Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2023; 19(4):1094-1109. doi:10.7150/ijbs.79666 This issue Cite
Review
Extracellular Vesicles in Mental Disorders: A State-of-art Review
1. Department of Psychiatry, the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China.
2. The Key Laboratory of Mental Disorder's Management in Zhejiang Province, Hangzhou 310003, China.
3. Brain Research Institute of Zhejiang University, Hangzhou 310003, China.
4. Zhejiang Engineering Center for Mathematical Mental Health, Hangzhou 310003, China.
5. Department of Neurobiology, NHC and CAMS Key Laboratory of Medical Neurobiology, School of Brain Science and Brian Medicine, and MOE Frontier Science Center for Brain Science and Brain-machine Integration, Zhejiang University School of Medicine, Hangzhou 310003, China.
6. Peking University Sixth Hospital, Peking University Institute of Mental Health, NHC Key Laboratory of Mental Health (Peking University), National Clinical Research Center for Mental Disorders (Peking University Sixth Hospital), Chinese Academy of Medical Sciences Research Unit (No.2018RU006), Peking University, Beijing, China.
7. Peking-Tsinghua Center for Life Sciences and PKU-IDG/McGovern Institute for Brain Research, Peking University, Beijing, China.
8. Department of Pathology, First Affiliated Hospital, Zhejiang University School of Medicine, Zhejiang, China.
9. National Health and Disease Human Brain Tissue Resource Center, Zhejiang University, Zhejiang, China.
*These authors contributed equally.
Received 2022-10-8; Accepted 2023-1-26; Published 2023-2-5
Abstract
Extracellular vesicles (EVs) are nanoscale particles with various physiological functions including mediating cellular communication in the central nervous system (CNS), which indicates a linkage between these particles and mental disorders such as schizophrenia, bipolar disorder, major depressive disorder, etc. To date, known characteristics of mental disorders are mainly neuroinflammation and dysfunctions of homeostasis in the CNS, and EVs are proven to be able to regulate these pathological processes. In addition, studies have found that some cargo of EVs, especially miRNAs, were significantly up- or down-regulated in patients with mental disorders. For many years, interest has been generated in exploring new diagnostic and therapeutic methods for mental disorders, but scale assessment and routine drug intervention are still the first-line applications so far. Therefore, underlying the downstream functions of EVs and their cargo may help uncover the pathogenetic mechanisms of mental disorders as well as provide novel biomarkers and therapeutic candidates. This review aims to address the connection between EVs and mental disorders, and discuss the current strategies that focus on EVs-related psychiatric detection and therapy.
Keywords: Extracellular vesicle, non-coding RNA, mental disorder, biomarker
Introduction
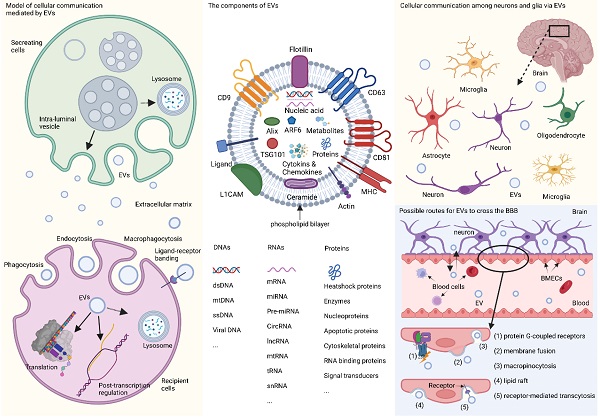
Extracellular vesicles (EVs) are minute phospholipid bilayer particles with diameters ranging from ~30 nm to 10 μm, and are typically classified into four sub-categories, exosomes (~30 nm to 150 nm), ectosomes (~100 nm to 1000 nm), apoptotic bodies (~1000 to 5000 nm), and oncosomes (~1 μm to 10 μm).[1, 2] The heterogeneity and role of EVs are predominantly determined by their cargo, such as nucleic acids, proteins, lipids, cytokines, chemokines, the end-stage neurotoxic and pathogenic metabolic products.[3-5] Among these contents, RNAs have drawn special attention. RNAs encapsulated in EVs mainly include non-coding RNAs such as mRNAs, long non-coding RNAs (lncRNAs), circular RNAs, and the widely-studied microRNAs (miRNAs or miR),[3] which have been proposed as emerging diagnostic or therapeutic candidates for many neuropsychiatric diseases.[6, 7] When transferred from cell to cell, RNAs in EVs can mediate the orientation, translation, and stability of mRNA in the target cells by combining with trans-acting factors, such as RNA-binding proteins, thus controlling cell development and differentiation.[8] Meanwhile, the pathophysiological characteristics of EV-derived lncRNAs, circular RNAs, and mRNA have also been gradually revealed over the years.[9-11] The identification of EVs is based on molecular biomarkers such as tetraspanins (e.g., CD9, CD63, CD81), syntenin-1, apoptosis-linked gene 2-interacting protein X (Alix), TSG101, neuronal origin marker L1 cell adhesion molecule (L1CAM), and adenosine diphosphate ribosylation factor 6 (ARF6).[12] Some of these markers can even indicate the different origination of EVs. For example, L1CAM is commonly recognized as the label of EVs derived from neurons in the central nervous system (CNS),[4, 5, 7] and other biomarkers such as glutamate aspartate transporter and transmembrane protein 119are indications of glia-derived EVs.[13] In recent years, studies on the classification, biological function, and protocols for isolation and detection of EVs are under rapid growth (Fig. 1).
In the CNS, EVs can be secreted by different cells and act as message carriers during neighboring and distal cellular communication between neurons, glia, and other types of cells,[14-23] thereby mediating a cascade of downstream reactions. EVs are capable of crossing the blood-brain barrier (BBB) via five regular routes (shown in Fig. 1) [16, 24] and can be detected in various body fluids, and have been proposed as possible biomarkers neurodegenerative disease and CNS tumors,[25-27] which open a window for us explore the physiological and morbid status of the brain non-invasively.[28]
EVs and their participation in cellular communication in the brain. EVs originate primordially as multivesicular bodies from intraluminal vesicles and are generated by inward budding, encapsulating nucleic acids, proteins, metabolites, and other components, some of which are recognized as detecting markers, such as tetraspanins, Alix, TSG101, ARF6, and L1CAM. Fusion of multivesicular bodies membrane with cell membrane facilitates the release of EVs, while fusion with lysosomal membrane leads to the degradation of components. When entering the recipient cells via macropinocytosis, phagocytosis, and endocytosis with or without ligand-receptor binding, EVs will end up in the lysosome, or participate in the post-transcription regulation, or translate their capsuled RNAs into functional proteins. In the brain, EVs are released by neurons and glia and mediate cellular communication between different neurons, or between glia and neurons. Furthermore, peripheral EVs can cross the BBB via five possible pathways theoretically, namely, association with a protein G-coupled receptor, surface adhesion and membrane fusion, macropinocytosis, lipid raft, and receptor-mediated transcytosis. EVs: extracellular vesicles; L1CAM: L1 cell adhesion molecule; TSG101: tumor susceptibility gene 101; CNS: central nervous system; BBB: blood-brain barrier; Alix: apoptosis-linked gene 2-interacting protein X; ARF6: adenosine diphosphate ribosylation factor 6; MHC: major histocompatibility complex
EVs and mental disorders
Up till now, the pathological mechanisms of most mental disorders remain largely unclear, thus hindering the innovation of strategies for diagnosis and treatment.[29] Inspiringly, EVs are a mirror that can reflect the real-time status of the brain and specific clinical symptoms like non-suicidal self-injury [30] and cognitive impairment.[31] Ongoing experimental and technological advances have been yielding remarkable findings on the physiological effects and heterogeneity of EVs and their cargoes,[3] and have shown the potential to harness EVs for prevention, prediction, precise diagnosis, and treatment of neuropsychiatric disorders. For example, insulin receptor substrate -1 (IRS-1), a protein associated with insulin resistance, was increased in neuron-delivered EVs from patients with MDD, thus indicating the relationship between insulin resistance in the CNS and clinical symptoms such as depressive feelings, suicide and anhedonia.[32] However, among various components in EVs, miRNA has been adequately explored and seems to be the most promising biomarker. Despite conflicting findings, accumulating studies of schizophrenia, major depressive disorder (MDD), bipolar disorder (BD), substance abuse (SA), and post-traumatic stress disorder (PTSD) have yielded early evidence on dysregulation in EV-derived miRNAs (Fig. 2).
Overlaps in peripheral miRNAs across different studies and their functions* *Only RNA families were identified, but not limited to specific homologous miRNAs as some of the research findings have not been replicated yet. All the miRNAs presented above are detected from serum/plasma. MDD: major depressive disorder; BD: bipolar disorder; SCZ: schizophrenia; SA: substance abuse; PTSD: post-traumatic stress disorder
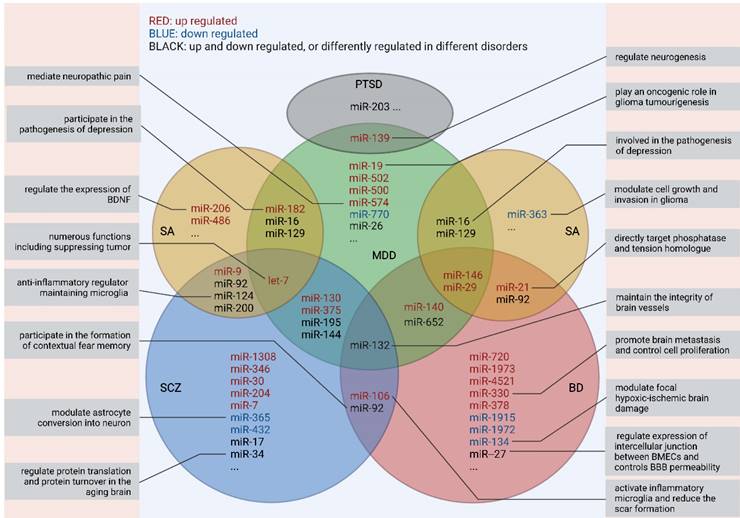
Notably, none of those dysregulated miRNAs showed specificity for a given disorder or has been repeatedly verified by different studies. Thus, more endeavor is needed to make further explorations of the EV-related RNA candidates before their clinical applications.
The neuropathological underpinnings
EVs implicated in the pathophysiological processes in the CNS
Mental disorders are not simply the results of brain dysfunction, they have pathophysiological underpinnings. EV-mediated myelin impairment of neurological diseases[33] are also implicated in different mental disorders.[6, 34-41] EVs participate in cellular communication between neurons and glia, which is known as “neuroimmune interactions”, and it is the basis of the pathophysiological processes including decreased synaptic activity, elimination of waste, and maintenance of myelin integrity.[19, 42] The most critical cell population participating in the neuroimmune interactions is microglia. Generally, EVs are important vehicles of microglia-related bioactive molecules which are delivered towards other CNS-resident cells, while the phenotype and physiological status of microglia could also be regulated by EVs from other cells.[43] EVs secreted by activated microglia are enriched in selective pathogenetic miRNAs and other bioactive factors that are entangled in intercellular interactions such as complement activation and neuroinflammation, which may disrupt innate immune signaling as well as neurotropism and synaptic signaling progress through regulating expression of neuron-specific phosphoproteins.[44-46] In addition, the differentiation and myelin deposition of oligodendrocytes that form a highly specialized functional entity could be influenced by microglia-derived EVs, which is critical for the remyelination process in neuroinflammatory diseases.[47]
EVs derived from astrocytes participate in neuroimmune interactions as well as the central-to-peripheral immune communication.[48] On the one hand, the astrocytes-derived pro-inflammatory EVs can be taken by neurons and lead to neuronal damage.[17, 48] Meanwhile, those EVs can mediate microglia activation, migration, and phagocytic capacity, accounting for neuron inflammation.[17, 49] On the other hand, in the physiological state, astrocyte-derived EVs prevent inflammation, facilitate tissue repair, and enhance physiological adaption to the inflammatory micro-environment during the acute morbid stage.[50-52] Furthermore, EVs could be produced by both CNS-resident and CNS-infiltrating leukocytes under the circumstance of neuroinflammation,[42] regulating the activity of neuron and glia cells in the CNS.
