10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2023; 19(9):2787-2802. doi:10.7150/ijbs.84570 This issue Cite
Research Paper
SLC5A3 is important for cervical cancer cell growth
1. Department of Obstetrics and Gynecology, Minhang Hospital, Fudan University, Shanghai, China.
2. Obstetrics and Gynecology Department, The First Affiliated Hospital of Soochow University, Suzhou, China.
# Equal contributors.
Received 2023-3-22; Accepted 2023-5-16; Published 2023-5-27
Abstract
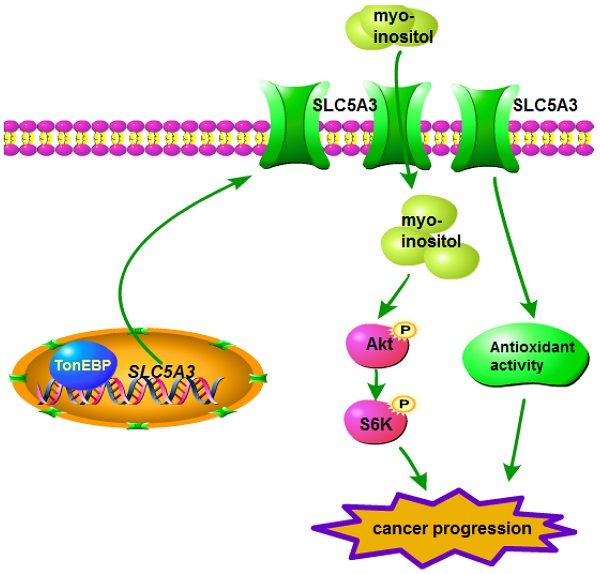
Novel molecular targets for cervical cancer must be identified. This study examined the role of SLC5A3, a myo-inositol transporter, in the pathogenesis of cervical cancer. Through boinformatics analysis, we showed that the SLC5A3 mRNA levels were upregulated in cervical cancer tissues. The upregulated SLC5A3 mRNA levels were negatively correlated with survival and progression-free interval. Genes co-expressed with SLC5A3 were enriched in multiple signaling cascades involved in cancer progression. In primary/established cervical cancer cells, SLC5A3 shRNA/knockout (KO) exerted growth-inhibitory effects and promoted cell death/apoptosis. Furthermore, SLC5A3 knockdown or KO downregulated myo-inositol levels, induced oxidative injury, and decreased Akt-mTOR activation in cervical cancer cells. In contrast, supplementation of myo-inositol or n-acetyl-L-cysteine or transduction of a constitutively active Akt1 construct mitigated SLC5A3 KO-induced cytotoxicity in cervical cancer cells. Lentiviral SLC5A3 overexpression construct transduction upregulated the cellular myo-inositol level and promoted Akt-mTOR activation, enhancing cervical cancer cell proliferation and migration. The binding of TonEBP to the SLC5A3 promoter was upregulated in cervical cancer. In vivo studies showed that intratumoral injection of SLC5A3 shRNA-expressing virus arrested cervical cancer xenograft growth in mice. SLC5A3 KO also inhibited pCCa-1 cervical cancer xenograft growth. The SLC5A3-depleted xenograft tissues exhibited myo-inositol downregulation, Akt-mTOR inactivation, and oxidative injury. Transduction of sh-TonEBP AAV construct downregulated SLC5A3 expression and inhibited pCCa-1 cervical cancer xenograft growth. Together, overexpressed SLC5A3 promotes growth of cervical cancer cells, representing as a novel therapeutic oncotarget for the devastating disease.
Introduction
Globally, cervical cancer is estimated to be associated with more than 320,000 mortalities every year [1, 2]. The incidence (13.1%) and mortality (6.1%) rates of cervical cancer in women are the fourth highest among cancers [3, 4].
Currently, a curative therapeutic strategy is not available for patients with advanced, recurrent, or metastatic cervical cancer [3-5]. The efficacy of current treatments, including traditional cisplatin/paclitaxel chemotherapy, bevacizumab targeted therapy [6, 7], and immunotherapy (immune checkpoint inhibitors) [8], is < 50% in these patients [4, 9]. Thus, there is a need to identify novel signaling targets and develop corresponding targeted therapies for advanced, recurrent, and metastatic cervical cancer [6, 10].
SLC5A3, which encodes a Na+/myo-inositol (MI) cotransporter, is located at q22 on chromosome 21 and contains one promoter and two exons spanning 2157 nucleotides [11, 12]. The SLC5A3 protein comprises 718 amino acid residues and is expressed in different human tissues [12]. The expression of SLC5A3 is reported to be upregulated in patients with Down's syndrome [12-14]. Andronic et al. demonstrated that hypotonic stress upregulated SLC5A3 expression and MI transport in HEK293 cells, suggesting that SLC5A3 regulates mammalian cell hypotonic volume [15].
Some studies have examined the function of SLC5A3 in the pathogenesis of human cancer. For example, SLC5A3-dependent MI transport, which promotes nutrient dependency, is required for acute myeloid leukemia (AML) cell proliferation [16]. SLC5A3 silencing decreased MI contents and arrested AML cell proliferation [16]. This study demonstrated that SLC5A3 overexpression promoted cervical cancer cell growth.
Materials and methods
Reagents
CCK-8, puromycin, cell culture medium, serum, MI, N-Acetyl-L-cysteine (NAC) as well as RNA reagents, fluorescence dyes, antibodies and the caspase inhibitors were provided by Dr. Cui [17] or purchased from Sigma (St. Louis, Mo).
Cells
“pCCa-1”, “pCCa-2”, “pCCa-3” primary cancer cells (derived from three patients), the primary human cervical epithelial cells (“HCerEpC1”), HeLa immortalized cells and Ect1/E6E7 epithelial cells were from Dr. Cao [18]. Cells were cultivated under the described high glucose (4.5g/L glucose) medium [19]. The protocols of this study were reviewed by the Ethics Committee of Minhang Hospital, Fudan University (Shanghai, China).
Human tissues
The cervical cancer tissues along with the matched adjacent normal cervical epithelial tissues were from twenty (20) cervical cancer patients (written-informed consent, administrated at authors' institution). The immunohistochemistry (IHC) staining in four μm-thick tissue slides were reported previously [20].
shRNA or overexpression
The sequences encoding three different shRNAs targeting human SLC5A3, shSLC5A3 (seq1, targeting: GCACTTACACTTATGATTATT), shSLC5A3 (seq2, targeting: GACTAATTCTGAAGCATATTT) and shSLC5A3 (seq3, targeting: ACCCTAACCATTGGCATATAT), or the SLC5A3 cDNA sequence (hSLC5A3[NM_006933.7]), were individually inserted into GV248 lentiviral construct (Genechem). Each of the construct was co-transfected to HEK-293 cells along with lentivirus envelope constructs (Genechem). The generated lentiviral particles were added to cultured cervical cancer cells or epithelial cells at MOI=11.6 for 48h. Afterwards, puromycin was added for another 96h to select stable cells. For animal xenograft studies, the two SLC5A3 shRNA (-seq1/3) sequence or the scramble control shRNA sequence (shC) was inserted into an AAV9 construct (Genechem). The shRNA AAV was then generated. Silencing or overexpression (hSLC5A3[NM_006933.7]) of TonEBP (tonicity-responsive enhancer-binding protein) was through exact the same procedure in vitro and in vivo. Two different shRNAs against TonEBP, shTonEBP-seq1 (targeting: GCAGCAGATTTCATCAAATAT) and shTonEBP-seq2 (targeting: GCAGAGTAACTGGACGAAATA), were utilized. Parental cells (“Pare”) were always utilized as the control cells.
CRISPR/Cas9
Cells were transfected with lentiviral particles with Cas9-expressing construct and stable cells were formed following selection. The two different sequences encoding small-guide (sgRNA) targeting SLC5A3 were each inserted into a lenti-CRISPR/Cas9-KO-puro construct (Genechem). sg-1 targets ATCCCAATTTACATCCGGTC with AGG PAM sequence. sg-2 targets TCCCAATTTACATCCGGTCA with GGG PAM sequence. The construct was transfected to HEK-293 cells to generate lentiviral particles. The particles were then added to Cas9 stable cells, and puromycin added to select stable colonies. SLC5A3 KO screening was carried out.
Immunofluorescence and ChIP assays
In the cell culture plate, the glass slide was first fixed and washed with PBS for three times. Next, the 0.5% Triton X-100 was added for permeabilization. Different fluoresce dyes were added to the glass slide and the redundant fluoresce dyes washed off four times with PBS. Water absorbent paper was utilized to suck up the liquid and the fluoresce signalings were observed and collected under a fluorescence microscope (Zeiss). TonEBP chromosome immunoprecipitation (ChIP)'s protocols were described previously [21].
Myo-inositol (MI) detection and superoxide dismutase (SOD) activity
In brief, MI contents were detected through a MI assay kit (ab252896, abcam, Shanghai, China). The SOD activity in fresh tumor tissues was measured under the SOD ELISA kit (Thermo-Fisher Invitrogen, Shanghai, China).
