Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2023; 19(2):484-501. doi:10.7150/ijbs.78654 This issue Cite
Review
Novel Role of the SIRT1 in Endocrine and Metabolic Diseases
1. Department of Cardiology, Xi'an No.3 Hospital, The Affiliated Hospital of Northwest University. Faculty of Life Sciences and Medicine, Northwest University, Xi'an, China.
2. Key Laboratory of Resource Biology and Biotechnology in Western China, Ministry of Education. Faculty of Life Sciences and Medicine, Northwest University, Xi'an, China.
3. Department of General Surgery, Tangdu Hospital, The Airforce Medical University, 1 Xinsi Road, Xi'an 710038, China.
#These authors contributed equally to this work.
Received 2022-9-5; Accepted 2022-11-15; Published 2023-1-1
Abstract
Silent information regulator 1 (SIRT1), a highly conserved NAD+-dependent deacetylase, is a cellular regulator that has received extensive attention in recent years and regarded as a sensor of cellular energy and metabolism. The accumulated evidence suggests that SIRT1 is involved in the development of endocrine and metabolic diseases. In a variety of organisms, SIRT1 regulates gene expression through the deacetylation of histone, transcription factors, and lysine residues of other modified proteins including several metabolic and endocrine signal transcription factors, thereby enhancing the therapeutic effects of endocrine and metabolic diseases. These evidences indicate that targeting SIRT1 has promising applications in the treatment of endocrine and metabolic diseases. This review focuses on the role of SIRT1 in endocrine and metabolic diseases. First, we describe the background and structure of SIRT1. Then, we outline the role of SIRT1 in endocrine and metabolic diseases such as hyperuricemia, diabetes, hypertension, hyperlipidemia, osteoporosis, and polycystic ovarian syndrome. Subsequently, the SIRT1 agonists and inhibitors in the above diseases are summarized and future research directions are proposed. Overall, the information presents here may highlight the potential of SIRT1 as a future biomarker and therapeutic target for endocrine and metabolic diseases.
Keywords: SIRT1, endocrine metabolic, agonists, inhibitors
Introduction
SIRT1 is a nicotinamide adenine dinucleotide (NAD+)-dependent deacetylase [1], which deacetylates histone and non-histone proteins [2] and is involved in the regulation of many physiological functions, including endocrine, metabolic regulation, immune response, oxidative stress, inflammation, and ageing [3-6]. As a key regulator of energy, SIRT1 affects glucose and lipid metabolism by stimulating endocrine signaling, which is associated with many molecules related to glucose/lipid metabolism, such as Adenosine 5'-monophosphate (AMP)-activated protein kinase (AMPK), Forkhead box O3 (FOXO3), Glucose Transporter 4 (GLUT4), Peroxisome proliferators-activated receptor γ (PPARγ), and Proliferator-activated receptor-gamma co-activator-1α (PGC-1α) [7-10]. The activation of SIRT1 have been reported to improve insulin secretion [11]. In contrast, SIRT1 deficiency leads to the decrease in the secretion of thyroid hormone [12], estrogen [13], testosterone[14], and pituitary hormone [15], causing disorders of endocrine and metabolic system, thereby developing into obesity [16], diabetes [17], hyperuricemia [18], hyperlipidemia [19], hypertension [20]. According to current research on SIRT1, this review focuses on the role of SIRT1 in endocrine and metabolic diseases. First, we sketch out the background and structure of SIRT1. Then, we highlight the relationship between SIRT1 and endocrine and metabolic diseases. Subsequently, we summarize the agonists/inhibitors of SIRT1 in endocrine and metabolic diseases. Finally, we propose future research directions between SIRT1 and endocrine and metabolic diseases. Although mature researches have been appeared on SIRT1, it is still an attractive node in the field of endocrine and metabolic diseases and provides new target for the treatment of the above diseases.
Sirtuins is also known as silent information regulator 2 (Sir2) [21, 22]. Increasing the dosage or activity of Sir2 has been shown to extend the life spans of yeast, worms, and flies, while deletions or mutations of the Sir2 reverses the result [21, 23, 24]. Sirtuins family consists of 7 members (SIRT1-SIRT7), which can be divided into four categories according to their structural similarity. SIRT1, SIRT2 and SIRT3 belong to type Ⅰ, SIRT4 belongs to type Ⅱ, SIRT5 belongs to type Ⅲ, as well as SIRT6 and SIRT7 belong to IVa and IVb subtypes of type IV respectively. In addition, according to their distribution, they are also divided into nuclear proteins (SIRT1, SIRT3, SIRT6 and SIRT7), plasmic proteins (SIRT2) and mitochondrial proteins (SIRT4 and SIRT5). The deacetylation activity of SIRT1 and other sirtuins need the help of NAD+, which is a cofactor also involved in DNA damage repair. Through deacetylation, the acetyl group of the acetylated protein substrate is transferred to the ADP-ribose (ADPR) of NAD+, and products such as deacetylation protein, Nicotinamide (NAM) and 2-O-acetyl-ADP-ribose [25, 26]. As a classic member of Sirtuins family, SIRT1 is more conservative than other members in structure, which is of great concern [27].
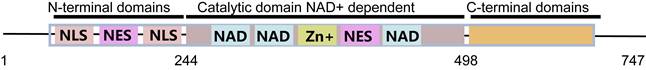
The SIRT1 gene is located on human chromosome 10 with a total length of approximately 33 Kb and contains 9 exons, 8 introns and untranslated regions [28]. The human SIRT1 protein is composed of 747 amino acids and mainly contains the three structures (central nuclear catalytic structural domain, nuclear localization signals (NLS), and nuclear export signals (NES)) [4, 25], there into the catalytic structural domain covers the substrate binding pocket and the NAD+ binding pocket (Figure 1) [29]. The histidine at position 363 of SIRT1, in which structural activity is dependent on the NAD+/NADH ratio in the cytoplasm, is an essential motif for deacetylation activity and involved in regulating the redox state and metabolic homeostasis [30]. SIRT1 acts as an important transcriptional regulator with lysine deacetylation on histones regulating chromatin structural stability and protein activity, thus participating in maintaining normal cellular functions [31]. For example, the deacetylation of SIRT1 affects the transcription of FOXO family members and PGC-1α and directly regulates lipid metabolism [32-34]. SIRT1 also deacetylates lysine at position 382 of p53 protein, preventing its transcriptional activation and p53-dependent apoptosis induction [35]. In addition to acetylation, other modifications of SIRT1, such as phosphorylation, ubiquitination, also partake in important physiological and pathophysiological functions. AMPK directly interacts with the deacetylase active domain of SIRT1 and phosphorylates Thr344 of SIRT1, which inhibits the deacetylation activity of SIRT1 [36]. Ubiquitination stabilizes proteins by covalently adding SUMO proteins to lysine residues. The Lys734of SIRT1 is modified by ubiquitin to increase the activity and stability of the protein [37]. Due to its unique structure and function, SIRT1 has also been confirmed to play a positive role in other aspects of mammalian health. SIRT1 deficiency leads to energy imbalance, endocrine and metabolic disorders [38]. Meanwhile, SIRT1 deficiency also promotes the occurrence of immunodeficiency, cardiovascular diseases, and aging-related diseases [39-42]. Therefore, the systematic summary and review of the role of SIRT1 in endocrine and metabolism-related cells and diseases is crucial for future research.
The role of SIRT1 in endocrine and metabolism-related cells
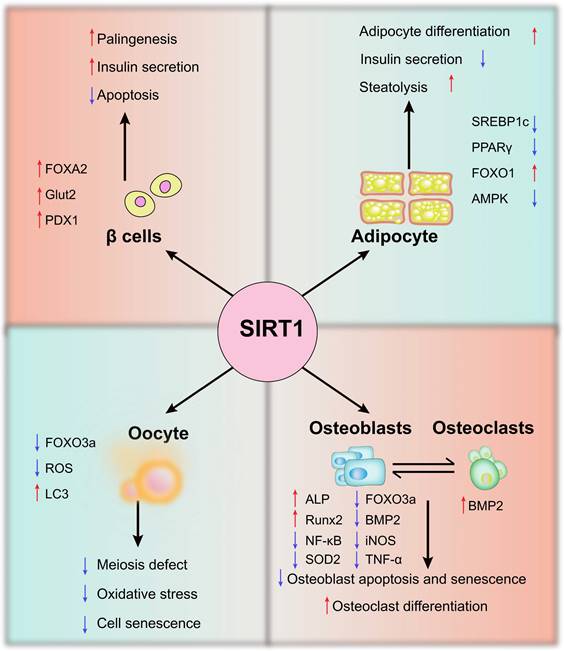
Endocrine and metabolic system are interrelated and influence each other. The endocrine system consists of endocrine glands, endocrine tissues, and endocrine cells, which includes beta cells, oocytes, osteoblasts, osteoclasts, and adipocytes (Figure 2).
The structure and functional domains of SIRT1 protein. SIRT1 protein mainly contains central nuclear catalytic domain (substrate binding bag and NAD+ binding bag), nuclear localization signal (NLS) and nuclear outlet signal.
Beta cells
Beta cells are insulin secreting cells in pancreatic islets, also known as B cells, accounting for about 60% of islet cells. Due to morphology and function, islet cells also contain alpha cells, delta cells, and pancreatic polypeptide cells [43]. It is well known that the impairment of beta cell function leads to insulin deficiency, which increases the level of blood glucose and diabetes. In addition, pancreatic beta cell canceration promotes the generation of insulinoma, which causes symptoms of malignant hypoglycemia [44]. The dynamic expression of SIRT1 was observed in endocrine progenitors both beta cell regeneration in neonatal rats and the second transition phase of mouse pancreas development. SIRT1 activation promotes beta cell regeneration by activating endocrine progenitor cells [45]. Similarly, SIRT1 enhances the secretion of insulin through NAD+-dependent deacetylation, and counteracts inflammatory signals to avoid islet cell apoptosis [46]. In addition, palmitate also decreased the expression of SIRT1 in INS-1 cells and isolated rat islets, while the result can be reversed by SIRT1. This is because SIRT1 overexpression enhances PDX1(Pancreatic and Duodenal Homeobox 1) stimulation and antagonizes FOXO1-inhibited insulin promoter activity [47]. PDX1 is essential for the pancreas development and beta cells formation. Wang et al., found that pancreas-specific knockout of SIRT1 inhibits PDX1 expression and impairs islet development. SIRT1 mutant mice develop progressive hyperglycemia, glucose intolerance, and insulin insufficiency, which directly correlate with SIRT1 deletion. They further confirmed that SIRT1 interacts with and deacetylates FOXA2 on the promoter of the PDX1 gene, and positively regulates its transcription. The above results suggest that SIRT1 is involved in the regulation of beta cell formation and pancreatic development [48]. Interestingly, Pinho et al., found that impairing the function of the beta cell without SIRT1 results in reduced insulin secretion, but it is not accompanied by elevated blood glucose. This may be a unique compensatory mechanism associated with decreased expression of the glucose transporter Slc2a2/Glut2 and glucagon like peptide-1 receptor as well as marked downregulation of endoplasmic reticulum [49].
The potential mechanisms of SIRT1 in endocrine - and metabolism-related cells. In β cells, adipocytes, oocytes, osteoblasts, and osteoclasts, SIRT1 regulates oxidative stress, apoptosis and senescence by regulating AMPK, FOXO1, PDX1 and other molecules
Oocytes
Endocrine system affects the development and maturity of female reproductive system and the function of female reproductive system [50]. Polycystic ovary syndrome (PCOS), primary ovarian insufficiency (POI), and other diseases are frequently accompanied by oocyte damage and senescence, follicular development, and atresia dysfunction [51, 52]. The ovaries are the source of oocytes and many reproductive hormones, including sex steroids, which is vital for female lifelong reproductive health. Ageing or damaged oocytes show elevated levels of reactive oxygen species (ROS) and impaired mitochondrial function, accompanied by the increased number of meiotic errors, unregulated autophagy-related proteins and early apoptosis, resulting in decreased oocyte quality and abnormal hormone secretion [53]. Notably, SIRT1 regulates key transcription factors involved in aging and longevity, and is closely related to oxidative stress and autophagy [54-56]. During the activation of pregranulosa cells (PGCs), oocytes and primordial follicles, the level of SIRT1 is memorably increased [57]. In addition, about half of the female mice with SIRT1 deficiency in oocytes become prematurely sterile between 9-11 months of age, the reduced ability or quality of oocyte development leads to increased oxidative stress in preimplantation embryos, which inhibits cleavage divisions. In view of that, all of that originate from defiance of oocyte-SIRT1 [58]. On the contrary, the activation of SIRT1 not only partially prevents the deficient phenotypes of ageing oocytes, but also alleviates meiosis abnormalities and oxidative stress of oocytes [59]. Autophagy degrades proteins and organelles which is degenerated and recycle their components in the cytoplasm, which is essential for the preimplantation process of early embryonic development in mammals [60]. Moreover, melatonin attenuates meiotic defects in oocytes by activating SIRT1 and regulating autophagy [61]. Tamura's study also confirmed that melatonin promotes the recovery of oocytes from the fallopian tube and allows normal fertilization in vitro. Further studies showed that the mRNA expression of SIRT1, light chain 3 (LC3), and telomere length are enhanced after melatonin treatment. Xu et al. showed that the significant increase of ROS during the postovulatory ageing inhibits SIRT1 expression, promotes the deacetylation of FOXO3a and inhibits Superoxide dismutase 2 (SOD2) expression, leading to the decrease in mitochondrial function and autophagy [62]. In conclusion, these studies show that the activation of SIRT1 is essential for the development of PCOS.
