10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2023; 19(13):4181-4203. doi:10.7150/ijbs.85158 This issue Cite
Review
IκB kinase β (IKKβ): Structure, transduction mechanism, biological function, and discovery of its inhibitors
1. School of Chinese Materia Medica, State Key Laboratory of Component-Based Chinese Medicine, Tianjin State Key Laboratory of Modern Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin 301617, China.
2. College of Pharmacy, Second Affiliated Hospital, Dalian Medical University, Dalian 116044, China.
3. School of Pharmaceutical Sciences, Health Science Center, Shenzhen University, Shenzhen 518061, China.
4. Faculty of Pharmaceutical Sciences, Toho University, Chiba 274-8510, Japan.
*These authors contributed equally to this work.
Received 2023-4-11; Accepted 2023-7-26; Published 2023-8-6
Abstract
The effective approach to discover innovative drugs will ask natural products for answers because of their complex and changeable structures and multiple biological activities. Inhibitory kappa B kinase beta (IKKβ), known as IKK2, is a key regulatory kinase responsible for the activation of NF-κB through its phosphorylation at Ser177 and Ser181 to promote the phosphorylation of inhibitors of kappa B (IκBs), triggering their ubiquitination and degradation to active the nuclear factor kappa-B (NF-κB) cascade. Chemical inhibition of IKKβ or its genetic knockout has become an effective method to block NF-κB-mediated proliferation and migration of tumor cells and inflammatory response. In this review, we summarized the structural feature and transduction mechanism of IKKβ and the discovery of inhibitors from natural resources (e.g. sesquiterpenoids, diterpenoids, triterpenoids, flavonoids, and alkaloids) and chemical synthesis (e.g. pyrimidines, pyridines, pyrazines, quinoxalines, thiophenes, and thiazolidines). In addition, the biosynthetic pathway of novel natural IKKβ inhibitors and their biological potentials were discussed. This review will provide inspiration for the structural modification of IKKβ inhibitors based on the skeleton of natural products or chemical synthesis and further phytochemistry investigations.
Keywords: IKKβ, Inhibitor, Natural products, chemical synthesis, Bioactivity
Introduction
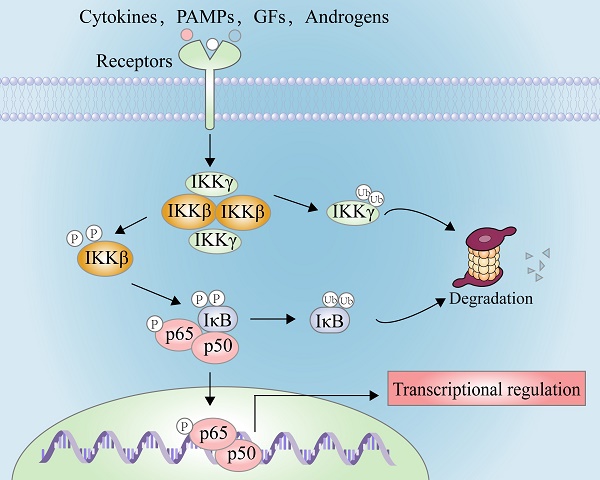
In 1986, Sen and co-workers focused on the work to discover novel nuclear factors that regulated the expression of immunoglobulin G (IgG) in B cells [1]. Fortunately, they found that a nuclear factor specifically bound to the promoter region of the Ig κ light chain, namely nuclear factor kappa-B (NF-κB) [2]. During this initial pioneering work and the subsequent recognition of transcription factors, NF-κB has been served as the most well-studied signal transduction paradigm in the latest three decades [2, 3].
NF-κB comprises five members of the family Rel, such as RelA (p65), RelB, c-Rel, NF-κB1 (p50), and NF-κB2 (p52) [4]. In normal cells, they form the homo- or hetero-complex with inhibitor of kappa B (IκB, including IκBα, IκBβ, and IκBε) and are anchored in the cytoplasm [4]. After the stimulation of exogenous substances, e.g. lipopolysaccharide (LPS), or inflammatory factors, e.g. interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α), IκBα is phosphorylated at Ser32 and Ser36, resulting in the rapid ubiquitination by the proteosome in the dependent manner to release NF-κB [5, 6]. Furthermore, activation of NF-κB leads to its nuclear translocation from the cytoplasm, allowing the binding of the promoter of its response elements and enhancer regions of its responsive genes, such as inflammatory cytokines, adhesion molecules, chemokines, and transcription factors [7]. However, it is very difficult to identify which can promote the phosphorylation of IκBs, which is not achieved until the presence of inhibitory kappa B kinases (IKKs) [3]. IKKβ, also known as IKK2, is a key regulatory kinase responsible for the NF-κB activation. In the classical pathway, the phosphorylation of IKKβ at Ser177 and Ser181, through the co-localization with TGF-beta-activated kinase 1 (TAK1) and mitogen-activated protein kinase kinase kinase 3 (MEKK3), promotes the phosphorylation of IκBs, triggering their ubiquitination and degradation to worsen the NF-κB cascade [3, 8, 9].
Because activated IKKβ can rapidly phosphorylate IκBs, chemical inhibition of IKKβ or its genetic knockout (KO) has become an experimental approach to block NF-κB mediated proliferation and migration of tumor cells and inflammatory response [3, 8, 9]. For example, rectal carcinoma can be alleviated via suppressing the IKKβ activity. Furthermore, the phosphorylation level of IKKβ is upregulated in melanoma tumor, and its genetic deletion noteworthily suppresses the development of melanoma tumor [10]. Herein, focused on the regulation and function of IKKβ, we summarized its inhibitors from natural products and chemical synthesis, which provided a useful guidance for the future development of potential IKKβ inhibitors, and the relationship between IKKβ and cancer, central nervous system (CNS), and metabolic diseases.
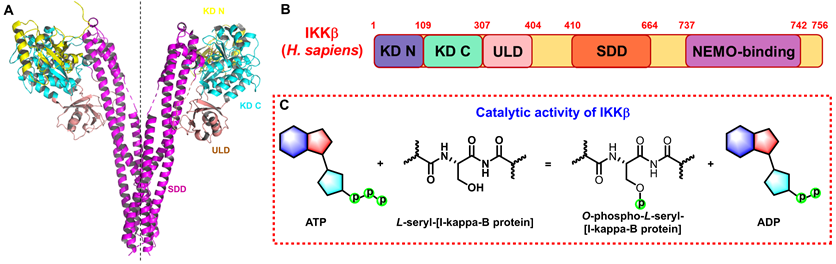
Structure and function of IKKβ. (A) 3D structure of human IKKβ dimmer (PDB: 4KIK). (B) Structural domain of human IKKβ. (C) Catalytic function of IKKβ.
Structure of IKKβ
The IKK complex includes IKKα, IKKβ, and IKKγ (NEMO), of which IKKα and IKKβ are the catalytic subunits, and IKKγ is the regulatory subunit [11]. IKKβ is ubiquitously expressed serine-threonine protein kinase comprising of 756 amino acids, it has 52% sequence identity and 70% homology with IKKα [12], and they share highly similar domain and tertiary structure as well [13-15]. As shown in Figure 1A (PDB: 4KIK), IKKβ contains the N-terminal kinase domain (KD N; residues 1-109), the C-terminal kinase domain (KD C; residues 110-307), the ubiquitin-like domain (ULD; residues 308-404), the scaffold dimerization domain (SDD; residues 410-664), and the NEMO-binding domain (residues 737-742, Figure 1B). Compared with IKKα, IKKβ has a ULD following the KD C, which is necessary for IKKβ functional activity and is important for its substrate specificity [16]. IKKβ activity requires the activation of phosphorylation of Ser177 and Ser181 in the activation loop (Figure 1C) [17]. However, until now, the exact sequence involving IKKβ activation has not been fully determined. Recent evidence for IKK complexes has demonstrated that oligomerization-mediated trans-autophosphorylation of the IKK subunit is the primary form of IKKβ activation [18]. Under the action of external stimuli, TAK1 first phosphorylates Ser177 of IKKβ, which primes subsequent IKK-catalyzed autophosphorylation of Ser181 [3, 8, 9]. Subsequently, Ser32 and Ser36 of IκB are phosphorylated by the activated IKK complex, resulting in its ubiquitylation by the S phase kinase-associated protein 1 (SKP1)-cullin 1-F-box protein (SCF)/beta-transducing repeat-containing protein (β-TrCP) E3 ubiquitin ligase complex and degradation by the proteasome [19-21].
Single nucleotide polymorphisms (SNPs) are the most abundant form of deoxyribonucleic acid (DNA) variation in the human genome, which influences disease-related genes and non-coding RNAs via enhancing the promoter activity and specific nuclear protein-binding affinity [22]. IKBKB is the gene responsible for encoding IKKβ with 21 exons localized in the chromosomal region 8p11.21. A variety of IKKβ SNPs have been reported, such as rs2272736, rs3747811, rs5029748, rs5029748, rs11986055, rs4560769, and rs6474386, and associated with the risk of hypertension, gastric and colorectal cancers, recurrent wheezing, systemic lupus erythematosus, obesity, and myelogenous leukemia [23-27]. Tessier et al. found that rs3747811 served as a high risk to have an increased waist circumference in South Asian population and increase the body mass index in Caucasian population, indicating the risk between this SNP with obesity [27]. Notably, SNP rs5029748 could decrease eighty percent risk for colon cancer [28]. In addition, Li's group from China analyzed the association of two SNPs for IKKβ, rs12676482 and rs2272733, with systemic lupus erythematosus, and demonstrated that there is no genetic predisposition to risk of systemic lupus erythematosus in Chinese Han population [24]. Therefore, the detailed relationship of SNPs with diseases requires further studies.
The IKKβ mechanism
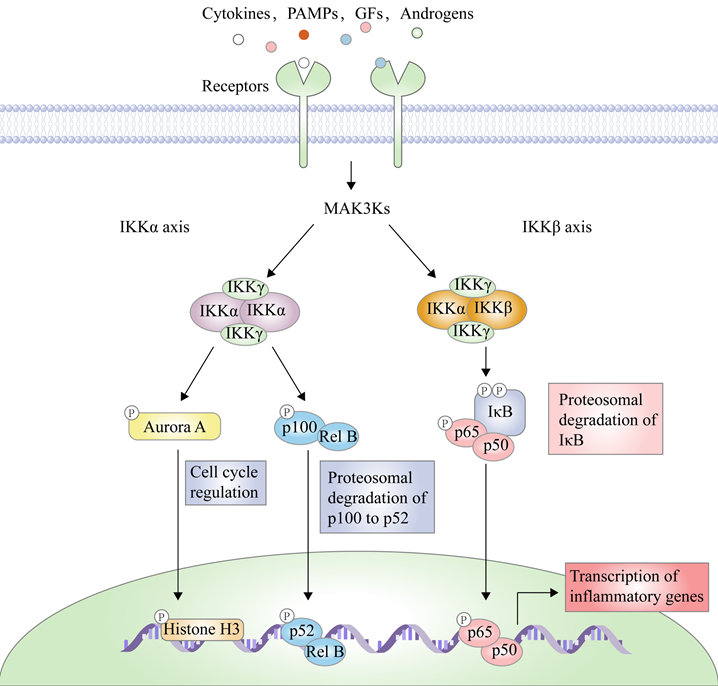
There are two principal pathways to activate NF-κB (Figure 2), including canonical pathway depended on IKKβ and non-canonical pathway depended on IKKα [29]. IKKβ-mediated signaling pathway is activated by inflammatory cytokines or exogenous substances, such as TNF-α and LPS [30], to promote the recruitment of key enzymes, such as TAK1, MEKK1, MEKK3, NF-κB-inducing kinase (NIK), NF-κB activating kinase (NAK), and transforming growth factor kinases [31]. For example, under the IL-1 stimulation, the protein domain of TNF receptor-associated factor 6 (TRAF6) finger catalyzes the polyubiquitination of Lys63-linked NEMO, and mediates the binding of TAK1 and TAB2/3 through the interaction between ubiquitin chains, specifically activating IKKβ through the phosphorylation of Ser177 and Ser181 [32]. Subsequently, Ser32 and Ser36 of IκB are phosphorylated by the activated IKK complex, resulting in its ubiquitylation by the SCF/β-TrCP E3 ubiquitin ligase complex and degradation by the proteasome [19-21, 33]. Because IκB normally binds to NF-κB, the latter's nuclear localization sequence (NLS) is masked, resulting in its main location in the cytoplasm, while removal of IκB exposes the masked site to induce the nuclear translocation of NF-κB, making it bind to the promoter regions of its downstream genes [34], such as cyclooxygenase-2 (COX-2), IL-6, and inducible nitric oxide synthase (iNOS). Furthermore, activated IKKβ mediates the Ser536 phosphorylation of NF-κB p65 to initiate reverse transcription, resulting in increased transcriptional activation [35, 36]. In the non-canonical pathway, IKKα activates NF-κB as well. Although IKKα and IKKβ have contain similar structural domains and possesses 70% homology, the kinase activity of IKKβ towards IκB is 20-50-fold higher than that of IKKα [37], therefore, IKKβ have a higher status in activation of NF-κB [38].
IKKβ-mediated NF-κB pathway is a key signal transduction pathway involved in inflammatory response, angiogenesis, invasiveness, metastasis, and immune escape [39-46]. After this signaling pathway is activated by inflammatory factors, it promotes the expression of inflammatory factors at the transcriptional level as well, thus forming a loop and producing an amplification effect to aggravate inflammation response [47-51]. In the case of the colitis-associated cancer (CAC) mouse model, IKKβ genetic deletion in intestinal epithelial cells greatly reduces the incidence of tumors through the increase of apoptosis [52], while its KO in the macrophage significantly reduces tumor size by suppressing the expression of pro-inflammatory factors [53, 54]. Therefore, IKKβ has be considered a potential target to develop drugs in the treatment of inflammation and caners.