Constitutively-active Akt1
The viral particles with the S473D constitutively-active Akt1 (caAkt1) were from Dr. Xu [22] and added to pCCa-1 primary cancer cells, with stable cells formed following selection [22].
Other assays
CCK-8 viability assay, Trypan blue staining of cell death, Caspase-3 activity detection, “Transwell” assay of cell migration, nuclear EdU staining (quantifying cell proliferation), nuclear TUNEL staining of cell death, thiobarbituric acid reactive substance (TBAR) assay, CellROX staining assay of ROS (reactive oxygen species) production and mitochondrial depolarization (JC-1 staining) were reported in other studies [23]. qRT-PCR and Western blotting protocols were reported early [17]. mRNA primers for SLC5A3 and SLC5A11 were described early [24, 25]. Figure S1 included the uncropped blotting images.
Xenograft study
The female nude mice, weighing 18.4-19.1g, were provided by Changzhou Cavens Experimental Animal Co (Changzhou, China). pCCa-1 cells in non-serum medium, at 5 × 10 6 cells per mouse, were subcutaneously (s.c.) injected to the mice. The xenografts were formed afterwards. Mice were thereafter intratumorally injected with AAV-packed shRNAs (at 1.2 × 10 9 PFU, 8.5 μL). The recordings of the xenografts and mice were reported previously [20].
Statistical difference
All the in vitro cellular experiments were repeated five times (n = 5). The statistical methods were described early [23]. P values < 0.05 indicated statistically significant. “N. S.” stands for non-statistical difference (P > 0.05).
Results
SLC5A3 is upregulated in cervical cancer
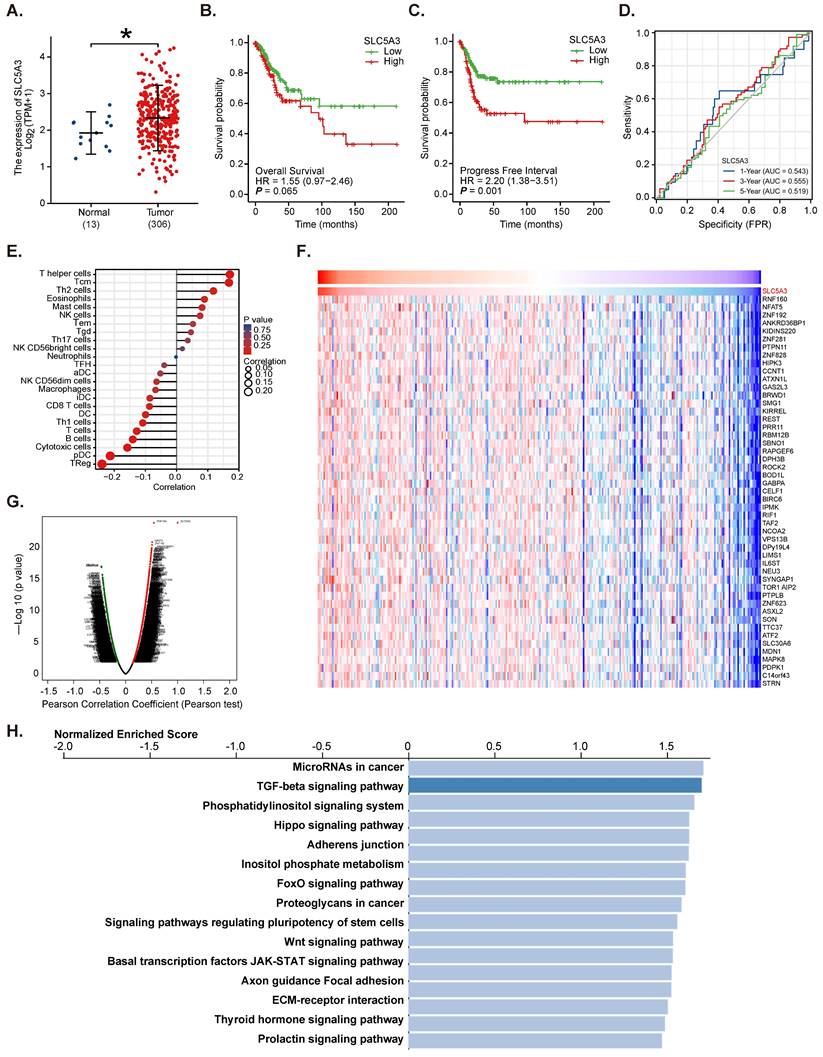
The Cancer Genome Atlas (TCGA) database and Genotype-Tissue Expression (GTEx) dataset, which comprise RNA sequencing data of 10 healthy tissues, three paracancerous tissues, and 306 cervical cancer tissues, were analyzed. The SLC5A3 mRNA expression levels in tumor tissues were markedly higher than those in paracancerous and healthy tissues (P < 0.05) (Figure 1A). Analysis of TCGA cervical cancer datasets revealed that patients with cervical cancer exhibiting SLC5A3 upregulation were associated with poor overall survival (P = 0.065) (Figure 1B) and progression-free interval (PFI) (P = 0.001, Figure 1C). The receiver operating characteristic curve analysis suggested that SLC5A3 upregulation can predict the 1-5 year survival rates of cervical cancer patients (Figure 1D).
Immune cell infiltration analysis based on transcriptome and other omics data revealed that SLC5A3 upregulation was correlated with the infiltration of different immune cells (Figure 1E). The LinkedOmics functional model was used to examine genes co-expressed with SLC5A3 in TCGA cervical cancer cohort. The top 50 significant genes positively correlated with SLC5A3 were retrieved (Figure 1F-G). Kyoto Encyclopedia of Genes and Genomes analyses revealed that genes co-expressed with SLC5A3 were enriched in multiple pathways involved in cancer progression, including Hippo, FOXO, JAK-STAT, and extracellular matrix pathways (Figure 1H). Thus, bioinformatics analysis revealed that SLC5A3 is upregulated in cervical cancer.
SLC5A3 is upregulated in clinical cervical cancer tissues and patient-derived or established cervical cancer cells
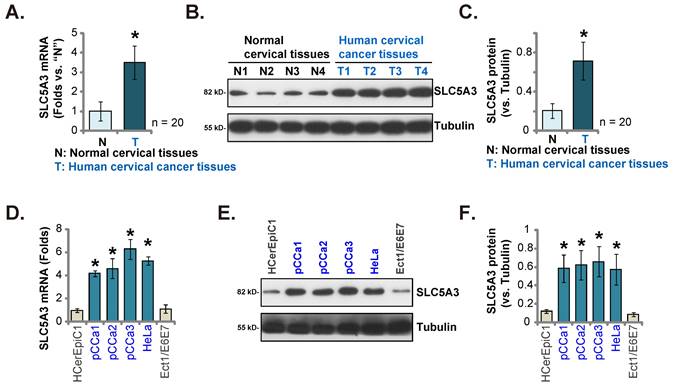
Next, the clinical specimens of cervical cancer (n = 20) were obtained. The SLC5A3 mRNA levels in cancer tissues (“T”) were higher than those in healthy epithelial tissues (N”) (Figure 2A). The protein levels of SLC5A3 were upregulated in the cancer tissue lysates prepared from four representative clinical specimens (Figure 2B). Western blotting analysis results of all 20 sets of tissue lysates were combined, which revealed significant SLC5A3 protein upregulation in cancer tissues (Figure 2C). SLC5A3 mRNA and protein expression was significantly upregulated in the primary human cervical cancer cells (pCCa-1/-2/-3 cells, which were obtained from Dr. Cao [18]) and the immortalized HeLa cells (Figure 2D-F), but were low in the cervical epithelial cells (HCerEpC1 [18] and Ect1/E6E7 cells) (Figure 2D-F).
