10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2024; 20(1):15-28. doi:10.7150/ijbs.83586 This issue Cite
Review
Role of YAP Signaling in Regulation of Programmed Cell Death and Drug Resistance in Cancer
1. Department of Medicine, Cedars-Sinai Medical Center, Los Angeles, California, USA.
2. Department of Pancreatic Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Received 2023-2-16; Accepted 2023-9-29; Published 2024-1-1
Abstract
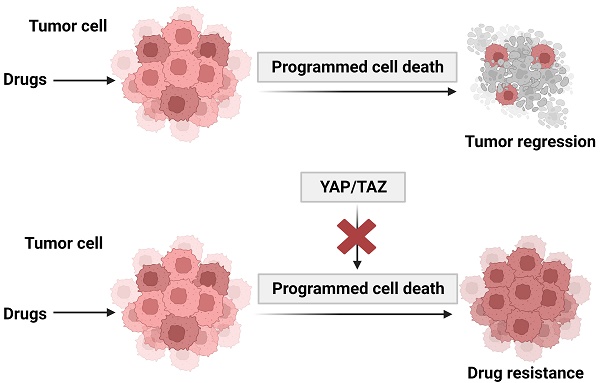
Although recent advances in cancer treatment significantly improved the prognosis of patients, drug resistance remains a major challenge. Targeting programmed cell death is a major approach of antitumor drug development. Deregulation of programmed cell death (PCD) contributes to resistance to a variety of cancer therapeutics. Yes-associated protein (YAP) and its paralog TAZ, the main downstream effectors of the Hippo pathway, are aberrantly activated in a variety of human malignancies. The Hippo-YAP pathway, which was originally identified in Drosophila, is well conserved in humans and plays a defining role in regulation of cell fate, tissue growth and regeneration. Activation of YAP signaling has emerged as a key mechanism involved in promoting cancer cell proliferation, metastasis, and drug resistance. Understanding the role of YAP/TAZ signaling network in PCD and drug resistance could facilitate the development of effective strategies for cancer therapeutics.
Keywords: Programmed cell death, YAP/TAZ, Apoptosis, Ferroptosis, Autophagy, Drug resistance
Current landscape of cancer therapy and challenge
Surgery combined with chemotherapy or radiotherapy are currently the first-line treatment modalities for localized tumors. However, many patients suffer from tumor recurrence and development of acquired resistance despite success in initial treatment [1, 2]. Most patients with advanced solid tumors, such as hepatocellular carcinoma, pancreatic adenocarcinoma and glioblastoma, show intrinsic resistance to chemotherapy and other antitumor agents at the initial stage [3-5]. Drug resistance in cancer is multifaceted and can be mediated by mechanisms that impact on drug availability, cell proliferation and response to DNA damage or metabolic pathways. Deregulation of cell death signaling represents an important mechanism of drug resistance because most of the anti-tumor therapy agents aim to trigger programmed cell death [6]. Thus, understanding the signaling pathways involved in regulating PCD and drug resistance can provide insights into development of new therapeutic targets.
The Hippo-YAP signaling pathway
The Hippo-YAP signaling plays an important role in aspects of malignant transformation, including cell proliferation, tumor progression, metastasis, and drug resistance [7-10]. YAP and its paralog TAZ are the main downstream effectors of the Hippo-YAP pathway and act as a transcriptional coactivator [11-13]. The YAP signaling can translocate into the nucleus and mediates gene transcription by binding to transcription factors, such as the TEA domain family (TEAD) proteins [12]. YAP exhibits oncogenic activities [14, 15] and is upregulated in most solid tumors [16-22]. YAP signaling target genes participate in regulation of development, cell proliferation, migration and survival [23-27].
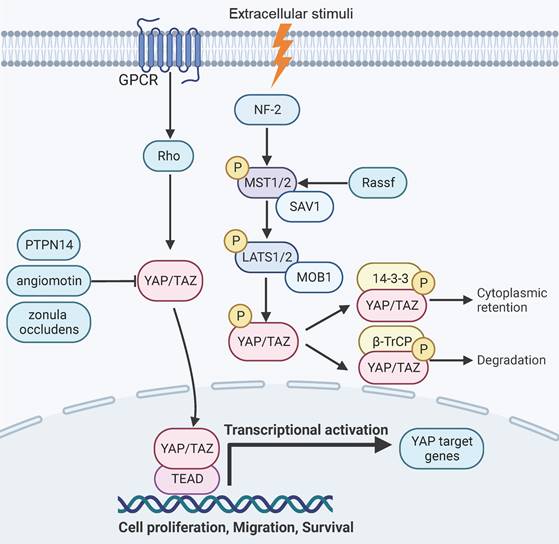
YAP signaling is responsive to intercellular adhesion, cell density, and mechanical stiffness of the extracellular matrix [7-10]. YAP can be negatively regulated by a cascade of phosphorylation events that are mediated by mammalian Ste20-like kinases1/2 (MST1/2, the mammalian homolog of Hippo) and large tumor suppressor 1/2 (LATS1/2) [11, 28, 29]. The scaffold proteins of adherens junctions, such as NF2 or KIBRA/WWC1, can recruit MST and LATS kinases to the plasma membrane and mediates their activation [30-33]. The kinase activity of MST1/2 can be activated by binding to the Salvador Family WW Domain Containing Protein 1 (SAV1), a scaffold protein that also forms a complex with LATS1/2[11, 28, 29, 34]. The Ras Association Domain Family Members (RASSFs) can also associate with MST1 and enhance its kinase activity [35-37].MST1/2 subsequently activates LATS1/2 by phosphorylating LATS1/2 and its regulatory protein Mps one binder kinase activator-1 (MOB-1) [38, 39]. In parallel to MST1/2, the MAP4K family kinases can also phosphorylate and activate LATS1/2 [40-42]. The activated LATS1/2 then phosphorylates YAP/TAZ [43, 44]. The phosphorylated YAP can associate with the 14-3-3 proteins and is rendered transcriptionally inactive due to retention in the cytoplasm [45, 46]. Alternatively, the phosphorylated forms of the YAP/TAZ proteins can also be primed for β-TrCP-mediated ubiquitination and degradation [13, 43, 47]. In addition, YAP can be inactivated by binding to a series of proteins, such as angiomotin, Protein Tyrosine Phosphatase Non-Receptor Type 14 (PTPN14) and tight junction protein zonula occludens [48-51]. Conversely, YAP can be activated by G-protein coupled receptors or the mevalonate pathway through rho GTPase signaling [52-55]. Epithelial cell transforming 2 (ECT2), a guanine nucleotide exchange factor for Rho-like GTPases that activates Rho signaling, can positively regulate YAP function and is reciprocally regulated by YAP [56]. The SRC family tyrosine kinases and the c-ABL kinase have also been reported to promote YAP signaling by tyrosine phosphorylation of YAP [57-59].