Biological potentials for IKKβ inhibition
IKKβ serves as a role in biological functions, such as immunity and inflammation, because of IKKβ responsible for NF-κB pathway. Accumulating evidence has demonstrated that caners, CNS, and metabolic diseases, including pancreatic cancer, Parkinson's disease (PD), diabetes, non-alcoholic fatty liver disease (NAFLD), are associated with IKKβ [55-57]. In addition, chemical inhibition of IKKβ or its genetic KO has beneficial effects to block NF-κB mediated above-mentioned diseases [3, 8, 9].
Cancers
Cancer is a terrible disease that has existed for more than 200 million years and first appeared in humans more than one million years ago. Cancer, different from infectious, parasitic, and other diseases caused by many environmental factors, is mainly caused by abnormal changes of its own normal cells under the long-term action of internal and external factors [58, 59]. IKKβ is overexpressed in tumors, including pancreatic, breast, ovarian, lung, myeloma, rectal, and leukemia cancers, and IKKβ-mediated NF-κB pathway is a key signal transduction pathway involved in the occurrence and development of tumors [39]. The phosphorylation of IKKβ at Ser177 and Ser181 promotes the IκBα phosphorylation to trigger the ubiquitination and degradation of the latter to worsen the NF-κB cascade [3, 8, 9]. Accumulating evidence demonstrates that IKKβ genetic KO and its chemical inhibition both block the NF-κB transduction to alleviate the tumor growth, such as pancreatic, colorectal, lung, and myeloma cancer cells [60-62]. Therefore, IKKβ plays a critical role in the treatment of cancers via blocking the NF-κB cascade.
Pancreatic cancer, first described in 1761, belongs to the member of malignant tumors with a high mortality [55]. Although enhancing survival rates of other cancers, that of pancreatic cancer still remains invariability since 1960s. Because of its insidious onset, the early detection is difficult, and symptoms generally appear in the middle and late stages, such as abdominal pain and jaundice, therefore, most treatment regimens are ineffective [55]. The present series of studies provide evidence that IKKβ is overexpressed in pancreatic cancer cells, such as Panc-1, MIA-PaCa-2, and AsPc-1 [63, 64], revealing its role in pancreatic cancer. Wu and co-workers treated pancreatic cancer cells AsPc-1 with apigenin and found its potential in the inhibition of the growth of pancreatic cancer cells [63]. Subsequent experiments demonstrated that apigenin inhibited the IKKβ activity to promote the cleaved caspase 3 expression, allowing the apoptosis of pancreatic cancer cells [63]. Similarly, Tong et al. demonstrated that an IKKβ inhibitor emodin suppressed the growth of gemcitabine resistant pancreatic cancer cell through apoptosis [64]. Inhibition of IKKβ by a quinoxaline urea inhibitor, synthesized by Radhakrishnan et al. in 2021, also displayed the antiproliferative effect against pancreatic cancer cells T3M4 and MiaPaCa2 [65].
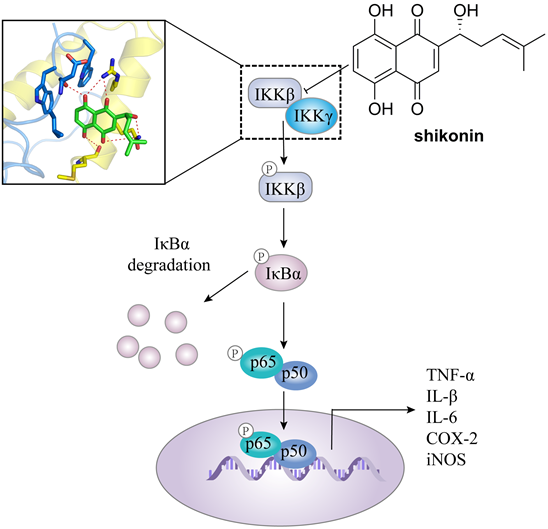
In addition, IKKβ inhibitors, e.g. abietic acid, alantolactone (16), MLN120B, and shikonin, have been used in the treatment of other cancers as well [60, 66, 67]. For colorectal cancer, Yu et al. found the antitumor effect of shikonin against colorectal cancer cells. Shikonin effectively blocked the IKKγ/IKKβ complex formation in vitro and in vivo via preventing the IKKγ-binding domain (NBD) of IKKβ from entering the hydrophobic pocket of IKKγ (Figure 3), resulting the inhibition of proliferation, migration, and invasiveness [60].
The transduction mechanism of IKKβ.
The mechanism of action of shikonin through suppressing the binding of IKKβ with IKKγ in against colorectal cancer.
CNS diseases
CNS diseases are one of the major problems threatening human health, and out of balance of the redox system and intense neuroinflammation are the major causes of CNS diseases [68]. In the CNS immune system, activation of microglial cells results in the neuroinflammation responsible for the elimination of pathogens, toxic components, and dead cells. Activated IKKβ promotes the NF-κB cascade to magnify the activization of microglial cells, allowing the release of pro-inflammatory factors and chemokines to worsen CNS diseases, therefore, suppressing the IKKβ activity is considered the strategy for the treatment of this type disease.
PD is a neurodegenerative disease characterized by the loss of dopamine neurons in substantia nigra, which often occurs in the elderly [69]. The current drugs for treating this disease can only control its further deterioration but accompanied by other adverse reactions. Recently, it has been reported that IKKβ-mediated NF-κB pathway also plays a key role in regulating neuroinflammation for PD. In LPS- or 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-mediated PD animals, the IKKβ activity is increased in substantia nigra because of the overexpression of its phosphorylation [70-72], which significantly promoted LPS-induced neurotoxicity [71], such as dopaminergic and tyrosine hydroxylase positive cell death, while its genetic abolishment or inhibition of IKKβ attenuated the neuroinflammation [70, 73-76]. For example, Zhang et al. has reported that administration of IKKβ inhibitors BAY-65-1942 inhibited the microglial activation, attenuated the loss of dopamine neurons, and enhanced levels of neurotransmitters [71], and the similar result was observed after administration of IKKβ inhibitors, such as BAY-65-1942, metformin, and ginsenoside Rb1, and peptide [70, 74, 75]. Accordingly, IKKβ functions as a therapeutic target for PD.
Alzheimer's disease (AD) is a neurodegenerative disease represented by impaired learning, memory, and cognitive abilities [77]. In AD, the extracellular amyloid beta (Aβ) deposition leads to the activation of microglia to trigger the neuroinflammation that causes the neuronal death and cognitive deficits [78]. Accumulating clinical evidences demonstrate that 20% of AD patients are associated with severe neuroinflammation [77, 79], meanwhile regulation of neuroinflammation is closely related to the immune and nervous system serves as a therapeutic strategy for AD [78]. A large number of data reasoned the activation of IKKβ/NF-κB by stimulators (e.g. TNF-α and IL-6) in neurons, microglia, and astrocytes to trigger the malignant cascade of AD [78, 80, 81]. Meanwhile, increasing of preclinical studies focused on IKKβ demonstrated the relationship between IKKβ and AD [82]. A study by Liu et al. suggested that the IKKβ deficiency in myeloid cells attenuated AD-related symptoms and pathology, such as cognitive deficits, inflammation, and Aβ load, by enhancing microglial and macrophage recruitment towards Aβ deposits and internalization [83]. Karpagam et al. has reported that celastrol (26), a natural product from Tripterygium wilfordii, inhibited the IKKβ activity to attenuate the Aβ cytotoxicity, triggering the neuroprotective effect in AD [56]. In addition, its analogue depended on the inhibition of IKKβ not only displayed therapeutic effects in AD, but also in metabolic diseases, amyotrophic lateral sclerosis, PD, and Huntington's disease [84-86].
Spinal cord injury (SCI) is a permanent motor and sensory deficits characterized by the invasive degeneration of the spinal cord tissue caused by the mechanical trauma to the neurons and surrounding vasculature [87]. Recently, Lee and co-workers revealed the role of IKKβ in neuroinflammation, one of the mechanisms of secondary SCI [88, 89]. This study found that IKKβ KO reduced the infiltration of neutrophils and macrophages and the pro-inflammatory factor expression through suppressing the myeloid cell activation, allowing the attenuation of neuronal loss and behavioral deficits in SCI [89]. Consistently, emerging evidence has indicated that targeting IKKβ with drugs or microRNAs alleviates the course of SCI [90-94]. For example, Li et al. demonstrated that tamoxifen administration inhibited the infiltration of inflammatory cells and neuron apoptosis via regulating the IKKβ pathway [94]. In addition, inhibition of IKKβ alleviated IL-1β-mediated NF-κB activation to trigger the blocking of DNA binding, resulting in the remission of tactile and cold allodynia [95].
Metabolic diseases
Metabolic diseases are becoming increasingly common in modern society, such as diabetes, NAFLD, and non-alcoholic steatohepatitis (NASH) due to people's lifestyles and irregular circadian rhythms [96]. Obesity is a low-grade sustained inflammatory disease, affecting about 30% of population all over the world [97, 98]. Furthermore, obesity has become an inducement to cause other diseases, and increases the risk of diabetes, NAFLD, and NASH under the stimulation of the chronic inflammation as well [98, 99]. Recently, increasing studies have demonstrated the effect of IKKβ in the obesity-mediated inflammation [100]. Douglass et al. found that special IKKβ genetic deletion in the astrocyte attenuated phenotypes of obesity, such as weight gain, glucose intolerance, and insulin resistance, in obesity mice [100]. Similarly, IKKβ inhibitor IMD-0354 not only promoted the adiponectin secretion in vitro, but also attenuated insulin resistance and enhanced plasma levels of adiponectin in the high-fat diet (HFD)-induced animal model [101]. In addition, SA18 and SA32, other IKKβ inhibitors, displayed similar effects in the obesity [102].
As a major public health issue, diabetes belongs to one of the family metabolic diseases characterized by hyperglycemia caused by insulin resistance [103]. A previous study by Salem et al. has reported that the specific IKKβ activation in β cells could cause to immune-mediated diabetes [104]. Furthermore, a great body of evidences have demonstrated that IKKβ genetic KO or its inhibitors displayed the therapeutic effect on diabetes [105-107]. For example, the IKKβ overexpression could suppress the insulin pathway, and IKKβ genetic deletion alleviated the course of insulin resistance in the obese animal model, such as HFD-induced and Lepob/ob obese mice [108]. Collectively, inhibition of IKKβ by salicylate or celastrol (26) decreased insulin resistance and lipid abnormalities and increased the adiponectin level to attenuate the adiposity [108, 109].
Besides, emerging evidence has indicated the overexpression of IKKβ in liver diseases, including NAFLD, NASH, and liver fibrosis [110]. NAFLD is a series of liver diseases to cause hepatic steatosis and death of liver cells, eventually resulting in cirrhosis and liver failure [57]. Over past few decades, the prevalence of NAFLD had increased year by year, and the prevalence of obesity and diabetes had increased as well. Because NAFLD was related to obesity and diabetes, it had attracted widespread attention [111-113]. Although the treatment of NAFLD had made great progress during the past decades, the mechanism of its development is still unclear [57, 114]. So far, two hypotheses on the mechanism of NAFLD are approved by academic community. One is the imbalance of fatty acid metabolism, which leads to the accumulation of triglycerides in the liver and steatosis; other is oxidative or metabolic stress and cytokine unbalance [114]. Dou et al. found that the IKKβ S-glutathionylation by glutathione disulfide amplified TNF-α mediated hepatotoxicity, revealing that IKKβ S-glutathionylation was the potential mechanism of NAFLD [57].
Others
Chemical inhibition of IKKβ is to the benefit of many other diseases, such as osteolysis, arthritis, premature birth, radiation injury, and allergy [59, 115-117]. McIntyre and co-workers investigated the protective effect of BMS-345541, an IKKβ inhibitor, and found that it could suppress the IL-1β level to attenuate inflammation and joint destruction, contributing to alleviate collagen-induced arthritis in the mouse model [117]. The similar result was observed in the rheumatoid arthritis through inhibiting the IKKβ activity by 4-[6-(cyclobutylamino)imidazo[1,2-b]pyridazin-3-yl]-2-fluoro-N-([(2S,4R)-4-fluoropyrrolidin-2-yl])methylbenzamide [118]. Furthermore, the selective IKKβ inhibitor SC-514 inhibited the IκBα degradation to inactivate the NF-κB signal, further triggering to impair RANKL (receptor activator of nuclear factor-κB ligand)-induced osteoclastogenesis [119], which was further supported by Thummuri's study showing that inhibition of IKKβ by abietic acid attenuated RANKL-induced osteoporosis [120]. The abovementioned findings all suggested that that targeting IKKβ was a potential treatment for osteoclast-related disorders. In addition, some IKKβ inhibitors, including tetrandrine, IMD-0560, and IMD-0354, were applied for the treatment of preterm delivery, radiation damage, and hyperalgesia [59, 115, 116]. So far, many IKKβ inhibitors displayed significant therapeutic effects in pre-clinical animal experiments, and some inhibitors, such as MLN-120B, IMD-2560, and SAR-113945, have finished the phase I or II clinical trial for the safety, tolerability, and pharmacokinetics. However, IKKβ deficient mice die on 14th day of gestation because of massive apoptosis of hepatocytes, therefore, these clinical trials have to be terminated [38, 121]. Therefore, discovery of new IKKβ inhibitors has sharply declined in the recent decade [121].
Targeting IKKβ with pharmacological small-molecule compounds
IKKβ potentials have been paid more and more attentions in the prevention and treatment of various diseases as the discovery of the first IKKβ inhibitor SPC839 (57) in 2001, therefore, its development has become a hot topic. To date, IKKβ inhibitors have been found from various pathways, including natural products and chemical synthesis, herein, advances of IKKβ inhibitors are summarized.
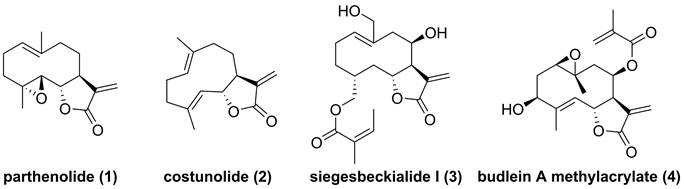
Chemical structure of parthenolide (1), costunolide (2), siegesbeckialide I (3), and budlein A methylacrylate (4).
