10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2023; 19(3):832-851. doi:10.7150/ijbs.75963 This issue Cite
Research Paper
Acquired resistance to EGFR-TKIs in NSCLC mediates epigenetic downregulation of MUC17 by facilitating NF-κB activity via UHRF1/DNMT1 complex
1. Cancer Research Center, Beijing Chest Hospital, Capital Medical University, Beijing Tuberculosis and Thoracic Tumor Research Institute, Beijing 101149, China.
2. Institute of Resources and Environment, Beijing Academy of Science and Technology, Beijing, 100089, China.
3. Department of Endoscopic Diagnosis and Treatment, Beijing Chest Hospital, Capital Medical University, Beijing Tuberculosis and Thoracic Tumor Institute, Beijing 101149, China.
* These authors contributed equally to this work.
Abstract
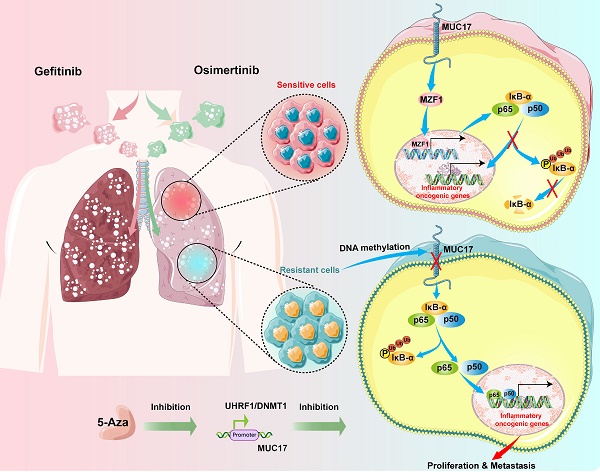
Treatment with epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) has brought significant benefits to non-small cell lung cancer (NSCLC) patients with EGFR mutations. However, most patients eventually develop acquired resistance after treatment. This study investigated the epigenetic effects of mucin 17 (MUC17) in acquired drug-resistant cells of EGFR-TKIs. We found that GR/OR (gefitinib/osimertinib-resistance) cells enhance genome-wide DNA hypermethylation, mainly in 5-UTR associated with multiple oncogenic pathways, in which GR/OR cells exerted a pro-oncogenic effect by downregulating mucin 17 (MUC17) expression in a dose- and time-dependent manner. Gefitinib/osimertinib acquired resistance mediated down-regulation of MUC17 by promoting DNMT1/UHRF1 complex-dependent promoter methylation, thereby activating NF-κB activity. MUC17 increased the generation of IκB-α and inhibit NF-κB activity by promoting the expression of MZF1. In vivo results also showed that DNMT1 inhibitor (5-Aza) in combination with gefitinib/osimertinib restored sensitivity to OR/GR cells. Acquired drug resistance of gefitinib/osimertinib promoted UHRF1/DNMT1 complex to inhibit the expression of MUC17. MUC17 in GR/OR cells may act as an epigenetic sensor for biomonitoring the resistance to EGFR-TKIs.
Keywords: DNA methylation, mucin 17, myeloid zinc-finger 1, NF-κB, EGFR-TKIs resistance

 Global reach, higher impact
Global reach, higher impact