Hippo-YAP pathway signaling and function. YAP signaling is negatively regulated through a cascade of phosphorylation events mediated by MST1/2 and LATS1/2, which leads to phosphorylation of YAP and subsequent proteasomal degradation or retention in the cytoplasm. Alternatively, YAP can be inactivated by binding to angiomotin, PTPN14 and zonula occludens. Signaling events triggered by GPCR and Rho can activate YAP by inducing its nuclear translocation. YAP target genes are involved in aspects of organ development, regeneration, and malignant transformation.
In addition to phosphorylation, YAP signaling can be regulated by other forms of post-transcriptional modifications. For example, YAP can be methylated by the SET Domain Containing 1A (SET1A) methyltransferase complex, which promotes its oncogenic activities by blocking nuclear export [60]. YAP can also be modified by O-GlcNAcylation that prevents its phosphorylation by LATS1, leading to nuclear localization and enhanced tumorigenic functions [61]. Moreover, O-GlcNAcylation of LAST2 has been reported to cause YAP/TAZ activation and promote tumor growth [62]. Acetylation of LAST1 inhibits YAP phosphorylation and degradation and promotes cancer cell invasion and growth [63]. Thus, post-transcriptional modification is an important mechanism in regulating YAP signaling.
YAP signaling activation and Drug Resistance
A growing number of studies reveal that activation of YAP signaling contributes to resistance to chemotherapy, targeted therapy and immunotherapy.
(1) YAP signaling and Chemotherapy
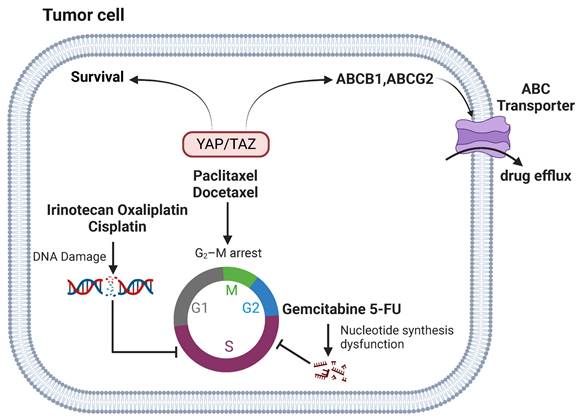
DNA-damaging agents, such as doxorubicin, irinotecan, oxaliplatin, and cisplatin, aim at DNA replication as a target to induce cytotoxic effects and are widely used in the clinics. YAP signaling is closely linked to the resistance of DNA-damaging agents. YAP is a key regulator of doxorubicin resistance in thyroid cancer and is regulated by tripartite motif-containing protein 11 (TRIM11) [64]. Overexpression of YAP confers resistance to doxorubicin by regulating bcl-xl [65]. Activation of YAP also promotes doxorubicin chemoresistance in cholangiocarcinoma and osteosarcoma cells [20, 66]. Inhibition of YAP enhances oxaliplatin and irinotecan sensitivity [67, 68]. YAP activation induces cisplatin resistance in small cell lung cancer (SCLC) cells [69], whereas knockdown of YAP increases the sensitivity of cisplatin in ovarian cancer cells [48, 70]. In addition, overexpression of TAZ is involved in regulation of cisplatin resistance of cervical, gastric, lung and ovarian cancer [22, 71-73].
Agents inhibiting metabolic pathways represent another major class of chemotherapy agents that can sabotage DNA or RNA synthesis and inhibit cell division and survival. Gemcitabine and 5-fluorouracil (5-FU) are cytidine and uracil nucleoside analogues, respectively, which are widely used for the treatment of multiple tumors. Knockdown of YAP enhances gemcitabine sensitivity [74]. In addition, high levels of YAP are associated with poor survival in patients following 5-FU treatment, which is accompanied with an increase of M2 polarization of macrophage in the tumor [75]. However, a recent study reported that overexpression of an activating mutant form of YAP appears to enhance cancer cell sensitivity to gemcitabine and 5-FU, by reducing drug efflux [76]. It should be noted that these findings remain to be corroborated with knockdown studies.
Anti-microtubule agents block mitosis by interfering with microtubules dynamics and induce apoptosis. Examples of classical anti-microtubule agents include taxanes (paclitaxel and docetaxel), which are widely used for the treatment of breast, ovarian, gastric, pancreatic and colorectal cancer [77]. YAP has been reported to confer resistance to paclitaxel in cancer cells [78, 79]. Similarly, TAZ and TAZ/TEAD-mediated expression of Cyr61 and CTGF are also vital in paclitaxel response [21, 80]. Moreover, down-regulation of YAP has been found to enhance sensitivity to docetaxel [81]. The mechanism by which YAP signaling modulates resistance to taxanes may involve YAP- and TEAD-regulated expression of ATP Binding Cassette Subfamily B Member 1 (ABCB1), which encodes the multidrug resistance protein 1 and is implicated in paclitaxel resistance [82]. In addition, YAP mediates the expression of an array of mitotic genes and deregulation YAP signaling can lead to aberrant mitotic checkpoint control [83, 84]. The mitotic regulator cyclin-dependent kinase 1 (CDK1) phosphorylates YAP in response to paclitaxel-induced G2/M arrest [85]. But its role in drug resistance remains to be clarified.
Thus, an increasing body of evidence indicate that activation of YAP signaling results in chemoresistance and inhibition of this pathway may enhance cancer cell sensitivity to chemotherapeutic drugs. Targeting YAP signaling pathway represents a potential strategy to overcome chemotherapy resistance. Indeed, the combination of YAP inhibitor with chemotherapy has shown increased efficacy in chemo-resistant tumors [86, 87].
(2) YAP signaling and Targeted Therapy
Targeted therapy is designed to block molecules and pathways that are vital for cancer cell proliferation, survival, invasion and metastasis and rewrote the paradigm of leukemia treatment [88]. Targeted therapy for epidermal growth factor receptor (EGFR), human epidermal growth factor receptor 2 (HER-2), vascular endothelial growth factor receptor (VEGFR), and BRAF have been developed for clinical use [88]. In this section, we will briefly discuss how YAP signaling regulates targeted therapies.
YAP signaling and chemotherapy. YAP/TAZ-mediated tumor chemoresistance by increasing survival in DNA damage and cell cycle arrest and reducing drug efflux. Anti-microtubule chemotherapeutic agents can induce G2/M cell cycle blockade. YAP signaling regulates their resistance through pathways such as CTGF/Cyr61, ABCB1, and CDKs. YAP signaling modulates resistance to DNA damage agents through TRIM11, p53, IL-8 pathways. Gemcitabine and 5-FU affect DNA and RNA synthesis in tumor cells. YAP signaling regulates their resistance through M2 polarization.
