10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2023; 19(13):4223-4241. doi:10.7150/ijbs.82287 This issue Cite
Research Paper
microRNA-130b-3p Attenuates Septic Cardiomyopathy by Regulating the AMPK/mTOR Signaling Pathways and Directly Targeting ACSL4 against Ferroptosis
1. Department of Cardiovascular Surgery, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China.
2. Department of Cardiology, West China Second University Hospital, Sichuan University, Chengdu, Sichuan, China.
3. Department of Cardiology, Seventh People's Hospital of Shanghai University of Traditional Chinese Medicine, Shanghai, China.
4. Nanjing Drum Tower Hospital Clinical College of Traditional Chinese and Western Medicine, Nanjing University of Chinese Medicine, Nanjing, China.
5. Department of Critical Care Medicine, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China.
6. Cardiology Department of Ministry of Education, Sichuan University, Chengdu, Sichuan, China.
*These authors contributed equally to this work
Abstract
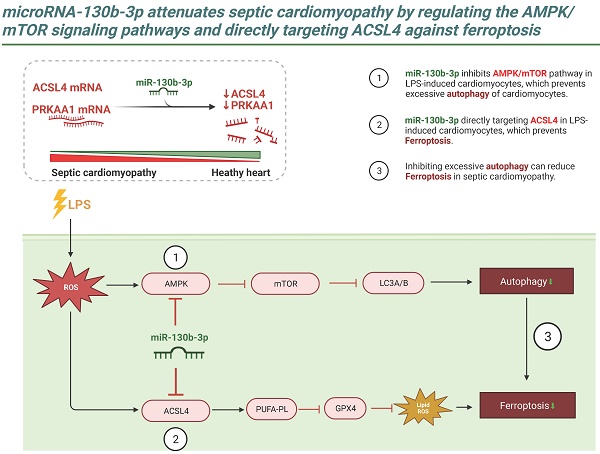
Ferroptosis is a newly identified type of programmed cell death that has been shown to contribute to the progression of septic cardiomyopathy. Although the role of miR-130b-3p as an oncogene that accelerates cancer progression by suppressing ferroptosis has been demonstrated, its role in the regulation of ferroptosis and cardiac injury in Lipopolysaccharide (LPS)-induced cardiomyopathy has not been fully clarified. In this study, we demonstrated that miR-130b-3p remarkably improved cardiac function and ameliorated morphological damage to heart tissue in LPS-induced mice. miR-130b-3p also improved cell viability and mitochondrial function and reduced the production of lipid ROS and ferroptosis in LPS-treated H9c2 cells. In addition, miR-130b-3p significantly upregulated GPX4 expression and suppressed ACSL4 activity in LPS-induced mouse heart tissue and H9c2 cells. Mechanistically, we used database analysis to locate miR-130b-3p and confirmed its inhibitory effects on the ferroptosis-related gene ACSL4 and autophagy-related gene PRKAA1 using a dual-luciferase reporter assay. In addition, we found that miR-130b-3p inhibited the activation of autophagy by downregulating the expression of the AMPK/mTOR signaling pathway. Meanwhile, our results show that RAPA (an autophagy activator) reverses the protective effect of miR-130b-3p mimic against LPS-induced ferroptosis, while CQ (an autophagy inhibitor) plays a facilitative role, suggesting that miR-130b-3p plays an important role in the development of ferroptosis by regulating autophagy in vitro. The findings reveal a novel function of miR-130b-3p in attenuating ferroptosis in cardiomyocytes, providing a new therapeutic target for ameliorating septic cardiomyopathy injury.
Keywords: microRNA-130b-3p, Ferroptosis, Autophagy, Septic cardiomyopathy, ACSL4, PRKAA1.

 Global reach, higher impact
Global reach, higher impact