Silence of SLC5A3 produces anti-cervical cancer cell activity
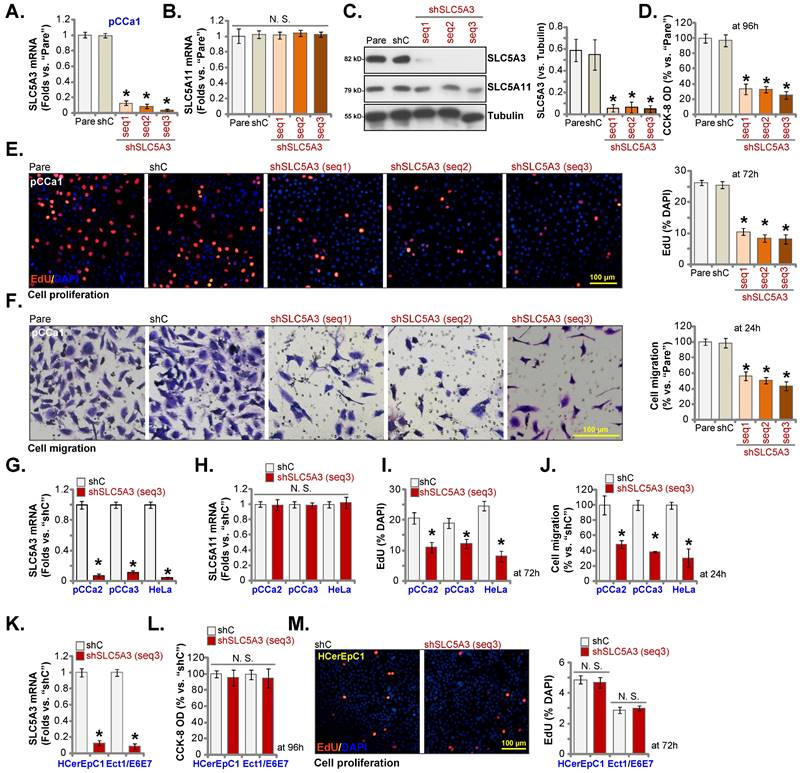
SLC5A3 was knocked down using lentiviral particles encoding three different shRNAs targeting SLC5A3. sh-SLC5A3 (seq1/2/3) constructs were transduced into pCCa-1 primary cells. The infected cells were further selected using a puromycin-supplemented medium to obtain stable colonies. As shown in Figure 3A, transduction with sh-SLC5A3 (seq1/2/3) constructs markedly downregulated the SLC5A3 mRNA levels in pCCa-1 cells but did not affect the SLC5A11 mRNA levels (Figure 3B). Consistently, transduction with sh-SLC5A3 (seq1/2/3) constructs downregulated the SLC5A3 protein levels in pCCa-1 cells (Figure 3C) but did not affect the SLC5A11 protein levels (Figure 3C).
CCK-8 assay showed that SLC5A3 knockdown decreased the viability of pCCa-1 cells (Figure 3D). The number of 5-ethynyl-2′-deoxyuridine (EdU)-positive nuclei was markedly downregulated in SLC5A3 knockdown pCCa-1 cells, suggesting that SLC5A3 knockdown inhibited cell proliferation (Figure 3E). Additionally, SLC5A3 knockdown suppressed the in vitro migration of pCCa-1 primary cells (Figure 3F).
SLC5A3 is upregulated in cervical cancer. TCGA cervical cancer cohort combining Genotype-Tissue Expression (GTEx) program revealed SLC5A3 mRNA transcripts in cervical cancer tissues (“Tumor”, n = 306) and ten (10) normal tissues plus three (3) parecancer normal tissues (total 13, “Normal”) (A).The overall survival (B) and progress free interval (C) in SLC5A3-low and SLC5A3-high cervical cancer patients from TCGA cervical cancer cohort. ROC curve showed the potential value of SLC5A3 overexpression in predicting cervical cancer patients' survival (1-5 years) (D). The multiple omics and immune infiltration analysis of the association between the SLC5A3 expression and immune cells infiltration (E). The heat map (F) and volcano map (G) of DEGs with SLC5A3 in TCGA cervical cancer cohort were presented; KEGG of top 15 enriched pathways of SLC5A3-associated DEGs were presented (H).
SLC5A3 is upregulated in clinical cervical cancer tissues and patient-derived or established cervical cancer cells. SLC5A3 expression in the described tissues of twenty (20) cervical cancer patients (n = 20) was tested (A-C). SLC5A3 expression in the described cells was also tested (D-F). *P < 0.05 versus “N” tissues/“HCerEpiC1” cells.
Transduction with sh-SLC5A3 (seq3) lentiviral particles downregulated the expression of SLC5A3 mRNA by more than 90% in pCCa-2/pCCa-3 primary cells or HeLa cells (Figure 3G) but did not affect the expression of SLC5A11 mRNA (Figure 3H). SLC5A3 knockdown markedly inhibited the proliferation (Figure 3I) and in vitro migration of cancer cells (Figure 3J). Similarly, transduction with sh-SLC5A3 (seq3) lentiviral particles downregulated the SLC5A3 mRNA levels in HCerEpC1 and Ect1/E6E7 epithelial cells (Figure 3K). However, SLC5A3 knockdown did not significantly reduce the viability (Figure 3L) and number of EdU-positive nuclei (Figure 3M) in the epithelial cells.
Apoptosis is induced in SLC5A3 knockdown cervical cancer cells
Cervical cancer cell growth arrest and proliferation inhibition can activate cell apoptosis [26-28]. Thus, the effect of SLC5A3 knockdown on cervical cancer cell apoptosis was examined. Transduction with sh-SLC5A3 (seq1/2/3) upregulated the CASP3 activity (Figure 4A) and the levels of cleaved CASP3 and PARP1 in pCCa-1 cells (Figure 4B). The number of TUNEL-positive nuclei was upregulated in SLC5A3 knockdown pCCa-1 cells (Figure 4C). Furthermore, SLC5A3 knockdown significantly increased the number of trypan blue-positive pCCa-1 cells (Figure 4D), indicating the upregulation of cell death. Treatment with caspase-apoptosis inhibitors, including z-DEVD-fmk and z-VAD-fmk, mitigated the SLC5A3 knockdown-induced downregulation of cell viability (Figure 4E) and upregulation of cell death (Figure 4F) in pCCa-1 cells.
Transduction with sh-SLC5A3 (seq3) significantly induced apoptosis and increased the number of TUNEL-positive nuclei in pCCa-2 and pCCa-3 primary cervical cancer cells and HeLa cells (Figure 4G) but did not promote CASP3 activation (Figure 4H) or induce cell apoptosis (TUNEL assays, Figure 4I) in HCerEpC1 and Ect1/E6E7 epithelial cells.
SLC5A3 knockout causes remarkable anti-cervical cancer activity
SLC5A3 was knocked out using CRISPR/ Cas9 system. Two CRISPR/Cas9-SLC5A3 knockout (KO) constructs, which encoded different single-guide RNAs (sgRNAs) against SLC5A3 [koSLC5A3 (sg1) and koSLC5A3 (sg2)], were individually transfected into Cas9-expressing pCCa-1 cells. After SLC5A3 KO screening, stable SLC5A3 KO pCCa-1 primary cells (“koSLC5A3” cells) were established. The expression of SLC5A3 was depleted in koSLC5A3 cells (Figure 5A-B) although SLC5A11 expression was unaltered (Figure 5A-B). The percentage of EdU-positive nuclei was downregulated in koSLC5A3 pCCa-1 cells (Figure 5C), suggesting the inhibition of cell proliferation. Furthermore, SLC5A3 KO markedly suppressed pCCa-1 cell migration (Figure 5D). The expression levels of cleaved CASP3, PARP1, and CASP9 (apoptotic marker proteins) were upregulated in koSLC5A3 pCCa-1 cells (Figure 5E). Consistently, the percentage of TUNEL-positive nuclei was upregulated in koSLC5A3 pCCa-1 cells (Figure 5F). Thus, SLC5A3 KO exerted anti-cervical cancer effects.
Silence of SLC5A3 produces anti-cervical cancer cell activity. pCCa-1 primary cells expressing the applied lentiviral SLC5A3 shRNA [shSLC5A3 (seq1/2/3)] or the control shRNA (shC) were established, with listed mRNAs and proteins tested (A-C). The above pCCa-1 cells were cultivated , cell viability (D), proliferation (E) and migration (F) were examined by the described methods. pCCa-2 and pCCa-3 primary cancer cells, HeLa cells, HCerEpC1 cells or Ect1/E6E7 epithelial cells were infected with lentiviral SLC5A3 shRNA (seq3) or shC, and stable cells formed. Listed mRNAs were shown (G, H and K). EdU nuclear incorporation (I and M), cell migration (J) and viability (L) were examined, and results quantified. * P < 0.05 vs. “shC” cells.
SLC5A3 knockdown/KO promotes MI depletion and oxidative injury in cervical cancer cells
SLC5A3 is involved in transporting MI [16, 29, 30]. The cellular MI contents were downregulated in sh-SLC5A3 (seq1)-transduced pCCa-1 cells (Figure 6A). Consistently, transduction with the koSLC5A3 (sg1) construct (Figure 5) downregulated the cellular MI contents (Figure 6A). SLC5A3 exerts anti-oxidant effects and downregulates reactive oxygen species (ROS) production in neuronal cells [31]. In this study, SLC5A3 knockdown or KO upregulated oxidative stress in cervical cancer cells. The CellROX dye intensity was upregulated in pCCa-1 cervical cancer cells transduced with sh-SLC5A3 (seq1) or koSLC5A3 (sg1) (Figure 6B). The thiobarbituric acid reactive substance (TBAR) values were upregulated in SLC5A3 knockdown or KO pCCa-1 cells, suggesting the upregulation of lipid peroxidation (Figure 6C). JC-1 monomer formation further supported the depolarization of mitochondria in SLC5A3-depleted pCCa-1 cells (Figure 6D). Transduction with shC and Cas9C (“shC+Cas9C”) did not affect MI contents (Figure 6A) and ROS production/oxidative injury (Figure 6B-D) in pCCa-1 cells.