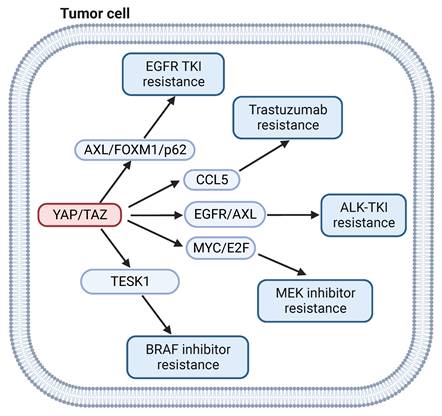
BRAF, a serine/threonine protein kinase of the RAF kinase family, is a key regulator of the MAP kinase signal transduction pathway. BRAF gene mutations occur in a large percentage of cancers, including approximately 50% of melanomas, 20% to 40% of thyroid cancers, and 10% of colorectal cancers [89]. Although BRAF inhibitors have shown benefit in melanoma patients with the oncogenic BRAFV600E mutant, acquired drug resistance remains a significant obstacle [90]. NF-2, a negative regulator of YAP signaling, was identified as a gene associated with cancer cell sensitivity to BRAF inhibitor, which indicates that YAP signaling could participate in BRAF inhibitor resistance [91]. In a separate study, activation of YAP was shown to induce resistance to BRAF inhibitor in melanoma cells through the actin dynamic regulator testis associated actin remodeling kinase 1 (TESK1) [92]. In addition, YAP confers immune evasion in BRAF inhibitor resistant melanoma cells by promoting PD-L1 expression, which can be targeted by immune checkpoint therapy [93]. This finding suggests that the combination of BRAF inhibitor with immunotherapy may represent a viable approach to treat BRAF inhibitor resistant melanoma.
EGFR is a receptor tyrosine kinase that is frequently mutated in many tumors [94]. Small-molecule tyrosine kinase inhibitors (TKIs) for EGFR have shown efficacy in treatment of EGFR-mutated tumors [95]. However, resistance remains a problem for clinicians. Numerous studies have indicated that activation of YAP/TAZ is widely associated with in EGFR TKI resistance [96-98]. YAP regulates epithelial-to-mesenchymal transition (EMT)-induced resistance to EGFR TKI in non-small cell lung cancer (NSCLC) via FOXM1/SAC pathway [99]. YAP could also mediate EGFR TKI-resistant through upregulation of AXL receptor tyrosine kinase [100] or the autophagy mediator p62[101]. Combination of YAP inhibitor and EGFR TKI improved response in EGFR inhibitor resistant NSCLC [102].
YAP signaling is also implicated in resistance to targeted therapies for HER-2, MEK, RAS, ALK and BET inhibitors. YAP1 dephosphorylation and TEAD2 overexpression are closely related to trastuzumab resistance by regulating cytokines like CCL5 in HER-2 positive breast cancer cell lines [103]. YAP deletion sensitizes the MEK inhibitor trametinib by depletion of MYC/MYCN and E2F transcriptional output in neuroblastoma cells [104]. Dasatinib can enhance the antitumor effect of trametinib in KRAS-mutant cancer models by inhibiting the expression of TAZ protein [105]. YAP activation promotes resistance to ALK-TKI through induction of p21 expression [106]. TAZ nuclear localization and transcriptional activity induces resistance to inhibitors of BET family proteins [107]. In summary, activation of YAP/TAZ signaling contributes to the resistance to an array of targeted therapy agents in various tumors. Targeting YAP signaling may improve the outcomes of targeted therapy.
(3) YAP signaling and Immunotherapy
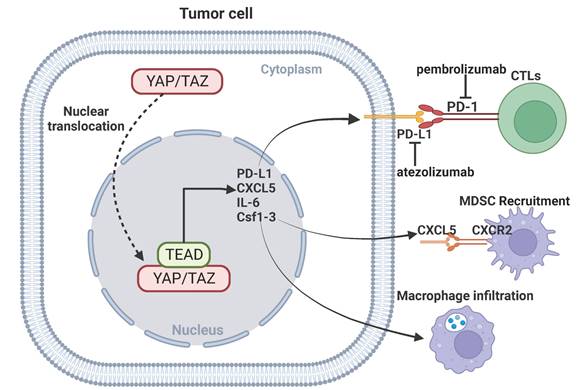
The recent success of using immune checkpoint inhibitors for the treatment of certain human cancers represents a breakthrough in immunotherapy. Immune checkpoint receptors play a critical role in the maintenance of immune homeostasis. The classic immune checkpoint receptors include PD-1, CTLA-4, and TIGIT. The engagement of the immune checkpoint receptors can result in anergy of CD8+ T cells and enhance tumorigenesis and invasiveness of tumors. Immune checkpoint inhibitors remove inhibitory signals of T-cell activation, which enables tumor-reactive T cells to mount an effective antitumor response [108, 109]. The anti-PD-1/PD-L1 agents, the most used immune checkpoint inhibitors, has shown promising outcomes in the treatment of certain cancer types, significantly extending the overall survival of patients [110]. Monoclonal antibodies against PD-1/PD-L1 or CTLA-4 have shown promising efficacy in certain tumors [111-113]. Emerging evidence indicates an important role for YAP signaling in modulating anti-PD-1/PD-L1 immunotherapy. PD-L1 is a direct transcriptional target of YAP signaling, knockdown of YAP inhibits expression of PD-L1 and reverses resistance to EGFR-TKI [114]. TAZ also upregulates PD-L1 expression in pancreatic cancer, leading to immune evasion and immunotherapy resistance [115]. Activation of YAP-mediated transcriptional hubs in the nuclei is associated with resistance of anti-PD-1 in a mouse model of lung cancer cells, and inhibition of YAP can enhance the efficacy of anti-PD-1 therapy [116]. In addition, YAP signaling in cancer cells can facilitate recruitment of macrophages or myeloid-derived suppressor cells (MDSC) to the tumor microenvironment by regulating CXCL5, IL-6 and Csf1-3, and inhibition of YAP-mediated immune cell infiltration impairs tumor growth [117, 118]. These findings indicate that targeting YAP signaling may improve the efficacy of immunotherapy.
YAP signaling and Programmed Cell Death
Apoptosis, ferroptosis and other forms of PCD are associated with cancer drug resistance [119, 120]. Recent studies discovered that YAP signaling plays an important role in the regulation of PCD in cancer [121]. Understanding the relationship between YAP signaling and PCD can assist the development of more effective cancer therapeutic strategies.
YAP signaling and targeted therapy. YAP signaling drives resistance of BRAF inhibitors by regulating TESK1. YAP signaling mediates EGFR TKI resistance by via FOXM1/p62. YAP signaling mediates Trastuzumab, ALK TKI and MEK inhibitors resistance by via CCL5, EGFR/AXL and MYC/E2F, respectively.
YAP signaling and immunotherapy. YAP mediates immunotherapeutic resistance by regulating cytotoxic T cells through upregulation of PD-L1 expression in cancer cells. YAP-mediated transcription of CXCL5, IL-6 and Csf1-3 promotes MDSC recruitment and macrophage infiltration.
