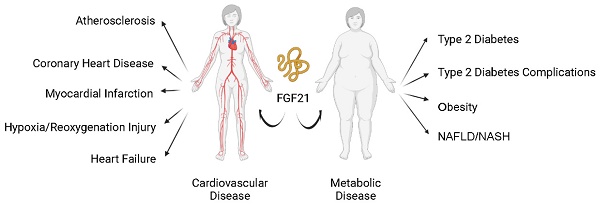
Modern human society is burdened with the pandemic of cardiovascular diseases (CVD, such as atherosclerosis, myocardial infarction (MI), and heart failure (HF)) and metabolic diseases (such as diabetes mellitus (DM), obesity, and metabolic syndrome (MS)). Cardiovascular and metabolic disease (CVMD) is the leading cause of mortality and morbidity worldwide and is mostly caused by abnormal metabolic processes [1, 2]. Fibroblast growth factor 21 (FGF21), which has protective effects against damage induced by abnormal metabolic conditions, has been intensively studied (Figure 1) and widely identified as a therapeutic candidate for CVMDs (Figure 2) [3, 4].
FGF21, a signaling protein of 208 amino acids, belongs to the fibroblast growth factor (FGF) family [5]. The human FGF family includes 22 members and has been divided into seven subfamilies. Subfamily 19 comprises three endocrine factors: FGF21, FGF19, and FGF23. The rest of the other subfamilies work in an intracrine or paracrine manner. Unlike conventional functions of promoting fibroblast proliferation and growth mediated by other FGFs, subfamily 19 mainly focuses on regulating metabolism. FGF19 regulates bile acid homeostasis, FGF23 regulates phosphate homeostasis, and FGF21 attracts the most attention due to its multiple beneficial effects on regulating glucose and lipid homeostasis as well as energy metabolism [6]. The way that transmembrane tyrosine kinase FGF receptor (FGFR) signaling activity is activated between subfamily 19 and other subfamilies is also different. Nonendocrine FGFs require heparan sulfate as a coreceptor to activate FGFR. However, endocrine FGFs lack the canonical heparin-binding domain (which also permits them to escape into the blood and be similar to endocrine hormones). They form a cell surface receptor complex with FGFR and another coreceptor, β-Klotho (KLB) [7].
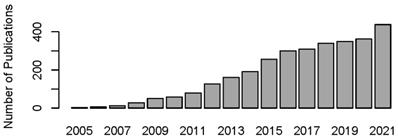
Year publications of FGF21 in PubMed (date of search, March 1, 2022). The number of publications on FGF21 is increasing year by year.
2. FGF21: tissue expression and biological functions
FGF21 is expressed after being induced by various stress conditions, such as cold exposure, nutritional stresses, exercise, and some pathologic conditions [8-11]. Tissues expressing FGF21 include the liver, brown adipose tissue (BAT), white adipose tissue (WAT), muscle, pancreas, heart, and brain [5, 12]. The liver is the major source of serum FGF21 under physiological conditions [13]. The process of hepatic FGF21 production is presented in Figure 3A. Different FGF21 transcription factors (including peroxisome proliferator-activated receptor (PPAR) α, activating transcription factor 4 (ATF4), carbohydrate response element-binding protein (ChREBP), and CCR4-NOT transcription complex subunit 6-like (CNOT6 L)) can be regulated under different states to promote FGF21 expression. Hepatic FGF21 can be induced in both states of nutrient deficiency (starvation, ketogenic diet, and methionine/choline-deficient diet) and forms of nutrient excess (simple sugars). Under nutrient-deficient states, fatty acids activate the transcription factor PPAR α, whose downstream target is FGF21 [8]. ATF4 is another effector that induces FGF21 expression under nutrient deficiency (amino acid limitation) [14] and oxidative stress [15, 16]. However, under conditions of carbohydrate load, in the liver and adipose tissues, another factor named ChREBP induces FGF21 to regulate de novo lipogenesis [17]. Notably, hepatic PPARα is required for the carbohydrate load-induced induction of FGF21 by ChREBP [18]. Moreover, CNOT6L is the catalytic subunit of the CCR4-NOT deadenylase, which can promote FGF21 mRNA decay. Disruption of CNOT6L activity in the liver can increase serum FGF21 levels [19].
FGF21-based drugs have also been investigated to increase FGF21 levels, which are summarized in Table 1 and Figure 3B. FGF21-based drugs can be divided into four categories: agents that increase FGF21 expression, FGF21-degrading protease inhibitors, FGF21 analogs, and FGF21-receptor agonists. For agents that increase FGF21 expression, in addition to chemicals such as neohesperidin [20] that increase FGF21 expression directly, the expression of FGF21 is increased by FGF21-transcription factor modulators, such as PPARα agonists (Pemafibrate) [21], cAMP-responsive element-binding protein H modulator (MS-275) [22], ATF4 modulator (Carbon monoxide) [23], and CNOT6L inhibitor (iD1) [24]. In addition, agents used for DM treatment, such as renal sodium/glucose cotransporter-2 inhibitors (Canagliflozin) [25], glucagon-like peptide-1 (GLP-1) analog (Liraglutide) [26], and metformin [27], also increase the expression of FGF21. Regarding FGF21-degrading protease inhibitors, fibroblast activation protein inhibitors can increase endogenous FGF21 levels in cynomolgus monkeys [28]. FGF21 analogs and FGF21-receptor agonists are the most studied FGF21-based drugs in preclinical and clinical studies. The preclinical and clinical studies of FGF21-based drugs in CVMDs will be discussed later.
FGF21 and cardiovascular and metabolic diseases. Current studies support that FGF21 plays an important role in cardiovascular and metabolic diseases. Nonalcoholic fatty liver disease (NAFLD); nonalcoholic steatohepatitis (NASH).
Molecular functions of FGF21 in cardiovascular and metabolic diseases. (A) The process of hepatic FGF21 production with different activating transcription factors, including peroxisome proliferator-activated receptors (PPARs), activating transcription factor 4 (ATF4), carbohydrate response element-binding protein (ChREBP), and CCR4-NOT transcription complex subunit 6-like (CNOT6 L). (B) FGF21-based drugs that can increase FGF21 levels. (C) FGF21 forms a cell surface receptor complex with FGFR and another coreceptor, β-Klotho (KLB). FGF21 exerts its metabolic protective effects by coordinating target tissues of FGF21, including the brain, liver, adipose tissues, pancreas, cardiac tissue and vasculature, muscles, and intestines. The brain can regulate food preferences and assist in coordinating FGF21-mediated interorgan responses. The liver and adipose tissues are the main targets of FGF21. The direct action of hepatic FGF21 is to regulate lipid and free fatty acid metabolism in the liver. The actions of FGF21 in white adipose tissue (WAT) and brown adipose tissue (BAT) are mainly focused on lipolysis, increasing glucose uptake, and thermogenesis.
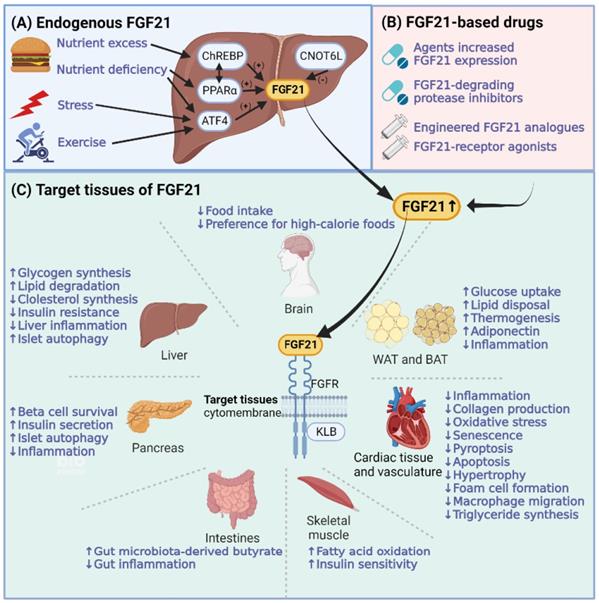
Target tissues of FGF21 comprise the liver, adipose tissues, brain, pancreas, heart, and kidney. FGF21 mediates biological functions in target tissues with the assistance of KLB and FGFR [29]. The molecular functions of FGF21 in CVMDs are summarized in Figure 3C. As a hepatoprotective factor, the direct action of hepatic FGF21 is to regulate lipid and free fatty acid metabolism in the liver, protecting against steatosis caused by nutrient stressors [30]. Adipose tissue is another essential target of hepatic FGF21. The endocrine actions of FGF21 in adipocytes are mainly focused on lipolysis and increasing glucose uptake. Weight loss caused by increased fatty acid oxidation was found to be as expected. However, the level of blood glucose is not decreased as expected in humans, which indicates the possibility of FGF21 as a lipid regulator analogous to insulin as a glucose regulator under the underlying different glucose control modes between animals and humans [31]. In addition, hepatic FGF21 can penetrate the blood-brain barrier (BBB). FGF21 derived from the liver can act on neurons, decrease sweet and alcohol preferences and stimulate water intake to maintain balanced nutritional conditions [32]. Moreover, FGF21 has nonmetabolic effects on daily wheel-running behavior as well as infertility and growth, which is associated with FGF21 signal transduction in the hypothalamus and brainstem [33]. Notably, muscle is another important source of serum FGF21. Myocytic FGF21 can act on WAT to induce the browning of WAT [34], on skeletal muscle to protect against insulin resistance [35], and on the heart to protect against cardiac hypertrophy [36].
Beyond its endocrine actions, FGF21 acts in an autocrine/paracrine way in BAT, WAT, the heart, and the pancreas. In adipose tissue, autocrine-derived FGF21 can induce cold-induced thermogenesis by uncoupling protein 1 (Ucp1)-dependent or Ucp1-independent mechanisms [37]. Cold exposure-induced FGF21 in BAT and WAT also promotes adipocyte browning, which can increase thermogenesis, with the assistance of coactivator peroxisome proliferator-activated receptor-gamma coactivator-1alpha and cytokine C-C motif chemokine ligand 11 [38]. In the pancreas, the secretion of FGF21 and digestive enzymes in pancreatic acinar cells is synchronized, which can minimize proteotoxic stress and prevent protein overload. Increased FGF21 also helps the pancreas protect against injury caused by pancreatitis in mice, such as endoplasmic reticulum stress and inflammation [39]. FGF21 can protect β cells from stress-induced dysfunction and induce insulin gene expression [40]. In addition, the autocrine action of FGF21 in the heart is stimulated by cardiomyopathy. In this way, FGF21 protects against heart disease injuries such as cardiac hypertrophy and infarction [36].
Overview of FGF21-based drugs in cardiometabolic disease
| FGF21-based drugs | Target | Effect | Species | Reference |
|---|---|---|---|---|
| Agents that increase FGF21 expression | ||||
| The renal sodium/glucose cotransporter-2 inhibitors (Canagliflozin) | AMP-activated protein kinase (AMPK) ↑- FGF21 ↑ | Fat mass reduction and lipolysis activation | Mouse | [25] |
| Glucagon-like peptide 1 analogue (Liraglutide) | FGF21↑ | Triglycerides synthesis ↓ | Mouse | [26] |
| Metformin | Activating transcription factor 4 (ATF4) ↑-FGF21 ↑ | Anti-obesity and anti-diabetes effects | Mouse and human | [27] |
| Intestinal serine protease inhibition (Camostat) | FGF21 ↑ | Food intake and body weight ↓ | Mouse | [230] |
| Peroxisome proliferator-activated receptor alpha (PPARα) modulator (Pemafibrate/K-877) | PPARα ↑-FGF21 ↑ | Management of atherogenic dyslipidaemia | Type 2 diabetes (T2DM) patients | [21] |
| Class I-specific histone deacetylase inhibitor (MS-275) | cAMP-responsive element-binding protein H (CREBH) ↑-FGF21 ↑ | Lipid metabolism-related gene expressions ↑ | Mouse | [22] |
| Dietary Cyanidin-3-Glucoside | FGF21 ↑ | Body weight and impairment of glucose tolerance ↓ | Mouse | [231] |
| Dichloroacetate | AMPK ↑- FGF21 ↑ | Against atherosclerosis | Mouse | [232] |
| Ishige okamurae extract | FGF21 ↑ | Hyperglycemia and Body weight ↓ | Mouse | [233] |
| Mediterranean tomato-based sofrito sauce | FGF21 ↑ | Healthy effects on glucose metabolism | Rat | [234] |
| Carbon monoxide | ATF4 ↑-FGF21 ↑ | Anti-inflammatory, antiproliferative, and antiapoptotic effects | Mouse | [23] |
| Berberine | AMPK ↑-FGF21↑ | The beneficial metabolic effect | Primary mouse hepatocytes | [235] |
| Berberine | Sirtuin1 (SIRT1) ↑-FGF21↑ | Hepatic lipid metabolism and energy expenditure ↑ | Mouse | [236] |
| Neohesperidin | FGF21↑ | Lipid-regulating effects | Mouse | [20] |
| Diet polyphenol curcumin | FGF21↑ | Metabolic beneficial effects | Mouse | [237] |
| A combined docosahexaenoic acid-thyroid hormone protocol | FGF21↑ | Energy expenditure ↑ | Rat | [238] |
| The TAZ activator 2-butyl-5-methyl-6-(pyridine-3-yl)-3-[2'-(1H-tetrazole-5-yl)-biphenyl-4-ylmethyl]-3H-imidazo[4,5-b] pyridine] (TM-25659) | ATF4 ↑-FGF21↑ | Insulin resistance and inflammation ↑ | Mouse | [239] |
| CCR4-NOT Transcription Complex Subunit 6 Like (CNOT6L) inhibitor | CNOT6L↓-FGF21↑ | Treat metabolic syndrome | Mouse | [24] |
| Haem-regulated eukaryotic translation initiation factor 2α kinase (HRI) activators | FGF21↑ | Treat T2DM | Mouse | [240] |
| Lentivirus-Mediated human FGF21 | FGF21↑ | Treat T2DM | Mouse | [131] |
| FGF21-degrading protease inhibitors | ||||
| Fibroblast activation protein (FAP) inhibition | FGF21↑ | Treat obesity-related metabolic disorders | Cynomolgus monkeys | [28] |
| Engineered FGF21 analogues | ||||
| Pegbelfermin | Binding to FGFR1 and β-Klotho | Improved metabolic parameters | Obesity and T2DM patients | [126] |
| Efruxifermin | Binding to FGFR1 and β-Klotho | Hepatic fat fraction ↓ | NASH patients | [194] |
| LLF580 | Binding to FGFR1 and β-Klotho | Triglycerides and hepatic fat ↓ | Obesity patients | [199] |
| BIO89-100 | Binding to FGFR1 and β-Klotho | Improve serum triglycerides | Hypertriglyceridemia patients | [241] |
| FGF21-receptor agonists | ||||
| C3201 | Binding to FGFR1 and β-Klotho | Treat obesity | Monkeys | [242] |
| The agonistic anti-FGFR1 antibody (R1MAb1) | Binding to FGFR1 | Treat obesity and diabetes | Mouse | [243] |
| FGF21-mimetic monoclonal antibodies (mimAb1) | Binding to β-Klotho | Treat obesity | Cynomolgus monkeys | [244] |
| GLP-1 and FGF21 dual agonist | Binding to β-Klotho | Treat diabetes | Mouse | [135] |
3. FGF21 and CVD
3.1 Atherosclerosis and coronary artery disease (CAD)
3.1.1 Relationship between FGF21 and CAD
CAD is a critical form of CVD. The elevated level of FGF21 is a biomarker for higher CVD risk in statin-treated patients [41]. In DM patients with coronary artery calcification, a lower level of FGF21 predicts a better long-term prognosis [42]. One study indicated that the baseline level of serum FGF21 can predict incident CAD in people with type 2 diabetes mellitus (T2DM) without known CVD [43]. However, another multiethnic study did not support FGF21 as a CVD biomarker in atherosclerosis patients without known CVD [44]. Although population characteristics for FGF21 as an incident CVD biomarker are controversial, FGF21 levels may still be utilized as a CAD risk biomarker for primary prevention [43]. The CAD primary prevention effect of FGF21 has been represented in subclinical atherosclerosis patients without nonalcoholic fatty liver disease (NAFLD) [45] and patients without a history of DM [46]. Moreover, FGF21 can be a biomarker to predict subclinical atherosclerosis, the pathological basis of CAD, in women without a history of CVD [47].
3.1.2 Mechanisms of FGF21 against atherosclerosis
The elevated FGF21 plays a protective role in CAD. Atherosclerosis is an important pathological basis of CAD. FGF21 can protect against atherosclerosis by modulating various proatherogenic cellular events. First, FGF21 can alleviate endothelial dysfunction via multiple pathways. For example, coculture of human umbilical vascular endothelial cells (HUVECs) with 5 ng/mL FGF21 delayed endothelial senescence by increasing silent information regulator 1 (SIRT1) [48]. One in vitro study investigated the impact of FGF21 on HUVEC pyroptosis stimulated by oxidized low-density lipoprotein, and the authors found that FGF21 can inhibit HUVEC pyroptosis through the tet methylcytosine dioxygenase 2- ubiquinol cytochrome c reductase core protein I-reactive oxygen species pathway [49]. In apolipoprotein E-/- (apoE-/-) mice, FGF21 inhibits the Fas signaling pathway to decrease endothelial apoptosis [50], and the effects on endothelial pyroptosis were mediated by inhibition of the NOD-, LRR- and pyrin domain-containing 3 (NLRP3) inflammasome pathway [51]. In addition, one in vivo study reported that FGF21 inhibits the nuclear factor kappa light chain enhancer of the activated B-cell (NF-κappaB) pathway in endothelial cells to improve atherosclerosis [52].
Second, macrophages are also crucial to the development of atherosclerosis. In vitro experiments proved that FGF21 can inhibit macrophage-derived foam cell formation to improve atherosclerosis [53]. FGF21-induced autophagy can reduce cholesterol accumulation and increase cholesterol efflux from foam cells via the activated kinase C receptor 1-adenosine 5'monophosphate-activated protein kinase (AMPK) pathway in vivo [54]. In an in vitro study, researchers also found that the cholesterol efflux-promoting effects of FGF21 are mediated by inducting ATP binding cassettes A1 and G1 in foam cells [55]. Moreover, FGF21 can affect macrophage-related cholesterol efflux by regulating the microRNA-33-sterol regulatory element-binding protein pathway [56]. Apart from inhibiting foam cell formation, FGF21 also suppresses macrophage apoptosis to improve atherosclerosis in vitro [57].