Natural IKKβ inhibitors
Sesquiterpenoids
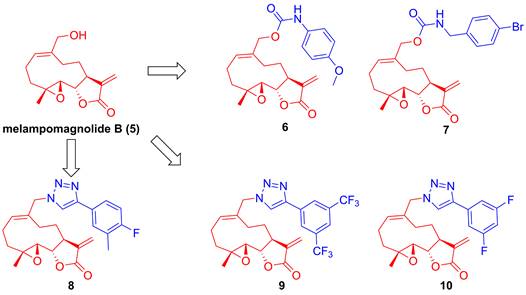
Parthenolide (1, Figure 4), naturally occurred in Tanacetum parthenium, belongs to the family of 5,10-seco-eudesmane type sesquiterpenoids, and its inhibitory effect towards IKKβ was first reported by Benjamin in 2001 [122, 123]. Researchers from China also found that a naturally occurring parthenolide analogue, costunolide (2), could covalently bind to Cys179 of IKKβ, and inhibit its activity, triggering its protective effect toward atherosclerosis in HFD-fed ApoE-/- mice [124]. Sigesbeckia glabrescens is a species of the genus Sigesbeckia first recorded in Xinxiu Herba written by Su Jing (Tang Dynasty) [125]. Based on its chemical constituents, Gao and colleagues obtained twenty-four sesquiterpenoids, including ten new compounds. They found that siegesbeckialide I (3, Figure 4) significantly displayed an inhibitory effect against IKKβ via covalently binding to amino acid residue Cys46 in the KD N region of IKKβ with the olefinic bond of an α,β-unsaturated lactone [125]. Budlein A methylacrylate (4), a natural sesquiterpenoid isolated from the genus Helianthus, displayed an anti-triple-negative breast cancer effect [126]. Based on the result of pull down depended on its probe biotinylated 4 (Bio-4, Figure 4), Wang et al. found that 4 could bind to IKKβ through the covalently binding of Cys179 in IKKβ [126]. Subsequently, Crooks's group demonstrated the potential of a parthenolide analogue, melampomagnolide B (5, Figure 5), towards IKKβ, and optimized its structural to afford a library of its analogues, such as 6-10 [127-129]. Because of their binding to the IKKβ ULD, 6 and 7 displayed significantly inhibitory effects [127].
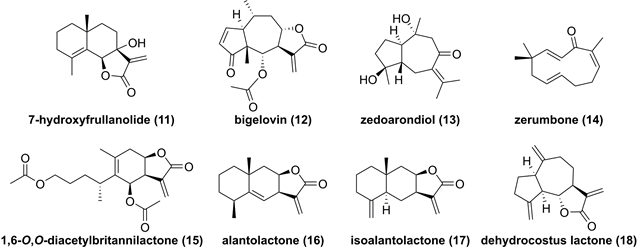
7-Hydroxyfrullanolide (11, Figure 6), isolated from Sphaeranthus indicus, is a sesquiterpene lactone with a 6/6/5 skeleton and possesses various bioactive activities [130]. Fonseca et al. found that 7-hydroxyfrullanolide (11) could suppress LPS-mediated NF-κB activation [130]. In-depth investigation on its mechanism revealed its anti-inflammatory effect depended on IKKβ, which was supported by the IKKβ kinase assay [130]. Feng and co-workers demonstrated that bigelovin (12), a pseudoguaiane-type sesquiterpenoid from the genus Inula, could induce the IKKβ degradation to suppress the NF-κB pathway, thus causing apoptosis of colon cancer cell [131]. Recent studies reported potentials of zedoarondiol (13), zerumbone (14), and 1,6-O,O-diacetylbritannilactone (15) against IKKβ as well [132-134]. In addition, our group also investigated chemical constituents of Inula helenium known as “Tu mu xiang” in Chinese, and found that alantolactone (16), isoalantolacton (17), and dehydrocostus lactone (18) exerted antitumor activities against glioblastoma through targeting IKKβ [66, 135, 136].
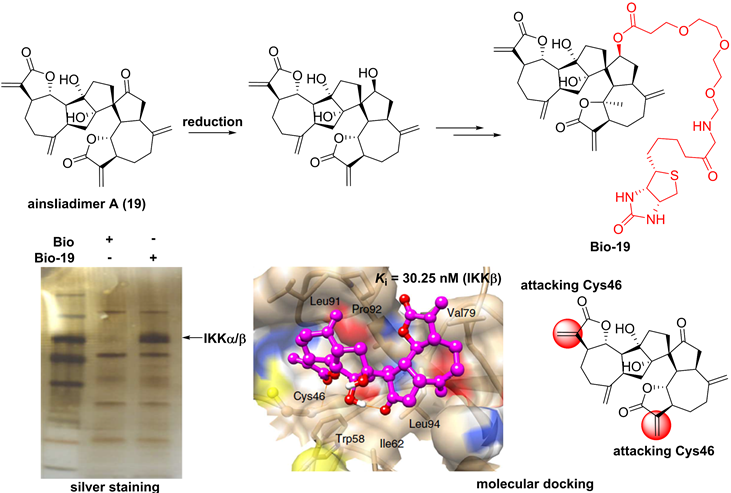
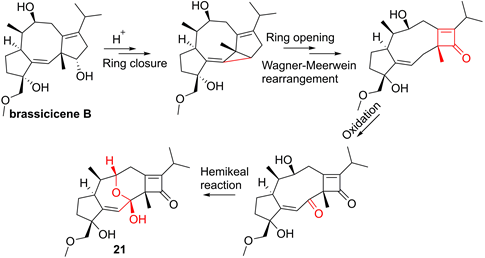
For the sesquiterpenoid dimer, Lei's group found that ainsliadimer A (19, Figure 7), isolated from Ainsliaea macrocephala, displayed an anti-inflammatory potential in Raw264.7 cells after exposure to LPS [137]. In order to reveal its direct cellular target, Lei and co-workers reduced the ketone carbonyl moiety of ainsliadimer A (19) and linked a biotin to afford a probe Bio-19. Using this probe to fish the binding proteins, Lei et al. found that ainsliadimer A (19) could selectively suppress IKKα/β via covalently binding to Cys46 in the activation loop of IKKα/β, meanwhile ainsliadimer A (19) had a good affinity (Ki = 30.25 nM) and specific reactivity (Kinact = 7.74 min-1) with IKKβ [137].
Diterpenoids
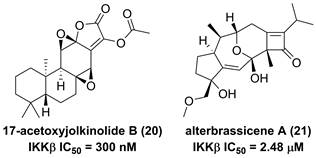
The root of dried Euphorbia fischeriana Steud is a traditional Chinese medicine first recorded 2,000 years ago, known as Langdu in Chinese, to treat cancer, edema, and ascites [138]. Yan and co-workers afforded seven diterpenoids from E. fischeriana, and found that 17-acetoxyjolkinolide B (20, Figure 8) displayed a remarkable inhibitory activity against TNF-α-medicated NF-κB activation. The investigation on its action mechanism demonstrated that 17-acetoxyjolkinolide B (20) could suppress the phosphorylation of TNF-α-medicated IκB and the nuclear translocation of p65 via inhibiting IKKβ (IC50 = 300 nM) [139]. In addition, a study by Hu et al. focused on fusicoccanes from Alternaria brassicicola found alterbrassicene A (21) sharing a 5/9/4-fused carbocyclic skeleton with a rare fused 2-cyclobuten-1-one motif [140], and its biosynthetic pathway was proposed as shown in Figure 9. It is worth noting that alterbrassicene A (21) displayed an inhibitory effect against IKKβ (IC50 = 2.48 μM), and it could bind to KD N and C lobes of IKKβ through hydrogen bond interactions with Leu21 and Arg31 [140]. Andrographolide (22, Figure 10) is a characteristic ent-labdane diterpenoid of Andrographis paniculata (Burm. f) Nees, a traditional medicinal plant in China, and possesses multiple bioactivities, such as antitumor and anti-inflammatory effects [141]. Chao and co-workers demonstrated that andrographolide (22) inhibited the IKKα/β activity to reduce the increase of TNF-α mediated ICAM-1 expression and the activation of TNF-α mediated NF-κB [141]. Cryptotanshinone (23), a diterpene quinone from traditional Chinese herb Salvia miltiorrhiza, has multiple potentials on various diseases [142]. In 2016, Wu et al. reported its anticancer activity towards acute lymphoblastic leukemia, and revealed its mechanism involved in the binding to the ATP binding region of IKKβ as same as MG132 (an IKKβ inhibitor) through interactions of Val29, Glu97, Tyr98, Cys99, Glu100, and Gly102 [142]. Phytochemical investigation focused on Sagittaria trifolia resulted in 11 diterpenoids, and subsequent study on anti-proliferative effects demonstrated the inhibitory potential of sclareol (24) toward IKKβ [143]. In addition, triptolidenol (25) was reported as well, and the result of molecular docking demonstrated its mechanism of action towards IKKβ [144].
Triterpenoids
Triterpenoids are common type compounds in natural products, so far, some of triterpenoids have been reported from Celastrus orbiculatus, Azadirachta indica, and Codonopsis lanceolata. Celastrol (26, Figure 11) is a quinone methide triterpenoid isolated from Celastrus orbiculatus, and showed inhibitory effects against TNF-α-, IKKβ-, or MEKK1-mediated NF-κB activation on the basis of NF-κB luciferase activity [145]. Subsequently, Lee and colleagues found that celastrol (26) could dose-dependently suppress the IKKβ activity, and the mutation of Cys179 in the activation loop of IKKβ abolished its effect, indicating that it targeted amino acid residue Cys179 of IKKβ to inhibit the activation of NF-κB [145]. In 2006, Ahmad et al. also reported that 2-cyano-3,12-dioxooleana-1,9,-dien-28-oic acid methyl ester (27), a pentacyclic triterpene, interacted with Cys179 to inhibit the IKKβ activity [146]. Subsequent studies focused on the structural modification of 27 afforded a new synthetic triterpenoid inhibitor 2-cyano-3,12-dioxooleana-1,9,-dien-28-oic acid imidazolide (28) [147]. In 2010, Gupta et al. found that the characteristic constituent of Azadirachta indica, nimbolide (29), promoted the apoptosis induced by chemotherapeutic agents in tumor cells [148].
Structural modification of melampomagnolide B (5)
Chemical structure of sequiterpnoids 11-18.
Chemical structure of ainsliadimer A (19) and the discovery of its covalently binding target.
Chemical structures of diterpenoids 20 and 21.
Plausible Biosynthetic Pathway for 21.
Chemical structures of diterpenoids 22-25.
Chemical structures of diterpenoids 26-37.
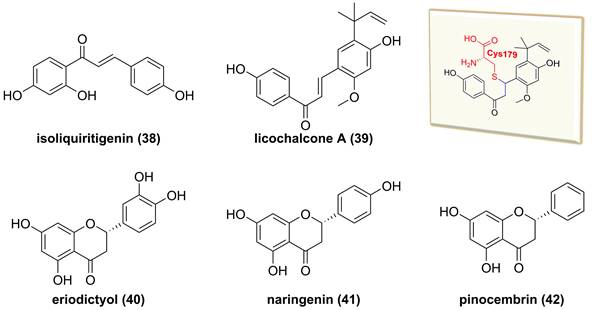
Chemical structures of diterpenoids 38-42 and interaction of 39 with IKKβ.
The study on the mechanism of action of 29 demonstrated that it could modify Cys179 of IKKβ to suppress the IKKβ activity, which was supported by amino acid mutation Cys179Ala [148]. In the past decade, pachymic acid (30) from Poria cocos, escin (31), acetyl-11-keto-β-boswellic acid (32) from the genus Boswellia, pristimerin (33) from Menispermum dauricum, oleanolic acid acetate (34) from Vigna angularis, deacetyl ganoderic acid (35) from Ganoderma lucidum, 3-O-acetylrubianol C (36) from Rubia philippinesis, and lancemaside (37) from Codonopsis lanceolata inhibited the IKKβ activity or regulated the phosphorylation of IKKβ [149-155]. For example, oleanolic acid acetate (34) exerted an anti-inflammatory effect via inhibiting IKKα/β to reduce the production of embryonic alkaline phosphatase and pro-inflammatory cytokines, including MCP-1, IL-1, IL-8, VCAM-1, and ICAM-1 [152]. In addition, lancemaside A (37), a pentacyclic triterpenoid glucose isolated from Codonopsis lanceolata, inhibited the LPS-mediated inflammatory response in Raw264.7 cells through blocking the IKKβ activation [156].
Flavonoids
Glycyrrhiza inflata is a traditional Chinese medicine to treat inflammatory related diseases, and its major chemical constituents are flavonoids and triterpenoids [157]. Isoliquiritigenin (38, Figure 12) and licochalcone A (39), isolated from the genus Glycyrrhiza, belong to the chalcone family and possess multiple bioactive activities, such as anti-inflammatory and anti-tumor effects [158, 159]. A study by Yan and co-workers reported the inhibitory potential of isoliquiritigenin (38) against human T lymphocyte activation [158]. Its mechanism involved in the covalently binding Cys46 of IKKβ via Michael addition because of the presence of an α,β-unsaturated ketone moiety in isoliquiritigenin (38) [158]. Similarly, Megumi et al. found that licochalcone A (39) inhibited IKK complex activation and IκB degradation via the covalently binding to amino acid residue Cys179 of IKKβ [159]. Additionally, some of dihyflavones, such as erioddictyol (40), naringenin (41), and pinocembrin (42), could interact with amino acid residues Thr23, Glu97, Cys99, and Asp166 in the ATP binding domain through hydrogen bonds to suppress the IKKβ activity [160].