Osteoblasts and osteoclasts
Bone as an endocrine organ, which contributes to physiological regulation, cognition, glucose metabolism and hormone balance. The balance between osteoblasts and osteoclasts determines the quality of bone [63]. SIRT1 expression is reduced in cartilage and subchondral bone plate in patients with osteoarthritis [64]. In addition, SIRT1 maintains the balance between bone formation and resorption by regulating the ratio of osteoblasts to osteoclasts [65]. A recent study also found that resveratrol (Res), the SIRT1 agonist, significantly improved bone quality and reduced serum alkaline phosphatase and osteocalcin levels in the rats with osteoporosis [66]. Osteocalcin is a hormone secreted by osteoblasts and has been shown to be involved in insulin secretion, insulin resistance and energy expenditure [67]. In MC3T3-E1 cells, inflammation increase apoptosis and decrease alkaline phosphatase (ALP) activity. While overexpression of SIRT1 inhibits osteoblast apoptosis, increases ALP activity and the expression of runt-related transcription factor 2 (Runx2) and osteocalcin. It was also found that overexpression of SIRT1 protects osteoblasts against tumor necrosis factor-α (TNF-α)-induced cell injury, at least in part, by repressing NF-κB activity and genes downstream of NF-κB, including iNOS. Specifically, TNF-α promotes iNOS expression and NO production by mediating NF-κB signaling pathway, while SIRT1 overexpression can reverse these results [68]. Notably, Runx2 is the gene that encodes for the protein involved in the osteogenic differentiation process from mesenchymal precursors [69]. Runx2 haploinsufficiency leads to the skeletal disorder characterized by bone and dental abnormalities known as cleidocranial dysplasia [70]. Hong et al. found that atorvastatin increases bone mass and promotes osteogenesis in ageing apolipoprotein E-deficient mice by activating SIRT1-Runx2 axis [71]. Additionally, Res increases the formation of SIRT1 and FOXO3a complex, regulates the activity of Runx2 promoter, and promotes ossification of human MSCs [72]. Bone morphogenetic protein 2 (BMP2) is the key factor in inducing cartilage differentiation. SIRT1 can promote the BMP2-induced cartilage differentiation of MSCs and reduce the apoptosis and decomposition of extracellular matrix under oxidative stress [73]. Zhao et al. also highlighted that Res dose-dependently increases both ALP and endothelial nitric oxide synthases (eNOS) levels, increase ALP, Runx2 and BMP2 and stimulate bone formation. On the contrary, SIRT1defiance reduces eNOS, BMP2 and ALP. The evidences describe above suggest that SIRT1 is the key molecule which regulates osteoblast differentiation and bone homeostasis [74].
Adipocytes
Adipocytes are essential for regulating pathological conditions such as obesity, diabetes and metabolic syndrome [75]. SIRT1 knockout porcine preadipocytes are reduced and apoptosis is increased [76]. Additionally, the mice with adipocyte-selective deletion of SIRT1 are more susceptible to diet-induced insulin resistance, which is associated with the increase in the number of adipose-resident macrophages and their polarization to the pro-inflammatory M1 subtype [77]. It has been reported that SIRT1 inhibits the transcriptional activities of PPARγ (the key factors in adipocyte differentiation) and sterol regulatory element-binding protein 1c (SREBP1c) through deacetylation, thus suppressing adipocyte differentiation, reducing fat accumulation, and promoting fat mobilization [78, 79]. SIRT1 overexpression can deacetylate Lys293 and Lys268 of PAPRγ and induce white adipose tissue remodeling [80]. However, in SIRT1 knockout mice, fatty acid mobilization of white adipocytes is disrupted after fasting, and SIRT1 also significantly inhibits PPARγ in adipocytes. Repression of PPAR-γ by SIRT1 is also evident in adipocytes, where overexpression of SIRT1 attenuates adipogenesis, and RNA interference of SIRT1 enhances it [79]. FOXO1 can be localized to the nuclear, cytoplasmic and mitochondrial compartments of adipocytes, affecting the source of ROS [81]. The level of FOXO1 is decreased in adipocytes of db/db mice. Res can transport FOXO1 to the nucleus by activating SIRT1 and increase the level of FOXO1 in adipocytes [82]. In addition, SIRT1 controls acetylation status and functional activity of FOXO1 that directly binds to the adipose triglyceride lipase (ATGL) promoter and regulates the transcription of ATGL gene, reducing the expression of AMPK in adipocytes [83] Activated SIRT1 reduces phosphorylated-FOXO1 expression, thereby activating FOXO1 and inhibiting adipogenesis of adipocytes [84]. Taken together, the above results indicate that SIRT1 is a novel adipocyte regulator that is associated with multiple signaling pathways such as PPARγ and FOXO1 to regulate adipocyte differentiation, fat accumulation and energy metabolism.
The role of the SIRT1 in endocrine and metabolic diseases
Under pathological conditions, endocrine disorders lead to abnormal function of endocrine glands, and then endocrine and metabolic diseases. SIRT1 in mammals can regulate the expression of target genes through various modifications, and plays an important role in endocrine and metabolic diseases [85, 86].
Hyperuricemia
Uric acid is a metabolite of purine, and it can increase and eventually lead to gout/hyperuricemia when there is a disorder of purine metabolism or uric acid excretion [87]. Hyperuricemia is a common endocrine and metabolic disease in middle-aged and elderly men, which has seriously harmed human health [88]. The traditional Chinese herb Smilax china L (effective component Res) has been used to treat hyperuricemia, gout and related kidney diseases. Moreover, studies have found that Res can reduce xanthine oxidase (XO), serum uric acid level, uric acid excretion fraction and blood urea nitrogen to normal state [89, 90]. In the mouse with hyperuricemia, the expression of ATP-binding cassette subfamily G member 2 in ileum was activated by acetylation PGC-1α/PPARγ pathway after SIRT1 activation by Res, reducing the level of serum uric acid, thus exerting an anti-hyperuricemia effect [91]. Additionally, hyperuricemia promotes the proliferation of vascular smooth muscle cells (VSMCs) via activating the renin-angiotensin-aldosterone system, causing renal vasoconstriction and glomerular arterial wall thickening [92, 93]. Ma et al. found that Simiao pill restores high fructose-induced hyperuricemia and metabolic syndrome by up-regulating SIRT1 in glomerular of mice with high-fructose, inhibiting NF-κB pathway and the activation of NOD-like receptor pyrin domain containing 3 (NLRP3) inflammasome, improving interstitial infiltration of nephritis cells and glomerular injury and reducing urinary albumin level [94]. Polydatin has also been shown to inhibit NF-κB/NLRP3 through the AMPK/SIRT1 pathway, thereby reducing potassium oxonate-induced hyperuricemia and renal inflammation [18]. Xu et al. also found that hyperuricemia was associated with decreased SIRT1, phosphorylation of downstream target molecules FOXO3a, increased expression of androgen receptor, XO and deacetylation of NF-κB subunit p65. Importantly, they further found that hyperuricemia is more frequent and widespread in men with nonalcoholic fatty liver disease (NAFLD) than in women, which is intimately contributed to the inhibition of SIRT1 signaling pathway induced by hyperuricemia [95, 96]. Interestingly, elevated estrogen levels caused by SIRT1 may also be responsible for treatment of hyperuricemia. H Sumino pointed out that postmenopausal women with hyperuricemia are treated with hormone replacement therapy to reduce serum uric acid level [97, 98]. In conclusion, these studies suggest that SIRT1 may be a potential target of hyperuricemia and its complications, which is of great significance for further clinical research and application.
Hypertension
Hypertension is a major risk factor for premature death and disability worldwide [99, 100]. The pathogenesis of hypertension can also lead to dysfunction of the nervous system [101], endocrine system [102] and immune system [103]. The information connection between these systems is mainly accomplished by neuropeptides and endocrine hormones, including neuropeptide Y [104], angiotensin II (Ang II) [105], arginine-vasopressin [106], endothelin [107] and NO [108]. Ryohei et al. reported that Res suppresses the expression of AT1R in the mouse aorta by activating SIRT1 and ameliorates Ang II-induced hypertension. Meanwhile, overexpression of SIRT1 reduces the expression of AT1R, while SIRT1 inhibitor (nicotinamide) reverses this effect. Further studies showed that the suppression of AT1R depends on the most proximal promoter region, which contains the Sp1 binding site (GC box). GC box mutation of luciferase construct failed to respond to Res, suggesting that Sp1 site plays an important role in Res-induced AT1R downregulation [109]. Nicotinamide phosphoribosyl transferase (NAMPT) is a potential cardiovascular protective effect of adipose cytokines, which plays an important role in DNA damage repair and prevent premature VSMCs in aging, and is also a key enzyme regulating NAD+ biosynthesis and SIRT1 activity [110, 111]. The overexpression of NAMPT partially inhibits Ang II-induced elevated ROS levels by regulating SIRT1 and the concentration of NAD+, thus relieving Ang II-induced hypertension. This observation suggests that NAMPT may regulate the occurrence of hypertension through SIRT1 [112]. In addition, endothelial dysfunction is also considered as a possible early key link in the occurrence of hypertension [113]. Extracellular vesicles collected from induced pluripotent stem cell-derived mesenchymal stem cells, may reduce age-related endothelial dysfunction, arteriosclerosis, and hypertension by activating the SIRT1-AMPKα-eNOS pathway [114]. Grape seed proanthocyanidin extracts (GSPE) indirectly up-regulates SIRT1 and inhibits aortic NO production disorder, improving hypertension and showing the potential of anti-inflammatory, antioxidant, anti-ageing and regulation of endothelial function [115-117]. Klotho, an ageing-suppressor gene, whose mutations results in significant increases in pulse wave velocity and blood pressure. Importantly, Gao et al., pointed out that serum Klotho deficiency in hypertensive patients is associated with significantly reduced activity of AMPKα, SIRT1, and eNOS in aortic endothelial cells (ECs), along with collagen expression and elastin breakdown. Conversely, activation of SIRT1 functionally interacts with AMPKα, up-regulates phosphorylation of AMPKα and then activates eNOS, inhibits ROS accumulation and oxidative stress in aortic ECs and leads to vascular remodeling, down-regulates collagen expression and elastin breakage, predicting the improvement of aortic sclerosis and hypertension [118].
Polycystic ovarian syndrome
PCOS is the most common endocrine and metabolic disordered disease in the women [119, 120]. This may be the result of hypothalamo-pituitary ovarian axis disorder, follicular membrane cells or granulosa cells (GC) dysfunction, and metabolic abnormalities [121-123]. PCOS is associated with specific reproductive health complications, including lower oocyte quality and clinical pregnancy rates in assisted conception cycles. Metformin, an anti-aging agent, is approved for the treatment of PCOS [124]. Clinical trials and observational studies have found that metformin can prevent or mitigate PCOS through SIRT1-related pathways [125]. Increased testosterone level is the pathological feature of PCOS patients, and when the PCOS patients are treated with Res, the level of serum testosterone decreases, the number of secondary and closed follicles increases, while Graafian follicles decrease. It was further found that the combination of metformin and Res induce the antioxidant and anti-inflammatory systems of PCOS by activating SIRT1 and AMPK, thereby improving the weight gain, hormone levels and follicular cell structure of PCOS [126]. Tao et al. found that SIRT1 expression in PCOS group is significantly lower than that in the control group in the establishment of PCOS rat induced by dehydroepiandrosterone, and shows the loss of estrous cycle, saccular dilatation of the follicle, reduce luteal number, and thickens follicular membrane cell layer [127]. The researcher also found that both metformin and exenatide improve reproductive endocrine function in PCOS rats via the AMPKα-SIRT1 pathway [128]. It is suggested that the deficiency of SIRT1 and estrogen promotes the occurrence of diseases related to female reproductive development. In addition, quercetin can up-regulate the expressions of AMPK and SIRT1 in ovarian tissues, and reverse the changes of adiponectin, visfatin and resistin in adipose tissues of PCOS rats, thus maintaining hormone and metabolic balance [129]. Interestingly, SIRT1 knockdown in human ovarian GC inhibits estrogen synthesis activity and aromatase [130]. Previous studies have pointed out that SIRT1 regulates non-histone activity and affects aromatase transcription regulation [131]. However, enhanced aromatase activity will increase PCOS susceptibility [52]. SIRT1 plays an important role in the genesis and development of PCOS, which provides a basis for the development of potential therapeutic methods to improve the metabolism and reproductive function of PCOS.
Osteoporosis
Osteoporosis is the disease induced by genetic and environmental interference with the endocrine system [132]. With aging, the decrease of NAD+ leads to the decrease in bone progenitor cells and bone mass, accompanied by the imbalance in the number and activity of osteoblasts and osteoclasts [133, 134]. Menopausal, ovariectomized female mice and aged male mice exhibit decreased SIRT1, osteoporosis and bone injury [135]. Meanwhile, SIRT1 knockout mice, as well as osteoblast and osteoclast specific knockout, show a low bone mass phenotype [136]. Significantly reduced bone mass, reduced bone formation and increased bone marrow adipose formation are observed in female SIRT1 haplo-insufficient (Sirt1+/-) mice, along with osteoarthritis [137, 138]. Importantly, clinical studies have identified the potential of SIRT1 in predicting and treating related diseases such as osteoporosis and osteonecrosis. SIRT1 expression in femoral neck was significantly reduced in patients with osteoporosis [139]. Additionally, in 16 female patients with osteoporosis, there is a negative correlation between SIRT1 activity in peripheral blood mononuclear cells and C-terminal cross-linking telopeptide of type I collagen, the marker of bone resorption in serum [140].
Besides, overexpression of SIRT1 inhibits H2O2-induced osteoblast apoptosis by activating the FOXO1/β-catenin pathway [141]. One estrogen, 17β-E2 (10-6 M) up-regulates SIRT1, p-AMPK and FOXO3a in osteoblasts, thereby inhibiting osteoblast apoptosis by promoting autophagy [142], while the protective effect of autophagy may be attributed to reducing intracellular oxidative damage and maintaining cell structure and function [143]. Res and endoplasmic reticulum stress (ERS) inhibitor 4-PBA significantly inhibit osteoclast differentiation and osteolysis [144]. Activation of SIRT1 can regulate the activity of osteoclasts and osteoblasts and improve bone metabolism, thereby reducing osteoporosis [145]. In conclusion, SIRT1 plays a positive role in maintaining bone homeostasis, bone mineralization and bone resorption, which also brings hope for the treatment of osteoporosis.
