Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2023; 19(15):4931-4947. doi:10.7150/ijbs.86869 This issue Cite
Review
Precise Orchestration of Gasdermins' Pore-Forming Function by Posttranslational Modifications in Health and Disease
1. Department of General Surgery, Nanjing BenQ Medical Center, The Affiliated BenQ Hospital of Nanjing Medical University, Nanjing 210000, China.
2. Research Institute of General Surgery, Affiliated Jinling Hospital, Medical School of Nanjing University, Nanjing 210000, China.
3. Jiangsu Provincial Key Laboratory of Critical Care Medicine, Department of Critical Care Medicine, Affiliated Zhongda Hospital, School of Medicine, Southeast University, Nanjing, China.
4. Department of General Surgery, The First Affiliated Hospital of Xi'an Jiaotong University, Xi'an 710061, Shaanxi Province, China.
Received 2023-6-6; Accepted 2023-9-4; Published 2023-9-18
Abstract
Gasdermins (GSDMs) serve as pivotal executors of pyroptosis and play crucial roles in host defence, cytokine secretion, innate immunity, and cancer. However, excessive or inappropriate GSDMs activation is invariably accompanied by exaggerated inflammation and results in tissue damage. In contrast, deficient or impaired activation of GSDMs often fails to promptly eliminate pathogens, leading to the increasing severity of infections. The activity of GSDMs requires meticulous regulation. The dynamic modulation of GSDMs involves many aspects, including autoinhibitory structures, proteolytic cleavage, lipid binding and membrane translocation (oligomerization and pre-pore formation), oligomerization (pore formation) and pore removal for membrane repair. As the most comprehensive and efficient regulatory pathway, posttranslational modifications (PTMs) are widely implicated in the regulation of these aspects. In this comprehensive review, we delve into the complex mechanisms through which a variety of proteases cleave GSDMs to enhance or hinder their function. Moreover, we summarize the intricate regulatory mechanisms of PTMs that govern GSDMs-induced pyroptosis.
Keywords: gasdermin, alternative splicing, pyroptosis, posttranslational modifications, dynamical regulation
Introduction
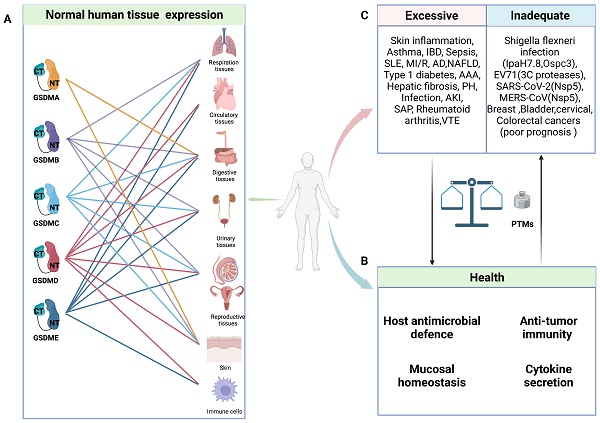
Two decades ago, gasdermins (GSDMs) were initially identified as risk genes associated with several alopecia-like skin diseases in mice and hearing loss in humans [1-4]. The role of GSDMs in health and disease has been progressively elucidated since then. In humans, there are six GSDM genes: GSDMA, GSDMB, GSDMC, GSDMD, GSDME (also known as DFNA5), and PJVK (also known as DFNB59) [5, 6]. Mice have three Gsdma genes (Gsdma1, Gsdma2, and Gsdma3), no Gsdmb gene, four Gsdmc genes (Gsdmc1, Gsdmc2, Gsdmc3, and Gsdmc4), and Gsdmd, Gsdme and Pjvk (Table 1). Previous research has established a strong correlation between GSDMs and sterile inflammation, as indicated by the association of Gsdma3 with alopecia induced by skin inflammation and that of GSDMB with childhood asthma [7-10]. Further investigations have revealed that the deletion of exon 8 in GSDME is associated with multiple mutations that trigger programmed cell death in cochlear cells, indicating the potential cytotoxic activity of GSDMs [11-13]. However, the specific type of cell death mediated by GSDMs and their underlying mechanisms remained unclear. Until 2015, GSDMD was considered executor of pyroptosis, which is a lytic form of cell death, and the substrate of proinflammatory caspases (caspase-1/-4/-5/-11) [14-16]. The study of GSDMD activation and cleavage has led to the identification of activation mechanisms for other GSDMs, such as the caspase-3-GSDME and caspase-8-GSDMC pyroptotic axes [17, 18]. Interestingly, several recent studies have revealed that cytotoxic lymphocytes interact with cells expressing GSDMB or GSDME to ultimately induce pyroptosis in these cells [19, 20]. Exotoxin B (SpeB) is secreted by Group A Streptococcus and can cleave GSDMA and induce pyroptosis in keratinocytes [21, 22]. These findings expand the scope of proteases activating GSDMs beyond intracellular caspases and further confirm that GSDMs serve as the executors of pyroptosis. The pore-forming and pyroptotic functions of GSDMs have gradually gained recognition.
Due to their unique tissue expression and activation mechanisms, GSDMs can have multifaceted roles in health and disease (Figure 1). Numerous studies have demonstrated the pivotal roles of GSDMs in host defence. On the one hand, GSDMs can induce pyroptosis in infected cells to eliminate pathogen replication niches and subsequently recruit immune cells through the release of proinflammatory cytokines to combat pathogens [23, 24]. On the other hand, GSDMB and GSDMD exhibit direct bactericidal activity in vitro by binding with bacterial membrane cardiolipin [25, 26]. Furthermore, GSDMD and GSDME play crucial roles in mediating the release of IL-1β and NETosis, thereby promoting the elimination of S. Typhimurium and Yersinia in infected neutrophils [27-29].
Summary of Human and mouse GSDMs functions
| GSDMs (Mouse ortholog) | Protease (Cleavage site) | Disease models | Biological function | Reference | |
|---|---|---|---|---|---|
| GSDMA (Gsdma1, Gsdma2, Gsdma3) | SpeB (GSDMA: Q246; Gsdma1:Q246; Gsdma2 and Gsdma3: Unknown) | Systemic sclerosis, Alopecia, Group A Streptococcus infection | Pyroptosis, mitochondrial damage | [21, 22, 38, 57, 60] | |
| GSDMB (None) | Isoform1(403aa): Lack exon 6 | GzmA (K231) Caspase-1/3/4/6/7/8/9 (D91) NE (M220) | Asthma, Sepsis, Inflammatory bowel disease, Colorectal, Breast, cervical cancers, Shigella flexneri infection | Peri-bronchial smooth muscle and collagen deposition, rs11078928 variant links to deceased asthma risk, Negative regulation the pore-forming function of pyroptotic isoforms | [20, 25, 38, 60, 63-73] |
| Isoform2(394aa): Lack exon 6,7 | GzmA (K222) Caspase-1/3/4/6/7/8/9 (D91) NE (M220) | Negative regulation the pore-forming function of pyroptotic isoforms | |||
| Isoform3(416aa): Normal | GzmA (K229, K244) Caspase-1/3/4/6/7/8/9 (D91) NE (M220) Caspase-1(D236) Der p3(K244) | Pyroptosis, Antitumor immunity, Adjuvant GSDMD-mediated pyroptosis (FL) | |||
| Isoform4(407aa): Lack exon 7 | GzmA (K229, K235) Caspase-1/3/4/6/7/8/9(D91) NE (M220 exon 5) | Pyroptosis, Antitumor immunity | |||
| IsoformU(411aa): Non-canonical splice junction | GzmA (K233, K239) Caspase-1/3/4/6/7/8/9(D91) NE (M220) | Cell proliferation, Migration, Adhesion (FL), Bacteria killing | |||
| GSDMC (Gsdmc1, Gsdmc2, Gsdmc3, Gsdmc4) | Caspase-8 (GSDMC: D240, D365; Gsdmc1, Gsdmc2 and Gsdmc3: Unknown; Gsdmc4: D233) Caspase-6(Unknown) | Breast cancer, Helminth infection | Pyroptosis, Antitumor Immunity, Type 2 immune response, Cytokines secretion | [17, 38, 60, 74-76] | |
| GSDMD(Gsdmd) | Caspase-1,4,5,11(GSDMD: D275; Gsdmd: D276) Caspase-8 (GSDMD: D275; Gsdmd: D276) NE (GSDMD: C268; Gsdmd: V251) CG (Gsdmd: L274) Caspase-3,7 (GSDMD: D87; Gsdmd: D88) 3C protease (GSDMD: Q193) Nsp5 (GSDMD: Q193) | Sepsis, Inflammatory bowel disease, Bacterial/viral/fungal infection, Kidney diseases, myocardial Ischemia/reperfusion, Non-alcoholic fatty liver disease, Abdominal aortic aneurysm, Rheumatoid Arthritis, Alzheimer's disease, Cancer | Pyroptosis, NETosis, Mitochondrial damage, Bacteria killing, Sublytic release Of inflammatory mediators, Regulate mucus granule exocytosis | [15, 16, 31, 38, 41, 60, 77-85, 87-95] | |
| GSDME(Gsdme) | Caspase-3 (GSDME: D270; Gsdme: D270) GzmB (GSDME: D270;Gsdme: D270) | Autosomal dominant nonsyndromic hearing loss, Neurologic diseases, Atherosclerosis, Aasopharyngeal carcinoma | Pyroptosis, Antitumor Immunity, Sublytic release of inflammatory mediators, Mitochondrial damage | [18, 19, 38, 57, 58, 60, 97-104, 153] | |
| PJVK(Pjvk) | Unknown | Recessive nonsyndromic hearing impairment | Unknown | [1, 38, 48, 60] | |
Table 1: Proteases cleaving and activating GSDMs are marked in bold. The sites in parentheses are proteases cleavage sites. Abbreviations: GzmA: granzyme A; GzmB: granzyme B; NE: neutrophil elastase; CG: cathepsin G
General characteristics of GSDMs in health and diseases. A: Schematic representation of normal expression of GSDMs in human tissues. B: In normal circumstance, GSDMs play a vital role in host anti-microbial defence, anti-tumor immunity, mucosal homeostasis and cytokine secretion. C: The pore-forming dysfunction of GSDMs is involved in the occurrence of various diseases. Abbreviations: IBD: inflammatory bowel disease; SLE: systemic lupus erythematosus; MI/R: myocardial ischemia/reperfusion; AD: Alzheimer's disease; NAFLD: non-alcoholic fatty liver disease; AAA: abdominal aortic aneurysm; PH: pulmonary hypertension; AKI: acute kidney injury; SAP: severe acute pancreatitis; VTE: venous thromboembolism; EV71: Enterovirus 71; PTMs: posttranslational modifications. Figures created with BioRender.com.
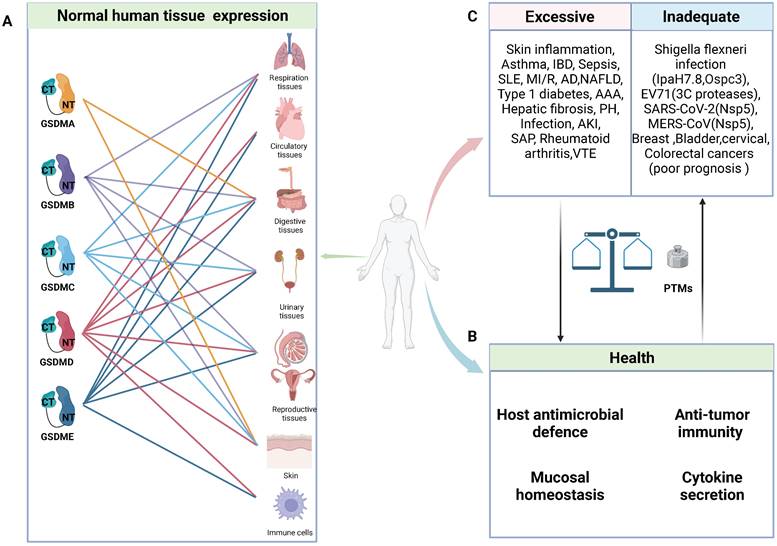
Notably, certain invasive pathogens have developed intricate mechanisms to impair pyroptosis and sustain their replication, such as the truncation of pore-forming GSDMs and posttranslational modifications (PTMs) of GSDMs and their upstream caspases [25, 30-34]. Nevertheless, every coin has two sides, and GSDM-induced pyroptosis is no exception. In animal experiments, pyroptosis inhibition or GSDMD knockout can effectively protect mice from fatal sepsis [35-37]. This effect may be attributed to the excessive pyroptosis in epithelial cells leading to dysfunction in the intestinal barrier, ultimately resulting in worsened inflammation and injury. Furthermore, emerging evidence has unveiled a robust correlation between inflammatory ailments and pyroptosis. Various single-nucleotide polymorphisms (SNPs) in GSDMs have been shown to be linked with susceptibility to asthma, rheumatoid arthritis, and inflammatory bowel disease (IBD) (Table 1). The crucial roles of GSDMs as mediators of inflammation have been increasingly recognized, and targeting their function has become an attractive strategy for inhibiting inflammatory responses [38]. However, cancer cell lysis and the intense inflammatory response mediated by GSDMs confer numerous benefits for antitumour therapy [39-42]. In addition to directly inducing cancer cell lysis, the subsequent release of immunostimulatory cellular contents can effectively recruit and activate immune cells such as cytotoxic T cells, thereby facilitating tumour eradication [42]. Thus, maintaining a delicate balance of pyroptosis is crucial in health and disease.