YAP signaling and Apoptosis
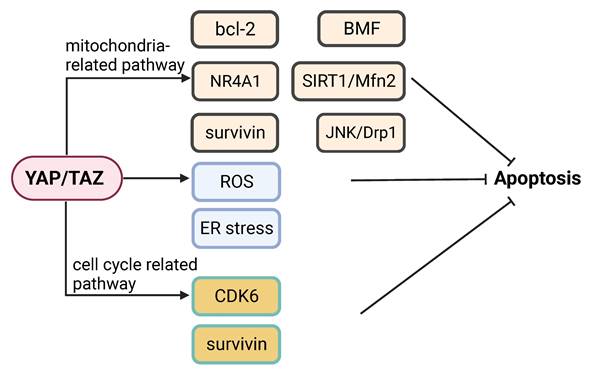
Apoptosis is a major cancer cell response to most therapeutic drugs. Dysregulation of apoptosis contributes to tumorigenesis and drug resistance [119]. YAP signaling participates in the regulation of apoptosis and inhibition of YAP signaling can promote apoptosis via multiple pathways. Inhibition of YAP signaling can promote apoptosis in multiple pathways. Knockdown of YAP and TAZ can enhance apoptosis under hypoxic condition [122]. YAP appears to modulate cancer cell susceptibility to apoptosis triggered by an ER stress inducing agent [123]. Knockdown of YAP can sensitize colon cancer cells to inhibitors of the MAPK pathway, which may involve YAP-mediated expression of CDK6 [124]. YAP is implicated in playing a role in determining the switch between apoptotic and survival pathways following activation of G protein-coupled bile acid receptor (GPBAR) signaling [125]. Inhibition of YAP increases cancer cell apoptosis induced by genotoxic agents [126]. Knockdown of YAP enhances apoptosis in cancer cells treated with the Abl and Src family kinase inhibitor bosutinib, which is associated with mitochondrial fragmentation and reactive oxygen species (ROS) accumulation [127]. Indeed, knockdown of YAP increases mitochondrial fission via JNK-Drp1, which can lead to apoptosis [128].
Activation of YAP can inhibit apoptosis by mediating the pro-apoptotic function of nuclear receptor 4A1 (NR4A1) [129]. Knockdown of YAP in cancer cells increases the levels of ER stress and apoptosis [18]. Ras association domain family member 4 (RASSF4) enhances apoptosis by decreasing YAP-regulated bcl-2 expression [130]. Knockdown of YAP can induce apoptosis by reducing SIRT1- and Mfn2-mediated mitophagy [131]. The YAP/TEAD4 complex can inhibit apoptosis and promote cancer progression by activating kinesin family member 4A (KIF4A) expression [132]. Overexpression of YAP promotes proliferation and suppress apoptosis via increase of bcl-2 [133]. YAP knockdown sensitizes bladder cancer cells to cisplatin-induced apoptosis, which is accompanied with downregulation of surviving [134]. A recent study showed that YAP plays a vital role in evasion of apoptosis by mediating cancer cell dormancy, which involves recruitment of the EMT transcriptional factor SLUG and suppression of the expression of the pro-apoptotic protein bcl-2-modifying factor (BMF) [135].
Further investigation of the precise mechanisms regarding the regulation of apoptosis by YAP signaling may contribute to the discovery of potential therapeutic targets.
YAP signaling and Ferroptosis
Ferroptosis is a biochemically and morphologically distinct form of PCD characterized by iron-dependent lipid peroxidation and compromise of cell membrane integrity [136]. Lipid peroxidation is a process under which oxidants such as free radicals and intracellular ROS attack lipids especially polyunsaturated fatty acids, leads to lipid peroxidation and cell death. Imbalance of iron redox ability leads to the production of oxygen free radicals and damages various cellular components, eventually induces ferroptosis. Cancer cells have developed many defense mechanisms to prevent lipid peroxidation. The most well-known is the glutathione peroxidase 4 (GPX4)-glutathione (GSH) system. GPX4 can reduce peroxidized lipids to their corresponding alcohols by binding to its cofactor GSH [137]. Ferroptosis is associated with multiple diseases, such as cancer, inflammation, heart injury and sepsis [138-141]. A growing number of studies indicates that YAP signaling modulates ferroptosis by regulating expression of genes involved in keeping the balance of intracellular ROS and lipid peroxidation.
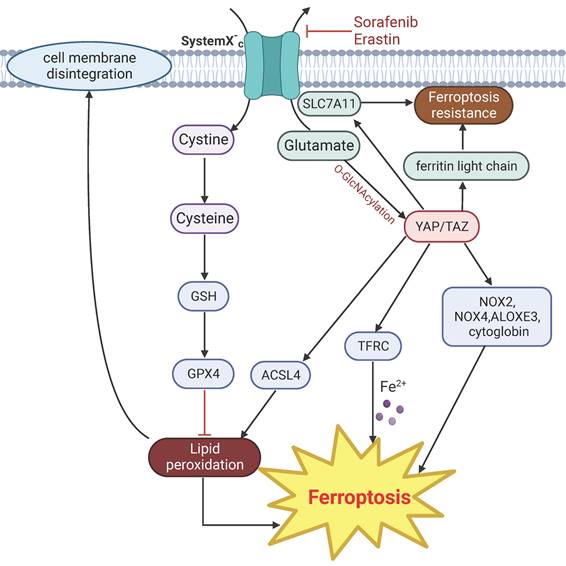
YAP can modulate iron concentration through the transcriptional regulation of transferrin receptor (TFRC) [142]. Activation of YAP also confers sensitivity to ferroptosis via regulation of the arachidonate lipoxygenase 3 (ALOXE3) [143], which promotes lipid peroxidation and ferroptosis [144], or by upregulating multiple regulators of ferroptosis, particularly TRFC and acyl-CoA synthetase long chain family member 4 (ACSL4) [145]. TAZ mediates ferroptosis through indirectly regulating the expression of the ROS-generating nicotinamide adenine dinucleotide phosphate oxidases (NOX) through angiopoietin-like 4 (ANGPLT4)-NOX2 and epithelial membrane protein 1 (EMP1)-NOX4 axis [146, 147]. YAP/p53 axis is required for lipid peroxidation and ferroptosis induced by cytoglobin, a heme-binding protein that mediates redox homeostasis in cells [148].
However, in a separate line of studies, YAP appears to have anti-ferroptosis effect. YAP/TAZ mediate the expression of solute carrier family 7 member 11 (SLC7A11), a subunit of the cystine/glutamate transporter that is important for maintaining intracellular cysteine and glutathione storage, and thus contributes to resistance to ferroptosis [149, 150]. Induction of ferroptosis by inhibition of the cystine/glutamate transporter system by erastin is accompanied with glutamate-induced O-GlcNAcylation and down regulation of YAP, and ectopic expression of a mutant form of YAP that cannot undergo O-GlcNAcylation reduces sensitivity to ferroptosis [151]. Moreover, YAP can also protect cells from ferroptosis by suppressing the expression of ferritin light chain, a major protein important for storing intracellular iron [152].
In summary, YAP signaling can regulate genes involved in different aspects of lipid peroxidation. The overall effect of YAP disruption on ferroptosis may depends on the genetic background of the cell types or the metabolic environment.
YAP signaling in regulation of apoptosis. YAP signaling can regulate apoptosis by modulating (1) Mitochondria-related events (e.g. bcl-2, BMF, NR4A1, SIRT1/Mfn2, survivin and JNK/Drp1); (2) ROS and ER stress responses; or (3) Mediators of the cell cycle, (e.g. CDK6 and survivin).