Third, neointimal hyperplasia, involving the proliferation and migration of vascular smooth muscle cells, is another essential pathological step for atherosclerosis. In high-fat diet (HFD) mice treated with a low dose of streptozotocin, FGF21 inhibits the migration and proliferation of vascular smooth muscle cells through the FGFR1-spleen tyrosine kinase-NLRP3 inflammasome pathway [58]. Notably, FGF21 can promote the secretion of adiponectin, which also exerts an atheroprotective effect by reducing endothelial dysfunction, thereby blocking the conversion of macrophages to foam cells and inhibiting vascular smooth muscle cell proliferation [59].
In conclusion, FGF21 can modulate proatherogenic cellular events, including vascular endothelial cells, macrophages, and vascular smooth muscle cells, to protect against atherosclerosis and CAD.
3.1.3 Preclinical and clinical trials of FGF21 in atherosclerosis
The protective effect of FGF21 against atherosclerosis has been proven in many preclinical trials. One study carried out on apoE-/- mice indicated that the administration of recombinant human FGF21 (rhFGF21) can protect against atherosclerotic plaque formation [60]. In another study, apoE-/- mice were treated with an FGF21 analog (LY2405319). LY2405319 treatment also reduces atheromatous plaque and lowers lipid profiles [61] in hypercholesterolaemic apoE*3-Leiden. In CETP mice (a model that has a lipid profile similar to that of human patients), rhFGF21 administration can reduce the severity and increase the stability index of atherosclerotic lesions [62]. However, there have been no clinical trials about the application of FGF21-based drugs in CVD treatment, and most clinical trials have investigated the application of FGF21 in metabolic diseases.
3.2 Myocardial infarction (MI) and ischemia/reperfusion (I/R) injury
3.2.1 Relationship between FGF21 and MI
MI is a severe ischemic heart disease with high mortality caused by coronary atherosclerosis and CAD. One study involving acute MI patients and stable angina pectoris (AP) patients showed that MI patients have higher serum FGF21 levels than stable AP patients [63]. Another study indicated that FGF21 has precise predictability for increased risks of unstable AP [64]. One study that enrolled 183 acute MI patients and 55 patients without acute MI suggested that circulating FGF21 levels might be a predictive marker for the clinical outcomes of acute MI patients [65]. Another study enrolled 348 MI patients with ST-segment elevation and found that elevated FGF21 is a powerful predictor of major adverse cardiovascular events [66].
3.2.2 Mechanisms of FGF21 against MI
FGF21 can regulate cardiac myocyte, H9c2 cell, and fibroblast actions to improve MI. In cardiac myocytes, FGF21 protects myocytes against ischemic injury [63]. In addition, FGF21 can effectively suppress inflammation and fibrosis in myocytes by regulating the FGFR-early growth response protein 1 pathway [67]. In H9c2 cells, apoptosis and oxidative stress can be alleviated via the FGF21-phosphatidylinositol 3-kinase (PI3K)-protein kinase B (Akt) pathway [68]. Regarding cardiac fibroblasts, FGFF21 can promote fibroblast apoptosis and reduce collagen production by inactivating the transforming growth factor-β1-drosophila mothers against the decapentaplegic protein 2/3-matrix metalloproteinase 2/9 signaling pathway in mice with myocardial infarction [69].
On the other hand, FGF21 can effectively suppress ventricular arrhythmia post MI by regulating the microRNA-143-early growth response protein 1 axis in vivo [70]. Another study confirmed the influence of FGF21 on decreasing the incidence of arrhythmia in rat and guinea pig models and showed that the incidence of ischemic arrhythmias in rhFGF21-treated models was decreased. Consistently, in hydrogen peroxide-treated AC16 cells, administration of FGF21 shortens cell action potential duration by ameliorating voltage-gated sodium channel 1.5 and potassium inwardly rectifying channel 2.1 dysregulations [71].
In conclusion, FGF21 can improve MI and ventricular arrhythmia post MI by regulating the inflammation, fibrosis, and action potential duration of myocytes.
3.2.3 Mechanisms of FGF21 against I/R injury
The rapid restoration of blood perfusion after MI may lead to I/R injury, which aggravates cell injury and death in the ischemic heart. FGF21 exhibits protective effects against heart I/R injury, and the mechanism has been investigated in animal and cell models. In the rat myocardial I/R model, researchers injected FGF21 into the rat myocardial I/R model and found that FGF21 can decrease infarction and inflammation after I/R [72]. In the H9c2 hypoxia/reoxygenation model, FGF21 can improve H9c2 cell I/R injury by promoting autophagy via different pathways, such as the FGF21-Beclin‑1/vacuolar protein sorting 34 proteins-autophagy pathway [73] and the FGF21-microRNA-145- autophagy pathway [72]. FGF21 can also decrease the expression levels of galectin-3 to alleviate hypoxia-induced cardiac myocyte injury in vivo [74]. In addition, FGF21 can improve the energy metabolism of H9c2 cells after I/R via the FGF21-angiopoietin-2-glucose transporter 1 pathway in vitro [75].
3.2.4 Preclinical and clinical trials of FGF21 in MI
In an animal model, intramuscular injection of FGF21-expressing adenoviral vectors into MI mice improved left ventricular systolic dysfunction after two weeks with the assistance of adiponectin [76]. In FGF21 knockout MI mice, FGF21 deficiency weakens the aerobic exercise-induced inhibition of oxidative stress and apoptosis [68]. Moreover, exercise training increased FGF21 expression and alleviated oxidative stress and cardiac fibrosis after MI [68, 69]. However, clinical trials about the application of FGF21 in CVD patients are still lacking and remain to be investigated.
3.3 Cardiac remodeling and HF
3.3.1 Relationship between FGF21 and HF
FGF21 has predictive value for the development and progression of HF. One study obtained blood and tissue samples from patients with end-stage HF with reduced ejection fraction (HFrEF) and found elevated serum FGF21 in HFrEF patients [77]. Another study that enrolled 128 HFrEF patients and 71 controls also found elevated FGF21 levels in patients with HFrEF [78]. In addition to HFrEF, patients with HF with preserved ejection fraction (HFpEF) also have higher FGF21 levels than patients without HF, which shows that FGF21 has the potential to diagnose HFpEF [79] and reflect diastolic dysfunction [80].
3.3.2 Mechanisms of FGF21 against HF
FGF21 can improve HF by regulating cardiac remodeling, which has been investigated in animal and cell models. Cardiac remodeling is a pathological process associated with cardiac hypertrophy, fibrosis, and other harmful cardiac derangement changes, which eventually cause HF. The protective effects of FGF21 on preventing cardiac hypertrophy have been evaluated in pathological animal models such as uremic cardiomyopathy rats [81], obesity-associated cardiomyopathy mice [82], and hypertension mice [51]. All of these pathological cardiomyopathies can result in HF. Moreover, in angiotensin II-induced cardiac hypertrophy mice, FGF21 can regulate the deacetylase activity of SIRT1 to improve cardiac hypertrophy [83]. For in vitro studies, FGF21 treatment can protect cardiomyocytes from hypertrophy and inflammation against oxidative stress [84], the possible factor that leads to HF [85]. Apart from improving cardiac hypertrophy, FGF21 can attenuate cardiac derangements to inhibit cardiac remodeling and HF. In FGF21-/- mice fed an HFD, the impaired ability of FGF21 promoted autophagy in cardiomyocytes, leading to lipid accumulation and cardiac derangements [82]. Pathological cardiac fibrosis is another critical step toward HF, and the protective effects of FGF21 against fibrosis are also observed in hypertensive heart disease mouse models [86].
3.3.3 Preclinical and clinical trials of FGF21 in HF
Preclinical and clinical trials of FGF21 in HF are limited. One study delivered 3 longevity-associated genes, FGF21, into HF mouse models and found increased heart function in aortic constriction [87]. Moreover, DPP-4 inhibitor-induced FGF21 also improves the contractile efficiency of pressure-overloaded mouse hearts [88].
4. FGF21 and metabolic diseases
4.1 T2DM
FGF21 can be a biomarker for predicting the onset of DM and has therapeutic effects on T2DM and its complications. Mechanisms of FGF21 against T2DM include improving glucose homeostasis, attenuating obesity, and alleviating inflammation. FGF21-based drugs have also been designed to treat T2DM in many preclinical and clinical studies.
4.1.1 Relationship between FGF21 and T2DM
A recent meta-analysis of 317 articles revealed that T2DM patients have a higher serum FGF21 level than control groups [89]. Moreover, serum FGF21 levels are lower in T2DM patients with higher urinary glucose excretion levels [90]. Another study assessed the association between FGF21 and glycohemoglobin, beta-cell function, and insulin resistance among 2 584 patients without hypoglycemic agent treatment. They found that patients with a higher FGF21 level have a higher risk of glycemic progression over five years [91]. The FGF21/adiponectin ratio is another valuable biomarker to predict the deterioration in glycemia [92]. Moreover, one study observed the DM incidence of 1 380 nondiabetic subjects over nine years and measured serum levels of FGF21. The results showed that serum FGF21 appears to be an alternative to the glucose tolerance test as a biomarker for DM prediction [93]. All of those studies [91-93] showed that FGF21 might be a biomarker in predicting the onset of prediabetes [92] and DM [93]. FGF21 is also related to the transition from prediabetes to DM. A recent study found that in WNIN/GR-Ob rats, the attenuation of FGF21 may aggravate glucose impairment and the transition from prediabetes to DM [94].
4.1.2 Mechanisms of FGF21 against T2DM
Elevated FGF21 plays a protective role in T2DM, which has been observed in animal models. In New Zealand obese mice, a T2DM and obesity model, data suggested that dietary methionine restriction-induced FGF21 can protect mice from glucose intolerance and T2DM [95]. Another study demonstrated that alternating-day fasting can inhibit insulin resistance and obesity in leptin receptor knockout mice, possibly depending on FGF21 [96].
The mechanism by which FGF21 protects against T2DM mainly involves improving glucose homeostasis in various ways. First, FGF21 can promote insulin expression and secretion to protect against T2DM. In that study, researchers found that FGF21 knockout exacerbates islet beta-cell failure and suppresses insulin secretion in pancreatic islets of db/db mice. Then, they found that inhibition of PI3K/Akt signaling can effectively suppress FGF21-induced insulin expression in mice, which indicated that FGF21-induced insulin expression and secretion occurred through the PI3K/Akt signaling pathway [97]. Notably, clamp studies in adults with T2D revealed that the secretion of insulin instead of glucose consumption induces a postprandial FGF21 increase [98], which indicated that FGF21 is also an insulin-dependent hormone in humans.
Second, FGF21 can improve glucose homeostasis by protecting β cells. FGF21 can downregulate islet cell lipid accumulation and thereby reduce the apoptosis and dysfunction of β-cells in vivo and in vitro, which is probably via activation of the AMPK-acetyl coenzyme A carboxylase (ACC) and PPARδ/γ signaling pathways [99]. Autophagy serves another essential role in protecting β cell survival and function. FGF21 can induce islet autophagy by inhibiting the AMPK-mammalian target of rapamycin (mTOR) signaling pathway in HFD C57/BL6J mice [100]. Apart from insulin-dependent ways to improve glucose homeostasis, FGF21 can take up glucose in adipocytes in an insulin-independent manner. Pegylated FGF21 can normalize plasma glucose in streptozotocin-treated mice and competitive insulin receptor antagonist-treated mice, whereas the function of pancreatic β-cells does not rest [101].
Third, FGF21 can reduce serum insulin and increase insulin sensitivity to protect against systemic insulin resistance. One study observed early-life exposure to chronic stress-induced long-term insulin sensitivity along with substantially increased FGF21 levels in C57Bl/6J mice [102]. To determine the specific target of FGF21 in improving hepatic insulin sensitivity, researchers administered FGF21 in mice and found that FGF21 can increase insulin sensitivity by inhibiting liver mTORC1. Consistently, mTORC1 is activated, and hepatic insulin resistance is exacerbated in FGF21-deficient mice [103]. Moreover, subcutaneous fat tissue is an essential tissue that FGF21 targets to regulate systemic insulin sensitivity. FGF21 can promote the expansion of subcutaneous fat and elevate adiponectin levels in subcutaneous fat to protect against systemic insulin resistance in vivo [104]. Adiponectin is an essential protein that can help FGF21 mediate insulin sensitivity. Researchers administered FGF21 to tunicamycin-induced 3T3-L1 adipocytes and HFD-induced obese mice. They found that the administration of FGF21 can inhibit endoplasmic reticulum stress in adipose tissue to release the suppression of adiponectin expression and restore insulin signaling [105].
In addition to improving glucose homeostasis, FGF21 can protect against T2DM in other ways. On the one hand, FGF21 can increase energy expenditure and attenuate obesity, as shown in obese mice [106]. However, the function of FGF21 in preventing diet-induced T2DM is not always accompanied by body fat mass change. In New Zealand obese mice, FGF21 treatment increases energy expenditure by WAT browning without changing body fat mass [107]. On the other hand, FGF-21 can alleviate inflammation while improving hyperglycemia. One study's results demonstrated the potent anti-inflammatory effect of FGF21 in the serum and WAT in db/db mice. In vitro experiments showed that FGF21 can ameliorate glucose uptake of tumor necrosis factor-α-induced insulin resistance by inhibiting the NF-κappaB signaling pathway in 3T3-L1 adipocytes [108].
4.1.3 Effects of FGF21 on complications of T2DM
FGF21 has therapeutic effects on complications of T2DM, including diabetic macroangiopathies and microangiopathies.
The heart is a vital target organ for T2DM, and the role of FGF21 in T2DM cardiovascular complications has been investigated in studies. Evidence suggests that FGF21 has therapeutic effects on diabetic cardiomyopathy in vitro and in vivo [109]. In T2D mice, FGF21 can prevent T2DM lipotoxicity-induced cardiomyopathy by activating both the AMPK-ACC-carnitine palmitoyltransferase-1-mediated lipid-lowering pathway and the AMPK-Akt2-nuclear transcription factor-E2-related factor 2 (Nrf2)-mediated antioxidative pathway in the heart [110]. Moreover, in T2DM patients with HFpEF, FGF21 showed its diagnostic potential for HFpEF [111]. Another study stratified 1132 DM patients with coronary artery calcification based on their FGF21 levels and recorded their major adverse cardiovascular events during 1.5-5.1 years of follow-up. Study results concluded that a lower baseline level of FGF21 predicts a better cardiovascular prognosis in DM patients [112]. Another study also supported that the level of circulating FGF21 is positively associated with human aortic stiffness, which was investigated in 130 T2DM patients [113]. In vitro, FGF21 can direct or activate the calcium/calmodulin-dependent protein kinase 2-AMPKα signaling pathway to attenuate high glucose-induced aortic endothelial function impairment and enhance aortic endothelium-dependent vasorelaxation [114].
Cerebrovascular disease is another severe complication of T2DM. The ischemic stroke mortality of T2DM patients is higher and neurological outcomes are worse than ordinary people [115]. Daily rhFGF21 administration lasting for two weeks post stroke in T2DM db/db mice can improve white matter and cerebrovascular impairment [116]. One study showed that FGF21 improves neurological outcomes after ischemic stroke in T2DM db/db mice, which may contribute to the antiproinflammatory effects by activating PPARγ in the brain [117]. In addition to cerebrovascular disease, DM tends to induce BBB dysfunction and cognitive impairment, including Alzheimer's disease [118]. In T2DM mice, massive BBB leakage was observed after ischemic stroke. FGF21 can protect against BBB disruption by activating PPARγ after ischemic stroke in T2DM db/db mice [119]. A study in T2DM mice demonstrated another potential mechanism of decreasing BBB permeability via the FGFR1-Kelch ECH-associated protein 1-Nrf2 activation pathway [120].
In addition to diabetic macroangiopathy, FGF21 also plays a role in diabetic microangiopathies such as diabetic retinopathy and diabetic nephropathy. One cross-sectional study recruited a total of 654 T2DM patients and found that the risk of sight-threatening diabetic retinopathy increased as the serum FGF21 level exceeded 554.69 pg/mL [121]. In type 1 diabetic mice, FGF21 can prevent early diabetic retinopathy by protecting photoreceptor function [122].
Regarding the association between FGF21 and diabetic nephropathy, a 6.3-year prospective cohort study in women with T2DM indicated that FGF21 can independently predict the progression to end-stage renal disease [123]. Another study found that the combination of FGF21 and soluble tumor necrosis factor receptor type 1 can better predict renal outcomes in a 3.5-year follow-up period for T2DM patients [124]. Moreover, from the aspect of genetic variants, study results indicated that FGF21-associated genetic variations are related to the estimated glomerular filtration rate in DM patients [125].
4.1.4 Preclinical and clinical trials of FGF21 in T2DM
Many FGF21-based drugs have been designed to treat T2DM, such as pegbelfermin (BMS-986036), a PEGylated FGF21. The results from a randomized phase 2 study in T2DM patients with obesity showed that pegbelfermin compared with placebo can improve triglycerides and increase adiponectin levels. However, there were no significant differences observed in glycated hemoglobin glycosylated hemoglobin A1c. The adverse effects of pegbelfermin are mild and mainly focused on injection-site bruising and diarrhea [126]. To overcome the weak binding affinity and instability of natural FGF21 [127], researchers analysed the structural and dynamic information of FGF21 and developed many FGF chimeras. For example, researchers devised a paracrine-endocrine FGF chimera that substituted the core of FGF21 with a stable and high receptor affinity core from paracrine FGF1 [128]. Another study using nuclear magnetic resonance spectroscopy found that the noncanonical flexible β-trefoil conformation of FGF21 affects protein stability, and they designed an FGF21-FGF19 chimera that can better decrease blood glucose in ob/ob mice [129]. FGF21 gene therapy is another way to overcome the unfavorable pharmacokinetic properties of native FGF21. Researchers administered an FGF21-overexpressing adeno-associated viral vector to mouse visceral adipose tissue, which decreases body weight and improves insulin sensitivity [130]. This study also highlights the potential of gene therapy to overexpress FGF21 in target tissues. Another study utilized lentivirus to mediate human FGF21 stable expression in T2DM rat liver tissue. The results showed that genes related to insulin sensitivity are increased, suggesting that injecting lentivirus-mediated rhFGF21 into rat livers might effectively treat T2DM [131].
