10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2023; 19(11):3383-3394. doi:10.7150/ijbs.82556 This issue Cite
Review
Interplay Between the Immune and Nervous Cognitive Systems in Homeostasis and in Malaria
1. Laboratório de Pesquisa em Malária, Instituto Oswaldo Cruz & Centro de Pesquisa, Diagnóstico e Treinamento em Malária (CPD-Mal) from Fundação Oswaldo Cruz (Fiocruz) and the Secretaria de Vigilância em Saúde (SVS), Ministério da Saúde, Brazil.
2. Laboratório de Biologia, campus Duque de Caxias, Colégio Pedro II, Brazil.
Received 2023-4-20; Accepted 2023-5-17; Published 2023-6-28
Abstract
The immune and nervous systems can be thought of as cognitive and plastic systems, since they are both involved in cognition/recognition processes and can be architecturally and functionally modified by experience, and such changes can influence each other's functioning. The immune system can affect nervous system function depending on the nature of the immune stimuli and the pro/anti-inflammatory responses they generate. Here we consider interactions between the immune and nervous systems in homeostasis and disease, including the beneficial and deleterious effects of immune stimuli on brain function and the impact of severe and non-severe malaria parasite infections on neurocognitive and behavioral parameters in human and experimental murine malaria. We also discuss the effect of immunization on the reversal of cognitive deficits associated with experimental non-severe malaria in a model susceptible to the development of the cerebral form of the illness. Finally, we consider the possibility of using human vaccines, largely exploited as immune-prophylactics for infectious diseases, as therapeutic tools to prevent or mitigate the expression of cognitive deficits in infectious and chronic degenerative diseases.
Keywords: immune system, nervous system, homeostasis, malaria, neurocognitive impairment.
Introduction
Both the immune and nervous systems perform cognitive tasks (“to know and to recognize”) by mobilizing processes endowed with specificity and memory and may, therefore, be considered “cognitive systems” [1-5]. Such systems organize themselves functionally and structurally, establishing new cellular connections in response to stimuli [1-3]. Following an antigenic or sensory experience, organisms begin to differ in relation to their previous arrangement, not only in their experiences and skills but also in their structures [1-3]. Cognitive systems can, thus, be characterized as plastic arrangements, and animals endowed with nervous and immune systems may be thought of as “cloneable organisms”, but not as “cloneable beings” [6].
The immune and nervous systems interact closely and constantly, and the influence of nervous system stimuli on the immune response, as well as the contribution of immune components to the homeostasis of cognitive function, are well documented and illustrative of this interplay [4,7-10]. The evidence for the nervous system's influence on the quality and fate of immune responses will not be considered further here.
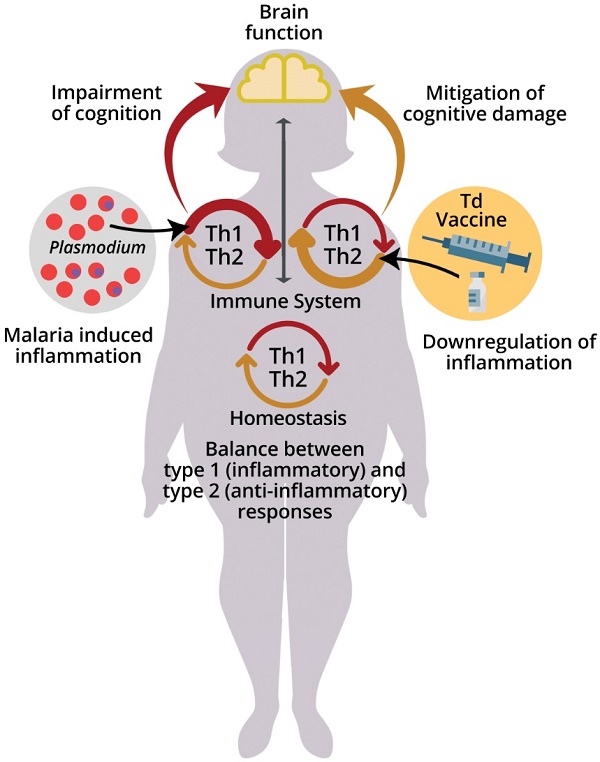
The immune system acquires new experiences (immunological learning) through contact with infectious or non-infectious foreign agents and their antigens or through active immunization [11]. Different stimulus strategies and immune responses can affect central nervous system (CNS) function in different ways (Fig. 1) [5,9,12-15].
Effects of malaria and immune system stimuli on the nervous system performance. Immune events are involved in brain function and neurocognitive homeostasis. T cells and microglia are classically described as important for the maintenance of neurogenesis in the hippocampus, being associated with the capacity for learning and spatial memory in adulthood [7]. The immune system influence is dependent upon the nature and intensity of the (activating or suppressing) stimuli it applies on the nervous system, benefitting or impairing its function. Infectious agents, such as malaria parasite, while promoting immune learning, may also exert signals perceived as disruptive to the immune response, differently from the positive properties of non-infectious stimuli, such as vaccines inducing type 2 immune responses. High levels of cytokines in the peripheral circulation and intracerebroventricular space, such as Interferon (IFN) and Tumor Necrosis Factor (TNF), produced by T cells in response to red blood cell infection (pRBC) and rupture, can impair the brain function and the cognitive ability [49]. On the other hand, the presence of T cells that produce anti-inflammatory cytokines, such as the interleukin 4 (IL-4), has been associated with improved cognitive ability and learning through the induction of brain-derived neurotrophic factor (BDNF) expression by astrocytes [12]. Neurocognitive impairment (learning and memory deficits and anxiety-like behavior) caused by a single episode of non-severe experimental murine malaria can be attenuated or even avoided by exposure to anti-inflammatory immune stimuli that included the largely used diphtheria tetanus human vaccine (Td) [123]. Increased numbers of regulatory T cells (Treg) in the spleen and interleukin 10 (IL-10) levels in the peripheral circulation observed in experimental studies may be involved in such effect [24].
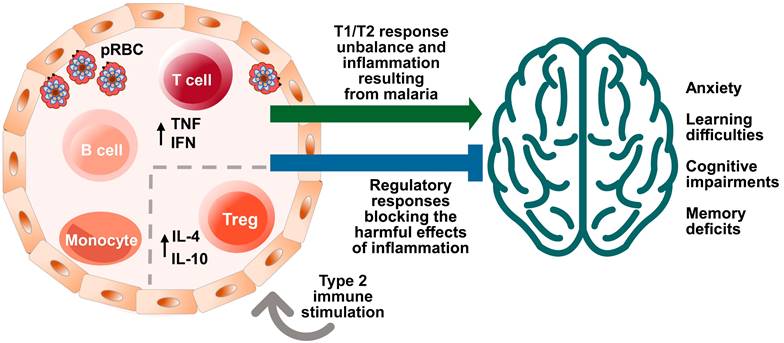
T cells and the proteins they produce play a critical role in regulating the immune response and influencing brain function [9]. The impact of immune stimuli on the nervous system can vary depending on their nature and context [9]. In general, proinflammatory immune responses are believed to have a negative effect on cognition, while anti-inflammatory immune responses may be protective or beneficial [9,12,13,15]. For example, cytokines, which function like neurotransmitters, can modulate synaptic activity and affect cognitive ability in the quad-partite synapse model (involving pre-synaptic and post-synaptic neurons, astrocytes and microglia). Furthermore, T cells can protect the nervous system from neuronal degeneration, and the IL-4 cytokine has been shown to maintain the M2 profile of meningeal myeloid cells and regulate astrocytic activity in brain-derived neurotrophic factor (BDNF) production, thus improving cognitive performance related to learning and memory [9,12,13,15].
Infectious diseases may manipulate or disrupt the immune response and have been associated with neurocognitive deficits [16]. Malaria, toxoplasmosis, leishmaniasis and other protozoan diseases can cause neurocognitive impairment and/or anxiety-like behavior in humans and/or in murine experimental models [17-24].
Malaria, a disease caused by parasites of the genus Plasmodium and transmitted through the bite of female Anopheles mosquitoes, is one of the main public health problems in the world [25]. Eight species of Plasmodium are now known to cause disease in man: P. malariae, P. vivax, P. falciparum, P. ovale curtisi and P. ovale wallikeri [26], P. knowlesi [27] and, as recently demonstrated, P. cynomolgi [28] and P. simium [29]. Although the latter three are to be classified as non-human primate parasites that can cause zoonotic infections in humans, it became recently clear that other species of Plasmodium can cause malaria in humans [30,31].
Malaria can cause cognitive-behavioral impairment, especially, but not exclusively, in its brain-injurious form (cerebral malaria, CM), in humans and mice [17,22-24,32,33].
This review focuses on the effect of the immune system on the nervous system and the influence of malaria parasite infection on neurocognitive performance. We consider CM as well as the non-severe form of the disease in both humans and mice.