Internalized by neurons in response to stimuli, EVs derived from oligodendrocytes have neuroprotective properties and are indispensable for the homeostasis of the CNS micro-environment.[53] Oligodendrocyte-specific genetically defective mice secreted fewer EVs-delivered proteins, resulting in a deficit of neuronal long-term stability.[54] In addition, many myelin proteins relevant to autoimmune encephalomyelitis were found in EVs cultured in vitro,[55] thus shedding light on drug delivery for CNS neuroimmune diseases such as multiple sclerosis.[56] Recently, myelin deficits have been documented as new neuroimaging characteristics in patients with recurrent MDD.[33] but the role of EVs in myelin deficits is still unclear.
EVs increase the permeability of the BBB
Leakage of the BBB, which is related to abnormalities in glutamatergic transmission and neuroinflammation,[57] has been observed in neurodegenerative diseases and some types of mental disorders.[24, 58-60] Proteomic studies also revealed that several neurodegenerative diseases biomarkers such as amyloid precursor protein and prion protein were enriched in neuron-derived or glia-derived EVs in human cerebrospinal fluid (CSF),[61] aligning with cognitive decline in several mental disorders including schizophrenia,[62] later-life MDD,[63] autism spectrum disorder (ASD),[64] sleep disorder (SD),[65] and PTSD.[65]Therefore, EVs might be involved in the dissemination of the pathological changes from brain to other tissues.
Notably, EVs may participate in regulating the permeability of the BBB. According to a study using a zebrafish model, EVs containing miR-132 derived from the brain could target directly at the tight junction between brain microvascular endothelial cells (BMECs), resulting in increasing BBB permeability and microhemorrhage risk in the CNS.[66]
Nevertheless, we should be cautious with the impact of EVs on the BBB. It has been demonstrated that brain-derived EVs could either protect BMECs from the hypoxia damage[67] and maintain the integrity of brain vessels,[66] or disrupt the homeostatic permeability of the BBB. Although relevant research is limited,[68] the increased BBB permeability acts as a bridge combining the dysregulation of EVs with the dysfunction of brain, indicating the potential of targeting EVs for the treatment of mental disorders.
EVs and major mental disorders
Schizophrenia
The clinical manifestations of schizophrenia, a prevalent mental disorder with complex etiology, are characterized by high heritability, early-onset, and debilitating course.[69,70] The pathological mechanisms of schizophrenia are still indistinct, but the linkage between schizophrenia and decreased dopamine in the prefrontal cortex, excessive dopaminergic activity in the mesolimbic tract, decreased mesocortical dopaminegic neurons and gamma-aminobutyric acid (GABA)-ergic inhibitory activities, has been widely recognized.[71] Increasing evidence also indicates that epigenetic modification may be associated with the pathogenesis of schizophrenia,[72] while EV and its encapsulated miRNAs may fitly act as epigenetic regulators.[73]
Neuron- or glia-derived EVs may contribute to the onset of schizophrenia by transporting toxic (or misfolded) proteins and neurotransmitters, resulting in clinical symptoms like cognitive deficits. 25 perturbed metabolites in EVs related to metabolism of glycerophospholipid, phenylalanine, tyrosine, or tryptophan have been found to be linked to schizophrenia.[74] In detail, proteins or small molecules such as amyloid-beta 42 (Aβ42) [62] and glial fibrillary acidic protein was elevated, while α-II-spectrin[75], subunits 1 and 6 of NADH-ubiquinone oxidoreductase and subunit 10 of cytochrome b-c1 oxidase were lower in patients with schizophrenia, which provides evidence on the astrocyte-related neuroinflammatory underpinnings of the association between the dysregulated protein expression and increased reactive oxygen species.[76] Other research discovered dysregulation of miRNAs in EVs from postmortem brain tissues, CSF and peripheral blood in patients with schizophrenia or other mental disorders with psychosis. Moreover, elevated phosphatidylserine-positive EVs were detected in the CSF of schizophrenia patients, which might be a signature of enhanced membrane shedding in the CNS.[77] Studies on blood EV-related miRNAs indicate that specific miRNAs might contribute to the pathogenesis of schizophrenia including abnormalities in protein glycosylation, neurotransmitter biosynthesis, and dendrite development, and eventually reshape the structural and functional phenotype of the brain.[78, 79] For example, miR-132 in EVs increased in MDD and BD while it decreased in SCZ, indicating the different pathological mechanism between mood disorders and SCZ, such as the CLOCK gene, a target gene of miR-132.[68] Besides, miR-132 has a broad impact on the nervous system. It not only regulates the differentiation, maturation and functioning of neuron, but also widely participates in axon growth and neural plasticity, and thus preventing hippocampal neurogenesis and relevant memory deficits.[80, 81] Other miRNAs are also important. miR-106b-5p, an inflammation- and tumor-associated miRNA, was reported in both schizophrenia and BD studies, while miR-195-5p, miR-181b, miR-144-5p, miR-130b, and let-7g were documented in both schizophrenia and MDD subjects, indicating the pathological mechanisms of dysregulation of AKT pathway, cytokine production, macrophage polarization, energy metabolism in those disorders,[68] thus providing a plausible explanation for the overlapping symptom spectrum across different mental disorders.
However, in-depth explorations on the role of EV-related miRNAs in accessing clinical symptoms and treatment outcomes of schizophrenia are limited. Patients with early-onset schizophrenia showed up-regulated exosomal miR-137 and decreased cytochrome c oxidase complex IV COX6A2, which is involved in the psychological functions of mitochondria.[82] This suggests that alternations of miR-137/COX6A2 EVs in plasma may represent a proxy marker of cortical microcircuit impairment, a pathological feature of schizophrenia.[82] Besides, the level of miR-223, an EV-related miRNA targeting glutamate receptors, was increased in the orbitofrontal cortex of patients with schizophrenia.[83] Following treatment with olanzapine or haloperidol, the expression of miR-223 was down-regulated while the expression of neuronal Grin2b was increased, thus revealing that miR-223 might be a promising treatment target of schizophrenia. Therefore, further investigation on the cargo of EVs may offer insights into the etiology of schizophrenia and new treatment strategies.
In terms of treatment, several studies revealed that EVs could alleviate the toxicity of glutamate overload in specific regions of the brain. In phencyclidine-induced schizophrenia mice model, mesenchymal stem cell-derived EVs can migrate to the prefrontal cortex by intranasal delivery, a brain area that is critically involved in the neuropathology of schizophrenia. Following EVs treatment, social interaction and disruption due to pre-pulse inhibition were significantly improved, parvalbumin-positive GABAergic interneurons were preserved, and the level of glutamate in the CSF was decreased,[84] thus indicating a potentially novel treatment strategy for schizophrenia.
Bipolar disorder
The pathophysiology of BD is complex. Neuroinflammation, disturbed neurogenesis and neuroplasticity are found to underline the recurrent depressive or manic/hypomanic attacks of BD.[85] Interestingly, EVs are closely bound up with the pathological mechanisms of BD, and act as emerging biomarkers for detecting BD clinically. Omics research has provided further evidence. Dozens of dysregulated miRNAs, among which some were involved in netrin-mediated axon guidance as well as signaling pathways of the endothelium, serotonin, and androgen, have been detected in BD cases, and a few miRNAs showed phase-specific alterations in BD patients.[86, 87]
Alternations in EVs detected from BD patients, as well as their cargoes, are potential biomarkers for identifying phases of BD. Metabolites produced by galactose or amino sugar metabolism like xylitol were decreased in serum EVs from patients with BD, and a random forest classifier constructed by 15 exosomal metabolites showed excellent performance in diagnosis and differential diagnosis for BD.[88] Astrocyte-derived EVs might transfer the stress signals such as cytokines and corticosteroids from peripheral blood, thereby causing deficits in neurogenesis.[89] The encapsulated miRNAs in EVs can regulate both synaptic plasticity and brain development. For example, miR-134 was associated with the development of dendritic spines and synapses, and was a biomarker for monitoring mania episodes in BD.[90] Moreover, miR-128 and miR-378 were also found to be linked to neurite outgrowth and neurogenesis in BD cases.[91]
Notably, the change of miR-142-3p was inconsistent in different studies, [86, 87] and in patients with type II BD, an elevated level of miR-142-3p was more likely to be detected in EVs from serum.[92, 93] miR-29c, which played a role in neural development and signal transduction in the CNS, was up-regulated in BD cases, and was proposed as a target of lithium treatment.[94] miR-29a, which was also up-regulated in BD patients, was critically involved in the functional and structural neurotoxic injuries.[95] miR-149 can inhibit glial proliferation.[96] It has been observed that miR-149 in EVs was altered in the anterior cingulate cortex (ACC) of BD patients,[97] and an increased miR-149 in EVs led to a reduced number of glial cell in the ACC of familial MDD and BD patients.[98, 99]
Besides, recent research have suggested that the gut-brain axis (GBA) may be implicated in the pathogenesis of BD,[100, 101] with increasing evidence revealing the disturbance of the intestinal microbiota and disruption of the bidirectional interaction between the brain and the gut microbes in BD individuals.[102-104] A significant discrepancy was found both in the serum and intestinal biomarkers of microbiome between BD cases and healthy controls.[105] A recently published review comprehensively discussed the involvement of brain- and gut-derived EV miRNAs in the GBA-related pathways (i.e., miR-375-3p, and miR-294-5p),[106-108] indicating a novel regulatory strategy targeting the GBA via EVs modulation.[109]
EVs also show a great potential in liquid biopsy in clinical practice. The levels of molecules in neuron origin EVs such as p-NF-κB and p-FADD were associated with anhedonia and treatment outcome of infliximab.[110] Moreover, EVs can reflect the cerebral status of insulin resistance, which are related to the cognitive function as well as the structure of the brain.[111] For example, the neuronal-enriched EV indicated that biomarkers of insulin signaling were associated with cognitive function and brain structures independently, and following infliximab treatment, improvement in anhedonic symptoms and decrease in inflammatory molecules was linked to an enhanced insulin signaling via neuron-derived EVs.[110, 112] Therefore, EVs provide a new insight into the pathological mechanisms of BD, which may facilitate the diagnosis and treatment in the future.
Major depressive disorder
Current knowledge on the pathophysiology of MDD includes alterations in neural and glial activity, hypo-connectivity of frontoparietal network, and overactivation in the hypothalamic-pituitary-adrenal axis, which contribute to disturbance in neruotransmitter transmission, as well as structural and functional abnormalities in the brain.[113, 114]
Findings of pre-clinical studies provide preliminary evidence that EVs are involved in the pathophysiological progression of MDD, of which the most well-known mechanisms are neuroinflammation and neuroplasticity. Activation of microglial cells in different regions of the CNS was observed in MDD patients, accompanied with a decrease in neurogenesis and an increase in glutamate toxicity.[115-117] EVs released by microglia encapsulate inflammatory cytokines and miRNAs that are crucial in neurogenesis, neurotransmission, and ion channel regulation.[118, 119] It has been reported that EV-related miR-9-5p promoted microglial M1 polarization and over-released cytokines, such as IL-1β, IL-6, and TNF-α, thus exacerbating nerve injury.[120] Fan et al.[121] observed that the overexpression of microglia-enriched miRNAs, such as miR-146a-5p in the hippocampal dentate gyrus of mice, can suppress neurogenesis and spontaneous discharge of excitatory neurons, and act on the contrary when it was down-regulated. Furthermore, miR-207 in the EVs derived from natural killer cells alleviated depressive symptoms mice by targeting Tril-NF-κB pathway in astrocytes.[122] The miR-26a and miR-186-5p were dysregulated in the hippocampus from animal models of MDD, which may be caused by the down-regulation of SERPINF1 encapsulated in EVs derived from the bone marrow mesenchymal cells and serum.[123, 124]
Clinical studies have also demonstrated the potential of cargoes in EVs for assisting the diagnosis of MDD. Molecules in EVs, such as pro-BDNF, miR-130b, miR-361-5p, miR-140-3p, miR-574-3p, miR-139-5p and miR-335-5p, were up-regulated in MDD cases, while BDNF, miR-34c-5p and miR-770-5p, miR-1292-3p, let-7b and let-7c were down-regulated.[125-127] Notably, higher miR-9-5p level was detected in serum- EVs of MDD patients, suggesting the connection between microglia-mediated neuroinflammation and MDD pathophysiology.[120] Additionally, the concentration of mitochondrial proteins and metabolic proteins such as insulin receptor substrate-1 in L1CAM enriched EVs, was increased in MDD patients, indicating dysregulation of neuronal mitochondrial activity and metabolic progress in CNS.[32, 128] Those findings suggest that changes of EVs and their cargoes can be used as disease markers for monitoring the pathological changes of MDD.