GSDMs-mediated pore formation is dynamically regulated at multiple levels through autoinhibitory structures, proteolytic cleavage, lipid binding and membrane translocation (oligomerization and pre-pore formation), oligomerization (pore formation) and pore removal for membrane repair [40, 43-51] (Figure 2A). Under normal circumstances, most GSDMs are located in the cytoplasm in an autoinhibited conformation [44]. Except PJVK, all GSDMs contain two conserved domains: a C-terminal repressor domain (CTD) and an N-terminal domain (NTD). Specific proteases cleave linker sites, leading to the activation of GSDMs [15, 16]. The GSDMs-NTD can specifically bind to lipids in the cell membrane, including phosphatidylinositol phosphates and phosphatidylserine (localized to the inner leaflet of the cell membrane), as well as cardiolipin (found in the inner and outer leaflets of bacterial membranes) [26, 48]. Then, GSDMs-NTD oligomerizes and forms pre-pores and finally functional pores within membranes without the assistance of membrane receptors [52, 53]. In contrast to mixed lineage kinase domain-like (MLKL)-mediated channel formation, which induces the influx of select ions to induce necroptosis, GSDMs-NTD oligomerizes and forms nonselective pores within membranes [54]. Following GSDMs-mediated pores formation, cytosolic increases in Ca2+ activate the endosomal sorting complex required for protein transport (ESCRT)-III, which then releases vesicles containing GSDMD pores to prevent further membrane rupture and pyroptosis [46]. In contrast, oligomers of the membrane surface protein ninjurin 1 (NINJ1), which contains two transmembrane domains, enhance plasma membrane rupture (PMR). In NINJ1-deficient cells, GSDMD pores are unable to induce cell rupture and typical pyroptosis [43, 47, 50]. The strong lipid binding at the outer leaflet of the lipid bilayer indicates the underlying mechanism of NINJ1-mediated PMR. Although it forms ring-like structures, NINJ2, a paralogue of NINJ1, failed to mediate PMR due to its binding to cholesterol in the inner leaflet [55]. Currently, the model of NINJ1-mediated PMR remains controversial [43, 47, 55, 56]. The release of NINJ1 oligomers into the supernatant challenges the amphipathic filament model or pore model with a hydrophilic conduit, which may support the cookie cutter model of cell lysis [55, 56]. Understanding whether NINJ1 forms rings with hydrophobic interiors and hydrophilic exteriors or pores containing a hydrophilic conduit is worth further investigation. In summary, these dynamic processes present promising targets for modulating pyroptosis.
The pore-forming mechanism and regulatory sites of GSDMs. A: Main steps of GSDMs pore-forming. Autoinhibition: Interaction between the CTD and NTD of most GSDMs impedes protein activation. Proteolytic cleavage: Specific proteases cleave the link sites between the CTD and NTD of GSDMs, releasing pore-forming fragments. Lipid selectivity: Various GSDMs demonstrate distinct lipid-binding capacities and lipid preference. Membrane Translocation (Oligomerization and pre-pores forming): The GSDMs-NTD preassembling on the target membrane surface before membrane insertion. Oligomerizations (Pore-forming): GSDMs-NTD oligomerize and then form pore-like structures on membrane surface. Membrane repair: After pore-forming, ESCRT-III will be activated by cytosolic upregulated Ca2+ to excrete vesicles containing GSDMD pores to prevent further membrane rupture. The oligomerization of the membrane surface protein NINJ1 is vital for regulating the rupture of the membrane. B: Refining the pore-forming capabilities of GSDMs through cleavage and PTMs. Schematic representation of the structure of GSDMs present the proteases, respective cleavage sites, PTMs and PTMs sites. The sites in parentheses are proteases cleavage sites. The cleavage sites of murine proteins are indicated in grey letters within parentheses. Red lines mean the proteases can produce pore-forming NTD through cleaving these sites. Black lines mean the proteases inactivate GSDMs by cleaving these sites, releasing non-pyroptotic NTD. The PTMs sites are shown in the GSDMs-NTD and -CTD. Distinct modification types are distinguished by the use of various colors. The conserved ubiquitination sites of GSDMB isoforms are indicated by asterisks. Abbreviations: NTD: N-terminal domains; CTD: C-terminal domains; PTMs: posttranslational modifications; NINJ1: ninjurin 1; CASP: caspase; GzmA: granzyme A; GzmB: granzyme B; NE: neutrophil elastase; CG: cathepsin G; SYVN1: Synoviolin; NSA: necrosulfonamide; PP1: Phosphatase1; ROS: reactive oxygen species; ZDHHC: zinc finger and DHHC motif-containing family. Figures created with BioRender.com.
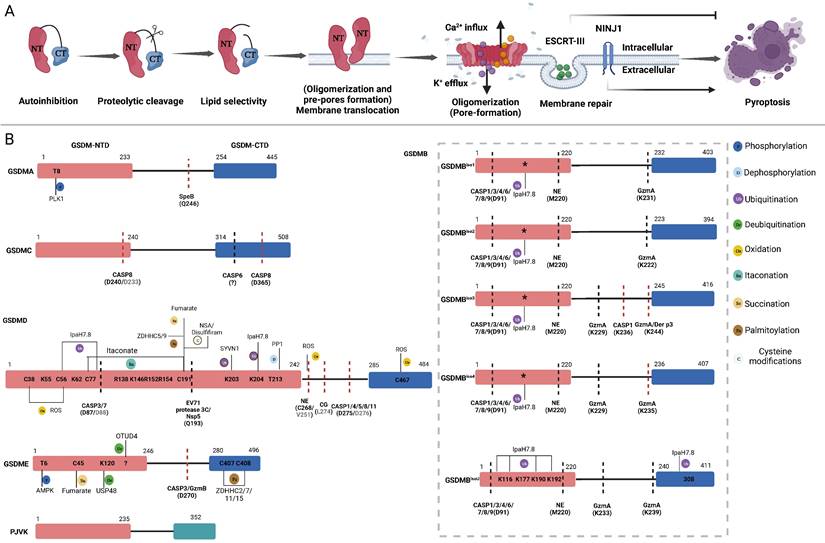
PTMs, which include but are not limited to phosphorylation, dephosphorylation, ubiquitination, deubiquitination, oxidation, itaconation, succination, and palmitoylation, exert profound effects on various aspects of GSDM-related protein function by precisely regulating specific amino acids within proteins. To date, various strategies for regulating GSDM signalling through PTMs have been elucidated, including GSDMs activation, proteolytic cleavage, membrane translocation, GSDMs-NTD oligomerization and other nonpyroptotic functions. This review will provide a comprehensive discussion on the fundamental biological mechanisms of GSDMs-mediated pyroptosis, recent advances in PTMs of GSDMs, and potential therapeutic applications based on PTMs for infectious diseases, cancer, and other inflammatory disorders.
Gasdermins' Characteristics and Cleavage Mechanisms
GSDMA
GSDMA is predominantly expressed in keratinocytes in the skin and epithelial cells in the gastrointestinal tract and has been linked to various dermatological conditions, systemic sclerosis, asthma, and IBD (Table 1). Previous research has indicated that GSDMA is linked to mitochondrial stress and dysfunction [57]. Consistently, a protein engineering study demonstrated that upon activation, GSDMA localized to mitochondria and showed delayed and decreased accumulation at the plasma membrane [58]. GSDMA-NTD induces early mitochondrial dysfunction prior to plasma membrane disruption, suggesting interplay between pyroptosis and cell death that centres on mitochondria.
Recent studies have uncovered a novel role of GSDMA in host recognition and the maintenance of defence barriers [21, 22]. Cleavage of the linker site of GSDMA at Q246 by SpeB leads to pyroptosis in keratinocytes (Figure 2B). Deng et al. and LaRock et al. used GAS to infect Gsdma1-knockout and Gsdma1-3-triple-knockout mice and confirmed that GSDMA plays a pivotal role in preventing colonization and infection by GAS. Following the transfection of GSDMs into HEK293T cells and incubation with SpeB, immunoblot analysis revealed signs of cleavage in GSDMC and GSDMD. In contrast, Deng et al. reported that coexpression of SpeB and GSDMA-E in 293T cells resulted in the specific cleavage of GSDMA but not GSDMB-E. It is worth exploring the potential of SpeB to cleave GSDMC and GSDMD, as well as elucidate the circumstances under which it exerts its proteolytic effects. Under normal conditions, autophagic machinery can effectively eliminate intracellular GAS through lysosomal degradation [59]. However, by secreting SpeB, GAS evades this innate immune defence mechanism. As an alternative strategy, SpeB-activated GSDMA induces lysis in infected host cells to restrict pathogen replication. Given their intracellular localization, GSDMs likely serve as sentinels against other internalized microbes.
GSDMB
GSDMB is predominantly expressed in the gastrointestinal tract, airway epithelium, lymphoid tissues, and various tumours[60, 61]. Genome-wide association studies have demonstrated a close correlation between GSDMB polymorphisms and susceptibility to asthma, IBD, and other chronic inflammatory diseases (Table 1).
Over the years, the functions and activation pathways of GSDMB have been a subject of dispute due to its unique lipid-binding properties and complex isoforms that result in differing crystal structures [62-64]. GSDMB is encoded by various splice variants, each with their own counterparts (Table 1). The isoforms of GSDMB exhibit variations in the presence or absence of exons 6 and 7. Specifically, GSDMBiso1 and GSDMBiso4 lack exons 6 and 7, respectively; GSDMBiso2 lacks both exons, while GSDMBiso3 contains both exons [65]. In addition to GSDMBiso1-4, several studies have used GSDMBisoU to investigate the nonpyroptotic functions of GSDMB. GSDMBisoU is similar to GSDMBiso4 but has an asparagine-to-aspartate substitution in the first residue in exon 6, in front of which is a four-residue insert [25, 65, 66]. Until recently, two pioneering studies used cryogenic electron microscopy to determine the indispensable role of exon 6 amino acids in the linker sequences of GSDMB isoforms in pore assembly and phospholipid binding [65, 67]. Several contemporaneous studies have also demonstrated that the presence of exon 6 is pivotal for defining the activity of GSDMB isoforms [68, 69]. Moreover, Kong et al. discovered that the coexpression of noncytotoxic isoforms (GSDMBiso1/2) could inhibit the pore-forming activity of cytotoxic isoforms (GSDMBiso3/4) [68]. This phenomenon may be attributed to the formation of hetero-oligomers, which inhibit crucial steps from intermediate assemblies to membrane pore formation (Figure 4). The composition of GSDMB isoforms varies in distinct cells and contexts and modulates the final pore-forming activity. Therefore, distinguishing between the pore-forming activity and tissue expression of different isoforms is crucial for elucidating the function of GSDMB.
Although GSDMBiso1 and GSDMBiso2 possess several protease cleavage sites, their ability to induce pyroptosis through pore formation is limited due to the absence of exon 6 (Table 1). Several earlier studies reported the noncytotoxic function of GSDMBiso1. Panganiban et al. found that the coding variant rs11078928 regulated the exon-5-8 transcript of the GSDMB gene and failed to induce pyroptosis via caspase-1 because exon 6 was skipped, which was associated with a decrease in asthma risk [70]. Consistently, a recent study reported that Der p3 from house dust mites (HDMs) can directly cleave GSDMBiso3 to induce human bronchial epithelial (HBE) cell pyroptosis. However, GSDMBiso1-NTD failed to mediate HBE cell pyroptosis [71]. In a separate study on asthma, GSDMBiso1 was identified as the predominant isoform and a transcriptional activator of TGFβ, MMP9, and chemokines in bronchial epithelial cells [72]. This finding suggests that GSDMB acts as a transcription activator rather than an executor of pyroptosis. Das et al. created a knock-in mouse model of human GSDMBiso1. After methacholine exposure, GSDMBiso1 knock-in mice exhibited heightened airway responsiveness and more severe airway remodelling. However, compared to wild-type mice, there was no change in inflammatory cells in bronchoalveolar lavage fluid [72]. These findings suggest that different isoforms of GSDMB may be involved in asthma through distinct mechanisms. Subsequent studies have provided compelling evidence that the cytoplasm and nuclei of HeLa and HEK293T cells contain GSDMBiso1 and its NTD. In contrast to its role in bronchial epithelial cells, GSDMBiso1-4 did not function as a transcription factor in HeLa cells [68]. These findings suggest that the nonpyroptotic role of GSDMB as a transcription factor is dependent on specific isoforms and conditions.