Apoptosis is induced in SLC5A3 knockdown cervical cancer cells. The pCCa-1/2/3 primary cells (A-D and G), HeLa cells (G), HCerEpC1 cells or Ect1/E6E7 epithelial cells (H and I), with the described SLC5A3 shRNA or the control shRNA (shC), were maintained in complete medium for designated time, caspase-PARP activation was examined (A, B, H); Cell apoptosis was examined by TUNEL nuclear staining (C, G and I) assays, with results quantified. Cell death was measured by the Trypan blue assay (D). The shC- or shSLC5A3 (seq3)-expressing pCCa-1 cells were co-treated with 40 μM of z-VAD-fmk (40 μM) or z-DEVD-fmk for 96h, cell viability and cell death were measured and quantified by CCK-8 (E) and Trypan blue staining (F) assays. * P < 0.05 vs. “shC” cells. # P < 0.05 vs. “Veh” treatment (E and F).
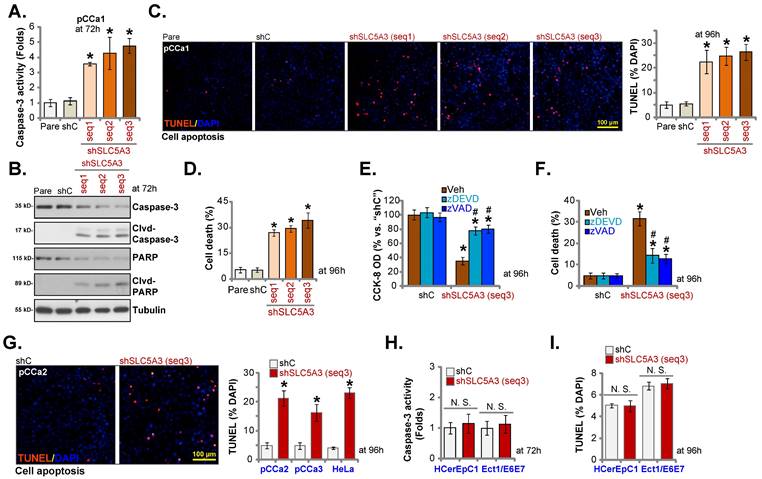
SLC5A3 knockout causes remarkable anti-cervical cancer activity. pCCa-1 cells, stably expressing the CRISPR/Cas9-SLC5A3-knockout (KO) construct encoded two different sgRNAs: koSLC5A3 (sg1) or koSLC5A3 (sg2), as well as the Cas9 control construct (“Cas9C”), were cultured, and listed mRNAs /proteins examined (A and B). The exact same number of above viable pCCa-1 cells were cultured for designated hours, and EdU nuclear incorporation (C), migration (D), caspase-3 activity (E) and TUNEL nuclear incorporation (F) were examined. * P < 0.05 vs. “Cas9C” cells.
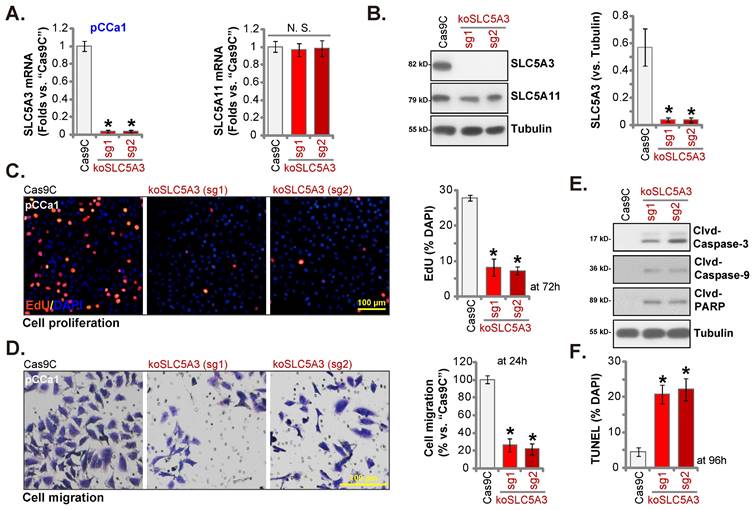
SLC5A3 knockdown/KO promotes MI depletion and oxidative injury in cervical cancer cells. pCCa-1 cells with described genetic treatments were cultured; Myo-inositol (MI) contents were examined (A). Cellular ROS contents were tested through quantifying the CellROX red fluorescence (B); TBAR intensity was measured (C); Mitochondrial depolarization was examined by quantifying the JC-1 green monomers (D). The above cells were also co-treated with MI (2.5 mM), NAC (400 μM) or the vehicle (“Veh”) control for 96h, cell viability (CCK-8,E), death (F) and apoptosis (TUNEL assays, G) were tested. * P < 0.05 vs. “Pare”/“shC” cells. # P < 0.05 vs. “Veh” treatment (E-G).
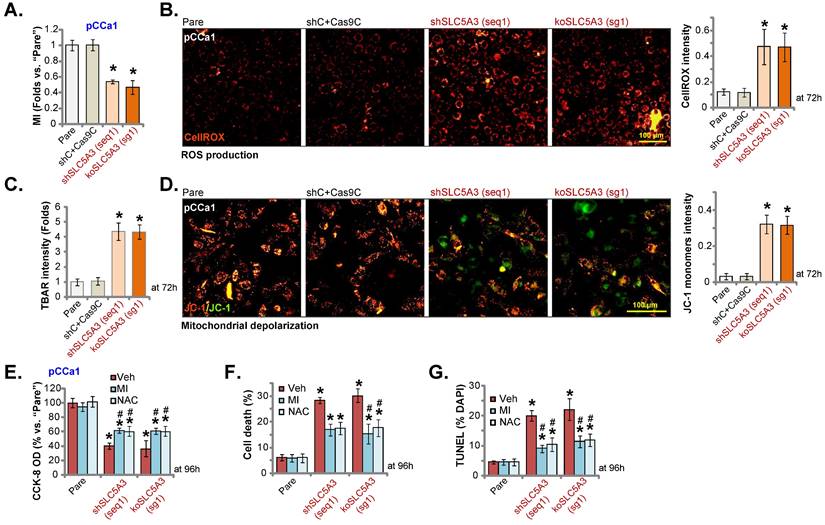
To investigate the contribution of MI downregulation and oxidative injury to the anti-cervical cancer effects of SLC5A3 depletion, cells were exogenously treated with MI or the anti-oxidant N-acetyl cysteine (NAC). Treatment with MI (2.5 mM) or NAC (400 μM) significantly mitigated the SLC5A3 knockdown/KO-induced downregulation of pCCa-1 cell viability (Figure 6E) and upregulation of pCCa-1 cell death and apoptosis (Figure 6F-G). However, treatment with MI or NAC alone did not decrease pCCa-1 cell viability and induce death/apoptosis (Figure 6E-G). These results indicate that SLC5A3 depletion promotes cervical cancer cell death by downregulating MI and ROS production.
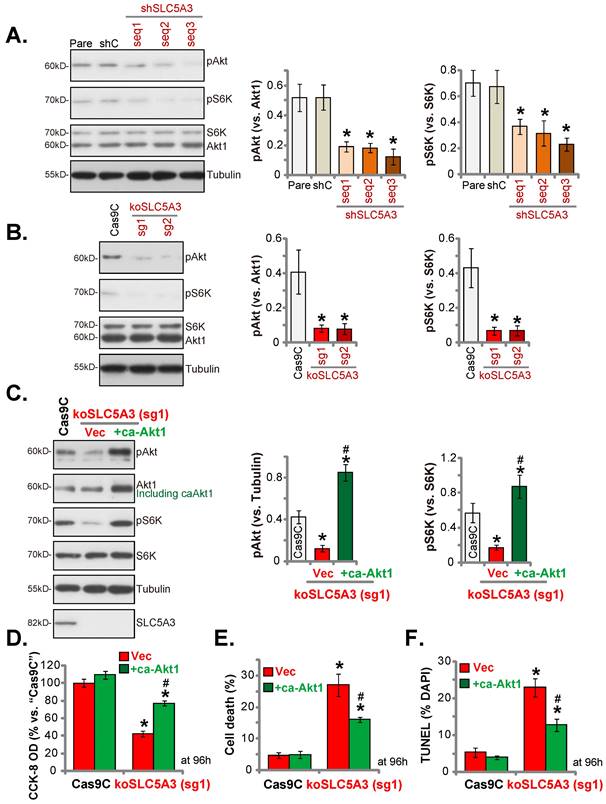
SLC5A3 knockdown/KO inhibits Akt-mTOR activation
Inositol second messengers can actively regulate multiple signaling cascades involved in cancer progression. In particular, inositols are reported to activate the Akt cascade [32]. Akt-mTOR cascade hyperactivation is important for cervical cancer growth [33, 34]. Transduction with sh-SLC5A3 (seq1/2/3) markedly downregulated the phosphorylation of Akt1 (Ser-473) and S6K (mTOR marker protein) (Figure 7A), resulting in Akt-mTOR cascade suppression. Similarly, transduction with koSLC5A3 (sg1) markedly downregulated the phosphorylation of Akt1 and S6K (Figure 7B). To activate the Akt-mTOR cascade, an S473D constitutively active Akt1 (“caAkt1”) construct was stably transduced into koSLC5A3 (sg1)-transduced pCCa-1 cells, which upregulated the phosphorylation of Akt1 and S6K (Figure 7C). Transduction with caAkt1 suppressed the SLC5A3 KO-induced downregulation of pCCa-1 cell viability (Figure 7D) and upregulation of cell death (Figure 7E) and apoptosis (Figure 7F).