Intriguingly, changes in behavioral phenotypes were also linked to the dysregulated level of EVs and their cargoes, showing the potential application of EVs in accessing the susceptibility, severity and symptoms of MDD. It was found that miRNAs in EVs could distinguish the susceptibility to chronic social defeat stress-induced social avoidance phenotype in mice, which may be due to the production of pro-inflammatory cytokines after injection.[129] Furthermore, downregulation of miR-146a-5p and Exosomal sigma-1 receptor was proven to ameliorate depressive-like behaviors in model mice.[121, 130] As for clinical findings, some molecules in EVs are associated with specific symptoms. For example, ISR-1 in neuron-derived EVs was related to the feeling of guilt, suicidality and anhedonia, while a higher level of pSer-IRS-1 was correlated to an aggravated severity of depression in females. [32] Furthermore, levels of miR-21-5p, miR-30d-5p, and miR-486-5p changed significantly in peripherally extracted neuron-derived EVs from patients who had favorable responses to antidepressant treatment, and the molecular targets of these miRNAs were also altered in the brain of depressed individuals,[131] suggesting the application of EVs in monitoring the treatment outcome of antidepressive treatment in the future.
In terms of the utility of EVs in MDD treatment, miR-146a-5p enriched EVs, RVG-circDYM engineered EVs, and miR-207 containing EVs showed the ability to alleviate depression-like behavior in mice. [121, 122, 132] However, there is still a long way to verify the accuracy and sensitivity of these biomarkers before clinical application.
EVs and other mental disorders
Relevant research has also indicated the critical role of EVs in other mental disorders including ASD, SA, SD, and PTSD. The latest and representative pre- and clinical studies are summarized in Table 1. It is noteworthy that overlaps of susceptibility between these mental disorders and aforementioned disorders including schizophrenia, MDD, and BD have been reported previously,[133-140] which could probably be medicated by the dysregulation of the same EVs or their cargoes.
Autism spectrum disorder
ASD is an early onset (usually in the first three years after birth) mental disorder which is characterized by impaired social communication as well as repetitive and restricted patterns of behaviors, interests, or activities.[141] Several molecules targeting on key synaptic genes were found to be related to ASD through pathological process including neuronal inflammation, microglial activation and abnormal growth.[142, 143] Emerging evidence indicates that dysfunction of synaptic and BBB-related gene expression regulated by miRNAs (i.e., miR-146a, miR-221, miR-654-5p, and miR-656) and other components such as mitochondrial DNA, lncRNA and mRNA derived from EVs originating in the CNS may contribute to the abnormalities in the growth of microglial and cause neuroinflammation, which was considered as a pathological landmark of ASD.[144-147] Notably, up-regulated expression of neuroinflammatory genes may also influence BBB integrity in ASD patients.[142] EVs are capable of mediating the increased permeability of the BBB, which further aggravates neuroinflammation. Therefore, EVs may be involved in the pathological mechanism of ASD via impairing the BBB function and inducing CNS neuroinflammation.
Interestingly, stem cell-derived EVs have the therapeutic potential for ASD since EVs can be directly delivered to the prefrontal cortex after nasal administration.[148, 149] Intranasal administration of mesenchymal stem cells derived EVs was found to relieve autistic-like behaviors in two mice models.[141, 150] Bone marrow-extracted mesenchymal stem cell (MSC)-derived exosomes showed the capacity to inhibit the release of pro-inflammatory molecules such as TNF-α and IL-1β,[151] which might in turn reduces the problem behaviors, such as irritability and decreased socialization in ASD patients.[143] However, clinical studies of EVs in ASD is still insufficient and calls for more endeavor.
Substance abuse
SA is a multifactorial syndrome resulting from a complex interplay between the reward circuitry. Research evidence revealed that SA might promote the release of endogenous EVs with changed cargoes, suggesting that exposure to addictive agents may disrupt the EV-mediated signal transduction.[152] A recent review comprehensively overviewed the current knowledge on EVs and their relationship with SA,[153] and concluded that cargo of EVs such as toll-like receptor 4, miR-146a, miR-182,[154] miR-124,[155] miR-21, miR-126,[156] miR-9-3p, [157] miR-21-3p,[158] miR-15b, miR-181, miR-125b,[159] and miR-29b[160] could be regarded as potential diagnostic biomarkers, and in the future may become therapeutic targets for the addiction to alcohol, cocaine, heroin, methamphetamine, nicotine, cannabidiol, or opiates. Interestingly, the levels of miR-16-5p, miR-129-5p, miR-363-3p, and miR-92a-3p from EVs in patients with heroin dependence and methamphetamine dependence showed a significantly negative correlation with the symptoms of anxiety,[161] indicating that EV-related miRNAs may serve as overlapping pathogenesis of these two disorders. Moreover, a recent study focusing on acute and protracted withdrawal showed that the alterations in cytokine level and imbalance of Th1/Th2/Th17 in patients addicted to heroin corresponded with abnormalities in EV-related lncRNA/mRNA expression,[162] which provided a novel explanation for the pathological mechanism of heroin addiction.
Typical pre-clinical and clinical studies on emerging EV-derived biomarkers for ASD, SA, SD, and PTSD*
| Year | Disease | Subjects | Sample source | Analysis methods | Results / Biomarkers | Ref. |
|---|---|---|---|---|---|---|
| 2018 | ASD | TG: BTBR T+tf/J mice, C57BL mice (MSC-derived EVs via intranasal administration) CG: BTBR T+tf/J mice, C57BL mice (saline via intranasal administration) | - | NanoSight analysis, NanoSight technology, western blot, etc. | Intranasal administration of MSC derived EVs increased male-to-male social interaction and reduced repetitive behaviors. | [145] |
| 2019 | NSC cell lines obtained from single IPSC clones from patients with ASD | Supernatants of spun media | qRT-PCR, TEM, western blot, northern blot, etc. | miR-1290 | [200] | |
| 2020 | TG: Male Shank3B C57BL/6J mice (20 μl MSC derived EVs, 107 particles/μl via intranasal administration) CG: Male wild-type littermate mice (placebo via intranasal administration) | Brain homogenates | qRT-PCR, etc. | Intranasal treatment with MSC-derived EVs improved the core ASD-like deficits of the mouse model of autism. | [150] | |
| 2018 | ASD | TG: children with ASD (n = 20) CG: healthy children (n = 8) | Serum | Western blot, TEM, ELISA, etc. | mtDNA, IL-1β | [146] |
| 2021 | TG: children with ASD (n=100) CG: healthy controls (n=60) | Immunosorbent assay, TEM, NTA, western blot, qRT-PCR, etc. | EDNRA, SLC17A6, HTR3A, OSTC, TMEM165, PC-5p-139289_26, and miR-193a-5p | [201] | ||
| 2021 | TG: children with ASD (n=14); women with healthy pregnancies (n=30) CG: healthy children (n=14); women with spontaneous preterm birth (n=38), preeclampsia (n=19), gestational diabetes mellitus (n=34) | Plasma | TEM, NTA, western blot, qRT-PCR, etc. | lncRNAs of SLC18A2, SYT9, STX8, and SYT15 genes mRNA of SLC18A2, SYT15, STX8, SV2C, SYT9, and SYP genes | [202] | |
| 2010 | SA | TG: Male ICR mice with morphine pellets implanted (1× 75 mg of morphine base/pellet/mouse) CG: Male ICR mice with placebo pellets implanted (1× 75 mg of placebo base/pellet/mouse) | Brain homogenates | Western blot, qRT-PCR, polysome analysis, etc. | miR-15b, miR-181, miR-125b | [159] |
| 2012 | TG: Indian strain rhesus macaques with simian immunodeficiency virus affected and Morphine injected CG: Indian strain rhesus macaques with simian immunodeficiency virus affected and saline-injected | Western blot, virtual northern blot, electron microscopy, luciferase activity assays, qRT-PCR, etc. | miR-29b | [160] | ||
| 2016 | TG: Adult male C57BL/6N mice with chronic cocaine intraperitoneal injection (20 mg/kg) CG: Adult male C57BL/6N mice with placebo intraperitoneal injection (20 mg/kg) | Percoll gradient centrifugation, flow cytometry analysis, etc. | miR-124 | [155] | ||
| 2019 | TG: 8-week-old male ApoE-/- C57BL/6 mice (high-fat diet), 8-week-old male ApoE-/- C57BL/6 mice (high-fat diet + nicotine) CG: 8-week-old male ApoE-/- C57BL/6 mice (normal chow diet) | vascular smooth muscle and macrophage cells | TEM, qRT-PCR, etc. | miR-21-3p | [158] | |
| Neurons and astrocytes derived from C57/BL6 wild-type, TLR4-Knock-out, and transgenic β actin DsRed mice | Supernatants of spun media | Flow cytometry analysis, NTA, TEM, etc. | TLR4, miR-146a, miR-182 | [154] | ||
| LN18 (chemo-resistant GBM cell line, grade IV glioblastoma derived from a male patient with a right temporal lobe glioma) LN229 (chemo-sensitive GBM cell line, glioblastoma derived from a female patient with right frontal parietal-occipital glioblastoma) | Supernatants of spun media | Western blot, NTA, miRNA analysis, etc. | miR-21, miR-126 | [156] | ||
| 2020 | SA | TG: heroin-dependent patients (n=42), methamphetamine-dependent patients (n=42) CG: health controls (n=42) | Serum | Microarray-based miRNA analysis, qRT-PCR, etc. | miR-9-3p | [157] |
| 2021 | TG: methamphetamine-dependent patients (n=10), heroin-dependent patients (n=10) CG: healthy controls (n=10) | Plasma | Western blot, TEM, NTA, qRT-PCR, etc. | miR-16-5p, miR-129-5p, miR-363-3p, miR-92a-3p | [161] | |
| 2019 | SD | TG: adult female mice with miR-155 knockout CG: female wild-type littermate control mice | Serum | Enzyme immunoassay, etc. | miR-155 | [169] |
| 2017 | SD | TG: narcolepsy patients (n=20) CG: healthy controls (n=17) | Peripheral blood | qRT-PCR, miRNA microarray analysis, etc. | miR-188-5p | [168] |
| 2020 | PTSD | TG: FKBP5 KO mice (male and female) CG: littermate wild type (male and female) | Dissected medial prefrontal cortex | miRNA extraction technology, etc. | Circulating EV-related miRNAs showed an altered expression in FKBP5 knockout mice | [203] |
| 2014 | PTSD | TG: PTSD patients (n=30) CG: healthy controls (n=42) | Peripheral blood | Flow cytometric analysis, miRNA array assays, qRT-PCR, etc. | miR-125a | [171] |
| 2019 | TG: male Iraq and Afghanistan combat veterans with PTSD (n=12) CG: male Iraq and Afghanistan combat veterans without PTSD (n=12) | Plasma EV plasma EV-depleted plasma | qRT-PCR, small RNA sequencing data analysis, etc. | miR-203a-3p, miR-339-5p | [172] | |
| TG: military personnel with mTBI (n=42) CG: healthy controls (n=22) | Plasma | Electro-chemiluminescent immunoassays, NTA, digital array technology, etc. | Exosomal IL-10 levels were related to PTSD symptoms in military personnel with mTBI. | [204] | ||
| 2020 | TG: patients with PTSD (n=48) CG: healthy controls (n=47) | Serum | Structural and perfusion magnetic resonance, arterial spin labeling, etc. | miRNA expression levels of composite markers may be associated with PTSD symptom severity | [203] | |
| 2021 | Patients with TBI and/or PTSD (n=144) | Plasma | miRNA profiling analysis, ELISA, etc. | Plasma neurofilament light chain, miR-139-5p | [205] |
* Yellow: pre-clinical studie; blue: clinical studies. All studies are listed in a chronological order.ASD: autism spectrum disorder; SA: substance abuse; SD: Sleep disturbance; PTSD: post-traumatic stress disorder; mTBI: mild traumatic brain injury; TG: test group; CG: control group; PCA: principal component analysis; MSC: mesenchymal stem cell; TEM: transmission electron microscope; ELISA: enzyme-linked immunosorbent assay; mtDNA: mitochondrial DNA; miRNA/miR: microRNA; lncRNA: long non-coding RNA; NTA: nanoparticle tracking analysis; qRT-PCR: quantitative real-time polymerase chain reaction; EV: extracellular vesicles; Ref: Reference.