Alkaloids
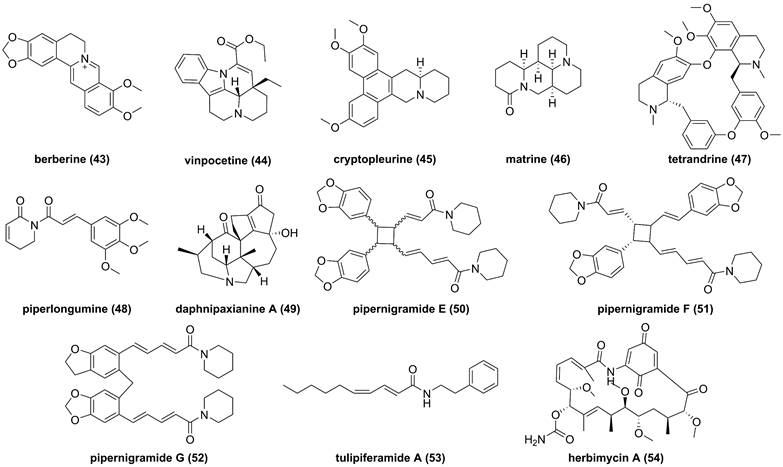
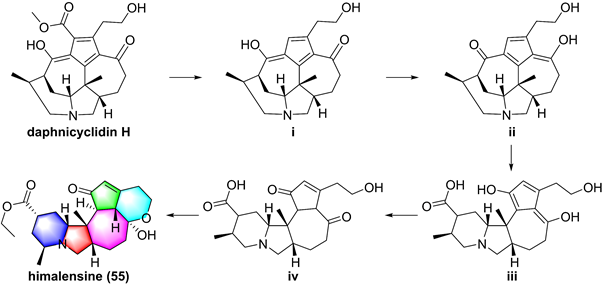
So far, fourteen natural alkaloids have been reported to possess inhibitory potentials towards IKKβ (Figure 13-15), including berberine (43), vinpocetine (44), cryptopleurin (45), matrine (46), tetrandrine (47), piperlongumine (48), daphnipaxianine A (49), pipernigamides E-G (50-52), tulipiferamide A (53), herbimycin A (54), himalesine (55), and ellipticine (56) [115, 161-169]. Among them, himalensine (55, Figure 14), isolated from Daphniphyllum himalense, possesses an unprecedented 6/5/6/7/5/6 skeleton, and its proposed biosynthetic pathway was discussed [164]. The decarboxylation of daphnicyclidin H, is a major constituent of Daphniphyllum himalense, yielded intermediate i, followed by Baeyer-Villager oxidation, ring opening, and esterification to afford 55 [164].
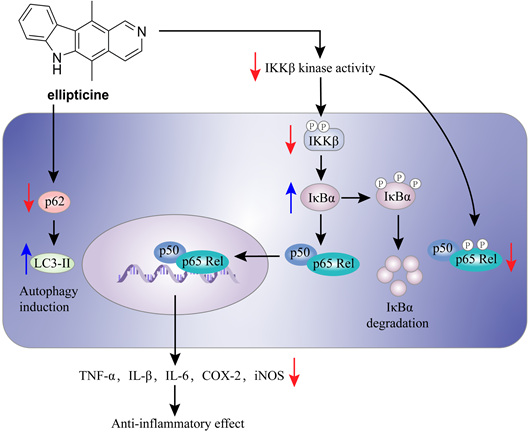
Herbimycin A (54) is an acetomycin antibiotic that possesses a potent Src tyrosine kinase inhibitory activity [170-172]. Ogino and colleagues found that herbimycin A (54) could reduce the iNOS expression and chemokine production via inactivating NF-κB. Subsequently study demonstrated that herbimycin A (54) could bind to Cys59 in the KD region of IKKβ, resulting in the inhibition of the IKKβ activity, which was supported by the experiment of amino acid mutation (Cys59Ala) [173]. A study by Chen et al. focused on the discovery of IKKβ inhibitors and their application in the inflammation demonstrated the potential of ellipticine (56, Figure 15), an alkaloid from Ochrosia elliptica [174]. Ellipticine (56) could suppress the IKKβ activity through directly binding to IKKβ, resulting in the inactivation of NF-κB signaling pathway on the basis of kinase and binding experiments, and the result of amino acid mutation suggested that Cys46 in the activation loop of IKKβ played a role in the inhibition of ellipticine (56) [174].
IKKβ inhibitors from chemical synthesis
Pyrimidines
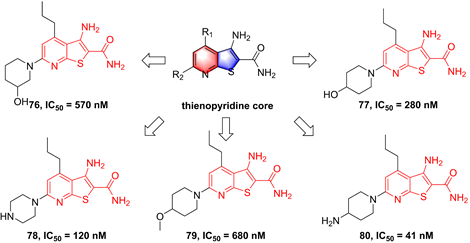
In 2001, Bhagwat et al. has reported a small molecule with an aminopyrimidine core, SPC839 (57, Figure 16), and its effect in an arthritis animal model [175]. The pharmacological study on its action mechanism demonstrated its effect was based on the inhibition of SPC839 (57) on the IKKβ activity, which led to a new era of the development of IKKβ inhibitors. Bingham et al. summarized the structural characteristic of SPC839 (57) and built the aminopyrimidine scaffold to afford a series of aminopyrimidine analogues, such as 58. Although this compound had a better potential than SPC839 (57), its selectivity was not satisfactory. Afterwards, they tried to modify the substituent moiety at the right of 58 through the induction of the electron-withdrawing (e.g. -CN, -NO2, and -Cl) or electron-donating (e.g. -OCH3 and -CH3) groups, and found that the electron-withdrawing group was in favor of the IKKβ selectivity (59, IKKα/IKKβ = 104.8; 60, IKKα/IKKβ = 5.7; 61, IKKα/IKKβ = 22.3) [175]. The potential interaction of this type inhibitor with IKKβ was reported as well that Cys99 interacted with the backbone NH and the aminopyrimidine-nitrogen [176]. Bingham and co-workers kept on the investigation of this type inhibitor, resulting in the production of 63 and 64 with an IKKβ selectivity [176]. Subsequently, pharmaceutical chemists from Korea also synthesized piperidinyl aminopyrimidine inhibitors, such as 65, whereas its potential had not been improved [177]. Crombie' group from American and Hong' group from Korea re-designed the benzenesulfonamido aminopyrimidine scaffold through the introduction of other substituent groups at R1, respectively, and got two representative inhibitors 66 and 67 that possessed a low nanomole level of inhibitory potentials [178, 179]. An analysis of their structures indicated that the sulfanilamide and amino groups formed three hydrogen bonds with Gly22, Asp103, and Lys106, respectively, except for the classical interactions of the aminopyrimidine backbone with Cys99. Additionally, 66 was applied for the investigation on LPS-mediated inflammation in vitro, and showed a remarkable anti-inflammatory effect [178].
Chemical structures of diterpenoids 43-54.
Proposed biosynthetic pathway of himalensine (55).
The mechanism of action of ellipticine (56) in the inflammation.
Pyridines
In 2002, Murata et al. performed the high-throughput screening based on the library of Bayer compounds, and found that 2-amino-3-cyano-4-aryl-6-(2-hydroxy-phenyl)pyridine analogue (68, Figure 17) displayed a potent inhibitory activity against IKKβ (IC50 = 1500 nM) [180]. Based on the skeleton of 2-amino-3-cyano-6-(2-hydroxy-phenyl)pyridine, a series of inhibitors were designed and synthesized, yielding compounds 69-74 [180, 181]. Among them, although compound 73 showed a low nanomole potential (IC50 = 40 nM), its performance was not as good as expected at cellular level (IC50 = 5000 nM) [181]. After detailed analysis of their structure-activity relationship (SAR), a cyclopropyl analog 75 afforded from the structure of 74 not only displayed a low nanomole inhibitory effect (IC50 = 8.5 nM), but also possessed reasonable aqueous solubility (0.12 mg/mL in buffer), excellent permeability, and orally bioavailability [182]. Based on previous studies, Wu et al. designed the thienopyridine skeleton (Figure 18) and optimized this type IKKβ inhibitor through the modification of R1 and R2 substituent groups [183]. The SAR investigation indicated the role of n-propyl in R1 substituent group, and followed by the modification to produce selective inhibitors 76-80 [183]. Meanwhile, 76-78 displayed anti-inflammatory effects against LPS-mediated TNF-α production in vivo, which provided a thought for the development of novel IKKβ inhibitors.
Aminopyrimidine type IKKβ inhibitors.
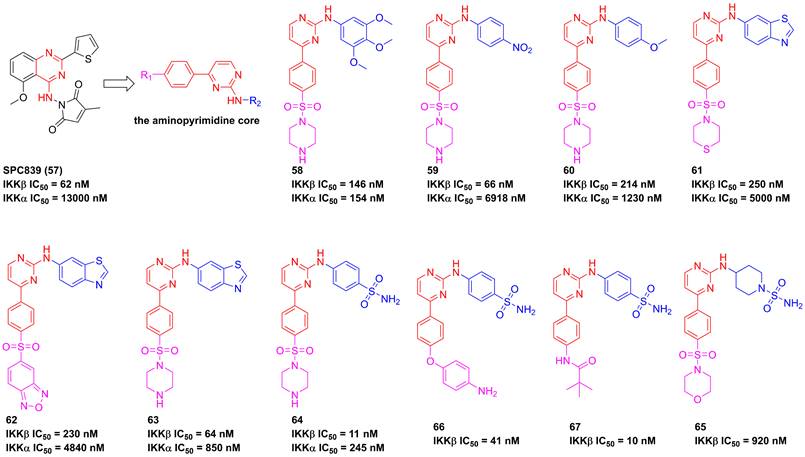
Optimization based on the skeleton of 2-amino-3-cyano-6-(2-hydroxy-phenyl)pyridine.
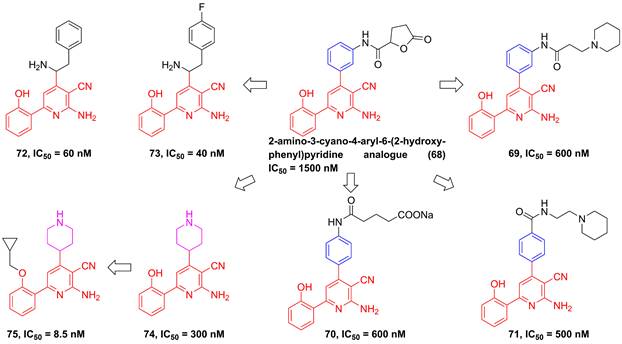
Optimization of thienopyridine core IKKβ inhibitors.
Pyrazine type IKKβ inhibitors.
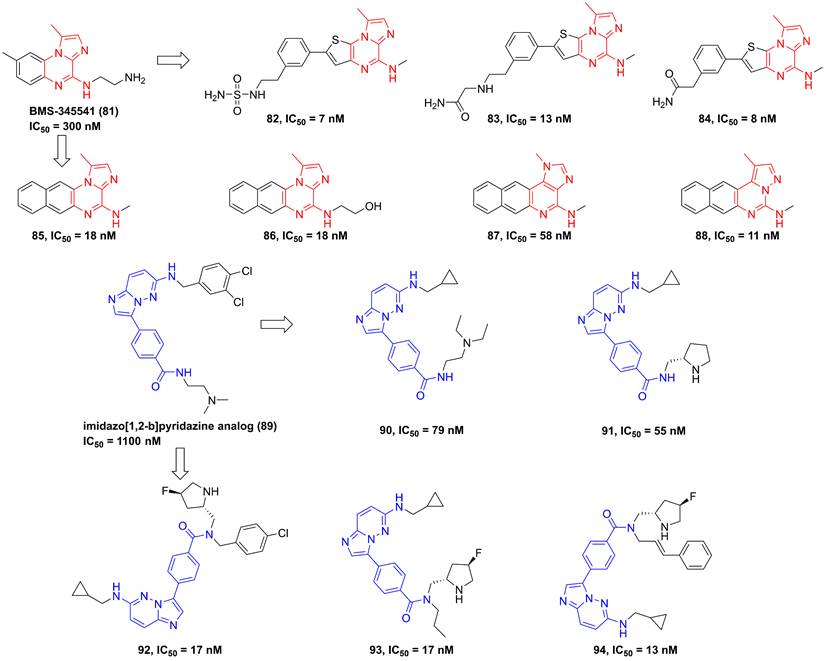
Pyrazines
In 2003, Burke and co-workers synthesized 4-(2′-aminoethyl)amino-1,8-dimethylimidazo(1,2-a)quinoxaline (BMS-345541, 81, Figure 19) that was a selective IKKβ inhibitor (IKKα IC50/IKKβ IC50 = 13.3) via binding to the allosteric site of IKKβ [35]. Furthermore, BMS-345541 (81) possessed an excellent pharmacokinetics and in vitro and in vivo anti-inflammatory effects through the release of LPS-mediated inflammatory factors [35], such as TNF-α, IL-1β, IL-8, or IL-6. According to the interaction with an allosteric site of IKKβ, a library of inhibitors with the imidazoquinoxaline core were developed and synthesized, such as 82-88 [184, 185]. Among them, 83 not only possessed a low nanomole potential, but also displayed a good selectivity (IKKα IC50/IKKβ IC50 = 30). Meanwhile, the delightful pharmacokinetic property of 83 led to its perfect anti-inflammatory effect for reducing the release of LPS-mediated TNF-α in vivo [185].
Upon the high-throughput screening, Shimizu et al. also found the imidazo[1,2-b]pyridazine scaffold of 89, and modified substituents in the 3-position of this type skeleton through the introduction of an electron-withdrawing group and hydrophobic substituents to the amide nitrogen to improve permeability and affinity for IKKβ. Therefore, they got many kinds of selective inhibitors 90-94 in 2010 and 2011, and summarized the interaction of this type inhibitor as well, requiring the role of Glu61 and Cys99 in the inhibition of IKKβ for pyrazine type IKKβ inhibitors [186, 187].
Quinoxalines and isoquinolines
Previously, Christopher's group disclosed 2-amino-3,5-diarylbenzamide inhibitors (Figure 20), and found the advantage of the sulfonamide moiety for the IKKβ inhibitory potency [188]. Therefore, they applied a 3D pharmacophore on the basis of interactions between IKKβ and phenylcarboxamide inhibitors to filter the database down to 289 halides. Depended on this result, they designed and synthesized a series of potential inhibitors, such as 95 and 96. These two compounds shared the same inhibitory potential against IKKβ and the special selectivity (IKKα IC50/IKKβ IC50, 25-fold for 95; 79-fold for 96), which was further supported by the molecular stimulation [188].
