10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2023; 19(11):3456-3471. doi:10.7150/ijbs.80760 This issue Cite
Research Paper
MiR-552-3p Regulates Multiple Fibrotic and Inflammatory genes Concurrently in Hepatic Stellate Cells Improving NASH-associated Phenotypes
1. Center for Drug Safety Evaluation and Research, State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 501 Haike Road, Shanghai 201203, China.
2. School of Life Science and Technology, ShanghaiTech University, 100 Haike Road, Shanghai 201210, China.
3. University of Chinese Academy of Sciences, No.19A Yuquan Road Beijing 100049, China.
4. Department of Pharmaceutics, College of Pharmaceutical Sciences, Soochow University, Suzhou 215123, China.
5. School of Chinese Materia Medica, Nanjing University of Chinese Medicine, Nanjing 210023, China.
6. Department of Infectious Diseases, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, 197 Ruijin 2nd Road, Shanghai 200025, China.
Abstract
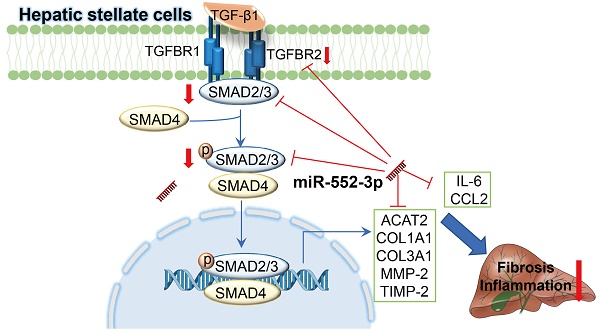
Non-alcoholic steatohepatitis (NASH) is a chronic liver disease characterized by hepatic steatosis, inflammation, and progressive fibrosis. Our previous study demonstrated that microRNA-552-3p (miR-552-3p) was down-regulated in the livers of patients with NASH and alleviated hepatic glycolipid metabolic disorders. However, whether miR-552-3p affects NASH progression remains unclear. In this current study, we found that hepatic miR-552-3p expression was negatively correlated with the degree of liver fibrosis and inflammation of NASH patients. Interestingly, the level of miR-552-3p was decreased during hepatic stellate cell (HSC) activation in vitro. Overexpression of miR-552-3p could not only inhibit the expression of fibrotic and inflammatory genes, but also restrain the activation of TGF-β1/Smad3 signaling pathway by down-regulating the expression of TGFBR2 and SMAD3 in HSCs, finally suppressing HSC activation. More importantly, overexpression of miR-552-3p ameliorated liver fibrosis and inflammation in two murine models: high fat/high fructose/high cholesterol diet-induced NASH model and carbon tetrachloride (CCl4)-treated liver fibrosis model. In conclusion, miR-552-3p plays a crucial role in the pathogenesis of NASH by limiting multiple fibrotic and inflammatory pathways in HSCs, which may shed light on its therapeutic potential in NASH.
Keywords: MiR-552-3p, NASH, Fibrosis, Inflammation, Synergistic multi-target suppression

 Global reach, higher impact
Global reach, higher impact