10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2023; 19(9):2695-2710. doi:10.7150/ijbs.80735 This issue Cite
Research Paper
Stroke by inducing HDAC9-dependent deacetylation of HIF-1 and Sp1, promotes TfR1 transcription and GPX4 reduction, thus determining ferroptotic neuronal death
1. Division of Pharmacology, Department of Neuroscience, Reproductive and Dentistry Sciences, School of Medicine, Federico II University of Naples, Via Pansini, 5, 80131, Naples, Italy.
2. Division of Pharmacology, Department of Science and Technology, University of Sannio, 82100 Benevento, Italy.
3. IRCCS Synlab SDN S.p.A, Via Gianturco 113, 80143 Naples, Italy.
Abstract
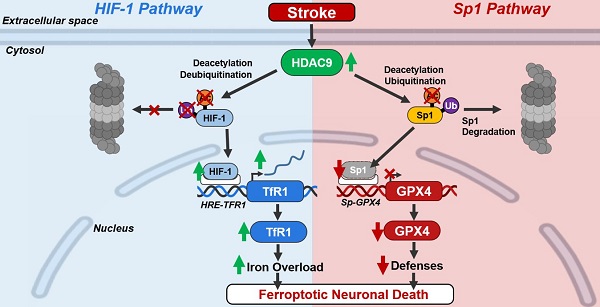
Background: The inhibition of histone deacetylase 9 (HDAC9) represents a promising druggable target for stroke intervention. Indeed, HDAC9 is overexpressed in neurons after brain ischemia where exerts a neurodetrimental role. However, mechanisms of HDAC9-dependent neuronal cell death are not yet well established.
Methods: Brain ischemia was obtained in vitro by primary cortical neurons exposed to glucose deprivation plus reoxygenation (OGD/Rx) and in vivo by transient middle cerebral artery occlusion. Western blot and quantitative real-time polymerase chain reaction were used to evaluate transcript and protein levels. Chromatin immunoprecipitation was used to evaluate the binding of transcription factors to the promoter of target genes. Cell viability was measured by MTT and LDH assays. Ferroptosis was evaluated by iron overload and 4-hydroxynonenal (4-HNE) release.
Results: Our results showed that HDAC9 binds to hypoxia-inducible factor 1 (HIF-1) and specificity protein 1 (Sp1), two transcription activators of transferrin 1 receptor (TfR1) and glutathione peroxidase 4 (GPX4) genes, respectively, in neuronal cells exposed to OGD/Rx. Consequently, HDAC9 induced: (1) an increase in protein level of HIF-1 by deacetylation and deubiquitination, thus promoting the transcription of the pro-ferroptotic TfR1 gene; and (2) a reduction in Sp1 protein levels by deacetylation and ubiquitination, thus resulting in a down-regulation of the anti-ferroptotic GPX4 gene. Supporting these results, the silencing of HDAC9 partially prevented either HIF-1 increase and Sp1 reduction after OGD/Rx. Interestingly, silencing of the neurodetrimental factors, HDAC9, HIF-1, or TfR1 or the overexpression of the prosurvival factors Sp1 or GPX4 significantly reduced a well-known marker of ferroptosis 4-HNE after OGD/Rx.
More important, in vivo, intracerebroventricular injection of siHDAC9 reduced 4-HNE levels after stroke by preventing: (1) HIF-1 and TfR1 increase and thus the augmented intracellular iron overload; and (2) a reduction of Sp1 and its target gene GPX4.
Conclusions: Collectively, results obtained suggest that HDAC9 mediates post-traslational modifications of HIF-1 and Sp1 that, in turn, increases TfR1 and decreases GPX4 expression, thus promoting neuronal ferroptosis in in vitro and in vivo models of stroke.
Keywords: HDAC9, Ferroptosis, Stroke, GPX4, TfR1

 Global reach, higher impact
Global reach, higher impact