Isoquinoline type IKKβ inhibitors and its interaction with IKKβ.
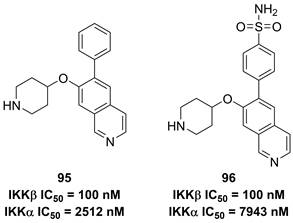
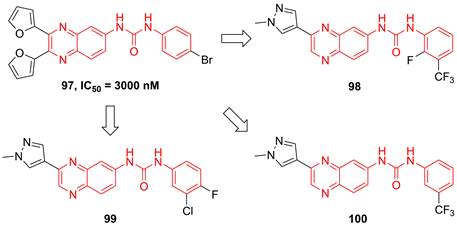
Recently, Radhakrishnan and co-workers found the hypothesis that IKKβ served as the signaling node to regulate the transcription and translation through the NF-kB and mTOR/p-S6K/p-eIF4EBP axis in tumors [189]. Based on the result of the kinase screen experiment, they demonstrated the potential of a quinoxaline urea 97 (Figure 21) in the inhibition of the IKKβ activity, and used it to confirmed the hypothesis [189]. In 2021, Radhakrishnan and colleges continued to optimize the structure of a quinoxaline urea inhibitor 97 and got the more potent inhibitors than the parent 97, such as 98-100 [65]. It was noteworthy that 98 not only showed the potent inhibitory effect but also possessed a good pharmacokinetics property. Preclinical results indicated the more potent anti-inflammatory (2.5 fold) and anti-tumor (4.3 fold) effects of 100 than the parent 97 [65], which provided a new direction for developing IKKβ inhibitors.
Benzamides
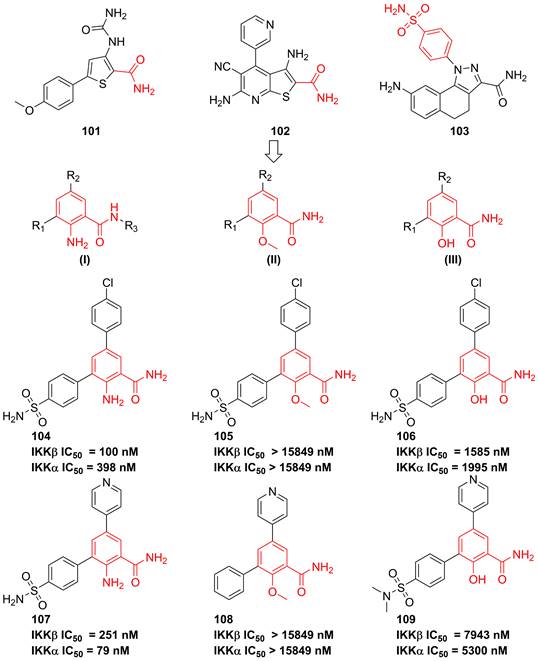
In 2007, Christopher and colleges analyzed recent IKK inhibitors, e.g. 101-103 (Figure 22), and found that 101-103 had a common motif, where the orientation of a primary amide was restricted by an adjacent hydrogen-bonding functionality, therefore, they tried to explore a new template based on the benzamide skeleton for developing IKKβ inhibitors [190]. The 2-amino-benzamide scaffold by modifying the C-2 substituents was constructed by introducing the amino, hydroxy, or methoxy group to afford potent inhibitors, such as 104-109 [190]. Although without the satisfactory result, this type skeleton displayed the potential as the temple of IKKβ inhibitors, which provided a valuable experience for developing IKKβ inhibitors.
Thiophenes and thiazolidines
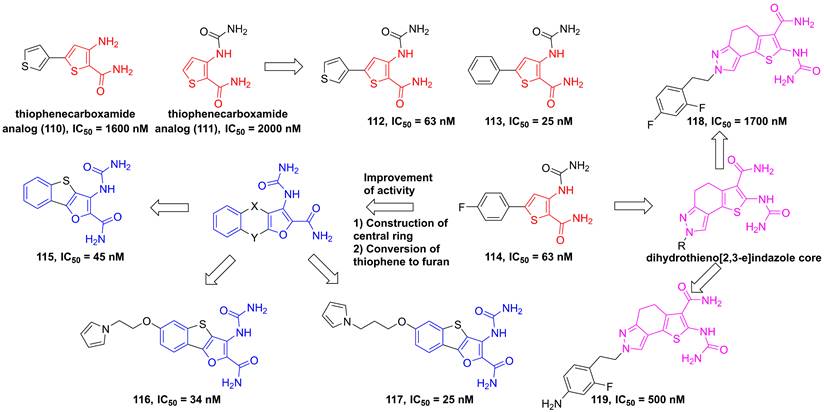
In 2004, through the high throughput screening to find two potential thiophenecarboxamides 110 and 111 (Figure 23), Baxter et al. optimized the thiophene core to produce a series of analogs [191]. Among them, compounds 112-114 showed low nanomole inhibitory activities against IKKβ and good pharmacokinetics [191]. Analysis of the structural characteristic of thiophenecarboxamide 114, a novel class of tricyclic furan derivatives were designed and synthesized by Sugiyama and colleagues, such as 3-[(aminocarbonyl)amino]-benzothieno-[3,2-b]furan-2-carboxamide derivatives 115-117 [192]. Introduction of substituents onto the benzothieno[3,2-b]furan to overcome the low metabolic stability yielded a series of 6-alkoxy derivatives, meanwhile improved oral bioavailabilities of inhibitors. The potent inhibitory activity of these derivatives resulted from an intramolecular non-bonded S…O interaction, except for four hydrogen bond interactions with Glu97, Tyr98, and Cys99, which was further supported by the co-crystal result [192]. A subsequent study by Takahashi et al. built a dihydrothieno[2,3-e]indazole core to afford a library of IKKβ inhibitors, such as 118 and 119, while having unsatisfying inhibitory effects [193].
Quinoxaline type IKKβ inhibitors.
Optimization of benzamide type IKKβ inhibitors.
Optimization of thiophene type inhibitors.
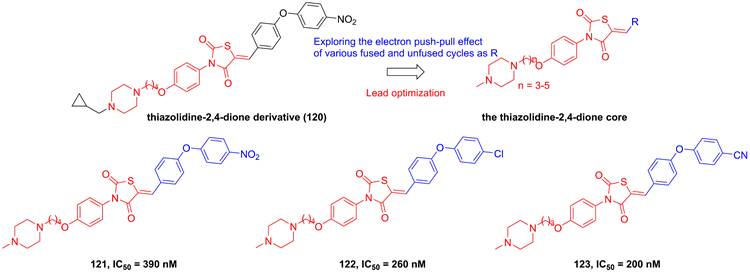
Roh's group found a lead compound (120, Figure 24) with a thiazolidine-2,4-dione skeleton and its potential in the inhibition of IKKβ (IC50 = 1500 nM), therefore, they tried to optimize this core via exploring the electron push-pull effect of the right moiety and analyze the SAR [194]. Fortunately, 121 and 122 showcased more potent inhibitory effects than the parent (about 4-6 fold) [194]. Subsequently, his group kept on the modification of the substituent depended on this skeleton, and got a library of the thiazolidine-2,4-dione derivatives. Among them, 123 with the cyano group at the right side possessed the sub nanomole level in the inhibition of IKKβ, meanwhile remaining the pharmacokinetics property and anti-inflammatory effect in vitro, which was further explained through the molecular stimulation showing the role of Arg47, Arg55, Trp58, and Leu91 for its potential [195]. In the in vivo experiment, 123 enhanced the mortality of septic shock induced mice (80% survival), demonstrating its protective potential for septic shock [195].
Rhodanines
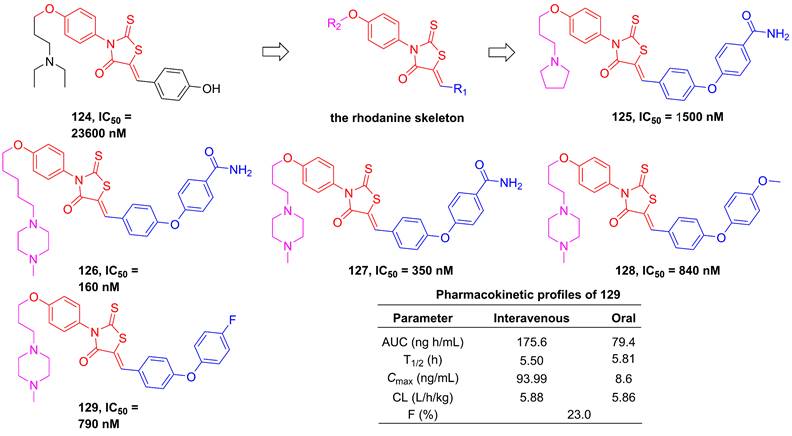
In 2012, Song et al. employed high-throughput screening to assay the inhibitory effect of the in-house library of compounds, in order to develop the new generation of IKKβ inhibitors [196]. They found a hit compound 124 (Figure 25) sharing the rhodamine scaffold from druggable and transformative points of view [196]. Therefore, the rhodanine core served as the basic skeleton to design IKKβ inhibitors, such as 125-127, and the result of the kinase assessment indicated the selectivity of 127 in the inhibition of the IKKβ inhibitors as well [196]. Although the good selectivity and inhibitory effect of this type compound, 125-127 did not display satisfactory pharmacokinetic property. Skin's group subsequently optimized its metabolic property through the modification of the right moiety based on the structure of 127, resulting in the production of 128 and 129 [197]. It was very excited that 129 remained a selectivity and inhibitory effect of the rhodanine type inhibitors, while possessed a good metabolic stability (T1/2 = 239 min in microsome; T1/2 = 89 min in plasma) and pharmacokinetic character [197].
Conclusion and perspective
In summary, we described the structure and transduction mechanism of IKKβ and its inhibitors from natural products (e.g. sesquiterpenoids, diterpenoids, flavonoids, and alkaloids) and chemically synthesized (e.g. pyrimidines, pyridines, pyrazines, and benzamides). Furthermore, inhibitory potentials of IKKβ are associated with in various diseases through regulating the NF-κB pathway, including cancer, PD, diabetes, SCI, NAFLD, osteolysis, and arthritis, therefore, it has been considered a potential target. Notably, for inhibitors from chemically synthesized, pyrimidine- and pyrazine-type compounds display low nanomole inhibitory effects, which attributes to the aromatic nitrogen atom in the skeleton of pyrimidine and pyrazine due to its hydrogen bond interaction with Cys99 of IKKβ, but their physical properties (water solubility), bioavailability, and pharmacokinetic parameters remains great improving spaces. Although the clinical trial of IKKβ inhibitors, such as IMD-2560, and SAR-113945, do not show satisfactory results for the safety, efficacy, and selectivity, natural products with more complex and changeable structural skeletons offer endless possibilities for discovering IKKβ inhibitors. Among naturally occurred inhibitors, sesquiterpenoids with an α,β-unsaturated lactone moiety have great potentials because they covalently bind to amino acid residues Cys46 and Cys179 of IKKβ. However, the total synthesis of natural products with multiply chiral centers has always been a difficult problem, therefore, simplification and extraction of effective structures of natural inhibitors may be an efficient means to synthetize new type IKKβ inhibitors in the future.
Optimization of thiazolidine IKKβ inhibitors based on the thiazolidine-2,4-dione core.
Rhodanine type inhibitors.