EVs also showed the potential of therapeutic use for SA. It has been found that intranasal administration of EVs with miR-124 alleviated cocaine-mediated microglial activation,[163] while astrocyte-derived EVs encapsulating siRNA restored phagocytic activity of microglia and restrained morphine addiction.[164] These studies shed light on the feasibility of EVs as a therapeutic option of SA.
Sleep disorder
SD, associated with various factors such as stress, endocrine dysfunction, and drugs, is constantly associated with neurodegenerative diseases and affective disorders, with overlapping molecular mechanisms such as dysregulation of BDNF.[125, 165, 166] Studies correlating EVs and sleep mainly focused on secondary sleep apnea, but herein we only discuss SD from the psychiatric perspective. EVs have been found to be involved in the pathogenesis of promoting Aβ formation, transferring tau protein, mediating neuroinflammation, and increasing BBB permeability in elderly patients with SD.[167] A series of pre- and clinical studies have shown that some EV-related miRNAs, including miR-188-5p[168] and miR-155,[169] may be responsible for the pathophysiological process of SD, but none showed reliable specificity.
Post-traumatic stress disorder
PTSD is a severe mental disorder caused by trauma and may affect neurodevelopment that result in structural and functional abnormalities in brain.[170] Significant alternations of EV-related miRNAs in veterans with PTSD were associated with immune disturbance, including immune response and pro-inflammatory cytokines secretion.[171] Therefore, these miRNAs may serve as candidates for monitoring the inflammatory status in PTSD individuals.[171] Additionally, veterans with PTSD showed alterations in plasma concentration of miR-203a-3p and miR-339-5p,[172] which would interact with genes involved in the pathogenesis of PTSD and other comorbidity conditions such as cardiovascular diseases, inflammatory reaction, and neurotransmitter system,[173, 174] suggesting these diseases may share similar pathophysiological mechanisms. Although growing number of emerging studies focusing on EV-related miRNAs and other biomarkers of PTSD, most findings still require further clinical validations.
Limitations
Research of EVs in mental disorders is still in its infant stage. Currently, most studies focus on improving the detecting techniques, identifying new EV candidates and validating their specificity in large sample size. In general, no clear conclusions have ever been drawn regarding the mechanisms of EVs underlying the pathogenesis of mental disorders.
One major limitation lies in the identification of tissue-specific EVs by liquid biopsy. In the CNS, EVs may originate from various types of cells including neurons, endothelial cells, glia, as well as peripheral immune cells migrating into the brain, or simply derive from the peripheral circulation.[19, 20, 34, 175] This accounts for the diversity in the components of EVs, making it difficult to identify the specific candidates for certain mental disorder. To overcome this difficulty, brain-derived EVs such as neuron- or -glia-specific EVs, or brain region-specific EVs with specific biomarkers are being developed.
Another limitation is that few EV-based biomarkers are disease-specific and may be identified across different diseases. Also, the exact function of these EV cargoes is unclear. For example, miR-132 was observed to be dysregulated in patients with different mental disorders, including schizophrenia, MDD, and BD,[68] and was known to participate in maintaining the integrity of brain vessels.[66] However, whether miR-132 contributes to the neuropathology of these mental disorders via damaging the brain vessels remains unknown. Controversial findings call for special concerns. For example, the miR-142-3p was increased in two BD cohorts from USA and China respectively, while was decreased in a cohort from Turkey.[86, 87] This inconsistence may generate from differences in ethnicity, size of cohorts or measurement methods. This phenomenon reminds researchers to establish standardized study design in EVs studies. Therefore, great endeavor is needed to clarify the molecular mechanisms of EVs and their cargoes in the CNS, which may further help to understand the role of EVs in mental disorders.
Future directions
Precise diagnosis and individualized treatment are two major directions for applying EVs in clinical psychiatry. Great challenge exists in the clinical practice of psychiatry, and most importantly, diagnosis of mental disorders is almost dependent on self-report symptoms and physicians' experience. Not a single experimental indicator has specificity for any mental disorder. Previous studies have demonstrated the diagnostic potential for EVs and their cargoes.[3] Furthermore, EVs with neuronal origin biomarkers provide novel, convenient, and safe approaches for exploring and identifying changes in the CNS. For example, neuron- and astrocyte-derived EVs in the peripheral blood and the encapsulated proteins including auto-lysosomal proteins, synaptic proteins, etc. were suggested as reliable biomarkers for early Alzheimer's disease (AD) diagnosis and even accurate predictors for the development of AD up to 10 years before its clinical onset.[176-178] However, there are still some issues that need to be considered. Firstly, the reliability of EVs as biomarkers for mental disorders should be verified, since the contents of EVs and their cargo in the peripheral blood are influenced by various factors including the interactions among different organ-originated EVs and the number of EVs crossing the BBB. The peripheral level of neuron-derived EVs and their cargo may not be sensitive enough to reflect the slight changes in the CNS, and in the early stage of many mental disorders, the pathobiological brain changes does not mean simultaneous change in EVs.[178] The inconsistency of experimental results should also be noted. Possible confounding factors include the process for extracting EVs, number of repeated freezing and thawing, antibody sensitivity, quantification methods, and contamination of blood on the extracted CSF.[178] Unfortunately, no standardized detection process has been widely used in different studies. Moreover, new evidence has raised question on the authenticity of L1CAM, a surface marker for neural EVs.[179] Indeed, except for plasma, the antibody of L1CAM is not necessarily neuron-specific but can originate from peripheral tissues.[180] Therefore, it is necessary to identify more specific protein markers originating from the brain or even certain cell types. Above all, although EVs have shown the potential to be developed as brand-new biomarkers for clinical diagnosis, differential diagnosis, and even rating the severity of symptoms of mental disorders, it is still in its infancy and needs more explorations.
In terms of gene therapy, EVs show advantages in targeting ability, therapeutic effect, high safety, and low immune response compared with traditional drug delivery systems including viruses, liposomes, and polyethyleneimine nanoparticles.[181] EVs are considered convenient and safe drug-delivering tools for treating mental disorders, because of its ability of crossing the BBB and being navigated to the target cells or receptors after being engineered.[42, 182] Indeed, pre-clinical and clinical studies on the treatment efficacy of EVs have provided preliminary evidence on its well tolerance and favorable response without severe toxic reaction.[183, 184] Surface ligands of EVs enable the development of receptor-mediated cellular communication, and specific ligands can be enriched by engineering manipulation, according to the need of inhibitory or excitatory effects on the downstream pathways.[3] EVs can act as carriers of nucleic acid fragments for the treatment of mental disorders, since miRNAs encapsulated in the EVs are protected from degradation by blood-derived ribonucleases,[185] thus helping keep their integrity when transported to distant tissues.[3] EV-siRNAs have been used to treat neurodegenerative diseases in mice,[14] while EV-derived miR-124a could enhance the expression of excitatory amino acid transporter 2 on the surface of astrocytes, which modulates synapse activity and alleviates neuronal apoptosis.[186] EVs derived from human mesenchymal stem cells are neuroprotective by inhibiting neuronal cell apoptosis, promoting nerve repair and regeneration, as well as restoring bioenergy following energy consumption induced by glutamate excitotoxicity.[187] EVs and their cargo such as cystatin C also assist in the process of degrading Aβ,[188, 189] which helps delay the progression of neurodegeneration. Given the complex origin of EVs, it is necessary to combine EVs biomarkers with other techniques such as MRI imaging.[190]
Notably, engineered EVs were not only a novel anticancer treatment via small non-coding RNAs , but also showed potential applications in the management of morphine addiction when modified with rabies viral glycoprotein or curcumin.[191-193] When modified with specific molecules such as brain homing peptide and neuropilin-1-targeted peptide, EVs can not only have the ability of targeted delivery but also obtain imaging features in vivo.[194, 195] Based on those techniques, similar pre-clinical studies have been carried out in neuropsychiatric diseases such as ASD,[145] and traumatic brain injury (TBI).[196]
Furthermore, since the complicated interactions in the extracellular micro-environment can hardly be reconstructed in vitro, and cultured cells cannot reflect the progression or fluctuation of mental disorders, novel strategies and more advanced techniques for culturing EVs in vitro and establishing a vivid environment for exploring the biological functions of EVs are urgently needed. One of the advanced techniques is the brain chip, a minute co-cultured system containing human neurons, BMECs, and glia under a biomimetic condition, that can be employed as an in vitro model for investigation of mental disorders.[197] In addition, with the help of human induced pluripotent stem cells, the brain‐derived EVs can be further used to explore the alterations in the cellular compositions and the specific stage of mental disorders. For example, a combination of 16 new marker candidates of brain-derived EVs and neural cell‐type‐specific EV proteins was recently detected for AD, which helps to expand our knowledge of molecular mechanisms of neurodegeneration.[198] Another promising method is the brain organoid, which helps to observe changes in brain phenotypes and provides new tools for developing new therapeutic strategies free from brain tissues of animal models or patients.[199] However, the feasibility of these methods remains to be validated, and a prospective design with early-stage mental disorder would be desirable to detect the objective biomarkers for timely diagnosis and treatment in the future.
Conclusion
With the rapid development of the EVs field, increasing interest has arisen regarding its potential role in the neuropathogenesis of mental disorders, such as neuroinflammation and dysfunction of the BBB. Cargoes in EVs derived from different cell types reflect the dynamic status of diseases and thus can act as monitor and reveal pathophysiological mechanisms. Therefore, EVs seem to be ideal biomarkers of mental status and can promote non-invasive, precise diagnosis and treatment for neuropsychiatric diseases. In the case of diseases, EVs can mediate neuroinflammation and increase the BBB permeability. While under physiological conditions, EVs are necessary mediators of cellular communication between neurons and glia. Changes in EVs from CSF and peripheral biofluids such as serum/plasm, as well as their cargoes, have been detected in patients with various mental disorders such as schizophrenia, MDD, BD, and ASD. Therefore, EVs and their cargoes may facilitate the early diagnosis and evaluation of mental disorders, thereby improving the disease prognosis. However, challenges are also great. Few EVs is disease-specific and their exact role in the pathogenesis of mental disorders is largely unclarified. Moreover, studies with large clinical cohorts were also warranted to verify previous findings. Taken together, identifying the functions of EVs with high histological or pathophysiological specificity will help shed light on the pathophysiological mechanisms of mental disorders and drive the development of psychiatry.