A previous clinical study indicated that the lipid-lowering effect of FGF21 is more significant than that of hypoglycemia in humans [126], enlightening us to explore the potential of applying FGF21 with clinical antidiabetic drugs instead of applying FGF21 alone. The beneficial effect of the combined application may be because antidiabetic clinical medicines can reduce FGF21 levels, which may improve FGF21 sensitivity [132]. Moreover, the performance of the antidiabetic drug exenatide is poor in T2DM patients with high baseline FGF21 levels, which was observed in a multicenter trial [133]. In addition, FGF21 may be the downstream target of antidiabetic drugs, such as metformin [27], GLP-1 analog [133], and sodium/glucose cotransporter-2 inhibitors [25]. In diabetic mouse models, researchers found that GLP-1 analogs can promote hepatocytes to produce FGF21, which inhibits hepatic glucose output [134]. Moreover, GLP-1 analogs regulate WAT lipid metabolism in T2DM mice through the FGF21- liver kinase B1 (LKB1)- AMPK- ACC1 pathway [26].
The combined application of FGF21 and clinical anti-diabetic drugs has been reported in many preclinical studies. GLP-1 and FGF21 are the most common combinations. In diabetic mouse models, researchers designed a GLP-1 and FGF21 dual agonist with enhanced KLB binding affinity, which shows a superior glucose-lowering effect compared to GLP-1 or FGF21 alone [135]. Another study also supported that sustained release of a GLP-1 and FGF21 dual agonist can protect diabetic mice from hyperglycemia [136]. In addition, a drug delivery system used for the delivery of GLP-1 and FGF21 in vitro and in vivo was developed [137]. Mesenchymal stem cells modified by FGF21 and GLP-1 transplantation are another way to combine GLP-1 and FGF21. Researchers transplanted mesenchymal stem cells into a T2DM mouse model and observed the therapeutic effects of T2DM [138]. Apart from combining FGF21 and GLP-1, FGF21 synergizes with Forkhead box protein O1 inhibition, revealing the impact on normalizing glucose control in diabetic mice [139]. In addition, studies have demonstrated that the combined application of FGF21 and insulin can increase the sensitization of FGF21 and insulin on metabolic effects in T2DM mice. Another study further investigated the effect of combined FGF21 and insulin on diabetic nephropathy and found better glucose and lipid amelioration effects than insulin or FGF21 alone [140].
In conclusion, many FGF21-based drugs, such as pegylated FGF21, FGF chimeras, and FGF21 gene therapy, have been designed to treat T2DM. Although the glucose-lowering effect of FGF21 is limited in humans, the combined application of FGF21 and clinical anti-diabetic drugs such as GLP-1 has shown a great glucose-lowering effect.
4.2 Obesity
FGF21 can be a biomarker that reflects the degree of obesity and the effectiveness of weight loss. Additionally, FGF21 can exhibit a weight loss effect by reducing energy intake and enhancing energy dissipation. FGF21-based drugs as well as FGF21 and GLP1 dual agonists, have been developed to treat obesity.
4.2.1 Relationship between FGF21 and obesity
Obesity is a complex metabolic disease that can contribute to other metabolic diseases, such as T2DM and CVD [141]. FGF21 has been considered a suitable biomarker for predicting many obesity-related situations. For example, recent studies have gradually focused on FGF21 levels in the pediatric population, not just adults. A cross-sectional and longitudinal analysis revealed that FGF21 was higher in obese hyperglycemic adolescents [142]. Another study supported that FGF21 can be a suitable biomarker to predict the total intrahepatic lipid content and fatty liver in obese children [143]. In addition, one study investigated the relationship between FGF21 and childhood obesity, and findings suggested that it is FGF21 deficiency instead of FGF21 resistance that results in insulin resistance in obese children [144]. Moreover, FGF21 genetic variants might influence qualitative changes in nutritional behavior, such as food preferences, in children [145].
On the other hand, the biomarker function of FGF21 in weight loss has been discussed in many studies. One analysis selected a cohort of healthy overweight or obese subjects to complete a 12-month energy restriction pattern. The results indicated that FGF21 levels are not influenced by weight loss in a healthy obesity population [146]. However, in obese adolescents, the level of FGF21 was increased after long-term interdisciplinary weight loss therapy, which can maintain the rest metabolic rate [147]. Interestingly, in obese mice, weight loss can lead to decreased FGF21, which can be used to predict improved behavior after weight loss [148]. Thus, the results on FGF21 levels post weight loss are not uniform. Consistently, one study that enrolled overweight and obese women found that changes in FGF21 levels differed among the subjects participating in the interdisciplinary intervention. Furthermore, they found that the adiponectin/leptin ratio was changed only in people with elevated FGF21 [149]. The changes in FGF21 levels after dietary interventions in different people can be used to assess the individual predisposition to weight gain over time. One study assessed FGF21 changes after 24 hours with different macronutrient contents and found that individuals with a blunted FGF21 response to a low-protein diet are at high risk for future weight gain [150].
Bariatric surgery is an essential weight-loss strategy that can effectively improve obesity and T2DM [151]. Laparoscopic sleeve gastrectomy (LSG) is a typical operation of bariatric surgery. The FGF21 levels after LSG are not certain. One study containing 40 severely obese participants showed no effect of bariatric surgery, including LSG and Roux-en-Y gastric bypass surgery, on serum FGF21 [152]. However, in HFD-fed male Wistar rats, LSG can reduce weight with decreased FGF21 [153]. Consistently, one study reported that three months after ileal transposition metabolic surgery, serum FGF21 levels were lower in obese Zucker rats [154]. Moreover, in research containing 137 obese patients with insulin resistance and high baseline FGF21, FGF21 concentrations were decreased after LSG-induced weight loss. They concluded that FGF21 concentrations appear to predict the insulin resistance change after weight loss [155]. However, another study showed increased FGF21 after bariatric surgery in obese patients compared with a diet-induced weight loss strategy. That study suggested that the increased FGF21 is independent of insulin sensitivity, which shows the predicted effect of FGF21 as a marker of severe nutritional stress [156].
In conclusion, the FGF21 levels in obese subjects after weight loss therapy, such as dietary interventions and bariatric surgery, are changed. Although the study result about whether FGF21 levels are increased or decreased after interventions is not certain, the value of FGF21 to predict the predisposition to weight gain over time is worthy of being investigated.
4.2.2 Mechanisms of FGF21-mediated anti-obesity effects
FGF21 can exhibit its weight loss effect by reducing energy intake and enhancing energy dissipation.
In obese minipigs, treatment with FGF21 leads to energy intake reduction and weight loss compared to the vehicle group [157]. The reducing energy intake effect of FGF21 may contribute to its impact on food preference. It has been shown that serum FGF21 levels are associated with food and drug craving in humans [158]. Although the effects of FGF21 on the preference for high-calorie foods are different by sex, FGF21 administration beneficially affects mice of both sexes [159]. Mechanistically, one study demonstrated that mice cannot respond to protein restriction when the brain FGF21 coreceptor is deleted, which indicated that FGF21 can reflect protein status to the brain and make adaptive food choices [160]. However, another in vivo study held that the effect of FGF21 administration on dietary protein restriction-induced weight loss is mediated by suppressing simple sugar consumption and promoting the intake of other macronutrients rather than altering protein preference [161]. FGF21 can also increase appetite and stimulate food intake under a fasting state in zebrafish. The expression of FGF21 increases before meals and decreases after meals, which shows the effect of FGF21 on balancing energy and metabolism in different situations [162].
The brain receives FGF21 signaling and regulates food preference, while FGF21-induced energy expenditure mostly occurs in adipose tissues. FGF21-mediated thermogenesis in adipose tissue is the main mechanism by which FGF21 enhances energy expenditure. KLB is decreased in WAT and BAT during DM in mice, indicating the impaired FGF21 thermogenic effect during DM and obesity [163]. In FGF21-deficient HFD mice, the expression of thermogenic-related genes such as Ucp1 is markedly reduced in BAT, leading to a reduction in the thermogenic response [164]. Researchers have also investigated the thermogenic effect of FGF21 in primates. Whole transcriptome analysis in primate subcutaneous adipose tissues revealed corresponding gene network changes in lipid storage and metabolism after FGF21-induced weight loss [165]. Mechanistically, beige fat thermogenesis was found to be related to the microRNA-182-5p-FGF21-acetylcholine pathway in rats. MicroRNA-182-5p is a crucial antiobesity molecule in adipocytes that promotes FGF21 expression and secretion in adipocytes. FGF21 stimulates acetylcholine synthesis and release from macrophages, which in turn activates the nicotine acetylcholine receptor of adipocytes, and thermogenic genes are consequently expressed [166]. Notably, although most activation of BAT thermogenesis requires the help of Ucp1, endogenous FGF21 can prevent obesity by browning WAT in the absence of Ucp1 in mice [167]. One recent in vivo study provided a different point of view that the Ucp1-independent pyrexic effect of FGF21 may not increase energy expenditure [168]. Nonetheless, mice can still lose weight by decreasing food intake and increasing physical activity without thermogenic effects [169], suggesting that the FGF21-mediated balance of energy and metabolism is not just one-dimensional.
Apart from weight loss, FGF21 can improve obesity-induced metabolic disorders. Previous studies have found that exogenous FGF21 administration can exhibit great metabolic effects on HFD obese mice [170], and FGF21 deficiency will aggravate obesity-induced inflammation and inflammation-mediated atrophy in obese mouse skeletal muscle [171]. Obesity-related inflammation is a critical element in the development of other metabolic diseases. One study found that FGF21 can efficiently ameliorate obesity-related inflammation in obese mice and 3 T3-L1 preadipocytes, which are mediated by the fibroblast growth factor receptor substrate 2-extracellular regulated protein kinases 1/2 signaling pathway [172].
4.2.3 Preclinical and clinical trials of FGF21 in obesity
Many FGF21-based drugs have also been developed and studied in obese mice. For example, researchers utilized Lactococcus lactis NZ3900 to produce rhFGF21, which can activate BAT to reduce the bodyweight of db/db mice [173]. Many studies have chosen to increase FGF21 expression through genetic plasmids. One study reported that injection with a liver-specific FGF21 expression nonviral plasmid in FGF21 knockout mice can restore FGF21 expression and regulate body mass [174]. Another study utilized an adeno-associated viral vector to express FGF21 in mouse visceral adipose tissue, which resulted in reduced whole-body adiposity and adipose tissue macrophage inflammation [130]. Moreover, researchers recently designed a novel FGF21 named LAPS-FGF21 with a longer half-life via conjugation to the Fc fragment of IgG4, which has shown an excellent therapeutic effect on obesity in mice [175]. In addition, the FGF21 and GLP1 dual-agonist, which was developed by using a polypeptide linker to fuse GLP-1 and FGF21, also shows excellent potent weight-reducing effects in diabetic mice [136]. Regarding clinical trials, in patients with obesity and T2DM, pegbelfermin (BMS-986036) has demonstrated its impact on improving high-density lipoprotein cholesterol and triglycerides in its randomized phase 2 study [126].
4.3 NAFLD/Nonalcoholic steatohepatitis (NASH)
FGF21 is a potential biomarker to reflect pathological processes of NAFLD, such as liver fat content and liver inflammation. The effect of FGF21 on protecting against NAFLD/NASH is targeted on the pathological characteristics of NAFLD/NASH, including reducing hepatocyte stress and hepatic steatosis as well as directly protecting against inflammation and fibrosis. Additionally, clinical trials of FGF21-based drugs, such as pegbelfermin, LLF580, and BIO89-100, have been performed on patients with NAFLD/NASH, and great effects have been observed.
4.3.1 Relationship between FGF21 and NAFLD/NASH
NAFLD is more than 5% of hepatocytes with cytoplasmic triglyceride droplets and no evidence of other chronic liver conditions. NASH is the severe stage of NAFLD [176]. The association between FGF21 and NAFLD has been confirmed among 3634 participants free of apparent CVD [177], and FGF21 is becoming a potential biomarker to reflect many pathological processes of NAFLD, such as liver fat content and liver inflammation. The FGF21 to adiponectin ratio has emerged as a biomarker to reflect the liver fat content of obese children [178]. Serum FGF21 alone is also supported for use as a biomarker for NAFLD liver fat content in a randomized trial that enrolled 220 NAFLD patients with central obesity [179]. Regarding the relationship between FGF21 and liver inflammation, researchers used a linear regression method to explore the impact of some liver inflammatory mediators on FGF21 and supported that FGF21 can be a biomarker for the liver inflammation process [180]. NASH is the higher stage of NAFLD with severe liver inflammation, and another cross-sectional study demonstrated that FGF21 is associated with the severity of NASH [181]. Apart from the potential to assist NAFLD/NASH diagnosis, FGF21 can help us predict NAFLD/NASH prognosis and treatment efficacy. Serum FGF21 levels can reflect the improvement of NAFLD in patients who received weight loss intervention in a prospective observational pilot study [182]. FGF21 levels can also be used to evaluate the effects of liraglutide, a drug that can reduce liver fat content, in overweight patients with NAFLD [183].
4.3.2 Mechanisms of FGF21 against NAFLD/NASH
FGF21 can protect against NAFLD/NASH by reducing hepatocyte stress and hepatic steatosis as well as directly protecting against inflammation and fibrosis, which is aimed at the pathological characteristics of NAFLD/NASH. For example, one in vitro study showed that dehydrovomifoliol-induced FGF21 can alleviate lipid accumulation in HepG2 cells [184]. In mouse models, the overexpression of hepatocyte-specific FGF21 can ameliorate HFD-induced liver steatosis [185]. Notably, the effect of FGF21 on liver steatosis is sex-specific in obese A(y) mice, showing more beneficial effects on males with melanocortin obesity [186]. Another mouse study pointed out that fasting-induced FGF21 can activate autophagy-mediated lipid degradation in a Jumonji domain containing-3 (JMJD3)-dependent manner. Mechanistically, JMJD3 upregulates autophagy-network genes upon FGF21 signaling, resulting in hepatic autophagy and lipid degradation. Consistently, hepatic expression of JMJD3 is substantially decreased in NAFLD patients [187]. In addition, one study suggested that hepatic six transmembrane protein of prostate 2 may be the downstream therapeutic target of FGF21 to improve NAFLD accompanying hepatic iron overload [188]. They observed that rhFGF21 treatment ameliorates hepatic steatosis and reduces iron exporters by upregulating hepatic six transmembrane protein of prostate 2 expressions in oleic acid-treated HepG2 cells.
Regarding the effect of FGF21 on improving liver inflammation, FGF21 can regulate hepatic metabolic gene expression and hepatic mitochondrial function to improve inflammation. One study compared the treatment effect for NAFLD between FGF21 administration and caloric restriction in diet-induced obese mice. They performed a high-throughput quantitative polymerase chain reaction and found that FGF21 administration affects more hepatic genes than caloric restriction to improve steatosis and inflammation [189]. Another study investigated the effects of the engineered FGF21 variant (LY2405319) in ob/ob mice with diet-induced NASH. The results showed that LY2405319 treatment reduces the symptoms of steatohepatitis and inflammatory markers by enhancing hepatic mitochondrial function [190]. Moreover, FGF21 can also exert its anti-inflammatory effect by inhibiting the hepatocyte-Toll-like receptor 4-interleukin-17A pathway to prevent NASH from transitioning into hepatocellular carcinoma [191].
In addition, cardiovascular disease is the leading cause of death among NAFLD/NASH patients, and an imbalanced metabolic state is an important mechanism underlying NAFLD/NASH. Compared with other potential NAFLD/NASH drugs, apart from the effects on the pathological characteristics of NAFLD/NASH itself, FGF21 also delivers improvements in whole-body metabolism and cardiovascular risk profile, as mentioned above.
4.3.3 Preclinical and clinical trials of FGF21 in NAFLD/NASH
There is no specific drug for NAFLD/NASH [192], and FGF21 has been a potential hot strategy for NAFLD/NASH. Drugs have been developed to treat NAFLD/NASH based on the beneficial pharmacological effects of FGF21. Efruxifermin is a long-acting FGF21 drug in clinical trials for NASH treatment. Researchers administered efruxifermin to Sprague Dawley rats weekly and found that it can reduce body weight without increasing sympathetic tone [193]. The BALANCED program is a clinical study with NASH patients conducted at 27 centres in America. It is also a phase 2a trial for eftuxifermin. Eighty patients were divided into receive efruxifermin treatment (28/50/70 mg or placebo, subcutaneous injection, weekly, 16 weeks), which showed reduced hepatic fat fraction in patients with F1-3 stage NASH. Regarding the adverse event of FGF21 in clinical trials, the most common adverse event is grade 1-2 gastrointestinal events [194].
Pegbelfermin is a PEGylated FGF21 drug that has advanced to phase 2 clinical trials for NASH treatment. In phase 2a trials, pegbelfermin shows improvements in liver fat fraction reduction and decreases liver damage/fibrosis markers [126, 195]. The FALCON program includes two phase 2b studies: subcutaneous injection with pegbelfermin to treat NASH, bridging fibrosis, and compensated cirrhosis [196]. Patients in these two studies were divided into receiving pegbelfermin (10/20/40 mg) or placebo treatment every week for 48 weeks and then followed for an additional four weeks. This program will provide information such as dose selection to support the phase 3 program, which can better observe hard outcomes of FGF21, such as long-term survival or cirrhosis [197]. Regarding safety issues, injection-site bruising and gastrointestinal conditions, such as diarrhea, are the most prevalent side effects, and most adverse events are mild [126]. In addition, B1344 is a novel PEGylated FGF21 analog designed in recent years. Researchers administered B1344 to cynomolgus monkeys with NAFLD and methionine-deficient mice with induced NAFLD via subcutaneous injection for 11 weeks. These preclinical results indicated that B1344 administration can improve hepatic steatosis, inflammation, and fibrosis [198].