Abbreviations
Aβ: amyloid beta; AD: Alzheimer's disease; CAC: colitis-associated cancer; CNS: central nervous system; COX-2: cyclooxygenase-2; DNA: deoxyribonucleic acid; HFD: high-fat diet; IgG: immunoglobulin G; IKK: inhibitory kappa B kinase; IKKβ: inhibitory kappa B kinase beta; IκB: inhibitors of kappa B; IL-6: interleukin-6; iNOS: inducible nitric oxide synthase; KD C: C-terminal kinase domain; KD N: N-terminal kinase domain; KO: knockout; LPS: lipopolysaccharide; MEKK3: mitogen-activated protein kinase kinase kinase 3; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; NAFLD: non-alcoholic fatty liver disease; NAK: NF-κB activating kinase; NASH: non-alcoholic steatohepatitis; NF-κB: nuclear factor kappa-B; NIK: NF-κB-inducing kinase; NLS: nuclear localization sequence; PD: Parkinson's disease; RANKL: receptor activator of nuclear factor-κB ligand; SCI: spinal cord injury; SDD: scaffold dimerization domain; SNP: single nucleotide polymorphism; TAK1: TGF-beta-activated kinase 1; TNF-α: tumor necrosis factor alpha; TRAF6: TNF receptor-associated factor 6; ULD: ubiquitin-like domain.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 82274069), China Postdoctoral Science Foundation (No. 2022M720095 and 2023T160434), and Young Elite Scientists Sponsorship Program by China Association of Chinese Medicine (No. 2022-QNRC2-B09).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46:705-716
2. Karin M, Yamamoto Y, Wang QM. The IKK NF-kappa B system: a treasure trove for drug development. Nat Rev Drug Discov. 2004;3:17-26
3. Gamble C, McIntosh K, Scott R. et al. Inhibitory kappa B Kinases as targets for pharmacological regulation. Br J Pharmacol. 2012;165:802-819
4. Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195-2224
5. Schmitz ML, Bacher S, Kracht M. I kappa B-independent control of NF-kappa B activity by modulatory phosphorylations. Trends Biochem Sci. 2001;26:186-190
6. Mao Y, Jiang F, Xu XJ. et al. Inhibition of IGF2BP1 attenuates renal injury and inflammation by alleviating m6A modifications and E2F1/MIF pathway. Int J Biol Sci. 2023;19:593-609
7. Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853-6866
8. Yang J, Lin Y, Guo Z. et al. The essential role of MEKK3 in TNF-induced NF-kappaB activation. Nat Immunol. 2001;2:620-624
9. Schmidt C, Peng B, Li Z. et al. Mechanisms of proinflammatory cytokine-induced biphasic NF-kappaB activation. Mol Cell. 2003;12:1287-1300
10. Yang J, Splittgerber R, Yull FE. et al. Conditional ablation of Ikkb inhibits melanoma tumor development in mice. J Clin Invest. 2010;120:2563-2574
11. Nardi M, Feinmark SJ, Hu L. et al. Complement-independent Ab-induced peroxide lysis of platelets requires 12-lipoxygenase and a platelet NADPH oxidase pathway. J Clin Invest. 2004;113:973-980
12. Xue N, Yang X, Wu R. et al. Synthesis and biological evaluation of imidazol-2-one derivatives as potential antitumor agents. Bioorg Med Chem. 2008;16:2550-2557
13. Baeuerle PA, Baichwal VR. NF-kappa B as a frequent target for immunosuppressive and anti-inflammatory molecules. Adv Immunol. 1997;65:111-137
14. Verma IM, Stevenson JK, Schwarz EM. et al. Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723-2735
15. Ehrnst A. Separate pathways of C activation by measles virus cytotoxic antibodies: subclass analysis and capacity of F(ab) molecules to activate C via the alternative pathway. J Immunol. 1978;121:1206-1212
16. Scheidereit C. IkappaB kinase complexes: gateways to NF-kappaB activation and transcription. Oncogene. 2006;25:6685-6705
17. Xu G, Lo YC, Li Q. et al. Crystal structure of inhibitor of kappaB kinase beta. Nature. 2011;472:325-330
18. Scholefield J, Henriques R, Savulescu AF. et al. Super-resolution microscopy reveals a preformed NEMO lattice structure that is collapsed in incontinentia pigmenti. Nat Commun. 2016;7:12629
19. Brown K, Gerstberger S, Carlson L. et al. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267:1485-1488
20. Henkel T, Machleidt T, Alkalay I. et al. Rapid proteolysis of I kappa B-alpha is necessary for activation of transcription factor NF-kappa B. Nature. 1993;365:182-185
21. Winston JT, Strack P, Beer-Romero P. et al. The SCFbeta-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IkappaBalpha and beta-catenin and stimulates IkappaBalpha ubiquitination in vitro. Genes Dev. 1999;13:270-283
22. Kwok PY, Chen X. Detection of single nucleotide polymorphisms. Curr Issues Mol Biol. 2003;5:43-60
23. Bruzzoni-Giovanelli H, Gonzalez JR, Sigaux F. et al. Genetic polymorphisms associated with increased risk of developing chronic myelogenous leukemia. Oncotarget. 2015;6:36269-36277
24. Li Y, Wu Z, Zhang S. et al. Genetic variants of IkappaB kinase beta (IKBKB) and polymerase beta (POLB) were not associated with systemic lupus rrythematosus risk in a Chinese han population. PLoS One. 2015;10:e0132556
25. Cerhan JR, Liu-Mares W, Fredericksen ZS. et al. Genetic variation in tumor necrosis factor and the nuclear factor-kappaB canonical pathway and risk of non-Hodgkin's lymphoma. Cancer Epidemiol Biomarkers Prev. 2008;17:3161-3169
26. Gong Y, Zhao W, Jia Q. et al. IKBKB rs2272736 is associated with gastric cancer survival. Pharmgenomics Pers Med. 2020;13:345-352
27. Tessier F, Fontaine-Bisson B, Lefebvre JF. et al. Investigating gene-gene and gene-environment interactions in the association between overnutrition and obesity-related phenotypes. Front Genet. 2019;10:151
28. Curtin K, Wolff RK, Herrick JS. et al. Exploring multilocus associations of inflammation genes and colorectal cancer risk using hapConstructor. BMC Med Genet. 2010;11:170
29. Riedlinger J, Reicke A, Zahner H. et al. Abyssomicins, inhibitors of the para-aminobenzoic acid pathway produced by the marine Verrucosispora strain AB-18-032. J Antibiot (Tokyo). 2004;57:271-279
30. Li H, Chen Y, Li M. et al. A C-type lectin (LvCTL4) from Litopenaeus vannamei is a downstream molecule of the NF-kappaB signaling pathway and participates in antibacterial immune response. Fish Shellfish Immunol. 2015;43:257-263
31. Li ZW, Chu W, Hu Y. et al. The IKKbeta subunit of IkappaB kinase (IKK) is essential for nuclear factor kappaB activation and prevention of apoptosis. J Exp Med. 1999;189:1839-1845
32. Li Q, Estepa G, Memet S. et al. Complete lack of NF-kappaB activity in IKK1 and IKK2 double-deficient mice: additional defect in neurulation. Genes Dev. 2000;14:1729-1733
33. Xiao G, Harhaj EW, Sun SC. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol Cell. 2001;7:401-409
34. Pandey MK, Sung B, Ahn KS. et al. Gambogic acid, a novel ligand for transferrin receptor, potentiates TNF-induced apoptosis through modulation of the nuclear factor-kappaB signaling pathway. Blood. 2007;110:3517-3525
35. Burke JR, Pattoli MA, Gregor KR. et al. BMS-345541 is a highly selective inhibitor of I kappa B kinase that binds at an allosteric site of the enzyme and blocks NF-kappa B-dependent transcription in mice. J Biol Chem. 2003;278:1450-1456
36. Luo W, Jin Y, Jiang Y. et al. Doublecortin-like kinase 1 activates NF-kappaB to induce inflammatory responses by binding directly to IKKbeta. Cell Death Differ. 2023;30:1184-1197
37. De Lucca GV, Shi Q, Liu Q. et al. Small molecule reversible inhibitors of bruton's tyrosine kinase (BTK): Structure-activity relationships leading to the identification of 7-(2-hydroxypropan-2-yl)-4-[2-methyl-3-(4-oxo-3,4-dihydroquinazolin-3-yl)phenyl]-9H-carbazole-1-carboxamide (BMS-935177). J Med Chem. 2016;59:7915-7935
38. Lee DF, Hung MC. Advances in targeting IKK and IKK-related kinases for cancer therapy. Clin Cancer Res. 2008;14:5656-5662
39. Tian F, Zhou P, Kang W. et al. The small-molecule inhibitor selectivity between IKKalpha and IKKbeta kinases in NF-kappaB signaling pathway. J Recept Signal Transduct Res. 2015;35:307-318
40. Wang S, Tian Z, Lu Y. et al. Sphingosine-1-phosphate receptor 4 attenuates neutrophilic airway inflammation in experimental asthma via repressing proinflammatory macrophage activation. Int J Biol Sci. 2023;19:1597-1615
41. Ansari J, Vital SA, Yadav S. et al. Regulating neutrophil PAD4/NOX-dependent cerebrovasular thromboinflammation. Int J Biol Sci. 2023;19:852-864
42. Huang Z, Shen S, Han X. et al. Macrophage DCLK1 promotes atherosclerosis via binding to IKKbeta and inducing inflammatory responses. EMBO Mol Med. 2023;15:e17198
43. Zhang J, Luan ZL, Huo XK. et al. Direct targeting of sEH with alisol B alleviated the apoptosis, inflammation, and oxidative stress in cisplatin-induced acute kidney injury. Int J Biol Sci. 2023;19:294-310
44. Zhang J, Zhang WH, Morisseau C. et al. Genetic deletion or pharmacological inhibition of soluble epoxide hydrolase attenuated particulate matter 2.5 exposure mediated lung injury. J Hazard Mater. 2023;458:131890
45. Zhang J, Zhang M, Huo XK. et al. Macrophage inactivation by small molecule wedelolactone via targeting sEH for the treatment of LPS-induced acute lung injury. ACS Cent Sci. 2023;9:440-456
46. Yang MJ, Oppong MB, Di JR. et al. Steroidal saponins with anti-inflammatory activity from Tribulus terrestris L. Acupuncture and Herbal Medicine. 2022;2:41-48
47. Liu Y, Sa KR, Xu W. et al. 1,7-Bis(4-hydroxyphenyl)-1,4,6-heptatrien-3-one inhibits SARS-CoV-2 by targeting the nucleocapsid protein. Acta Materia Medica. 2023;2:270-280
48. Ai J, Ren YS, Cheng L. et al. Identification of key genes and active anti-inflammatory ingredients in Panax medicinal plants by climate-regulated callus culture combined with gene-component-efficacy gray correlation analysis. Acupuncture and Herbal Medicine. 2022;2:261-273
49. Wang W, Xiong LL, Wu YL. et al. New lathyrane diterpenoid hybrids have anti-inflammatory activity through the NF-κB signaling pathway and autophagy. Acta Materia Medica. 2022;1:224-243
50. Zhang J, Zhang M, Zhang WH. et al. Total flavonoids of Inula japonica alleviated the inflammatory response and oxidative stress in LPS-induced acute lung injury via inhibiting the sEH activity: Insights from lipid metabolomics. Phytomedicine. 2022;107:154380
51. Zhang J, Zhang M, Zhang WH. et al. Total terpenoids of Inula japonica activated the Nrf2 receptor to alleviate the inflammation and oxidative stress in LPS-induced acute lung injury. Phytomedicine. 2022;107:154377
52. Zhang DD. The Nrf2-Keap1-ARE signaling pathway: The regulation and dual function of Nrf2 in cancer. Antioxid Redox Signal. 2010;13:1623-1626
53. Barrs VR, Giger U, Wilson B. et al. Erythrocytic pyruvate kinase deficiency and AB blood types in Australian Abyssinian and Somali cats. Aust Vet J. 2009;87:39-44
54. An HS, Yoo JW, Jeong JH. et al. Lipocalin-2 promotes acute lung inflammation and oxidative stress by enhancing macrophage iron accumulation. Int J Biol Sci. 2023;19:1163-1177
55. Ansari D, Tingstedt B, Andersson B. et al. Pancreatic cancer: yesterday, today and tomorrow. Future Oncol. 2016;12:1929-1946
56. Veerappan K, Natarajan S, Ethiraj P. et al. Inhibition of IKKbeta by celastrol and its analogues - an in silico and in vitro approach. Pharm Biol. 2017;55:368-373
57. Dou X, Li S, Hu L. et al. Glutathione disulfide sensitizes hepatocytes to TNFalpha-mediated cytotoxicity via IKK-beta S-glutathionylation: a potential mechanism underlying non-alcoholic fatty liver disease. Exp Mol Med. 2018;50:1-16
58. Curtius K, Wright NA, Graham TA. An evolutionary perspective on field cancerization. Nat Rev Cancer. 2018;18:19-32
59. Waga K, Yamaguchi M, Miura S. et al. IKKbeta inhibitor IMD-0354 attenuates radiation damage in whole-body X-irradiated mice. Oxid Med Cell Longev. 2019;2019:5340290
60. Yu Z, Gao J, Zhang X. et al. Characterization of a small-molecule inhibitor targeting NEMO/IKKbeta to suppress colorectal cancer growth. Signal Transduct Target Ther. 2022;7:71
61. Prescott JA, Cook SJ. Targeting IKKbeta in cancer: challenges and opportunities for the therapeutic utilisation of IKKbeta inhibitors. Cells. 2018;7:115
62. Spella M, Ntaliarda G, Skiadas G. et al. Non-oncogene addiction of KRAS-mutant cancers to IL-1beta via versican and mononuclear IKKbeta. Cancers (Basel). 2023;15:1866
63. Wu DG, Yu P, Li JW. et al. Apigenin potentiates the growth inhibitory effects by IKK-beta-mediated NF-kappaB activation in pancreatic cancer cells. Toxicol Lett. 2014;224:157-164
64. Tong H, Huang Z, Chen H. et al. Emodin reverses gemcitabine resistance of pancreatic cancer cell lines through inhibition of IKKbeta/NF-kappaB signaling pathway. Onco Targets Ther. 2020;13:9839-9848
65. Sagar S, Singh S, Mallareddy JR. et al. Structure activity relationship (SAR) study identifies a quinoxaline urea analog that modulates IKKbeta phosphorylation for pancreatic cancer therapy. Eur J Med Chem. 2021;222:113579