Abbreviations
EVs: extracellular vesicles; BBB: blood-brain barrier; BMECs: brain microvascular endothelial cells; miRNA /miR: microRNA; lncRNA: long non-coding RNA; mtDNA: mitochondrial DNA; CNS: central nervous system; CSF: cerebrospinal fluid; BD: bipolar disorder; MDD: major depressive disorder; ASD: autism spectrum disorder; SA: substance abuse; SD: sleep disorder; PTSD: post-traumatic stress disorder; TBI: traumatic brain injury; AD: Alzheimer's disease; L1CAM: L1 cell adhesion molecule; ALIX: apoptosis-linked gene 2-interacting protein X; TSG101: tumor susceptibility gene 101; ARF6: adenosine diphosphate ribosylation factor 6; MHC: major histocompatibility complex; Aβ: amyloid-beta; BDNF: brain-derived neurotrophic factor; MSC: mesenchymal stem cell; TEM: transmission electron microscope; NTA: nanoparticle tracking analysis; GABA: gamma-aminobutyric acid; GBA: gut-brain axis; TG: test group; CG: control group; PCA: principal component analysis; ELISA: enzyme-linked immunosorbent assay; qRT-PCR: quantitative real-time polymerase chain reaction; ACC: anterior cingulate cortex; Ref: Reference.
Acknowledgements
Funding
The writing of this manuscript was supported by funding from the Zhejiang Provincial Key Research and Development Program (No. 2021C03107) and the Leading Talent of Scientific and Technological Innovation - "Ten Thousand Talents Program" of Zhejiang Province (No. 2021R52016) and the Innovation team for precision diagnosis and treatment of major brain diseases (No. 2020R01001).
Role of the funding source
The funders had no role in the study design; collection, analysis, and interpretation of data; writing of the report; and decision to submit the article for publication.
Author contributions
LZK, DHZ, and SH carried out literature searches, prepared figures, did data interpretation and writing; JZ, LL, JBL, and SHH conceptualized and edited the paper. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Yates AG, Pink RC, Erdbrugger U, Siljander PR, Dellar ER, Pantazi P. et al. In sickness and in health: The functional role of extracellular vesicles in physiology and pathology in vivo: Part I: Health and Normal Physiology: Part I: Health and Normal Physiology. J Extracell Vesicles. 2022;11:e12151
2. Ciardiello C, Migliorino R, Leone A, Budillon A. Large extracellular vesicles: Size matters in tumor progression. Cytokine Growth Factor Rev. 2020;51:69-74
3. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020 367
4. Mathieu M, Martin-Jaular L, Lavieu G, Thery C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9-17
5. Bebelman MP, Smit MJ, Pegtel DM, Baglio SR. Biogenesis and function of extracellular vesicles in cancer. Pharmacol Ther. 2018;188:1-11
6. Huo L, Du X, Li X, Liu S, Xu Y. The Emerging Role of Neural Cell-Derived Exosomes in Intercellular Communication in Health and Neurodegenerative Diseases. Front Neurosci. 2021;15:738442
7. Guedes VA, Devoto C, Leete J, Sass D, Acott JD, Mithani S. et al. Extracellular Vesicle Proteins and MicroRNAs as Biomarkers for Traumatic Brain Injury. Front Neurol. 2020;11:663
8. Di Liegro CM, Schiera G, Di Liegro I. Extracellular Vesicle-Associated RNA as a Carrier of Epigenetic Information. Genes (Basel). 2017 8
9. Shan S, Yang Y, Jiang J, Yang B, Yang Y, Sun F. et al. Extracellular vesicle-derived long non-coding RNA as circulating biomarkers for endometriosis. Reprod Biomed Online. 2022;44:923-33
10. Yang L, Han B, Zhang Z, Wang S, Bai Y, Zhang Y. et al. Extracellular Vesicle-Mediated Delivery of Circular RNA SCMH1 Promotes Functional Recovery in Rodent and Nonhuman Primate Ischemic Stroke Models. Circulation. 2020;142:556-74
11. Tao Y, Wei X, Yue Y, Wang J, Li J, Shen L. et al. Extracellular vesicle-derived AEBP1 mRNA as a novel candidate biomarker for diabetic kidney disease. J Transl Med. 2021;19:326
12. Greening DW, Xu R, Gopal SK, Rai A, Simpson RJ. Proteomic insights into extracellular vesicle biology - defining exosomes and shed microvesicles. Expert Rev Proteomics. 2017;14:69-95
13. Kumar A, Kim S, Su Y, Sharma M, Kumar P, Singh S. et al. Brain cell-derived exosomes in plasma serve as neurodegeneration biomarkers in male cynomolgus monkeys self-administrating oxycodone. EBioMedicine. 2021;63:103192
14. Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341-5
15. Polanco JC, Li C, Durisic N, Sullivan R, Gotz J. Exosomes taken up by neurons hijack the endosomal pathway to spread to interconnected neurons. Acta Neuropathol Commun. 2018;6:10
16. Saeedi S, Israel S, Nagy C, Turecki G. The emerging role of exosomes in mental disorders. Transl Psychiatry. 2019;9:122
17. Dickens AM, Tovar YRLB, Yoo SW, Trout AL, Bae M, Kanmogne M. et al. Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci Signal. 2017 10
18. Fruhbeis C, Frohlich D, Kuo WP, Amphornrat J, Thilemann S, Saab AS. et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 2013;11:e1001604
19. Antonucci F, Turola E, Riganti L, Caleo M, Gabrielli M, Perrotta C. et al. Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J. 2012;31:1231-40
20. Bahrini I, Song JH, Diez D, Hanayama R. Neuronal exosomes facilitate synaptic pruning by up-regulating complement factors in microglia. Sci Rep. 2015;5:7989
21. Lopez-Verrilli MA, Picou F, Court FA. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia. 2013;61:1795-806
22. Ridder K, Keller S, Dams M, Rupp AK, Schlaudraff J, Del Turco D. et al. Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS Biol. 2014;12:e1001874
23. Balusu S, Van Wonterghem E, De Rycke R, Raemdonck K, Stremersch S, Gevaert K. et al. Identification of a novel mechanism of blood-brain communication during peripheral inflammation via choroid plexus-derived extracellular vesicles. EMBO Mol Med. 2016;8:1162-83
24. Saint-Pol J, Gosselet F, Duban-Deweer S, Pottiez G, Karamanos Y. Targeting and Crossing the Blood-Brain Barrier with Extracellular Vesicles. Cells. 2020 9
25. Chung CC, Chan L, Chen JH, Hung YC, Hong CT. Plasma Extracellular Vesicle alpha-Synuclein Level in Patients with Parkinson's Disease. Biomolecules. 2021 11
26. Su H, Rustam YH, Masters CL, Makalic E, McLean CA, Hill AF. et al. Characterization of brain-derived extracellular vesicle lipids in Alzheimer's disease. J Extracell Vesicles. 2021;10:e12089
27. Harshyne LA, Nasca BJ, Kenyon LC, Andrews DW, Hooper DC. Serum exosomes and cytokines promote a T-helper cell type 2 environment in the peripheral blood of glioblastoma patients. Neuro Oncol. 2016;18:206-15
28. Galazka G, Mycko MP, Selmaj I, Raine CS, Selmaj KW. Multiple sclerosis: Serum-derived exosomes express myelin proteins. Mult Scler. 2018;24:449-58
29. The Lancet P. Blood biomarkers in psychiatry. Lancet Psychiatry. 2016;3:693
30. Chen Q, Liu X, Xu R, Wang X, Zhou D, Tang Y. Circulating exosomal microRNAs in nonsuicidal self-injury. Asian J Psychiatr. 2022;80:103310
31. Chen B, Song L, Yang J, Zhou WY, Cheng YY, Lai YJ. Proteomics of serum exosomes identified fibulin-1 as a novel biomarker for mild cognitive impairment. Neural Regen Res. 2023;18:587-93
32. Nasca C, Dobbin J, Bigio B, Watson K, de Angelis P, Kautz M. et al. Insulin receptor substrate in brain-enriched exosomes in subjects with major depression: on the path of creation of biosignatures of central insulin resistance. Mol Psychiatry. 2021;26:5140-9
33. Hou G, Lai W, Jiang W, Liu X, Qian L, Zhang Y. et al. Myelin deficits in patients with recurrent major depressive disorder: An inhomogeneous magnetization transfer study. Neurosci Lett. 2021;750:135768
34. Gayen M, Bhomia M, Balakathiresan N, Knollmann-Ritschel B. Exosomal MicroRNAs Released by Activated Astrocytes as Potential Neuroinflammatory Biomarkers. Int J Mol Sci. 2020 21
35. Cao T, Zhen XC. Dysregulation of miRNA and its potential therapeutic application in schizophrenia. CNS Neurosci Ther. 2018;24:586-97
36. Kavalali ET, Monteggia LM. Targeting Homeostatic Synaptic Plasticity for Treatment of Mood Disorders. Neuron. 2020;106:715-26
37. Alam R, Abdolmaleky HM, Zhou JR. Microbiome, inflammation, epigenetic alterations, and mental diseases. Am J Med Genet B Neuropsychiatr Genet. 2017;174:651-60
38. Dean J, Keshavan M. The neurobiology of depression: An integrated view. Asian J Psychiatr. 2017;27:101-11
39. Penninx B, Lange SMM. Metabolic syndrome in psychiatric patients: overview, mechanisms, and implications. Dialogues Clin Neurosci. 2018;20:63-73
40. Quintero M, Stanisic D, Cruz G, Pontes JGM, Costa T, Tasic L. Metabolomic Biomarkers in Mental Disorders: Bipolar Disorder and Schizophrenia. Adv Exp Med Biol. 2019;1118:271-93
41. Kato T. Current understanding of bipolar disorder: Toward integration of biological basis and treatment strategies. Psychiatry Clin Neurosci. 2019;73:526-40
42. Ruan J, Miao X, Schluter D, Lin L, Wang X. Extracellular vesicles in neuroinflammation: Pathogenesis, diagnosis, and therapy. Mol Ther. 2021;29:1946-57
43. Paolicelli RC, Bergamini G, Rajendran L. Cell-to-cell Communication by Extracellular Vesicles: Focus on Microglia. Neuroscience. 2019;405:148-57
44. Leidal AM, Debnath J. Unraveling the mechanisms that specify molecules for secretion in extracellular vesicles. Methods. 2020;177:15-26
45. Mathews PM, Levy E. Exosome Production Is Key to Neuronal Endosomal Pathway Integrity in Neurodegenerative Diseases. Front Neurosci. 2019;13:1347
46. Federici C, Shahaj E, Cecchetti S, Camerini S, Casella M, Iessi E. et al. Natural-Killer-Derived Extracellular Vesicles: Immune Sensors and Interactors. Front Immunol. 2020;11:262
47. Gualerzi A, Lombardi M, Verderio C. Microglia-oligodendrocyte intercellular communication: role of extracellular vesicle lipids in functional signalling. Neural Regen Res. 2021;16:1194-5
48. Zhao S, Sheng S, Wang Y, Ding L, Xu X, Xia X. et al. Astrocyte-derived extracellular vesicles: A double-edged sword in central nervous system disorders. Neurosci Biobehav Rev. 2021;125:148-59
49. Neckles VN, Morton MC, Holmberg JC, Sokolov AM, Nottoli T, Liu D. et al. A transgenic inducible GFP extracellular-vesicle reporter (TIGER) mouse illuminates neonatal cortical astrocytes as a source of immunomodulatory extracellular vesicles. Sci Rep. 2019;9:3094
50. Pei X, Li Y, Zhu L, Zhou Z. Astrocyte-derived exosomes suppress autophagy and ameliorate neuronal damage in experimental ischemic stroke. Exp Cell Res. 2019;382:111474