Diabetes
As a metabolic disease, diabetes is characterized by defective insulin secretion or insulin dysfunction leading to impaired islet beta cell function [146]. Study confirmed that the SIRT1 level was always lower in patients with poor glycaemic control than in those with good glycaemic control [147]. In the isolated rat islets, SIRT1-mediated NF-κB deacetylation inhibits iNOS and cytokine-mediated beta cell damage [148]. SIRT1 activation also promotes beta cell recovery and endocrine progenitor differentiation [45]. These studies revealed that SIRT1 plays an active role in regulating insulin secretion. Notably, direct sequencing and exon sequencing of a patient with type 1 diabetes revealed t-to-C exchange in SIRT1 exon 1 and excessive production of nitric oxide, cytokines and chemokines, suggesting that SIRT1 mutation may be a potential weakness of patients with diabetes [149]. There are many strategies and drugs for the treatment of diabetes in clinic, among which metformin is the star of clinical drug. Hyperglycemia induced expression of inflammatory genes, NF-κB and the proapoptotic gene Bax in bovine retinal capillary endothelial cells (BRECs) and diabetic rat's retinas. BRECs with knockdown SIRT1 increases sensitivity to hyperglycemic stress, while SIRT1 overexpression or metformin activation inhibit mitochondrial ROS-mediated PARP activity and glyceraldehyde-3-phosphate dehydrogenase through upregulation of LKB1/AMPK, and ultimately inhibiting NF-κB and Bax expression. It was proved that metformin may be associated with the SIRT1/LKB1/AMPK pathway in inhibiting diabetic retinopathy [150]. High glucose treatment significantly reduces the expression of SIRT1 protein in mouse microvascular ECs. However, overexpression of SIRT1 or metformin can attenuate the decreased expression of SIRT1 induced by high glucose, thus regulating downstream targets FOXO1 and p53/p21, and protecting ECs from high glucose-induced premature aging [151]. In addition, SIRT1 is also involved in the occurrence of other diabetes-related diseases. Decreased hepatic glucose production in type 2 diabetes rats with reduced hepatic SIRT1 levels, and systemic SIRT1 activation induced by drugs or genes can prevent dietary diabetes [152]. Knockout of SIRT1 leads to hyperglycemia and insulin resistance in the liver [153]. Importantly, the positive effect of SIRT1 has also been confirmed in diabetic nephropathy [154, 155] and diabetic cardiomyopathy [156].
Hyperlipidemia
Hyperlipidemia is a common disease of dyslipidemia caused by endocrine and metabolic disorders [157, 158]. Hyperlipidemia is clinically divided into the following categories: hypercholesterolemia, hypertriglyceridemia, mixed hyperlipidemia and atherosclerotic dyslipidemia [159]. SIRT1 is the major regulatory factor of lipid and carbohydrate metabolism. The reduction of SIRT1 will cause metabolic disorders, fatty liver and obesity [160]. Reciprocally, enhancing the activity of SIRT1 may normalize abnormal fat morphology and abnormal expression of lipid metabolism markers, thus regulating cholesterol and lipid metabolism [161, 162]. SIRT1 is involved in the caspase-1 pathway in early hyperlipidemia and promotes ECs activation prior to monocyte recruitment [163]. In addition, SIRT1 is closely associated with lipid metabolism markers, including PPARα/γ [80], SREBP [164], Liver X Receptor α (LXRα) [165] and low-density lipoprotein (LDL) receptor [166]. For example, the accumulation of oxidized LDL (oxLDL) in peritoneal macrophages of SIRT1-deficient mice increases and promotes the formation of foam cells. SIRT1 reduces the expression of lectin-like oxLDL receptor-1 by inhibiting NF-κB signaling pathway, thus reducing oxLDL uptake and alleviating atherosclerosis [167]. SIRT1 also leads to lipolysis of mature adipocytes by enhancing the activity of PPARα [79] and directly deacetylates SREBP during fasting, which leads to inhibition of lipid synthesis and fat storage [164]. In addition, PPARα may indirectly affect lipid synthesis through cross-talk with SREBP and exploit the advantages to the full in regulating cellular fatty acid and cholesterol homeostasis [168, 169]. AMPK is a nutrition-sensing molecule which correspondingly reduces fatty acid synthesis [170]. Increased SIRT1 and AMPK activity inhibits dysregulation of lipids and obese phenotypes [171]. In the hyperlipidemia-induced hepatic steatosis and atherosclerotic mice, SIRT1 restores cholesterol efflux caused by hyperlipidemia through regulating the LXRα/β-PPARγ pathway [172]. Notably, there are several compounds which alleviates lipid metabolism diseases such as hyperlipidemia by activating SIRT1. As the cofactor of SIRT1, nicotinic acid increases p-AMPK and SIRT1 in adipocytes and myotube, reduces total cholesterol, cholesterol esters, plasma triglycerides, and lessens the size of atherosclerotic lesions and lipid area [173]. Ginsenoside Rb2 prevents hepatic lipid accumulation in vivo and in vitro through SIRT1-mediated autophagy induction [174]. Melatonin improves serum biochemical markers and liver morphological damage, and inhibits oxidative stress through its antioxidant properties and upregulation of SIRT1 [175]. Importantly, He et al. assessed the association between serum SIRT1 levels and coronary atherosclerotic plaque characteristics by computed tomography angiography (CTA) and Framingham Risk Score generation in each patient. The results showed that serum SIRT1 level is significantly reduced in the non-high-risk plaque group. It is suggested that SIRT1 may play a predictive role in coronary artery pre-CTA plaque screening [176]. These evidences confirm the positive effect of SIRT1 activation on hyperlipidemia.
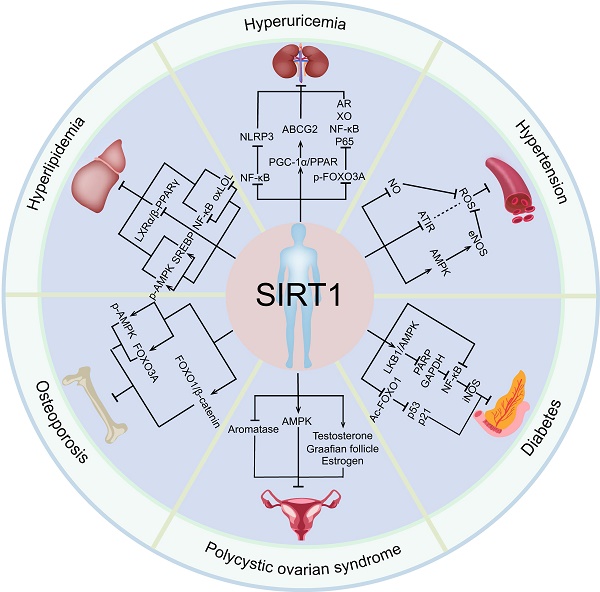
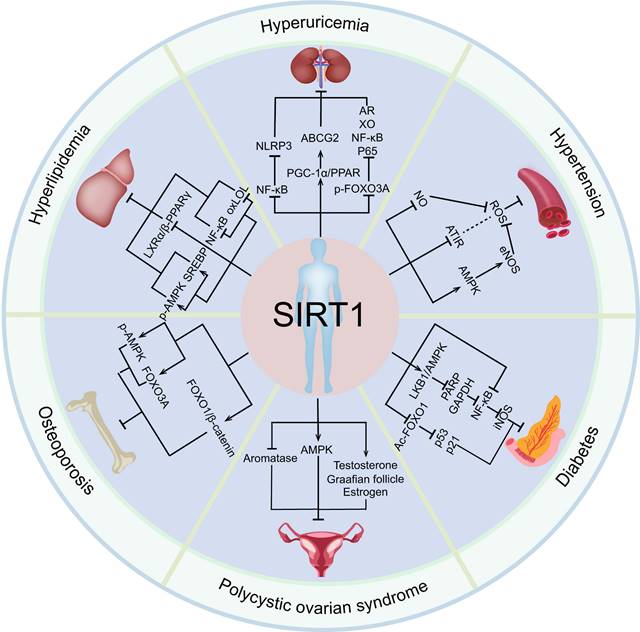
In conclusion, SIRT1 has a significant protective effect on a variety of endocrine diseases, such as hyperuricemia, hypertension, polycystic ovarian syndrome, osteoporosis, by regulating a variety of target genes (Figure 3). Importantly, SIRT1 have been widely studied in clinical practice and gradually become key molecules in the treatment of endocrine and metabolic diseases and aging related diseases [177].
Application of SIRT1 agonists in endocrine and metabolic diseases
A large number of studies have shown that SIRT1 has positive effects on a variety of diseases, along with the development of different SIRT1 activators, including Res [178], SRT2183 [179], SRT1460 [180], SRT1720 [181], SRT2104 [182] and SRT3025 [183] (Table 1).
Res
Res is a natural polyphenol with strong biological activity, also known as astragalus triphenol [184]. Res has been paid attention to by the medical community, with anti-oxidation, antibacterial, anti-inflammatory, anti-aging and estrogen-like activities [185-187]. Many formulations containing Res have been shown to be beneficial in healthy, obese male by reducing lipid content, circulating glucose, triglycerides and inflammatory markers in the liver [188-190]. The combination of Res with SIRT1 promotes the conformational change of SIRT1 and enhances its activity [191]. Res affects thyroid function by enhancing iodide ion capture, and increases thyrotropin secretion by activating SIRT1[192]. It also enhances insulin sensitivity and reduces hepatic glucose production [17]. In addition, Res promotes browning in a SIRT1-dependent manner and has a beneficial effect on excess fat utilization, suggesting potential therapeutic application of Res in the treatment of obesity and related metabolic disorders [54]. Sara et al. systematically analyzed the effects of Res in animal and clinical studies. Many animal studies have reported beneficial effects of Res on sex hormones, gonadotropins, and blood glucose. In particular, Res improves ovarian volume, high-quality oocyte rate, high-quality embryo rate, androgen, and gonadotropin concentration in PCOS patients [193]. A meta-analysis was conducted to review the effects of Res intake on weight loss. This study showed that the supplementation of Res significantly reduces body weight, body mass index, waist circumference, and fat mass, and increases lean mass [194]. It is worth noting that a clinical study assessed the effects of short-term high-dose Res administration on intestinal and hepatic lipoprotein turnover and insulin sensitivity in non-diabetic, overweight, and obese male who had mild hypertriglyceridemia. Res was given at doses of 1000 mg/day for one week and increased to 2000 mg/day the second week. In this study, Res improved glucose tolerance without causing any adverse reactions, however, there was no significant effect on insulin sensitivity and plasma triglyceride content, which may be related to the time and concentration of the drug [190]. Notably, Res has a positive effect on metabolism (URL: www.clinicaltrials.gov. Unique Identifier: NCT01451918.) [190]. Interestingly, Res reduced cerebrospinal fluid (CSF) metalloproteinase 9 (MMP9), modulated neuroinflammation, improved mild-to-moderate Alzheimer's disease, and induced adaptive immunity. SIRT1 activation may be a viable target for the treatment or prevention of neurodegenerative diseases [177]. Additionally, in vivo res pretreatment confers neuroprotection similar to ischemic preconditioning (IPC) via the SIRT1-UCP2 (mitochondrial uncoupling protein 2) pathway [195]. Furthermore, potential effect modifications by sex, smoking and vascular risk factors of the SIRT/UCP genes in the associations with atherosclerotic plaque [195]. Meanwhile, polymorphisms in SIRT6/UCP1 genes may be important for increased carotid plaque burden and echodensity, but translation of these findings to an individual risk of cerebrovascular events needs further investigation [196]. These basic studies and randomized controlled trials provide potential evidence for the clinical application and development of Res as a daily dietary supplement.
The relationship between SIRT1 and endocrine and metabolic diseases. Activated SIRT1 can prevent the occurrence and development of various endocrine and metabolic diseases, including hyperuricemia, hypertension, polycystic ovary syndrome, osteoporosis, diabetes, and hyperlipidemia.
The roles of SIRT1 agonists in endocrine and metabolic diseases.