Interaction between the immune and nervous cognitive systems in homeostasis
Both the immune and the nervous systems exhibit innate and adaptive (learned) responses. Innate responses of the nervous system are genetically controlled and linked to the physical structure of the brain and the CNS, while adaptive responses result from the interaction between experience and neuronal plasticity. Innate behavior refers to condition (structure, vocation and instinct), while adaptive behavior refers to learning resulting from experiences and living, which can include different types of learned behavior, such as habituation, imprinting, and classical and operant conditioning [34,35]. Both rely on neural circuits (that can be "overlapping", if they share some common neurons or pathways; "shared", if they involve the same neurons or pathways between different behaviors or processes; or "evolutionarily common", if they have been conserved across different species over time) being excited by intrinsic and extrinsic stimuli [36]. Therefore, we can consider that attributes traditionally considered characteristic of the immune system may also be true for the nervous system, such as the ability to change its cellular and biomolecular structures and organizations as they are stimulated [36].
In addition to being strategically and operationally similar, the immune and nervous systems are closely linked both in structural and functional terms, sharing molecular mechanisms and cellular ligands and receptors [37].
The fundamentals of “classical neuroimmunology” such as the existence of neuropeptide receptors on the lymphocyte membrane and of cytokine receptors on neurons, microglia and astrocytes, have been extensively described [14,38-41]. More recently it has been shown that, in addition to T lymphocytes, immune cells such as B lymphocytes, macrophages and dendritic cells express specific neurotransmitter receptors that affect the function of these cells upon receipt of information from the nervous system [37].
It is not surprising, therefore, that immune events influence brain function and neurocognitive homeostasis. CD4 T lymphocytes and microglia are important for the maintenance of neurogenesis in the hippocampus and are associated with the capacity for learning and spatial memory in adulthood [7].
More recently, a “brain super autoantigens theory” has been proposed, postulating autoimmunity as a physiological process naturally involved in brain function. According to this theory, the driving agents of cognitive evolution are pro-cognitive T cells reactive to CNS autoantigens [10]. Neurons expressing specific immunogenic antigens stimulate a repertoire of cognitive-promoting autoimmune T cells, and, as a result, the generation and diversity of brain autoimmune T cells can drive selective pressure on the neural genes that compile brain super autoantigens. Such reciprocal pressure stimulates neurocognition - formed by neurons, synapses and non-neuronal cells (such as glial cells) - orchestrating cognitive competence and resulting in the coevolution of both systems [10].
The reversal of cognitive deficits in adult mice and neonate mice lacking lymphocytes by adoptive transfer of T and lymphoid cells, respectively, illustrates the importance of adaptive immunity in brain maturity and cognitive ability [42,43]. Likewise, acute and peripheral T cell depletion, induced by the administration of FTY720 [a compound that promotes the internalization of the sphingosine 1-phosphate receptor 1 and, consequently, the sequestration of thymocytes in lymph nodes [44,45], results in impaired learning and memory in mice [12].
The nervous system was considered for many years an immune privileged site, with the exception of microglia, any immune signal in the parenchyma was considered to be the hallmark of pathology [4]. When T cells were first suggested as modulators of cognitive performance, questions were raised concerning the mechanisms that drive them to the brain compartment [46]. It is possible that T cells penetrate the CNS parenchyma, but rarely and for very short periods of time [46]. They may also affect the CNS through the release of cytokines into the bloodstream, penetrating the blood-brain barrier (BBB) by volume diffusion, transport systems [47] or CD4 T cells trafficking across the choroid plexus and meninges [8]. Another pathway proposed for the entry of immune cells and their products into the brain is via the circumventricular organs (CVOs) [48,49]. Brain-resident CD4 T cells have been identified in mice and humans and may be required for the maturation of microglia, proper synaptic pruning and behavior [50].
It is estimated that human cerebrospinal fluid (CSF) contains up to 500,000 T cells, the majority being memory cells (CD45RO+) [12,51] with the ability of returning to the lymph nodes, as suggested by their expression of CCR7 and L-selectin [51,52]. These cells are separated from the brain parenchyma by the pia mater and appear to influence and be influenced by events in the brain [9].
In addition to T cells, other immune components, such as B lymphocytes, granulocytes, macrophages, mast cells and dendritic cells, are present in the “meningeal structures” of the brain bathed by the CSF [12,8,53]. Thus, suppression or deprivation of T cells can promote a pro-inflammatory phenotype in myeloid cells such as monocytes and granulocytes, with induction of high levels of IL-1β, IL-12 and TNF-α in the peripheral circulation and in the intracerebroventricular space, potentially triggering impairment of brain function and cognitive ability [49]. The anti-inflammatory cytokine IL-4 is associated with cognitive ability by inducing the expression of BDNF by astrocytes, a factor fundamental for the maintenance of neurocognitive ability [12]. Thus, IL-4 maintains the M2 phenotype of meningeal myeloid cells and regulates the expression of BDNF by neuroglial cells [12].
Immune responses can trigger heterogeneous effects on brain functionality in experimental models
Stimulation of the immune system can have varying effects on cognitive function [54]. Studies aimed at assessing the effect of immune stimuli on brain function report: i) the influence of neonatal vaccination on neuronal plasticity and cognitive function under normal physiological conditions in adulthood [55]; ii) the impact of mitogenic and/or inflammatory stimulation of the immune system on the cognitive function of adult mice [56] and neuronal activation [57]; iii) the effect of maternal immune stimulation on the neurocognitive performance of offspring [58]; and iv) the increased risk of neurodegenerative disease development due to inflammatory process [59,60].
Immunization of C57BL/6 neonate mice with the BCG vaccine was shown to increase locomotivity as well as the distance travelled in the center of an open field and to improve spatial memory performance in the Morris water maze test. This was associated with increased peripheral and brain levels of IFN-γ and IL-4, neurogenesis and hippocampal BDNF, suggesting a beneficial immunomodulatory effect of immunization on cognitive function [55,61]. Additionally, activation of an anti-inflammatory state of microglia and improvements of cognitive ability have been reported in C57BL/6 mice [62] and rats [54] immunized with BCG.
Also, the study of Brombacher et al. reinforced the importance of Th2 cytokines (IL-13 and IL-4) in the cognitive function of BALB/c mice challenged with the helminth Nippostrongylus brasiliensis, demonstrating the involvement of these cytokines in the stimulation of BDNF production by meningeal and hippocampal astrocytes [15].
IL-4 knockout mice and those in which IL-4-producing T cells are depleted exhibit meningeal myeloid cells with pro-inflammatory phenotype and are cognitively impaired [12]. These data demonstrate a role for T cell derived IL-4 in the regulation of the pro- and anti-inflammatory phenotypes of meningeal myeloid cells and consequently in the expression of BDNF in the brain and the homeostasis of cognitive function [13].
Still on the beneficial effect of anti-inflammatory immune responses on the nervous system, it was reported that the non-inflammatory interleukin 17, IL-17, secreted by meningeal γδ T cells, is a promoter of short-term memory in mice [63].
Adverse effects of immune stimuli on cognitive function have also been demonstrated. Th1 cytokines activate neuroinflammatory processes and have a negative effect on brain function. Yang et al. [55] showed that neonate C57BL/6 mice immunized with the hepatitis B (HBV) vaccine performed poorly in the elevated plus maze and the Morris water maze. The mice had elevated levels of TNF-α, reduced levels of IFN-γ and demonstrated decreased neurogenesis and BDNF in the hippocampus at the eighth week of life. This suggests that neonatal HBV vaccination may result in detrimental behavioral effects in early adulthood. These data are corroborated by a study by Li et al. [54] who reported similar observations in rats. Inoculation of lipopolysaccharide (LPS) in C57BL/6 mice also increases levels of pro-inflammatory cytokines, mainly TNF-α and IL-1β, and induces infiltration of immune cells into the hippocampus, increased myeloperoxidase activity in the cortex and hippocampus and increased immunoreactivity of microglia and astrocytes, leading to neuronal damage and memory loss [64]. Behavioral deficiencies related to memory, decreased locomotivity and anxiety-like behavior have been observed 10 months post LPS injection in Wistar rats [65]. Even inoculation of minimum doses of LPS can cause spatial memory deficiencies in the Y-maze test in C57BL/6 mice [56].
More recently, experimental studies in mice have demonstrated that peripheral immune challenges with dextran sulfate sodium, which induce inflammatory bowel disease, can influence the activity of the insular cortex - a crucial cortical region involved in the perception of the body's physiological condition [66]. Such an immune challenge is associated with activation of neurons in the insular cortex. Furthermore, neuronal reactivation was capable of reactivating the peripheral immune response even in the absence of immune challenges, leading to an increase in the percentage of leukocytes, the proportions of activated CD4 and CD8 T cells and dendritic cells, and the levels of TNF-α, IL-6 and IL-17 in intestinal compartments. These findings suggest that the insular cortex receives information from peripheral neurons responsive to signals about peripheral immune status and is an important brain region for the recovery of specific immune responses [57].