66. Wang X, Yu Z, Wang C. et al. Alantolactone, a natural sesquiterpene lactone, has potent antitumor activity against glioblastoma by targeting IKKbeta kinase activity and interrupting NF-kappaB/COX-2-mediated signaling cascades. J Exp Clin Cancer Res. 2017;36:93
67. Liu X, Chen W, Liu Q. et al. Abietic acid suppresses non-small-cell lung cancer cell growth via blocking IKKbeta/NF-kappaB signaling. Onco Targets Ther. 2019;12:4825-4837
68. Li YQ, Guo C. A review on lactoferrin and central nervous system diseases. Cells. 2021;10:1810
69. Sun CP, Zhou JJ, Yu ZL. et al. Kurarinone alleviated Parkinson's disease via stabilization of epoxyeicosatrienoic acids in animal model. Proc Natl Acad Sci U S A. 2022;119:e2118818119
70. Li DW, Zhou FZ, Sun XC. et al. Ginsenoside Rb1 protects dopaminergic neurons from inflammatory injury induced by intranigral lipopolysaccharide injection. Neural Regen Res. 2019;14:1814-1822
71. Zhang F, Qian L, Flood PM. et al. Inhibition of IkappaB kinase-beta protects dopamine neurons against lipopolysaccharide-induced neurotoxicity. J Pharmacol Exp Ther. 2010;333:822-833
72. Mondal S, Roy A, Jana A. et al. Testing NF-kappaB-based therapy in hemiparkinsonian monkeys. J Neuroimmune Pharmacol. 2012;7:544-556
73. Popichak KA, Afzali MF, Kirkley KS. et al. Glial-neuronal signaling mechanisms underlying the neuroinflammatory effects of manganese. J Neuroinflammation. 2018;15:324
74. Tayara K, Espinosa-Oliva AM, Garcia-Dominguez I. et al. Divergent effects of metformin on an inflammatory model of Parkinson's disease. Front Cell Neurosci. 2018;12:440
75. Ghosh A, Roy A, Liu X. et al. Selective inhibition of NF-kappaB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson's disease. Proc Natl Acad Sci U S A. 2007;104:18754-18759
76. Flood PM, Qian L, Peterson LJ. et al. Transcriptional factor NF-kappaB as a target for therapy in Parkinson's disease. Parkinsons Dis. 2011;2011:216298
77. Sun CP, Zhang XY, Zhou JJ. et al. Inhibition of sEH via stabilizing the level of EETs alleviated Alzheimer's disease through GSK3beta signaling pathway. Food Chem Toxicol. 2021;156:112516
78. Leng F, Edison P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat Rev Neurol. 2021;17:157-172
79. Sun CP, Zhang XY, Morisseau C. et al. Discovery of soluble epoxide hydrolase inhibitors from chemical synthesis and natural products. J Med Chem. 2021;64:184-215
80. Cai Y, Hu J, He M. KL-FGF23-VD axis in improving late-onset Alzheimer's disease by modulating IKK/NF-kappaB signal pathway. Evid Based Complement Alternat Med. 2022;2022:3100621
81. Li Y, Jin T, Liu N. et al. A short peptide exerts neuroprotective effects on cerebral ischemia-reperfusion injury by reducing inflammation via the miR-6328/IKKbeta/NF-kappaB axis. J Neuroinflammation. 2023;20:53
82. Mattson MP, Camandola S. NF-kappaB in neuronal plasticity and neurodegenerative disorders. J Clin Invest. 2001;107:247-254
83. Liu Y, Liu X, Hao W. et al. IKKbeta deficiency in myeloid cells ameliorates Alzheimer's disease-related symptoms and pathology. J Neurosci. 2014;34:12982-12999
84. Cleren C, Calingasan NY, Chen J. et al. Celastrol protects against MPTP- and 3-nitropropionic acid-induced neurotoxicity. J Neurochem. 2005;94:995-1004
85. Ma X, Xu L, Alberobello AT. et al. Celastrol protects against obesity and metabolic dysfunction through activation of a HSF1-PGC1alpha transcriptional axis. Cell Metab. 2015;22:695-708
86. Zhang C, Zhao M, Wang B. et al. The Nrf2-NLRP3-caspase-1 axis mediates the neuroprotective effects of celastrol in Parkinson's disease. Redox Biol. 2021;47:102134
87. Sandrow-Feinberg HR, Houle JD. Exercise after spinal cord injury as an agent for neuroprotection, regeneration and rehabilitation. Brain Res. 2015;1619:12-21
88. Han X, Lu M, Wang S. et al. Targeting IKK/NF-kappaB pathway reduces infiltration of inflammatory cells and apoptosis after spinal cord injury in rats. Neurosci Lett. 2012;511:28-32
89. Kang J, Jiang MH, Min HJ. et al. IKK-beta-mediated myeloid cell activation exacerbates inflammation and inhibits recovery after spinal cord injury. Eur J Immunol. 2011;41:1266-1277
90. Li Y, Qiu H, Yao S. et al. Geniposide exerts protective effects on spinal cord injury in rats by inhibiting the IKKs/NF-kappaB signaling pathway. Int Immunopharmacol. 2021;100:108158
91. Lim H, Lee H, Noh K. et al. IKK/NF-kappaB-dependent satellite glia activation induces spinal cord microglia activation and neuropathic pain after nerve injury. Pain. 2017;158:1666-1677
92. Fei M, Li Z, Cao Y. et al. MicroRNA-182 improves spinal cord injury in mice by modulating apoptosis and the inflammatory response via IKKbeta/NF-kappaB. Lab Invest. 2021;101:1238-1253
93. Deng G, Gao Y, Cen Z. et al. miR-136-5p Regulates the Inflammatory Response by Targeting the IKKbeta/NF-kappaB/A20 Pathway After Spinal Cord Injury. Cell Physiol Biochem. 2018;50:512-524
94. Wei HY, Ma X. Tamoxifen reduces infiltration of inflammatory cells, apoptosis and inhibits IKK/NF-kB pathway after spinal cord injury in rats. Neurol Sci. 2014;35:1763-1768
95. Tegeder I, Niederberger E, Schmidt R. et al. Specific Inhibition of IkappaB kinase reduces hyperalgesia in inflammatory and neuropathic pain models in rats. J Neurosci. 2004;24:1637-1645
96. Xu S, Feng Y, He W. et al. Celastrol in metabolic diseases: Progress and application prospects. Pharmacol Res. 2021;167:105572
97. Ozcan U, Cao Q, Yilmaz E. et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457-461
98. Xu L, Nagata N, Ota T. Glucoraphanin: a broccoli sprout extract that ameliorates obesity-induced inflammation and insulin resistance. Adipocyte. 2018;7:218-225
99. Sudhakaran M, Doseff AI. The targeted impact of flavones on obesity-induced inflammation and the potential synergistic role in cancer and the gut microbiota. Molecules. 2020;25:2477
100. Douglass JD, Dorfman MD, Fasnacht R. et al. Astrocyte IKKbeta/NF-kappaB signaling is required for diet-induced obesity and hypothalamic inflammation. Mol Metab. 2017;6:366-373
101. Kamon J, Yamauchi T, Muto S. et al. A novel IKKbeta inhibitor stimulates adiponectin levels and ameliorates obesity-linked insulin resistance. Biochem Biophys Res Commun. 2004;323:242-248
102. Bhattarai BR, Ko JH, Shrestha S. et al. Inhibition of IKK-beta: a new development in the mechanism of the anti-obesity effects of PTP1B inhibitors SA18 and SA32. Bioorg Med Chem Lett. 2010;20:1075-1077
103. Okonkwo UA, DiPietro LA. Diabetes and wound angiogenesis. Int J Mol Sci. 2017 18
104. Salem HH, Trojanowski B, Fiedler K. et al. Long-term IKK2/NF-kappaB signaling in pancreatic beta-cells induces immune-mediated diabetes. Diabetes. 2014;63:960-975
105. Young MF, Findlay DM, Dominguez P. et al. Osteonectin promoter. DNA sequence analysis and S1 endonuclease site potentially associated with transcriptional control in bone cells. J Biol Chem. 1989;264:450-456
106. Eleftheriou P, Geronikaki A, Petrou A. PTP1B inhibition, a promising approach for the treatment of diabetes type II. Curr Top Med Chem. 2019;19:246-263
107. Shrestha S, Bhattarai BR, Cho H. et al. PTP1B inhibitor ertiprotafib is also a potent inhibitor of IkappaB kinase beta (IKK-beta). Bioorg Med Chem Lett. 2007;17:2728-2730
108. Yuan M, Konstantopoulos N, Lee J. et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673-1677
109. Kim JE, Lee MH, Nam DH. et al. Celastrol, an NF-kappaB inhibitor, improves insulin resistance and attenuates renal injury in db/db mice. PLoS One. 2013;8:e62068
110. Hao YR, Tang FJ, Zhang X. et al. Suppression of NF-kappaB activation by PDLIM2 restrains hepatic lipogenesis and inflammation in high fat diet induced mice. Biochem Biophys Res Commun. 2018;503:564-571
111. Choudhury J, Sanyal AJ. Insulin resistance and the pathogenesis of nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8:575-594 ix
112. Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842-845
113. James OF, Day CP. Non-alcoholic steatohepatitis (NASH): a disease of emerging identity and importance. J Hepatol. 1998;29:495-501
114. Jou J, Choi SS, Diehl AM. Mechanisms of disease progression in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:370-379
115. Zhao H, Luo F, Li H. et al. Antinociceptive effect of tetrandrine on LPS-induced hyperalgesia via the inhibition of IKKbeta phosphorylation and the COX-2/PGE(2) pathway in mice. PLoS One. 2014;9:e94586
116. Toda A, Sawada K, Fujikawa T. et al. Targeting inhibitor of kappaB kinase beta prevents inflammation-induced preterm delivery by inhibiting IL-6 production from amniotic cells. Am J Pathol. 2016;186:616-629
117. McIntyre KW, Shuster DJ, Gillooly KM. et al. A highly selective inhibitor of I kappa B kinase, BMS-345541, blocks both joint inflammation and destruction in collagen-induced arthritis in mice. Arthritis Rheum. 2003;48:2652-2659
118. Tanaka S, Toki T, Yokoyama M. et al. A novel inhibitor of I-kappaB kinase beta ameliorates experimental arthritis through downregulation of proinflammatory cytokines in arthritic joints. Biol Pharm Bull. 2014;37:87-95
119. Liu Q, Wu H, Chim SM. et al. SC-514, a selective inhibitor of IKKbeta attenuates RANKL-induced osteoclastogenesis and NF-kappaB activation. Biochem Pharmacol. 2013;86:1775-1783
120. Thummuri D, Guntuku L, Challa VS. et al. Abietic acid attenuates RANKL induced osteoclastogenesis and inflammation associated osteolysis by inhibiting the NF-KB and MAPK signaling. J Cell Physiol. 2018;234:443-453
121. Liu J, Zhuang Y, Wu J. et al. IKKbeta mediates homeostatic function in inflammation via competitively phosphorylating AMPK and IkappaBalpha. Acta Pharm Sin B. 2022;12:651-664
122. Kwok BH, Koh B, Ndubuisi MI. et al. The anti-inflammatory natural product parthenolide from the medicinal herb Feverfew directly binds to and inhibits IkappaB kinase. Chem Biol. 2001;8:759-766
123. Mathema VB, Koh YS, Thakuri BC. et al. Parthenolide, a sesquiterpene lactone, expresses multiple anti-cancer and anti-inflammatory activities. Inflammation. 2012;35:560-565
124. Huang ZQ, Luo W, Li WX. et al. Costunolide alleviates atherosclerosis in high-fat diet-fed ApoE(-/-) mice through covalently binding to IKKbeta and inhibiting NF-kappaB-mediated inflammation. Acta Pharmacol Sin. 2023;44:58-70
125. Gao X, Shen X, Zheng Y. et al. Sesquiterpene lactones from Sigesbeckia glabrescens possessing potent anti-inflammatory activity by directly binding to IKKalpha/beta. J Nat Prod. 2021;84:2808-2821
126. Wang XZ, Feng Y, Han YF. et al. Budlein A methylacrylate demonstrates potent activity against triple-negative breast cancer by targeting IkappaBalpha kinase and exportin-1. Toxicol Appl Pharmacol. 2020;408:115263
127. Penthala NR, Balasubramaniam M, Dachavaram SS. et al. Antitumor properties of novel sesquiterpene lactone analogs as NFkappaB inhibitors that bind to the IKKbeta ubiquitin-like domain (ULD). Eur J Med Chem. 2021;224:113675
128. Janganati V, Ponder J, Balasubramaniam M. et al. MMB triazole analogs are potent NF-kappaB inhibitors and anti-cancer agents against both hematological and solid tumor cells. Eur J Med Chem. 2018;157:562-581
129. Janganati V, Ponder J, Thakkar S. et al. Succinamide derivatives of melampomagnolide B and their anti-cancer activities. Bioorg Med Chem. 2017;25:3694-3705
130. Fonseca LC, Dadarkar SS, Lobo AS. et al. NF-kappaB-mediated anti-inflammatory activity of the sesquiterpene lactone 7-hydroxyfrullanolide. Eur J Pharmacol. 2011;657:41-50
131. Feng Y, Xia J, Xu X. et al. Sesquiterpene lactone bigelovin induces apoptosis of colon cancer cells through inducing IKK-beta degradation and suppressing nuclear factor kappa B activation. Anticancer Drugs. 2021;32:664-673
132. Liu YP, Wen JK, Wu YB. et al. 1,6-O,O-diacetylbritannilactones inhibits IkappaB kinase beta-dependent NF-kappaB activation. Phytomedicine. 2009;16:156-160
133. Fatima A, Abdul AB, Abdullah R. et al. Binding mode analysis of zerumbone to key signal proteins in the tumor necrosis factor pathway. Int J Mol Sci. 2015;16:2747-2766
134. Cho W, Nam JW, Kang HJ. et al. Zedoarondiol isolated from the rhizoma of Curcuma heyneana is involved in the inhibition of iNOS, COX-2 and pro-inflammatory cytokines via the downregulation of NF-kappaB pathway in LPS-stimulated murine macrophages. Int Immunopharmacol. 2009;9:1049-1057
135. Wang J, Yu Z, Wang C. et al. Dehydrocostus lactone, a natural sesquiterpene lactone, suppresses the biological characteristics of glioma, through inhibition of the NF-kappaB/COX-2 signaling pathway by targeting IKKbeta. Am J Cancer Res. 2017;7:1270-1284
136. Xing JS, Wang X, Lan YL. et al. Isoalantolactone inhibits IKKbeta kinase activity to interrupt the NF-kappaB/COX-2-mediated signaling cascade and induces apoptosis regulated by the mitochondrial translocation of cofilin in glioblastoma. Cancer Med. 2019;8:1655-1670
137. Dong T, Li C, Wang X. et al. Ainsliadimer A selectively inhibits IKKalpha/beta by covalently binding a conserved cysteine. Nat Commun. 2015;6:6522