LLF580 is a novel FGF21 analog with an additional disulfide bond and a fused Fc domain. The clinical study of LLF580 comprised 61 obese participants divided into two groups and treated with 300 mg LLF580 or placebo via once-month subcutaneous injections for three months [199]. LLF580 is beneficial in improving liver fat and injury, suggesting its potential to treat NAFLD. Notably, compared with other FGF21-based drugs administered once weekly, the administration frequency of LLF580 is once monthly, which provides additional patient convenience. However, this durable pharmacology was under a very high 300-mg single dose, and the cost-effective dose should be further investigated. In addition, GLP-1 and FGF21 dual agonists have also been studied in the treatment of NASH in recent years in HFD-induced obese mice [135].
4.4 Metabolic syndrome (MS)
MS is a series of risk symptoms for CVD and T2DM, including dyslipidemia, hypertension, hyperglycemia, and other metabolic disorders [200]. The core of MS is insulin resistance. FGF21 has been identified as an insulin-dependent postprandial hormone [98] and a metabolic regulator that can improve lipid and glucose homeostasis [107]. The levels of FGF21 in MS patients have been observed in many studies. Serum levels of FGF21 are positively associated with MS in patients with T2DM [201], and serum FGF21 levels in MS patients are higher than those in healthy volunteers after 12 weeks of supervised exercise [202]. In addition, one American multiethnic longitudinal study investigated the role of FGF21 in accessing MS incidents, finding that people with higher FGF21 levels tend to develop MS [203].
Current studies show that FGF21 was a repressor of adipocyte lipolysis in both mice and humans, and treatment with FGF21 can reduce free fatty acids [204, 205]. However, the glucose-lowering effects of FGF21 demonstrated in humans were attenuated compared with those in mice, which may account for the lack of BAT in humans compared with rodents. BAT is the target tissue of FGF21 that conducts acute insulin sensitization [206]. Circulating lipid profile improvement caused by FGF21 does not accompany improvement in glycemic control, which reminds us to reshape the view of FGF21 as prevention instead of cure. For instance, FGF21 analogs can be used in obesity before T2D develops to improve FGF21 responsiveness and maintain insulin sensitivity in the longer term. FGF21 can be used with other current glycemic control therapies to compensate for its shortage and better exert its metabolic regulation effects.
4.5 Other metabolic diseases
Apart from the abovementioned metabolic disease, FGF21 is associated with other metabolic disorders or metabolic states. Plasma FGF21 is elevated in inherited metabolic diseases such as mitochondrial disorders in both mice and humans [207]. In addition, in the acromegaly population, increased FGF21 levels are independently associated with the state of acromegaly [208]. Moreover, plasma FGF21 can predict the development of metabolic comorbidities in patients with other disorders. For example, the serum FGF21 levels in acute Kawasaki disease patients are evaluated to predict the development of coronary artery lesions [209]. FGF21 is used for psoriasis patients to predict the risk of cardiometabolic comorbidities [210]. FGF21 may also monitor the metabolic abnormalities induced by antiretroviral drugs in HIV infection patients [211] and psychotropic drugs in bipolar mania patients [212].
5. FGF21 in CVMD: a mechanistic overview
5.1 Reduction of cardiometabolic risk factors
FGF21 is a novel metabolic regulator of energy homeostasis. It was first recognized because of reduced plasma glucose and triglycerides in obese mice [213]. Subsequently, an increasing number of mechanisms by which FGF21 reduces cardiometabolic risk factors have been investigated. Table 1 shows the role of FGF21-based drugs in CVMDs. For metabolic disease, glucose and lipid profiles are fundamental factors in maintaining a healthy metabolism, which can be improved by FGF21 [26, 130, 135, 138]. Studies that investigated T2DM and FGF21 found that FGF21 can improve glucose homeostasis-related risk factors, such as glycated hemoglobin glycosylated hemoglobin A1c [142], insulin [97], and insulin resistance [108]. For obese animals and patients, preclinical and clinical trials have indicated the effect of FGF21 on improving the serum lipid profile and reducing body weight [173-175]. Regarding CVD, FGF21 can reduce the risk factors that influence the pathologic process of CVD, such as atherosclerosis [214], arterial stiffness [113], and blood pressure [86].
5.2 Molecular targets of FGF21
FGF21 improves CVMD by modulating multiple signaling pathways related to lipid and glucose metabolism (Table 2). The major molecular targets of FGF21 against CVMD are AMPK, SIRT1, autophagy-related molecules, and gut microbiota-related molecules.
Molecular targets of FGF21 in different cell types in CVMD
| Cell type | Molecular targets | Observed effects | References |
|---|---|---|---|
| Endothelial cells | FGF21-SIRT1 | Endothelial senescence↓ | [48] |
| FGF21-TET2-UQCRC1-ROS | Ox-LDL-induced HUVEC pyroptosis ↓ | [49] | |
| FGF21-Fas | HUVEC apoptosis ↓ | [50] | |
| FGF21-NFkappaB | Atheromatous plaques ↓ | [52] | |
| FGF21-CaMKK2-AMPKα | Antioxidative; dilate the aorta | [114] | |
| Macrophage | FGF21-NFkappaB; FGF21-RACK1-autophagy; FGF21-LKB1-AMPK-ACC1 | Foam cells formation and macrophage migration ↓; synthesis of TG ↓ | [53,54,26] |
| Smooth muscle cells | FGF21-Syk-NLRP3 inflammasome | Proliferation and migration ↓ | [58] |
| Cardiomyocytes | FGF21-SIRT1-LKB1; FoxO1; FGF21-Ucp2/3; Sod2 | ROS accumulation and cardiomyocyte apoptosis ↓ | [83,84] |
| FGF21-microRNA-143-EGR1-NaV1.5/Kir2.1; FGF21-NaV1.5/Kir2.1 | Ventricular arrhythmias in post-infarcted hearts ↓ | [70,71] | |
| FGF21-AMPK-Akt2-Nrf2; FGF21-AMPK-ACC | Antioxidative effect; lipid-lowering effect | [110] | |
| FGF21-PI3K-Akt; FGF21-ALCAT1 | Oxidative stress and apoptosis ↓ | [68] | |
| FGF21-EGR1; FGF21-galectin-3 | Collagen synthesis and inflammation after myocardial infarction ↓; pathological cardiac remodeling ↓ | [67,74] | |
| FGF21-Beclin1/Vps34-autophagy; FGF21-microRNA-145/ Angpt2/autophagy | Hypoxia/reoxygenation injury ↓ | [73,72,75] | |
| FGF21-autophagy | Cardiac steatosis ↓ | [82] | |
| Cardiac fibroblasts | FGF21-TGF-β1-Smad2/3-MMP2/9 | Cell apoptosis and collagen production ↓ | [69] |
| Beta cells | FGF21-ERK1/2; FGF21-Akt; FGF21-PI3K-Akt; FGF21-AMPK-ACC; FGF21-PPARδ/γ | Beta-cell survival and insulin secretion ↑ | [40,97,99] |
| FGF21-AMPK-mTOR | Islet autophagy ↑ | [100] | |
| Adipocytes | FGF21-ERK1/2-CCL11; FGF21-acetylcholine-acetylcholine receptor | Beiging of white adipose tissue ↑; anti-inflammatory effects ↑ | [38,166,172] |
| FGF21-adiponectin | Neointima formation and macrophage inflammation ↓; insulin resistance ↓ | [60,76,104,105] | |
| FGF21-NF-kappaB-TNF-α | Inflammation ↓; glucose uptake ↑ | [108] | |
| Hepatocyte | FGF21-hepatic sterol regulatory element-binding protein-2 | Cholesterol synthesis ↓ | [60] |
| FGF21-mTORC1 | Hepatic insulin resistance ↓; glycogen synthesis ↑; liver steatosis ↓ | [103,185] | |
| FGF21-JMJD3 histone demethylase | Hepatic autophagy and lipid degradation ↑ | [187] | |
| FGF21-hepatic six transmembrane protein of prostate 2 | Hepatic steatosis and insulin resistance ↓ | [188] | |
| FGF21-TLR4-IL-17A | Prevent nonalcoholic steatohepatitis- hepatocellular carcinoma transition | [191] |
Abbreviations: Fibroblast growth factor 21 (FGF21), silent information regulator 1 (SIRT1), tet methylcytosine dioxygenase (TET2), ubiquinol cytochrome c reductase core protein I (UQCRC1), reactive oxygen species (ROS), low density lipoprotein (LDL), human umbilical vein endothelial cell (HUVEC), nuclear factor kappa light chain enhancer of activated B cells (NF-κappaB), calcium/calmodulin-dependent protein kinase kinase 2 (CaMKK2, also known as CaMKKβ), adenosine 5'-monophosphate -activated protein kinase (AMPK), Activated kinase C receptor 1 (RACK1), liver kinase B1 (LKB1), acetyl coenzyme A carboxylase (ACC), triglyceride (TG), spleen tyrosine kinase (Syk), NOD-, LRR- and pyrin domain-containing 3 (NLRP3), forkhead box protein O1 (FoxO1), mitochondrial uncoupling proteins (Ucp2 and Ucp3), MnSOD (Sod2), early growth response protein 1 (EGR1), voltage-gated sodium channels 1.5 (NaV1.5), potassium inwardly-rectifying channels 2.1 (Kir2.1), nuclear transcription factor-E2-related factor 2 (Nrf2), phosphatidylinositol 3-kinase (PI3K), lysocardiolipinacyltransferase-1 (ALCAT1), vacuolar protein sorting 34 (Vps34), angiopoietin-2 (Angpt2), transforming growth factor-β1 (TGF-β1), drosophila mothers against decapentaplegic protein (Smad), matrix metalloproteinase (MMP), extracellular regulated protein kinases (ERK), peroxisome proliferators-activated receptors (PPAR), C-C Motif Chemokine Ligand 11 (CCL11), tumour necrosis factor-α (TNFα), mammalian target of rapamycin (mTOR), jumonji domain containing-3 (JMJD3), toll-like receptor 4 (TLR4), interleukin-17A (IL-17A).
An important molecular target of FGF21 involved in the cardiometabolic protective effects is AMPK, a decisive energy metabolism regulator [215]. AMPK-mediated signaling pathways can regulate many cell activities related to cardiometabolic processes. In endothelial cells, FGF21 can mediate the FGF21-calcium/calmodulin-dependent protein kinase 2-AMPKα pathway to exert antioxidative effects [114]. Moreover, the FGF21-LKB1-AMPK-ACC1 pathway can prohibit the synthesis of triglycerides in macrophages, which further prohibits foam cell formation [26]. AMPK-related antioxidative effects and lipid-lowering effects can also be observed in cardiomyocytes via the FGF21-AMPK-Akt2-Nrf2 pathway and FGF21-AMPK-ACC pathway [110], respectively. The AMPK-mediated cell activities mentioned above can inhibit the pathological process of atherosclerosis and consequently CVD. In addition, AMPK-mediated signaling pathways also influence the pathological process of metabolic disease. For example, in mice, the FGF21-AMPK-ACC pathway can increase beta cell survival and insulin secretion [97], and the FGF21-AMPK-mTOR pathway [100] can increase islet autophagy. Increased beta cells and improved beta cell function can regulate the serum glucose level and the process of T2DM.
Apart from the effect on AMPK, one in vitro study indicated that FGF21 can target SIRT to inhibit inflammatory oxidative stress in CVMD [216]. Inflammatory oxidative stress and related cell death are essential in the development of atherosclerosis. The FGF21-SIRT1 pathway can prohibit reactive oxygen species accumulation in cardiomyocytes, reducing apoptosis [48]. Consistently, FGF21 can reduce endothelial senescence by targeting SIRT1 [83].
Under nutrient deprivation, autophagy is essential for energy homeostasis and cellular survival, and studies on FGF21 mediating autophagy are increasing. FGF21 mediates autophagy-induced cholesterol efflux in macrophages to inhibit macrophages from becoming foam cells by increasing activated kinase C receptor 1 in apoE-/- mice [54]. FGF21-induced autophagy can also alleviate H/R injury and cardiac steatosis in cardiomyocytes [73]. In addition, islet autophagy and hepatic autophagy are essential to maintaining glucose and lipid homeostasis. FGF21 can mediate islet autophagy via the FGF21-AMPK-mTOR pathway in mice [100], and the effect of FGF21 on hepatic steatosis and insulin resistance in mice occurs via the FGF21-JMJD3 histone demethylase pathway [187].
The gut microbiota is another target by which FGF21 can reshape host inflammation and metabolic health. Many studies have reported that gut microbiota alterations are related to FGF21 levels. In a randomized controlled-feeding trial that enrolled 117 overweight adults, the results showed that fried meat consumption induced gut microbiota and metabolism changes that were associated with changes in FGF21 [217]. Similar results were also observed in gut microbiota transplantation trials. One study transplanted the gut microbiota into mice and found that the increase in gut butyrate is associated with elevated FGF21 in transplanted mice [218]. Another study used microbiota transplantation to treat human recurrent clostridioides difficile infection and observed gut microbiota and bile acid profile restoration associated with an upregulated bile acid-farnesoid X receptor-FGF pathway [219]. The FGF21-β-hydroxybutyrate-NLRP3 axis is another pathway in the gut microbiota. FGF21 can stimulate ketogenesis in the liver. Hepatic β-oxidation of fatty acids produces β-hydroxybutyrate, which further inhibits NLRP3 and modulates gut inflammation [220].
6. Summary of FGF21 in CVMD
6.1 FGF21: as a biomarker for metabolic and cardiovascular diseases
Although the FGF21 test is not a routine and standard test in hospital laboratories, current studies have observed that FGF21 is upregulated in metabolic and other disorders and realized its potential value as a diagnostic and prognostic biomarker for metabolic diseases. As mentioned earlier, FGF21 is considered a biomarker for predicting the presence of T2DM. In addition, FGF21 has the potential to assess the severity of the disease and predict long-term prognosis. For example, a lower level of FGF21 predicts a better prognosis in DM patients. FGF21 levels correlate with the severity of steatohepatitis, which can help identify patients with NASH at risk of disease progression [181]. Compared to the traditional biomarker used in clinical laboratories, FGF21 still showed potential. One study indicated that FGF21 seems superior to other adipokines and can be recommended as an alternative to the glucose tolerance test [93].
The role of FGF21 as a biomarker in CVD is controversial. In some clinical studies, FGF21 is considered a biomarker for predicting the presence of atherosclerosis and cardiovascular events [41-47]. One study also showed that the identification effect of FGF21, which identifies left ventricular systolic function and cardiac death, was not inferior to serum N-terminal pro-brain natriuretic peptide in patients [221]. In addition, mechanistic studies revealed that FGF21 can act directly on the cardiovascular system and may be used as an early biomarker of CVD. FGF21 can induce cardiac protection by preventing cardiac lipotoxicity, oxidative stress, and inflammation by directly binding to the FGFR of the heart [222]. However, in another multiethnic clinical study, FGF21 was not a CVD biomarker in atherosclerosis patients without known CVD [44]. Previous studies also showed that cardiac protection may contribute to the beneficial effects of FGF21 as a metabolic regulator, which can regulate systemic glucose-lipid metabolism disturbance, the key risk factor for CVD [3]. Moreover, although FGF21 can be produced by the heart and act on the heart in an autocrine way, circulating levels of FGF21 are now known to be derived primarily from the liver [13]. Therefore, the circulating levels of FGF21 should not directly reflect the CVD-related pathophysiological process and should be used as a biomarker of CVD.
In summary, FGF21 is a valuable biomarker for metabolic diseases and deserves further study to translate the biomarker potential into clinical laboratories. Regarding the role of FGF21 as a biomarker in CVD, more studies are needed to investigate the relationship between the effect of FGF21 on the systemic and the heart, and more careful thoughts are needed on the meaning of circulating liver-derived FGF21 as a biomarker in CVD.
6.2 Preclinical and clinical trials of FGF21 in CVMD
Preclinical studies about the role of FGF21 in CVMD are summarized in Table 3. As mentioned before, FGF21 can improve the development of CVMDs, including atherosclerosis, CAD, MI, H/R injury, HF, T2DM, obesity, and NASH, in vivo and in vitro. Moreover, clinical trials of FGF21-based drugs are mainly performed on patients with NAFLD/NASH and related hypertriglyceridaemia, which are summarized in Table 4. In addition to pegbelfermin [196] and LLF580 [199], BIO89-100 is another novel FGF21-based drug that was presented recently. The impact of BIO89-100 on reducing liver fat and lowering triglycerides is still under further investigation.