YAP signaling related mechanisms in regulation of ferroptosis. Drugs such as erastin and sorafenib directly target cystine transporter and trigger ferroptosis. Activation of YAP induce ferroptosis by upregulating the expression of ACSL4 and TRFC. Other regulators of YAP mediated ferroptosis include NOX2, NOX4, ALOXES3 and cytoglobin. Glutamate-induced O-GlcNAcylation of YAP inhibit ferroptosis. YAP signaling suppress ferroptosis by targeting SLC7A11 and ferritin light chain.
YAP signaling and Autophagy
Autophagy is a mechanism by which cells adapt to physiological or pathological changes by degrading and recycling parts of the cell in a lysosome-dependent manner. Autophagy is vital in maintaining organismal homeostasis [153, 154] and dysfunction of autophagy is implicated in multiple diseases [155-157]. The molecular mechanism of autophagy involves several autophagy-associated proteins (ATG). Various stimuli, such as nutrient deficiencies and hypoxia, can leads to the formation of phagocytic vesicles, a step regulated by two protein complexes. One is the Vps34 complex containing Vps34, ATG6, ATG14 and Vps15. The other is the unc-51 like autophagy activating kinase (ULK1)/ATG1 complex, which is an important positive regulator of autophagosome formation [158]. Autophagy is involved in aspects of tumorigenesis, including cancer cell survival, invasion and immune response [159-162]. Recent studies have shown complex interactions between YAP signaling and autophagic pathways [163].
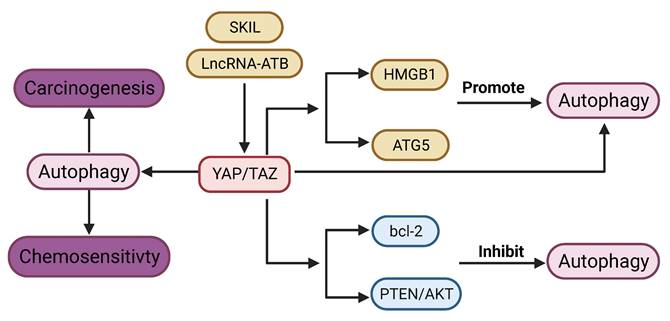
YAP signaling promotes autophagy in most tumors. YAP is required for lncRNA-ATB induced autophagy [164]. YAP activates autophagy by promoting ATG5 transcription, while autophagy in turn negatively regulates YAP through autophagic degradation [165]. Disruption of autophagy by genetic deletion of ATG7 in the hepatocytes leads to upregulation of YAP and malignant transformation in the liver, which can be reversed by concurrent deletion of YAP [166]. Silencing of YAP leads to impaired autophagy, which enhances cisplatin sensitivity in cancer cells [70]. Proto-oncogene SKIL could induce TAZ-dependent autophagy in NSCLC cell lines [167]. Cisplatin-induced autophagy can activate YAP by decreasing phosphorylation [168]. YAP promotes autophagy and tumor progression in glioblastoma through upregulation of high mobility group box 1 (HMGB1) [17].
Contrary to the above-mentioned studies, YAP signaling has also been shown to inhibit autophagy. YAP can suppress autophagy in sarcoma [169]. Autophagy induced by depletion of TAZ could inhibit migration and invasion [170]. Blockade of YAP induces autophagy‑related cell death and confers sensitivity of chemotherapy [19]. YAP inhibits autophagy through the suppression of phosphatase and tensin homolog (PTEN) and activation of the AKT/mTOR pathway [171]. Moreover, YAP inhibits autophagy by upregulating expression of bcl-2 [172].
In summary, YAP signaling appears to play opposing roles in regulation of autophagy, which may be dependent on the genetic background of the tumor. The mechanisms involving in the relationship between YAP signaling and autophagy needs further exploration.
YAP signaling and Pyroptosis
Pyroptosis, also known as inflammatory necrosis, is a type of PCD characterized by Gasdemin-mediated membrane rupture and release of inflammatory molecules [173]. Pyroptosis is usually initiated in response to viral and bacterial infections, accompanied by activation of the inflammasome and secretion of pro-inflammatory cytokines [174]. Pyroptosis facilitates inflammatory microenvironment, which promotes carcinogenesis and metastasis [175]. The role of pyroptosis in tumor development is complicated. In the initiation stage, pyroptosis can promote tumor development through inflammasome or the release of pro-inflammatory cytokines, such as interleukin-1β and IL-18 [176, 177]. In later stages, the inhibition of pyroptosis may promote tumor progression [178].
Pyroptosis is involved in the regulation of drug resistance in cancer. Downregulation of Gasdermin E (GSDME), a key regulator of pyroptosis, confers retinoblastoma cells resistance to chemotherapy [179]. Bioinformatics analysis reveals that four regulatory genes of pyroptosis are closely related to temozolomide resistance in glioma [180]. Caspase-1/GSDMD dependent pyroptosis is involved in cisplatin resistance of NSCLC cells [181]. Pyroptosis induced by STAT-3β enhances cisplatin sensitivity in esophageal squamous carcinoma cells [182]. In addition, pyroptosis-related gene signature has shown promise in predicting the efficacy of immunotherapy in multiple cancer types [183]. Pyroptosis improves the sensitivity of immunotherapy by remodeling tumor microenvironment [184].
Existing research regarding YAP signaling on the regulation of pyroptosis is mostly focused on non-cancerous diseases like infection, inflammation and diabetes [185-187] and only a few studies explored the role of YAP signaling in cancerous pyroptosis. Inactivation of YAP switch chemotherapy induced cell death from apoptosis to pyroptosis through upregulating the expression of GSDME [69]. A recent study showed that MST1 can promote ROS-induced pyroptosis, which is accompanied by inactivation of YAP via phosphorylation and results in suppression of tumor cell proliferation and invasion [188].
Connection between YAP signaling and Autophagy. lncRNA ATB and SKIL can promote autophagy via YAP signaling. YAP signaling promotes autophagy through regulation of HMGB1, ATG5. On the other hand, YAP signaling inhibits autophagy via bcl-2, and PTEN/AKT pathway. YAP signaling could regulate carcinogenesis and chemosensitivity via autophagy.
Conclusion and Future Perspectives
In summary, YAP signaling is engaged in the regulation of multiple forms of programmed cell death, including apoptosis, ferroptosis, autophagy, and pyroptosis. Activation of YAP/TAZ contributes to resistance to a variety of tumor therapeutic modalities, such as chemotherapy, targeted therapy and immunotherapy. Verteporfin (VP), originally used for treating fundus macular degeneration, possesses potency of effectively YAP inhibition [189]. Several studies indicate that VP could increase sensitivity to targeted or chemotherapy drugs by inhibition of YAP [190-194]. Clinical trials using VP for the treatment of pancreatic cancer are underway [195]. As such, VP has the potential to become an anti-tumor agent of multiple tumor types in the future. More recently, a pan-TEAD inhibitor has been developed and showed activity in blocking YAP signaling and overcoming KRAS G12C inhibitor resistance [196]. Moreover, small molecule inhibitors of TEAD auto-palmitoylation have also been reported to exhibit potency to inhibit NF2-deficient Mesothelioma [197]. Similarly, K-975, a TEAD inhibitor, can inhibit the proliferation of malignant pleural mesothelioma (MPM) cell lines and provide significant survival benefit in MPM xenograft model [198]. In addition, several other YAP/TEAD inhibitors are currently tested in the preclinical and clinical research stages, and may provide more drug choices for YAP/TEAD based anti-tumor therapy in the future [199]. The advancement of our understanding in YAP or YAP-mediated signaling events in cancer drug resistance may lead to development of new therapeutic regimen for cancer.