Ectopic SLC5A3 overexpression exerts pro-cervical cancer effects
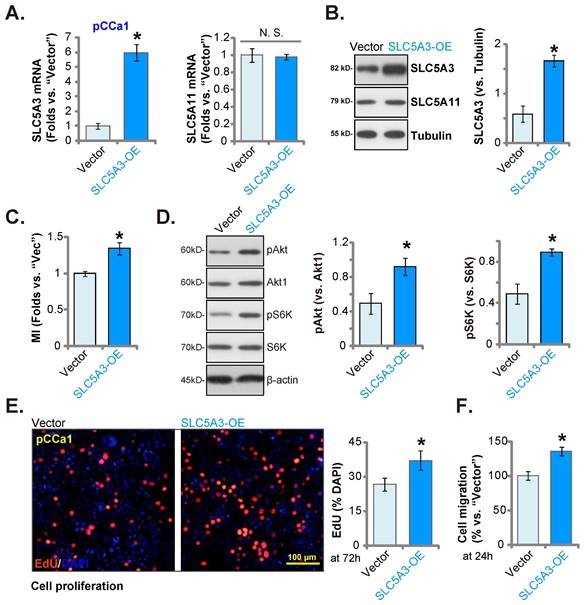
Next, a lentiviral SLC5A3 overexpression construct (encoding SLC5A3 cDNA) was stably transduced into pCCa-1 cells (“SLC5A3-OE” cells). The SLC5A3 mRNA levels were upregulated by 5-6 fold in SLC5A3-OE cells (Figure 8A) but the SLC5A11 mRNA levels were unaltered (Figure 8A). Consistently, the SLC5A3 protein levels were upregulated in SLC5A3-OE cells (Figure 8B) but the SLC5A11 protein levels were unaffected (Figure 8B). The cellular MI contents were upregulated in SLC5A3-OE cells (Figure 8C). Additionally, SLC5A3 overexpression upregulated Akt-mTOR cascade activation and promoted the phosphorylation of Akt1 and S6K in pCCa-1 cells (Figure 8D). Furthermore, ectopic SLC5A3 overexpression increased EdU incorporation, suggesting that SLC5A3 overexpression promoted pCCa-1 cell proliferation (Figure 8E). The in vitro migration of SLC5A3-OE cells was also strengthened (Figure 8F). These findings indicate that ectopic SLC5A3 overexpression enhanced cervical cancer cell proliferation and migration.
SLC5A3 silencing/KO inhibits Akt-mTOR activation. pCCa-1 cells with the applied lentiviral SLC5A3 shRNA [shSLC5A3 (seq1/2/3)], control shC, the CRISPR/Cas9-SLC5A3-knockout (KO) construct encoded two different sgRNAs: koSLC5A3 (sg1) and koSLC5A3 (sg2), or Cas9C were cultured, listed proteins were shown (A and B). The koSLC5A3 (sg1) pCCa-1 cells were further stably transduced with the S473D caAkt1 or vector (“Vec”), listed proteins were examined (C); After culture of 96h, cell viability (D), death (E) and cell apoptosis (TUNEL assays, F) were examined. * P < 0.05 vs. “Pare”/“Cas9C” cells. # P < 0.05 vs. “Vec” cells (C-F).
TonEBP-SLC5A3 promoter binding is upregulated in cervical cancer
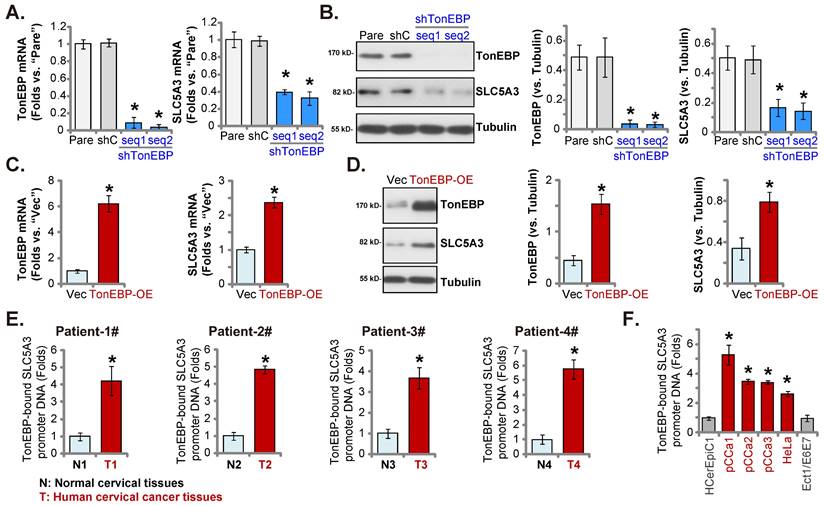
The upregulated SLC5A3 mRNA and protein levels in cervical cancer contribute to cancer cell growth. Next, the potential mechanism of SLC5A3 upregulation was examined by focusing on the transcriptional mechanism. Johnson et al. demonstrated that TonEBP (NFAT5) is an important transcription factor of SLC5A3 and that TonEBP expression was correlated with SLC5A3 expression [35]. TonEBP (NFAT5) was the second top differentially expressed gene (DEG) that was positively correlated with SLC5A3 expression in TCGA cervical cancer cohort (Figure 1F-G). The pCCa-1 primary cells were transduced with lentiviral particles encoding TonEBP shRNAs (sh-TonEBP-seq1 and sh-TonEBP-seq2). Stable TonEBP knockdown cells were obtained after selection. Transduction with TonEBP shRNAs markedly downregulated the expression of TonEBP (Figure 9A-B) and SLC5A3 (Figure 9A-B) in pCCa-1 cells.
Ectopic SLC5A3 overexpression exerts pro-cervical cancer effects. SLC5A3-overexpressing pCCa-1 cells (“SLC5A3-OE”) or empty vector (“Vector”) were established, the listed mRNAs and proteins were measured (A, B and D); Myo-inositol (MI) contents were measured (C). The exact same number of the pCCa-1 cells were cultured for designated hours, EdU incorporation (E) and migration (F) were examined. * P < 0.05 vs. “Vector” cells.
TonEBP cDNA sequence-encoding lentiviral particles were transduced into pCCa-1 cells to obtain stable TonEBP-overexpressing cells (“TonEBP-OE” cells). The TonEBP Mrna and protein levels, as well as the SLC5A3 mRNA and protein levels, were upregulated in TonEBP-OE pCCa-1 cells (Figure 9C-D). These results indicate that TonEBP is a key transcription factor of SLC5A3 in cervical cancer cells.
The results of ChIP assay (Figure 9E) revealed that the binding of TonEBP to the proposed SLC5A3 promoter [35] was significantly upregulated in the cervical cancer tissues of four patients but was relatively low in matched adjacent non-cancerous cervical epithelial tissues (Figure 9E). The binding of TonEBP to the SLC5A3 promoter was strong in pCCa-1/pCCa-2/pCCa-3 primary cells and immortalized HeLa cells (Figure 9F) but weak in HCerEpC1 and Ect1/E6E7 cells (Figure 9F). Therefore TonEBP-SLC5A3 promoter DNA binding increasing in cervical cancer is key for SLC5A3 upregulation.