Role of FGF21 in cardiometabolic disease in preclinical studies
| Animal models | Recombinant human FGF21 | Observed effects | References |
|---|---|---|---|
| apoE-/- mice | 1mg/kg/d, s.c., 14wk; 10mg/kg/d, s.c., 12wk; 0.1 mg/kg, i.p., 5wk | Anti-atherosclerotic effect | [51,54,61] |
| HFD induced- atherosclerosis mice | 6mg/kg/d, i.v., 40d | Anti-atherosclerotic effect | [52] |
| APOE*3-Leiden.CETP mice | 1mg/kg/times, 3 times/w, s.c., 16wk | Anti-atherosclerotic effect | [62] |
| SIRT1-iKO mice | 2.5mg/kg/d, i.p., 4wk | Ang II-induced cardiac hypertrophy ↓ | [83] |
| MI rats; FGF21 KO MI rats | 7μg/kg/d, i.p., 4wk; 1×109 pfu/mouse, i.m.; 1 μg/kg, 5 μg/kg, and 10 μg/kg, i.v. | Postinfarcted inflammation; fibrosis; arrhythmias ↓ | [67,76,71] |
| Wistar rat myocardial I/R injury model | 0.1 mg/kg/d, i.p., 4wk | Myocardial ischemia-reperfusion injury ↓ | [72] |
| FGF21-KO mice | 0.1 mg/kg/d, osmotic pump, 4wk | Restores subcutaneous adipose tissue mass and reverses insulin resistance | [104] |
| Obesity mice | 1 mg/kg/d, i.p., 4wk | Insulin resistance ↓ | [105] |
| Streptozotocin-treated mice | 5mg/kg, twice/w, s.c. | Normalizes blood glucose without improving β-cell function | [101] |
| 10mg/kg, twice/w, i.p., 4wk | Improve diabetic retinopathy | [122] | |
| Db/db mice | 1 mg/kg/d, i.p., 5wk; FGF21-FGF19 chimera: 0.6 mg/kg/d, s.c., 7d | Improving hyperglycemic and alleviating inflammation | [108,129] |
| 1.5mg/kg, twice/d, s.c., 2wk | Improves neurological outcomes following focal ischemic stroke | [117] | |
| 1mg/kg/w, s.c., 2wk | Therapeutic potential for diabetes and NASH | [135] | |
| T2DM rats | Injected the livers with lentivirus containing hFGF21 (50-μL volume with a titer of 5×109 viral particles/ml) | Liver lipid droplet content ↓; insulin sensitivity ↑; glycogen synthesis ↑ | [131] |
| Insulin-resistant BTBR mice | FGF21 gene therapy targeted visceral adipose tissue | Insulin sensitivity ↑; glycemic processing ↑; body weight ↓; hepatic steatosis ↓ | [130] |
| T2DM mouse model | FGF21 and GLP1 modified mesenchymal stem cells transplantation | Lipid metabolism ↑; blood glucose ↓ | [138] |
| T2DM cynomolgus monkeys | 1mg/kg/d, s.c., 4wk | Blood glucose ↓ | [128] |
| Obese Göttingen minipigs | 0.1 mg/kg/d for 9.5 weeks and 0.3 mg/kg/d for 4.5 weeks, s.c., 14wk | Food intake ↓; body weight ↓ | [157] |
| Db/db mice | Recombinant Lactococcus lactis NZ3900 expressing bioactive human FGF21: 200 μl/mice/d, p.o., 13wk | Body weight ↓ | [173] |
| Db/db mice; ob/ob mice | GLP-1 and FGF21 dual agonist: 1000nmol/kg; 0.5-1mg/kg | Body weight ↓; blood glucose ↓; NAFLD activity score ↓ | [136,135] |
| Diet-induced obesity mouse | Long-acting FGF21: 75nmol/kg | Body weight ↓; blood glucose ↓ | [175] |
| 0.6mg/kg/d, s.c., 18d | Improve liver steatosis and inflammation | [189] | |
| Leptin-deficient ob/ob mice | 5mg/kg, i.p., 3wk | Prevent NASH | [190] |
| FGF21-KO mice | Unknown dose, s.c., 4wk | Liver steatosis ↓; peroxidative damage ↓ | [30] |
| Cynomolgus monkeys with NAFLD | 0.5-2mg/kg, 1-2times/w, s.c., 11wk | Hepatic steatosis, inflammation, and fibrosis ↓ | [198] |
| Obesity, T2DM, HF, and renal failure mouse models | Gene therapies based on 3 longevity associated genes included FGF21 | Improve all 4 diseases | [87] |
Abbreviations: subcutaneous injection (s.c.); intravenous injection (i.v.); intraperitoneal injection (i.p.); intramuscular injection (i.m.); per os (p.o.); apolipoprotein E-/- (apoE-/-); high-fat diet (HFD); silent information regulator 1 (SIRT1); myocardial infarction (MI); ischemia/reperfusion (I/R); Angiotensin II (Ang II); nonalcoholic fatty liver disease (NAFLD); nonalcoholic steatohepatitis (NASH); type 2 diabetes (T2DM); heart failure (HF).
Clinical trials involving FGF21
| FGF21 | Condition or disease | Clinical trial ID | Outcome | References |
|---|---|---|---|---|
| Pegbelfermin | Obesity and T2DM | NCT02097277 | HbA1c -; high-density lipoprotein ↓; triglyceride ↓; adiponectin ↑ | [126] |
| Pegbelfermin | Obesity and NAFLD | NCT02413372 | Hepatic fat fraction↓ | [195] |
| Pegbelfermin | FALCON 1: NASH with stage 3 liver fibrosis; FALCON 2: NASH with compensated cirrhosis | FALCON 1: NCT03486899; FALCON 2: NCT03486912 | Improvement in fibrosis | [196] |
| Efruxifermin | NASH | NCT03976401 | Hepatic fat fraction↓ | [194] |
| LLF580 | Obese adults with modest hypertriglyceridemia | NCT03466203 | Triglycerides and hepatic fat↓ | [199] |
| BIO89-100 | Severe hypertriglyceridemia | NCT04541186 | Improvement in serum triglycerides | [241] |
Abbreviations: nonalcoholic fatty liver disease (NAFLD); nonalcoholic steatohepatitis (NASH); type 2 diabetes (T2DM); glycosylated haemoglobin A1c (HbA1c).
7. Conclusions and outlook
In the past decade, a great stride has been made in our understanding of the pivotal role of FGF21 in CVMD, the leading cause of death worldwide. Regardless of the molecular mechanisms or clinical applications, there are many contradictions regarding FGF21, which increases the difficulty of successful clinical application. First, the pharmacological effects of FGF21 are species specific. The most significant difference is that the glucose-lowering effects of FGF21 analogs in humans are attenuated compared with those in mice [126, 129], which may account for the lack of BAT in humans compared with rodents [206]. Another controversial impact of FGF21 is bone loss. Mouse studies have suggested that FGF21 promotes bone loss, but this effect in humans is conflicting [223, 224]. Second, the pharmacologic and physiological effects of FGF21 are different under different stimuli, tissue sources, and experimental models. For example, both fasting and feeding can induce the production of FGF21 [9]. FGF21 can decrease the preference for high-calorie foods [159] as well as increase appetite [162]. Moreover, FGF21 is significantly elevated in obesity and DM, which may be attributed to 'FGF21 resistance' [225]. However, obese rodents and humans in this potentially FGF21-resistant state can still respond to FGF21 at pharmacological levels [226]. Contradictions on FGF21 suggest that the metabolic balance mediated by FGF21 is dynamic and involves multiorgan crosstalk instead of one-dimensional crosstalk [227]. The nervous system plays an essential role in coordinating complex and dynamic FGF21-mediated responses. Identification of the neural circuits related to FGF21 may help to better understand the nuanced regulatory effects of FGF21 and will be an important future direction [228]. Further investigations related to this 'contradictory molecule' are needed to promote the application of FGF21.
Regarding the clinical application of FGF21, the focus of FGF21 clinical trials is on NAFLD/NASH based on its powerful lipid profile benefits, which have been processed in phase 2b studies [196]. However, a study on FGF21 clinical application did not meet the primary endpoints of glycemic control [126], suggesting that further studies are warranted to utilize FGF21 as a stand-alone medication for T2DM. Actually, FGF21 can act as a 'sensitizer' instead of a 'regulator' in CVMD. Combining FGF21 and clinical antidiabetic drugs is a potent therapeutic in T2DM [132]. In addition, FGF21 administration methods are continuously being developed to overcome the poor pharmacokinetics of natural FGF21, such as FGF21 gene therapy and tissue target therapy [130]. In addition to FGF21 analogs, FGF21-based drugs, such as FGF21 receptor agonists, FGF21-degrading protease inhibitors, and FGF21 transcription factor activators, have also been investigated (Figure 3B) [229]. Notably, beyond the clinical application of FGF21-based drugs, FGF21 has great potential as a biomarker for metabolic disease diagnosis and prognosis.
In conclusion, the performance of FGF21 between pharmacology and physiology as well as among different species is obviously different. Considering the complexity and heterogeneity of FGF21, personalized FGF21-based and combination pharmacotherapies used with other current metabolic therapies should be given more attention in the future.
Abbreviations
CVMD: cardiovascular and metabolic disease; FGF21: fibroblast growth factor 21; CVD: cardiovascular diseases; MI: myocardial infarction; HF: heart failure; DM: diabetes mellitus; MS: metabolic syndrome; FGF: fibroblast growth factor; FGFR: FGF receptor; KLB: β-Klotho; BAT: brown adipose tissue; PPAR: peroxisome proliferators-activated receptors; BBB: blood-brain barrier; WAT: white adipose tissue; Ucp1: uncoupling protein 1; CAD: coronary artery disease; T2DM: type 2 diabetes mellitus; NAFLD: nonalcoholic fatty liver disease; apoE-/-: apolipoprotein E-/-; rhFGF21: recombinant human FGF21; HUVECs: human umbilical vein endothelial cells; SIRT1: silent information regulator 1; NLRP3: NOD-, LRR- and pyrin domain-containing 3; NF-κappaB: the nuclear factor kappa light chain enhancer of the activated B-cell; AMPK: adenosine 5'-monophosphate-activated protein kinase; HFD: high-fat diet; AP: angina pectoris; PI3K: phosphatidylinositol 3-kinase; Akt: protein kinase B; I/R: Ischemia/reperfusion; HFrEF: HF with reduced ejection fraction; HFpEF: HF with preserved ejection fraction; ACC: Acetyl coenzyme A carboxylase; mTOR: mammalian target of rapamycin; Nrf2: nuclear transcription factor-E2-related factor 2; GLP-1: glucagon-like peptide-1; LKB1: liver kinase B1; LSG: Laparoscopic sleeve gastrectomy; JMJD3: Jumonji domain containing-3; MS: Metabolic syndrome.
Acknowledgements
This study was supported by grants from National Key R&D Program of China (No. 2021YFC2500500), National Natural Science Foundation of China (Grant No's. 82070464, 20 81941022, 81530025) and Strategic Priority Research Program of Chinese Academy of Sciences (Grant No. XDB38010100). This work was also supported by Program for Innovative Research Team of The First Affiliated Hospital of USTC (CXGG02), Anhui Provincial Key Research and Development Program (Grant No. 202104j07020051), Anhui Province Science Fund for Distinguished Young Scholars (Grant No. 2208085J08), Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (Grant No. 2017BT01S131).
Author contributions
Conceptualization: S.X. and J.W.; Writing: H.T.; Revision: S.X., J.W., Z.C., W.W., T.Y.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Saklayen MG. The Global Epidemic of the Metabolic Syndrome. Curr Hypertens Rep. 2018;20:12
2. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW. et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation. 2021;143:e254-e743
3. Cheng P, Zhang F, Yu L, Lin X, He L, Li X. et al. Physiological and Pharmacological Roles of FGF21 in Cardiovascular Diseases. J Diabetes Res. 2016;2016:1540267
4. Geng L, Lam KSL, Xu A. The therapeutic potential of FGF21 in metabolic diseases: from bench to clinic. Nat Rev Endocrinol. 2020;16:654-67
5. Fisher FM, Maratos-Flier E. Understanding the Physiology of FGF21. Annu Rev Physiol. 2016;78:223-41
6. Dolegowska K, Marchelek-Mysliwiec M, Nowosiad-Magda M, Slawinski M, Dolegowska B. FGF19 subfamily members: FGF19 and FGF21. J Physiol Biochem. 2019;75:229-40
7. Li X. The FGF metabolic axis. Front Med. 2019;13:511-30
8. Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell metabolism. 2007;5:426-37
9. Uebanso T, Taketani Y, Yamamoto H, Amo K, Ominami H, Arai H. et al. Paradoxical regulation of human FGF21 by both fasting and feeding signals: is FGF21 a nutritional adaptation factor? PloS one. 2011;6:e22976
10. Fazeli PK, Lun M, Kim SM, Bredella MA, Wright S, Zhang Y. et al. FGF21 and the late adaptive response to starvation in humans. J Clin Invest. 2015;125:4601-11
11. Kim KH, Jeong YT, Oh H, Kim SH, Cho JM, Kim YN. et al. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nature medicine. 2013;19:83-92
12. Geller S, Arribat Y, Netzahualcoyotzi C, Lagarrigue S, Carneiro L, Zhang L. et al. Tanycytes Regulate Lipid Homeostasis by Sensing Free Fatty Acids and Signaling to Key Hypothalamic Neuronal Populations via FGF21 Secretion. Cell Metab. 2019;30:833-44.e7
13. Markan KR, Naber MC, Ameka MK, Anderegg MD, Mangelsdorf DJ, Kliewer SA. et al. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes. 2014;63:4057-63
14. De Sousa-Coelho AL, Marrero PF, Haro D. Activating transcription factor 4-dependent induction of FGF21 during amino acid deprivation. Biochem J. 2012;443:165-71
15. Schaap FG, Kremer AE, Lamers WH, Jansen PL, Gaemers IC. Fibroblast growth factor 21 is induced by endoplasmic reticulum stress. Biochimie. 2013;95:692-9
16. Wan XS, Lu XH, Xiao YC, Lin Y, Zhu H, Ding T. et al. ATF4- and CHOP-dependent induction of FGF21 through endoplasmic reticulum stress. Biomed Res Int. 2014;2014:807874
17. Lundsgaard AM, Fritzen AM, Sjoberg KA, Myrmel LS, Madsen L, Wojtaszewski JFP. et al. Circulating FGF21 in humans is potently induced by short term overfeeding of carbohydrates. Mol Metab. 2017;6:22-9
18. Iroz A, Montagner A, Benhamed F, Levavasseur F, Polizzi A, Anthony E. et al. A Specific ChREBP and PPARalpha Cross-Talk Is Required for the Glucose-Mediated FGF21 Response. Cell Rep. 2017;21:403-16
19. Morita M, Siddiqui N, Katsumura S, Rouya C, Larsson O, Nagashima T. et al. Hepatic posttranscriptional network comprised of CCR4-NOT deadenylase and FGF21 maintains systemic metabolic homeostasis. Proc Natl Acad Sci U S A. 2019;116:7973-81
20. Wu H, Liu Y, Chen X, Zhu D, Ma J, Yan Y. et al. Neohesperidin Exerts Lipid-Regulating Effects in vitro and in vivo via Fibroblast Growth Factor 21 and AMP-Activated Protein Kinase/Sirtuin Type 1/Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1¦Á Signaling Axis. Pharmacology. 2017;100:115-26
21. Fruchart JC. Pemafibrate (K-877), a novel selective peroxisome proliferator-activated receptor alpha modulator for management of atherogenic dyslipidaemia. Cardiovasc Diabetol. 2017;16:124
22. Zhang Q, Zhu Q, Deng R, Zhou F, Zhang L, Wang S. et al. MS-275 induces hepatic FGF21 expression via H3K18ac-mediated CREBH signal. Journal of molecular endocrinology. 2019;62:187-96
23. Joe Y, Kim S, Kim HJ, Park J, Chen Y, Park HJ. et al. FGF21 induced by carbon monoxide mediates metabolic homeostasis via the PERK/ATF4 pathway. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2018;32:2630-43
24. Katsumura S, Siddiqui N, Goldsmith MR, Cheah JH, Fujikawa T, Minegishi G. et al. Deadenylase-dependent mRNA decay of GDF15 and FGF21 orchestrates food intake and energy expenditure. Cell Metab. 2022;34:564-80 e8
25. Osataphan S, Macchi C, Singhal G, Chimene-Weiss J, Sales V, Kozuka C. et al. SGLT2 inhibition reprograms systemic metabolism via FGF21-dependent and -independent mechanisms. JCI Insight. 2019 4
26. Zhang N, Liu C, Zhang Y, Xu D, Gui L, Lu Y. et al. Liraglutide regulates lipid metabolism via FGF21- LKB1- AMPK- ACC1 pathway in white adipose tissues and macrophage of type 2 diabetic mice. Biochem Biophys Res Commun. 2021;548:120-6
27. Kim KH, Jeong YT, Kim SH, Jung HS, Park KS, Lee HY. et al. Metformin-induced inhibition of the mitochondrial respiratory chain increases FGF21 expression via ATF4 activation. Biochem Biophys Res Commun. 2013;440:76-81
28. Dunshee DR, Bainbridge TW, Kljavin NM, Zavala-Solorio J, Schroeder AC, Chan R. et al. Fibroblast Activation Protein Cleaves and Inactivates Fibroblast Growth Factor 21. J Biol Chem. 2016;291:5986-96
29. Yang C, Jin C, Li X, Wang F, McKeehan WL, Luo Y. Differential specificity of endocrine FGF19 and FGF21 to FGFR1 and FGFR4 in complex with KLB. PLoS One. 2012;7:e33870
30. Fisher FM, Chui PC, Nasser IA, Popov Y, Cunniff JC, Lundasen T. et al. Fibroblast growth factor 21 limits lipotoxicity by promoting hepatic fatty acid activation in mice on methionine and choline-deficient diets. Gastroenterology. 2014;147:1073-83 e6