| Compound | Molecular formula | Molecular weight | Model | Vitro/Vivo | Dosage and Administration | Effects | Reference |
|---|---|---|---|---|---|---|---|
| Res | C14H12O3 | 228.24 | Obesity rats models | Rats | AIN93G supplemented with 2 g/kg or 4 g/kg diet, 12 weeks | Res reduces adipocyte browning and fat loss, and improves other metabolic phenotypes, including hyperglycemia and hyperlipidemia in mice, which are mediated through SIRT1. | [54] |
| Res | C14H12O3 | 228.24 | Yeast polysaccharide and potassium oxonate induced hyperuricemia model in C57BL/6 mice | C57BL/6 female mice | 20 or 40 mg/kg, intragastrically, 0, 7, 14, 21 and 28day | SIRT1 and its activator, Res, have clear anti-hyperuricemia functions in this mouse model, which possible mechanism is the activation of ABCG2 in the ileum through the PGC-1α/PPARγ pathway. | [91] |
| Res | C14H12O3 | 228.24 | Dehydroepiandrosterone-induced polycystic ovary syndrome rats | Rats | 20 or 40 mg/kg, intraperitoneal injection, 28day | Res combined therapy may improve the weight gain, hormone profile, and ovarian follicular cell architecture by inducing antioxidant and anti-inflammatory systems via SIRT1 and AMPK activation in polycystic ovary syndrome. | [126] |
| Res | C14H12O3 | 228.24 | Obesity model and nicotinamide-streptozotocin-high fat diet induced mild type 2 diabetes rats | Rats | 60 ng/kg min, infusion into the lumen of the duodenum, 50 min | Res reverses a 3d high fat diet-induced reduction in duodenal-mucosal Sirt1 protein levels while also enhancing insulin sensitivity and lowering hepatic glucose production. | [17] |
| Res | C14H12O3 | 228.24 | Particle-induced osteolysis (PIO) animal | C57BL/6 female mice | 60 mg/kg, intragastrically, 2 weeks. | Res inhibits endoplasmic reticulum stress and reduces polyethylene particle - induced osteoclast differentiation and osteolysis. | [144] |
| SRT3025 | C31H31N5O2S2 | 569.74 | Apolipoprotein E-deficient mouse of atherosclerosis | C57BL/6J mice | 3.18 g/kg diet, 12 weeks | SRT3025 can reduce plasma cholesterol levels, inflammation and atherosclerosis, and increase LDL receptor (Ldlr) and proprotein convertase subtilisin/kexin type 9 (Pcsk9) accumulation in the liver. | [183] |
| SRT3025 | C31H31N5O2S2 | 569.74 | Ovariectomy (OVX)-induced bone loss | C57BL/6J mice | 50 and 100 mg/kg/day, Oral administration, 6 weeks. | Treatment with SRT3025 decreased bone sclerostin expression and increased cortical periosteal mineralizing surface and serum propeptide of type I procollagen, a bone formation marker. | [197] |
| SRT3025 | C31H31N5O2S2 | 569.74 | Streptozotocin (STZ)-induced diabetes | Male CD1 mice | 3.18 g/kg, milled in chow, 20 weeks. | Treatment with SRT3025 diminished their glucagon secretion and proliferative activity in association with a reduction in the alpha cell associated transcription factor, Aristaless Related Homeobox (Arx) | [198] |
| SRT3025 | C31H31N5O2S2 | 569.74 | Advanced oxidation protein products (AOPPs) induce osteoporosis. | C57Bl/6 mice | 50 mg/kg, in drinking water, 16 weeks. | SRT3025 inhibits the expression of sclerosclerin in bone cells by activating SIRT1, and reduced the resorptive activity and formation activity of bone tissue, thereby alleviating age-related bone loss | [200] |
| SRT3025 | C31H31N5O2S2 | 569.74 | Diabetes mouse | Old male db/db mouse | 3.18 g/kg, milled in chow, 12 weeks. | While reducing hyperglycaemia and promoting beta cell expansion, enhancing the activity of SIRT1 facilitates a phenotypic change in a db/db mouse model of diabetes to one that more closely resembles the physiological state of torpor or hibernation. | [238] |
| Nicotinic acid | C6H5NO2 | 123.11 | Hyperlipidemia and atherosclerosis mice | LDL receptor knockout mice | 50, 250, or 1000 mg/kg, milled in chow, 8 weeks. | Nicotinic acid reduces total cholesterol, cholesterol esters, plasma triglycerides, atherosclerotic lesion size, lipid area, and aortic macrophage infiltration. | [239] |
| ginsenoside Rb2 | C53H90O22 | 1079.27 | Nonalcoholic fatty liver disease (NAFLD) and glucose tolerance model | C57BL/KsJ-Lepdb (db/db) mice | 10 mg/kg/day, intraperitoneal injection, 4 weeks. | Rb2 alleviates hepatic lipid accumulation by restoring autophagy via the induction of sirt1 and activation of AMPK, and resultes in improved NAFLD and glucose tolerance. | [174] |
| Melatonin | C13H16N2O2 | 232.282 | Apolipoprotein E-deficient mice (ApoE-/-) | C57BL/6 male mice and ApoE-/- male mice | 10 mg/kg/day, dissolved in 1% ethanol and then diluted in tap water, 9 weeks | Melatonin treatment improves serum biochemical markers and hepatic morphological impairment and inhibited oxidative stress through its antioxidant properties and also by SIRT1 upregulation. | [175] |
| Melatonin | C13H16N2O2 | 232.282 | Polycystic ovary syndrome (PCOS) and premature ovarian failure (POF) mice | ICR(CD-1) mice Liver Microsomes (Female) | 15 mg/kg/day, intraperitoneal injection, 2 days | Melatonin suppresses FOXO1 via the PI3K-AKT axis, improves granulosa cell resistance to oxidative stress, and abolishes the autophagic response, from genes expression to the formation of autophagic vacuoles. | [217] |
| Melatonin | C13H16N2O2 | 232.282 | chronic stress mice | Female BALB/c mice | 20 mg/kg/day, intraperitoneal injection, 28 days | Melatonin alleviates chronic stress-induced oxidative meiosis defects in mouse MII oocytes by regulating SIRT1 and autophagy | [61] |
| SRT1720 | C25H24ClN7OS | 506.02236 | Type 2 diabetic mice | Male C57BL/KsJ db/db mice | 50 mg/kg/day, gavage,10 weeks. | SRT1720 inhibits the expression of HF1 α, GLUT1 and SNAIL by activating SIRT1, and alleviates and prevents diabetic induced renal fibrosis | [211] |
| SRT1720 | C25H24ClN7OS | 506.02236 | High-fat diet mice | Adult female Kunming mice | 50 mg/kg/day, intraperitoneal injection, 6 weeks. | SRT1720 may improve the follicle pool reserve in high-fat diet-induced obese female mice via activating SIRT1 signaling and suppressing mTOR signaling, thus extending the ovarian lifespan. | [212] |
| Metformin (MF) | C₄HN₅ | 129.164 | polycystic ovary syndrome (PCOS) rats | Female rats | 300 mg/kg/day, subcutaneous injection, 4 weeks. | MF can improve the reproductive and endocrine functions of rats with PCOS via the AMPKα-SIRT1 pathway. | [128] |
| Quercetin | CHO7 | 302.236 | Letrozole-induced Polycystic ovary syndrome (PCOS) mice | Female Wistar rats | 100 mg/kg/day, intragastric administration, 30 days. | Treatment with quercetin improves the PCOS related disturbances in estrous cycle, lipid profile, serum levels of testosterone, estradiol and progesterone, and insulin resistance. Besides, the expression levels of AMPK and SIRT-1 in ovarian tissue are upregulated in the rats which received quercetin. Quercetin also reverses the PCOS-induced alteration in adipose tissue levels of adiponectin, visfatin, and resistin. | [129] |
SRT3025
As the SIRT1 small molecule activator with oral activity, SRT3025 increases the expression of hepatic LDL receptors and accumulation of proprotein convertase subtilisin/kexin type 9 (Pcsk9), reduces plasma cholesterol level, inhibits inflammatory response and atherosclerosis [183]. Estrogen deficiency can lead to rapid bone loss and skeletal fragility. Oral administration of SRT3025 (50 and 100 mg/kg/d) for 6 weeks completely reverses the harmful effects of ovariectomy on bone mass and bone structure. SRT3025 achieves its therapeutic effect by decreasing the expression of bone sclerostin, increasing cortical periosteal mineralizing surface and serum propeptide of type I procollagen (a bone formation marker). Additionally, SIRT1 inhibitor EX-527 reverses the positive effects of SRT3025 in vitro. This study provides a theoretical basis for SRT3025 in metabolic and age-related diseases such as osteoporosis [197]. Diabetic mice treated with SRT3025 has significantly improved blood glucose, reduced islet alpha cell mass and decreased plasma glucagon concentration. Consistent with the decrease in glucagon abundance, overexpression of key gluconeogenic enzymes, glucose-6-phosphatase and phosphoenolpyruvate carboxykinase (PCK1), which are associated with diabetes, are also decreased by SRT3025 [198]. In addition, pharmacological activation of SIRT1 by SRT3025 increases the expression of several thermogenic genes FOXC2, PGC-1α, Dio2, TFAM and Cyc1 in C3HT101/2 cells. Notably, SRT3025 treatment increases PGC-1α mRNA and protein levels through activating SIRT1 in femoral MSCs in female patients undergoing hip operations caused by fracture or osteoarthritis. These evidences confirm that SRT3025 activates SIRT1 and upregulates PGC-1α to stimulate a thermogenic gene program in mouse and human bone marrow adipocytes [199]. SRT3025 inhibits the expression of sclerotin in osteocytes and thus inhibits age-related bone loss [200]. Importantly, a phase I clinical trial of SRT3025 at different doses in the treatment of diabetes has been completed (NCT01340911). This evidence will provide outstanding guidance for the development and application of SIRT1 agonists.
SRT2183
SRT2183, a selective SIRT1 activator, can bind to SIRT1 enzym-peptide substrate complex, reduces the Michaelis constant for acetylated substrates, and directly activates SIRT1 through allosteric mechanism [181, 201]. The EC1.5 (the compound concentration required to increase enzyme activity by 50%) of SRT2183 is about 0.36 μM, and the maximum activation is 296%. SRT2183 reduces the acetylation of SIRT1 substrate p53. SRT2183 is used as a positive control in SIRT1 deacetylation because of its good tolerability [181]. SRT2183 activates the expression of AMPK and SIRT1 and reduces the acetylation level of lysine 310 of RelA/p65[202]. In bone marrow macrophages, SRT2183 inhibits RANKL-induced osteoclast formation and resorption ability, suggesting that SRT2183 plays a positive role in bone metabolism [202]. Moreover, high glucose exposure induced the expression of p53, SIRT1 and AMPK in HepG2 cells. SRT2183 reverses this result and reduces triglyceride accumulation and cytoplasmic oxidative stress [203]. In addition, many metabolic syndromes are associated with reduced kidney function, and the medulla is critical in regulating water and sodium balance and maintaining normal blood pressure. Activated SIRT1 by SRT2183 reduces apoptosis and increases fibrosis in unilateral ureteral obstruction [204]. Recent studies have shown that SRT2183 also inhibits the growth of ovarian cancer cells. In terms of mechanism, SRT2183 has anti-ovarian cancer effects by activating the apoptosis pathway and increasing the level of LC3II, enhancing the degradation of p62/SQSTM1, and inducing the maturation of autophagosomes [205]. However, compared with other SIRT1 agonists, there are few studies on SRT2183 at the present stage, especially in animal experiments. Further studies are needed to explore SIRT1-related agonist differences and feedback regulation in endocrine and metabolic pathways.
CAY10602
VASANTHA et al. performed a high-throughput screening of 147,000 compounds. Compounds with relative percentages greater than or equal to 150 are considered definitive SIRT1 activators. CAY10602 increases SIRT1 activation and significantly inhibits TNF-α expression [206]. As a cytokine generated by macrophages, TNF-α is involved in adipose tissue metabolism and endocrine function [207, 208]. High-fat-diet increases body weight, serum total cholesterol, triglycerides, aspartate aminotransferase, alanine aminotransferase, blood glucose, insulin levels, and liver malondialdehyde, while decreases liver superoxide dismutase activity. These changes are negatively correlated with SIRT1 and PGC-1α. HepG2 hepatocytes cell line exposed to oleic acid (OA) for 48 h shows decreased cell viability, apoptosis, lipid accumulation and ROS production, while pretreatment with CAY10602 at 20μM for 2h reverses this effect. On the contrary, pretreatment with Tenovin-6 aggravates the effect of OA on hepG2[209]. CAY10602 restores phosphorylation of eNOS (p-eNOS), p-AMPK, and phosphorylation of Akt (p-Akt) levels inhibited by high glucose in diabetic mice. These results suggest that CAY10602 contributes to the beneficial effects of SIRT1 on endothelial function in diabetes and obesity [210].
Others
In addition to Res, SRT3025, CAY10602, and SRT2183, there are many other small molecular compounds that can activate SIRT1, including SRT1460, SRT1720, and SRT2104. The EC1.5 of SRT1460 is 2.9μM and the maximum activation rate is 447%. The dissociation constant and reaction enthalpy of SRT1460 confirm that SRT1460 binds to a SIRT1-peptide substrate complex and promotes a more productive conformation that improves catalytic activity [181]. In addition, SRT1720 is also a small molecule activator of SIRT1 with an EC1.5 of 0.16 μM and a maximum activation value of 781%. SRT1720 has a clear protective effect on diabetic nephropathy, which originates from that SRT1720 inhibits the expression of HIF1α, GLUT1 and SNAIL, thereby reducing glomerular hypertrophy, mesangial expansion, glomerulosclerosis, and interstitial fibrosis [211]. SRT1720 also up-regulates the expressions of SIRT1, SIRT6, FOXO3a and NRF-1, inhibits the expressions of mTORC1, p-MTOR, p-P70S6K, NF-κB and p53, improves the follicular reserve of diet-induced obese female mice, and prolongs the ovarian life [212]. SRT2104 inhibits dysfunction in ECs treated with high glucose [213]. It is worth noting that natural products also have a positive effect on regulating endocrine and metabolic diseases. For example, naringenin and hesperetin help to improve impaired thyroid function in the old-aged rats [214]. Isoflavonoid can affect the expression of SIRT1 and regulate the electrophysiology of hypothalamic neurons related to the secretion of gonadotropin-releasing hormone (GnRH), controlling hormone release and reproductive maturation [215]. In addition, vitamin D [216], melatonin [217], natural carotene [218], berberine [219], ferulic acid [220] and other natural products affect the expression of SIRT1 to varying degrees, and then participate in the regulation of endocrine and metabolic systems.
Application of SIRT1 inhibitors EX527 in endocrine and metabolic diseases
In addition, inhibitors of SIRT1 have also been widely studied, including EX527 (Selisistat)[221], Sirtinol [222], Inauhzin [223], SIRT1/2 Inhibitor IV [224]. EX527 has been widely studied and applied in a variety of diseases. For example, In DM mice, EX527 inhibited promyelocytic zinc finger protein (PLZF) and insulin induced by Far-infrared (FIR) radiation, respectively. SIRT1 upregulation also increased Ca+V1.2 expression and calcium influx, promoting insulin secretion in β-cells [225]. In addition, studies have shown that fucoglycan (FO) isolated from brown algae can ameliorate pancreatic β-cell injury and impaired insulin synthesis under diabetic conditions, and improve hyperglycemia, lower expression of SIRT1, PDX-1, and GLP-1R in the pancreas of diabetic mice. EX527 plays an important auxiliary role in this study, which can significantly reverse the beneficial effects of FO [226]. Similarly, EX527 blocks the protective effect of curcumin on MIN6 (a mouse insulinoma cell line) cells exposed to HO [227]. The above evidence suggests a significant protective effect of SIRT1 in diabetes mellitus.
Conclusion and Perspectives
SIRT1, a protein deacetylase dependent on NAD+, has long been considered as an evolutionarily conserved life-regulating factor and is associated with many aging-related diseases [228]. With the further study of SIRT1, another potential role of SIRT1 has been discovered -- the energy sensor of the body. SIRT1 is involved in hormone regulation, energy uptake, circadian rhythm, and metabolism. It also has potential therapeutic applications in cardiovascular disease, cancer and age-related diseases. The loss of SIRT1 leads to abnormal secretion of some hormones and metabolic disorders. SIRT1 regulates the function of pancreatic beta cells, improves insulin sensitivity and increases insulin secretion [11, 12]. It has also been reported that Res, flavonoids and other compounds also affects insulin secretion by regulating the activity of SIRT1 in glucose-dependent insulin secretion [178, 229]. These evidences suggest that SIRT1 is a key target of glucose metabolism and insulin resistance. In an orthotopic transplantation rat cholangiocarcinoma (CCA) model, the SIRT1 inhibitor sirtinol reduced tumor size and tumorigenic proteins (glioma-associated oncogene 1, phosphorylated extracellular signal-regulated kinase, and IL-6) expression [230]. In addition, SIRT1 has been shown to promote progression of colorectal cancer [231]. SIRT1 also plays a key role in gout/hyperuricemia, hypertension, hyperlipidaemia and other diseases. For example, SIRT1 regulate metabolist-related target molecules, including PPARγ, SREBP and LXRα, to improve the lipid metabolic environment. It has been reported that SIRT1 is closely related to adiponectin, leptin or resistance derived from adipose tissue, and the level of adiponectin is positively correlated with SIRT1, which can be used as an endocrine signal to mediate the browning of white adipose tissues [232]. Myeloid-specific SIRT1 knockout increases hepatic steatosis and hypothalamic inflammation in mice fed a high-fat diet [233]. In addition, SIRT1 is also involved in regulating the secretion of thyroid hormones [234], testosterone [235], aldosterone [236], estrogen [13], glucagon [198] cortisol and pituitary hormone [15]. At the same time, a variety of small molecules compounds and natural products can act on SIRT1 to different degrees, and our current research has also confirmed that SIRT1 plays an important role in the development of endocrine and metabolic disorders. Notably, the above effects may be caused by different action mechanisms of SIRT1 streets, such as SIRT1/Keap1/Nrf2/HO-1 and PI3K/Akt/GSK-3β mediated oxidative stress, SIRT1/NF-κB mediated inflammatory response, SIRT1/PGC1α mediated mitochondrial damage, and SIRT1/FOXO mediated autophagy [237]. These evidences provide a theoretical basis for SIRT1 as a therapeutic target for endocrine and metabolic diseases.