138. Wang YB, Huang R, Wang HB. et al. Diterpenoids from the roots of Euphorbia fischeriana. J Nat Prod. 2006;69:967-970
139. Yan SS, Li Y, Wang Y. et al. 17-Acetoxyjolkinolide B irreversibly inhibits IkappaB kinase and induces apoptosis of tumor cells. Mol Cancer Ther. 2008;7:1523-1532
140. Hu Z, Sun W, Li F. et al. Fusicoccane-derived diterpenoids from Alternaria brassicicola: Investigation of the structure-stability relationship and discovery of an IKKbeta inhibitor. Org Lett. 2018;20:5198-5202
141. Chao CY, Lii CK, Tsai IT. et al. Andrographolide inhibits ICAM-1 expression and NF-kappaB activation in TNF-alpha-treated EA.hy926 cells. J Agric Food Chem. 2011;59:5263-5271
142. Wu CF, Klauck SM, Efferth T. Anticancer activity of cryptotanshinone on acute lymphoblastic leukemia cells. Arch Toxicol. 2016;90:2275-2286
143. Assani I, Du Y, Wang CG. et al. Anti-proliferative effects of diterpenoids from Sagittaria trifolia L. tubers on colon cancer cells by targeting the NF-kappaB pathway. Food Funct. 2020;11:7717-7726
144. Jin J, Zhou M, Wang X. et al. Triptolidenol, isolated from Tripterygium wilfordii, disrupted NF-kappaB/COX-2 pathway by targeting ATP-binding sites of IKKbeta in clear cell renal cell carcinoma. Fitoterapia. 2021;148:104779
145. Lee JH, Koo TH, Yoon H. et al. Inhibition of NF-kappa B activation through targeting I kappa B kinase by celastrol, a quinone methide triterpenoid. Biochem Pharmacol. 2006;72:1311-1321
146. Ahmad R, Raina D, Meyer C. et al. Triterpenoid CDDO-Me blocks the NF-kappaB pathway by direct inhibition of IKKbeta on Cys-179. J Biol Chem. 2006;281:35764-35769
147. Yore MM, Liby KT, Honda T. et al. The synthetic triterpenoid 1-[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole blocks nuclear factor-kappaB activation through direct inhibition of IkappaB kinase beta. Mol Cancer Ther. 2006;5:3232-3239
148. Gupta SC, Prasad S, Reuter S. et al. Modification of cysteine 179 of IkappaBalpha kinase by nimbolide leads to down-regulation of NF-kappaB-regulated cell survival and proliferative proteins and sensitization of tumor cells to chemotherapeutic agents. J Biol Chem. 2010;285:35406-35417
149. Kang K, Quan KT, Byun HS. et al. 3-O-acetylrubianol C (3AR-C) induces RIPK1-dependent programmed cell death by selective inhibition of IKKbeta. FASEB J. 2020;34:4369-4383
150. Sheng F, Zhang L, Wang S. et al. Deacetyl ganoderic acid F inhibits LPS-induced neural inflammation via NF-kappaB pathway both in vitro and in vivo. Nutrients. 2019;12:85
151. Harikumar KB, Sung B, Pandey MK. et al. Escin, a pentacyclic triterpene, chemosensitizes human tumor cells through inhibition of nuclear factor-kappaB signaling pathway. Mol Pharmacol. 2010;77:818-827
152. Lim HJ, Jang HJ, Kim MH. et al. Oleanolic acid acetate exerts anti-inflammatory activity via IKKalpha/beta suppression in TLR3-mediated NF-kappaB activation. Molecules. 2019;24:4002
153. Ling H, Zhang Y, Ng KY. et al. Pachymic acid impairs breast cancer cell invasion by suppressing nuclear factor-kappaB-dependent matrix metalloproteinase-9 expression. Breast Cancer Res Treat. 2011;126:609-620
154. Hui B, Yao X, Zhou Q. et al. Pristimerin, a natural anti-tumor triterpenoid, inhibits LPS-induced TNF-alpha and IL-8 production through down-regulation of ROS-related classical NF-kappaB pathway in THP-1 cells. Int Immunopharmacol. 2014;21:501-508
155. Wang H, Syrovets T, Kess D. et al. Targeting NF-kappa B with a natural triterpenoid alleviates skin inflammation in a mouse model of psoriasis. J Immunol. 2009;183:4755-4763
156. Kim E, Yang WS, Kim JH. et al. Lancemaside A from Codonopsis lanceolata modulates the inflammatory responses mediated by monocytes and macrophages. Mediators Inflamm. 2014;2014:405158
157. Wang C, Chen L, Xu C. et al. A comprehensive review for phytochemical, pharmacological, and biosynthesis studies on Glycyrrhiza spp. Am J Chin Med. 2020;48:17-45
158. Yan F, Yang F, Wang R. et al. Isoliquiritigenin suppresses human T Lymphocyte activation via covalently binding cysteine 46 of IkappaB kinase. Oncotarget. 2017;8:34223-34235
159. Funakoshi-Tago M, Tanabe S, Tago K. et al. Licochalcone A potently inhibits tumor necrosis factor alpha-induced nuclear factor-kappaB activation through the direct inhibition of IkappaB kinase complex activation. Mol Pharmacol. 2009;76:745-753
160. Charoensin S, Huang TC, Hsu JL. An innovative cell model revealed the inhibitory effect of flavanone structure on peroxynitrite production through interaction with the IKKbeta kinase domain at ATP binding site. Food Sci Nutr. 2020;8:2904-2912
161. Pei H, Xue L, Tang M. et al. Alkaloids from black pepper (Piper nigrum L.) exhibit anti-inflammatory activity in murine macrophages by inhibiting activation of NF-kappaB pathway. J Agric Food Chem. 2020;68:2406-2417
162. Pandey MK, Sung B, Kunnumakkara AB. et al. Berberine modifies cysteine 179 of IkappaBalpha kinase, suppresses nuclear factor-kappaB-regulated antiapoptotic gene products, and potentiates apoptosis. Cancer Res. 2008;68:5370-5379
163. Jin HR, Jin SZ, Cai XF. et al. Cryptopleurine targets NF-kappaB pathway, leading to inhibition of gene products associated with cell survival, proliferation, invasion, and angiogenesis. PLoS One. 2012;7:e40355
164. Zhang H, Shyaula SL, Li JY. et al. Himalensines A and B, alkaloids from Daphniphyllum himalense. Org Lett. 2016;18:1202-1205
165. Shao H, Yang B, Hu R. et al. Matrine effectively inhibits the proliferation of breast cancer cells through a mechanism related to the NF-kappaB signaling pathway. Oncol Lett. 2013;6:517-520
166. Han JG, Gupta SC, Prasad S. et al. Piperlongumine chemosensitizes tumor cells through interaction with cysteine 179 of IkappaBalpha kinase, leading to suppression of NF-kappaB-regulated gene products. Mol Cancer Ther. 2014;13:2422-2435
167. Chattopadhyay AK, Hanessian S. Recent progress in the chemistry of Daphniphyllum alkaloids dagger. Chem Rev. 2017;117:4104-4146
168. Park I, Byun HS, Hur GM. et al. Tulipiferamide A, an alkamide from Liriodendron tulipifera, exhibits an anti-inflammatory effect via targeting IKKbeta phosphorylation. J Nat Prod. 2021;84:1598-1606
169. Jeon KI, Xu X, Aizawa T. et al. Vinpocetine inhibits NF-kappaB-dependent inflammation via an IKK-dependent but PDE-independent mechanism. Proc Natl Acad Sci U S A. 2010;107:9795-9800
170. Uehara T, Baba I, Nomura Y. Induction of cytokine-induced neutrophil chemoattractant in response to various stresses in rat C6 glioma cells. Brain Res. 1998;790:284-292
171. Uehara Y, Fukazawa H, Murakami Y. et al. Irreversible inhibition of v-src tyrosine kinase activity by herbimycin A and its abrogation by sulfhydryl compounds. Biochem Biophys Res Commun. 1989;163:803-809
172. Uehara T, Matsuno J, Kaneko M. et al. Transient nuclear factor kappaB (NF-kappaB) activation stimulated by interleukin-1beta may be partly dependent on proteasome activity, but not phosphorylation and ubiquitination of the IkappaBalpha molecule, in C6 glioma cells. Regulation of NF-kappaB linked to chemokine production. J Biol Chem. 1999;274:15875-15882
173. Ogino S, Tsuruma K, Uehara T. et al. Herbimycin A abrogates nuclear factor-kappaB activation by interacting preferentially with the IkappaB kinase beta subunit. Mol Pharmacol. 2004;65:1344-1351
174. Chen Q, Liu J, Zhuang Y. et al. Identification of an IKKbeta inhibitor for inhibition of inflammation in vivo and in vitro. Pharmacol Res. 2019;149:104440
175. Bingham AH, Davenport RJ, Gowers L. et al. A novel series of potent and selective IKK2 inhibitors. Bioorg Med Chem Lett. 2004;14:409-412
176. Bingham AH, Davenport RJ, Fosbeary R. et al. Synthesis and structure-activity relationship of aminopyrimidine IKK2 inhibitors. Bioorg Med Chem Lett. 2008;18:3622-3627
177. Kim S, Jung JK, Lee HS. et al. Discovery of piperidinyl aminopyrimidine derivatives as IKK-2 inhibitors. Bioorg Med Chem Lett. 2011;21:3002-3006
178. Shin Y, Lim SM, Yan HH. et al. Optimization and biological evaluation of aminopyrimidine-based IkappaB kinase beta inhibitors with potent anti-inflammatory effects. Eur J Med Chem. 2016;123:544-556
179. Crombie AL, Sum FW, Powell DW. et al. Synthesis and biological evaluation of tricyclic anilinopyrimidines as IKKbeta inhibitors. Bioorg Med Chem Lett. 2010;20:3821-3825
180. Murata T, Shimada M, Sakakibara S. et al. Discovery of novel and selective IKK-beta serine-threonine protein kinase inhibitors. Part 1. Bioorg Med Chem Lett. 2003;13:913-918
181. Murata T, Shimada M, Kadono H. et al. Synthesis and structure-activity relationships of novel IKK-beta inhibitors. Part 2: Improvement of in vitro activity. Bioorg Med Chem Lett. 2004;14:4013-4017
182. Murata T, Shimada M, Sakakibara S. et al. Synthesis and structure-activity relationships of novel IKK-beta inhibitors. Part 3: Orally active anti-inflammatory agents. Bioorg Med Chem Lett. 2004;14:4019-4022
183. Wu JP, Fleck R, Brickwood J. et al. The discovery of thienopyridine analogues as potent IkappaB kinase beta inhibitors. Part II. Bioorg Med Chem Lett. 2009;19:5547-5551
184. Beaulieu F, Ouellet C, Ruediger EH. et al. Synthesis and biological evaluation of 4-amino derivatives of benzimidazoquinoxaline, benzimidazoquinoline, and benzopyrazoloquinazoline as potent IKK inhibitors. Bioorg Med Chem Lett. 2007;17:1233-1237
185. Belema M, Bunker A, Nguyen VN. et al. Synthesis and structure-activity relationship of imidazo(1,2-a)thieno(3,2-e)pyrazines as IKK-beta inhibitors. Bioorg Med Chem Lett. 2007;17:4284-4289
186. Shimizu H, Tanaka S, Toki T. et al. Discovery of imidazo[1,2-b]pyridazine derivatives as IKKbeta inhibitors. Part 1: Hit-to-lead study and structure-activity relationship. Bioorg Med Chem Lett. 2010;20:5113-5118
187. Shimizu H, Yasumatsu I, Hamada T. et al. Discovery of imidazo[1,2-b]pyridazines as IKKbeta inhibitors. Part 2: improvement of potency in vitro and in vivo. Bioorg Med Chem Lett. 2011;21:904-908
188. Christopher JA, Bamborough P, Alder C. et al. Discovery of 6-aryl-7-alkoxyisoquinoline inhibitors of IkappaB kinase-beta (IKK-beta). J Med Chem. 2009;52:3098-3102
189. Radhakrishnan P, Bryant VC, Blowers EC. et al. Targeting the NF-kappaB and mTOR pathways with a quinoxaline urea analog that inhibits IKKbeta for pancreas cancer therapy. Clin Cancer Res. 2013;19:2025-2035
190. Christopher JA, Avitabile BG, Bamborough P. et al. The discovery of 2-amino-3,5-diarylbenzamide inhibitors of IKK-alpha and IKK-beta kinases. Bioorg Med Chem Lett. 2007;17:3972-3977
191. Baxter A, Brough S, Cooper A. et al. Hit-to-lead studies: the discovery of potent, orally active, thiophenecarboxamide IKK-2 inhibitors. Bioorg Med Chem Lett. 2004;14:2817-2822
192. Sugiyama H, Yoshida M, Mori K. et al. Synthesis and structure activity relationship studies of benzothieno[3,2-b]furan derivatives as a novel class of IKKbeta inhibitors. Chem Pharm Bull (Tokyo). 2007;55:613-624
193. Takahashi H, Shinoyama M, Komine T. et al. Novel dihydrothieno[2,3-e]indazole derivatives as IkappaB kinase inhibitors. Bioorg Med Chem Lett. 2011;21:1758-1762
194. Elkamhawy A, Youn Kim N, Hassan AHE. et al. Optimization study towards more potent thiazolidine-2,4-dione IKK-beta modulator: Synthesis, biological evaluation and in silico docking simulation. Bioorg Chem. 2019;92:103261
195. Elkamhawy A, Kim NY, Hassan AHE. et al. Thiazolidine-2,4-dione-based irreversible allosteric IKK-beta kinase inhibitors: Optimization into in vivo active anti-inflammatory agents. Eur J Med Chem. 2020;188:111955
196. Song H, Lee YS, Roh EJ. et al. Discovery of potent and selective rhodanine type IKKbeta inhibitors by hit-to-lead strategy. Bioorg Med Chem Lett. 2012;22:5668-5674
197. Kim D, Kim YG, Seo JH. et al. Identification and characterization of potent, selective and metabolically stable IKKbeta inhibitor. Bioorg Med Chem Lett. 2016;26:1120-1123
Author contact
![]() Corresponding authors: School of Chinese Materia Medica, State Key Laboratory of Component-Based Chinese Medicine, Tianjin State Key Laboratory of Modern Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin, 301617, China. College of Pharmacy, Dalian Medical University, Dalian 116044, China. E-mail: suncp146com (C.P. Sun); Second Affiliated Hospital, Dalian Medical University, Dalian 116044, China. E-mail: maxc1978com (X.C. Ma).
Corresponding authors: School of Chinese Materia Medica, State Key Laboratory of Component-Based Chinese Medicine, Tianjin State Key Laboratory of Modern Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin, 301617, China. College of Pharmacy, Dalian Medical University, Dalian 116044, China. E-mail: suncp146com (C.P. Sun); Second Affiliated Hospital, Dalian Medical University, Dalian 116044, China. E-mail: maxc1978com (X.C. Ma).

 Global reach, higher impact
Global reach, higher impact