Whether caspases are capable of cleaving GSDMBiso3 and GSDMBiso4 to induce pyroptosis remains a controversial topic. Panganiban et al. demonstrated that caspase-1 could cleave GSDMBiso3 at D236, which is located in exon 7, leading to the induction of pyroptosis in HEK293T cells [70]. Conversely, several studies showed that caspase-1/3/4/6/7/8/9 and NE mediated proteolytic inactivation of GSDMBiso3 and GSDMBiso4 in THP-1 cells [63, 69, 73] (Figure 2B). Moreover, full-length GSDMBiso3 was shown to promote GSDMD-mediated noncanonical pyroptosis by strengthening the activity of caspase-4 in THP-1 macrophages [73]. The intricate interplay between GSDMB and caspases poses a challenge to determining the pyroptotic functions of GSDMBiso3 and GSDMBiso4. In 2020, Zhou and colleagues reported that granzyme A (GzmA) derived from natural killer (NK) and/or CD8+ T cells cleaved GSDMB at K244 (major cleavage site) and K229 (minor cleavage site), which was conserved in GSDMBiso1-4 in a colon cancer cell line, resulting in pyroptosis in cancer cells [20]. A subsequent study showed that only GzmA cleaved GSDMBiso3 at K244 and that GSDMBiso4 could exhibit pore-forming activity. Compared to that of GSDMBiso3, the cytotoxicity of GSDMBiso4 was relatively weak [68]. Analogously, another study showed that GSDMBiso4 and GSDMBiso3 derivative fragments that lacked exon 7 exhibited only partial pore-forming activity [65]. After being attacked by NK cells, GSDMBiso1-2-expressing cells tended to undergo apoptosis, while GSDMBiso3-expressing cells predominantly underwent pyroptosis. In contrast, GSDMBiso4-expressing cells underwent mixed pyroptosis and apoptosis within tumours [68]. Increasing the expression of GSDMBiso3 and/or GSDMBiso4 is a crucial strategy for antitumour therapy due to the immunogenic inertness of apoptosis compared to pyroptosis. Indeed, recent studies have reported distinct functions of GSDMBiso1-4 in various cancers. Notably, the high expression of pyroptotic GSDMBiso3 or GSDMBiso4 but not GSDMBiso1 or GSDMBiso2 has been associated with a more favourable prognosis among patients with bladder, breast and cervical cancers [68, 69]. Furthermore, GSDMBiso3 and GSDMBiso4 account for 75% of total GSDMB transcripts in small intestinal mucosal and rectal epithelial cells, as well as over 38% of total GSDMB transcripts in colonic epithelial cells, indicating that GSDMB-mediated pyroptosis may play a pivotal role in gastrointestinal diseases such as IBD [65].
Two recent studies reported the nonpyroptotic functions of GSDMB using a GSDMB isoform recorded in the UniProt database (termed GSDMBisoU) [25, 66]. Hansen et al. discovered that GSDMBisoU was cleaved by GzmA and preferred to form pores in bacterial-derived membranes by constructing bacterial-mimetic liposomes and mammalian liposomes in vitro. The authors reached the same conclusion in GSDMBisoU knock-in mice that GSDMBisoU was cleaved by GzmA and directly killed intracellular bacteria instead of inducing host cell pyroptosis [25]. However, GSDMBisoU has negligible pore-forming activity compared with GSDMBiso3 due to a four-residue insertion before exon 6 that disarranges the β10 structure of GSDMBisoU-NTD [65]. This finding suggests that GSDMB may exert significant effects on host antimicrobial defence, although the pore-forming activity of GSDMB isoforms differs. Subsequent studies showed that different NTDs of GSDMB isoforms similarly bound to cardiolipin and other lipids. However, only GSDMBiso3-NTD244 (cleaved at K224) and GSDMBiso4-NTD killed bacteria in vitro [68]. Currently, investigations of the bactericidal functions of GSDMB isoforms are primarily based on in vitro studies, and further animal experiments are required to improve our understanding. Moreover, GSDMBisoU affects epithelial maintenance and repair by promoting the proliferation, migration, and adhesion of intestinal epithelial cells (IECs) in full-length (FL) form, which regulates PDGF-A-dependent FAK phosphorylation [66]. However, the expression of GSDMBisoU is minimal in normal IECs [65]. Whether and how isoform composition changes in IBD remain unknown, indicating that the nonpyroptotic function of GSDMB in IBD requires further study. In addition, the association between changes in the GSDMB isoform and different disease phenotypes of IBD, such as active intestinal inflammation and fibrosis, is also worth exploring.
The isoforms collectively play a crucial role in the biological functions of GSDMB in various contexts. It is imperative to determine which GSDMB isoform is predominant in specific cells and how isoform composition changes in health and disease conditions.
GSDMC
GSDMC is widely expressed in various tissues, including the upper gastrointestinal and airway epithelium, skin, and spleen [60]. In addition to inducing pyroptosis, GSDMC has been shown to participate in the type 2 immune response to helminth infections by promoting unconventional secretion of IL-33 through GSDMC pores [61, 74, 75] (Table 1 and Figure 4).
Recently, Hou et al. reported that caspase-8 cleaves GSDMC at D365 in breast cancer cells, leading to a switch from TNFα-induced apoptosis to pyroptosis [17] (Figure 1B). Under hypoxic conditions, STAT3 and PD-L1 translocate to the nucleus to upregulate GSDMC expression. Ultimately, caspase-8 cleaves GSDMC to generate GSDMC-NTD, inducing pyroptosis in breast cancer cells. In addition to that of caspase-8, the ability of caspase-6 to cleave GSDMC has also been discovered. Interestingly, GSDMC cleaved by caspase-6 failed to induce pyroptosis in response to treatment with TNFα plus CHX. It would be worthwhile to investigate whether caspase-6 can induce pyroptosis by cleaving GSDMC under conditions of caspase-6 activation [17]. Additionally, the metabolite α-ketoglutarate (α-KG) has been reported to increase intracellular ROS levels and activate the plasma membrane-localized death receptor DR6, which serves as a protein‒protein interaction platform for caspase-8 and GSDMC [76] (Figure 4). Under different conditions, including different cell lines and stimuli, caspase-8 may cleave GSDMC at alternative sites (D240) to release the pyroptotic NTD [76]. However, the underlying regulatory mechanism requires further investigation.
GSDMD
GSDMD is widely expressed in various tissues and is involved in multiple inflammatory diseases and cancers [41, 61, 77-79]. GSDMD pores not only induce lytic cell death but also serve as channels for the release of inflammatory cytokines [80-82]. Additionally, GSDMD plays a crucial role in maintaining intestinal mucosal homeostasis by promoting mucus layer formation to defend against various pathogens [83] (Table 1).
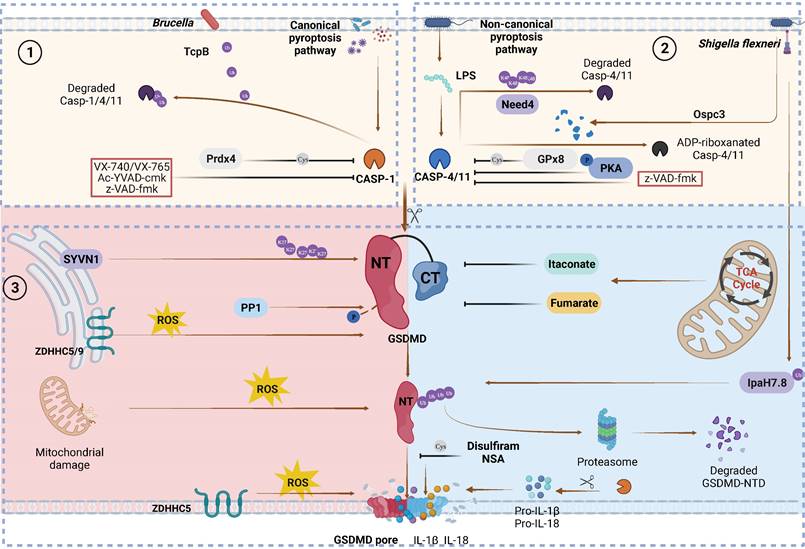
In canonical inflammasome pathways, cytosolic pattern recognition receptors (PRRs) specifically monitor pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) from intracellular and extracellular sources (Figure 3). Subsequently, these receptors directly recruit downstream pro-caspase-1 or apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) to further recruit pro-caspase-1, resulting in the formation of inflammasome complexes [84]. Inflammasome complexes activate caspase-1, which in turn promotes the maturation of proinflammatory precursor cytokines (pro-IL-1β and pro-IL-18) and proteolytic cleavage of GSDMD [85]. In the noncanonical inflammasome pathway, caspase-11 in mice (caspase-4 and caspase-5 in humans) can be activated by direct recognition of intracellular lipopolysaccharide (LPS) from gram-negative bacteria [86].
In addition to proinflammatory caspases (caspase-1/-4/-5/-11), other caspases can process GSDMD into active or inactive fragments (Figure 4). Recent studies have revealed that the Yersinia effector protein YopJ can inhibit TGFβ-associated kinase 1 (TAK1) to promote apoptotic caspase-8 cleavage of GSDMD at D276 (human D275) in murine macrophages, ultimately leading to pyroptosis[87, 88]. This finding suggests that the caspase-8-GSDMD pathway serves as an emergency host defence mechanism that induces pyroptosis in the absence of proinflammatory caspases. Furthermore, in the event of gram-negative bacterial infection of neutrophils, the granule-associated proteases cathepsin G (CatG) and ELANE (neutrophil elastase), which are specific to neutrophils, can also process GSDMD into active GSDMD-NTD (Figure 2B), thereby inducing pyroptosis in neutrophils and leading to the formation of neutrophil extracellular traps (NETs) as a means of preventing further infection [89, 90].
Canonical and non-canonical GSDMD cleavage and PTMs regulation. Caspase PTMs regulation. (1) TcpB secreted by Brucella to ubiquitylates caspase-1/4/11 and promotes their degradation, leading to inactivate GSDMD signaling. Prdx4 inactivates caspase-1 by modifying its C397. VX740, VX765, Ac-YVAD-cmk and z-VAD-fmk covalently modify the catalytic cysteine residues to inactivate caspases. (2) Nedd4 induces the K48-linked polyubiquitination of caspase-11 and succeeding degradation. PKA phosphorylates caspase-11, inhibiting non-canonical pyroptosis. GPx8 negatively regulated activation of GSDMD via disulfide bounding to the C118 site in caspase-4/11. Shigella flexneri can secrete OspC3 to inhibit pyroptosis via the ADP-ribosylation modification of caspase-4 and caspase-1l respectively at R314 and R310. GSDMD PTMs regulation. (3) SYVN1 induces K27-linked polyubiquitination of GSDMD at K203 and K204, promoting pyroptosis. PP1 enhances pyroptosis by dephosphorylating multiple sites on GSDMD. ZDHHC5/9 facilitates GSDMD ROS-dependent palmitoylation at C191 human (C192 mouse), promoting plasma membrane localization. ROS promote macrophage pyroptosis by oxidizing GSDMD. Endogenous itaconate covalently binds to GSDMD at C77, reducing caspase-1-mediated GSDMD cleavage. Fumarate can bind to GSDMD at multiple sites called succination to reduce proteolytic cleavage and inhibit NTD oligomerization. Disulfiram /NSA can inhibit NTD oligomerization through cys-modifying. Shigella flexneri can secrete IpaH7.8 to ubiquitylate hGSDMD and promote it degradation to inhibit pryroptosis. Abbreviations: Prdx4: thiol-specific peroxidase peroxiredoxin-4; PKA: Protein kinase A; GPx8: Glutathione peroxidase 8. Figures created with BioRender.com.
In addition to cleaving GSDMD to induce pyroptosis, certain caspases or proteases from pathogens can mediate the proteolytic inactivation of GSDMD. Apoptotic caspase-3 and caspase-7 can cleave D87 (mouse D88), which is located within the pore-forming GSDMD-NTD, resulting in a shortened GSDMD-NTD that has lost its ability to induce pyroptosis [91-93] (Figure 2B). In addition to host-derived proteases, GSDMD is also inactivated by exogenous proteases produced by pathogens to inhibit pyroptosis. 3C proteases, which are viral proteins produced by enterovirus 71 (EV71), process GSDMD at Q193 to inhibit pyroptosis, suggesting a strategy by which invading pathogens evade the host immune system [31]. Likewise, the 3C-like protease Nsp5 produced by coronaviruses (CoVs) such as SARS-CoV-2, MERS-CoV, PDCoV, and PEDV can cleave human GSDMD (hGSDMD) at Q193 to generate two nonpyroptotic fragments. This results in an inability to impair viral replication [94] (Figure 4).