However, endocrine and metabolic regulation is a complex process, and the above two aspects interact with each other. Moreover, the treatment targeting SIRT1 is still in the preliminary stage. More basic research and more clinical trials are needed before patients can benefit from SIRT1-targeted therapies. Further research may focus on, 1) comprehensively expounding the complex regulatory mechanism of SIRT1 in endocrine and metabolic systems. 2) Exploring the degree of SIRT1's involvement in regulating energy homeostasis and its sensitivity to energy under physiological conditions. 3) Developing standards for assessing SIRT1 expression, especially SIRT1 content in blood and hormone, sugar and lipid metabolism levels, so as to predict endocrine and metabolic diseases. 4) Endocrine and metabolic diseases are closely related to daily diet. How can the body maintain appropriate SIRT1 levels to protect the body from lipid and glucose metabolism disorders? 5) Studying whether SIRT1 targeted therapy has suboptimal efficacy in clinical application. In conclusion, this review provides a comprehensive overview of SIRT1's role in endocrine and metabolic diseases and provides theoretical basis for SIRT1's potential as a novel therapeutic target.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81871607 and 82070422), Youth Science and Technology Rising Star Project of Shaanxi Province (2020KJXX-036), Innovation Capability Strong Foundation Plan of Xi'an City (Medical Research Project, 21YXYJ0037), Key Research and Development Program of Shaanxi (2020ZDLSF04-03), and Major Research Projects of Xi'an Science and Technology Plan [201805104YX12SF38(2)].
Competing Interests
The authors have declared that no competing interest exists.
References
1. Cantó C, Auwerx J. Targeting sirtuin 1 to improve metabolism: all you need is NAD(+)? Pharmacological reviews. 2012;64:166-87
2. Molina-Serrano D, Kyriakou D, Kirmizis A. Histone Modifications as an Intersection Between Diet and Longevity. Frontiers in genetics. 2019;10:192
3. Guarente L, Picard F. Calorie restriction-the SIR2 connection. Cell. 2005;120:473-82
4. Nogueiras R, Habegger KM, Chaudhary N, Finan B, Banks AS, Dietrich MO. et al. Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiological reviews. 2012;92:1479-514
5. Daenthanasanmak A, Iamsawat S, Chakraborty P, Nguyen HD, Bastian D, Liu C. et al. Targeting Sirt-1 controls GVHD by inhibiting T-cell allo-response and promoting Treg stability in mice. Blood. 2019;133:266-79
6. Chen HZ, Wang F, Gao P, Pei JF, Liu Y, Xu TT. et al. Age-Associated Sirtuin 1 Reduction in Vascular Smooth Muscle Links Vascular Senescence and Inflammation to Abdominal Aortic Aneurysm. Circulation research. 2016;119:1076-88
7. Zhang H, Liu D, Wang X, Chen X, Long Y, Chai W. et al. Melatonin improved rat cardiac mitochondria and survival rate in septic heart injury. Journal of pineal research. 2013;55:1-6
8. Mayoral R, Osborn O, McNelis J, Johnson AM, Oh DY, Izquierdo CL. et al. Adipocyte SIRT1 knockout promotes PPARγ activity, adipogenesis and insulin sensitivity in chronic-HFD and obesity. Molecular metabolism. 2015;4:378-91
9. Chen H, Hu X, Yang R, Wu G, Tan Q, Goltzman D. et al. SIRT1/FOXO3a axis plays an important role in the prevention of mandibular bone loss induced by 1,25(OH)(2)D deficiency. International journal of biological sciences. 2020;16:2712-26
10. Chen YR, Lai YL, Lin SD, Li XT, Fu YC, Xu WC. SIRT1 interacts with metabolic transcriptional factors in the pancreas of insulin-resistant and calorie-restricted rats. Molecular biology reports. 2013;40:3373-80
11. Duan X, Sun W, Sun H, Zhang L. Perfluorooctane sulfonate continual exposure impairs glucose-stimulated insulin secretion via SIRT1-induced upregulation of UCP2 expression. Environmental pollution (Barking, Essex: 1987). 2021;278:116840
12. Thakran S, Sharma P, Attia RR, Hori RT, Deng X, Elam MB. et al. Role of sirtuin 1 in the regulation of hepatic gene expression by thyroid hormone. The Journal of biological chemistry. 2013;288:807-18
13. Sasaki Y, Ikeda Y, Miyauchi T, Uchikado Y, Akasaki Y, Ohishi M. Estrogen-SIRT1 Axis Plays a Pivotal Role in Protecting Arteries Against Menopause-Induced Senescence and Atherosclerosis. Journal of atherosclerosis and thrombosis. 2020;27:47-59
14. Khawar MB, Liu C, Gao F, Gao H, Liu W, Han T. et al. Sirt1 regulates testosterone biosynthesis in Leydig cells via modulating autophagy. Protein & cell. 2021;12:67-75
15. Monteserin-Garcia J, Al-Massadi O, Seoane LM, Alvarez CV, Shan B, Stalla J. et al. Sirt1 inhibits the transcription factor CREB to regulate pituitary growth hormone synthesis. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2013;27:1561-71
16. Braud L, Pini M, Stec DF, Manin S, Derumeaux G, Stec DE. et al. Increased Sirt1 secreted from visceral white adipose tissue is associated with improved glucose tolerance in obese Nrf2-deficient mice. Redox biology. 2021;38:101805
17. Côté CD, Rasmussen BA, Duca FA, Zadeh-Tahmasebi M, Baur JA, Daljeet M. et al. Resveratrol activates duodenal Sirt1 to reverse insulin resistance in rats through a neuronal network. Nature medicine. 2015;21:498-505
18. Chen L, Lan Z. Polydatin attenuates potassium oxonate-induced hyperuricemia and kidney inflammation by inhibiting NF-κB/NLRP3 inflammasome activation via the AMPK/SIRT1 pathway. Food & function. 2017;8:1785-92
19. Shao D, Han J, Hou X, Fry J, Behring JB, Seta F. et al. Glutaredoxin-1 Deficiency Causes Fatty Liver and Dyslipidemia by Inhibiting Sirtuin-1. Antioxidants & redox signaling. 2017;27:313-27
20. Carnevale D, Pallante F, Fardella V, Fardella S, Iacobucci R, Federici M. et al. The angiogenic factor PlGF mediates a neuroimmune interaction in the spleen to allow the onset of hypertension. Immunity. 2014;41:737-52
21. Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes & development. 1999;13:2570-80
22. Ivy JM, Klar AJ, Hicks JB. Cloning and characterization of four SIR genes of Saccharomyces cerevisiae. Molecular and cellular biology. 1986;6:688-702
23. Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227-30
24. Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:15998-6003
25. Jiao F, Gong Z. The Beneficial Roles of SIRT1 in Neuroinflammation-Related Diseases. Oxidative medicine and cellular longevity. 2020;2020:6782872
26. Yu J, Auwerx J. The role of sirtuins in the control of metabolic homeostasis. Annals of the New York Academy of Sciences. 2009;1173(Suppl 1):E10-9
27. Yamamoto H, Schoonjans K, Auwerx J. Sirtuin functions in health and disease. Molecular endocrinology (Baltimore, Md). 2007;21:1745-55
28. Mahlknecht U, Voelter-Mahlknecht S. Chromosomal characterization and localization of the NAD+-dependent histone deacetylase gene sirtuin 1 in the mouse. International journal of molecular medicine. 2009;23:245-52
29. Sauve AA, Wolberger C, Schramm VL, Boeke JD. The biochemistry of sirtuins. Annual review of biochemistry. 2006;75:435-65
30. Ma Y, Nie H, Chen H, Li J, Hong Y, Wang B. et al. NAD⁺/NADH metabolism and NAD⁺-dependent enzymes in cell death and ischemic brain injury: current advances and therapeutic implications. Current medicinal chemistry. 2015;22:1239-47
31. Vaquero A, Scher M, Lee D, Erdjument-Bromage H, Tempst P, Reinberg D. Human SirT1 interacts with histone H1 and promotes formation of facultative heterochromatin. Molecular cell. 2004;16:93-105
32. Gallardo-Montejano VI, Saxena G, Kusminski CM, Yang C, McAfee JL, Hahner L. et al. Nuclear Perilipin 5 integrates lipid droplet lipolysis with PGC-1α/SIRT1-dependent transcriptional regulation of mitochondrial function. Nature communications. 2016;7:12723
33. Yun JM, Chien A, Jialal I, Devaraj S. Resveratrol up-regulates SIRT1 and inhibits cellular oxidative stress in the diabetic milieu: mechanistic insights. The Journal of nutritional biochemistry. 2012;23:699-705
34. Cho JH, Kim GY, Pan CJ, Anduaga J, Choi EJ, Mansfield BC. et al. Downregulation of SIRT1 signaling underlies hepatic autophagy impairment in glycogen storage disease type Ia. PLoS genetics. 2017;13:e1006819
35. Magni M, Buscemi G, Maita L, Peng L, Chan SY, Montecucco A. et al. TSPYL2 is a novel regulator of SIRT1 and p300 activity in response to DNA damage. Cell death and differentiation. 2019;26:918-31
36. Lee CW, Wong LL, Tse EY, Liu HF, Leong VY, Lee JM. et al. AMPK promotes p53 acetylation via phosphorylation and inactivation of SIRT1 in liver cancer cells. Cancer research. 2012;72:4394-404
37. Yu L, Dong L, Li H, Liu Z, Luo Z, Duan G. et al. Ubiquitination-mediated degradation of SIRT1 by SMURF2 suppresses CRC cell proliferation and tumorigenesis. Oncogene. 2020;39:4450-64
38. Rasha F, Mims BM, Castro-Piedras I, Barnes BJ, Grisham MB, Rahman RL. et al. The Versatility of Sirtuin-1 in Endocrinology and Immunology. Frontiers in cell and developmental biology. 2020;8:589016
39. Yang Y, Duan W, Li Y, Jin Z, Yan J, Yu S. et al. Novel role of silent information regulator 1 in myocardial ischemia. Circulation. 2013;128:2232-40
40. Qiu Y, Zhou X, Liu Y, Tan S, Li Y. The Role of Sirtuin-1 in Immune Response and Systemic Lupus Erythematosus. Frontiers in immunology. 2021;12:632383
41. Liang F, Kume S, Koya D. SIRT1 and insulin resistance. Nature reviews Endocrinology. 2009;5:367-73
42. Brooks CL, Gu W. How does SIRT1 affect metabolism, senescence and cancer? Nature reviews Cancer. 2009;9:123-8
43. Lawlor N, George J, Bolisetty M, Kursawe R, Sun L, Sivakamasundari V. et al. Single-cell transcriptomes identify human islet cell signatures and reveal cell-type-specific expression changes in type 2 diabetes. Genome research. 2017;27:208-22
44. Duvillié B, Kourdoughli R, Druillennec S, Eychène A, Pouponnot C. Interplay Between Diabetes and Pancreatic Ductal Adenocarcinoma and Insulinoma: The Role of Aging, Genetic Factors, and Obesity. Frontiers in endocrinology. 2020;11:563267
45. Wu SY, Liang J, Yang BC, Leung PS. SIRT1 Activation Promotes β-Cell Regeneration by Activating Endocrine Progenitor Cells via AMPK Signaling-Mediated Fatty Acid Oxidation. Stem cells (Dayton, Ohio). 2019;37:1416-28
46. Prud'homme GJ, Glinka Y, Udovyk O, Hasilo C, Paraskevas S, Wang Q. GABA protects pancreatic beta cells against apoptosis by increasing SIRT1 expression and activity. Biochemical and biophysical research communications. 2014;452:649-54
47. Wu L, Zhou L, Lu Y, Zhang J, Jian F, Liu Y. et al. Activation of SIRT1 protects pancreatic β-cells against palmitate-induced dysfunction. Biochimica et biophysica acta. 2012;1822:1815-25
48. Wang RH, Xu X, Kim HS, Xiao Z, Deng CX. SIRT1 deacetylates FOXA2 and is critical for Pdx1 transcription and β-cell formation. International journal of biological sciences. 2013;9:934-46
49. Pinho AV, Bensellam M, Wauters E, Rees M, Giry-Laterriere M, Mawson A. et al. Pancreas-Specific Sirt1-Deficiency in Mice Compromises Beta-Cell Function without Development of Hyperglycemia. PloS one. 2015;10:e0128012
50. Sen A, Kushnir VA, Barad DH, Gleicher N. Endocrine autoimmune diseases and female infertility. Nature reviews Endocrinology. 2014;10:37-50
51. Qiao J, Feng HL. Extra- and intra-ovarian factors in polycystic ovary syndrome: impact on oocyte maturation and embryo developmental competence. Human reproduction update. 2011;17:17-33
52. Dewailly D, Robin G, Peigne M, Decanter C, Pigny P, Catteau-Jonard S. Interactions between androgens, FSH, anti-Müllerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Human reproduction update. 2016;22:709-24
53. Yang Q, Dai S, Luo X, Zhu J, Li F, Liu J. et al. Melatonin attenuates postovulatory oocyte dysfunction by regulating SIRT1 expression. Reproduction (Cambridge, England). 2018;156:81-92
54. Li Z, Zhang Z, Ke L, Sun Y, Li W, Feng X. et al. Resveratrol promotes white adipocytes browning and improves metabolic disorders in Sirt1-dependent manner in mice. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2020;34:4527-39
55. Zhang W, Huang Q, Zeng Z, Wu J, Zhang Y, Chen Z. Sirt1 Inhibits Oxidative Stress in Vascular Endothelial Cells. Oxidative medicine and cellular longevity. 2017;2017:7543973
56. Xu C, Wang L, Fozouni P, Evjen G, Chandra V, Jiang J. et al. SIRT1 is downregulated by autophagy in senescence and ageing. Nature cell biology. 2020;22:1170-9
57. Zhang T, Du X, Zhao L, He M, Lin L, Guo C. et al. SIRT1 facilitates primordial follicle recruitment independent of deacetylase activity through directly modulating Akt1 and mTOR transcription. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2019;33:14703-16
58. Iljas JD, Wei Z, Homer HA. Sirt1 sustains female fertility by slowing age-related decline in oocyte quality required for post-fertilization embryo development. Aging cell. 2020;19:e13204