51. Guo YS, Liang PZ, Lu SZ, Chen R, Yin YQ, Zhou JW. Extracellular alphaB-crystallin modulates the inflammatory responses. Biochem Biophys Res Commun. 2019;508:282-8
52. Chen W, Zheng P, Hong T, Wang Y, Liu N, He B. et al. Astrocytes-derived exosomes induce neuronal recovery after traumatic brain injury via delivering gap junction alpha 1-20 k. J Tissue Eng Regen Med. 2020;14:412-23
53. Kramer-Albers EM. Extracellular vesicles in the oligodendrocyte microenvironment. Neurosci Lett. 2020;725:134915
54. Fruhbeis C, Kuo-Elsner WP, Muller C, Barth K, Peris L, Tenzer S. et al. Oligodendrocytes support axonal transport and maintenance via exosome secretion. PLoS Biol. 2020;18:e3000621
55. Casella G, Rasouli J, Boehm A, Zhang W, Xiao D, Ishikawa LLW. et al. Oligodendrocyte-derived extracellular vesicles as antigen-specific therapy for autoimmune neuroinflammation in mice. Sci Transl Med. 2020 12
56. Riazifar M, Mohammadi MR, Pone EJ, Yeri A, Lasser C, Segaliny AI. et al. Stem Cell-Derived Exosomes as Nanotherapeutics for Autoimmune and Neurodegenerative Disorders. ACS Nano. 2019;13:6670-88
57. Pollak TA, Drndarski S, Stone JM, David AS, McGuire P, Abbott NJ. The blood-brain barrier in psychosis. Lancet Psychiatry. 2018;5:79-92
58. Chen CY, Chao YM, Lin HF, Chen CJ, Chen CS, Yang JL. et al. miR-195 reduces age-related blood-brain barrier leakage caused by thrombospondin-1-mediated selective autophagy. Aging Cell. 2020;19:e13236
59. Kamintsky L, Cairns KA, Veksler R, Bowen C, Beyea SD, Friedman A. et al. Blood-brain barrier imaging as a potential biomarker for bipolar disorder progression. Neuroimage Clin. 2020;26:102049
60. Welcome MO. Cellular mechanisms and molecular signaling pathways in stress-induced anxiety, depression, and blood-brain barrier inflammation and leakage. Inflammopharmacology. 2020;28:643-65
61. Chiasserini D, van Weering JR, Piersma SR, Pham TV, Malekzadeh A, Teunissen CE. et al. Proteomic analysis of cerebrospinal fluid extracellular vesicles: a comprehensive dataset. J Proteomics. 2014;106:191-204
62. Lee EE, Winston-Gray C, Barlow JW, Rissman RA, Jeste DV. Plasma Levels of Neuron- and Astrocyte-Derived Exosomal Amyloid Beta1-42, Amyloid Beta1-40, and Phosphorylated Tau Levels in Schizophrenia Patients and Non-psychiatric Comparison Subjects: Relationships With Cognitive Functioning and Psychopathology. Front Psychiatry. 2020;11:532624
63. Inoue M, Baba H, Yamamoto K, Shimada H, Yamakawa Y, Suzuki T. et al. Serum Levels of Albumin-beta-Amyloid Complex in Patients with Depression. Am J Geriatr Psychiatry. 2016;24:764-72
64. Sokol DK, Maloney B, Westmark CJ, Lahiri DK. Novel Contribution of Secreted Amyloid-beta Precursor Protein to White Matter Brain Enlargement in Autism Spectrum Disorder. Front Psychiatry. 2019;10:165
65. Calderon-Garciduenas L, Rajkumar RP, Stommel EW, Kulesza R, Mansour Y, Rico-Villanueva A. et al. Brainstem Quadruple Aberrant Hyperphosphorylated Tau, Beta-Amyloid, Alpha-Synuclein and TDP-43 Pathology, Stress and Sleep Behavior Disorders. Int J Environ Res Public Health. 2021 18
66. Xu B, Zhang Y, Du XF, Li J, Zi HX, Bu JW. et al. Neurons secrete miR-132-containing exosomes to regulate brain vascular integrity. Cell Res. 2017;27:882-97
67. Yuan X, Wu Q, Wang P, Jing Y, Yao H, Tang Y. et al. Exosomes Derived From Pericytes Improve Microcirculation and Protect Blood-Spinal Cord Barrier After Spinal Cord Injury in Mice. Front Neurosci. 2019;13:319
68. Gruzdev SK, Yakovlev AA, Druzhkova TA, Guekht AB, Gulyaeva NV. The Missing Link: How Exosomes and miRNAs can Help in Bridging Psychiatry and Molecular Biology in the Context of Depression, Bipolar Disorder and Schizophrenia. Cell Mol Neurobiol. 2019;39:729-50
69. Keller WR, Kum LM, Wehring HJ, Koola MM, Buchanan RW, Kelly DL. A review of anti-inflammatory agents for symptoms of schizophrenia. J Psychopharmacol. 2013;27:337-42
70. Hilker R, Helenius D, Fagerlund B, Skytthe A, Christensen K, Werge TM. et al. Heritability of Schizophrenia and Schizophrenia Spectrum Based on the Nationwide Danish Twin Register. Biol Psychiatry. 2018;83:492-8
71. Debnath M, Venkatasubramanian G, Berk M. Fetal programming of schizophrenia: select mechanisms. Neurosci Biobehav Rev. 2015;49:90-104
72. Cariaga-Martinez A, Saiz-Ruiz J, Alelu-Paz R. From Linkage Studies to Epigenetics: What We Know and What We Need to Know in the Neurobiology of Schizophrenia. Front Neurosci. 2016;10:202
73. Mingardi J, Musazzi L, De Petro G, Barbon A. miRNA Editing: New Insights into the Fast Control of Gene Expression in Health and Disease. Mol Neurobiol. 2018;55:7717-27
74. Du Y, Chen L, Li XS, Li XL, Xu XD, Tai SB. et al. Metabolomic Identification of Exosome-Derived Biomarkers for Schizophrenia: A Large Multicenter Study. Schizophr Bull. 2021;47:615-23
75. Ranganathan M, Rahman M, Ganesh S, D'Souza DC, Skosnik PD, Radhakrishnan R. et al. Analysis of circulating exosomes reveals a peripheral signature of astrocytic pathology in schizophrenia. World J Biol Psychiatry. 2021:1-13
76. Goetzl EJ, Srihari VH, Guloksuz S, Ferrara M, Tek C, Heninger GR. Decreased mitochondrial electron transport proteins and increased complement mediators in plasma neural-derived exosomes of early psychosis. Transl Psychiatry. 2020;10:361
77. Mobarrez F, Nybom R, Johansson V, Hultman CM, Wallen H, Landen M. et al. Microparticles and microscopic structures in three fractions of fresh cerebrospinal fluid in schizophrenia: case report of twins. Schizophr Res. 2013;143:192-7
78. Green MJ, Matheson SL, Shepherd A, Weickert CS, Carr VJ. Brain-derived neurotrophic factor levels in schizophrenia: a systematic review with meta-analysis. Mol Psychiatry. 2011;16:960-72
79. Du Y, Yu Y, Hu Y, Li XW, Wei ZX, Pan RY. et al. Genome-Wide, Integrative Analysis Implicates Exosome-Derived MicroRNA Dysregulation in Schizophrenia. Schizophr Bull. 2019;45:1257-66
80. Walgrave H, Balusu S, Snoeck S, Vanden Eynden E, Craessaerts K, Thrupp N. et al. Restoring miR-132 expression rescues adult hippocampal neurogenesis and memory deficits in Alzheimer's disease. Cell Stem Cell. 2021;28:1805-21 e8
81. Qian Y, Song J, Ouyang Y, Han Q, Chen W, Zhao X. et al. Advances in Roles of miR-132 in the Nervous System. Front Pharmacol. 2017;8:770
82. Khadimallah I, Jenni R, Cabungcal JH, Cleusix M, Fournier M, Beard E. et al. Mitochondrial, exosomal miR137-COX6A2 and gamma synchrony as biomarkers of parvalbumin interneurons, psychopathology, and neurocognition in schizophrenia. Mol Psychiatry. 2021
83. Amoah SK, Rodriguez BA, Logothetis CN, Chander P, Sellgren CM, Weick JP. et al. Exosomal secretion of a psychosis-altered miRNA that regulates glutamate receptor expression is affected by antipsychotics. Neuropsychopharmacology. 2020;45:656-65
84. Tsivion-Visbord H, Perets N, Sofer T, Bikovski L, Goldshmit Y, Ruban A. et al. Mesenchymal stem cells derived extracellular vesicles improve behavioral and biochemical deficits in a phencyclidine model of schizophrenia. Transl Psychiatry. 2020;10:305
85. Woelfer M, Kasties V, Kahlfuss S, Walter M. The Role of Depressive Subtypes within the Neuroinflammation Hypothesis of Major Depressive Disorder. Neuroscience. 2019;403:93-110
86. Fries GR, Lima CNC, Valvassori SS, Zunta-Soares G, Soares JC, Quevedo J. Preliminary investigation of peripheral extracellular vesicles' microRNAs in bipolar disorder. J Affect Disord. 2019;255:10-4
87. Ceylan D, Tufekci KU, Keskinoglu P, Genc S, Ozerdem A. Circulating exosomal microRNAs in bipolar disorder. J Affect Disord. 2020;262:99-107
88. Du Y, Dong JH, Chen L, Liu H, Zheng GE, Chen GY. et al. Metabolomic Identification of Serum Exosome-Derived Biomarkers for Bipolar Disorder. Oxid Med Cell Longev. 2022;2022:5717445
89. Luarte A, Cisternas P, Caviedes A, Batiz LF, Lafourcade C, Wyneken U. et al. Astrocytes at the Hub of the Stress Response: Potential Modulation of Neurogenesis by miRNAs in Astrocyte-Derived Exosomes. Stem Cells Int. 2017;2017:1719050
90. Rong H, Liu TB, Yang KJ, Yang HC, Wu DH, Liao CP. et al. MicroRNA-134 plasma levels before and after treatment for bipolar mania. J Psychiatr Res. 2011;45:92-5
91. Kidnapillai S, Wade B, Bortolasci CC, Panizzutti B, Spolding B, Connor T. et al. Drugs used to treat bipolar disorder act via microRNAs to regulate expression of genes involved in neurite outgrowth. J Psychopharmacol. 2020;34:370-9
92. Lee SY, Wang TY, Lu RB, Wang LJ, Chang CH, Chiang YC. et al. Peripheral BDNF correlated with miRNA in BD-II patients. J Psychiatr Res. 2021;136:184-9
93. Lee SY, Lu RB, Wang LJ, Chang CH, Lu T, Wang TY. et al. Serum miRNA as a possible biomarker in the diagnosis of bipolar II disorder. Sci Rep. 2020;10:1131
94. Banigan MG, Kao PF, Kozubek JA, Winslow AR, Medina J, Costa J. et al. Differential expression of exosomal microRNAs in prefrontal cortices of schizophrenia and bipolar disorder patients. Plos One. 2013;8:e48814
95. Zhang H, Cai X, Xiang C, Han Y, Niu Q. miR-29a and the PTEN-GSK3beta axis are involved in aluminum-induced damage to primary hippocampal neuronal networks. Ecotoxicol Environ Saf. 2021;224:112701
96. Choi JL, Kao PF, Itriago E, Zhan Y, Kozubek JA, Hoss AG. et al. miR-149 and miR-29c as candidates for bipolar disorder biomarkers. Am J Med Genet B Neuropsychiatr Genet. 2017;174:315-23
97. Delalle I. MicroRNAs as Candidates for Bipolar Disorder Biomarkers. Psychiatr Danub. 2021;33:451-5
98. Drevets WC, Price JL, Simpson JR Jr, Todd RD, Reich T, Vannier M. et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824-7
99. Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95:13290-5
100. Zhang P, Kong L, Huang H, Pan Y, Zhang D, Jiang J. et al. Gut Microbiota - A Potential Contributor in the Pathogenesis of Bipolar Disorder. Front Neurosci. 2022;16:830748
101. Flowers SA, Ward KM, Clark CT. The Gut Microbiome in Bipolar Disorder and Pharmacotherapy Management. Neuropsychobiology. 2020;79:43-9
102. Flowers SA, Baxter NT, Ward KM, Kraal AZ, McInnis MG, Schmidt TM. et al. Effects of Atypical Antipsychotic Treatment and Resistant Starch Supplementation on Gut Microbiome Composition in a Cohort of Patients with Bipolar Disorder or Schizophrenia. Pharmacotherapy. 2019;39:161-70
103. Bengesser SA, Morkl S, Painold A, Dalkner N, Birner A, Fellendorf FT. et al. Epigenetics of the molecular clock and bacterial diversity in bipolar disorder. Psychoneuroendocrinology. 2019;101:160-6
