ISSN: 1449-2288
Int J Biol Sci 2023; 19(1):104-119. doi:10.7150/ijbs.75106 This issue Cite
Research Paper
Inhibition of androgen receptor enhanced the anticancer effects of everolimus through targeting glucose transporter 12
1. Department of General Surgery, First Medical Center, Chinese PLA General Hospital, Beijing 100853, China.
2. Medical School of Chinese PLA, Beijing 100853, China.
* These authors contributed equally to this work.
Abstract
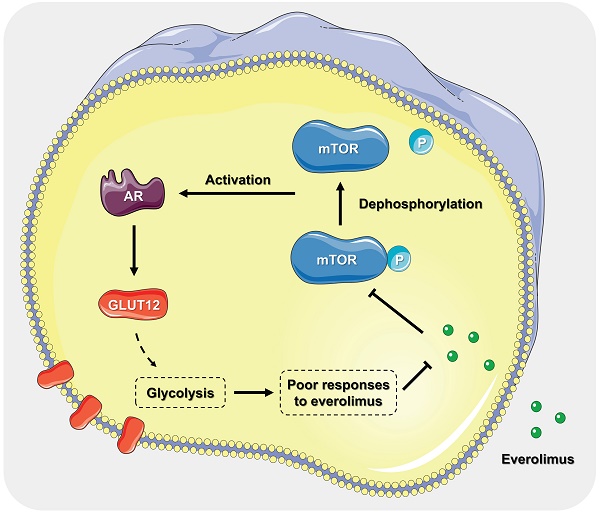
Everolimus was designed as a mammalian target of rapamycin (mTOR) inhibitor. It has been proven as a targeted drug for gastric cancer (GC) therapy. However, long-term treatment with everolimus may cause severe side effects for recipients. Decreasing the dosage and attenuating the associated risks are feasible to promote clinical translation of everolimus. This study aimed to identify the underlying mechanisms of responses to everolimus and develop novel regimens for GC treatment. Our findings proved that there was a significant dose-dependent relationship of everolimus-induced GC cell apoptosis and glycolysis inhibition. Then, we found that a member of glucose transporter (GLUT12) family, GLUT12, was actively upregulated to counteract the anticancer effects of everolimus. GLUT12 might be overexpressed in GC. High expression of GLUT12 might be correlated with tumor progression and short survival time of GC patients. Bioinformatic analysis suggested that GLUT12 might be involved in regulating cancer development and metabolism. The experiments proved that GLUT12 significantly promoted GC growth, glycolysis and impaired the anticancer effects of everolimus. Androgen receptor (AR) is a classical oncogenic factor in many types of cancer. Everolimus elevated GLUT12 expression in an AR-dependent manner. Inhibition of AR activity abrogated the promotive effects on GLUT12 expression. Both in-vitro and in-vivo experiments demonstrated that GLUT12 knockdown augmented anticancer effects of everolimus. Enzalutamide, an AR inhibitor, or AR knockdown was comparable to GLUT12 suppression. This study identified the role of the AR/GLUT12 pathway in the development of poor responses to everolimus. Interference with AR/GLUT12 pathway may serve as a promising approach to promoting the translational application of everolimus in GC therapy.
Keywords: Androgen receptor, Apoptosis, Everolimus, Gastric cancer, Glucose transporter 12

 Global reach, higher impact
Global reach, higher impact