ISSN: 1449-2288
Int J Biol Sci 2023; 19(14):4672-4688. doi:10.7150/ijbs.81595 This issue Cite
Research Paper
Low expression of m6A reader YTHDC1 promotes progression of ovarian cancer via PIK3R1/STAT3/GANAB axis
1. The State Key Laboratory of Pharmaceutical Biotechnology, Division of Immunology, Medical School, Nanjing University, Nanjing, China.
2. Jiangsu Key Laboratory of Molecular Medicine, Division of Immunology, Medical School, Nanjing University, Nanjing, China.
3. General Clinical Research Center, Nanjing First Hospital, Nanjing Medical University, Nanjing, China.
Abstract
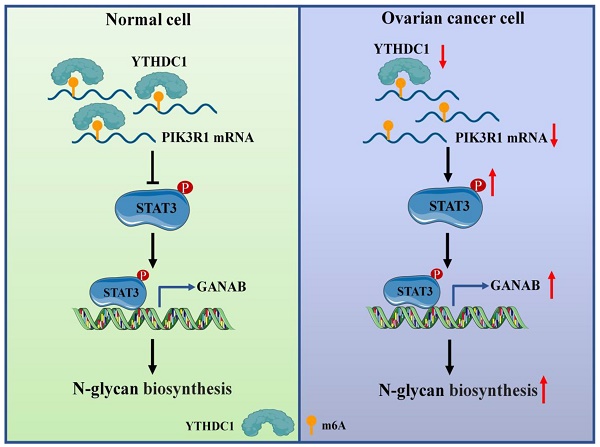
Background: N6-Methyladenosine (m6A) is considered to be the most prevalent and abundant internal modification observed in mRNA between viruses and mammals. As a reversible epigenetic modification, m6A controls gene expression in diverse physiological and pathological processes. Accumulating evidence in recent years reveals that aberrant expression of m6A reader proteins may have tumor-suppressing or carcinogenic functions. However, the biological role and mechanism of m6A reader YTH Domain Containing 1 (YTHDC1) in ovarian cancer progression remain inadequately understood.
Methods: Quantitative RT-PCR, immunohistochemistry, Western blot, and bioinformatics analyses were undertaken for studying the YTHDC1 expression in ovarian cancer. In vitro and in vivo models were used to examine the role of YTHDC1. RNA sequencing, RNA immunoprecipitation sequencing, m6A-modified RNA immunoprecipitation, actinomycin-D assay, chromatin immunoprecipitation, and Western blot were used in the investigation the regulatory mechanisms among YTHDC1, Signal Transducer and Activator of Transcription 3 (STAT3), Phosphoinositide-3-Kinase Regulatory Subunit 1 (PIK3R1), and Glucosidase II Alpha Subunit (GANAB).
Results: Here, we found that YTHDC1 expression is decreased in ovarian cancer. Overexpression of YTHDC1 inhibited ovarian cancer development both in vivo and in vitro. Mechanistically, PIK3R1 was identified to be the direct target for YTHDC1. YTHDC1 enhanced PIK3R1 stability in an m6A-dependent manner, which subsequently inhibited GANAB expression in the N-glycan biosynthesis via the STAT3 signaling.
Conclusions: Our findings unveil YTHDC1 as a tumor suppressor in the progression of ovarian cancer and as a potential prognostic biomarker that could serve as a target in ovarian cancer treatment.
Keywords: m6A reader, YTHDC1, PIK3R1, RNA stability, ovarian cancer

 Global reach, higher impact
Global reach, higher impact