Activated GSDMD can also bind cardiolipin, which is primarily expressed in the inner and outer leaflets of bacterial membranes, to directly kill bacteria [26]. Similarly, GSDMD-NTD regulates mitochondrial function by binding to cardiolipin and forming pores in the mitochondrial membrane structure, resulting in mitochondrial oxidative stress and the release of mitochondrial dsDNA (mtDNA) [53, 57, 95] (Figure 4). Recently, the identification of GSDMD pore formation in mitochondria and the release of mtDNA have been recognized as prerequisites for Nur77-mediated detection of intracellular LPS and dsDNA, thereby activating the noncanonical NLRP3 inflammasome [96]. This finding highlights the extensive interactions between GSDMD and inflammation, as well as various cell death types, including pyroptosis, apoptosis, and necroptosis. Regulating pore formation by GSDMD is crucial for maintaining immune homeostasis and treating inflammatory diseases.
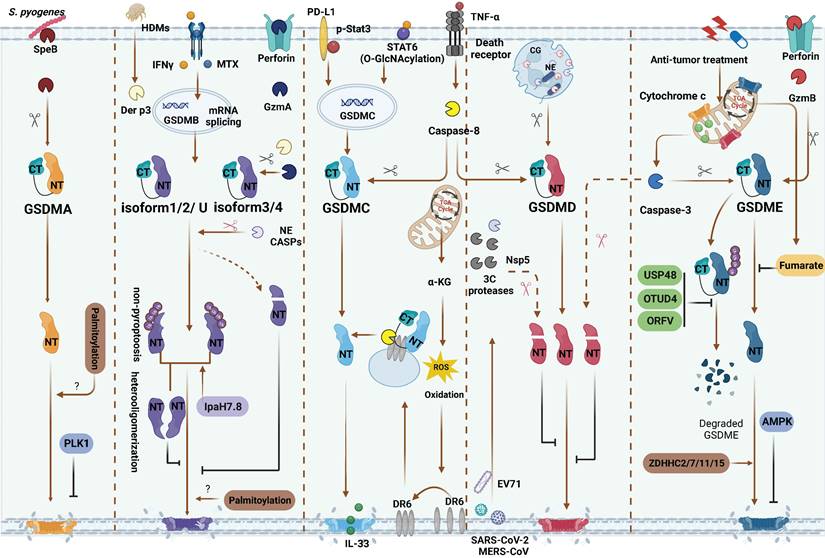
Inflammasome-independent GSDMs cleavage and PTMs regulation. GSDMA: SpeB directly activate GSDMA. PLK1 mediate GSDMA phosphorylation and inhibit GSDMA-NTD oligomerization. GSDMB: Methotrexate and IFNγ are identified as inflammatory or non-inflammatory stimuli of upregulating various GSDMB isoforms, respectively. GzmA from NK and/or CD8+ T cells and Der p3 from HDM can cleave different GSDMB isoforms. Only GzmA cleave GSDMBiso3,4 and Der p3 cleave GSDMBiso3 at K244 can produce pyroptotic NTD. Caspase-1 also can cleave GSDMBiso3 at D236 to induce pyroptosis. Caspase-1/3/4/6/7/8/9 and NE mediated proteolytic inactivation of GSDMBiso1-4. Non-pyroptotic GSDMB-NTD can inhibit the pore-forming activity of pyroptotic GSDMB-NTD by heterooligomerization. Shigella flexneri can secrete IpaH7.8 to ubiquitylate almost GSDMB isoforms and induce them degradation or directly inhibit the pyroptosis functions of GSDMB. GSDMC: In hypoxia situation, STAT3 and PD-L1 migrated to nucleus, enhancing GSDMC expression. TNFα-induced caspase-8 cleaves GSDMC in cancer cells. In response to helminth infection, STAT6 O-GlcNAcylation induces GSDMC expression. Then caspase-8 activates GSDMC and forming the pores for IL-33 releasing. α-KG can elevate intercellular ROS level to activate plasma membrane-localized death receptor DR6 serving as a platform caspase-8 and GSDMC interaction. GSDMD: In neutrophils, CG and NE activate GSDMD to induce NETosis. 3C protease from EV71, Nsp5 from SARS-CoV-2, MERS-CoV and caspase-3 can inactivate GSDMD. GSDME: Caspase-3 and GzmB can cleave GSDME at the same site. AMPK phosphorylates GSDME at T6 and inhibits pyroptosis. Fumarate can bind to GSDME at multiple sites called succination to inhibit pyroptosis. In anti-tumor therapies, USP48, OTUD4 and ORFV can reduce GSDME ubiquitination and degradation. During chemotherapy, ZDHHC2/7/11/15 can promote GSDME palmitoylation and further pyroptrosis. Red scissors represent inactivation and black scissors represent activation. Abbreviations: SpeB: Streptococcus pyogenes exotoxin B; PLK1: Polo-like kinase 1; CASP: caspase; HDMs: house dust mites; NE: neutrophil elastase; CG: cathepsin G; α-KG: α-ketoglutarate; DR6: death receptor 6; EV71: Enterovirus 71; AMPK: AMP-activated protein kinase; OTUD4: ovarian tumor family deubiquitinase 4; ORFV: oncolytic parapoxvirus ovis. Figures created with BioRender.com.
GSDME
GSDME is extensively expressed in various tissues, including the cochlea, skin, and gut. Initially, identified as a contributor to human hearing loss, GSDME has since been linked to inflammatory diseases such as atherosclerosis, skin inflammation, and IBD [60, 61]. Additionally, its unique ability to switch from apoptosis to pyroptosis holds great promise for tumour immunotherapy (Table 1).
Caspase-3 can cleave GSDME at D270, thereby generating the pyroptotic GSDME-NTD and inducing cell death. In response to chemotherapy drugs or apoptotic triggers, cells expressing GSDME undergo a transition from apoptosis to pyroptosis [18, 97]. Zhang et al. revealed that granzyme B (GzmB), which is s serine protease secreted by cytotoxic T lymphocytes and NK cells, triggers GSDME-dependent pyroptosis in the tumour microenvironment by cleaving GSDME at the same site as caspase-3 [19] (Figure 2B). Recent studies have revealed the pivotal role of GSDME in IBD [98, 99]. Increased levels of GSDME are cleaved by caspase-3, leading to IEC pyroptosis and the subsequent release of proinflammatory cytokines that augment immune responses [99]. Moreover, in a mouse model that was intravenously injected with TNFα, GSDME and upstream interferon regulating factor 1 (IRF1) were crucial for IEC shedding in IBD [98]. Moreover, the importance of GSDME in defending against viral and Candida albicans infections has been demonstrated [100-102]. In T cells, keratinocytes and alveolar epithelial cells, pathogen invasion results in mitochondrial damage, which subsequently triggers caspase-3 cleavage of GSDME and culminates in IL-1α release and cell death. Mitochondrial damage is a ubiquitous process that eukaryotes undergo in response to endogenous or exogenous stimuli, suggesting that the caspase-3-GSDME axis may be a general mechanism during abnormal conditions [103, 104]. Furthermore, GSDMA and GSDMD can target the mitochondrial membrane, and GSDMB induces pyroptosis with mitochondrial damage [53, 57, 58, 69, 95]. Whether these GSDMs interact with GSDME remains unclear, and more studies are needed to further explore the possible relationships.
PTMs: The regulators of GSDMs functions
The precise regulation of GSDMs-mediated pore formation plays a pivotal role in determining cell fate. As the most ubiquitous and efficient regulatory pathway, PTMs extensively participate in the modulation of protein stability, localization, and protein‒protein interactions by covalently attaching specific chemical groups to amino acid side chains of target proteins [105-107]. PTMs exert a range of regulatory effects on pyroptosis. In the subsequent sections, we will examine the distinct PTMs of each GSDM and elucidate their specific roles in various pathophysiological contexts.
Phosphorylation and dephosphorylation
Phosphorylation and dephosphorylation are common mechanisms for regulating protein activity [108]. GSDMA and GSDME undergo phosphorylation at T8 and T6, respectively, by unidentified kinases (Table 2). Phosphorylation of the GSDMA and GSDME NTDs inhibits the oligomerization of GSDMs and pyroptotic activity [109]. A PLK1-dependent phosphoproteome was identified, indicating that Polo-like kinase 1 (PLK1), a serine-threonine kinase, may be involved in the phosphorylation of GSDMA [110] (Figure 4). However, it remains unclear whether PLK1 phosphorylates GSDME in a similar manner. In addition, some metabolites with kinase activity may exert broad functions and regulate pyroptosis. AMP-activated protein kinase (AMPK) is activated by the metabolite N-acetylglucosamine-6-phosphate (GlcNAc-6P) and antagonizes GSDME-mediated pyroptosis by phosphorylating GSDME at T6 [111]. Recently, Li et al. reported that the phosphorylation of GSDMD at T213 could regulate oligomerization and suppress pyroptosis through steric hindrance (inhibiting the interaction between GSDMD monomers), while phosphatase 1 (PP1) can dephosphorylate multiple sites on GSDMD including T213 to increase pyroptosis [112]. Based on the phosphoproteome and proteomic mass spectrometry, GSDMD has several potential phosphorylation sites whose impact on its function remains unclear (Table 2). The pore-forming activity of GSDMs may be limited by phosphorylation, which is dependent on the structural characteristics of GSDMs.
Ubiquitination and deubiquitination
Protein ubiquitination is a dynamic PTM involving the covalent attachment of ubiquitin to a target protein and includes monoubiquitination and polyubiquitination [113]. The effects of this modification vary depending on the type of ubiquitination and substrate involved.
Stabilizing or inhibiting GSDM degradation can enhance the effects of pyroptosis. Shi et al. found that Synoviolin (SYVN1) promotes pyroptosis by inducing K27-linked polyubiquitination of GSDMD at K203 and K204 [114] (Figure 3). Ubibrowser software was used to investigate other potential E3 ubiquitin ligases that interact with GSDMD, such as Mindbomb Homologue 2 (MIB2) and Nedd4. Another study showed that sodium arsenite (NaAsO2) prevented GSDMD degradation by inhibiting K48- and K63-linked ubiquitination, leading to GSDMD accumulation and pyroptosis [115]. Consequently, uncontrolled pyroptosis worsens liver damage and insulin resistance. Moreover, some E3 ligase proteins can interact with GSDMs without using their ligase activity. For example, the PRY-SPRY domain of TRIM21 binds to GSDMD to stabilize and promote NTD oligomerization independently of its E3 ligase activity [116].
In cancer treatments, the deubiquitination of GSDMs through activation and stabilization is a valuable strategy (Figure 4). Ovarian tumour family deubiquitinase 4 (OTUD4) can enhance radiosensitivity in nasopharyngeal carcinoma cells by positively regulating pyroptosis via GSDME deubiquitination [117]. Similarly, USP48 can deubiquitinate GSDME at K120 and K189 by removing the K48-linked ubiquitin chains, thereby augmenting cancer cell susceptibility to pyroptosis in response to treatment [118]. Another investigation demonstrated that oncolytic parapoxvirus ovis (ORFV), a promising biotherapeutic antitumour agent, promotes tumour cell pyroptosis by decreasing GSDME ubiquitination [119]. While some deubiquitinating enzymes can stabilize and promote GSDME to induce lytic cell death, others may have the opposite effect on cancer cells. For example, in bladder cancer, USP24 stabilizes the nonpyroptotic GSDMB isoform, which enhances STAT3 phosphorylation and promotes cancer cell proliferation [120].