Murine models of maternal immune activation are widely used to study cognitive deficiencies (memory) and intrinsic changes involved in depressive- and anxiety-like behavior linked to neurodevelopmental alterations, and it is now known that stimuli that induce increased levels of pro-inflammatory cytokines in pregnant mice can cause neurocognitive deficits in the offspring. Although not demonstrated by Brabi et al. [67] at gestational day (GD) 17, the challenge of pregnant C57BL/6 mice at GD 9 with LPS causes anxiety and depression related behavior (revealed by elevated plus maze, open field, light-dark, forced swimming and tail suspension tasks) in the offspring at 8 and 10 weeks of age [68]. In C57BL/6 pregnant mice immunized with Poly (I:C), increased levels of IL-1β were observed in the hippocampus of the offspring, a cytokine related to behavioral disorders [69]. Under this immunization scenario, it has been reported that maternal pathway of Th17 cells and IL17A cytokine can induce autism-like phenotype in offspring [70]. Additionally, Mueller et al. [58] reported deficiencies in working memory and changes in social behavior in the adult offspring at 9-12 weeks. The effects of maternal immune stimulation on the neurodevelopment of offspring in murine experimental models were recently systematized by Woods et al. [71].
One particular detrimental effect of maternal immune activation may be associated with exacerbation of Th2 cytokines levels. C57BL/6 mice desensitized with ovalbumin (OVA) present attenuated allergic asthma when challenged intranasally. Female C57BL/6 mice exposed to aerosolized OVA before and during pregnancy and their offspring showed social interaction deficiencies and increased object-burying behavior when compared to the offspring of mothers not exposed to OVA [72]. Using this same model, it was shown that chronic OVA-induced asthma causes learning and memory deficiencies as assessed by the Morris water maze test [73]. Similarly, neonatal overexposure to IL-4 is associated with neuroinflammation and cognitive impairment in C57BL/6 mice [74]. Such negative effects of IL-4 on cognitive-behavioral performance were also observed following neonatal HBV vaccination [55].
Events characteristic of the inflammatory process such as induction of cytokines and activation of immune cells, as well as dysfunctions of the immune system can increase the risks of development of neurodegenerative diseases such as multiple sclerosis and Parkinson's disease [10,59,60]. Some reports support the "theory of depression induced by cytokines", by Maes [75], demonstrating that depressive disorders, in the absence of somatic comorbidities, such as heart disease and obesity, may be associated with increased concentrations of pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6 in the CNS and periphery [76-79]. These inflammatory mediators may impact the cognitive functioning of individuals causing impairment of learning and memory, loss of visual and spatial perception abilities, loss of verbal and executive fluency, attention deficits, and inhibition of reaction, planning and problem solving - effects seen even in mild cases of depression [78].
As described earlier, peripheral immune challenges inducing pro-inflammatory immune responses may be associated with alterations in brain function, triggered by neurochemical and neuroinflammatory events that can negatively impact brain plasticity and cognitive ability. These changes include the reduction of important neurotrophins for cognition, such as BDNF, as well as decreased neurogenesis.
Plasmodium infection can cause neurocognitive impairment in humans
Given the above, changes in physiological neuroimmune communication as a result of the immune response triggered by infectious diseases such as malaria, may be expected to result in cognitive changes.
Around 92% of malaria cases in the world are due to P. falciparum; 1 to 2% of which progress to CM, which causes approximately 80% of the deaths due to malaria [80]. In humans, the main cellular event of CM is the sequestration of red blood cells, mostly parasitized, platelets and leukocytes in cerebral blood vessels, triggering microvasculature obstruction, inflammation and petechial hemorrhage in the brain and cerebellum [81]. The onset of CM can be gradual or sudden and the condition can evolve from simple headache to deep coma within a couple of hours in children and non-immune adults. Adult patients with CM may present symptoms that mimic those of acute delirium, brain abscess, hypertensive encephalopathy, viral encephalitis, epilepsy, heat stroke, intoxication with drugs and poisons and bacterial, fungal or protozoan meningoencephalitis [82-84].
Approximately 15-25% of individuals diagnosed with CM die, even with prompt treatment with artemisinin derivatives that promote a very sharp decline in parasitemia, and survivors may have neurocognitive sequelae [85]. CM is, therefore, a subject of great interest in malariology [22,32].
Neurocognitive sequelae of CM can occur in the short or long-term and may include ataxia, hemiparesis, monoparesis, hemiplegia, severe motor deficit, dysphasia, behavioral difficulties, severe learning difficulties and visual, auditory or linguistic impairment, and even epilepsy [86]. In addition to these, hyperactivity and aggressive, self-injurious or destructive behaviors, anxiety and depression have also been reported [87-89].
Several studies indicate the existence of long-term neurocognitive sequelae that may lead to childhood cognitive impairments, especially (but not only) in Africa, where CM and its complications are more prevalent, particularly among children under five years old [22,32,80,86,88,90-95].
Severe neurological manifestations, such as coma [96], as well as levels of TNF-α, IL-6, IL-8 and granulocyte colony stimulating factor (G-CSF) [32], kynurenine and kynurenic acid [97] and angiopoietin-2 [98] are increased in the CSF being associated with persistent neurocognitive sequelae of Ugandan child recovering from CM. High levels of Tau proteins in CSF and plasma are also associated with neurocognitive impairment related to attention, associative memory and working memory following CM [93,99]. These proteins are equally associated with malaria retinopathy [94], and with dementia in Alzheimer's disease [100].
There is, furthermore, a clear relationship between episodes of malaria and reduced cognitive function associated with memory and learning in non-severe forms of malaria [18,95,101-104]. In Sri Lanka, for example, where the most prevalent species were P. falciparum and P. vivax, children with a history of more than five episodes of non-severe malaria performed less well in writing, language and mathematics than those with a history of three or less episodes. Secondary factors such as socio-economic and nutritional status of the children were also considered, but the main factor influencing the academic performance of these children was the number of previous malaria episodes [101].
In Zambia, exposure of children under five years of age to Plasmodium infection has a negative effect on pre-school and educational development, indicating that exposure to the parasite exerts an impact not only on children's health, but on their cognitive development as well [103].
The first evidence of an association between non-severe malaria and learning impairments in Latin America was reported by Vitor-Silva et al. [102] who observed reduced performance in school tests (final average in Portuguese and mathematics) after at least one non-severe P. falciparum and/or P. vivax infection, in children in the Brazilian Amazon. Cognitive impairment, assessed by an indicator of intellectual function, Intelligence-IV (WPPSI-IV) which measures parameters such as verbal comprehension and working memory, was reported in children between 2 and 7 years old who had suffered at least one P. vivax malaria episode compared to children who had never had malaria [104]. More recently it was reported that acute P. vivax malaria may be associated with long-term impairment of executive and cognitive functions in the elderly [105].
Evidence of neurocognitive impairment related to motor ability and fitness in the TEA-Ch test (Everyday Attention Test for Children) was found in individuals infected with Plasmodium but asymptomatic for malaria in the Republic of Yemen and Uganda, respectively, where P. falciparum is the most prevalent species [106].
In addition to the aforementioned sequelae, retinopathy - although rarely documented when compared to P. falciparum malaria [107] - is a possible sequela of non-severe P. vivax malaria.
Plasmodium infection can cause neurocognitive impairment in mice
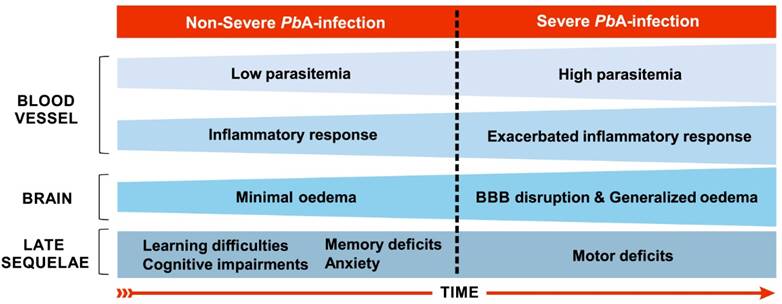
In the murine experimental model of CM (ECM), involving the infection of C57BL/6 mice with the ANKA strain of Plasmodium berghei (PbA), the evolution and establishment of ECM occurs between the fifth and sixth days of infection [108] and is characterized by the accumulation of leukocytes, mainly T cells and macrophages, in the cerebral microvasculature [81], although parasitized red blood cells may also be observed in the cerebral microenvironment [109]. Consistent with CM in humans, hemorrhage and inflammation also occur with a consequent breakdown of the BBB (Fig. 2), causing brain damage in regions that are important for cognitive performance such as the cortex and hippocampus [110].
The initial neuropathogenesis of C57BL/6 mice infection with PbA occurs in the olfactory bulb where the early expression of chemokines, especially CCL21, on the third day after infection, could explain the impaired smelling function recorded from the fourth day on, as assessed by the buried food test [111]. Potter et al. [108] describe the kinetics of histopathological events associated with ECM development in this murine model. On the fourth day of infection low numbers of leukocytes are recorded adhering to vessels in the brain, along with low parasitaemia and minimal edema. On the fifth day of infection, such events appear throughout the whole brain and an increase of the parasitemia occurs. Generalized and severe hemorrhage, edema and adherence of leukocytes throughout the brain occur on the sixth day of infection.