Abbreviations
PCD: programmed cell death; YAP: yes-associated protein; TEAD: TEA domain family; MST: mammalian Ste20-like kinases; LATS: large tumor suppressor; MOB-1: Mps one binder kinase activator-1; KIBRA: KIdney and BRAin expressed protein; WWC1: WW and C2 domain containing 1; GPCR: G-protein coupled receptors; PTPN14: protein tyrosine phosphatase non-receptor type 14; SET1A: set domain containing 1A; HCC: hepatocellular carcinoma; SCLC: small cell lung cancer; HIF: hypoxia-inducible factor; TRIM11: tripartite motif-containing protein 11; CTGF: connective tissue growth factor; ABCB1: ATP binding cassette subfamily B member 1; CYR61: cysteine-rich angiogenic inducer 61; CDK: cyclin-dependent kinase; COX-2: cyclooxygenase 2; 5-FU: 5-fluorouracil; RAR: retinoic acid receptors; RXR: retinoid X receptors; ALDH1A3: aldehyde dehydrogenase 1 family member A3; OCT4: octamer-binding transcription factor 4; EGFR: epidermal growth factor receptor; HER-2: human epidermal growth factor receptor 2; VEGFR: vascular endothelial growth factor receptor; TESK1: testis associated actin remodeling Kinase 1; TKI: tyrosine kinase inhibitor; EMT: epithelial-to-mesenchymal transition; NSCLC: non-small cell lung cancer; FOXM1: forkhead box protein M1; SAC: sacsin molecular chaperone; MEK: mitogen-activated protein kinase kinase; BET: bromodomain and extra-terminal motif; PD-1: Programmed death protein 1; PD-L1: programmed cell death ligand 1; CTLA-4: cytotoxic T-lymphocyte-associated antigen 4; TIGIT: T cell immunoglobulin and ITIM domain; MDSC: myeloid-derived suppressor cells; IL-6: Interleukin 6; CSF: colony stimulating factor; CXCL5: c-x-c motif chemokine ligand 5; ER: endoplasmic reticulum; PERK: protein kinase RNA-like ER kinase; EIF2a: eukaryotic translation initiation factor 2A; MAPK: mitogen-activated protein kinase; GPBAR: G protein-coupled bile acid receptor; JNK: c-Jun n-terminal kinase; NR4A1: nuclear receptor 4A1; KIF4A: kinesin family member 4A; BMF: Bcl-2-modifying factor; GPX4: glutathione peroxidase 4; GSH: glutathione; TFRC: transferrin receptor; ACSL4: acyl-CoA synthetase long chain family member 4; NOX: nicotinamide adenine dinucleotide phosphate oxidases; SLC7A11: solute carrier family 7 member 11; ALOXE3: Arachidonate Lipoxygenase 3; SUFU: suppressor of fused homolog; ANGPLT4: angiopoietin-like 4; EMP1: epithelial membrane protein 1; ATG: autophagy-associated proteins; ULK1: unc-51 like autophagy activating kinase; TNBC: triple-negative breast cancer; ANKRD1: ankyrin repeat domain 1; ERK: extracellular signal-regulated kinase; HMGB1: high mobility group box 1; UPR: unfolded protein response; RAC1: Rac family small GTPase 1; mTOR: mechanistic target of rapamycin kinase; PTEN: phosphatase and tensin homolog; GSDME: gasdermin E; MST1: macrophage stimulating 1; VP: verteporfin.
Acknowledgements
This work is supported by NIH grants 5P01CA233452.
All figures were created with BioRender.com.
Author Contributions
Wei Zhou and Qiang Wang initiated and drafted the manuscript. Adrian Lim participated in collecting related studies. Heshui Wu, Mouad Edderkaoui and Arsen Osipov participated in the design of the review. Stephen Pandol revised the manuscript. All authors have read and agreed on the published version of the manuscript.
ORCID
Wei Zhou https://orcid.org/0000-0003-2004-277X.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Schoenfeld AJ, Hellmann MD. Acquired Resistance to Immune Checkpoint Inhibitors. Cancer Cell. 2020;37(4):443-55
2. Lim ZF, Ma PC. Emerging insights of tumor heterogeneity and drug resistance mechanisms in lung cancer targeted therapy. J Hematol Oncol. 2019;12(1):134
3. Yu S, Zhang C, Xie KP. Therapeutic resistance of pancreatic cancer: Roadmap to its reversal. Biochim Biophys Acta Rev Cancer. 2021;1875(1):188461
4. Gusyatiner O, Hegi ME. Glioma epigenetics: From subclassification to novel treatment options. Semin in Cancer Biol. 2018;51:50-8
5. Bao MH, Wong CC. Hypoxia, Metabolic Reprogramming, and Drug Resistance in Liver Cancer. Cells. 2021;10(7):1715
6. Housman G, Byler S, Heerboth S, Lapinska K, Longacre M, Snyder N. et al. Drug resistance in cancer: an overview. Cancers (Basel). 2014;6(3):1769-92
7. Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J. et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21(21):2747-61
8. Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N. et al. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154(5):1047-59
9. Panciera T, Azzolin L, Cordenonsi M, Piccolo S. Mechanobiology of YAP and TAZ in physiology and disease. Nat Rev Mol Cell Biol. 2017;18(12):758-70
10. Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI. et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol. 2013;15(6):637-46
11. Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122(3):421-34
12. Zhao B, Ye X, Yu J, Li L, Li W, Li S. et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22(14):1962-71
13. Zhang H, Liu CY, Zha ZY, Zhao B, Yao J, Zhao S. et al. TEAD transcription factors mediate the function of TAZ in cell growth and epithelial-mesenchymal transition. J Bio Chem. 2009;284(20):13355-62
14. Zhao B, Kim J, Ye X, Lai ZC, Guan KL. Both TEAD-binding and WW domains are required for the growth stimulation and oncogenic transformation activity of yes-associated protein. Cancer Res. 2009;69(3):1089-98
15. Li H, Wu BK, Kanchwala M, Cai J, Wang L. et al. YAP/TAZ drives cell proliferation and tumour growth via a polyamine-eIF5A hypusination-LSD1 axis. Nat Cell Biol. 2022;24(3):373-83
16. Yuan Y, Li D, Li H, Wang L, Tian G, Dong Y. YAP overexpression promotes the epithelial-mesenchymal transition and chemoresistance in pancreatic cancer cells. Mol Med Rep. 2016;13(1):237-42