SLC5A3 knockdown arrests the growth of cervical cancer xenografts
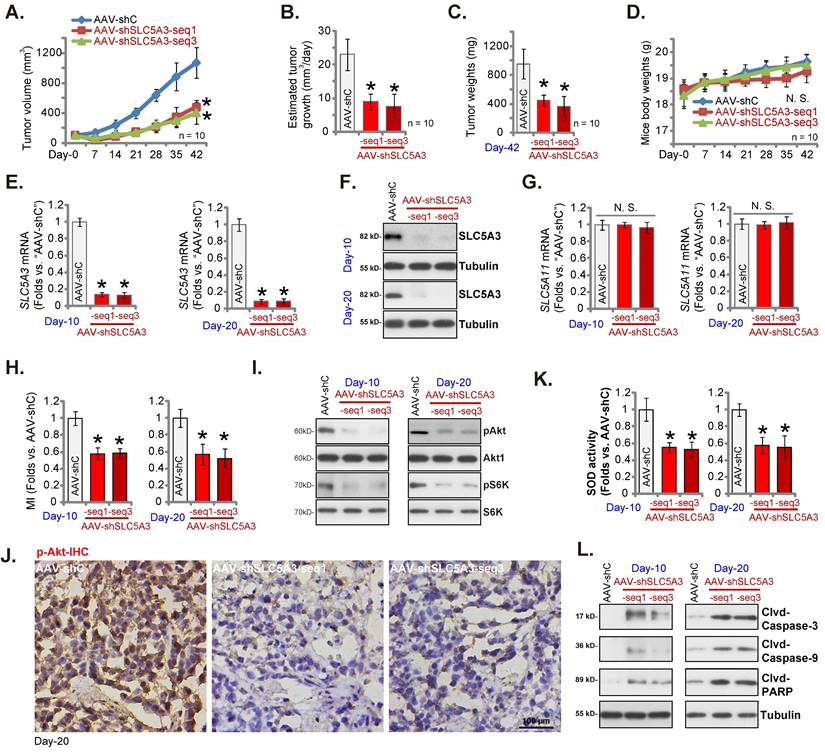
Nude mice were subcutaneously injected with pCCa-1 primary cells, resulting in the formation of xenograft tumors after three weeks (day 0). The SLC5A3 shRNA-encoding adeno-associated virus (AAV) constructs [AAV-sh-SLC5A3 (seq1) and AAV-sh-SLC5A3 (seq3)] were intratumorally injected into mice. Mice in the control group were intratumorally injected with shC-encoding AAV (“AAV-shC”) construct. The AAV constructs were injected once every two days five times. As shown in Figure 10A, the weekly xenograft growth curve revealed that AAV-sh-SLC5A3 injection impaired pCCa-1 xenograft growth. The volume of AAV-sh-SLC5A3 (seq1/3)-injected tumors was lower than that of the AAV-shC-injected tumors (Figure 10A). Moreover, AAV-sh-SLC5A3 injection suppressed daily pCCa-1 xenograft growth (estimated in mm3 per day) (Figure 10B). All pCCa-1 xenografts were carefully isolated on day 42. The weight of AAV-sh-SLC5A3-injected pCCa-1 xenografts significantly decreased (Figure 10C). The mean bodyweight was not significantly different between the three groups (Figure 10D).
TonEBP-SLC5A3 promoter binding is upregulated in cervical cancer. pCCa-1 cells with the applied lentiviral TonEBP shRNA (shTonEBP-seq1/shTonEBP-seq2, with two different sequences), shC, TonEBP-overexpressing construct (“TonEBP-OE”) or control vector ( “Vec”) were formed, with listed mRNAs/proteins examined (A-D). ChIP assay results revealed the relative level of the SLC5A3 promoter DNA-bound to TonEBP in listed tissues (E) and cells (F). * P < 0.05 vs. “shC”/“Vec”/“N”/“HCerEpiC1”.
Next, the signaling cascades in the AAV-sh-SLC5A3-injected tumors were analyzed. On days 10 and 20, one pCCa-1 xenograft was isolated from each group. The SLC5A3 mRNA (Figure 10E) and protein (Figure 10F) levels were markedly downregulated in AAV-sh-SLC5A3 (seq1/3)-injected tumors but the SLC5A11 mRNA levels were unchanged (Figure 10G). Additionally, the MI contents (Figure 10H) and the phosphorylation of Akt1 and S6K (Figure 10I) were downregulated in AAV-sh-SLC5A3 (seq1/3)-injected pCCa-1 xenograft tissues. IHC analysis further confirmed the suppression of Akt1 phosphorylation in AAV-sh-SLC5A3 (seq1/3)-injected pCCa-1 xenografts (Figure 10J).
The superoxide dismutase (SOD) activity was significantly downregulated (Figure 10K) in AAV-sh-SLC5A3 (seq1/3)-injected pCCa-1 xenograft tissues, indicating the induction of oxidative injury. The levels of the cleaved apoptosis marker proteins were upregulated in AAV-sh-SLC5A3 (seq1/3)-injected pCCa-1 xenograft tissues (Figure 10L). Thus, SLC5A3 knockdown downregulated MI contents, suppressed Akt-mTOR cascade activation, promoted oxidative injury, and induced apoptosis in pCCa-1 xenografts.
SLC5A3 KO hinders pCCa-1 cervical cancer xenograft growth
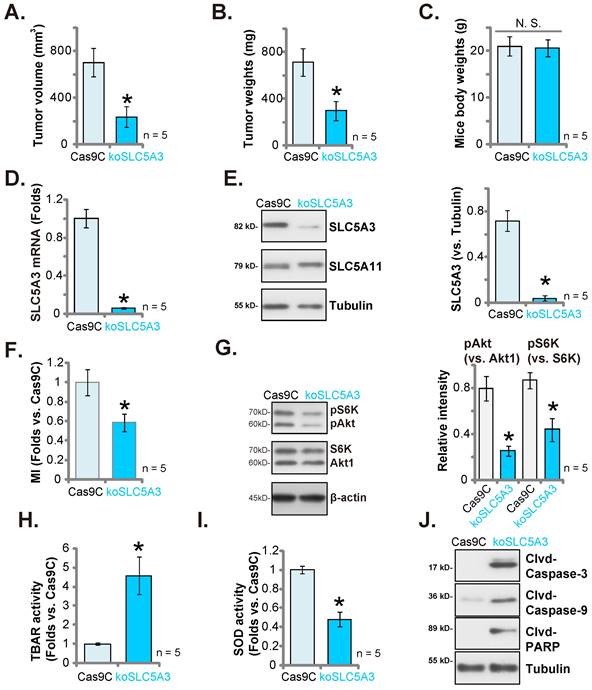
Next the primary pCCa-1 cells with the CRISPR/Cas9-SLC5A3-knockout (KO) construct (“koSLC5A3”, with sg1, see Figure 5) or Cas9C (see Figure 5) were s.c. injected to nude mice. Sixty days later, the pCCa-1 xenografts were isolated. The sizes and weights of koSLC5A3 (sg1)-injected pCCa-1 xenografts were markedly downregulated (Figure 11A-B) when compared with those of Cas9C pCCa-1 xenografts. The bodyweight was not significantly different between the groups (Figure 11C). Tumor lysates were prepared from koSLC5A3 (sg1)-injected and Cas9C-injected pCCa-1 xenografts. The SLC5A3 expression levels were downregulated in the lysates of koSLC5A3 (sg1)-injected xenograft tissues (Figure 11D-E) but the SLC5A11 protein levels were unaffected (Figure 11E). Additionally, the MI contents (Figure 11F) and the phosphorylation of Akt1 and S6K (Figure 11G) were downregulated in koSLC5A3 (sg1)-injected pCCa-1 xenograft tissues. Furthermore, the TBAR values were upregulated in koSLC5A3 (sg1)-injected pCCa-1 xenograft tissues (Figure 11H), indicating upregulation of lipid peroxidation. The SOD activity was downregulated in koSLC5A3 (sg1)-injected xenograft tissues, indicating upregulation of oxidative injury (Figure 11I). The levels of the cleaved apoptosis marker proteins were upregulated in koSLC5A3 (sg1)-injected xenograft tissues, suggesting apoptosis activation (Figure 11J). Thus, SLC5A3 KO significantly impaired pCCa-1 cervical cancer xenograft growth.
SLC5A3 knockdown arrests the growth of cervical cancer xenografts. The nude mice bearing pCCa-1 xenografts were intratumorally injected with SLC5A3 shRNA-expressing AAV [“AAV-shSLC5A3 (seq1)/AAV-shSLC5A3 (seq3)”] or the shC-expressing AAV (“AAV-shC”), every 48h for 10 days; Weekly pCCa-1 xenograft volumes (A) and animal weights (D) were measured; Daily pCCa-1 xenograft growth was estimated (B). All pCCa-1 xenografts were isolated at Day-42 and tumor weights recorded (C). In the described pCCa-1 xenograft tissues, expression of listed mRNAs/proteins (E-G, I and L), myo-inositol (MI) contents (H) and SOD activity (K) were measured; The pCCa-1 xenograft tissue slides were subject to IHC staining of p-Akt (J). *P < 0.05 versus “AAV-shC” group.
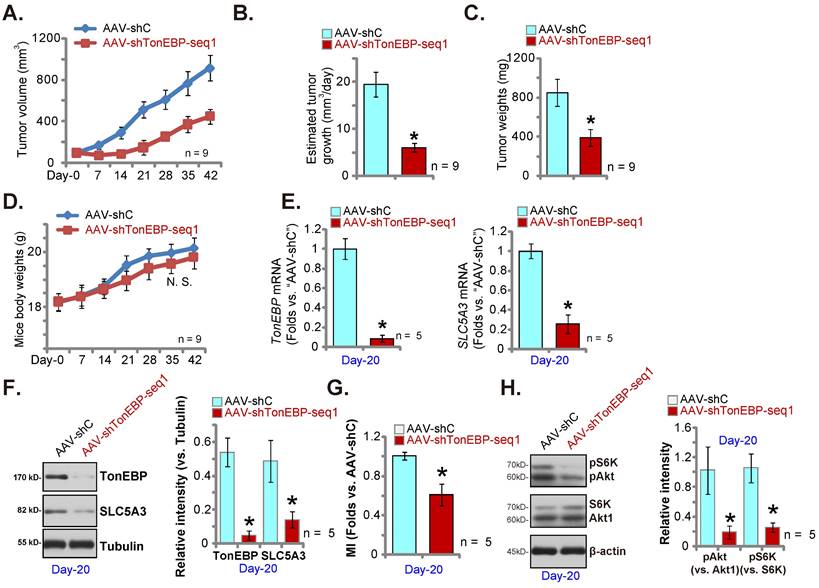
TonEBP silencing inhibits pCCa-1 cervical cancer xenograft growth
We further hypothesized that silencing of the transcription factor TonEBP should downregulate SLC5A3 expression and inhibit cervical cancer cell in vivo growth. Therefore, TonEBP shRNA AAV (“AAV-shTonEBP-seq1”) were intratumorally injected to mice bearing pCCa-1 tumors. The control group mice received AAV-shC (see Figure 10). AAV-shTonEBP-seq1 hindered pCCa-1 xenograft growth in vivo (Figure 12A). The daily pCCa-1 xenograft growth was significantly decreased after AAV-shTonEBP-seq1 injection (Figure 12B). Analyzing the isolated pCCa-1 xenografts at Day-42 found that AAV-shTonEBP-seq1-treated pCCa-1 xenografts were lighter than AAV-shC xenografts (Figure 12C), with no difference in animal weights detected (Figure 12D).