The pathophysiological processes of ECM are dependent on the host's immune response. Howland et al. [112] considered CD8 T cells to be the main mediators of death in ECM. Approximately 90% of lymphocytes sequestered in the brain express CXCR3, associated with type 1 immune responses and inflammation, and mice deficient in this chemokine receptor have reduced traffic of CD8 T cells to the brain and 70-90% protection against the development of ECM. There is a strong association between the expression of this receptor and migration of T cells to the organs and the development of ECM [113].
In summary, recruitment of CD8 T cells to the brain results in increased expression of pro-inflammatory cytokines such as IFN-γ, TNF-α and chemokines that promote the activation of cerebral microvasculature endothelial cells and expression of receptors and ligands that favor leukocyte adhesion [114].
The neurological syndrome of ECM is characterized by paralysis, ataxia, convulsions, and coma [82]. Animals that survive this syndrome experience neurocognitive sequelae such as motor impairments and memory-related disorders, apparent both immediately and 30-40 days after infection [17,22,33,95,115]. Anxiety-like behavior has also been reported on the fifth day of infection [116], but may not represent a behavioral sequela, since animals were assessed during an active infection.
Although observed in the short or medium term after non-severe P. falciparum and P. vivax malaria, cognitive impairments have not been recorded in the experimental murine models considered standard for the study of non-severe malaria, such as BALB/c, C57BL/6 and Swiss mice infected by PbA, P. chabaudi chabaudi and P. yoelii 17NL, respectively [17,116]. These animals were evaluated using behavioral tasks such as open field and novel object recognition test, which analyze short-term and long-term memory.
More recently, however, de Sousa et al. [23] reported cognitive-behavioral impairments related to long-term memory and anxiety-like behavior late after a single episode of non-severe experimental malaria (Fig. 2), if the model susceptible to the development of ECM - C57BL/6 mice infected with PbA - is used, with mice treated before the appearance of any sign of neurological impairment. These data suggest that the use of the PbA infection model, with treatment prior to the appearance of the clinical signs of ECM, may create an experimental condition that somehow simulates the cases of non-severe P. falciparum human malaria that can potentially evolve to CM. The data of de Sousa et al. [23] also seem to indicate that even if signs of neurological impairment are not immediately measurable, malaria parasite infection may induce early changes in the physiology of the brain indirectly through the dissemination of inflammatory mediators released during systemic inflammation. For this reason, we have been using the expression “non-severe malaria” or even non-complicated malaria, instead of “non-cerebral malaria”, to describe the early events of PbA infection in C57BL/6 mice [23,24,95].
Table S1 illustrates studies reporting behavioral changes studied in PbA infected C57BL/6 mice, indicating the brain anatomic regions related to the neurocognitive impairment in the referred experimental model or found affected in the quoted study. The reported changes have been observed during infection (both severe and non-severe) and/or after the end of treatment, being of short or long-term duration.
Main features of severe and non-severe malaria in C57BL/6 mice infected by Plasmodium berghei ANKA. On the fourth day of infection, when the disease can still be characterized as a non-severe malaria, low inflammatory response (few leukocytes adhered to blood vessels) in the brain are recorded, along with low parasitemia (about 2.5%) and oedema [108]. Between the fifth and sixth days of infection, murine cerebral malaria may start to establish. On the fifth day, such events expand throughout the brain and an increase in parasitaemia occurs [108]. Although parasitized red blood cells can also be observed in the brain microenvironment [109], there is a predominance of leukocyte accumulation, mainly T cells, in the cerebral microvasculature [81]. Generalized and severe bleeding, elevated levels of edema and leukocyte adhesion throughout the brain, and damage to the BBB occur from the sixth day of infection on [108]. Long-term cognitive and behavioral deficits, such as learning and memory difficulties and anxiety-like behavior, are observed as sequelae using this model of infection, including in mice treated the day before the onset of cerebral malaria establishment, the fourth day of infection [23,24]. These sequelae are also evident after cerebral malaria along with motor system impairment [17,33].
Re-establishment of neurocognitive function by immune responses in mice
Immunization procedures can reverse disease-associated neurocognitive deficiencies. Zuo et al. [117] observed a beneficial effect of BCG vaccination in the murine model of Alzheimer's Disease (AD). Immunization promoted an increase in levels of IL-4, IL-10, TGF-β1 and neurotrophic factors in the brain. This procedure did not decrease the concentration of β-amyloid (Aβ) in the brain, but it was able to induce a beneficial immunomodulatory effect by attenuating cognitive deficits as measured by the Morris water maze test. Using the same experimental model and behavioral assessment, Xing et al. [118] reported that immunization with a DNA vaccine (encoding ten tandem repeats of Aβ3-10 fused with mouse IL-4), performed when AD was already established in the mouse, reduced cerebral inflammation and attenuated cognitive impairment.
An increase in the Aβ peptide is seen in Down Syndrome together with cognitive difficulties. In the experimental model of segmental trisomy 16 [119], Belichenko et al. [120] reported improvements in cognitive ability after immunization of adult Ts65Dn mice with the Aβ peptide. Immunization minimally reduced Aβ levels in the brain, but significantly restricted the atrophy of cholinergic neurons and improved short-term memory.
Anxiety-like behavior shares some characteristics with depressive behavior, such as a reduction of BDNF and impairment of neurogenesis in the hippocampus. Lewitus et al. [121] investigated the impact of immunization with a myelin-derived peptide in rats with depressive behavior and found that immunization restored levels of BDNF and neurogenesis in the hippocampus and attenuated anxious and depressive behavior.
Recently, de Sousa et al. [24] reported a beneficial modulatory effect of stimuli with immunogens able to induce type 2 immune responses having as one of its components the Td vaccine used in humans, on cognition of healthy adult C57BL/6 mice. These stimuli were able to reverse the cognitive-behavioral deficiencies characteristic of PbA infection in which mice are chloroquine treated before the onset of ECM [23] (Fig. 1). This reversal effect of cognitive-behavioral damage was also observed when using only the Td vaccine as immunogen [122]. These results offer an additional potential approach for improving cognition and for recovering cognitive behavioral damage caused by chronic or infectious diseases, including malaria, as we have recently proposed [123].
Conclusions
The immune and nervous systems are interrelated cognitive systems that interact both in homeostasis and in abnormal conditions. Exogenous stimuli such as infectious agents and vaccines stimulate the immune system, modifying it structurally and operationally. Depending on the nature of the immune responses they trigger, stimuli can heterogeneously impact the functioning of the CNS and consequent cognitive behavioral responses. The studies discussed here demonstrate both the benefits and harm that may be exerted by the immune response on neurocognitive performance. Severe and even non-severe malaria can result in memory deficiencies and anxiety-like phenotypes in humans and mice. Immune-stimulation of P. berghei ANKA infected and treated mice can reverse these sequelae. Further studies on the interplay between the immune and nervous systems in infectious and chronic degenerative diseases and on the properties and composition of the immune stimuli capable of influencing neurocognitive parameters are needed. A better understanding of how these immunomodulatory agents operate to improve neurocognitive behavioral performance may open up a range of possibilities for the use of vaccines beyond their classic use as infectious disease prophylactics.
Supplementary Material
Supplementary table S1.
Acknowledgements
LPS and PRG are grateful to the Postgraduate Program in Parasite Biology (PPBP) of the Instituto Oswaldo Cruz, Fiocruz, for the support, and to the Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior (Capes) for doctoral scholarships. LPS also thanks the Fundação Carlos Chagas Filho de Apoio à Pesquisa do Rio de Janeiro (Faperj) for a PhD fellowship. The authors are grateful to Professor Richard Culleton (Ehime University, Ehime, Japan) for carefully reading the final version of this review manuscript and for the valuable comments and suggestions and Fernando Vasconcelos for the final versions of figures and graphical abstract. FLRG and CTDR are supported by CNPq, Brazil, through a Productivity Research Fellowship and CTDR is a “Cientista do Nosso Estado” by Faperj. The Laboratório de Pesquisa em Malária (IOC, Fiocruz) is an Associated Laboratory of the Instituto Nacional de Ciência e Tecnologia (INCT) in Neuroimmunomodulation supported by the CNPq and also of the Faperj Neuroinflammation Network.