31. Xiao M, Tang Y, Wang S, Wang J, Wang J, Guo Y. et al. The Role of Fibroblast Growth Factor 21 in Diabetic Cardiovascular Complications and Related Epigenetic Mechanisms. Front Endocrinol (Lausanne). 2021;12:598008
32. Hsuchou H, Pan W, Kastin AJ. The fasting polypeptide FGF21 can enter brain from blood. Peptides. 2007;28:2382-6
33. Owen BM, Bookout AL, Ding X, Lin VY, Atkin SD, Gautron L. et al. FGF21 contributes to neuroendocrine control of female reproduction. Nat Med. 2013;19:1153-6
34. Keipert S, Ost M, Johann K, Imber F, Jastroch M, van Schothorst EM. et al. Skeletal muscle mitochondrial uncoupling drives endocrine cross-talk through the induction of FGF21 as a myokine. Am J Physiol Endocrinol Metab. 2014;306:E469-82
35. Lindegaard B, Hvid T, Grondahl T, Frosig C, Gerstoft J, Hojman P. et al. Expression of fibroblast growth factor-21 in muscle is associated with lipodystrophy, insulin resistance and lipid disturbances in patients with HIV. PLoS One. 2013;8:e55632
36. Planavila A, Redondo I, Hondares E, Vinciguerra M, Munts C, Iglesias R. et al. Fibroblast growth factor 21 protects against cardiac hypertrophy in mice. Nat Commun. 2013;4:2019
37. Keipert S, Lutter D, Schroeder BO, Brandt D, Stahlman M, Schwarzmayr T. et al. Endogenous FGF21-signaling controls paradoxical obesity resistance of UCP1-deficient mice. Nat Commun. 2020;11:624
38. Huang Z, Zhong L, Lee JTH, Zhang J, Wu D, Geng L. et al. The FGF21-CCL11 Axis Mediates Beiging of White Adipose Tissues by Coupling Sympathetic Nervous System to Type 2 Immunity. Cell Metab. 2017;26:493-508 e4
39. Johnson CL, Weston JY, Chadi SA, Fazio EN, Huff MW, Kharitonenkov A. et al. Fibroblast growth factor 21 reduces the severity of cerulein-induced pancreatitis in mice. Gastroenterology. 2009;137:1795-804
40. Wente W, Efanov AM, Brenner M, Kharitonenkov A, Koster A, Sandusky GE. et al. Fibroblast growth factor-21 improves pancreatic beta-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes. 2006;55:2470-8
41. Ong KL, Hui N, Januszewski AS, Kaakoush NO, Xu A, Fayyad R. et al. High plasma FGF21 levels predicts major cardiovascular events in patients treated with atorvastatin (from the Treating to New Targets [TNT] Study). Metabolism. 2019;93:93-9
42. Gan F, Huang J, Dai T, Li M, Liu J. Serum level of fibroblast growth factor 21 predicts long-term prognosis in patients with both diabetes mellitus and coronary artery calcification. Annals of palliative medicine. 2020;9:368-74
43. Lee CH, Woo YC, Chow WS, Cheung CYY, Fong CHY, Yuen MMA. et al. Role of Circulating Fibroblast Growth Factor 21 Measurement in Primary Prevention of Coronary Heart Disease Among Chinese Patients With Type 2 Diabetes Mellitus. Journal of the American Heart Association. 2017 6
44. Ong KL, Campbell S, Wu BJ, McClelland RL, Kokkinos J, Szklo M. et al. Relationship of fibroblast growth factor 21 with subclinical atherosclerosis and cardiovascular events: Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2019;287:46-53
45. Wu L, Qian L, Zhang L, Zhang J, Zhou J, Li Y. et al. Fibroblast Growth Factor 21 is Related to Atherosclerosis Independent of Nonalcoholic Fatty Liver Disease and Predicts Atherosclerotic Cardiovascular Events. Journal of the American Heart Association. 2020;9:e015226
46. Wang JS, Sheu WH, Lee WJ, Lee IT, Lin SY, Lee WL. et al. Associations of fibroblast growth factor 21 with cardiovascular risk and beta-cell function in patients who had no history of diabetes. Clinica chimica acta; international journal of clinical chemistry. 2017;472:80-5
47. Basurto L, Gregory MA, Hernández SB, Sánchez-Huerta L, Martínez AD, Manuel-Apolinar L. et al. Monocyte chemoattractant protein-1 (MCP-1) and fibroblast growth factor-21 (FGF-21) as biomarkers of subclinical atherosclerosis in women. Exp Gerontol. 2019;124:110624
48. Yan J, Wang J, Huang H, Huang Y, Mi T, Zhang C. et al. Fibroblast growth factor 21 delayed endothelial replicative senescence and protected cells from H(2)O(2)-induced premature senescence through SIRT1. American journal of translational research. 2017;9:4492-501
49. Chen JJ, Tao J, Zhang XL, Xia LZ, Zeng JF, Zhang H. et al. Inhibition of the ox-LDL-Induced Pyroptosis by FGF21 of Human Umbilical Vein Endothelial Cells Through the TET2-UQCRC1-ROS Pathway. DNA and cell biology. 2020;39:661-70
50. Yan X, Gou Z, Li Y, Wang Y, Zhu J, Xu G. et al. Fibroblast growth factor 21 inhibits atherosclerosis in apoE-/- mice by ameliorating Fas-mediated apoptosis. Lipids in health and disease. 2018;17:203
51. Zeng Z, Zheng Q, Chen J, Tan X, Li Q, Ding L. et al. FGF21 mitigates atherosclerosis via inhibition of NLRP3 inflammasome-mediated vascular endothelial cells pyroptosis. Exp Cell Res. 2020;393:112108
52. Zhang Y, Liu Z, Zhou M, Liu C. Therapeutic effects of fibroblast growth factor21 against atherosclerosis via the NFkappaB pathway. Mol Med Rep. 2018;17:1453-60
53. Wang N, Li JY, Li S, Guo XC, Wu T, Wang WF. et al. Fibroblast growth factor 21 regulates foam cells formation and inflammatory response in Ox-LDL-induced THP-1 macrophages. Biomed Pharmacother. 2018;108:1825-34
54. Xiaolong L, Dongmin G, Liu M, Zuo W, Huijun H, Qiufen T. et al. FGF21 induces autophagy-mediated cholesterol efflux to inhibit atherogenesis via RACK1 up-regulation. J Cell Mol Med. 2020;24:4992-5006
55. Shang W, Yu X, Wang H, Chen T, Fang Y, Yang X. et al. Fibroblast growth factor 21 enhances cholesterol efflux in THP-1 macrophage-derived foam cells. Mol Med Rep. 2015;11:503-8
56. Guo Y, Luo F, Yi Y, Xu D. Fibroblast growth factor 21 potentially inhibits microRNA-33 expression to affect macrophage actions. Lipids Health Dis. 2016;15:208
57. Li E, Wang T, Wang F, Wang T, Sun LQ, Li L. et al. FGF21 protects against ox-LDL induced apoptosis through suppressing CHOP expression in THP1 macrophage derived foam cells. BMC Cardiovasc Disord. 2015;15:80
58. Wei W, Li XX, Xu M. Inhibition of vascular neointima hyperplasia by FGF21 associated with FGFR1/Syk/NLRP3 inflammasome pathway in diabetic mice. Atherosclerosis. 2019;289:132-42
59. Jin L, Lin Z, Xu A. Fibroblast Growth Factor 21 Protects against Atherosclerosis via Fine-Tuning the Multiorgan Crosstalk. Diabetes Metab J. 2016;40:22-31
60. Lin Z, Pan X, Wu F, Ye D, Zhang Y, Wang Y. et al. Fibroblast growth factor 21 prevents atherosclerosis by suppression of hepatic sterol regulatory element-binding protein-2 and induction of adiponectin in mice. Circulation. 2015;131:1861-71
61. Maeng HJ, Lee GY, Bae JH, Lim S. Effect of Fibroblast Growth Factor 21 on the Development of Atheromatous Plaque and Lipid Metabolic Profiles in an Atherosclerosis-Prone Mouse Model. Int J Mol Sci. 2020 21
62. Liu C, Schönke M, Zhou E, Li Z, Kooijman S, Boon MR. et al. Pharmacological treatment with FGF21 strongly improves plasma cholesterol metabolism to reduce atherosclerosis. Cardiovasc Res. 2022;118:489-502
63. Sunaga H, Koitabashi N, Iso T, Matsui H, Obokata M, Kawakami R. et al. Activation of cardiac AMPK-FGF21 feed-forward loop in acute myocardial infarction: Role of adrenergic overdrive and lipolysis byproducts. Sci Rep. 2019;9:11841
64. Cheng J, Su X, Qiao L, Zhai C, Chen W. Circulating level of fibroblast growth factor 21 is independently associated with the risks of unstable angina pectoris. Bioscience reports. 2018 38
65. Chen H, Lu N, Zheng M. A high circulating FGF21 level as a prognostic marker in patients with acute myocardial infarction. Am J Transl Res. 2018;10:2958-66
66. Gu L, Jiang W, Qian H, Zheng R, Li W. Elevated serum FGF21 predicts the major adverse cardiovascular events in STEMI patients after emergency percutaneous coronary intervention. PeerJ. 2021;9:e12235
67. Li J, Gong L, Zhang R, Li S, Yu H, Liu Y. et al. Fibroblast growth factor 21 inhibited inflammation and fibrosis after myocardial infarction via EGR1. Eur J Pharmacol. 2021;910:174470
68. Bo W, Ma Y, Xi Y, Liang Q, Cai M, Tian Z. The Roles of FGF21 and ALCAT1 in Aerobic Exercise-Induced Cardioprotection of Postmyocardial Infarction Mice. Oxid Med Cell Longev. 2021;2021:8996482
69. Ma Y, Kuang Y, Bo W, Liang Q, Zhu W, Cai M. et al. Exercise Training Alleviates Cardiac Fibrosis through Increasing Fibroblast Growth Factor 21 and Regulating TGF-β1-Smad2/3-MMP2/9 Signaling in Mice with Myocardial Infarction. Int J Mol Sci. 2021 22
70. Li J, Xu C, Liu Y, Li Y, Du S, Zhang R. et al. Fibroblast growth factor 21 inhibited ischemic arrhythmias via targeting miR-143/EGR1 axis. Basic Res Cardiol. 2020;115:9
71. Li J, Li Y, Liu Y, Yu H, Xu N, Huang D. et al. Fibroblast Growth Factor 21 Ameliorates Na(V)1.5 and Kir2.1 Channel Dysregulation in Human AC16 Cardiomyocytes. Front Pharmacol. 2021;12:715466
72. Hu S, Cao S, Tong Z, Liu J. FGF21 protects myocardial ischemia-reperfusion injury through reduction of miR-145-mediated autophagy. Am J Transl Res. 2018;10:3677-88
73. Ren Z, Xiao W, Zeng Y, Liu MH, Li GH, Tang ZH. et al. Fibroblast growth factor-21 alleviates hypoxia/reoxygenation injury in H9c2 cardiomyocytes by promoting autophagic flux. Int J Mol Med. 2019;43:1321-30
74. Sun M, Jin L, Bai Y, Wang L, Zhao S, Ma C. et al. Fibroblast growth factor 21 protects against pathological cardiac remodeling by modulating galectin-3 expression. J Cell Biochem. 2019;120:19529-40
75. Hu S, Cao S, Liu J. Role of angiopoietin-2 in the cardioprotective effect of fibroblast growth factor 21 on ischemia/reperfusion-induced injury in H9c2 cardiomyocytes. Exp Ther Med. 2017;14:771-9
76. Joki Y, Ohashi K, Yuasa D, Shibata R, Ito M, Matsuo K. et al. FGF21 attenuates pathological myocardial remodeling following myocardial infarction through the adiponectin-dependent mechanism. Biochem Biophys Res Commun. 2015;459:124-30
77. Sommakia S, Almaw NH, Lee SH, Ramadurai DKA, Taleb I, Kyriakopoulos CP. et al. FGF21 (Fibroblast Growth Factor 21) Defines a Potential Cardiohepatic Signaling Circuit in End-Stage Heart Failure. Circ Heart Fail. 2021: Circheartfailure121008910.
78. Fan L, Gu L, Yao Y, Ma G. Elevated Serum Fibroblast Growth Factor 21 Is Relevant to Heart Failure Patients with Reduced Ejection Fraction. Comput Math Methods Med. 2022;2022:7138776
79. Ianos RD, Pop C, Iancu M, Rahaian R, Cozma A, Procopciuc LM. Diagnostic Performance of Serum Biomarkers Fibroblast Growth Factor 21, Galectin-3 and Copeptin for Heart Failure with Preserved Ejection Fraction in a Sample of Patients with Type 2 Diabetes Mellitus. Diagnostics (Basel). 2021 11
80. Chou RH, Huang PH, Hsu CY, Chang CC, Leu HB, Huang CC. et al. Circulating Fibroblast Growth Factor 21 is Associated with Diastolic Dysfunction in Heart Failure Patients with Preserved Ejection Fraction. Sci Rep. 2016;6:33953
81. Suassuna PGA, Cherem PM, de Castro BB, Maquigussa E, Cenedeze MA, Lovisi JCM. et al. αKlotho attenuates cardiac hypertrophy and increases myocardial fibroblast growth factor 21 expression in uremic rats. Experimental biology and medicine. 2020;245:66-78
82. Rup¨¦rez C, Lerin C, Ferrer-Curriu G, Cairo M, Mas-Stachurska A, Sitges M. et al. Autophagic control of cardiac steatosis through FGF21 in obesity-associated cardiomyopathy. International journal of cardiology. 2018;260:163-70
83. Li S, Zhu Z, Xue M, Yi X, Liang J, Niu C. et al. Fibroblast growth factor 21 protects the heart from angiotensin II-induced cardiac hypertrophy and dysfunction via SIRT1. Biochim Biophys Acta Mol Basis Dis. 2019;1865:1241-52
84. Planavila A, Redondo-Angulo I, Ribas F, Garrabou G, Casademont J, Giralt M. et al. Fibroblast growth factor 21 protects the heart from oxidative stress. Cardiovasc Res. 2015;106:19-31
85. Mancini A, Vergani E, Bruno C, Olivieri G, Di Segni C, Silvestrini A. et al. Oxidative stress as a possible mechanism underlying multi-hormonal deficiency in chronic heart failure. Eur Rev Med Pharmacol Sci. 2018;22:3936-61
86. Ferrer-Curriu G, Redondo-Angulo I, Guitart-Mampel M, Ruperez C, Mas-Stachurska A, Sitges M. et al. Fibroblast growth factor-21 protects against fibrosis in hypertensive heart disease. J Pathol. 2019;248:30-40
87. Davidsohn N, Pezone M, Vernet A, Graveline A, Oliver D, Slomovic S. et al. A single combination gene therapy treats multiple age-related diseases. Proc Natl Acad Sci U S A. 2019;116:23505-11
88. Furukawa N, Koitabashi N, Matsui H, Sunaga H, Umbarawan Y, Syamsunarno M. et al. DPP-4 inhibitor induces FGF21 expression via sirtuin 1 signaling and improves myocardial energy metabolism. Heart Vessels. 2021;36:136-46
89. Wang YS, Ye J, Cao YH, Zhang R, Liu Y, Zhang SW. et al. Increased serum/plasma fibroblast growth factor 21 in type 2 diabetes mellitus: a systematic review and meta-analysis. Postgrad Med J. 2019;95:134-9
90. Zhang R, Cai X, Du Y, Liu L, Han X, Liu W. et al. Association of serum fibroblast growth factor 21 and urinary glucose excretion in hospitalized patients with type 2 diabetes. J Diabetes Complications. 2021;35:107750
91. Ong KL, O'Connell R, Januszewski AS, Jenkins AJ, Xu A, Sullivan DR. et al. Baseline Circulating FGF21 Concentrations and Increase after Fenofibrate Treatment Predict More Rapid Glycemic Progression in Type 2 Diabetes: Results from the FIELD Study. Clin Chem. 2017;63:1261-70
92. Liu D, Wu L, Gao Q, Long X, Hou X, Qian L. et al. FGF21/adiponectin ratio predicts deterioration in glycemia: a 4.6-year prospective study in China. Cardiovasc Diabetol. 2021;20:157
93. Woo YC, Lee CH, Fong CH, Xu A, Tso AW, Cheung BM. et al. Serum fibroblast growth factor 21 is a superior biomarker to other adipokines in predicting incident diabetes. Clinical endocrinology. 2017;86:37-43
94. Kondeti S, D MD, Mn M, S MVKP, Nemani H, Kalashikam RR. Attenuation of FGF21 signalling might aggravate the impairment of glucose homeostasis during the high sucrose diet induced transition from prediabetes to diabetes in WNIN/GR-Ob rats. Biomed Pharmacother. 2021;137:111252
95. Castaño-Martinez T, Schumacher F, Schumacher S, Kochlik B, Weber D, Grune T. et al. Methionine restriction prevents onset of type 2 diabetes in NZO mice. Faseb j. 2019;33:7092-102
96. Zhang H, Zhang W, Yun D, Li L, Zhao W, Li Y. et al. Alternate-day fasting alleviates diabetes-induced glycolipid metabolism disorders: roles of FGF21 and bile acids. J Nutr Biochem. 2020;83:108403
97. Pan Y, Wang B, Zheng J, Xiong R, Fan Z, Ye Y. et al. Pancreatic fibroblast growth factor 21 protects against type 2 diabetes in mice by promoting insulin expression and secretion in a PI3K/Akt signaling-dependent manner. J Cell Mol Med. 2019;23:1059-71
98. Samms RJ, Lewis JE, Norton L, Stephens FB, Gaffney CJ, Butterfield T. et al. FGF21 Is an Insulin-Dependent Postprandial Hormone in Adult Humans. The Journal of clinical endocrinology and metabolism. 2017;102:3806-13
99. Xie T, So WY, Li XY, Leung PS. Fibroblast growth factor 21 protects against lipotoxicity-induced pancreatic β-cell dysfunction via regulation of AMPK signaling and lipid metabolism. Clinical science. 2019;133:2029-44
100. Cheng STW, Li SYT, Leung PS. Fibroblast Growth Factor 21 Stimulates Pancreatic Islet Autophagy via Inhibition of AMPK-mTOR Signaling. Int J Mol Sci. 2019 20
101. Diener JL, Mowbray S, Huang WJ, Yowe D, Xu J, Caplan S. et al. FGF21 Normalizes Plasma Glucose in Mouse Models of Type 1 Diabetes and Insulin Receptor Dysfunction. Endocrinology. 2021 162
102. Jelenik T, Dille M, Muller-Luhlhoff S, Kabra DG, Zhou Z, Binsch C. et al. FGF21 regulates insulin sensitivity following long-term chronic stress. Mol Metab. 2018;16:126-38
103. Gong Q, Hu Z, Zhang F, Cui A, Chen X, Jiang H. et al. Fibroblast growth factor 21 improves hepatic insulin sensitivity by inhibiting mammalian target of rapamycin complex 1 in mice. Hepatology. 2016;64:425-38