17. Zhao M, Zhang Y, Jiang Y, Wang K, Wang X, Zhou D. et al. YAP promotes autophagy and progression of gliomas via upregulating HMGB1. J Exp Clin Cancer Res. 2021;40(1):99
18. Liu H, Mei D, Xu P, Wang H, Wang Y. YAP promotes gastric cancer cell survival and migration/invasion via the ERK/endoplasmic reticulum stress pathway. Oncol Lett. 2019;18(6):6752-8
19. Zhou Y, Wang Y, Zhou W, Chen T, Wu Q, Chutturghoon VK. et al. YAP promotes multi-drug resistance and inhibits autophagy-related cell death in hepatocellular carcinoma via the RAC1-ROS-mTOR pathway. Cancer Cell Int. 2019;19:179
20. Marti P, Stein C, Blumer T, Abraham Y, Dill MT, Pikiolek M. et al. YAP promotes proliferation, chemoresistance, and angiogenesis in human cholangiocarcinoma through TEAD transcription factors. Hepatology. 2015;62(5):1497-510
21. Bartucci M, Dattilo R, Moriconi C, Pagliuca A, Mottolese M, Federici G. et al. TAZ is required for metastatic activity and chemoresistance of breast cancer stem cells. Oncogene. 2015;34(6):681-90
22. Ge L, Li DS, Chen F, Feng JD, Li B, Wang TJ. TAZ overexpression is associated with epithelial-mesenchymal transition in cisplatin-resistant gastric cancer cells. Int J Oncol. 2017;51(1):307-15
23. Zanconato F, Forcato M, Battilana G, Azzolin L, Quaranta E, Bodega B. et al. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat Cell Biol. 2015;17(9):1218-27
24. Chan SW, Lim CJ, Loo LS, Chong YF, Huang C, Hong W. TEADs mediate nuclear retention of TAZ to promote oncogenic transformation. J Biol Chem. 2009;284(21):14347-58
25. Shao D, Zhai P, Del Re DP, Sciarretta S, Yabuta N, Nojima H. et al. A functional interaction between Hippo-YAP signalling and FoxO1 mediates the oxidative stress response. Nat Commun. 2014;5:3315
26. Chen Q, Zhang N, Gray RS, Li H, Ewald AJ, Zahnow CA. et al. A temporal requirement for Hippo signaling in mammary gland differentiation, growth, and tumorigenesis. Genes Dev. 2014;28(5):432-7
27. Zhang Y, Wang Y, Zhou D, Wang K, Wang X, Wang X. et al. Radiation-induced YAP activation confers glioma radioresistance via promoting FGF2 transcription and DNA damage repair. Oncogene. 2021;40(27):4580-91
28. Udan RS, Kango-Singh M, Nolo R, Tao C, Halder G. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat Cell Biol. 2003;5(10):914-20
29. Chan EH, Nousiainen M, Chalamalasetty RB, Schäfer A, Nigg EA, Silljé HH. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene. 2005;24(12):2076-86
30. Yin F, Yu J, Zheng Y, Chen Q, Zhang N, Pan D. Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell. 2013;154(6):1342-55
31. Genevet A, Wehr MC, Brain R, Thompson BJ, Tapon N. Kibra is a regulator of the Salvador/Warts/Hippo signaling network. Dev Cell. 2010;18(2):300-8
32. Yu J, Zheng Y, Dong J, Klusza S, Deng WM, Pan D. Kibra functions as a tumor suppressor protein that regulates Hippo signaling in conjunction with Merlin and Expanded. Dev Cell. 2010;18(2):288-99
33. Baumgartner R, Poernbacher I, Buser N, Hafen E, Stocker H. The WW domain protein Kibra acts upstream of Hippo in Drosophila. Dev Cell. 2010;18(2):309-16
34. Callus BA, Verhagen AM, Vaux DL. Association of mammalian sterile twenty kinases, Mst1 and Mst2, with hSalvador via C-terminal coiled-coil domains, leads to its stabilization and phosphorylation. FEBS J. 2006;273(18):4264-76
35. Praskova M, Khoklatchev A, Ortiz-Vega S, Avruch J. Regulation of the MST1 kinase by autophosphorylation, by the growth inhibitory proteins, RASSF1 and NORE1, and by Ras. Biochem J. 2004;381(Pt 2):453-62
36. Oh HJ, Lee KK, Song SJ, Jin MS, Song MS, Lee JH. et al. Role of the tumor suppressor RASSF1A in Mst1-mediated apoptosis. Cancer Res. 2006;66(5):2562-9
37. Dhanaraman T, Singh S, Killoran RC, Singh A, Xu X, Shifman JM. et al. RASSF effectors couple diverse RAS subfamily GTPases to the Hippo pathway. Sci Signal. 2020;13(653):eabb4778
38. Hoa L, Kulaberoglu Y, Gundogdu R, Cook D, Mavis M, Gomez M. et al. The characterisation of LATS2 kinase regulation in Hippo-YAP signalling. Cell Signal. 2016;28(5):488-97
39. Hergovich A, Schmitz D, Hemmings BA. The human tumour suppressor LATS1 is activated by human MOB1 at the membrane. Biochem Biophys Res Commun. 2006;345(1):50-8
40. Zheng Y, Wang W, Liu B, Deng H, Uster E, Pan D. Identification of Happyhour/MAP4K as Alternative Hpo/Mst-like Kinases in the Hippo Kinase Cascade. Dev Cell. 2015;34(6):642-55
41. Meng Z, Moroishi T, Mottier-Pavie V, Plouffe SW, Hansen CG, Hong AW. et al. MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat Commun. 2015;6:8357
42. Li Q, Li S, Mana-Capelli S, Roth Flach RJ, Danai LV, Amcheslavsky A. et al. The conserved misshapen-warts-Yorkie pathway acts in enteroblasts to regulate intestinal stem cells in Drosophila. Dev Cell. 2014;31(3):291-304
43. Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev. 2010;24(1):72-85
44. Li FL, Fu V, Liu G, Tang T, Konradi AW, Peng X. et al. Hippo pathway regulation by phosphatidylinositol transfer protein and phosphoinositides. Nat Chem Biol. 2022;18(10):1076-1086
45. Kanai F, Marignani PA, Sarbassova D, Yagi R, Hall RA, Donowitz M. et al. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J. 2000;19(24):6778-91
46. Vassilev A, Kaneko KJ, Shu H, Zhao Y, DePamphilis ML. TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev. 2001;15(10):1229-41
47. Liu CY, Zha ZY, Zhou X, Zhang H, Huang W, Zhao D. et al. The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCF{beta}-TrCP E3 ligase. J Biol Chem. 2010;285(48):37159-69
48. Huang JM, Nagatomo I, Suzuki E, Mizuno T, Kumagai T, Berezov A. et al. YAP modifies cancer cell sensitivity to EGFR and survivin inhibitors and is negatively regulated by the non-receptor type protein tyrosine phosphatase 14. Oncogene. 2013;32(17):2220-9