At Day-20, one pCCa-1 xenograft per group was isolated. TonEBP and SLC5A3 mRNA levels were both decreased in AAV-shTonEBP-seq1-treated pCCa-1 xenograft tissues (Figure 12E). TonEBP and SLC5A3 protein expression was decreased as well (Figure 12F). As a consequence, MI contents (Figure 12G) and Akt-S6K phosphorylation (Figure 12H) were reduced in TonEBP-silenced pCCa-1 xenograft tissues. These results together supported that silencing the transcription factor TonEBP downregulated SLC5A3 and inhibited pCCa-1 cervical cancer xenograft growth.
SLC5A3 KO suppresses cervical cancer xenograft growth. The koSLC5A3 pCCa-1 cells (with sg1) orCas9C control were s.c. injected to the nude mice, after 60 days pCCa-1 xenograft volumes (A) and weights (B) as well as animal body weights (C) were recorded. In the fresh pCCa-1 xenograft tissues listed mRNAs/proteins were measured (D, E, G and J); Myo-inositol (MI) contents (F), TBAR intensity (H) and SOD activity (I) were tested. *P < 0.05 versus “Cas9C” group.
Discussion
Human papillomavirus (HPV) vaccines, cervical cancer screening, and advances in diagnosis and adjuvant treatments have contributed to the decreased incidence of cervical cancer, especially in developed countries [3, 36, 37]. Currently, cervical cancer does not feature among the top 10 malignant tumors in the United States. However, in China and other developing countries, cervical cancer is the second most common tumor among women and is associated with high cancer-related mortality rates each year [3, 36, 37].
We hypothesized that SLC5A3 is an important oncogenic gene for cervical cancer. Bioinformatics analysis revealed that SLC5A3 is upregulated in cervical cancer tissues. The upregulated SLC5A3 expression was correlated with both poor survival and poor PFI. SLC5A3 upregulation was confirmed in clinical cancer tissues and different cervical cancer cells. SLC5A3 knockdown or KO suppressed cervical cancer cell viability, proliferation, and migration and induced cell death and apoptosis. In contrast, SLC5A3 overexpression promoted cancer cell proliferation and migration. Transduction with sh-SLC5A3 AAV constructs suppressed pCCa-1xenograft growth in nude mice. Similarly, SLC5A3 KO also inhibited pCCa-1 cervical cancer xenograft growth.
MI is used to treat reproductive and metabolic disorders [38]. Previous studies have demonstrated that MI decreases body mass index and increases insulin sensitivity in female patients with polycystic ovary syndrome [39]. Additionally, MI regulates steroidogenesis and modulates androgen and estrogen contents [39]. Lin et al. demonstrated that SLC5A3 is a key metabolic factor for AML using an in vivo CRISPR screening platform [40]. SLC5A3 is critical for AML cell proliferation. CRISPR-mediated SLC5A3 KO markedly suppressed AML orthotopic xenograft growth in vivo and induced apoptosis [40]. SLC5A3-induced MI import promotes AML cell proliferation [40]. Wei et al. demonstrated that MI promotes nutrient dependency in AML and that MI imported via SLC5A3 maintained AML cell proliferation [16]. This study demonstrated that cellular MI contents were upregulated in SLC5A3 knockdown/KO cervical cancer cells but were upregulated in SLC5A3-overexpressing cells. MI was also downregulated in sh-SLC5A3 AAV-injected or koSLC5A3 (sg1)-injected cervical cancer xenograft tissues. MI supplementation alleviated SLC5A3 KO-induced apoptosis and cell death in cervical cancer cells. Thus, SLC5A3-dependent MI import is critical for cervical cancer cell growth.
TonEBP silencing inhibits pCCa-1 cervical cancer xenograft growth. The nude mice bearing pCCa-1 xenografts were intratumorally injected with TonEBP shRNA-expressing AAV (“AAV-shTonEBP-seq1”) or AAV-shC, every 48h for 10 days; Weekly pCCa-1 xenograft volumes (A) and body weights of the mice (D) were recorded; Daily pCCa-1 xenograft growth was estimated (B). All pCCa-1 xenografts were isolated at Day-42 and xenograft weights were recorded (C). At Day-20, one pCCa-1 xenograft per group was isolated; Listed mRNAs/proteins (E, F and H) and myo-inositol (MI) contents (G) were tested. *P < 0.05 versus “AAV-shC” group.
Akt-mTOR cascade hyperactivation is a driving factor for cervical cancer growth and progression [33, 34, 41]. Various pharmacological inhibitors or monoclonal antibodies against the Akt-mTOR cascade-related factors exerted therapeutic effects on cervical cancer [33, 34, 41]. This study proposed that SLC5A3 is essential for Akt-mTOR signaling activation. SLC5A3 knockdown/KO significantly decreased the phosphorylation of Akt1 and S6K in primary cervical cancer cells. The Akt-mTOR cascade was inhibited in sh-SLC5A3 AAV-injected cervical cancer xenograft tissues and SLC5A3 KO xenograft tissues. In contrast, SLC5A3 overexpression upregulated the phosphorylation of Akt1 and S6K. The reactivation of Akt-mTOR by caAkt1 suppressed SLC5A3 KO-induced cervical cancer cell death. Thus, SLC5A3 mediates cervical cancer cell growth, at least partially, by promoting Akt-mTOR activation.
SLC5A3, which is widely expressed in different human tissues, is important for cellular osmoregulation [11]. Previous studies have reported that SLC5A3 regulates inflammatory cell infiltration during the progression of sporadic inclusion body myositis [29]. Zhou et al. revealed the potential anti-oxidant activity of SLC5A3 and demonstrated that SLC5A3 knockdown augmented oxidative stress in human neuroblastoma cells [31]. Long non-coding RNA NORAD upregulated SLC5A3 expression by sponging microRNA-204-5p and consequently alleviates oxidative injury and suppressed cell death in neuroblastoma cells [31]. The ROS and oxidative stress levels were upregulated in SLC5A3 knockdown cervical cancer cells and SLC5A3 knockdown/KO xenograft tissues. The anti-oxidant NAC suppressed SLC5A3 KO-induced cytotoxicity against cervical cancer cells. Thus, SLC5A3 depletion exerts anti-cervical cancer effects by inducing oxidative stress.
This study demonstrated that TonEBP is the key transcription factor for SLC5A3 in cervical cancer. TonEBP was the second top DEG that was positively correlated with SLC5A3 in TCGA cohort. The SLC5A3 mRNA and protein expression levels were downregulated upon TonEBP knockdown but were upregulated upon ectopic TonEBP overexpression in cervical cancer cells. The binding of TonEBP to the SLC5A3 promoter was upregulated in various cervical cancer tissues/cells and may contribute to SLC5A3 upregulation. TonEBP knockdown suppressed SLC5A3 expression and inhibited pCCa-1 tumor growth in vivo. Thus, TonEBP upregulates SLC5A3 expression and promotes cervical cancer cell growth.
Conclusion
The upregulated SLC5A3 promotes cervical cancer cell growth.
Supplementary Material
Supplementary figure data.
Acknowledgements
Funding
This work was generously supported by grants from Hospital-level disciplines-Chronic disease Group-Cervical Diagnosis and Treatment Centre and obstetrics Endocrinology (YJXK-2021-11).
Author contributions
All authors conceived, designed, and supervised the study, performed the experiments and analyzed the data, and wrote the paper. All authors reviewed and approved the final manuscript.
Ethical Approval and Consent to participate
This study was approved by the Ethics Committee of Minhang Hospital, Fudan University.