PTMs regulation of GSDMs
| GSDMs | PTM | Factors | PTM sites | PTM effects | Reference |
|---|---|---|---|---|---|
| GSDMA | Phosphorylation | PLK1 | T8(H) | Inhibit NTD oligomerization | [109, 110] |
| Palmitoylation | Unknown | Unknown | Unknown | [146] | |
| GSDMB | Ubiquitination | IpaH7.8 | K166, K308(H) | Proteasomal degradation | [65, 67, 126] |
| K177, K190, K192(H) | Inhibit membrane translocation (membrane insertion) | ||||
| Dephosphorylation | USP24 | Unknown | Unknown | [120] | |
| Palmitoylation | Unknown | Unknown | Unknown | [146] | |
| GSDMC | Phosphorylation | Unknown | S197(H) | Unknown (303 human Ser/Thr kinases potential interaction sites) | [154] |
| GSDMD | Phosphorylation | Unknown | S30, S31, T32 (H) | Triple mutation suppresses pryroptosis | [112, 154] |
| S45, S46, S47 (H) | Triple mutation suppresses pryroptosis | ||||
| T213(H) | Enhance steric hindrance to inhibit NTD oligomerization and pyroptosis | ||||
| S181, S185, S201, S250, S252, T251(H) | Unknown (303 human Ser/Thr kinases potential interaction sites) | ||||
| Dephosphorylation | PP1 | T213(H) | Promote NTD oligomerization | [112] | |
| Ubiquitination | IpaH7.8 | K55, K62, K204(H) | Proteasomal degradation | [33, 126] | |
| K27-Polyubiquitination | SYVN1 | K203, K204(H) | Promote pyroptosis | [114] | |
| Oxidation | ROS | C38, C56, C268, C467(H) C39, C57, C265, C487(M) | Promote cleavage by caspase-1 | [131, 132] | |
| C192(M) | Promote NTD oligomerization | ||||
| Itaconation | Endogenous itaconate | C77(M) | Reduce proteolytic cleavage | [141, 144] | |
| ITalk | C77, C192(M) | Unknown (Similar to Itaconate) | |||
| Succination | Fumarate derivatives (MMF, DMF) | C191(H)/C192(M) | Reduce proteolytic cleavage and inhibit NTD oligomerization | [138] | |
| Palmitoylation | ZDHHC5/9 | C191(H)/C192(M) | Promote plasma membrane localization | [145, 146] | |
| Cysteine-modification | Disulfiram | C191(H)/C192(M) | Inhibit NTD oligomerization | [36] | |
| Cysteine-modification | Necrosulfonamide | C191(H)/C192(M) | Inhibit NTD oligomerization | [37] | |
| GSDME | Phosphorylation | AMPK | T6(H) | Inhibit cleavage by caspase-3 and NTD oligomerization | [109, 111, 154] |
| Unknown | S252, T117, T6(H) | Unknown (303 human Ser/Thr kinases potential interaction sites) | |||
| Deubiquitination | OTUD4 | Unknown | Maintain the stability of GSDME | [117] | |
| Stabilization (Deubiquitination) | ORFV | Unknown | Maintain the stability of GSDME | [119] | |
| K48-link deubiquitination | USP48 | K120, K189(H) | Maintain the stability of GSDME | [118] | |
| Succination | Fumarate derivatives (MMF, DMF) | C45(H) | Inhibit Pyroptosis (Similar to GSDMD) | [138] | |
| Palmitoylation | ZDHHC2/7/11/15 | C407/C408(H) | Promote pyroptosis (CTD is palmitoylation) | [147] | |
| Palmitoylation | Unknown | Unknown | Most likely promote plasma membrane localization (NTD is palmitoylation) | [146] |
Table 2: The PTMs sites of human or murine proteins are indicated in H or M in parentheses. Abbreviations: Polo-like kinase 1: PLK1; PP1: Phosphatase1; SYVN1: Synoviolin; ROS: reactive oxygen species; ITalk: a specific and cell permeable bioorthogonal probe; MMF: Monomethyl fumarate; DMF: Dimethyl fumarate; OTUD4: ovarian tumor family deubiquitinase 4; ORFV: oncolytic parapoxvirus ovis
In response to pathogenic bacteria invading the host, GSDMs quickly execute pyroptosis in infected cells such as epithelial cells, neutrophils, and macrophages to prevent intracellular pathogen replication [23, 28, 121-123]. However, certain microorganisms have developed specific mechanisms to counteract pyroptosis [124, 125] (Figure 3,4). Hansen et al. discovered that IpaH7.8, an E3 ubiquitin ligase produced by Shigella flexneri, targets K166 and K308, as well as other ubiquitinated residues of GSDMBisoU, to induce 26S proteasomal degradation, thereby protecting Shigella flexneri from the bactericidal activity of NK cells [25]. A subsequent study showed that ubiquitination of GSDMB at K177, K190 and K192 by IpaH7.8 was sufficient to block GSDMB-induced pyroptosis without the involvement of the proteasome [67]. Interestingly, several studies demonstrated that IpaH7.8 could inhibit the pyroptotic activity of hGSDMD but not mouse GSDMD (mGSDMD) through the ubiquitination of K55, K62, K204 and other residues [33, 126]. The presence of R20 in mGSDMD creates a bulge in its crystal structure, which prevents IpaH7.8-mediated ubiquitination compared to hGSDMD [126]. Moreover, several studies have reported that pathogens can disrupt host defence by inducing ubiquitination, ADP-ribosylation, and ADPR-deacylization in GSDM-mediated pyroptosis pathway factors, suggesting that PTMs may be exploited by invading pathogens [30, 32, 34] (Figure 3).
Oxidation
Oxidation‒reduction reactions play a crucial role in cellular metabolism, and their by-products regulate various biological processes, such as stress responses, inflammation, and cell death, through multiple pathways [127]. Exogenous and endogenous ROS can activate inflammasome pathways and stimulate pyroptosis [128, 129]. During pore formation, the GSDMD-NTD cannot induce pyroptosis without the Ragulator-Rag components RagA or RagC. However, this can be remedied with ROS [130]. Moreover, ROS can exert their effects through the covalent modification of proteins. Recent studies have revealed that oxidative PTMs involving cysteines within GSDMD are closely associated with pyroptosis (Figure 3). In 2019, Wang et al. discovered that mitochondrial ROS (mtROS) induced macrophage pyroptosis by oxidizing hGSDMD at C38, C56, and C268 and mGSDMD at C39, C57, C265 and C487 [131]. Another recent study reported that during pyroptosis, the C57, C77, C192 and C265 sites of mGSDMD-NTD underwent oxidation [132] (Table 2). Among these sites, C192 was indispensable for ROS responsiveness.
In addition to activating inflammasome pathways and inducing oligomerization through oxidative modification of GSDMD-NTD, mtROS may possess other mechanisms by which they regulate pyroptosis. (Table 2). A recent study showed that oxidized mitochondrial DNA (Ox-mtDNA) in the cytoplasm binds to GSDMD-NTD at R138, K146, R152, and R154, thereby promoting the oligomerization of GSDMD-NTD in neutrophils and NETs formation [133]. Furthermore, activation of the caspase-3-GSDME axis by ROS has been documented in the context of antitumour therapy and chemotherapeutic drug-induced nephrotoxicity [134, 135]. Whether ROS modulate pore formation by other GSDMs through oxidative modification requires further investigation.
Succination
Many studies have revealed that cell metabolism regulates inflammatory responses [136, 137]. After being stimulated by LPS, macrophages tend to switch their metabolic characteristics from oxidative phosphorylation to aerobic glycolysis [138, 139]. Some metabolic intermediates, such as itaconate, succinate and fumarate, correct aberrant inflammatory responses through transcriptional and metabolic regulation, as well as PTMs [138-140]. Moreover, studies have shown that metabolic intermediates regulate various types of cell death, including pyroptosis [138, 141-143]. Humphries et al. showed that dimethyl fumarate (DMF) or endogenous fumarate could bind to pivotal cysteine residues on GSDMD and GSDME, resulting in an irreversible process called succination, which forms S-(2-succinyl)-cysteine [138]. There are many succination sites on GSDMD and GSDME according to mass spectrometry results. To date, researchers have identified many succination sites in hGSDMD (C56, C191, C268, C309, and C467), mGSDMD (C39, C57, C77, C122, C192, C265, C299, C434, C448, C487, and C489), and human GSDME (C45, C156, C168, C180, C235, C371, C408, C417, and C489) (Table 2). Similarly, succination limits GSDME-induced cell death [138]. However, the specific succination sites on GSDME and their impact on its interaction with upstream proteases require further investigation.
Itaconation
Itaconate contains an electrophilic α, β-unsaturated carboxylic acid moiety that can undergo Michael addition with the cysteine residues of proteins, leading to itaconation [144]. Itaconate-associated PTMs have been reported to play pivotal roles in modulating many pathological processes [137]. In 2021, Bambouskova et al. showed that endogenous itaconate covalently bound to GSDMD at C77, which was termed itaconation, thereby reducing caspase-1-mediated GSDMD cleavage and establishing tolerance to prolonged periods of LPS exposure in macrophages [141]. Significantly, octyl-itaconate or similar itaconate compounds exhibit many PTM sites for the same substrate. Qin and colleagues used the specific and cell-permeable bio-orthogonal probe ITalk to identify additional potential itaconate modification sites for GSDMD (Table 2). Intriguingly, C77 and C192 of GSDMD were shown to be susceptible to itaconate modification. Previous studies have identified C191 in hGSDMD (hC191) and C192 in mGSDMD (mC192) as crucial sites for GSDMD-NT oligomerization [26]. These derivatives of itaconate may have a wider range of potential applications than endogenous itaconate in the regulation of pyroptosis. Similarly, further research is needed to determine whether itaconate can modify other GSDMs.
Palmitoylation
Protein palmitoylation refers to the reversible attachment of palmitic acid to cysteine residues, which participates in multiple intracellular physiological processes. During this process, palmitoyl acyl transferase (PAT) enzymes (zinc finger and DHHC motif-containing) in the ZDHHC family play an indispensable role in transferring palmitate to cysteines [145-147]. Accumulating evidence indicates that the palmitoylation of GSDMs is a key regulatory mechanism (Figures 3 and 4). During chemotherapy, pyroptosis induced by GSDME can be enhanced by palmitoylation of the C407 and C408 residues of GSDME-CTD via ZDHHC2, 7, 11 and 15. Palmitoylation inhibitors and palmitoylation site mutation reinforce autoinhibition between GSDME-CTD and GSDME-NTD [147]. Recent studies have shown that ZDHHC5/9 mediates ROS-dependent palmitoylation of GSDMD at hC191/mC192 to promote plasma membrane localization, which is indispensable for pyroptosis [145, 146]. In addition to GSDMD, GSDMB-FL, GSDME-FL and GSDME-NTD can also be palmitoylated. This means that palmitoylation may serve as a ubiquitous PTM, governing the membrane localization of GSDMs-NTD and thereby regulating pyroptosis. However, the interacting enzymes and the particular modification sites require further study.
Cysteine modification
hC191/mC192 is essential for GSDMD to mediate pyroptosis, which may also be a target for modulation by other small molecules [26]. Disulfiram, an FDA-approved medication for alcohol abuse treatment, can covalently bind to hC191/mC192 within GSDMD through cysteine modification, thereby reducing pore formation in an animal model of LPS-induced sepsis [36] (Figure 3). The Cys-modifying drug necrosulfonamide (NSA) was reported to block pyroptosis by directly binding to hC191/mC192 in GSDMD [37]. Collectively, these findings indicate that the modification of key cysteine sites in GSDMs may serve as a pivotal regulatory mechanism of pore formation. This finding highlights the potential therapeutic opportunities of targeting this pathway in various human diseases.
Among these modification residues of GSDMD, hC191/mC192 has been identified as the indispensable target for various PTMs, including oxidation, succination, itaconation and palmitoylation [132, 138, 144-146]. Furthermore, several Cys-modifying drugs have shown therapeutic effects in animal models [36, 37]. In fact, different PTMs at hC191/mC192 can occur in a context-dependent manner. For example, DMF had little effect on preventing liposome leakage [36]. However, DMF inhibited THP-1 cell and BMDM death. In mice, the administration of DMF protected against LPS-induced shock and other inflammatory diseases by targeting GSDMD [138]. In addition, there may be interactions between different PTMs during the dynamic process of pyroptosis [145, 146]. The palmitoylation of GSDMD-FL and -NTD at hC191/mC192 occurred in a ROS-dependent manner and promoted membrane localization [146]. Although multiple PTMs can occur at GSDMD hC191/mC192, the final effect on pyroptosis and the dominant PTMs seems to be associated with the treatment of cells or animals. The competition between different PTMs at GSDMD hC191/mC192 may be the underlying mechanism of pyroptosis regulation.
Therapeutic strategies involving PTMs
Due to their potential in antimicrobial defence, inflammatory diseases, and cancer treatment, GSDM-mediated pyroptosis has gained significant attention. NSA and disulfiram have shown promising potential in several mouse models, including DSS-induced colitis, LPS-induced sepsis, and vascular remodelling induced by chronic hypoxia [36, 148-151]. Itaconate, fumarate and their derivatives exert potential therapeutic effects against inflammatory and pyroptosis-related diseases, as demonstrated by cell and animal models [137, 152]. Moreover, several fumarate analogues, including DMF, diroximel fumarate, and tepilamide fumarate, have been FDA-approved for the treatment of multiple sclerosis and autoimmune encephalitis [138]. Further exploration is warranted to determine whether these PTM drugs targeting GSDMD could be applied to other diseases caused by pyroptosis. In antitumour therapy, GSDME protein levels are crucial for caspase-3/GSDME-mediated tumour cell pyroptosis. In cases of low GSDME expression, chemotherapy tends to induce apoptosis rather than pyroptosis, thereby attenuating its therapeutic efficacy [119]. Furthermore, tumour patients with elevated levels of the pyroptotic GSDMB isoform exhibit improved survival outcomes [68, 69]. Activating and stabilizing pyroptotic GSDMs is a valuable strategy for cancer treatment due to their role in antitumour immunity. Achieving the proper balance between cell death and survival is important for antimicrobial defence. To avoid being eliminated, some pathogens release specific effectors that inhibit pyroptosis via PTMs. Therefore, enhancing the activity of GSDMs or eliminating negative effects of pathogens on pyroptosis may exert significant protective effects during bacterial infection.