104. Zheng P, Yang J, Li Y, Wu J, Liang W, Yin B. et al. Gut Microbial Signatures Can Discriminate Unipolar from Bipolar Depression. Adv Sci (Weinh). 2020;7:1902862
105. Kamada N, Seo SU, Chen GY, Nunez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13:321-35
106. Peck BC, Mah AT, Pitman WA, Ding S, Lund PK, Sethupathy P. Functional Transcriptomics in Diverse Intestinal Epithelial Cell Types Reveals Robust MicroRNA Sensitivity in Intestinal Stem Cells to Microbial Status. J Biol Chem. 2017;292:2586-600
107. Dalmasso G, Nguyen HTT, Fais T, Massier S, Barnich N, Delmas J. et al. Crohn's Disease-Associated Adherent-Invasive Escherichia coli Manipulate Host Autophagy by Impairing SUMOylation. Cells. 2019 8
108. Hoban AE, Stilling RM, G MM, Moloney RD, Shanahan F, Dinan TG. et al. Microbial regulation of microRNA expression in the amygdala and prefrontal cortex. Microbiome. 2017;5:102
109. Zhao L, Ye Y, Gu L, Jian Z, Stary CM, Xiong X. Extracellular vesicle-derived miRNA as a novel regulatory system for bi-directional communication in gut-brain-microbiota axis. J Transl Med. 2021;19:202
110. Lee Y, Mansur RB, Brietzke E, Kapogiannis D, Delgado-Peraza F, Boutilier JJ. et al. Peripheral inflammatory biomarkers define biotypes of bipolar depression. Mol Psychiatry. 2021;26:3395-406
111. Cuperfain AB, Kennedy JL, Goncalves VF. Overlapping mechanisms linking insulin resistance with cognition and neuroprogression in bipolar disorder. Neurosci Biobehav Rev. 2020;111:125-34
112. Mansur RB, Delgado-Peraza F, Subramaniapillai M, Lee Y, Iacobucci M, Nasri F. et al. Exploring brain insulin resistance in adults with bipolar depression using extracellular vesicles of neuronal origin. J Psychiatr Res. 2021;133:82-92
113. Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA Psychiatry. 2015;72:603-11
114. Zhang HF, Mellor D, Peng DH. Neuroimaging genomic studies in major depressive disorder: A systematic review. CNS Neurosci Ther. 2018;24:1020-36
115. Singhal G, Baune BT. Microglia: An Interface between the Loss of Neuroplasticity and Depression. Front Cell Neurosci. 2017;11:270
116. Kim YK, Na KS. Role of glutamate receptors and glial cells in the pathophysiology of treatment-resistant depression. Prog Neuropsychopharmacol Biol Psychiatry. 2016;70:117-26
117. Kim YK, Na KS, Myint AM, Leonard BE. The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:277-84
118. Brites D, Fernandes A. Neuroinflammation and Depression: Microglia Activation, Extracellular Microvesicles and microRNA Dysregulation. Front Cell Neurosci. 2015;9:476
119. Ilgin C, Topuzoglu A. Extracellular Vesicles in Psychiatry Research in the Context of RDoC Criteria. Psychiatry Investig. 2018;15:1011-8
120. Xian X, Cai LL, Li Y, Wang RC, Xu YH, Chen YJ. et al. Neuron secrete exosomes containing miR-9-5p to promote polarization of M1 microglia in depression. J Nanobiotechnology. 2022;20:122
121. Fan C, Li Y, Lan T, Wang W, Long Y, Yu SY. Microglia secrete miR-146a-5p-containing exosomes to regulate neurogenesis in depression. Mol Ther. 2022;30:1300-14
122. Li D, Wang Y, Jin X, Hu D, Xia C, Xu H. et al. NK cell-derived exosomes carry miR-207 and alleviate depression-like symptoms in mice. J Neuroinflammation. 2020;17:126
123. Guo H, Huang B, Wang Y, Zhang Y, Ma Q, Ren Y. Bone marrow mesenchymal stem cells-derived exosomes improve injury of hippocampal neurons in rats with depression by upregulating microRNA-26a expression. Int Immunopharmacol. 2020;82:106285
124. Jiang M, Gu YF, Cai JF, Wang A, He Y, Feng YL. MiR-186-5p Dysregulation Leads to Depression-like Behavior by De-repressing SERPINF1 in Hippocampus. Neuroscience. 2021;479:48-59
125. Gelle T, Samey RA, Plansont B, Bessette B, Jauberteau-Marchan MO, Lalloue F. et al. BDNF and pro-BDNF in serum and exosomes in major depression: Evolution after antidepressant treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2021;109:110229
126. Fang K, Xu JX, Chen XX, Gao XR, Huang LL, Du AQ. et al. Differential serum exosome microRNA profile in a stress-induced depression rat model. J Affect Disord. 2020;274:144-58
127. Bocchio-Chiavetto L, Maffioletti E, Bettinsoli P, Giovannini C, Bignotti S, Tardito D. et al. Blood microRNA changes in depressed patients during antidepressant treatment. Eur Neuropsychopharmacol. 2013;23:602-11
128. Goetzl EJ, Wolkowitz OM, Srihari VH, Reus VI, Goetzl L, Kapogiannis D. et al. Abnormal levels of mitochondrial proteins in plasma neuronal extracellular vesicles in major depressive disorder. Mol Psychiatry. 2021;26:7355-62
129. Sakamoto S, Mallah D, Medeiros DJ, Dohi E, Imai T, Rose IVL. et al. Alterations in circulating extracellular vesicles underlie social stress-induced behaviors in mice. FEBS Open Bio. 2021;11:2678-92
130. Wang Y, Gao C, Gao T, Zhao L, Zhu S, Guo L. Plasma exosomes from depression ameliorate inflammation-induced depressive-like behaviors via sigma-1 receptor delivery. Brain Behav Immun. 2021;94:225-34
131. Saeedi S, Nagy C, Ibrahim P, Theroux JF, Wakid M, Fiori LM. et al. Neuron-derived extracellular vesicles enriched from plasma show altered size and miRNA cargo as a function of antidepressant drug response. Mol Psychiatry. 2021;26:7417-24
132. Yu X, Bai Y, Han B, Ju M, Tang T, Shen L. et al. Extracellular vesicle-mediated delivery of circDYM alleviates CUS-induced depressive-like behaviours. J Extracell Vesicles. 2022;11:e12185
133. Pezzimenti F, Han GT, Vasa RA, Gotham K. Depression in Youth with Autism Spectrum Disorder. Child Adolesc Psychiatr Clin N Am. 2019;28:397-409
134. Chisholm K, Lin A, Abu-Akel A, Wood SJ. The association between autism and schizophrenia spectrum disorders: A review of eight alternate models of co-occurrence. Neurosci Biobehav Rev. 2015;55:173-83
135. Gandal MJ, Zhang P, Hadjimichael E, Walker RL, Chen C, Liu S. et al. Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science. 2018 362
136. Salloum IM, Brown ES. Management of comorbid bipolar disorder and substance use disorders. Am J Drug Alcohol Abuse. 2017;43:366-76
137. Hunt GE, Large MM, Cleary M, Lai HMX, Saunders JB. Prevalence of comorbid substance use in schizophrenia spectrum disorders in community and clinical settings, 1990-2017: Systematic review and meta-analysis. Drug Alcohol Depend. 2018;191:234-58
138. Ferrarelli F. Sleep Abnormalities in Schizophrenia: State of the Art and Next Steps. Am J Psychiatry. 2021;178:903-13
139. Duncan LE, Ratanatharathorn A, Aiello AE, Almli LM, Amstadter AB, Ashley-Koch AE. et al. Largest GWAS of PTSD (N=20 070) yields genetic overlap with schizophrenia and sex differences in heritability. Mol Psychiatry. 2018;23:666-73
140. Cerimele JM, Bauer AM, Fortney JC, Bauer MS. Patients With Co-Occurring Bipolar Disorder and Posttraumatic Stress Disorder: A Rapid Review of the Literature. J Clin Psychiatry. 2017;78:e506-e14
141. Dean DD, Agarwal S, Muthuswamy S, Asim A. Brain exosomes as minuscule information hub for Autism Spectrum Disorder. Expert Rev Mol Diagn. 2021;21:1323-31
142. Fiorentino M, Sapone A, Senger S, Camhi SS, Kadzielski SM, Buie TM. et al. Blood-brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Mol Autism. 2016;7:49
143. Alessio N, Brigida AL, Peluso G, Antonucci N, Galderisi U, Siniscalco D. Stem Cell-Derived Exosomes in Autism Spectrum Disorder. Int J Environ Res Public Health. 2020 17
144. Nguyen LS, Lepleux M, Makhlouf M, Martin C, Fregeac J, Siquier-Pernet K. et al. Profiling olfactory stem cells from living patients identifies miRNAs relevant for autism pathophysiology. Mol Autism. 2016;7:1
145. Perets N, Hertz S, London M, Offen D. Intranasal administration of exosomes derived from mesenchymal stem cells ameliorates autistic-like behaviors of BTBR mice. Mol Autism. 2018;9:57
146. Tsilioni I, Theoharides TC. Extracellular vesicles are increased in the serum of children with autism spectrum disorder, contain mitochondrial DNA, and stimulate human microglia to secrete IL-1beta. J Neuroinflammation. 2018;15:239
147. Guang S, Pang N, Deng X, Yang L, He F, Wu L. et al. Synaptopathology Involved in Autism Spectrum Disorder. Front Cell Neurosci. 2018;12:470
148. Gillet V, Hunting DJ, Takser L. Turing Revisited: Decoding the microRNA Messages in Brain Extracellular Vesicles for Early Detection of Neurodevelopmental Disorders. Curr Environ Health Rep. 2016;3:188-201
149. Mazurskyy A, Howitt J. Extracellular Vesicles in Neurological Disorders. Subcell Biochem. 2021;97:411-36
150. Perets N, Oron O, Herman S, Elliott E, Offen D. Exosomes derived from mesenchymal stem cells improved core symptoms of genetically modified mouse model of autism Shank3B. Mol Autism. 2020;11:65
151. Chen W, Huang Y, Han J, Yu L, Li Y, Lu Z. et al. Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol Res. 2016;64:831-40
152. Carone C, Genedani S, Leo G, Filaferro M, Fuxe K, Agnati LF. In vitro effects of cocaine on tunneling nanotube formation and extracellular vesicle release in glioblastoma cell cultures. J Mol Neurosci. 2015;55:42-50
153. Chivero ET, Dagur RS, Peeples ES, Sil S, Liao K, Ma R. et al. Biogenesis, physiological functions and potential applications of extracellular vesicles in substance use disorders. Cell Mol Life Sci. 2021;78:4849-65
154. Ibanez F, Montesinos J, Urena-Peralta JR, Guerri C, Pascual M. TLR4 participates in the transmission of ethanol-induced neuroinflammation via astrocyte-derived extracellular vesicles. J Neuroinflammation. 2019;16:136
155. Guo ML, Periyasamy P, Liao K, Kook YH, Niu F, Callen SE. et al. Cocaine-mediated downregulation of microglial miR-124 expression involves promoter DNA methylation. Epigenetics. 2016;11:819-30
156. Kosgodage US, Uysal-Onganer P, MacLatchy A, Mould R, Nunn AV, Guy GW. et al. Cannabidiol Affects Extracellular Vesicle Release, miR21 and miR126, and Reduces Prohibitin Protein in Glioblastoma Multiforme Cells. Transl Oncol. 2019;12:513-22
157. Gu WJ, Zhang C, Zhong Y, Luo J, Zhang CY, Zhang C. et al. Altered serum microRNA expression profile in subjects with heroin and methamphetamine use disorder. Biomed Pharmacother. 2020;125:109918
158. Zhu J, Liu B, Wang Z, Wang D, Ni H, Zhang L. et al. Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation. Theranostics. 2019;9:6901-19
159. He Y, Yang C, Kirkmire CM, Wang ZJ. Regulation of opioid tolerance by let-7 family microRNA targeting the mu opioid receptor. J Neurosci. 2010;30:10251-8