59. Wu X, Hu F, Zeng J, Han L, Qiu D, Wang H. et al. NMNAT2-mediated NAD(+) generation is essential for quality control of aged oocytes. Aging cell. 2019;18:e12955
60. Song S, Guo Q, Zhu Y, Yuan P, Yan Z, Yan L. et al. Exploring the role of autophagy during early human embryonic development through single-cell transcriptome and methylome analyses. Science China Life sciences. 2022;65:940-52
61. Guo Y, Sun J, Bu S, Li B, Zhang Q, Wang Q. et al. Melatonin protects against chronic stress-induced oxidative meiotic defects in mice MII oocytes by regulating SIRT1. Cell cycle (Georgetown, Tex). 2020;19:1677-95
62. Xu W, Li L, Sun J, Zhu S, Yan Z, Gao L. et al. Putrescine delays postovulatory aging of mouse oocytes by upregulating PDK4 expression and improving mitochondrial activity. Aging. 2018;10:4093-106
63. Al-Bari MAA, Hossain S, Mia U, Al Mamun MA. Therapeutic and Mechanistic Approaches of Tridax Procumbens Flavonoids for the Treatment of Osteoporosis. Current drug targets. 2020;21:1687-702
64. Abed É, Delalandre A, Lajeunesse D. Beneficial effect of resveratrol on phenotypic features and activity of osteoarthritic osteoblasts. Arthritis research & therapy. 2017;19:151
65. Da L, Xueqing W, Wei W, Chenghao M, Baoqing P, Shuqin W. et al. Synthesis and Application of Iron Oxide Nanoparticles in Bone Tissue Repair %J Journal of Nanomaterials. 2021. 2021
66. Yang X, Jiang T, Wang Y, Guo L. The Role and Mechanism of SIRT1 in Resveratrol-regulated Osteoblast Autophagy in Osteoporosis Rats. Scientific reports. 2019;9:18424
67. Ducy P. The role of osteocalcin in the endocrine cross-talk between bone remodelling and energy metabolism. Diabetologia. 2011;54:1291-7
68. Huang W, Shang WL, Wang HD, Wu WW, Hou SX. Sirt1 overexpression protects murine osteoblasts against TNF-α-induced injury in vitro by suppressing the NF-κB signaling pathway. Acta pharmacologica Sinica. 2012;33:668-74
69. Dalle Carbonare L, Innamorati G, Valenti MT. Transcription factor Runx2 and its application to bone tissue engineering. Stem cell reviews and reports. 2012;8:891-7
70. Jonason JH, Xiao G, Zhang M, Xing L, Chen D. Post-translational Regulation of Runx2 in Bone and Cartilage. Journal of dental research. 2009;88:693-703
71. Hong W, Wei Z, Qiu Z, Li Z, Fu C, Ye Z. et al. Atorvastatin promotes bone formation in aged apoE(-/-) mice through the Sirt1-Runx2 axis. Journal of orthopaedic surgery and research. 2020;15:303
72. Tseng PC, Hou SM, Chen RJ, Peng HW, Hsieh CF, Kuo ML. et al. Resveratrol promotes osteogenesis of human mesenchymal stem cells by upregulating RUNX2 gene expression via the SIRT1/FOXO3A axis. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2011;26:2552-63
73. Lu Y, Zhou L, Wang L, He S, Ren H, Zhou N. et al. The role of SIRT1 in BMP2-induced chondrogenic differentiation and cartilage maintenance under oxidative stress. Aging. 2020;12:9000-13
74. Zhao M, Ko SY, Garrett IR, Mundy GR, Gutierrez GE, Edwards JR. The polyphenol resveratrol promotes skeletal growth in mice through a sirtuin 1-bone morphogenic protein 2 longevity axis. British journal of pharmacology. 2018;175:4183-92
75. Li X, Easley CJ. Microfluidic systems for studying dynamic function of adipocytes and adipose tissue. Analytical and bioanalytical chemistry. 2018;410:791-800
76. Pang WJ, Xiong Y, Wang Y, Tong Q, Yang GS. Sirt1 attenuates camptothecin-induced apoptosis through caspase-3 pathway in porcine preadipocytes. Experimental cell research. 2013;319:670-83
77. Hui X, Zhang M, Gu P, Li K, Gao Y, Wu D. et al. Adipocyte SIRT1 controls systemic insulin sensitivity by modulating macrophages in adipose tissue. EMBO reports. 2017;18:645-57
78. Eseberri I, Miranda J, Lasa A, Churruca I, Portillo MP. Doses of Quercetin in the Range of Serum Concentrations Exert Delipidating Effects in 3T3-L1 Preadipocytes by Acting on Different Stages of Adipogenesis, but Not in Mature Adipocytes. Oxidative medicine and cellular longevity. 2015;2015:480943
79. Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R. et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771-6
80. Qiang L, Wang L, Kon N, Zhao W, Lee S, Zhang Y. et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Pparγ. Cell. 2012;150:620-32
81. Ioannilli L, Ciccarone F, Ciriolo MR. Adipose Tissue and FoxO1: Bridging Physiology and Mechanisms. Cells. 2020 9
82. Subauste AR, Burant CF. Role of FoxO1 in FFA-induced oxidative stress in adipocytes. American journal of physiology Endocrinology and metabolism. 2007;293:E159-64
83. Chakrabarti P, English T, Karki S, Qiang L, Tao R, Kim J. et al. SIRT1 controls lipolysis in adipocytes via FOXO1-mediated expression of ATGL. Journal of lipid research. 2011;52:1693-701
84. Lee JH, Jung HA, Kang MJ, Choi JS, Kim GD. Fucosterol, isolated from Ecklonia stolonifera, inhibits adipogenesis through modulation of FoxO1 pathway in 3T3-L1 adipocytes. The Journal of pharmacy and pharmacology. 2017;69:325-33
85. Chen Z, Peng IC, Cui X, Li YS, Chien S, Shyy JY. Shear stress, SIRT1, and vascular homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:10268-73
86. Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo R. Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circulation research. 2008;102:519-28
87. Sato Y, Feig DI, Stack AG, Kang DH, Lanaspa MA, Ejaz AA. et al. The case for uric acid-lowering treatment in patients with hyperuricaemia and CKD. Nature reviews Nephrology. 2019;15:767-75
88. Shih CY, Chen CY, Wen CJ, Liu HM, Kuo HK. Relationship between serum uric acid and cerebral white matter lesions in the elderly. Nutrition, metabolism, and cardiovascular diseases: NMCD. 2012;22:154-9
89. Xu WA, Yin L, Pan HY, Shi L, Xu L, Zhang X. et al. Study on the correlation between constituents detected in serum from Rhizoma Smilacis Glabrae and the reduction of uric acid levels in hyperuricemia. Journal of ethnopharmacology. 2013;150:747-54
90. Chen L, Yin H, Lan Z, Ma S, Zhang C, Yang Z. et al. Anti-hyperuricemic and nephroprotective effects of Smilax china L. Journal of ethnopharmacology. 2011;135:399-405
91. Wang J, Zhu XX, Liu L, Xue Y, Yang X, Zou HJ. SIRT1 prevents hyperuricemia via the PGC-1α/PPARγ-ABCG2 pathway. Endocrine. 2016;53:443-52
92. Lytvyn Y, Bjornstad P, Lovshin JA, Singh SK, Boulet G, Farooqi MA. et al. Association between uric acid, renal haemodynamics and arterial stiffness over the natural history of type 1 diabetes. Diabetes, obesity & metabolism. 2019;21:1388-98
93. Milanesi S, Verzola D, Cappadona F, Bonino B, Murugavel A, Pontremoli R. et al. Uric acid and angiotensin II additively promote inflammation and oxidative stress in human proximal tubule cells by activation of toll-like receptor 4. Journal of cellular physiology. 2019;234:10868-76
94. Ma CH, Kang LL, Ren HM, Zhang DM, Kong LD. Simiao pill ameliorates renal glomerular injury via increasing Sirt1 expression and suppressing NF-κB/NLRP3 inflammasome activation in high fructose-fed rats. Journal of ethnopharmacology. 2015;172:108-17
95. Xu K, Liu S, Zhao X, Zhang X, Fu X, Zhou Y. et al. Treating hyperuricemia related non-alcoholic fatty liver disease in rats with resveratrol. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2019;110:844-9
96. Xu K, Zhao X, Fu X, Xu K, Li Z, Miao L. et al. Gender effect of hyperuricemia on the development of nonalcoholic fatty liver disease (NAFLD): A clinical analysis and mechanistic study. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2019;117:109158
97. Sumino H, Ichikawa S, Kanda T, Nakamura T, Sakamaki T. Reduction of serum uric acid by hormone replacement therapy in postmenopausal women with hyperuricaemia. Lancet (London, England). 1999;354:650
98. Bendale DS, Karpe PA, Chhabra R, Shete SP, Shah H, Tikoo K. 17-β Oestradiol prevents cardiovascular dysfunction in post-menopausal metabolic syndrome by affecting SIRT1/AMPK/H3 acetylation. British journal of pharmacology. 2013;170:779-95
99. Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nature reviews Nephrology. 2020;16:223-37
100. Ungvari Z, Toth P, Tarantini S, Prodan CI, Sorond F, Merkely B. et al. Hypertension-induced cognitive impairment: from pathophysiology to public health. Nature reviews Nephrology. 2021;17:639-54
101. Mahfoud F, Schlaich MP, Lobo MD. Device Therapy of Hypertension. Circulation research. 2021;128:1080-99
102. Rossi GP, Ceolotto G, Caroccia B, Lenzini L. Genetic screening in arterial hypertension. Nature reviews Endocrinology. 2017;13:289-98
103. Madhur MS, Elijovich F, Alexander MR, Pitzer A, Ishimwe J, Van Beusecum JP. et al. Hypertension: Do Inflammation and Immunity Hold the Key to Solving this Epidemic? Circulation research. 2021;128:908-33
104. Calvillo L, Gironacci MM, Crotti L, Meroni PL, Parati G. Neuroimmune crosstalk in the pathophysiology of hypertension. Nature reviews Cardiology. 2019;16:476-90
105. Sharma RK, Yang T, Oliveira AC, Lobaton GO, Aquino V, Kim S. et al. Microglial Cells Impact Gut Microbiota and Gut Pathology in Angiotensin II-Induced Hypertension. Circulation research. 2019;124:727-36
106. Kim YB, Kim YS, Kim WB, Shen FY, Lee SW, Chung HJ. et al. GABAergic excitation of vasopressin neurons: possible mechanism underlying sodium-dependent hypertension. Circulation research. 2013;113:1296-307
107. von Lueder TG, Atar D, Krum H. Current role of neprilysin inhibitors in hypertension and heart failure. Pharmacology & therapeutics. 2014;144:41-9
108. Wu Y, Ding Y, Ramprasath T, Zou MH. Oxidative Stress, GTPCH1, and Endothelial Nitric Oxide Synthase Uncoupling in Hypertension. Antioxidants & redox signaling. 2021;34:750-64
109. Miyazaki R, Ichiki T, Hashimoto T, Inanaga K, Imayama I, Sadoshima J. et al. SIRT1, a longevity gene, downregulates angiotensin II type 1 receptor expression in vascular smooth muscle cells. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:1263-9
110. Yoon MJ, Yoshida M, Johnson S, Takikawa A, Usui I, Tobe K. et al. SIRT1-Mediated eNAMPT Secretion from Adipose Tissue Regulates Hypothalamic NAD+ and Function in Mice. Cell metabolism. 2015;21:706-17
111. Watson A, Nong Z, Yin H, O'Neil C, Fox S, Balint B. et al. Nicotinamide Phosphoribosyltransferase in Smooth Muscle Cells Maintains Genome Integrity, Resists Aortic Medial Degeneration, and Is Suppressed in Human Thoracic Aortic Aneurysm Disease. Circulation research. 2017;120:1889-902
112. Zhou L, Zhang S, Bolor-Erdene E, Wang L, Tian D, Mei Y. NAMPT/SIRT1 Attenuate Ang II-Induced Vascular Remodeling and Vulnerability to Hypertension by Inhibiting the ROS/MAPK Pathway. Oxidative medicine and cellular longevity. 2020;2020:1974265
113. Kho J, Tian X, Wong WT, Bertin T, Jiang MM, Chen S. et al. Argininosuccinate Lyase Deficiency Causes an Endothelial-Dependent Form of Hypertension. American journal of human genetics. 2018;103:276-87
114. Feng R, Ullah M, Chen K, Ali Q, Lin Y, Sun Z. Stem cell-derived extracellular vesicles mitigate ageing-associated arterial stiffness and hypertension. Journal of extracellular vesicles. 2020;9:1783869
115. Preuss HG, Bagchi D, Bagchi M. Protective effects of a novel niacin-bound chromium complex and a grape seed proanthocyanidin extract on advancing age and various aspects of syndrome X. Annals of the New York Academy of Sciences. 2002;957:250-9
116. Zhang FL, Gao HQ, Wu JM, Ma YB, You BA, Li BY. et al. Selective inhibition by grape seed proanthocyanidin extracts of cell adhesion molecule expression induced by advanced glycation end products in endothelial cells. Journal of cardiovascular pharmacology. 2006;48:47-53
117. Cui X, Liu X, Feng H, Zhao S, Gao H. Grape seed proanthocyanidin extracts enhance endothelial nitric oxide synthase expression through 5'-AMP activated protein kinase/Surtuin 1-Krüpple like factor 2 pathway and modulate blood pressure in ouabain induced hypertensive rats. Biological & pharmaceutical bulletin. 2012;35:2192-7
118. Gao D, Zuo Z, Tian J, Ali Q, Lin Y, Lei H. et al. Activation of SIRT1 Attenuates Klotho Deficiency-Induced Arterial Stiffness and Hypertension by Enhancing AMP-Activated Protein Kinase Activity. Hypertension (Dallas, Tex: 1979). 2016;68:1191-9
119. Kumariya S, Ubba V, Jha RK, Gayen JR. Autophagy in ovary and polycystic ovary syndrome: role, dispute and future perspective. Autophagy. 2021;17:2706-33
120. de Medeiros SF, Rodgers RJ, Norman RJ. Adipocyte and steroidogenic cell cross-talk in polycystic ovary syndrome. Human reproduction update. 2021;27:771-96
121. Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nature reviews Endocrinology. 2018;14:270-84
122. Franks S, Stark J, Hardy K. Follicle dynamics and anovulation in polycystic ovary syndrome. Human reproduction update. 2008;14:367-78
123. Estienne A, Bongrani A, Ramé C, Kurowska P, Błaszczyk K, Rak A. et al. Energy sensors and reproductive hypothalamo-pituitary ovarian axis (HPO) in female mammals: Role of mTOR (mammalian target of rapamycin), AMPK (AMP-activated protein kinase) and SIRT1 (Sirtuin 1). Molecular and cellular endocrinology. 2021;521:111113