Author contributions
The text of this manuscript originated in a theoretical chapter of the PhD thesis of LPS, who wrote the draft of this review article. LPS participated in all stages of designing the review article, as well as in the discussions of ideas for drawing up figures and tables. PRG participated in the revision of the text, after draft correction, adding new information and preparing the table. FLRG participated in all stages of discussion, correction and revision of the text since the draft and creation of figures. CTDR (LPS's PhD thesis supervisor) is responsible for draft, text after draft, figures and table correction and finalizing the manuscript review together with LPS, PRG and FLRG. All authors read, reviewed and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Jerne NK. Idiotypic networks theory and other preconceived ideas. Immunol Rev. 1984;79:5-24 doi: 10.1111/j.1600-065x.1984.tb00484.x
2. Cohen IR. The cognitive paradigm and the immunological homunculus. Immunol Today. 1992;13(12):490-4 doi: 10.1016/0167-5699(92)90024-2
3. Cohen IR. The cognitive principle challenges clonal selection. Immunol Today. 1992;13(11):441-4 doi: 10.1016/0167-5699(92)90071-E
4. Kipnis J. Multifaceted interactions between adaptive immunity and the central nervous system. Science. 2016;353(6301):766-71 doi: 10.1126/science.aag2638
5. Nutma E, Willison H, Martino G, Amor S. Neuroimmunology - the past, present and future. Clin Exp Immunol. 2019;197(3):278-293 doi: 10.1111/cei.13279
6. Barcinski MA. Prefácio. In: Imagens, Micróbios e Espelhos: os sistemas imune e nervoso e nossa relação com o ambiente, 1ed. Rio de Janeiro: Fiocruz. 2017;3:161-162
7. Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N. et al. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9(2):268-75 doi: 10.1038/nn1629
8. Kivisäkk P, Imitola J, Rasmussen S, Elyaman W, Zhu B, Ransohoff RM. et al. Localizing central nervous system immune surveillance: meningeal antigenpresenting cells activate T cells during experimental autoimmune encephalomyelitis. Ann Neurol. 2009;65(4):457-69 doi: 10.1002/ana.21379
9. Kipnis J, Gandini S, Derecki NC. Pro-cognitive properties of T cells. Nat Rev Immunol. 2012;12(9):663-9 doi: 10.1038/nri3280
10. Nataf S. Autoimmunity as a Driving Force of Cognitive Evolution. Front Neurosci. 2017;11:582. doi: 10.3389/fnins.2017.00582
11. Parkin J, Cohen B. An overview of the immune system. Lancet. 2021;357(9270):1777-89 doi: 10.1016/S0140-6736(00)04904-7
12. Derecki NC, Cardini AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR. et al. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J. Exp. Med. 2010;207(5):1067-80 doi: 10.1084/jem.20091419
13. Gandini SP, Cronk JC, Norris GT, Kipnis J. IL-4 in the brain: a cytokine to remember. J Immunol. 2012;189(9):4213-9 doi: 10.4049/jimmunol.1202246
14. Kenney MJ, Ganta CK. Autonomic nervous system and immune system interactions. Compr Psysiol. 2014;4(3):1177-200 doi: 10.1002/cphy.c130051
15. Brombacher TM, De Gouveia KS, Cruywagen L, Makena N, Booley F, Tamgue O. et al. Nippostrongylus brasiliensis infection leads to impaired reference memory and myeloid cell interference. Sci Rep. 2018;8(1):2958. doi: 10.1038/s41598-018-20770-x
16. Stefano GB. Historical Insight into infections and disorders associated with neurological and psychiatric sequelae similar to long covid. Med Sci Monit. 2021;27:e931447. doi: 10.12659/MSM.931447
17. Reis PA, Comim CM, Hermani F, Silva B, Barichello T, Portella AC. et al. Cognitive dysfunction is sustained after rescue therapy in experimental cerebral malaria and is reduced by additive antioxidant therapy. PloS Pathog. 2010;6:e1000963. doi: 10.1371/journal.ppat.1000963
18. Thuilliez J, Sissoko MS, Toure OB, Kamate P, Berthélemy JC, Doumbo OK. Malaria and primary education in Mali: a longitudinal study in the village of Donéguébougou. Soc Sci Med. 2010;71(2):324-334 doi: 10.1016/j.socscimed.2010.02.027
19. Kannan G, Pletnikov MV. Toxoplasma gondii and cognitive deficits in schizophrenia: an animal model perspective. Schizophr Bull. 2012;38(6):1155-61 doi: 10.1093/schbul/sbs079
20. Portes A, Giestal-de-Araujo E, Fagundes A, Pandolfo P, Geraldo AS, Lira MLF. et al. Leishmania amazonensis infection induces behavioral and modulates cytokine and neurotrophin production in the murine cerebral cortex. J Neuroimmunol. 2016;301:65-73 doi: 10.1016/j.jneuroim.2016.11.003
21. Ssenkusu JM, Hodges JS, Opoka RO, Idro R, Shapiro E, John CC. et al. Long-term behavioral problems in children with severe malaria. Pediatrics. 2016;138(5):e20161965. doi: 10.1542/peds.2016-1965
22. Reverchon F, Mortaud S, Sivoyon M, Maillet I, Laugeray A, Palomo J. et al. IL-33 receptor ST2 regulates the cognitive impairments associated with experimental cerebral malaria. PloS Pathog. 2017;13(4):e1006322. doi: 10.1371/journal.ppat.1006322
23. de Sousa LP, de Almeida RF, Ribeiro-Gomes FL, Carvalho LJM, Souza TM, Souza DOG. et al. Long-term effect of uncomplicated Plasmodium berghei ANKA malaria on memory and anxiety-like behaviour in C57BL/6 mice. Parasit Vectors. 2018;11(1):191. doi: 10.1186/s13071-018-2778-8
24. de Sousa LP, Ribeiro-Gomes FL, de Almeida RF, Mello e Souza T, Werneck GL, Souza DO. et al. Immune system challenge improves recognition memory and reverses malaria-induced cognitive impairment in mice. Scient Report. 2021;11(1):14857. doi: 10.1038/s41598-021-94167-8
25. Malaria Report, 2022. World Health Organization. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022
26. Sutherland CJ, Tanomsing N, Nolder D, Oguike M, Jennison C, Pukrittayakamee S. Two nonrecombining sympatric forms of the human malaria parasite plasmodium ovale occur globally. J Infect Dis. 2010;201(10):1544-1550 doi: 10.1086/652240
27. Singh B, Sung LK, Matusop A, Radhakrishnan A, Shamsul SSG, Cox-Singh J. et al. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet. 2004;363(9414):1017-24 doi: 10.1016/S0140-6736(04)15836-4
28. Ta TH, Hisam S, Lanza M, Jiram AI, Ismail N, Rubio JM. First case of a naturally acquired human infection with Plasmodium cynomolgi. Malar J. 2014;13:68. doi: 10.1186/1475-2875-13-68
29. Brasil P, Zalis MG, Pina-Costa A, Siqueira AM, Júnior CB, Silva S, Areas ALL. et al. Outbreak of human malaria caused by Plasmodium simium in the Atlantic Forest in Rio de Janeiro: a molecular epidemiological investigation. Lancet Glob Health. 2017;5(10):e1038-e1046 doi: 10.1016/S2214-109X(17)30333-9
30. Yap NJ, Hossain H, Nada-Raja T, Ngui R, Muslim A, Hoh BP. et al. Natural Human Infections with Plasmodium cynomolgi, P. inui, and 4 other Simian Malaria Parasites, Malaysia. Emerg Infect Dis. 2021;27(8):2187-2191 doi: 10.3201/eid2708.204502
31. Liew JWK, Bukhari FDM, Jeyaprakasam NK, Phang WK, Vythilingam I, Lau YL. Natural Plasmodium inui Infections in Humans and Anopheles cracens Mosquito, Malaysia. Emerg Infect Dis. 2021:27-10 doi: 10.3201/eid2710.210412
32. John CC, Bangirana P, Byarugaba J, Opoka RO, Idro R, Jurek AM. et al. Cerebral malaria in children is associated with long-term cognitive impairment. Pediatrics. 2008;122(1):e92-9 doi: 10.1542/peds.2007-3709
33. Dai M, Reznik SE, Spray DC, Weiss LM, Tanowitz HB, Gulinello M. et al. Persistent cognitive and motor deficits after successful antimalarial treatment in murine cerebral malaria. Microbes Infect. 2010;12(14-15):1198-207 doi: 10.1016/j.micinf.2010.08.006
34. Carew TJ, Sahley CL. Invertebrate learning and memory: from behavior to molecules. Ann Rev Neurosci. 1986;9:435-87 doi: 10.1146/annurev.ne.09.030186.002251
