ISSN: 1449-2288
Int J Biol Sci 2023; 19(4):1284-1298. doi:10.7150/ijbs.74985 This issue Cite
Research Paper
Apolipoprotein L3 enhances CD8+ T cell antitumor immunity of colorectal cancer by promoting LDHA-mediated ferroptosis
1. Department of General Surgery, Zhongshan Hospital, Fudan University, Shanghai, China
2. Cancer Center, Zhongshan Hospital, Fudan University, Shanghai, China
3. Shanghai Engineering Research Center of Colorectal Cancer Minimally Invasive Surgery, Shanghai, China
# Yang Lv, Wentao Tang, YuQiu Xu, WenJu Chang and ZhiYuan Zhang contributed equally to this paper.
Abstract
Aim: Colorectal cancer (CRC) is the leading cause of cancer associated death worldwide and immune checkpoint blockade therapy only benefit a small set of CRC patients. Tumor ferroptosis of CRC reflected immune-activation in our previous findings. Understanding the mechanisms underlying how to bolster CD8+ T cells function through ferroptosis in CRC tumor microenvironment (TME) will greatly benefit cancer immunotherapy.
Methods: Genes between ferroptosis and CD8+ T cell function in CRC were screened through Cox, WGCNA and differential expression analysis. Immunohistochemistry and Immunofluorescence analysis were performed. Co-immunoprecipitation were performed to determine protein-protein interaction, mRNA level was determined by qRT-PCR. RSL3 was used to induce ferroptosis, and ferroptosis levels were evaluated by measuring Transmission Electron Microscope analysis, MDA, Fe2+level and cell viability.
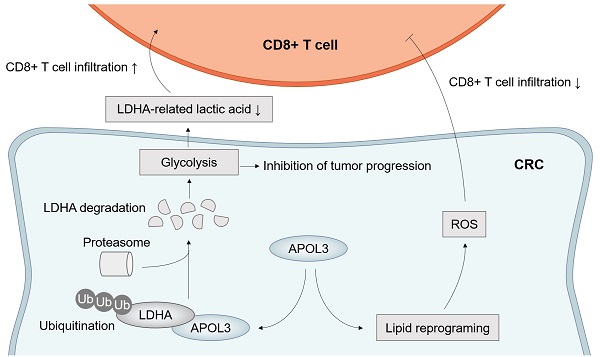
Results: We screened APOL3 as the significant modulator for ferroptosis-related CD8+ infiltration in CRC. Next, by in vitro and in vivo, we found that increased APOL3 expression was positively correlated with sensitivity to ferroptosis and antitumor ability of CD8+ T cells. Next, we demonstrated that APOL3 can binds LDHA and promote its ubiquitylation-related degradation. Then, based on in vivo analysis and tumor specimen, we discovered the APOL3-LDHA axis can facilitate the tumor ferroptosis and cytotoxic ability of CD8+ T cells through increased IFNγ and decreased lactic acid concentration.
Conclusion: The present study demonstrated that APOL3 promotes ferroptosis and immunotherapy in colorectal cancer cells. The present work provides us with a novel target to overcome drug resistance to ferroptosis and immunotherapy.
Keywords: colorectal cancer, ferroptosis, CD8+ T cell infiltration, ubiquitin

 Global reach, higher impact
Global reach, higher impact