124. Nestler JE. Metformin for the treatment of the polycystic ovary syndrome. The New England journal of medicine. 2008;358:47-54
125. Ala M, Ala M. Metformin for Cardiovascular Protection, Inflammatory Bowel Disease, Osteoporosis, Periodontitis, Polycystic Ovarian Syndrome, Neurodegeneration, Cancer, Inflammation and Senescence: What Is Next? ACS pharmacology & translational science. 2021;4:1747-70
126. Furat Rencber S, Kurnaz Ozbek S, Eraldemır C, Sezer Z, Kum T, Ceylan S. et al. Effect of resveratrol and metformin on ovarian reserve and ultrastructure in PCOS: an experimental study. Journal of ovarian research. 2018;11:55
127. Tao X, Zhang X, Ge SQ, Zhang EH, Zhang B. Expression of SIRT1 in the ovaries of rats with polycystic ovary syndrome before and after therapeutic intervention with exenatide. International journal of clinical and experimental pathology. 2015;8:8276-83
128. Tao X, Cai L, Chen L, Ge S, Deng X. Effects of metformin and Exenatide on insulin resistance and AMPKα-SIRT1 molecular pathway in PCOS rats. Journal of ovarian research. 2019;12:86
129. Mihanfar A, Nouri M, Roshangar L, Khadem-Ansari MH. Therapeutic potential of quercetin in an animal model of PCOS: Possible involvement of AMPK/SIRT-1 axis. European journal of pharmacology. 2021;900:174062
130. Zhang J, Liu J, Zhu K, Hong Y, Sun Y, Zhao X. et al. Effects of BMAL1-SIRT1-positive cycle on estrogen synthesis in human ovarian granulosa cells: an implicative role of BMAL1 in PCOS. Endocrine. 2016;53:574-84
131. Parakh TN, Hernandez JA, Grammer JC, Weck J, Hunzicker-Dunn M, Zeleznik AJ. et al. Follicle-stimulating hormone/cAMP regulation of aromatase gene expression requires beta-catenin. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12435-40
132. Riddle RC, Clemens TL. Insulin, osteoblasts, and energy metabolism: why bone counts calories. The Journal of clinical investigation. 2014;124:1465-7
133. Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet (London, England). 2011;377:1276-87
134. Kim HN, Ponte F, Warren A, Ring R, Iyer S, Han L. et al. A decrease in NAD(+) contributes to the loss of osteoprogenitors and bone mass with aging. NPJ aging and mechanisms of disease. 2021;7:8
135. Li Q, Cheng JC, Jiang Q, Lee WY. Role of sirtuins in bone biology: Potential implications for novel therapeutic strategies for osteoporosis. Aging cell. 2021;20:e13301
136. Zainabadi K, Liu CJ, Caldwell ALM, Guarente L. SIRT1 is a positive regulator of in vivo bone mass and a therapeutic target for osteoporosis. PloS one. 2017;12:e0185236
137. Cohen-Kfir E, Artsi H, Levin A, Abramowitz E, Bajayo A, Gurt I. et al. Sirt1 is a regulator of bone mass and a repressor of Sost encoding for sclerostin, a bone formation inhibitor. Endocrinology. 2011;152:4514-24
138. Gabay O, Oppenhiemer H, Meir H, Zaal K, Sanchez C, Dvir-Ginzberg M. Increased apoptotic chondrocytes in articular cartilage from adult heterozygous SirT1 mice. Annals of the rheumatic diseases. 2012;71:613-6
139. El-Haj M, Gurt I, Cohen-Kfir E, Dixit V, Artsi H, Kandel L. et al. Reduced Sirtuin1 expression at the femoral neck in women who sustained an osteoporotic hip fracture. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2016;27:2373-8
140. Godfrin-Valnet M, Khan KA, Guillot X, Prati C, Baud L, Abbas W. et al. Sirtuin 1 activity in peripheral blood mononuclear cells of patients with osteoporosis. Medical science monitor basic research. 2014;20:142-5
141. Yao H, Yao Z, Zhang S, Zhang W, Zhou W. Upregulation of SIRT1 inhibits H2O2-induced osteoblast apoptosis via FoxO1/β-catenin pathway. Molecular medicine reports. 2018;17:6681-90
142. Wang Y, Mei R, Hao S, Luo P, Wang P, Almatari Y. et al. Up-regulation of SIRT1 induced by 17beta-estradiol promotes autophagy and inhibits apoptosis in osteoblasts. Aging. 2021;13:23652-71
143. Bai SC, Xu Q, Li H, Qin YF, Song LC, Wang CG. et al. NADPH Oxidase Isoforms Are Involved in Glucocorticoid-Induced Preosteoblast Apoptosis. Oxidative medicine and cellular longevity. 2019;2019:9192413
144. Zhang L, Bao D, Li P, Lu Z, Pang L, Chen Z. et al. Particle-induced SIRT1 downregulation promotes osteoclastogenesis and osteolysis through ER stress regulation. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2018;104:300-6
145. Shakibaei M, Buhrmann C, Mobasheri A. Resveratrol-mediated SIRT-1 interactions with p300 modulate receptor activator of NF-kappaB ligand (RANKL) activation of NF-kappaB signaling and inhibit osteoclastogenesis in bone-derived cells. The Journal of biological chemistry. 2011;286:11492-505
146. Kaneko K, Ueki K, Takahashi N, Hashimoto S, Okamoto M, Awazawa M. et al. Class IA phosphatidylinositol 3-kinase in pancreatic β cells controls insulin secretion by multiple mechanisms. Cell metabolism. 2010;12:619-32
147. Balestrieri ML, Servillo L, Esposito A, D'Onofrio N, Giovane A, Casale R. et al. Poor glycaemic control in type 2 diabetes patients reduces endothelial progenitor cell number by influencing SIRT1 signalling via platelet-activating factor receptor activation. Diabetologia. 2013;56:162-72
148. Schug TT, Li X. Sirtuin 1 in lipid metabolism and obesity. Annals of medicine. 2011;43:198-211
149. Biason-Lauber A, Böni-Schnetzler M, Hubbard BP, Bouzakri K, Brunner A, Cavelti-Weder C. et al. Identification of a SIRT1 mutation in a family with type 1 diabetes. Cell metabolism. 2013;17:448-55
150. Zheng Z, Chen H, Li J, Li T, Zheng B, Zheng Y. et al. Sirtuin 1-mediated cellular metabolic memory of high glucose via the LKB1/AMPK/ROS pathway and therapeutic effects of metformin. Diabetes. 2012;61:217-28
151. Arunachalam G, Samuel SM, Marei I, Ding H, Triggle CR. Metformin modulates hyperglycaemia-induced endothelial senescence and apoptosis through SIRT1. British journal of pharmacology. 2014;171:523-35
152. Erion DM, Yonemitsu S, Nie Y, Nagai Y, Gillum MP, Hsiao JJ. et al. SirT1 knockdown in liver decreases basal hepatic glucose production and increases hepatic insulin responsiveness in diabetic rats. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:11288-93
153. Wang RH, Kim HS, Xiao C, Xu X, Gavrilova O, Deng CX. Hepatic Sirt1 deficiency in mice impairs mTorc2/Akt signaling and results in hyperglycemia, oxidative damage, and insulin resistance. The Journal of clinical investigation. 2011;121:4477-90
154. Liu R, Zhong Y, Li X, Chen H, Jim B, Zhou MM. et al. Role of transcription factor acetylation in diabetic kidney disease. Diabetes. 2014;63:2440-53
155. Kitada M, Kume S, Imaizumi N, Koya D. Resveratrol improves oxidative stress and protects against diabetic nephropathy through normalization of Mn-SOD dysfunction in AMPK/SIRT1-independent pathway. Diabetes. 2011;60:634-43
156. Ding M, Feng N, Tang D, Feng J, Li Z, Jia M. et al. Melatonin prevents Drp1-mediated mitochondrial fission in diabetic hearts through SIRT1-PGC1α pathway. Journal of pineal research. 2018;65:e12491
157. Houten SM, Watanabe M, Auwerx J. Endocrine functions of bile acids. The EMBO journal. 2006;25:1419-25
158. McCarthy MI. Susceptibility gene discovery for common metabolic and endocrine traits. Journal of molecular endocrinology. 2002;28:1-17
159. Burkholder GA, Tamhane AR, Salinas JL, Mugavero MJ, Raper JL, Westfall AO. et al. Underutilization of aspirin for primary prevention of cardiovascular disease among HIV-infected patients. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2012;55:1550-7
160. Li SJ, Liu CH, Chang CW, Chu HP, Chen KJ, Mersmann HJ. et al. Development of a dietary-induced metabolic syndrome model using miniature pigs involvement of AMPK and SIRT1. European journal of clinical investigation. 2015;45:70-80
161. Feng T, Liu P, Wang X, Luo J, Zuo X, Jiang X. et al. SIRT1 activator E1231 protects from experimental atherosclerosis and lowers plasma cholesterol and triglycerides by enhancing ABCA1 expression. Atherosclerosis. 2018;274:172-81
162. Nguyen LT, Saad S, Chen H, Pollock CA. Parental SIRT1 Overexpression Attenuate Metabolic Disorders Due to Maternal High-Fat Feeding. International journal of molecular sciences. 2020 21
163. Yin Y, Li X, Sha X, Xi H, Li YF, Shao Y. et al. Early hyperlipidemia promotes endothelial activation via a caspase-1-sirtuin 1 pathway. Arteriosclerosis, thrombosis, and vascular biology. 2015;35:804-16
164. Walker AK, Yang F, Jiang K, Ji JY, Watts JL, Purushotham A. et al. Conserved role of SIRT1 orthologs in fasting-dependent inhibition of the lipid/cholesterol regulator SREBP. Genes & development. 2010;24:1403-17
165. Hammer SS, Vieira CP, McFarland D, Sandler M, Levitsky Y, Dorweiler TF. et al. Fasting and fasting-mimicking treatment activate SIRT1/LXRα and alleviate diabetes-induced systemic and microvascular dysfunction. Diabetologia. 2021;64:1674-89
166. Huang X, Cai H, Li H, Su Y, Li H, Li W. et al. Cinnamon as Dietary Supplement Caused Hyperlipidemia in Healthy Rats. Evidence-based complementary and alternative medicine: eCAM. 2021;2021:9892088
167. Stein S, Lohmann C, Schäfer N, Hofmann J, Rohrer L, Besler C. et al. SIRT1 decreases Lox-1-mediated foam cell formation in atherogenesis. European heart journal. 2010;31:2301-9
168. van der Meer DL, Degenhardt T, Väisänen S, de Groot PJ, Heinäniemi M, de Vries SC. et al. Profiling of promoter occupancy by PPARalpha in human hepatoma cells via ChIP-chip analysis. Nucleic acids research. 2010;38:2839-50
169. Lee JH, Kang HS, Park HY, Moon YA, Kang YN, Oh BC. et al. PPARα-dependent Insig2a overexpression inhibits SREBP-1c processing during fasting. Scientific reports. 2017;7:9958
170. Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y. et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. The Journal of biological chemistry. 2008;283:20015-26
171. Caton PW, Kieswich J, Yaqoob MM, Holness MJ, Sugden MC. Metformin opposes impaired AMPK and SIRT1 function and deleterious changes in core clock protein expression in white adipose tissue of genetically-obese db/db mice. Diabetes, obesity & metabolism. 2011;13:1097-104
172. Barbalata T, Deleanu M, Carnuta MG, Niculescu LS, Raileanu M, Sima AV. et al. Hyperlipidemia Determines Dysfunctional HDL Production and Impedes Cholesterol Efflux in the Small Intestine: Alleviation by Ginger Extract. Molecular nutrition & food research. 2019;63:e1900029
173. Bruckbauer A, Banerjee J, Cao Q, Cui X, Jing J, Zha L. et al. Erratum: Leucine-nicotinic acid synergy stimulates AMPK/Sirt1 signaling and regulates lipid metabolism and lifespan in Caenorhabditis elegans, and hyperlipidemia and atherosclerosis in mice. American journal of cardiovascular disease. 2017;7:97
174. Huang Q, Wang T, Yang L, Wang HY. Ginsenoside Rb2 Alleviates Hepatic Lipid Accumulation by Restoring Autophagy via Induction of Sirt1 and Activation of AMPK. International journal of molecular sciences. 2017 18
175. Bonomini F, Favero G, Rodella LF, Moghadasian MH, Rezzani R. Melatonin Modulation of Sirtuin-1 Attenuates Liver Injury in a Hypercholesterolemic Mouse Model. BioMed research international. 2018;2018:7968452
176. He X, Zheng J, Liu C. Low serum level of sirtuin 1 predicts coronary atherosclerosis plaques during computed tomography angiography among an asymptomatic cohort. Coronary artery disease. 2019;30:621-5
177. Moussa C, Hebron M, Huang X, Ahn J, Rissman RA, Aisen PS. et al. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer's disease. Journal of neuroinflammation. 2017;14:1
178. Jimenez-Gomez Y, Mattison JA, Pearson KJ, Martin-Montalvo A, Palacios HH, Sossong AM. et al. Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high-fat, high-sugar diet. Cell metabolism. 2013;18:533-45
179. He W, Wang Y, Zhang MZ, You L, Davis LS, Fan H. et al. Sirt1 activation protects the mouse renal medulla from oxidative injury. The Journal of clinical investigation. 2010;120:1056-68
180. Chini CC, Espindola-Netto JM, Mondal G, Guerrico AM, Nin V, Escande C. et al. SIRT1-Activating Compounds (STAC) Negatively Regulate Pancreatic Cancer Cell Growth and Viability Through a SIRT1 Lysosomal-Dependent Pathway. Clinical cancer research: an official journal of the American Association for Cancer Research. 2016;22:2496-507
181. Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ. et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712-6
182. van der Meer AJ, Scicluna BP, Moerland PD, Lin J, Jacobson EW, Vlasuk GP. et al. The Selective Sirtuin 1 Activator SRT2104 Reduces Endotoxin-Induced Cytokine Release and Coagulation Activation in Humans. Critical care medicine. 2015;43:e199-202
183. Miranda MX, van Tits LJ, Lohmann C, Arsiwala T, Winnik S, Tailleux A. et al. The Sirt1 activator SRT3025 provides atheroprotection in Apoe-/- mice by reducing hepatic Pcsk9 secretion and enhancing Ldlr expression. European heart journal. 2015;36:51-9