35. Solomonia RO, McCabe BJ. Molecular mechanisms of memory in imprinting. Neurosci Biobehav Ver. 2015;50:56-69 doi: 10.1016/j.neubiorev.2014.09.013
36. Kadon ICG. State-dependent plasticity of innate behavior in fruit flies. Curr Opin Neurobiol. 2019;54:60-65 doi: 10.1016/j.conb.2018.08.014
37. Reardon C, Murray K, Lomax AE. Neuroimmune Communication in Health and Disease. Physiol Rev. 2018;98(4):2287-2316 doi: 10.1152/physrev.00035.2017
38. Pert CB, Ruff MR, Weber RJ, Herkenham M. Neuropeptides and their receptors: a psychosomatic network. J Immunol. 1985;135(2 Suppl):820s-826
39. Sawada Y, Kawai R, McManaway M, Otsuki H, Rice KC, Patlak CS. et al. Kinetic analysis of transport and opioid receptor binding of [3H](-)-cyclofoxy in rat brain in vivo: implications for human studies. J Cereb Blood Flow Metab. 1991;11(2):183-203 doi: 10.1038/jcbfm.1991.51
40. Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve-an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52(4):595-638
41. Zhang ZJ, Jiang BC, Gao YJ. Chemokines in neuron-glial cell interaction and pathogenesis of neuropathic pain. Cell Mol Life Sci. 2017;74(18):3275-3291 doi: 10.1007/s00018-017-2513-1
42. Brynskikh A, Warren T, Zhu J, Kipnis J. Adaptive immunity affects learning behavior in mice. Brain Behav. Immun. 2008;22(6):861-9 doi: 10.1016/j.bbi.2007.12.008
43. Clark KR. Learning Theories: Behaviorism. Radiol Technol. 2018;90(2):172-175
44. Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R. et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J. Biol. Chem. 2002;277(24):21453-7 doi: 10.1074/jbc.C200176200
45. Yang Z, Chen M, Fialkow LB, Ellett JD, Wu R, Brinkmann V. et al. The immune modulator FYT720 prevents autoimmune diabetes in nonobese diabetic mice small star, filled. Clin. Immunol. 2003;107(1):30-5 doi: 10.1016/s1521-6616(02)00054-2
46. Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M. T cell deficiency leads to cognitive dysfunction: Implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc Natl Acad Sci U S A. 2004;101(21):8180-5 doi: 10.1073/pnas.0402268101
47. Vitkovic L, Konsman JP, Bockaert J, Dantzer R, Homburger V, Jacque C. Cytokine signals propagate through the brain. Mol Psychiatry. 2000;5(6):604-15 doi: 10.1038/sj.mp.4000813
48. Dantzer R, Konsman JP, Bluthé RM, Kelley KW. Neural and humoral pathways of communication from the immune system to the brain: parallel or convergent? Auton Neurosci. 2000;85(1-3):60-5 doi: 10.1016/S1566-0702(00)00220-4
49. Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Rev Neurosci. 2008;9(1):46-56 doi: 10.1038/nrn2297
50. Pasciuto E, Burton OT, Roca CP, Lagou V, Rajan WD, Theys T. et al. Microglia Require CD4 T Cells to Complete the Fetal-to-Adult Transition. Cell. 2020;182(3):625-640.e24 doi: 10.1016/j.cell.2020.06.026
51. Kivisäkk P, Mahad DJ, Callahan MK, Trebst C, Tucky B, Wei T. et al. Human cerebrospinal fluid central memory CD4+ T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci USA. 2003;100(14):8389-94 doi: 10.1073/pnas.1433000100
52. Kivisäkk P, Mahad DJ, Callahan MK, Sikora K, Trebst C, Tucky B. et al. Expression of CCR7 in multiple sclerosis: implications for CNS immunity. Ann Neurol. 2004;55(5):627-38 doi: 10.1002/ana.20049
53. Anandasabapathy N, Victora GD, Meredith M, Feder R, Dong B, Kluger C. et al. Flt3L controls the development of radiosensitive dendritic cells in the meninges and choroid plexus of the steady-state mouse brain. J. Exp. Med. 2011;208(8):1695-1705 doi: 10.1084/jem.20102657
54. Li Q, Qi F, Yang J, Zhang L, Gu H, Zou J. et al. Neonatal vaccination with bacillus Calmette-Guérin and hepatitis B vaccines modulates hippocampal synaptic plasticity in rats. J. Neuroimmunol. 2015;288:1-12 doi: 10.1016/j.jneuroim.2015.08.019
55. Yang J, Qi F, Gu H, Zou J, Yang Y, Yuan Q. et al. Neonatal BCG vaccination of mice improves neurogenesis and behavior in early life. Brain Res Bull. 2016;120:25-33 doi: 10.1016/j.brainresbull.2015.10.012
56. Mohammadi F, Rahimian R, Fakhraei N, Rezayat SM, Javadi-Paydar M, Dehpour AR. et al. Effect of glatiramer acetate on short-term memory impairment induced by lipopolysaccharide in male mice. Fudam Clin Pharmacol. 2016;30(4):347-56 doi: 10.1111/fcp.12202
57. Koren T, Yifa R, Amer M, Krot M, Boshnak N, Ben-Shaanan TL. et al. Insular cortex neurons encode and retrieve specific immune responses. Cell. 2021;184(24):5902-5915.e17 doi: 10.1016/j.cell.2021.10.013
58. Mueller M, Polesel J, Richetto U, Meyer U, Weber-Stadlbauer U. Mouse models of maternal immune activation: mind your caging system!. Brain Behav Immun. 2018;73:643-660 doi: 10.1016/j.bbi.2018.07.014
59. de Virgilio, Greco A, Fabbrini G, Inghilleri M, Rizzo MI, Gallo A. et al. Parkinson's disease: Autoimmunity and neuroinflammation. Autoimmum Rev. 2016;5(10):1005-11 doi: 10.1016/j.autrev.2016.07.022
60. Cai L & Huang. Neurofilament light chain as a biological marker for multiple sclerosis: a meta-analysis study. Neuropsychiatr Dis Treat. 2018;14:2241-2254 doi: 10.2147/NDT.S173280
61. Xu Y, He F, Qi F, Yang G, Zheng F, Yang J. et al. Remodeling the Th1 polarized systemic environment contributes to neurogenesis and cognitive function via the Wnt7a pathway in neonatal mice. Neurobiol Learn Mem. 2017;141:60-71 doi: 10.1016/j.nlm.2017.03.002
62. Qi F, Zuo Z, Yang J, Hu S, Yang Y, Yuan Q. et al. Combined effect of BCG vaccination and enriched environment promote neurogenesis and spatial cognition via a shift in meningeal macrophage M2 polarization. Neuroinflamation. 2017;14(1):32. doi: 10.1186/s12974-017-0808-7
63. Ribeiro M, Brigas HC, Temido-Ferreira M, Pousinha PA, Regen T, Santa C. Meningeal γδ T cell-derived IL-17 controls synaptic plasticity and short-term memory. Sci Immunol. 2019;4(40):eaay5199. doi: 10.1126/sciimmunol.aay5199
64. Carvalho FB, Gutierres JM, Bueno A, Agostinho P, Zago AM, Vieira J. et al. Anthocyanins control neuroinflammation and consequent memory dysfunction in mice exposed to lipopolysaccharide. Mol Neurobiol. 2017;54(5):3350-3367 doi: 10.1007/s12035-016-9900-8
65. Bossù P, Cutuli D, Palladino I, Caporali P, Angelucci F, Laricchiuta D. et al. A single intraperitoneal injection of endotoxin in rats induces long-lasting modifications in behavior and brain protein levels of TNF-α and IL-18. J Neuroinflammation. 2012;9:101. doi: 10.1186/1742-2094-9-101
66. Prilutski Y, Livneh Y. Physiological Needs: Sensations and Predictions in the Insular Cortex. Physiology (Bethesda). 2023;38(2):0. doi: 10.1152/physiol.00019.2022
67. Brabi S, Doosti M-H, Salari A-A. Strain-dependent effects of prenatal maternal immune activation on anxiety- and depression-like behaviors in offspring. Brain, Behav and Immun. 2014; 164-176. doi: 10.1016/j.bbi. 2013 12.003
68. Depino AM. Early prenatal exposure to LPS results in anxiety- and depression-related behaviors in adulthood. Neuroscience. 2015;299:56-65 doi: 10.1016/j.neuroscience.2015.04.065
69. Giovanoli S, Notter T, Richetto J, Labouesse MA, Vuillermot S, Riva MA. et al. Late prenatal immune activation causes hippocampal deficits in the absence of persistent inflammation across aging. J Neuroinflammation. 2015;12:221. doi: 10.1186/s12974-015-0437-y
70. Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016;351(6276):933-9 doi: 10.1126/science.aad0314
71. Woods RM, Lorusso JM, Potter HG, Neill JC, Glazier JD, Hager R. Maternal immune activation in rodent models: A systematic review of neurodevelopmental changes in gene expression and epigenetic modulation in the offspring brain. Neurosci Biobehav Rev. 2021;129:389-421 doi: 10.1016/j.neubiorev.2021.07.015