Overall, applying approved drugs or designing new drugs to target the PTMs of GSDMs to affect signalling shows promising prospects in cancer, infectious diseases and inflammatory diseases.
Conclusions and future perspectives
In the past two decades, many mysteries of GSDMs have been gradually unveiled. Current studies have demonstrated the underlying mechanism of GSDMs in many diseases. Increasing the activity of pyroptosis aggravates tissue damage and induces an exaggerated inflammatory response, but weakening pyroptosis typically fails to eliminate invading pathogens. Pyroptosis activity is closely related to the sites of cleavage, the types of proteases, and PTMs, which will become optional targets for the future regulation of pyroptosis.
This review focused on describing the latest studies on protease-mediated GSDM cleavage, summarized the underlying sites and described the mechanisms of PTMs. Currently, several questions exist about the cleavage and PTMs of GSDMs. First, as a vital regulatory method, PTMs are involved in most processes associated with GSDM function, including autoinhibition, proteolytic cleavage, oligomerization and even pyroptosis-independent biological effects. Palmitoylation of GSDMD at mC192 occurred in a ROS-dependent manner to regulate membrane translocation, indicating an interplay between different PTMs. Which PTM predominates in specific intermediate steps of pyroptosis and how these PTMs interact remain unclear. Second, it is important to identify what types of GSDMs are expressed in different cells and the changes in GSDMs between normal and diseased states. For GSDMB, it is necessary to determine the main subtype in different cells and tissues. Third, whether we can apply what we know about the PTMs of one GSDM to other GSDMs is worth further examination.
Although the PTMs of GSDMs have been gradually discovered in various diseases, the potential modification pathways and mechanisms are still unclear. Moreover, the PTMs of GSDMs may occur in a context-dependent manner, which indicates that the same PTM may not have the same effects in vivo or in vitro. For clinical translation, more experiments on the PTMs of GSDMs are needed to determine their effects. Generally speaking, the PTMs of GSDMs are newly emerging targets for modulating cell death and the resulting immune responses.
Abbreviations
GSDMs: gasdermins; PTMs: posttranslational modifications; NTD: N-terminal domains; CTD: C-terminal domains; ; MLKL: mixed lineage kinase domain-like; SpeB: Streptococcus pyogenes exotoxin B; GzmA: granzyme A; GzmB: granzyme B; NE: neutrophil elastase; CG: cathepsin G; SYVN1: Synoviolin; NSA: necrosulfonamide; PP1: Phosphatase1; ROS: reactive oxygen species; ZDHHC: zinc finger and DHHC motif-containing family; PKA: Protein kinase A; AMPK: AMP-activated protein kinase; Ox-mtDNA: Oxidized mitochondrial DNA; PLK1: Polo-like kinase 1; HDMs: house dust mites; NE: neutrophil elastase; CG: cathepsin G; α-KG: α-ketoglutarate; DR6: death receptor 6; EV71: Enterovirus 71; OTUD4: ovarian tumor family deubiquitinase 4; ORFV: oncolytic parapoxvirus ovis; IBD: inflammatory bowel disease.
Acknowledgements
This project was funded by National Natural Science Foundation of China (82072149, 82072223, 82272209, 82272237), Jiangsu Provincial Medical Innovation Center (CXZX202217), Key Research and Development Program of Jiangsu Province (BE2022823).
Author contributions
H.J., J.K., J.W., and J.R. made contributions to the conception or design of the study. H.J., P.L., X.L., C.N., and B.L. made the figures and tables. P.L., J.K., J.L., Y.L., and W.L. revised pictures and tables with input from all authors. H.J. wrote the initial draft of the manuscript. J.W., X.W., Y.Z., and J.R. read and revised early drafts of the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Delmaghani S, del Castillo FJ, Michel V, Leibovici M, Aghaie A, Ron U. et al. Mutations in the gene encoding pejvakin, a newly identified protein of the afferent auditory pathway, cause DFNB59 auditory neuropathy. Nat Genet. 2006;38:770-8
2. Lunny DP, Weed E, Nolan PM, Marquardt A, Augustin M, Porter RM. Mutations in gasdermin 3 cause aberrant differentiation of the hair follicle and sebaceous gland. J Invest Dermatol. 2005;124:615-21
3. Schwander M, Sczaniecka A, Grillet N, Bailey JS, Avenarius M, Najmabadi H. et al. A forward genetics screen in mice identifies recessive deafness traits and reveals that pejvakin is essential for outer hair cell function. J Neurosci. 2007;27:2163-75
4. Zhou Y, Jiang X, Gu P, Chen W, Zeng X, Gao X. Gsdma3 mutation causes bulge stem cell depletion and alopecia mediated by skin inflammation. Am J Pathol. 2012;180:763-74
5. Saeki N, Usui T, Aoyagi K, Kim DH, Sato M, Mabuchi T. et al. Distinctive expression and function of four GSDM family genes (GSDMA-D) in normal and malignant upper gastrointestinal epithelium. Genes Chromosomes Cancer. 2009;48:261-71
6. Tamura M, Tanaka S, Fujii T, Aoki A, Komiyama H, Ezawa K. et al. Members of a novel gene family, Gsdm, are expressed exclusively in the epithelium of the skin and gastrointestinal tract in a highly tissue-specific manner. Genomics. 2007;89:618-29
7. Tanaka S, Mizushina Y, Kato Y, Tamura M, Shiroishi T. Functional conservation of Gsdma cluster genes specifically duplicated in the mouse genome. G3 (Bethesda). 2013;3:1843-50
8. Wu H, Romieu I, Sienra-Monge JJ, Li H, del Rio-Navarro BE, London SJ. Genetic variation in ORM1-like 3 (ORMDL3) and gasdermin-like (GSDML) and childhood asthma. Allergy. 2009;64:629-35
9. Yu J, Kang MJ, Kim BJ, Kwon JW, Song YH, Choi WA. et al. Polymorphisms in GSDMA and GSDMB are associated with asthma susceptibility, atopy and BHR. Pediatr Pulmonol. 2011;46:701-8
10. Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S. et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363:1211-21
11. Op de Beeck K, Van Camp G, Thys S, Cools N, Callebaut I, Vrijens K. et al. The DFNA5 gene, responsible for hearing loss and involved in cancer, encodes a novel apoptosis-inducing protein. Eur J Hum Genet. 2011;19:965-73
12. Van Rossom S, Op de Beeck K, Franssens V, Swinnen E, Schepers A, Ghillebert R. et al. The splicing mutant of the human tumor suppressor protein DFNA5 induces programmed cell death when expressed in the yeast Saccharomyces cerevisiae. Front Oncol. 2012;2:77
13. Van Rossom S, Op de Beeck K, Hristovska V, Winderickx J, Van Camp G. The deafness gene DFNA5 induces programmed cell death through mitochondria and MAPK-related pathways. Front Cell Neurosci. 2015;9:231
14. He WT, Wan H, Hu L, Chen P, Wang X, Huang Z. et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015;25:1285-98
15. Kayagaki N, Stowe IB, Lee BL, O'Rourke K, Anderson K, Warming S. et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666-71
16. Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660-5
17. Hou J, Zhao R, Xia W, Chang CW, You Y, Hsu JM. et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat Cell Biol. 2020;22:1264-75
18. Rogers C, Fernandes-Alnemri T, Mayes L, Alnemri D, Cingolani G, Alnemri ES. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun. 2017;8:14128
19. Zhang Z, Zhang Y, Xia S, Kong Q, Li S, Liu X. et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature. 2020;579:415-20
20. Zhou Z, He H, Wang K, Shi X, Wang Y, Su Y. et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science. 2020 368
21. Deng W, Bai Y, Deng F, Pan Y, Mei S, Zheng Z. et al. Streptococcal pyrogenic exotoxin B cleaves GSDMA and triggers pyroptosis. Nature. 2022;602:496-502
22. LaRock DL, Johnson AF, Wilde S, Sands JS, Monteiro MP, LaRock CN. Group A Streptococcus induces GSDMA-dependent pyroptosis in keratinocytes. Nature. 2022;605:527-31
23. Deets KA, Vance RE. Inflammasomes and adaptive immune responses. Nat Immunol. 2021;22:412-22
24. Nozaki K, Li L, Miao EA. Innate Sensors Trigger Regulated Cell Death to Combat Intracellular Infection. Annu Rev Immunol. 2022;40:469-98
25. Hansen JM, de Jong MF, Wu Q, Zhang LS, Heisler DB, Alto LT. et al. Pathogenic ubiquitination of GSDMB inhibits NK cell bactericidal functions. Cell. 2021;184:3178-91.e18
26. Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H. et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153-8
27. Chen KW, Demarco B, Ramos S, Heilig R, Goris M, Grayczyk JP. et al. RIPK1 activates distinct gasdermins in macrophages and neutrophils upon pathogen blockade of innate immune signaling. Proc Natl Acad Sci U S A. 2021 118
28. Chen KW, Monteleone M, Boucher D, Sollberger G, Ramnath D, Condon ND. et al. Noncanonical inflammasome signaling elicits gasdermin D-dependent neutrophil extracellular traps. Sci Immunol. 2018 3
29. Sollberger G, Choidas A, Burn GL, Habenberger P, Di Lucrezia R, Kordes S. et al. Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci Immunol. 2018 3
30. Jakka P, Namani S, Murugan S, Rai N, Radhakrishnan G. The Brucella effector protein TcpB induces degradation of inflammatory caspases and thereby subverts non-canonical inflammasome activation in macrophages. J Biol Chem. 2017;292:20613-27
31. Lei X, Zhang Z, Xiao X, Qi J, He B, Wang J. Enterovirus 71 Inhibits Pyroptosis through Cleavage of Gasdermin D. J Virol. 2017 91
32. Li Z, Liu W, Fu J, Cheng S, Xu Y, Wang Z. et al. Shigella evades pyroptosis by arginine ADP-riboxanation of caspase-11. Nature. 2021;599:290-5
33. Luchetti G, Roncaioli JL, Chavez RA, Schubert AF, Kofoed EM, Reja R. et al. Shigella ubiquitin ligase IpaH7.8 targets gasdermin D for degradation to prevent pyroptosis and enable infection. Cell Host Microbe. 2021;29:1521-30.e10
34. Peng T, Tao X, Xia Z, Hu S, Xue J, Zhu Q. et al. Pathogen hijacks programmed cell death signaling by arginine ADPR-deacylization of caspases. Mol Cell. 2022;82:1806-20.e8
35. Chen H, Li Y, Wu J, Li G, Tao X, Lai K. et al. RIPK3 collaborates with GSDMD to drive tissue injury in lethal polymicrobial sepsis. Cell Death Differ. 2020;27:2568-85
36. Hu JJ, Liu X, Xia S, Zhang Z, Zhang Y, Zhao J. et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat Immunol. 2020;21:736-45
37. Rathkey JK, Zhao J, Liu Z, Chen Y, Yang J, Kondolf HC. et al. Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci Immunol. 2018 3
38. Liu X, Xia S, Zhang Z, Wu H, Lieberman J. Channelling inflammation: gasdermins in physiology and disease. Nat Rev Drug Discov. 2021;20:384-405
39. Hou J, Hsu JM, Hung MC. Molecular mechanisms and functions of pyroptosis in inflammation and antitumor immunity. Mol Cell. 2021;81:4579-90