184. Maepa M, Razwinani M, Motaung S. Effects of resveratrol on collagen type II protein in the superficial and middle zone chondrocytes of porcine articular cartilage. Journal of ethnopharmacology. 2016;178:25-33
185. Ahmad I, Hoda M. Molecular mechanisms of action of resveratrol in modulation of diabetic and non-diabetic cardiomyopathy. Pharmacological research. 2020;161:105112
186. Zhang LX, Li CX, Kakar MU, Khan MS, Wu PF, Amir RM. et al. Resveratrol (RV): A pharmacological review and call for further research. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2021;143:112164
187. Zhou DD, Luo M, Huang SY, Saimaiti A, Shang A, Gan RY. et al. Effects and Mechanisms of Resveratrol on Aging and Age-Related Diseases. Oxidative medicine and cellular longevity. 2021;2021:9932218
188. van der Spuy WJ, Pretorius E. Is the use of resveratrol in the treatment and prevention of obesity premature? Nutrition research reviews. 2009;22:111-7
189. Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH. et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell metabolism. 2011;14:612-22
190. Dash S, Xiao C, Morgantini C, Szeto L, Lewis GF. High-dose resveratrol treatment for 2 weeks inhibits intestinal and hepatic lipoprotein production in overweight/obese men. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:2895-901
191. Borra MT, Smith BC, Denu JM. Mechanism of human SIRT1 activation by resveratrol. The Journal of biological chemistry. 2005;280:17187-95
192. Sebai H, Hovsépian S, Ristorcelli E, Aouani E, Lombardo D, Fayet G. Resveratrol increases iodide trapping in the rat thyroid cell line FRTL-5. Thyroid: official journal of the American Thyroid Association. 2010;20:195-203
193. Shojaei-Zarghani S, Rafraf M. Resveratrol and Markers of Polycystic Ovary Syndrome: a Systematic Review of Animal and Clinical Studies. Reproductive sciences (Thousand Oaks, Calif). 2021
194. Tabrizi R, Tamtaji OR, Lankarani KB, Akbari M, Dadgostar E, Dabbaghmanesh MH. et al. The effects of resveratrol intake on weight loss: a systematic review and meta-analysis of randomized controlled trials. Critical reviews in food science and nutrition. 2020;60:375-90
195. Della-Morte D, Dave KR, DeFazio RA, Bao YC, Raval AP, Perez-Pinzon MA. Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience. 2009;159:993-1002
196. Dong C, Della-Morte D, Cabral D, Wang L, Blanton SH, Seemant C. et al. Sirtuin/uncoupling protein gene variants and carotid plaque area and morphology. International journal of stroke: official journal of the International Stroke Society. 2015;10:1247-52
197. Artsi H, Cohen-Kfir E, Gurt I, Shahar R, Bajayo A, Kalish N. et al. The Sirtuin1 activator SRT3025 down-regulates sclerostin and rescues ovariectomy-induced bone loss and biomechanical deterioration in female mice. Endocrinology. 2014;155:3508-15
198. Zhang Y, Thai K, Jin T, Woo M, Gilbert RE. SIRT1 activation attenuates α cell hyperplasia, hyperglucagonaemia and hyperglycaemia in STZ-diabetic mice. Scientific reports. 2018;8:13972
199. Artsi H, Gurt I, El-Haj M, Müller R, Kuhn GA, Ben Shalom G. et al. Sirt1 Promotes a Thermogenic Gene Program in Bone Marrow Adipocytes: From Mice to (Wo)Men. Frontiers in endocrinology. 2019;10:126
200. Zeng J, Xiao Q, Li X, Chen J. Advanced oxidation protein products aggravate age-related bone loss by increasing sclerostin expression in osteocytes via ROS-dependent downregulation of Sirt1. International journal of molecular medicine. 2021 47
201. Duan W. Targeting sirtuin-1 in Huntington's disease: rationale and current status. CNS drugs. 2013;27:345-52
202. Gurt I, Artsi H, Cohen-Kfir E, Hamdani G, Ben-Shalom G, Feinstein B. et al. The Sirt1 Activators SRT2183 and SRT3025 Inhibit RANKL-Induced Osteoclastogenesis in Bone Marrow-Derived Macrophages and Down-Regulate Sirt3 in Sirt1 Null Cells. PloS one. 2015;10:e0134391
203. Nelson LE, Valentine RJ, Cacicedo JM, Gauthier MS, Ido Y, Ruderman NB. A novel inverse relationship between metformin-triggered AMPK-SIRT1 signaling and p53 protein abundance in high glucose-exposed HepG2 cells. American journal of physiology Cell physiology. 2012;303:C4-c13
204. Kitada M, Kume S, Takeda-Watanabe A, Kanasaki K, Koya D. Sirtuins and renal diseases: relationship with aging and diabetic nephropathy. Clinical science (London, England: 1979). 2013;124:153-64
205. Sun T, Hu Y, He W, Shang Y, Yang X, Gong L. et al. SRT2183 impairs ovarian cancer by facilitating autophagy. Aging. 2020;12:24208-18
206. Nayagam VM, Wang X, Tan YC, Poulsen A, Goh KC, Ng T. et al. SIRT1 modulating compounds from high-throughput screening as anti-inflammatory and insulin-sensitizing agents. Journal of biomolecular screening. 2006;11:959-67
207. Uno S, Imagawa A, Okita K, Sayama K, Moriwaki M, Iwahashi H. et al. Macrophages and dendritic cells infiltrating islets with or without beta cells produce tumour necrosis factor-alpha in patients with recent-onset type 1 diabetes. Diabetologia. 2007;50:596-601
208. Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. European heart journal. 2008;29:2959-71
209. Jiang Y, Chen D, Gong Q, Xu Q, Pan D, Lu F. et al. Elucidation of SIRT-1/PGC-1α-associated mitochondrial dysfunction and autophagy in nonalcoholic fatty liver disease. Lipids in health and disease. 2021;20:40
210. Cheang WS, Wong WT, Wang L, Cheng CK, Lau CW, Ma RCW. et al. Resveratrol ameliorates endothelial dysfunction in diabetic and obese mice through sirtuin 1 and peroxisome proliferator-activated receptor δ. Pharmacological research. 2019;139:384-94
211. Han W, Wang C, Yang Z, Mu L, Wu M, Chen N. et al. SRT1720 retards renal fibrosis via inhibition of HIF1α /GLUT1 in diabetic nephropathy. The Journal of endocrinology. 2019
212. Zhou XL, Xu JJ, Ni YH, Chen XC, Zhang HX, Zhang XM. et al. SIRT1 activator (SRT1720) improves the follicle reserve and prolongs the ovarian lifespan of diet-induced obesity in female mice via activating SIRT1 and suppressing mTOR signaling. Journal of ovarian research. 2014;7:97
213. Wu H, Wu J, Zhou S, Huang W, Li Y, Zhang H. et al. SRT2104 attenuates diabetes-induced aortic endothelial dysfunction via inhibition of P53. The Journal of endocrinology. 2018;237:1-14
214. Miler M, Živanović J, Ajdžanović V, Milenkovic D, Jarić I, Šošić-Jurjević B. et al. Citrus Flavanones Upregulate Thyrotroph Sirt1 and Differently Affect Thyroid Nrf2 Expressions in Old-Aged Wistar Rats. Journal of agricultural and food chemistry. 2020;68:8242-54
215. Xiong J, Tian Y, Ling A, Liu Z, Zhao L, Cheng G. Genistein affects gonadotrophin-releasing hormone secretion in GT1-7 cells via modulating kisspeptin receptor and key regulators. Systems biology in reproductive medicine. 2022;68:138-50
216. Abiri B, Sarbakhsh P, Vafa M. Randomized study of the effects of vitamin D and/or magnesium supplementation on mood, serum levels of BDNF, inflammation, and SIRT1 in obese women with mild to moderate depressive symptoms. Nutritional neuroscience. 2021:1-13
217. Shen M, Cao Y, Jiang Y, Wei Y, Liu H. Melatonin protects mouse granulosa cells against oxidative damage by inhibiting FOXO1-mediated autophagy: Implication of an antioxidation-independent mechanism. Redox biology. 2018;18:138-57
218. Di Emidio G, Rossi G, Bonomo I, Alonso GL, Sferra R, Vetuschi A. et al. The Natural Carotenoid Crocetin and the Synthetic Tellurium Compound AS101 Protect the Ovary against Cyclophosphamide by Modulating SIRT1 and Mitochondrial Markers. Oxidative medicine and cellular longevity. 2017;2017:8928604
219. Chen DL, Yang KY. Berberine Alleviates Oxidative Stress in Islets of Diabetic Mice by Inhibiting miR-106b Expression and Up-Regulating SIRT1. Journal of cellular biochemistry. 2017;118:4349-57
220. El-Mesallamy HO, Gawish RA, Sallam AM, Fahmy HA, Nada AS. Ferulic acid protects against radiation-induced testicular damage in male rats: impact on SIRT1 and PARP1. Environmental science and pollution research international. 2018;25:6218-27
221. Peck B, Chen CY, Ho KK, Di Fruscia P, Myatt SS, Coombes RC. et al. SIRT inhibitors induce cell death and p53 acetylation through targeting both SIRT1 and SIRT2. Molecular cancer therapeutics. 2010;9:844-55
222. Villalba JM, Alcaín FJ. Sirtuin activators and inhibitors. BioFactors (Oxford, England). 2012;38:349-59
223. Zhang Q, Zeng SX, Zhang Y, Zhang Y, Ding D, Ye Q. et al. A small molecule Inauhzin inhibits SIRT1 activity and suppresses tumour growth through activation of p53. EMBO molecular medicine. 2012;4:298-312
224. Botta G, De Santis LP, Saladino R. Current advances in the synthesis and antitumoral activity of SIRT1-2 inhibitors by modulation of p53 and pro-apoptotic proteins. Current medicinal chemistry. 2012;19:5871-84
225. Hsu YH, Chen YC, Chen YW, Chiu TH, Kuo YT, Chen CH. Far-infrared radiation prevents decline in β-cell mass and function in diabetic mice via the mitochondria-mediated Sirtuin1 pathway. Metabolism: clinical and experimental. 2020;104:154143
226. Yu WC, Chen YL, Hwang PA, Chen TH, Chou TC. Fucoidan ameliorates pancreatic β-cell death and impaired insulin synthesis in streptozotocin-treated β cells and mice via a Sirt-1-dependent manner. Molecular nutrition & food research. 2017 61
227. Cao Z, Wu Z, Duan T, Tang C, Huang D, Hu X. Curcumin ameliorates HO-induced injury through SIRT1-PERK-CHOP pathway in pancreatic beta cells. Acta biochimica et biophysica Sinica. 2022;54:370-7
228. Imperatore F, Maurizio J, Vargas Aguilar S, Busch CJ, Favret J, Kowenz-Leutz E. et al. SIRT1 regulates macrophage self-renewal. The EMBO journal. 2017;36:2353-72
229. Chen L, Hu FB, Yeung E, Tobias DK, Willett WC, Zhang C. Prepregnancy consumption of fruits and fruit juices and the risk of gestational diabetes mellitus: a prospective cohort study. Diabetes care. 2012;35:1079-82
230. Pant K, Peixoto E, Richard S, Biswas A, O'Sullivan MG, Giama N. et al. Histone Deacetylase Sirtuin 1 Promotes Loss of Primary Cilia in Cholangiocarcinoma. Hepatology (Baltimore, Md). 2021;74:3235-48
231. Wang TW, Chern E, Hsu CW, Tseng KC, Chao HM. SIRT1-Mediated Expression of CD24 and Epigenetic Suppression of Novel Tumor Suppressor miR-1185-1 Increases Colorectal Cancer Stemness. Cancer research. 2020;80:5257-69
232. Liu L, Zhang T, Hu J, Ma R, He B, Wang M. et al. Adiponectin/SIRT1 Axis Induces White Adipose Browning After Vertical Sleeve Gastrectomy of Obese Rats with Type 2 Diabetes. Obesity surgery. 2020;30:1392-403
233. Jeon BT, Kim KE, Heo RW, Shin HJ, Yi CO, Hah YS. et al. Myeloid-specific deletion of SIRT1 increases hepatic steatosis and hypothalamic inflammation in mice fed a high-fat diet. Metabolic brain disease. 2014;29:635-43
234. Hao CM, Haase VH. Sirtuins and their relevance to the kidney. Journal of the American Society of Nephrology: JASN. 2010;21:1620-7
235. Toorie AM, Cyr NE, Steger JS, Beckman R, Farah G, Nillni EA. The Nutrient and Energy Sensor Sirt1 Regulates the Hypothalamic-Pituitary-Adrenal (HPA) Axis by Altering the Production of the Prohormone Convertase 2 (PC2) Essential in the Maturation of Corticotropin-releasing Hormone (CRH) from Its Prohormone in Male Rats. The Journal of biological chemistry. 2016;291:5844-59
236. Motonishi S, Nangaku M, Wada T, Ishimoto Y, Ohse T, Matsusaka T. et al. Sirtuin1 Maintains Actin Cytoskeleton by Deacetylation of Cortactin in Injured Podocytes. Journal of the American Society of Nephrology: JASN. 2015;26:1939-59
237. Cui Z, Zhao X, Amevor FK, Du X, Wang Y, Li D. et al. Therapeutic application of quercetin in aging-related diseases: SIRT1 as a potential mechanism. Frontiers in immunology. 2022;13:943321
238. Gilbert RE, Thai K, Advani SL, Cummins CL, Kepecs DM, Schroer SA. et al. SIRT1 activation ameliorates hyperglycaemia by inducing a torpor-like state in an obese mouse model of type 2 diabetes. Diabetologia. 2015;58:819-27
239. Bruckbauer A, Banerjee J, Cao Q, Cui X, Jing J, Zha L. et al. Leucine-nicotinic acid synergy stimulates AMPK/Sirt1 signaling and regulates lipid metabolism and lifespan in Caenorhabditis elegans, and hyperlipidemia and atherosclerosis in mice. American journal of cardiovascular disease. 2017;7:33-47
Author contact
![]() Corresponding authors: Yang Yang: yang200214yyedu.cn. Huan Zhang: huanzhangnwcom. Department of Cardiology, Xi'an No.3 Hospital, The Affiliated Hospital of Northwest University. Faculty of Life Sciences and Medicine, Northwest University, 10 Fengcheng Three Road, Xi'an, China.
Corresponding authors: Yang Yang: yang200214yyedu.cn. Huan Zhang: huanzhangnwcom. Department of Cardiology, Xi'an No.3 Hospital, The Affiliated Hospital of Northwest University. Faculty of Life Sciences and Medicine, Northwest University, 10 Fengcheng Three Road, Xi'an, China.

 Global reach, higher impact
Global reach, higher impact