72. Schwartzer J, Careaga M, Coburn MA, Rose DR, Hughes HK, Ashwood P. Behavioral impact of maternal allergic-asthma in two genetically distinct mouse strains. Brain Behav Immun. 2017;63:99-107 doi: 10.1016/j.bbi.2016.09.007
73. Guo R-B, Sun P-L, Zhao A-P, Gu J, Ding X, Qi J. et al. Chronic asthma results in cognitive dysfunction in immature mice. Exp Neurol. 2013;247:209-17 doi: 10.1016/j.expneurol.2013.04.008
74. Wang X, Yang J, Xing Z, Zhang H, Wen Y, Qi F. et al. IL-4 mediates the delayed neurobehavioral impairments induced by neonatal hepatitis B vaccination that involves the downregulation of the IL-4 receptor in the hippocampus. Cytokine. 2018;110:137-149 doi: 10.1016/j.cyto.2018.04.037
75. Maes M. Major depression and activation of the inflammatory response system. Adv Exp Med Biol. 1999;461:25-46 doi: 10.1007/978-0-585-37970-8_2
76. Loftis JM, Huckans M, Morasco BJ. Neuroimmune mechanisms of cytokine-induced depression: current theories and novel treatment strategies. Neurobiol Dis. 2010;37(3):519-33 doi: 10.1016/j.nbd.2009.11.015
77. Maes M, Galecki P, Chang YS, Berk M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro) degenerative processes in that illness. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(3):676-92 doi: 10.1016/j.pnpbp.2010.05.004
78. Galecki P, Talarowska M. Inflammatory theory of depression. Psychiatr Pol. 2018;52(3):437-447 doi: 10.12740/PP/76863
79. Quinn ME, Stanton CH, Slavich GM, Joormann J. Executive Control, Cytokine Reactivity to Social Stress, and Depressive Symptoms: Testing the Social Signal Transduction Theory of Depression. Stress. 2020;23(1):60-68 doi: 10.1080/10253890.2019.1641079
80. Malaria Report, 2021. World Health Organization. https://www.who.int/publications/i/item/9789240040496
81. Ghazanfari N, Mueller SN, Heath WR. Cerebral Malaria in Mouse and Man. Front Immunol. 2018;9:2016. doi: 10.3389/fimmu.2018.02016
82. Martins YC, Carvalho LJM, Daniel-Ribeiro CT. Challenges in the determination of early predictors of cerebral malaria: lessons from the human disease and the experimental murine models. Neuroimmunomodul. 2009;16:134-45 doi: 10.1159/000180268
83. Christensen SS & Eslick GD. Cerebral malaria as a risk factor for the development of epilepsy and other long-term neurological conditions: a meta-analysis. Tran R Soc Trop Med Hyg. 2015;109(4):233-8 doi: 10.1093/trstmh/trv005
84. Postels DG, Soldatos A, LaRovere KL. Outcomes measures in children after acute central nervous system infections and malaria. Curr Opin Pediatr. 2019;31(6):756-762 doi: 10.1097/MOP.0000000000000823
85. Bruneel F. Human cerebral malaria: 2019 mini review. Rev Neurol (Paris). 2019;175(7-8):445-450 doi: 10.1016/j.neurol.2019.07.008
86. Odera VM, Snow RW, Newton CRJC. The burden of the neurocognitive impairment associated with Plasmodium falciparum malaria in sub-Saharan Africa. Am J Trop Med Hyg. 2004;71:64-70
87. Dugbartey AT, Dugbartey MT, Apedo MY. Delayed neuropsychiatric effects of malaria in Ghana. J Nerv Ment Dis. 1998;186(3):183-6 doi: 10.1097/00005053-199803000-00007
88. Idro R, Kakooza-Mwesige A, Balyejjussa S, Mirembe G, Mugasha C, Tugumisirize J. et al. Severe neurological sequelae and behaviour problems after cerebral malaria in Ugandan children. BMC Res Notes. 2010;3:104. doi: 10.1186/1756-0500-3-104
89. Idro R, Kakooza-Mwesige A, Asea B, Ssebyala K, Bangirana P, Opoka RO. et al. Cerebral malaria is associated with long-term mental health disorders: a cross sectional survey of a long-term cohort. Malar J. 2016;15:184. doi: 10.1186/s12936-016-1233-6
90. Boivin MJ, Bangirana P, Byarugaba J, Opoka RO, Idro R, Jurek AM. et al. Cognitive impairment after cerebral malaria in children: a prospective study. Pediatrics. 2007;119:e360-6 doi: 10.1542/peds.2006-2027
91. Kihara M, Carter JA, Holding PA, Vargha-Khadem F, Scott RC, Idro R. et al. Impaired everyday memory associated with encephalopathy of severe malaria: the role of seizures and hippocampal damage. Malar J. 2009;8:273. doi: 10.1186/1475-2875-8-273
92. Bangirana P, Allebeck P, Boivin MJ, John CC, Page C, Ehnvall A. et al. Cognition, behaviour and academic skills after cognitive rehabilitation in Uganda children surviving severe malaria: a randomised trial. BMC Neurol. 2011;11:96. doi: 10.1186/1471-2377-11-96
93. Datta D, Conroy AL, Castelluccio PF, Ssenkusu JM, Park GS, Opoka RO. et al. Elevated Cerebrospinal Fluid Tau Protein Concentrations on Admission Are Associated with Long-term Neurologic and Cognitive Impairment in Ugandan Children with Cerebral Malaria. Clin Infect Dis. 2020;70(6):1161-1168 doi: 10.1093/cid/ciz325
94. Tu Z, Gormley J, Sheth V, Seydel KB, Taylor T, Beare N. et al. Cerebral malaria: insight into pathology from optical coherence tomography. Sci Rep. 2021;11(1):15722. doi: 10.1038/s41598-021-94495-9
95. Rosa-Gonçalves P, Ribeiro-Gomes FL, Daniel-Ribeiro CT. Malaria Related Neurocognitive Deficits and Behavioral Alterations. Front Cell Infect Microbiol. 2022;12:829413. doi: 10.3389/fcimb.2022.829413
96. Bangirana P, Opoka RO, Boivin MJ, Idro R, Hodges JS, John CC. Neurocognitive domains affected by cerebral malaria and severe malarial anemia in children. Learn Individ Differ. 2016;46:38-44 doi: 10.1016/j.lindif.2015.01.010
97. Holmberg D, Franzén-Röhl E, Idro R, Opoka RO, Bangirana P, Sellgren CM. et al. Cerebrospinal fluid kynurenine and kynurenic acid concentrations are associated with coma duration and long-term neurocognitive impairment in Ugandan children with cerebral malaria. Malar J. 2017;16(1):303. doi: 10.1186/s12936-017-1954-1
98. Ouma BJ, Bangirana P, Ssenkusu JM, Datta D, Opoka RO, Idro R. et al. Plasma angiopoietin-2 is associated with age-related deficits in cognitive sub-scales in Ugandan children following severe malaria. Malar J. 2021;20(1):17. doi: 10.1186/s12936-020-03545-6
99. Datta D, Bangirana P, Opoka RO, Conroy AL, Co K, Bond C. et al. Association of Plasma Tau With Mortality and Long-term Neurocognitive Impairment in Survivors of Pediatric Cerebral Malaria and Severe Malarial Anemia. Jamma Netw Open. 2021;4(12):e2138515. doi: 10.1001/jamanetworkopen.2021.38515
100. Naseri NN, Wang H, Guo J, Sharma M, Luo W. The complexity of tau in Alzheimer's disease. Neurosc Lett. 2019;705:183-194 doi: 10.1016/j.neulet.2019.04.022
101. Fernando SD, Gunawardena DM, Bandara MR, De Silva D, Carter R, Mendis KN. et al. The impact of repeated malaria attacks on the school performance of children. Am J Trop Med Hyg. 2003;69:582-8
102. Vitor-Silva S, Reyes-Lecca RC, Pinheiro TR, Lacerda MV. Malaria is associated with poor school performance in endemic area of the Brazilian Amazon. Malar J. 2009;8:230. doi: 10.1186/1475-2875-8-230
103. Fink G, Olgiati A, Hawela M, Miller JM, Matafwali B. Association between early childhood exposure to malaria and children's pre-school development evidence from Zambia early childhood development project. Malar J. 2013;12:12. doi: 10.1186/1475-2875-12-12
104. Tapajós R, Castro D, Gisely Melo G, Balogun S, James M, Pessoa R. et al. Malaria impact on cognitive function of children in a peri-urban community in the Brazilian Amazon. Malar J. 2019;18:173. doi: 10.1186/s12936-019-2802-2
105. Pessoa RC, Oliveira-Pessoa GF, Souza BKA, Sampaio VS, Pinto ALCB, Barboza LL. et al. Impact of Plasmodium vivax malaria on executive and cognitive functions in elderlies in the Brazilian Amazon. Sci Rep. 2022;12(1):10361. doi: 10.1038/s41598-022-14175-0