40. Rao Z, Zhu Y, Yang P, Chen Z, Xia Y, Qiao C. et al. Pyroptosis in inflammatory diseases and cancer. Theranostics. 2022;12:4310-29
41. Sarrió D, Martínez-Val J, Molina-Crespo Á, Sánchez L, Moreno-Bueno G. The multifaceted roles of gasdermins in cancer biology and oncologic therapies. Biochim Biophys Acta Rev Cancer. 2021;1876:188635
42. Kong Q, Zhang Z. Cancer-associated pyroptosis: A new license to kill tumor. Front Immunol. 2023;14:1082165
43. Kayagaki N, Kornfeld OS, Lee BL, Stowe IB, O'Rourke K, Li Q. et al. NINJ1 mediates plasma membrane rupture during lytic cell death. Nature. 2021;591:131-6
44. Liu Z, Wang C, Yang J, Zhou B, Yang R, Ramachandran R. et al. Crystal Structures of the Full-Length Murine and Human Gasdermin D Reveal Mechanisms of Autoinhibition, Lipid Binding, and Oligomerization. Immunity. 2019;51:43-9.e4
45. Mari SA, Pluhackova K, Pipercevic J, Leipner M, Hiller S, Engel A. et al. Gasdermin-A3 pore formation propagates along variable pathways. Nat Commun. 2022;13:2609
46. Rühl S, Shkarina K, Demarco B, Heilig R, Santos JC, Broz P. ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science. 2018;362:956-60
47. Degen M, Santos JC, Pluhackova K, Cebrero G, Ramos S, Jankevicius G. et al. Structural basis of NINJ1-mediated plasma membrane rupture in cell death. Nature. 2023
48. Ding J, Wang K, Liu W, She Y, Sun Q, Shi J. et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111-6
49. Johnson AG, Mayer ML, Schaefer SL, McNamara-Bordewick NK, Hummer G, Kranzusch PJ. Structure and assembly of a bacterial gasdermin pore. bioRxiv. 2023
50. Kayagaki N, Stowe IB, Alegre K, Deshpande I, Wu S, Lin Z. et al. Inhibiting membrane rupture with NINJ1 antibodies limits tissue injury. Nature. 2023
51. Korn V, Pluhackova K. Not sorcery after all: Roles of multiple charged residues in membrane insertion of gasdermin-A3. Front Cell Dev Biol. 2022;10:958957
52. Sborgi L, Rühl S, Mulvihill E, Pipercevic J, Heilig R, Stahlberg H. et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. Embo j. 2016;35:1766-78
53. Aglietti RA, Estevez A, Gupta A, Ramirez MG, Liu PS, Kayagaki N. et al. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc Natl Acad Sci U S A. 2016;113:7858-63
54. Chen X, He WT, Hu L, Li J, Fang Y, Wang X. et al. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 2016;26:1007-20
55. Sahoo B, Mou Z, Liu W, Dubyak G, Dai X. How NINJ1 mediates plasma membrane rupture and why NINJ2 cannot. bioRxiv. 2023. 2023 05.31.543175
56. David L, Borges JP, Hollingsworth LR, Volchuk A, Jansen I, Steinberg BE. et al. NINJ1 mediates plasma membrane rupture through formation of nanodisc-like rings. bioRxiv. 2023. 2023 06.01.543231
57. Lin PH, Lin HY, Kuo CC, Yang LT. N-terminal functional domain of Gasdermin A3 regulates mitochondrial homeostasis via mitochondrial targeting. J Biomed Sci. 2015;22:44
58. Kondolf HC, D'Orlando DA, Dubyak GR, Abbott DW. Protein engineering reveals that gasdermin A preferentially targets mitochondrial membranes over the plasma membrane during pyroptosis. J Biol Chem. 2023;299:102908
59. Nakagawa I, Amano A, Mizushima N, Yamamoto A, Yamaguchi H, Kamimoto T. et al. Autophagy defends cells against invading group A Streptococcus. Science. 2004;306:1037-40
60. Broz P, Pelegrín P, Shao F. The gasdermins, a protein family executing cell death and inflammation. Nat Rev Immunol. 2020;20:143-57
61. De Schutter E, Roelandt R, Riquet FB, Van Camp G, Wullaert A, Vandenabeele P. Punching Holes in Cellular Membranes: Biology and Evolution of Gasdermins. Trends Cell Biol. 2021;31:500-13
62. Cardamone G, Paraboschi EM, Rimoldi V, Duga S, Soldà G, Asselta R. The Characterization of GSDMB Splicing and Backsplicing Profiles Identifies Novel Isoforms and a Circular RNA That Are Dysregulated in Multiple Sclerosis. Int J Mol Sci. 2017 18
63. Chao KL, Kulakova L, Herzberg O. Gene polymorphism linked to increased asthma and IBD risk alters gasdermin-B structure, a sulfatide and phosphoinositide binding protein. Proc Natl Acad Sci U S A. 2017;114:E1128-e37
64. Ivanov AI, Rana N, Privitera G, Pizarro TT. The enigmatic roles of epithelial gasdermin B: Recent discoveries and controversies. Trends Cell Biol. 2023;33:48-59
65. Zhong X, Zeng H, Zhou Z, Su Y, Cheng H, Hou Y. et al. Structural mechanisms for regulation of GSDMB pore-forming activity. Nature. 2023;616:598-605
66. Rana N, Privitera G, Kondolf HC, Bulek K, Lechuga S, De Salvo C. et al. GSDMB is increased in IBD and regulates epithelial restitution/repair independent of pyroptosis. Cell. 2022;185:283-98.e17
67. Wang C, Shivcharan S, Tian T, Wright S, Ma D, Chang J. et al. Structural basis for GSDMB pore formation and its targeting by IpaH7.8. Nature. 2023;616:590-7
68. Kong Q, Xia S, Pan X, Ye K, Li Z, Li H. et al. Alternative splicing of GSDMB modulates killer lymphocyte-triggered pyroptosis. Sci Immunol. 2023;8:eadg3196
69. Oltra SS, Colomo S, Sin L, Pérez-López M, Lázaro S, Molina-Crespo A. et al. Distinct GSDMB protein isoforms and protease cleavage processes differentially control pyroptotic cell death and mitochondrial damage in cancer cells. Cell Death Differ. 2023;30:1366-81
70. Panganiban RA, Sun M, Dahlin A, Park HR, Kan M, Himes BE. et al. A functional splice variant associated with decreased asthma risk abolishes the ability of gasdermin B to induce epithelial cell pyroptosis. J Allergy Clin Immunol. 2018;142:1469-78.e2
71. Panganiban RA, Mwase C, Park JA, Lu Q. Direct cleavage and activation of gasdermin B by allergens. Allergy. 2023
72. Das S, Miller M, Beppu AK, Mueller J, McGeough MD, Vuong C. et al. GSDMB induces an asthma phenotype characterized by increased airway responsiveness and remodeling without lung inflammation. Proc Natl Acad Sci U S A. 2016;113:13132-7
73. Chen Q, Shi P, Wang Y, Zou D, Wu X, Wang D. et al. GSDMB promotes non-canonical pyroptosis by enhancing caspase-4 activity. J Mol Cell Biol. 2019;11:496-508
74. Zhao M, Ren K, Xiong X, Xin Y, Zou Y, Maynard JC. et al. Epithelial STAT6 O-GlcNAcylation drives a concerted anti-helminth alarmin response dependent on tuft cell hyperplasia and Gasdermin C. Immunity. 2022;55:623-38.e5
75. Xi R, Montague J, Lin X, Lu C, Lei W, Tanaka K. et al. Up-regulation of gasdermin C in mouse small intestine is associated with lytic cell death in enterocytes in worm-induced type 2 immunity. Proc Natl Acad Sci U S A. 2021 118
76. Zhang JY, Zhou B, Sun RY, Ai YL, Cheng K, Li FN. et al. The metabolite α-KG induces GSDMC-dependent pyroptosis through death receptor 6-activated caspase-8. Cell Res. 2021;31:980-97
77. Burdette BE, Esparza AN, Zhu H, Wang S. Gasdermin D in pyroptosis. Acta Pharm Sin B. 2021;11:2768-82
78. Fang Y, Tian S, Pan Y, Li W, Wang Q, Tang Y. et al. Pyroptosis: A new frontier in cancer. Biomed Pharmacother. 2020;121:109595
79. Xia S. Biological mechanisms and therapeutic relevance of the gasdermin family. Mol Aspects Med. 2020;76:100890
80. Heilig R, Dick MS, Sborgi L, Meunier E, Hiller S, Broz P. The Gasdermin-D pore acts as a conduit for IL-1β secretion in mice. Eur J Immunol. 2018;48:584-92
81. Tsuchiya K, Hosojima S, Hara H, Kushiyama H, Mahib MR, Kinoshita T. et al. Gasdermin D mediates the maturation and release of IL-1α downstream of inflammasomes. Cell Rep. 2021;34:108887
82. Xia S, Zhang Z, Magupalli VG, Pablo JL, Dong Y, Vora SM. et al. Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature. 2021;593:607-11
83. Zhang J, Yu Q, Jiang D, Yu K, Yu W, Chi Z. et al. Epithelial Gasdermin D shapes the host-microbial interface by driving mucus layer formation. Sci Immunol. 2022;7:eabk2092
84. Pandey A, Shen C, Feng S, Man SM. Cell biology of inflammasome activation. Trends Cell Biol. 2021;31:924-39
85. Afonina IS, Müller C, Martin SJ, Beyaert R. Proteolytic Processing of Interleukin-1 Family Cytokines: Variations on a Common Theme. Immunity. 2015;42:991-1004
86. Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P. et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187-92
87. Orning P, Weng D, Starheim K, Ratner D, Best Z, Lee B. et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science. 2018;362:1064-9
88. Sarhan J, Liu BC, Muendlein HI, Li P, Nilson R, Tang AY. et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc Natl Acad Sci U S A. 2018;115:E10888-e97
89. Burgener SS, Leborgne NGF, Snipas SJ, Salvesen GS, Bird PI, Benarafa C. Cathepsin G Inhibition by Serpinb1 and Serpinb6 Prevents Programmed Necrosis in Neutrophils and Monocytes and Reduces GSDMD-Driven Inflammation. Cell Rep. 2019;27:3646-56.e5
90. Kambara H, Liu F, Zhang X, Liu P, Bajrami B, Teng Y. et al. Gasdermin D Exerts Anti-inflammatory Effects by Promoting Neutrophil Death. Cell Rep. 2018;22:2924-36
91. Chen KW, Demarco B, Heilig R, Shkarina K, Boettcher A, Farady CJ. et al. Extrinsic and intrinsic apoptosis activate pannexin-1 to drive NLRP3 inflammasome assembly. Embo j. 2019 38
92. Demarco B, Grayczyk JP, Bjanes E, Le Roy D, Tonnus W, Assenmacher CA. et al. Caspase-8-dependent gasdermin D cleavage promotes antimicrobial defense but confers susceptibility to TNF-induced lethality. Sci Adv. 2020 6
93. Taabazuing CY, Okondo MC, Bachovchin DA. Pyroptosis and Apoptosis Pathways Engage in Bidirectional Crosstalk in Monocytes and Macrophages. Cell Chem Biol. 2017;24:507-14.e4
94. Shi F, Lv Q, Wang T, Xu J, Xu W, Shi Y. et al. Coronaviruses Nsp5 Antagonizes Porcine Gasdermin D-Mediated Pyroptosis by Cleaving Pore-Forming p30 Fragment. mBio. 2022;13:e0273921
95. Platnich JM, Chung H, Lau A, Sandall CF, Bondzi-Simpson A, Chen HM. et al. Shiga Toxin/Lipopolysaccharide Activates Caspase-4 and Gasdermin D to Trigger Mitochondrial Reactive Oxygen Species Upstream of the NLRP3 Inflammasome. Cell Rep. 2018;25:1525-36.e7
96. Zhu F, Ma J, Li W, Liu Q, Qin X, Qian Y. et al. The orphan receptor Nur77 binds cytoplasmic LPS to activate the non-canonical NLRP3 inflammasome. Immunity. 2023;56:753-67.e8
97. Wang Y, Gao W, Shi X, Ding J, Liu W, He H. et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547:99-103
98. Tan G, Huang C, Chen J, Chen B, Shi Y, Zhi F. An IRF1-dependent Pathway of TNFα-induced Shedding in Intestinal Epithelial Cells. J Crohns Colitis. 2022;16:133-42
99. Tan G, Huang C, Chen J, Chen B, Zhi F. Gasdermin-E-mediated pyroptosis participates in the pathogenesis of Crohn's disease by promoting intestinal inflammation. Cell Rep. 2021;35:109265
100. Wan X, Li J, Wang Y, Yu X, He X, Shi J. et al. H7N9 virus infection triggers lethal cytokine storm by activating gasdermin E-mediated pyroptosis of lung alveolar epithelial cells. Natl Sci Rev. 2022;9:nwab137