104. Li H, Wu G, Fang Q, Zhang M, Hui X, Sheng B. et al. Fibroblast growth factor 21 increases insulin sensitivity through specific expansion of subcutaneous fat. Nature communications. 2018;9:272
105. Guo Q, Xu L, Liu J, Li H, Sun H, Wu S. et al. Fibroblast growth factor 21 reverses suppression of adiponectin expression via inhibiting endoplasmic reticulum stress in adipose tissue of obese mice. Exp Biol Med (Maywood). 2017;242:441-7
106. Goto T, Hirata M, Aoki Y, Iwase M, Takahashi H, Kim M. et al. The hepatokine FGF21 is crucial for peroxisome proliferator-activated receptor-α agonist-induced amelioration of metabolic disorders in obese mice. The Journal of biological chemistry. 2017;292:9175-90
107. Laeger T, Baumeier C, Wilhelmi I, Wurfel J, Kamitz A, Schurmann A. FGF21 improves glucose homeostasis in an obese diabetes-prone mouse model independent of body fat changes. Diabetologia. 2017;60:2274-84
108. Wang N, Xu TY, Zhang X, Li JY, Wang YX, Guo XC. et al. Improving hyperglycemic effect of FGF-21 is associated with alleviating inflammatory state in diabetes. International immunopharmacology. 2018;56:301-9
109. Zhang X, Yang L, Xu X, Tang F, Yi P, Qiu B. et al. A review of fibroblast growth factor 21 in diabetic cardiomyopathy. Heart Fail Rev. 2019;24:1005-17
110. Yang H, Feng A, Lin S, Yu L, Lin X, Yan X. et al. Fibroblast growth factor-21 prevents diabetic cardiomyopathy via AMPK-mediated antioxidation and lipid-lowering effects in the heart. Cell Death Dis. 2018;9:227
111. Ianoș RD, Pop C, Iancu M, Rahaian R, Cozma A, Procopciuc LM. Diagnostic Performance of Serum Biomarkers Fibroblast Growth Factor 21, Galectin-3 and Copeptin for Heart Failure with Preserved Ejection Fraction in a Sample of Patients with Type 2 Diabetes Mellitus. Diagnostics (Basel). 2021 11
112. Gan F, Huang J, Dai T, Li M, Liu J. Serum level of fibroblast growth factor 21 predicts long-term prognosis in patients with both diabetes mellitus and coronary artery calcification. Ann Palliat Med. 2020;9:368-74
113. Huang SY, Wu DA, Tsai JP, Hsu BG. Serum Levels of Fibroblast Growth Factor 21 Are Positively Associated with Aortic Stiffness in Patients with Type 2 Diabetes Mellitus. Int J Environ Res Public Health. 2021 18
114. Ying L, Li N, He Z, Zeng X, Nan Y, Chen J. et al. Fibroblast growth factor 21 Ameliorates diabetes-induced endothelial dysfunction in mouse aorta via activation of the CaMKK2/AMPKα signaling pathway. Cell death & disease. 2019;10:665
115. Munoz-Rivas N, Mendez-Bailon M, Hernandez-Barrera V, de Miguel-Yanes JM, Jimenez-Garcia R, Esteban-Hernandez J. et al. Time Trends in Ischemic Stroke among Type 2 Diabetic and Non-Diabetic Patients: Analysis of the Spanish National Hospital Discharge Data (2003-2012). PLoS One. 2015;10:e0145535
116. Jiang Y, Han J, Li Y, Wu Y, Liu N, Shi SX. et al. Delayed rFGF21 Administration Improves Cerebrovascular Remodeling and White Matter Repair After Focal Stroke in Diabetic Mice. Transl Stroke Res. 2021
117. Jiang Y, Liu N, Wang Q, Yu Z, Lin L, Yuan J. et al. Endocrine Regulator rFGF21 (Recombinant Human Fibroblast Growth Factor 21) Improves Neurological Outcomes Following Focal Ischemic Stroke of Type 2 Diabetes Mellitus Male Mice. Stroke. 2018;49:3039-49
118. Banks WA. The Blood-Brain Barrier Interface in Diabetes Mellitus: Dysfunctions, Mechanisms and Approaches to Treatment. Curr Pharm Des. 2020;26:1438-47
119. Jiang Y, Lin L, Liu N, Wang Q, Yuan J, Li Y. et al. FGF21 Protects against Aggravated Blood-Brain Barrier Disruption after Ischemic Focal Stroke in Diabetic db/db Male Mice via Cerebrovascular PPARgamma Activation. Int J Mol Sci. 2020 21
120. Yu Z, Lin L, Jiang Y, Chin I, Wang X, Li X. et al. Recombinant FGF21 Protects Against Blood-Brain Barrier Leakage Through Nrf2 Upregulation in Type 2 Diabetes Mice. Molecular neurobiology. 2019;56:2314-27
121. Jin S, Xia N, Han L. Association between serum fibroblast growth factor 21 level and sight-threatening diabetic retinopathy in Chinese patients with type 2 diabetes. BMJ Open Diabetes Res Care. 2021 9
122. Fu Z, Wang Z, Liu CH, Gong Y, Cakir B, Liegl R. et al. Fibroblast Growth Factor 21 Protects Photoreceptor Function in Type 1 Diabetic Mice. Diabetes. 2018;67:974-85
123. Liu JJ, Liu S, Choo RWM, Wee SL, Xu A, Lim SC. Sex modulates the association of fibroblast growth factor 21 with end-stage renal disease in Asian people with Type 2 diabetes: a 6.3-year prospective cohort study. Diabet Med. 2018;35:880-6
124. Chang LH, Hwu CM, Chu CH, Lin YC, Huang CC, You JY. et al. The combination of soluble tumor necrosis factor receptor type 1 and fibroblast growth factor 21 exhibits better prediction of renal outcomes in patients with type 2 diabetes mellitus. J Endocrinol Invest. 2021;44:2609-19
125. Yu W, Zhu H, Chen X, Gu X, Zhang X, Shen F. et al. Genetic Variants Flanking the FGF21 Gene Were Associated with Renal Function in Chinese Patients with Type 2 Diabetes. J Diabetes Res. 2019;2019:9387358
126. Charles ED, Neuschwander-Tetri BA, Pablo Frias J, Kundu S, Luo Y, Tirucherai GS. et al. Pegbelfermin (BMS-986036), PEGylated FGF21, in Patients with Obesity and Type 2 Diabetes: Results from a Randomized Phase 2 Study. Obesity (Silver Spring). 2019;27:41-9
127. Degirolamo C, Sabba C, Moschetta A. Therapeutic potential of the endocrine fibroblast growth factors FGF19, FGF21 and FGF23. Nat Rev Drug Discov. 2016;15:51-69
128. Zhao L, Niu J, Lin H, Zhao J, Liu Y, Song Z. et al. Paracrine-endocrine FGF chimeras as potent therapeutics for metabolic diseases. EBioMedicine. 2019;48:462-77
129. Zhu L, Zhao H, Liu J, Cai H, Wu B, Liu Z. et al. Dynamic folding modulation generates FGF21 variant against diabetes. EMBO Rep. 2021;22:e51352
130. Queen NJ, Bates R, Huang W, Xiao R, Appana B, Cao L. Visceral adipose tissue-directed FGF21 gene therapy improves metabolic and immune health in BTBR mice. Mol Ther Methods Clin Dev. 2021;20:409-22
131. Lu S, Liu G, Chen T, Wang W, Hu J, Tang D. et al. Lentivirus-Mediated hFGF21 Stable Expression in Liver of Diabetic Rats Model and Its Antidiabetic Effect Observation. Hum Gene Ther. 2020;31:472-84
132. Guo C, Zhao L, Li Y, Deng X, Yuan G. Relationship between FGF21 and drug or nondrug therapy of type 2 diabetes mellitus. J Cell Physiol. 2021;236:55-67
133. Yang K, Wang H, Wei R, Xiao W, Tian Q, Wang C. et al. High baseline FGF21 levels are associated with poor glucose-lowering efficacy of exenatide in patients with type 2 diabetes. Acta Diabetol. 2021;58:595-602
134. Liu J, Yang K, Yang J, Xiao W, Le Y, Yu F. et al. Liver-derived fibroblast growth factor 21 mediates effects of glucagon-like peptide-1 in attenuating hepatic glucose output. EBioMedicine. 2019;41:73-84
135. Pan Q, Lin S, Li Y, Liu L, Li X, Gao X. et al. A novel GLP-1 and FGF21 dual agonist has therapeutic potential for diabetes and non-alcoholic steatohepatitis. EBioMedicine. 2021;63:103202
136. Gilroy CA, Capozzi ME, Varanko AK, Tong J, D'Alessio DA, Campbell JE. et al. Sustained release of a GLP-1 and FGF21 dual agonist from an injectable depot protects mice from obesity and hyperglycemia. Sci Adv. 2020;6:eaaz9890
137. Geng S, Qin L, He Y, Li X, Yang M, Li L. et al. Effective and safe delivery of GLP-1AR and FGF-21 plasmids using amino-functionalized dual-mesoporous silica nanoparticles in vitro and in vivo. Biomaterials. 2021;271:120763
138. Xue B, Xiao X, Yu T, Xiao X, Xie J, Ji Q. et al. Mesenchymal stem cells modified by FGF21 and GLP1 ameliorate lipid metabolism while reducing blood glucose in type 2 diabetic mice. Stem Cell Res Ther. 2021;12:133
139. Lee YK, Diaz B, Deroose M, Lee SX, Belvedere S, Accili D. et al. FOXO1 inhibition synergizes with FGF21 to normalize glucose control in diabetic mice. Mol Metab. 2021;49:101187
140. Meng F, Cao Y, Khoso MH, Kang K, Ren G, Xiao W. et al. Therapeutic effect and mechanism of combined use of FGF21 and insulin on diabetic nephropathy. Arch Biochem Biophys. 2021;713:109063
141. Apovian CM. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care. 2016;22:s176-85
142. Wolf RM, Jaffe AE, Rodriguez S, Lei X, Sarver DC, Straub AT. et al. Altered adipokines in obese adolescents: a cross-sectional and longitudinal analysis across the spectrum of glycemia. Am J Physiol Endocrinol Metab. 2021;320:E1044-e52
143. Flisiak-Jackiewicz M, Bobrus-Chociej A, Wasilewska N, Tarasow E, Wojtkowska M, Lebensztejn DM. Can hepatokines be regarded as novel non-invasive serum biomarkers of intrahepatic lipid content in obese children? Adv Med Sci. 2019;64:280-4
144. Li G, Yin J, Fu J, Li L, Grant SFA, Li C. et al. FGF21 deficiency is associated with childhood obesity, insulin resistance and hypoadiponectinaemia: The BCAMS Study. Diabetes & metabolism. 2017;43:253-60
145. Ruiz-Padilla AJ, Morales-Hernandez G, Ruiz-Noa Y, Alonso-Castro AJ, Lazo-de-la-Vega-Monroy ML, Preciado-Puga MDC. et al. Association of the 3'UTR polymorphism (rs11665896) in the FGF21 gene with metabolic status and nutrient intake in children with obesity. J Pediatr Endocrinol Metab. 2019;32:921-8
146. Headland ML, Clifton PM, Keogh JB. Effects of Weight Loss on FGF-21 in Human Subjects: An Exploratory Study. Int J Environ Res Public Health. 2019 16
147. Kravchychyn ACP, Campos R, Ferreira YAM, Vicente S, Corgosinho FC, Oyama LM. et al. Higher increase degree of FGF21 post long-term interdisciplinary weight loss therapy preserves the free fat mass and rest metabolic rate in adolescents with obesity. Arch Endocrinol Metab. 2020;64:479-82
148. Power Guerra N, Parveen A, Bühler D, Brauer DL, Müller L, Pilz K. et al. Fibroblast Growth Factor 21 as a Potential Biomarker for Improved Locomotion and Olfaction Detection Ability after Weight Reduction in Obese Mice. Nutrients. 2021 13
149. Dâmaso AR, Machado PP, Rhein SO, Masquio DCL, Oyama LM, Boldarine VT. et al. Effects of an interdisciplinary weight loss program on fibroblast growth factor 21 and inflammatory biomarkers in women with overweight and obesity. Arch Endocrinol Metab. 2021;65:821-31
150. Vinales KL, Begaye B, Bogardus C, Walter M, Krakoff J, Piaggi P. FGF21 Is a Hormonal Mediator of the Human "Thrifty" Metabolic Phenotype. Diabetes. 2019;68:318-23
151. Mechanick JI, Apovian C, Brethauer S, Timothy Garvey W, Joffe AM, Kim J. et al. Clinical Practice Guidelines for the Perioperative Nutrition, Metabolic, and Nonsurgical Support of Patients Undergoing Bariatric Procedures - 2019 Update: Cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic and Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Obesity (Silver Spring). 2020;28:O1-O58
152. Nielsen MS, Ritz C, Chenchar A, Bredie WLP, Gillum MP, Sjödin A. Does FGF21 Mediate the Potential Decrease in Sweet Food Intake and Preference Following Bariatric Surgery? Nutrients. 2021 13
153. Pei E, Liu Y, Jiang W, Lin S, Huang L, Lin M. et al. Sleeve gastrectomy attenuates high fat diet-induced non-alcoholic fatty liver disease. Lipids Health Dis. 2018;17:243
154. Stygar D, Sawczyn T, Dulska A, Chełmecka E, Mielańczyk Ł, Matysiak N. et al. Ileal transposition helps to regulate plasma hepatokine levels in obese Zucker (Crl:ZUC(ORL)-Lepr(fa)) rats. Sci Rep. 2021;11:7774
155. G¨®mez-Ambrosi J, Gallego-Escuredo JM, Catal¨¢n V, Rodr¨ªguez A, Domingo P, Moncada R. et al. FGF19 and FGF21 serum concentrations in human obesity and type 2 diabetes behave differently after diet- or surgically-induced weight loss. Clinical nutrition: official journal of the European Society of Parenteral and Enteral Nutrition. 2017;36:861-8
156. Crujeiras AB, Gomez-Arbelaez D, Zulet MA, Carreira MC, Sajoux I, de Luis D. et al. Plasma FGF21 levels in obese patients undergoing energy-restricted diets or bariatric surgery: a marker of metabolic stress? Int J Obes (Lond). 2017;41:1570-8
157. Christoffersen B, Straarup EM, Lykkegaard K, Fels JJ, Sass-Ørum K, Zhang X. et al. FGF21 decreases food intake and body weight in obese Göttingen minipigs. Diabetes Obes Metab. 2019;21:592-600
158. Epperlein S, Gebhardt C, Rohde K, Chakaroun R, Patt M, Schamarek I. et al. The Effect of FGF21 and Its Genetic Variants on Food and Drug Cravings, Adipokines and Metabolic Traits. Biomedicines. 2021 9
159. Makarova E, Kazantseva A, Dubinina A, Jakovleva T, Balybina N, Baranov K. et al. The Same Metabolic Response to FGF21 Administration in Male and Female Obese Mice Is Accompanied by Sex-Specific Changes in Adipose Tissue Gene Expression. Int J Mol Sci. 2021 22
160. Hill CM, Laeger T, Dehner M, Albarado DC, Clarke B, Wanders D. et al. FGF21 Signals Protein Status to the Brain and Adaptively Regulates Food Choice and Metabolism. Cell Rep. 2019;27:2934-47.e3
161. Flippo KH, Jensen-Cody SO, Claflin KE, Potthoff MJ. FGF21 signaling in glutamatergic neurons is required for weight loss associated with dietary protein dilution. Sci Rep. 2020;10:19521
162. Blanco AM, Bertucci JI, Unniappan S. FGF21 Mimics a Fasting-Induced Metabolic State and Increases Appetite in Zebrafish. Sci Rep. 2020;10:6993
163. Moure R, Cairó M, Morón-Ros S, Quesada-López T, Campderrós L, Cereijo R. et al. Levels of β-klotho determine the thermogenic responsiveness of adipose tissues: involvement of the autocrine action of FGF21. Am J Physiol Endocrinol Metab. 2021;320:E822-e34
164. Mutsnaini L, Kim CS, Kim J, Joe Y, Chung HT, Choi HS. et al. Fibroblast growth factor 21 deficiency aggravates obesity-induced hypothalamic inflammation and impairs thermogenic response. Inflamm Res. 2019;68:351-8
165. Murray SA, Dalbøge LS, Baquero K, Sanford CA, Misquith A, Mercer AJ. et al. Whole transcriptome analysis and validation of metabolic pathways in subcutaneous adipose tissues during FGF21-induced weight loss in non-human primates. Sci Rep. 2020;10:7287
166. Meng W, Xiao T, Liang X, Wen J, Peng X, Wang J. et al. The miR-182-5p/FGF21/acetylcholine axis mediates the crosstalk between adipocytes and macrophages to promote beige fat thermogenesis. JCI Insight. 2021 6
167. Keipert S, Lutter D, Schroeder BO, Brandt D, Ståhlman M, Schwarzmayr T. et al. Endogenous FGF21-signaling controls paradoxical obesity resistance of UCP1-deficient mice. Nat Commun. 2020;11:624
168. Zouhar P, Janovska P, Stanic S, Bardova K, Funda J, Haberlova B. et al. A pyrexic effect of FGF21 independent of energy expenditure and UCP1. Mol Metab. 2021;53:101324
169. Challa TD, Dapito DH, Kulenkampff E, Kiehlmann E, Moser C, Straub L. et al. A Genetic Model to Study the Contribution of Brown and Brite Adipocytes to Metabolism. Cell Rep. 2020;30:3424-33 e4
170. Geng L, Liao B, Jin L, Huang Z, Triggle CR, Ding H. et al. Exercise Alleviates Obesity-Induced Metabolic Dysfunction via Enhancing FGF21 Sensitivity in Adipose Tissues. Cell Rep. 2019;26:2738-52.e4
171. Kim CS, Joe Y, Choi HS, Back SH, Park JW, Chung HT. et al. Deficiency of fibroblast growth factor 21 aggravates obesity-induced atrophic responses in skeletal muscle. J Inflamm (Lond). 2019;16:17
172. Wang N, Zhao TT, Li SM, Sun X, Li ZC, Li YH. et al. Fibroblast Growth Factor 21 Exerts its Anti-inflammatory Effects on Multiple Cell Types of Adipose Tissue in Obesity. Obesity (Silver Spring). 2019;27:399-408
173. Cao WY, Dong M, Hu ZY, Wu J, Li YC, Xu HD. Recombinant Lactococcus lactis NZ3900 expressing bioactive human FGF21 reduced body weight of Db/Db mice through the activity of brown adipose tissue. Benef Microbes. 2020;11:67-78
174. Girer NG, Rontoyanni VG, Joshi A, Patrikeev I, Murton AJ, Porter C. et al. Liver-Specific Nonviral Gene Delivery of Fibroblast Growth Factor 21 Protein Expression in Mice Regulates Body Mass and White/Brown Fat Respiration. J Pharmacol Exp Ther. 2021;378:157-65