160. Hu G, Yao H, Chaudhuri AD, Duan M, Yelamanchili SV, Wen H. et al. Exosome-mediated shuttling of microRNA-29 regulates HIV Tat and morphine-mediated neuronal dysfunction. Cell Death Dis. 2012;3:e381
161. Chen F, Zou L, Dai Y, Sun J, Chen C, Zhang Y. et al. Prognostic plasma exosomal microRNA biomarkers in patients with substance use disorders presenting comorbid with anxiety and depression. Sci Rep. 2021;11:6271
162. Zhang Z, Wu H, Peng Q, Xie Z, Chen F, Ma Y. et al. Integration of Molecular Inflammatory Interactome Analyses Reveals Dynamics of Circulating Cytokines and Extracellular Vesicle Long Non-Coding RNAs and mRNAs in Heroin Addicts During Acute and Protracted Withdrawal. Front Immunol. 2021;12:730300
163. Chivero ET, Liao K, Niu F, Tripathi A, Tian C, Buch S. et al. Engineered Extracellular Vesicles Loaded With miR-124 Attenuate Cocaine-Mediated Activation of Microglia. Front Cell Dev Biol. 2020;8:573
164. Hu G, Liao K, Niu F, Yang L, Dallon BW, Callen S. et al. Astrocyte EV-Induced lincRNA-Cox2 Regulates Microglial Phagocytosis: Implications for Morphine-Mediated Neurodegeneration. Mol Ther Nucleic Acids. 2018;13:450-63
165. Wadhwa M, Prabhakar A, Ray K, Roy K, Kumari P, Jha PK. et al. Inhibiting the microglia activation improves the spatial memory and adult neurogenesis in rat hippocampus during 48 h of sleep deprivation. J Neuroinflammation. 2017;14:222
166. Rosa E, Fahnestock M. CREB expression mediates amyloid beta-induced basal BDNF downregulation. Neurobiol Aging. 2015;36:2406-13
167. Holth JK, Fritschi SK, Wang C, Pedersen NP, Cirrito JR, Mahan TE. et al. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science. 2019;363:880-4
168. Mosakhani N, Sarhadi V, Panula P, Partinen M, Knuutila S. Narcolepsy patients' blood-based miRNA expression profiling: miRNA expression differences with Pandemrix vaccination. Acta Neurol Scand. 2017;136:462-9
169. Surbhi Borniger JC, Russart KLG Zhang N, Magalang UJ Nelson RJ. miR-155 deletion modulates lipopolysaccharide-induced sleep in female mice. Chronobiol Int. 2019;36:188-202
170. Herringa RJ. Trauma, PTSD, and the Developing Brain. Curr Psychiatry Rep. 2017;19:69
171. Zhou J, Nagarkatti P, Zhong Y, Ginsberg JP, Singh NP, Zhang J. et al. Dysregulation in microRNA expression is associated with alterations in immune functions in combat veterans with post-traumatic stress disorder. Plos One. 2014;9:e94075
172. Lee MY, Baxter D, Scherler K, Kim TK, Wu X, Abu-Amara D. et al. Distinct Profiles of Cell-Free MicroRNAs in Plasma of Veterans with Post-Traumatic Stress Disorder. J Clin Med. 2019 8
173. Blessing EM, Reus V, Mellon SH, Wolkowitz OM, Flory JD, Bierer L. et al. Biological predictors of insulin resistance associated with posttraumatic stress disorder in young military veterans. Psychoneuroendocrinology. 2017;82:91-7
174. Swartzman S, Booth JN, Munro A, Sani F. Posttraumatic stress disorder after cancer diagnosis in adults: A meta-analysis. Depress Anxiety. 2017;34:327-39
175. Dietz AG, Goldman SA, Nedergaard M. Glial cells in schizophrenia: a unified hypothesis. Lancet Psychiatry. 2020;7:272-81
176. Yin Q, Ji X, Lv R, Pei JJ, Du Y, Shen C. et al. Targetting Exosomes as a New Biomarker and Therapeutic Approach for Alzheimer's Disease. Clin Interv Aging. 2020;15:195-205
177. Winston CN, Goetzl EJ, Akers JC, Carter BS, Rockenstein EM, Galasko D. et al. Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimers Dement (Amst). 2016;3:63-72
178. Zhang T, Ma S, Lv J, Wang X, Afewerky HK, Li H. et al. The emerging role of exosomes in Alzheimer's disease. Ageing Res Rev. 2021;68:101321
179. Norman M, Ter-Ovanesyan D, Trieu W, Lazarovits R, Kowal EJK, Lee JH. et al. L1CAM is not associated with extracellular vesicles in human cerebrospinal fluid or plasma. Nat Methods. 2021;18:631-4
180. Fowler CD. NeuroEVs: Characterizing Extracellular Vesicles Generated in the Neural Domain. J Neurosci. 2019;39:9262-8
181. Liu R, Liu J, Ji X, Liu Y. Synthetic nucleic acids delivered by exosomes: a potential therapeutic for generelated metabolic brain diseases. Metab Brain Dis. 2013;28:551-62
182. Tang TT, Wang B, Wu M, Li ZL, Feng Y, Cao JY. et al. Extracellular vesicle-encapsulated IL-10 as novel nanotherapeutics against ischemic AKI. Sci Adv. 2020;6:eaaz0748
183. Witwer KW, Van Balkom BWM, Bruno S, Choo A, Dominici M, Gimona M. et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J Extracell Vesicles. 2019;8:1609206
184. Kordelas L, Rebmann V, Ludwig AK, Radtke S, Ruesing J, Doeppner TR. et al. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28:970-3
185. Cheng L, Sharples RA, Scicluna BJ, Hill AF. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J Extracell Vesicles. 2014 3
186. Morel L, Regan M, Higashimori H, Ng SK, Esau C, Vidensky S. et al. Neuronal exosomal miRNA-dependent translational regulation of astroglial glutamate transporter GLT1. J Biol Chem. 2013;288:7105-16
187. Hao P, Liang Z, Piao H, Ji X, Wang Y, Liu Y. et al. Conditioned medium of human adipose-derived mesenchymal stem cells mediates protection in neurons following glutamate excitotoxicity by regulating energy metabolism and GAP-43 expression. Metab Brain Dis. 2014;29:193-205
188. Jiang L, Dong H, Cao H, Ji X, Luan S, Liu J. Exosomes in Pathogenesis, Diagnosis, and Treatment of Alzheimer's Disease. Med Sci Monit. 2019;25:3329-35
189. Mi W, Pawlik M, Sastre M, Jung SS, Radvinsky DS, Klein AM. et al. Cystatin C inhibits amyloid-beta deposition in Alzheimer's disease mouse models. Nat Genet. 2007;39:1440-2
190. Deng Y, Gong P, Han S, Zhang J, Zhang S, Zhang B. et al. Reduced cerebral cortex thickness is related to overexpression of exosomal miR-146a-5p in medication-free patients with major depressive disorder. Psychol Med. 2022:1-8
191. Katakowski M, Buller B, Zheng X, Lu Y, Rogers T, Osobamiro O. et al. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013;335:201-4
192. Liu Y, Li D, Liu Z, Zhou Y, Chu D, Li X. et al. Targeted exosome-mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse. Sci Rep. 2015;5:17543
193. Wang H, Sui H, Zheng Y, Jiang Y, Shi Y, Liang J. et al. Curcumin-primed exosomes potently ameliorate cognitive function in AD mice by inhibiting hyperphosphorylation of the Tau protein through the AKT/GSK-3beta pathway. Nanoscale. 2019;11:7481-96
194. Jia G, Han Y, An Y, Ding Y, He C, Wang X. et al. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials. 2018;178:302-16
195. Yuan D, Zhao Y, Banks WA, Bullock KM, Haney M, Batrakova E. et al. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials. 2017;142:1-12
196. Williams AM, Dennahy IS, Bhatti UF, Halaweish I, Xiong Y, Chang P. et al. Mesenchymal Stem Cell-Derived Exosomes Provide Neuroprotection and Improve Long-Term Neurologic Outcomes in a Swine Model of Traumatic Brain Injury and Hemorrhagic Shock. J Neurotrauma. 2019;36:54-60
197. Wang P, Wu Y, Chen W, Zhang M, Qin J. Malignant Melanoma-Derived Exosomes Induce Endothelial Damage and Glial Activation on a Human BBB Chip Model. Biosensors (Basel). 2022 12
198. You Y, Muraoka S, Jedrychowski MP, Hu J, McQuade AK, Young-Pearse T. et al. Human neural cell type-specific extracellular vesicle proteome defines disease-related molecules associated with activated astrocytes in Alzheimer's disease brain. J Extracell Vesicles. 2022;11:e12183
199. de Jong JO, Llapashtica C, Genestine M, Strauss K, Provenzano F, Sun Y. et al. Cortical overgrowth in a preclinical forebrain organoid model of CNTNAP2-associated autism spectrum disorder. Nat Commun. 2021;12:4087
200. Moore D, Meays BM, Madduri LSV, Shahjin F, Chand S, Niu M. et al. Downregulation of an Evolutionary Young miR-1290 in an iPSC-Derived Neural Stem Cell Model of Autism Spectrum Disorder. Stem Cells Int. 2019;2019:8710180
201. Yannan Qin LC HZ, Shuang Cai, Jinyuan Zhang, Bo Guo, Fei Wu, Lingyu Zhao, Wen Li, Lei Ni, Liying Liu, Xiaofei Wang, Yanni Chen, Chen Huang. Whole-transcriptome analysis of serum neuron-derived exosomes reveals specific signature for autism spectrum disorder in Chinese children. Research Square. 2021
202. Fang Y, Wan C, Wen Y, Wu Z, Pan J, Zhong M. et al. Autism-associated synaptic vesicle transcripts are differentially expressed in maternal plasma exosomes of physiopathologic pregnancies. J Transl Med. 2021;19:154
203. Kang HJ, Yoon S, Lee S, Choi K, Seol S, Park S. et al. FKBP5-associated miRNA signature as a putative biomarker for PTSD in recently traumatized individuals. Sci Rep. 2020;10:3353
204. Gill J, Mustapic M, Diaz-Arrastia R, Lange R, Gulyani S, Diehl T. et al. Higher exosomal tau, amyloid-beta 42 and IL-10 are associated with mild TBIs and chronic symptoms in military personnel. Brain Inj. 2018;32:1277-84
205. Guedes VA, Lai C, Devoto C, Edwards KA, Mithani S, Sass D. et al. Extracellular Vesicle Proteins and MicroRNAs Are Linked to Chronic Post-Traumatic Stress Disorder Symptoms in Service Members and Veterans With Mild Traumatic Brain Injury. Front Pharmacol. 2021;12:745348
Author contact
![]() Corresponding authors: Shaohua Hu, Department of Psychiatry, the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China Email: dorhushaohuaedu.cn. Jing Zhang, Department of Pathology, First Affiliated Hospital, Zhejiang University School of Medicine, Zhejiang, China. Email: jzhang1989edu.cn. Lin Lu, Institute of Mental Health/National Clinical Research Center for Mental Disorders, Peking University Sixth Hospital, Peking University, Beijing 100191, China Email: linluedu.cn. Jianbo Lai, Department of Psychiatry, the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China Email: laijianboedu.cn.
Corresponding authors: Shaohua Hu, Department of Psychiatry, the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China Email: dorhushaohuaedu.cn. Jing Zhang, Department of Pathology, First Affiliated Hospital, Zhejiang University School of Medicine, Zhejiang, China. Email: jzhang1989edu.cn. Lin Lu, Institute of Mental Health/National Clinical Research Center for Mental Disorders, Peking University Sixth Hospital, Peking University, Beijing 100191, China Email: linluedu.cn. Jianbo Lai, Department of Psychiatry, the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China Email: laijianboedu.cn.

 Global reach, higher impact
Global reach, higher impact