101. Orzalli MH, Prochera A, Payne L, Smith A, Garlick JA, Kagan JC. Virus-mediated inactivation of anti-apoptotic Bcl-2 family members promotes Gasdermin-E-dependent pyroptosis in barrier epithelial cells. Immunity. 2021;54:1447-62.e5
102. Chao YY, Puhach A, Frieser D, Arunkumar M, Lehner L, Seeholzer T. et al. Human T(H)17 cells engage gasdermin E pores to release IL-1α on NLRP3 inflammasome activation. Nat Immunol. 2023;24:295-308
103. Vitale I, Pietrocola F, Guilbaud E, Aaronson SA, Abrams JM, Adam D. et al. Apoptotic cell death in disease-Current understanding of the NCCD 2023. Cell Death Differ. 2023;30:1097-154
104. Harrington JS, Ryter SW, Plataki M, Price DR, Choi AMK. Mitochondria in Health, Disease, and Ageing. Physiol Rev. 2023
105. Deribe YL, Pawson T, Dikic I. Post-translational modifications in signal integration. Nat Struct Mol Biol. 2010;17:666-72
106. Lee JM, Hammarén HM, Savitski MM, Baek SH. Control of protein stability by post-translational modifications. Nat Commun. 2023;14:201
107. Wu X, Xu M, Geng M, Chen S, Little PJ, Xu S. et al. Targeting protein modifications in metabolic diseases: molecular mechanisms and targeted therapies. Signal Transduct Target Ther. 2023;8:220
108. Nguyen H, Kettenbach AN. Substrate and phosphorylation site selection by phosphoprotein phosphatases. Trends Biochem Sci. 2023
109. Rogers C, Erkes DA, Nardone A, Aplin AE, Fernandes-Alnemri T, Alnemri ES. Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat Commun. 2019;10:1689
110. Santamaria A, Wang B, Elowe S, Malik R, Zhang F, Bauer M. et al. The Plk1-dependent phosphoproteome of the early mitotic spindle. Mol Cell Proteomics. 2011;10:M110.004457
111. Ai YL, Wang WJ, Liu FJ, Fang W, Chen HZ, Wu LZ. et al. Mannose antagonizes GSDME-mediated pyroptosis through AMPK activated by metabolite GlcNAc-6P. Cell Res. 2023
112. Li Y, Pu D, Huang J, Zhang Y, Yin H. Protein phosphatase 1 regulates phosphorylation of gasdermin D and pyroptosis. Chem Commun (Camb). 2022;58:11965-8
113. Roberts JZ, Crawford N, Longley DB. The role of Ubiquitination in Apoptosis and Necroptosis. Cell Death Differ. 2022;29:272-84
114. Shi Y, Yang Y, Xu W, Shi D, Xu W, Fu X. et al. E3 ubiquitin ligase SYVN1 is a key positive regulator for GSDMD-mediated pyroptosis. Cell Death Dis. 2022;13:106
115. Zhu Y, Zhang J, Yao X, Qiu T, Jiang L, Wang N. et al. Ubiquitinated gasdermin D mediates arsenic-induced pyroptosis and hepatic insulin resistance in rat liver. Food Chem Toxicol. 2022;160:112771
116. Gao W, Li Y, Liu X, Wang S, Mei P, Chen Z. et al. TRIM21 regulates pyroptotic cell death by promoting Gasdermin D oligomerization. Cell Death Differ. 2022;29:439-50
117. Di M, Miao J, Pan Q, Wu Z, Chen B, Wang M. et al. OTUD4-mediated GSDME deubiquitination enhances radiosensitivity in nasopharyngeal carcinoma by inducing pyroptosis. J Exp Clin Cancer Res. 2022;41:328
118. Ren Y, Feng M, Hao X, Liu X, Li J, Li P. et al. USP48 Stabilizes Gasdermin E to Promote Pyroptosis in Cancer. Cancer Res. 2023;83:1074-93
119. Lin J, Sun S, Zhao K, Gao F, Wang R, Li Q. et al. Oncolytic Parapoxvirus induces Gasdermin E-mediated pyroptosis and activates antitumor immunity. Nat Commun. 2023;14:224
120. He H, Yi L, Zhang B, Yan B, Xiao M, Ren J. et al. USP24-GSDMB complex promotes bladder cancer proliferation via activation of the STAT3 pathway. Int J Biol Sci. 2021;17:2417-29
121. Churchill MJ, Mitchell PS, Rauch I. Epithelial Pyroptosis in Host Defense. J Mol Biol. 2022;434:167278
122. de Sá KSG, Amaral LA, Rodrigues TS, Ishimoto AY, de Andrade WAC, de Almeida L. et al. Gasdermin-D activation promotes NLRP3 activation and host resistance to Leishmania infection. Nat Commun. 2023;14:1049
123. Doerflinger M, Deng Y, Whitney P, Salvamoser R, Engel S, Kueh AJ. et al. Flexible Usage and Interconnectivity of Diverse Cell Death Pathways Protect against Intracellular Infection. Immunity. 2020;53:533-47.e7
124. Lamkanfi M, Dixit VM. Manipulation of host cell death pathways during microbial infections. Cell Host Microbe. 2010;8:44-54
125. Verdonck S, Nemegeer J, Vandenabeele P, Maelfait J. Viral manipulation of host cell necroptosis and pyroptosis. Trends Microbiol. 2022;30:593-605
126. Yin H, Zheng J, He Q, Zhang X, Li X, Ma Y. et al. Insights into the GSDMB-mediated cellular lysis and its targeting by IpaH7.8. Nat Commun. 2023;14:61
127. Sies H, Berndt C, Jones DP. Oxidative Stress. Annu Rev Biochem. 2017;86:715-48
128. Liu S, Huang B, Cao J, Wang Y, Xiao H, Zhu Y. et al. ROS fine-tunes the function and fate of immune cells. Int Immunopharmacol. 2023;119:110069
129. Weindel CG, Ellzey LM, Martinez EL, Watson RO, Patrick KL. Gasdermins gone wild: new roles for GSDMs in regulating cellular homeostasis. Trends Cell Biol. 2023
130. Evavold CL, Hafner-Bratkovič I, Devant P, D'Andrea JM, Ngwa EM, Boršić E. et al. Control of gasdermin D oligomerization and pyroptosis by the Ragulator-Rag-mTORC1 pathway. Cell. 2021;184:4495-511.e19
131. Wang Y, Shi P, Chen Q, Huang Z, Zou D, Zhang J. et al. Mitochondrial ROS promote macrophage pyroptosis by inducing GSDMD oxidation. J Mol Cell Biol. 2019;11:1069-82
132. Devant P, Boršić E, Ngwa EM, Xiao H, Chouchani ET, Thiagarajah JR. et al. Gasdermin D pore-forming activity is redox-sensitive. Cell Rep. 2023;42:112008
133. Miao N, Wang Z, Wang Q, Xie H, Yang N, Wang Y. et al. Oxidized mitochondrial DNA induces gasdermin D oligomerization in systemic lupus erythematosus. Nat Commun. 2023;14:872
134. Dai B, Zhang R, Qi S, Liu L, Zhang X, Deng D. et al. Intravital molecular imaging reveals that ROS-caspase-3-GSDME-induced cell punching enhances humoral immunotherapy targeting intracellular tumor antigens. Theranostics. 2022;12:7603-23
135. Shen X, Wang H, Weng C, Jiang H, Chen J. Caspase 3/GSDME-dependent pyroptosis contributes to chemotherapy drug-induced nephrotoxicity. Cell Death Dis. 2021;12:186
136. Gupta S, Sarangi PP. Inflammation driven metabolic regulation and adaptation in macrophages. Clin Immunol. 2023;246:109216
137. Shi X, Zhou H, Wei J, Mo W, Li Q, Lv X. The signaling pathways and therapeutic potential of itaconate to alleviate inflammation and oxidative stress in inflammatory diseases. Redox Biol. 2022;58:102553
138. Humphries F, Shmuel-Galia L, Ketelut-Carneiro N, Li S, Wang B, Nemmara VV. et al. Succination inactivates gasdermin D and blocks pyroptosis. Science. 2020;369:1633-7
139. Liao ST, Han C, Xu DQ, Fu XW, Wang JS, Kong LY. 4-Octyl itaconate inhibits aerobic glycolysis by targeting GAPDH to exert anti-inflammatory effects. Nat Commun. 2019;10:5091
140. Li W, Li Y, Kang J, Jiang H, Gong W, Chen L. et al. 4-octyl itaconate as a metabolite derivative inhibits inflammation via alkylation of STING. Cell Rep. 2023;42:112145
141. Bambouskova M, Potuckova L, Paulenda T, Kerndl M, Mogilenko DA, Lizotte K. et al. Itaconate confers tolerance to late NLRP3 inflammasome activation. Cell Rep. 2021;34:108756
142. Daniels BP, Kofman SB, Smith JR, Norris GT, Snyder AG, Kolb JP. et al. The Nucleotide Sensor ZBP1 and Kinase RIPK3 Induce the Enzyme IRG1 to Promote an Antiviral Metabolic State in Neurons. Immunity. 2019;50:64-76.e4
143. He R, Liu B, Xiong R, Geng B, Meng H, Lin W. et al. Itaconate inhibits ferroptosis of macrophage via Nrf2 pathways against sepsis-induced acute lung injury. Cell Death Discov. 2022;8:43
144. Qin W, Zhang Y, Tang H, Liu D, Chen Y, Liu Y. et al. Chemoproteomic Profiling of Itaconation by Bioorthogonal Probes in Inflammatory Macrophages. J Am Chem Soc. 2020;142:10894-8
145. Balasubramanian A, Ghimire L, Hsu AY, Kambara H, Liu X, Hasegawa T. et al. Palmitoylation of gasdermin D directs its membrane translocation and pore formation in pyroptosis. bioRxiv. 2023
146. Du G, Healy LB, David L, Walker C, Fontana P, Dong Y. et al. ROS-dependent palmitoylation is an obligate licensing modification for GSDMD pore formation. bioRxiv. 2023
147. Hu L, Chen M, Chen X, Zhao C, Fang Z, Wang H. et al. Chemotherapy-induced pyroptosis is mediated by BAK/BAX-caspase-3-GSDME pathway and inhibited by 2-bromopalmitate. Cell Death Dis. 2020;11:281
148. Hu S, Wang L, Xu Y, Li F, Wang T. Disulfiram attenuates hypoxia-induced pulmonary hypertension by inhibiting GSDMD cleavage and pyroptosis in HPASMCs. Respir Res. 2022;23:353
149. Tang S, Yang C, Li S, Ding Y, Zhu D, Ying S. et al. Genetic and pharmacological targeting of GSDMD ameliorates systemic inflammation in macrophage activation syndrome. J Autoimmun. 2022;133:102929
150. Yang S, Feng Y, Chen L, Wang Z, Chen J, Ni Q. et al. Disulfiram accelerates diabetic foot ulcer healing by blocking NET formation via suppressing the NLRP3/Caspase-1/GSDMD pathway. Transl Res. 2023;254:115-27
151. Yang W, Tao K, Wang Y, Huang Y, Duan C, Wang T. et al. Necrosulfonamide ameliorates intestinal inflammation via inhibiting GSDMD-medicated pyroptosis and MLKL-mediated necroptosis. Biochem Pharmacol. 2022;206:115338
152. Li Y, Gong W, Li W, Liu P, Liu J, Jiang H. et al. The IRG1-Itaconate axis: A regulatory hub for immunity and metabolism in macrophages. Int Rev Immunol. 2022:1-15
153. Lu H, Zhang S, Wu J, Chen M, Cai MC, Fu Y. et al. Molecular Targeted Therapies Elicit Concurrent Apoptotic and GSDME-Dependent Pyroptotic Tumor Cell Death. Clin Cancer Res. 2018;24:6066-77
154. Johnson JL, Yaron TM, Huntsman EM, Kerelsky A, Song J, Regev A. et al. An atlas of substrate specificities for the human serine/threonine kinome. Nature. 2023;613:759-66
Author contact
![]() Corresponding authors: Xiuwen Wu, Department of Surgery, Jinling Hospital, 305 East Zhongshan Rd, Nanjing 210002 China. E-mail: wuxiuwenedu.cn; Yun Zhao, Department of General Surgery, Nanjing BenQ Medical Center, The Affiliated BenQ Hospital of Nanjing Medical University, Nanjing 210000, China E-mail: zhaoyuncn or zhaoyun0562019com; Jianan Ren, Department of Surgery, Jinling Hospital, 305 East Zhongshan Road, Nanjing, 210002, China. E-mail: jiananredu.cn.
Corresponding authors: Xiuwen Wu, Department of Surgery, Jinling Hospital, 305 East Zhongshan Rd, Nanjing 210002 China. E-mail: wuxiuwenedu.cn; Yun Zhao, Department of General Surgery, Nanjing BenQ Medical Center, The Affiliated BenQ Hospital of Nanjing Medical University, Nanjing 210000, China E-mail: zhaoyuncn or zhaoyun0562019com; Jianan Ren, Department of Surgery, Jinling Hospital, 305 East Zhongshan Road, Nanjing, 210002, China. E-mail: jiananredu.cn.

 Global reach, higher impact
Global reach, higher impact