Availability of data and material
All data needed to evaluate the conclusions in the paper are present in the paper and the Supplementary Materials.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30
3. Arbyn M, Weiderpass E, Bruni L, de Sanjose S, Saraiya M, Ferlay J. et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8:e191-e203
4. Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet. 2019;393:169-82
5. Canfell K, Kim JJ, Brisson M, Keane A, Simms KT, Caruana M. et al. Mortality impact of achieving WHO cervical cancer elimination targets: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. 2020;395:591-603
6. Vora C, Gupta S. Targeted therapy in cervical cancer. ESMO Open. 2018;3:e000462
7. Crafton SM, Salani R. Beyond Chemotherapy: An Overview and Review of Targeted Therapy in Cervical Cancer. Clin Ther. 2016;38:449-58
8. Ferrall L, Lin KY, Roden RBS, Hung CF, Wu TC. Cervical Cancer Immunotherapy: Facts and Hopes. Clin Cancer Res. 2021;27:4953-73
9. Meijer C, Steenbergen RDM. Gynaecological cancer: Novel molecular subtypes of cervical cancer - potential clinical consequences. Nat Rev Clin Oncol. 2017;14:397-8
10. Duenas-Gonzalez A, Serrano-Olvera A, Cetina L, Coronel J. New molecular targets against cervical cancer. Int J Womens Health. 2014;6:1023-31
11. Mallee JJ, Atta MG, Lorica V, Rim JS, Kwon HM, Lucente AD. et al. The structural organization of the human Na+/myo-inositol cotransporter (SLC5A3) gene and characterization of the promoter. Genomics. 1997;46:459-65
12. Berry GT, Mallee JJ, Kwon HM, Rim JS, Mulla WR, Muenke M. et al. The human osmoregulatory Na+/myo-inositol cotransporter gene (SLC5A3): molecular cloning and localization to chromosome 21. Genomics. 1995;25:507-13
13. Raveau M, Lignon JM, Nalesso V, Duchon A, Groner Y, Sharp AJ. et al. The App-Runx1 region is critical for birth defects and electrocardiographic dysfunctions observed in a Down syndrome mouse model. PLoS Genet. 2012;8:e1002724
14. Berry GT, Wang ZJ, Dreha SF, Finucane BM, Zimmerman RA. In vivo brain myo-inositol levels in children with Down syndrome. J Pediatr. 1999;135:94-7
15. Andronic J, Shirakashi R, Pickel SU, Westerling KM, Klein T, Holm T. et al. Hypotonic activation of the myo-inositol transporter SLC5A3 in HEK293 cells probed by cell volumetry, confocal and super-resolution microscopy. PLoS One. 2015;10:e0119990
16. Wei Y, Huang YH, Skopelitis DS, Iyer SV, Costa ASH, Yang Z. et al. SLC5A3-Dependent Myo-inositol Auxotrophy in Acute Myeloid Leukemia. Cancer Discov. 2022;12:450-67
17. Cui Z, Mu C, Wu Z, Pan S, Cheng Z, Zhang ZQ. et al. The sodium/myo-inositol co-transporter SLC5A3 promotes non-small cell lung cancer cell growth. Cell Death Dis. 2022;13:569
18. Zhang J, Yin DP, Zhang Y, Zhang JN, Yang Y, Zhang ZQ. et al. Identification of Galphai3 as a novel molecular therapeutic target of cervical cancer. Int J Biol Sci. 2022;18:5667-80
19. Li G, Zhou LN, Yang H, He X, Duan Y, Wu F. Ninjurin 2 overexpression promotes human colorectal cancer cell growth in vitro and in vivo. Aging (Albany NY). 2019;11:8526-41
20. Liu YY, Chen MB, Cheng L, Zhang ZQ, Yu ZQ, Jiang Q. et al. microRNA-200a downregulation in human glioma leads to Galphai1 over-expression, Akt activation, and cell proliferation. Oncogene. 2018;37:2890-902
21. Yao J, Wu XY, Yu Q, Yang SF, Yuan J, Zhang ZQ. et al. The requirement of phosphoenolpyruvate carboxykinase 1 for angiogenesis in vitro and in vivo. Sci Adv. 2022;8:eabn6928
22. Zha JH, Xia YC, Ye CL, Hu Z, Zhang Q, Xiao H. et al. The Anti-Non-Small Cell Lung Cancer Cell Activity by a mTOR Kinase Inhibitor PQR620. Front Oncol. 2021;11:669518
23. Li SP, Ou L, Zhang Y, Shen FR, Chen YG. A first-in-class POLRMT specific inhibitor IMT1 suppresses endometrial carcinoma cell growth. Cell Death Dis. 2023;14:152
24. Lu X, Fan Y, Li M, Chang X, Qian J. HTR2B and SLC5A3 Are Specific Markers in Age-Related Osteoarthritis and Involved in Apoptosis and Inflammation of Osteoarthritis Synovial Cells. Front Mol Biosci. 2021;8:691602
25. Diez-Sampedro A, Hirayama BA, Osswald C, Gorboulev V, Baumgarten K, Volk C. et al. A glucose sensor hiding in a family of transporters. Proc Natl Acad Sci U S A. 2003;100:11753-8
26. Kong L, Hao Q, Wang Y, Zhou P, Zou B, Zhang YX. Regulation of p53 expression and apoptosis by vault RNA2-1-5p in cervical cancer cells. Oncotarget. 2015;6:28371-88
27. Kim HS, Yoon G, Ryu JY, Cho YJ, Choi JJ, Lee YY. et al. Sphingosine kinase 1 is a reliable prognostic factor and a novel therapeutic target for uterine cervical cancer. Oncotarget. 2015;6:26746-56
28. Bai X, Ma Y, Zhang G. Butein suppresses cervical cancer growth through the PI3K/AKT/mTOR pathway. Oncol Rep. 2015;33:3085-92
29. De Paepe B, Merckx C, Jarosova J, Cannizzaro M, De Bleecker JL. Myo-Inositol Transporter SLC5A3 Associates with Degenerative Changes and Inflammation in Sporadic Inclusion Body Myositis. Biomolecules. 2020 10
30. Vawter MP, Hamzeh AR, Muradyan E, Civelli O, Abbott GW, Alachkar A. Association of Myoinositol Transporters with Schizophrenia and Bipolar Disorder: Evidence from Human and Animal Studies. Mol Neuropsychiatry. 2019;5:200-11
31. Zhou S, Zhang D, Guo J, Chen Z, Chen Y, Zhang J. Long non-coding RNA NORAD functions as a microRNA-204-5p sponge to repress the progression of Parkinson's disease in vitro by increasing the solute carrier family 5 member 3 expression. IUBMB Life. 2020;72:2045-55
32. D'Oria R, Laviola L, Giorgino F, Unfer V, Bettocchi S, Scioscia M. PKB/Akt and MAPK/ERK phosphorylation is highly induced by inositols: Novel potential insights in endothelial dysfunction in preeclampsia. Pregnancy Hypertens. 2017;10:107-12
33. Bossler F, Hoppe-Seyler K, Hoppe-Seyler F. PI3K/AKT/mTOR Signaling Regulates the Virus/Host Cell Crosstalk in HPV-Positive Cervical Cancer Cells. Int J Mol Sci. 2019 20
34. Bahrami A, Hasanzadeh M, Hassanian SM, ShahidSales S, Ghayour-Mobarhan M, Ferns GA. et al. The Potential Value of the PI3K/Akt/mTOR Signaling Pathway for Assessing Prognosis in Cervical Cancer and as a Target for Therapy. J Cell Biochem. 2017;118:4163-9
35. Johnson ZI, Doolittle AC, Snuggs JW, Shapiro IM, Le Maitre CL, Risbud MV. TNF-alpha promotes nuclear enrichment of the transcription factor TonEBP/NFAT5 to selectively control inflammatory but not osmoregulatory responses in nucleus pulposus cells. J Biol Chem. 2017;292:17561-75
36. Falcaro M, Castanon A, Ndlela B, Checchi M, Soldan K, Lopez-Bernal J. et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. Lancet. 2021;398:2084-92
37. Brisson M, Kim JJ, Canfell K, Drolet M, Gingras G, Burger EA. et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. 2020;395:575-90
38. Dinicola S, Unfer V, Facchinetti F, Soulage CO, Greene ND, Bizzarri M. et al. Inositols: From Established Knowledge to Novel Approaches. Int J Mol Sci. 2021 22
39. Fruzzetti F, Perini D, Russo M, Bucci F, Gadducci A. Comparison of two insulin sensitizers, metformin and myo-inositol, in women with polycystic ovary syndrome (PCOS). Gynecol Endocrinol. 2017;33:39-42
40. Lin S, Larrue C, Scheidegger NK, Seong BKA, Dharia NV, Kuljanin M. et al. An In vivo CRISPR Screening Platform for Prioritizing Therapeutic Targets in AML. Cancer Discov. 2022;12:432-49
41. Zagouri F, Sergentanis TN, Chrysikos D, Filipits M, Bartsch R. Molecularly targeted therapies in cervical cancer. A systematic review. Gynecol Oncol. 2012;126:291-303
Author contact
![]() Corresponding authors: Dr. Ling Xu (xu_lingedu.cn); Baohua Yang (baohuayangcom); Fang-rong Shen (shenfredu.cn); Lifeng Wang (wanglifengcom).
Corresponding authors: Dr. Ling Xu (xu_lingedu.cn); Baohua Yang (baohuayangcom); Fang-rong Shen (shenfredu.cn); Lifeng Wang (wanglifengcom).

 Global reach, higher impact
Global reach, higher impact