106. Nankabirwa J, Wandera B, Kiwanuka N, Staedke SG, Kamya MR, Brooker SJ. Asymptomatic Plasmodium Infection and Cognition among Primary Schoolchildren in a High Malaria Transmission Setting in Uganda. Am. J. Trop. Med. Hyg. 2013;88(6):1102-1108 doi: 10.4269/ajtmh.12-0633
107. Padhy SK, Sahu S, Govindahari V. Retinopathy Secondary to Uncomplicated Plasmodium vivax Malaria. Case Reports. 2021;52(1):50-51 doi: 10.3928/23258160-20201223-10
108. Potter S, Chan-Ling T, Ball HJ, Mansour H, Mitchell A, Maluish L. et al. Perforin mediated apoptosis of cerebral microvascular endotelial cells during experimental cerebral malaria. Int J Parasitol. 2006;36:485-96 doi: 10.1016/j.ijpara.2005.12.005
109. Khandare AV, Bobade D, Deval M, Patil T, Saha B, Prakash D. Expression of negative immune regulatory molecules, pro-inflammatory chemokine and cytokines in immunopathology of ECM developing mice. Acta Trop. 2017;172:58-63 doi: 10.1016/j.actatropica.2017.04.025
110. Freeman BD, Martins YC, Akide-Ndunge OB, Bruno FP, Wang H, Tanowitz HB. et al. Endothelin-1 Mediates Brain Microvascular Dysfunction Leading to Long-Term Cognitive Impairment in a Model of Experimental Cerebral Malaria. Plos Pathog. 2016;12(3):e1005477. doi: 10.1371/journal.ppat.1005477
111. Zhao H, Aoshi T, Kawai S, Mori Y, Konishi A, Ozkan M. et al. Olfactory plays a key role in spatiotemporal pathogenesis of cerebral malaria. Cell Host Microbe. 2014;15(5):551-63 doi: 10.1016/j.chom.2014.04.008
112. Howland SW, Claser C, Poh CM. Pathogenic CD8+ T cells in experimental cerebral malaria. Sem. Immunop. 2015;37:221-231 doi: 10.1007/s00281-015-0476-6
113. Hansen DS, Siomos MA, Buckingham L, Scalzo AA, Schofield L. Regulation of Murine Cerebral Malaria Pathogenesis by CD1d-restricted NKT cells and the natural killer complex. Immunity. 2003;18(3):391-402 doi: 10.1016/s1074-7613(03)00052-9
114. Villegas-Mendez A, Strangward P, Shaw TN, Rajkovic I, Tosevski V, Forman R. et al. Gamma Interferon Mediates Experimental Cerebral Malaria by Signaling within Both the Hematopoietic and Nonhematopoietic Compartments. Infect Immun. 2017;85(11):e01035-16 doi: 10.1128/IAI.01035-16
115. Campos AC, Brant F, Miranda AS, Machado FS, Teixeira AL. Cannabidiol increases survival and promotes rescue of cognitive function in a murine model of cerebral malaria. Neuroscience. 2015;289:166-80 doi: 10.1016/j.neuroscience.2014.12.051
116. Guha SK, Tillu R, Sood A, Patgaonkar M, Nanavaty IN, Sengupta A. et al. Single episode of mild murine malaria induces neurinflammation, alters microglial profile, impairs adult neurogenesis, and causes deficits in social and anxiety-like behavior. Brain Behav Immun. 2014;42:123-37 doi: 10.1016/j.bbi.2014.06.009
117. Zuo Z, Qi F, Yang J, Wang X, YaruWen Y, Yuan Q. Immunization with Bacillus Calmette-Guérin (BCG) alleviates neuroinflammation and cognitive deficits in APP / PS1 mice via the recruitment of inflammation-resolving monocytes to the brain. Neurob of Disea. 2017;101:27-39 doi: 10.1016/j.nbd.2017.02.001
118. Xing XN, Sha S, Chen XH, Guo WS, Guo R, Jiang TZ. et al. Active Immunization with DNA Vaccine Reduced Cerebral Inflammation and Improved Cognitive Ability in APP/PS1 Transgenic Mice by In vivo Electroporation. Neurochem Res. 2015;40(5):1032-41 doi: 10.1007/s11064-015-1559-4
119. Davisson MT, Schmidt C, Reeves RH, Irving NG, Akeson EC, Harris BS. et al. Segmental trisomy as a mouse model for Down syndrome. Prog Clin Biol Res. 1993;384:117-33
120. Belichenko PV, Madani R, Rey-Bellet L, Pihlgren M, Becker A, Plassard A. et al. An Anti-β-Amyloid Vaccine for Treating Cognitive Deficits in a Mouse Model of Down Syndrome. Plos One. 2016;11(3):e0152471. doi: 10.1371/journal.pone.0152471
121. Lewitus MG, Yarkoni AW, Ziv Y, Shabat-Simon M, Gersner R, Zangen A. et al. Vaccination as a Novel Approach for Treating Depressive Behavior. Bio Psych. 2009;65:283-288 doi: 10.1016/j.biopsych.2008.07.014
122. Rosa-Gonçalves P, de Sousa LP, Maia AB, Ribeiro-Gomes FL, Gress CCTL, Werneck GL. et al. Dynamics and immunomodulation of cognitive deficits and behavioral changes in non-severe experimental malaria. Front Immunol. 2022;13:1021211. doi: 10.3389/fimmu.2022.1021211
123. Rosa-Gonçalves P, de Sousa LP, Ribeiro-Gomes FL, Carvalho LJM, Daniel-Ribeiro CT. Immunomodulation through vaccination as a promising therapeutic strategy to mitigate malaria-related neurocognitive sequelae. Brain Behav Immun. 2023;109:102-104 doi: 10.1016/j.bbi.2023.01.007
124. Desruisseaux MS, Gulinello M, Smith DN, Lee SC, Tsuji M, Weiss LM. et al. Cognitive dysfunction in mice infected with Plasmodium berghei strain ANKA. J Infect Dis. 2008;197(11):1621-7 doi: 10.1086/587908
125. de Miranda AS, Lacerda-Queiroz N, de Carvalho Vilela M, Rodrigues DH, Rachid MA, Quevedo J. et al. Anxiety-like behavior and proinflammatory cytokine levels in the brain of C57BL/6 mice infected with Plasmodium berghei (strain ANKA). Neurosci Lett. 2011;491(3):202-6 doi: 10.1016/j.neulet.2011.01.038
126. Reis PA, Estato V, da Silva TI, d'Avila JC, Siqueira LD, Assis EF. et al. Statins decrease neuroinflammation and prevent cognitive impairment after cerebral malaria. PLoS Pathog. 2012(12):e1003099. doi: 10.1371/journal.ppat.1003099
127. Miranda AS, Brant F, Rocha NP, Cisalpino D, Rodrigues DH, Souza DG. et al. Further evidence for an anti-inflammatory role of artesunate in experimental cerebral malaria. Malar J. 2013;12:388. doi: 10.1186/1475-2875-12-388
128. Miranda AS, Brant F, Campos AC, Vieira LB, Rocha NP, Cisalpino D. et al. Evidence for the contribution of adult neurogenesis and hippocampal cell death in experimental cerebral malaria cognitive outcome. Neuroscience. 2015;284:920-933 doi: 10.1016/j.neuroscience.2014.10.062
129. Miranda AS, Brant F, Vieira LB, Rocha NP, Vieira ÉLM, Rezende GHS. et al. A Neuroprotective Effect of the Glutamate Receptor Antagonist MK801 on Long-Term Cognitive and Behavioral Outcomes Secondary to Experimental Cerebral Malaria. Mol Neurobiol. 2017;54(9):7063-7082 doi: 10.1007/s12035-016-0226-3
130. Souza TL, Grauncke ACB, Ribeiro LR, Mello FK, Oliveira SM, Brant F. et al. Cerebral Malaria Causes Enduring Behavioral and Molecular Changes in Mice Brain Without Causing Gross Histopathological Damage. Neuroscience. 2018;369:66-75 doi: 10.1016/j.neuroscience.2017.10.043
131. Lima MN, Oliveira HA, Fagundes PM, Estato V, Silva AYO, Freitas RJRX. et al. Mesenchymal stromal cells protect against vascular damage and depression-like behavior in mice surviving cerebral malaria. Stem Cell Res Ther. 2020;11(1):367. doi: 10.1186/s13287-020-01874-6
132. Ataide BJA, Kauffmann N, Mendes NSF, Torres MLM, Dos Anjos LM, Passos ADCF. et al. Melatonin Prevents Brain Damage and Neurocognitive Impairment Induced by Plasmodium Berghei ANKA Infection in Murine Model of Cerebral Malaria. Front Cell Infect Microbiol. 2020;10:541624. doi: 10.3389/fcimb.2020.541624
133. Duan H, Zhao S, Xiang J, Ju C, Chen X, Gramaglia I. et al. Targeting the CD146/Galectin-9 axis protects the integrity of the blood-brain barrier in experimental cerebral malaria. Cell Mol Immunol. 2021;18(10):2443-2454 doi: 10.1038/s41423-020-00582-8
134. Kumar SP, Babu PP. NADPH Oxidase: a Possible Therapeutic Target for Cognitive Impairment in Experimental Cerebral Malaria. Mol Neurobiol. 2022;59(2):800-820 doi: 10.1007/s12035-021-02598-1
Author contact
![]() Corresponding authors: Daniel-Ribeiro, E-mail: malariabr; de Sousa, E-mail: luciana.sousafiocruz.br.
Corresponding authors: Daniel-Ribeiro, E-mail: malariabr; de Sousa, E-mail: luciana.sousafiocruz.br.

 Global reach, higher impact
Global reach, higher impact