175. Kim D, Lee J, Bae I, Kim M, Huh Y, Choi J. et al. Preparation, characterization, and pharmacological study of a novel long-acting FGF21 with a potential therapeutic effect in obesity. Biologicals. 2021;69:49-58
176. Sanyal AJ, Brunt EM, Kleiner DE, Kowdley KV, Chalasani N, Lavine JE. et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54:344-53
177. Tucker B, McClelland RL, Allison MA, Budoff MJ, Wu BJ, Barter PJ. et al. Relationship of fibroblast growth factor 21 levels with inflammation, lipoproteins and non-alcoholic fatty liver disease. Atherosclerosis. 2020;299:38-44
178. Tas E, Bai S, Ou X, Mercer K, Lin H, Mansfield K. et al. Fibroblast Growth Factor-21 to Adiponectin Ratio: A Potential Biomarker to Monitor Liver Fat in Children With Obesity. Front Endocrinol (Lausanne). 2020;11:654
179. Xiao F, Shi X, Huang P, Zeng X, Wang L, Zeng J. et al. Dose-response relationship between serum fibroblast growth factor 21 and liver fat content in non-alcoholic fatty liver disease. Diabetes Metab. 2021;47:101221
180. Cantero I, Abete I, Bullón-Vela V, Crujeiras AB, Casanueva FF, Zulet MA. et al. Fibroblast growth factor 21 levels and liver inflammatory biomarkers in obese subjects after weight loss. Arch Med Sci. 2022;18:36-44
181. Barb D, Bril F, Kalavalapalli S, Cusi K. Plasma Fibroblast Growth Factor 21 Is Associated With Severity of Nonalcoholic Steatohepatitis in Patients With Obesity and Type 2 Diabetes. J Clin Endocrinol Metab. 2019;104:3327-36
182. Watanabe M, Risi R, Camajani E, Contini S, Persichetti A, Tuccinardi D. et al. Baseline HOMA IR and Circulating FGF21 Levels Predict NAFLD Improvement in Patients Undergoing a Low Carbohydrate Dietary Intervention for Weight Loss: A Prospective Observational Pilot Study. Nutrients. 2020 12
183. Li X, Wu X, Jia Y, Fu J, Zhang L, Jiang T. et al. Liraglutide Decreases Liver Fat Content and Serum Fibroblast Growth Factor 21 Levels in Newly Diagnosed Overweight Patients with Type 2 Diabetes and Nonalcoholic Fatty Liver Disease. J Diabetes Res. 2021;2021:3715026
184. Xi Y, Zheng J, Xie W, Xu X, Cho N, Zhou X. et al. (+)-Dehydrovomifoliol Alleviates Oleic Acid-Induced Lipid Accumulation in HepG2 Cells via the PPARα-FGF21 Pathway. Front Pharmacol. 2021;12:750147
185. Yano K, Yamaguchi K, Seko Y, Okishio S, Ishiba H, Tochiki N. et al. Hepatocyte-specific fibroblast growth factor 21 overexpression ameliorates high-fat diet-induced obesity and liver steatosis in mice. Lab Invest. 2021
186. Makarova E, Kazantseva A, Dubinina A, Denisova E, Jakovleva T, Balybina N. et al. Fibroblast Growth Factor 21 (FGF21) Administration Sex-Specifically Affects Blood Insulin Levels and Liver Steatosis in Obese A(y) Mice. Cells. 2021 10
187. Byun S, Seok S, Kim YC, Zhang Y, Yau P, Iwamori N. et al. Fasting-induced FGF21 signaling activates hepatic autophagy and lipid degradation via JMJD3 histone demethylase. Nat Commun. 2020;11:807
188. Kim HY, Kwon WY, Park JB, Lee MH, Oh YJ, Suh S. et al. Hepatic STAMP2 mediates recombinant FGF21-induced improvement of hepatic iron overload in nonalcoholic fatty liver disease. Faseb j. 2020;34:12354-66
189. Keinicke H, Sun G, Mentzel CMJ, Fredholm M, John LM, Andersen B. et al. FGF21 regulates hepatic metabolic pathways to improve steatosis and inflammation. Endocr Connect. 2020;9:755-68
190. Lee JH, Kang YE, Chang JY, Park KC, Kim HW, Kim JT. et al. An engineered FGF21 variant, LY2405319, can prevent non-alcoholic steatohepatitis by enhancing hepatic mitochondrial function. American journal of translational research. 2016;8:4750-63
191. Zheng Q, Martin RC, Shi X, Pandit H, Yu Y, Liu X. et al. Lack of FGF21 promotes NASH-HCC transition via hepatocyte-TLR4-IL-17A signaling. Theranostics. 2020;10:9923-36
192. Tokushige K, Ikejima K, Ono M, Eguchi Y, Kamada Y, Itoh Y. et al. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis 2020. J Gastroenterol. 2021;56:951-63
193. Tillman EJ, Brock WJ, Rolph T. Efruxifermin, a long-acting Fc-fusion FGF21 analogue, reduces body weight gain but does not increase sympathetic tone or urine volume in Sprague Dawley rats. Br J Pharmacol. 2021
194. Harrison SA, Ruane PJ, Freilich BL, Neff G, Patil R, Behling CA. et al. Efruxifermin in non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled, phase 2a trial. Nat Med. 2021;27:1262-71
195. Sanyal A, Charles ED, Neuschwander-Tetri BA, Loomba R, Harrison SA, Abdelmalek MF. et al. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet. 2019;392:2705-17
196. Abdelmalek MF, Charles ED, Sanyal AJ, Harrison SA, Neuschwander-Tetri BA, Goodman Z. et al. The FALCON program: Two phase 2b randomized, double-blind, placebo-controlled studies to assess the efficacy and safety of pegbelfermin in the treatment of patients with nonalcoholic steatohepatitis and bridging fibrosis or compensated cirrhosis. Contemp Clin Trials. 2021;104:106335
197. Verzijl CRC, Van De Peppel IP, Struik D, Jonker JW. Pegbelfermin (BMS-986036): an investigational PEGylated fibroblast growth factor 21 analogue for the treatment of nonalcoholic steatohepatitis. Expert Opin Investig Drugs. 2020;29:125-33
198. Cui A, Li J, Ji S, Ma F, Wang G, Xue Y. et al. The Effects of B1344, a Novel Fibroblast Growth Factor 21 Analog, on Nonalcoholic Steatohepatitis in Nonhuman Primates. Diabetes. 2020;69:1611-23
199. Rader DJ, Maratos-Flier E, Nguyen A, Hom D, Ferriere M, Li Y. et al. LLF580, an FGF21 Analog, Reduces Triglycerides and Hepatic Fat in Obese Adults With Modest Hypertriglyceridemia. J Clin Endocrinol Metab. 2022;107:e57-e70
200. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA. et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640-5
201. Gao RY, Hsu BG, Wu DA, Hou JS, Chen MC. Serum Fibroblast Growth Factor 21 Levels Are Positively Associated with Metabolic Syndrome in Patients with Type 2 Diabetes. Int J Endocrinol. 2019;2019:5163245
202. Chang JS, Namkung J. Effects of Exercise Intervention on Mitochondrial Stress Biomarkers in Metabolic Syndrome Patients: A Randomized Controlled Trial. Int J Environ Res Public Health. 2021 18
203. Ong KL, McClelland RL, Allison MA, Kokkinos J, Wu BJ, Barter PJ. et al. Association of elevated circulating fibroblast growth factor 21 levels with prevalent and incident metabolic syndrome: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2019;281:200-6
204. Arner P, Pettersson A, Mitchell PJ, Dunbar JD, Kharitonenkov A, Ryden M. FGF21 attenuates lipolysis in human adipocytes - a possible link to improved insulin sensitivity. FEBS Lett. 2008;582:1725-30
205. Li X, Ge H, Weiszmann J, Hecht R, Li YS, V¨¦niant MM. et al. Inhibition of lipolysis may contribute to the acute regulation of plasma FFA and glucose by FGF21 in ob/ob mice. FEBS letters. 2009;583:3230-4
206. Lee P, Greenfield JR, Ho KK, Fulham MJ. A critical appraisal of the prevalence and metabolic significance of brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2010;299:E601-6
207. Forsstr?m S, Jackson CB, Carroll CJ, Kuronen M, Pirinen E, Pradhan S. et al. Fibroblast Growth Factor 21 Drives Dynamics of Local and Systemic Stress Responses in Mitochondrial Myopathy with mtDNA Deletions. Cell metabolism. 2019;30:1040-54.e7
208. Yurekli BS, Kutbay NO, Aksit M, Suner A, Simsir IY, Seckiner S. et al. Acromegaly is associated with high fibroblast growth factor-21 levels. Journal of endocrinological investigation. 2019;42:53-60
209. Peng Y, Pei Q, Feng S, Su Y, Liu R, Yi Q. et al. Increased levels of circulating fibroblast growth factor 21 in children with Kawasaki disease. Clin Exp Med. 2019;19:457-62
210. Kiluk P, Baran A, Kaminski TW, Maciaszek M, Flisiak I. The Level of FGF 21 as a New Risk Factor for the Occurrence of Cardiometabolic Disorders amongst the Psoriatic Patients. Journal of clinical medicine. 2019 8
211. Moure R, Domingo P, Villarroya J, Gasa L, Gallego-Escuredo JM, Quesada-L¨®pez T. et al. Reciprocal Effects of Antiretroviral Drugs Used To Treat HIV Infection on the Fibroblast Growth Factor 21/α-Klotho System. Antimicrobial agents and chemotherapy. 2018 62
212. Hu Q, Wang C, Liu F, He J, Wang F, Wang W. et al. High serum levels of FGF21 are decreased in bipolar mania patients during psychotropic medication treatment and are associated with increased metabolism disturbance. Psychiatry Res. 2019;272:643-8
213. Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ. et al. FGF-21 as a novel metabolic regulator. The Journal of clinical investigation. 2005;115:1627-35
214. Tabari FS, Karimian A, Parsian H, Rameshknia V, Mahmoodpour A, Majidinia M. et al. The roles of FGF21 in atherosclerosis pathogenesis. Rev Endocr Metab Disord. 2019;20:103-14
215. Shao M, Lu X, Cong W, Xing X, Tan Y, Li Y. et al. Multiple low-dose radiation prevents type 2 diabetes-induced renal damage through attenuation of dyslipidemia and insulin resistance and subsequent renal inflammation and oxidative stress. PloS one. 2014;9:e92574
216. Wang H, Qiang L, Farmer SR. Identification of a domain within peroxisome proliferator-activated receptor gamma regulating expression of a group of genes containing fibroblast growth factor 21 that are selectively repressed by SIRT1 in adipocytes. Molecular and cellular biology. 2008;28:188-200
217. Gao J, Guo X, Wei W, Li R, Hu K, Liu X. et al. The Association of Fried Meat Consumption With the Gut Microbiota and Fecal Metabolites and Its Impact on Glucose Homoeostasis, Intestinal Endotoxin Levels, and Systemic Inflammation: A Randomized Controlled-Feeding Trial. Diabetes Care. 2021;44:1970-9
218. Kundu P, Lee HU, Garcia-Perez I, Tay EXY, Kim H, Faylon LE. et al. Neurogenesis and prolongevity signaling in young germ-free mice transplanted with the gut microbiota of old mice. Sci Transl Med. 2019 11
219. Monaghan T, Mullish BH, Patterson J, Wong GK, Marchesi JR, Xu H. et al. Effective fecal microbiota transplantation for recurrent Clostridioides difficile infection in humans is associated with increased signalling in the bile acid-farnesoid X receptor-fibroblast growth factor pathway. Gut Microbes. 2019;10:142-8
220. Golonka RM, Xiao X, Abokor AA, Joe B, Vijay-Kumar M. Altered nutrient status reprograms host inflammation and metabolic health via gut microbiota. J Nutr Biochem. 2020;80:108360
221. Shen Y, Zhang X, Pan X, Xu Y, Xiong Q, Lu Z. et al. Contribution of serum FGF21 level to the identification of left ventricular systolic dysfunction and cardiac death. Cardiovasc Diabetol. 2017;16:106
222. Zhang Y, Liu D, Long XX, Fang QC, Jia WP, Li HT. The role of FGF21 in the pathogenesis of cardiovascular disease. Chin Med J (Engl). 2021;134:2931-43
223. Wei W, Dutchak PA, Wang X, Ding X, Wang X, Bookout AL. et al. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor gamma. Proc Natl Acad Sci U S A. 2012;109:3143-8
224. Andersen B, Straarup EM, Heppner KM, Takahashi DL, Raffaele V, Dissen GA. et al. FGF21 decreases body weight without reducing food intake or bone mineral density in high-fat fed obese rhesus macaque monkeys. Int J Obes (Lond). 2018;42:1151-60
225. Satapati S, He T, Inagaki T, Potthoff M, Merritt ME, Esser V. et al. Partial resistance to peroxisome proliferator-activated receptor-alpha agonists in ZDF rats is associated with defective hepatic mitochondrial metabolism. Diabetes. 2008;57:2012-21
226. Markan KR, Naber MC, Small SM, Peltekian L, Kessler RL, Potthoff MJ. FGF21 resistance is not mediated by downregulation of beta-klotho expression in white adipose tissue. Mol Metab. 2017;6:602-10
227. Badakhshi Y, Jin T. Current understanding and controversies on the clinical implications of fibroblast growth factor 21. Crit Rev Clin Lab Sci. 2021;58:311-28
228. Flippo KH, Potthoff MJ. Metabolic Messengers: FGF21. Nat Metab. 2021;3:309-17
229. Sonoda J, Chen MZ, Baruch A. FGF21-receptor agonists: an emerging therapeutic class for obesity-related diseases. Hormone molecular biology and clinical investigation. 2017 30
230. Albarazanji K, Jennis M, Cavanaugh CR, Lang W, Singh B, Lanter JC. et al. Intestinal serine protease inhibition increases FGF21 and improves metabolism in obese mice. American journal of physiology Gastrointestinal and liver physiology. 2019;316:G653-G67
231. Tian L, Ning H, Shao W, Song Z, Badakhshi Y, Ling W. et al. Dietary Cyanidin-3-Glucoside Attenuates High-Fat-Diet-Induced Body-Weight Gain and Impairment of Glucose Tolerance in Mice via Effects on the Hepatic Hormone FGF21. The Journal of nutrition. 2020;150:2101-11
232. Min BK, Oh CJ, Park S, Lee JM, Go Y, Park BY. et al. Therapeutic effect of dichloroacetate against atherosclerosis via hepatic FGF21 induction mediated by acute AMPK activation. Experimental & molecular medicine. 2019;51:1-12
233. Seo YJ, Lee K, Chei S, Jeon YJ, Lee BY. Ishige okamurae Extract Ameliorates the Hyperglycemia and Body Weight Gain of db/db Mice through Regulation of the PI3K/Akt Pathway and Thermogenic Factors by FGF21. Marine drugs. 2019 17
234. Sandoval V, Rodr¨ªguez-Rodr¨ªguez R, Mart¨ªnez-Garza, Rosell-Cardona C, Lamuela-Ravent¨®s RM, Marrero PF. et al. Mediterranean Tomato-Based Sofrito Sauce Improves Fibroblast Growth Factor 21 (FGF21) Signaling in White Adipose Tissue of Obese ZUCKER Rats. Molecular nutrition & food research. 2018 62
235. Zhou F, Bai M, Zhang Y, Zhu Q, Zhang L, Zhang Q. et al. Berberine-induced activation of AMPK increases hepatic FGF21 expression via NUR77. Biochemical and biophysical research communications. 2018;495:1936-41
236. Sun Y, Xia M, Yan H, Han Y, Zhang F, Hu Z. et al. Berberine attenuates hepatic steatosis and enhances energy expenditure in mice by inducing autophagy and fibroblast growth factor 21. British journal of pharmacology. 2018;175:374-87
237. Zeng K, Tian L, Patel R, Shao W, Song Z, Liu L. et al. Diet Polyphenol Curcumin Stimulates Hepatic Fgf21 Production and Restores Its Sensitivity in High-Fat-Diet-Fed Male Mice. Endocrinology. 2017;158:277-92
238. Vargas R, Riquelme B, Fern¨¢ndez J, Videla LA. A combined docosahexaenoic acid-thyroid hormone protocol upregulates rat liver ¦Â-Klotho expression and downstream components of FGF21 signaling as a potential novel approach to metabolic stress conditions. Food & function. 2017;8:3980-8
239. Jung JG, Yi SA, Choi SE, Kang Y, Kim TH, Jeon JY. et al. TM-25659-Induced Activation of FGF21 Level Decreases Insulin Resistance and Inflammation in Skeletal Muscle via GCN2 Pathways. Mol Cells. 2015;38:1037-43
240. Zarei M, Pujol E, Quesada-L¨®pez T, Villarroya F, Barroso E, V¨¢zquez S. et al. Oral administration of a new HRI activator as a new strategy to improve high-fat-diet-induced glucose intolerance, hepatic steatosis, and hypertriglyceridaemia through FGF21. British journal of pharmacology. 2019;176:2292-305
241. Study to Explore the Efficacy and Safety of BIO89-100 in Subjects With Severe Hypertriglyceridemia. 2021
242. Smith R, Duguay A, Bakker A, Li P, Weiszmann J, Thomas MR. et al. FGF21 can be mimicked in vitro and in vivo by a novel anti-FGFR1c/beta-Klotho bispecific protein. PLoS One. 2013;8:e61432
243. Wu AL, Kolumam G, Stawicki S, Chen Y, Li J, Zavala-Solorio J. et al. Amelioration of type 2 diabetes by antibody-mediated activation of fibroblast growth factor receptor 1. Sci Transl Med. 2011;3:113ra26
244. Foltz IN, Hu S, King C, Wu X, Yang C, Wang W. et al. Treating diabetes and obesity with an FGF21-mimetic antibody activating the betaKlotho/FGFR1c receptor complex. Sci Transl Med. 2012;4:162ra53
Author contact
![]() Corresponding authors: E-mail: sxu1984edu.cn; wengjpedu.cn.
Corresponding authors: E-mail: sxu1984edu.cn; wengjpedu.cn.

 Global reach, higher impact
Global reach, higher impact