49. Lian I, Kim J, Okazawa H, Zhao J, Zhao B, Yu J. et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010;24(11):1106-18
50. Liu X, Yang N, Figel SA, Wilson KE, Morrison CD, Gelman IH. et al. PTPN14 interacts with and negatively regulates the oncogenic function of YAP. Oncogene. 2013;32(10):1266-73
51. Oka T, Remue E, Meerschaert K, Vanloo B, Boucherie C, Gfeller D. et al. Functional complexes between YAP2 and ZO-2 are PDZ domain-dependent, and regulate YAP2 nuclear localization and signalling. Biochem J. 2010;432(3):461-72
52. Feng X, Degese MS, Iglesias-Bartolome R, Vaque JP, Molinolo AA, Rodrigues M. et al. Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry. Cancer Cell. 2014;25(6):831-45
53. Sorrentino G, Ruggeri N, Specchia V, Cordenonsi M, Mano M, Dupont S. et al. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol. 2014;16(4):357-66
54. Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH. et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150(4):780-91
55. Park HW, Kim YC, Yu B, Moroishi T, Mo JS, Plouffe SW. et al. Alternative Wnt Signaling Activates YAP/TAZ. Cell. 2015;162(4):780-94
56. Li C, Peng Z, Wang Y, Lam G, Nissen N, Tang J. et al. Epithelial cell transforming 2 is regulated by Yes-associated protein 1 and mediates pancreatic cancer progression and metastasis. Am J Physiol Gastrointest Liver Physiol. 2021;320(3):G380-G395
57. Taniguchi K, Wu LW, Grivennikov SI, de Jong PR, Lian I, Yu FX. et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. 2015;519(7541):57-62
58. Levy D, Adamovich Y, Reuven N, Shaul Y. Yap1 phosphorylation by c-Abl is a critical step in selective activation of proapoptotic genes in response to DNA damage. Mol Cell. 2008;29(3):350-61
59. Rosenbluh J, Nijhawan D, Cox AG, Li X, Neal JT, Schafer EJ. et al. β-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151(7):1457-73
60. Fang L, Teng H, Wang Y, Liao G, Weng L, Li Y. et al. SET1A-Mediated Mono-Methylation at K342 Regulates YAP Activation by Blocking Its Nuclear Export and Promotes Tumorigenesis. Cancer Cell. 2018;34(1):103-18
61. Peng C, Zhu Y, Zhang W, Liao Q, Chen Y, Zhao X. et al. Regulation of the Hippo-YAP Pathway by Glucose Sensor O-GlcNAcylation. Mol Cell. 2017;68(3):591-604
62. Kim E, Kang JG, Kang MJ, Park JH, Kim YJ, Kweon TH. et al. O-GlcNAcylation on LATS2 disrupts the Hippo pathway by inhibiting its activity. Proc Natl Acad Sci U S A. 2020;117(25):14259-69
63. Yang S, Xu W, Liu C, Jin J, Li X, Jiang Y. et al. LATS1 K751 acetylation blocks activation of Hippo signalling and switches LATS1 from a tumor suppressor to an oncoprotein. Sci China Life Sci. 2022;65(1):129-41
64. Tang J, Tian Z, Liao X, Wu G. SOX13/TRIM11/YAP axis promotes the proliferation, migration and chemoresistance of anaplastic thyroid cancer. Int J Biol Sci. 2021;17(2):417-29
65. Huo X, Zhang QI, Liu AM, Tang C, Gong Y, Bian J. et al. Overexpression of Yes-associated protein confers doxorubicin resistance in hepatocellullar carcinoma. Oncol Rep. 2013;29(2):840-6
66. Wang DY, Wu YN, Huang JQ, Wang W, Xu M, Jia JP. et al. Hippo/YAP signaling pathway is involved in osteosarcoma chemoresistance. Chin J Cancer. 2016;35:47
67. Tao Y, Shan L, Xu X, Jiang H, Chen R, Qian Z. et al. Huaier Augmented the Chemotherapeutic Sensitivity of Oxaliplatin via Downregulation of YAP in Hepatocellular Carcinoma. J Cancer. 2018;9(21):3962-70
68. Dai XY, Zhuang LH, Wang DD, Zhou TY, Chang LL, Gai RH. et al. Nuclear translocation and activation of YAP by hypoxia contributes to the chemoresistance of SN38 in hepatocellular carcinoma cells. Oncotarget. 2016;7(6):6933-47
69. Wu Q, Guo J, Liu Y, Zheng Q, Li X, Wu C. et al. YAP drives fate conversion and chemoresistance of small cell lung cancer. Sci Adv. 2021;7(40):eabg1850
70. Xiao L, Shi XY, Zhang Y, Zhu Y, Zhu L, Tian W. et al. YAP induces cisplatin resistance through activation of autophagy in human ovarian carcinoma cells. Onco Targets Ther. 2016;9:1105-14
71. Bi L, Ma F, Tian R, Zhou Y, Lan W, Song Q. et al. AJUBA increases the cisplatin resistance through hippo pathway in cervical cancer. Gene. 2018;644:148-54
72. Xu W, Wei Y, Li Y, Yin Y, Yuan W, Yang Y. et al. TAZ inhibition restores sensitivity of cisplatin via AKT/mTOR signaling in lung adenocarcinoma. Oncol Rep. 2017;38(3):1815-21
73. Li C, Wang Q, Luo Y, Xiang J. TAZ Regulates the Cisplatin Resistance of Epithelial Ovarian Cancer Cells via the ANGPTL4/SOX2 Axis. Anal Cell Pathol (Amst). 2022;2022:5632164
74. Jiang Z, Chen X, Chen K, Sun L, Gao L, Zhou C. et al. YAP Inhibition by Resveratrol via Activation of AMPK Enhances the Sensitivity of Pancreatic Cancer Cells to Gemcitabine. Nutrients. 2016;8(10):546
75. He Z, Chen D, Wu J, Sui C, Deng X, Zhang P. et al. Yes associated protein 1 promotes resistance to 5-fluorouracil in gastric cancer by regulating GLUT3-dependent glycometabolism reprogramming of tumor-associated macrophages. Arch Biochem Biophys. 2021;702:108838
76. Gujral TS, Kirschner MW. Hippo pathway mediates resistance to cytotoxic drugs. Proc Natl Acad Sci U S A. 2017;114(18):E3729-38
77. Mosca L, Ilari A, Fazi F, Assaraf YG, Colotti G. Taxanes in cancer treatment: Activity, chemoresistance and its overcoming. Drug Resist Updat. 2021;54:100742
78. Zhang X, George J, Deb S, Degoutin JL, Takano EA, Fox SB. et al. The Hippo pathway transcriptional co-activator, YAP, is an ovarian cancer oncogene. Oncogene. 2011;30(25):2810-22
79. Li W, Cao Y, Xu J, Wang Y, Li W, Wang Q. et al. YAP transcriptionally regulates COX-2 expression and GCCSysm-4 (G-4), a dual YAP/COX-2 inhibitor, overcomes drug resistance in colorectal cancer. J Expe Clin Cancer Res. 2017;36(1):144
80. Lai D, Ho KC, Hao Y, Yang X. Taxol resistance in breast cancer cells is mediated by the hippo pathway component TAZ and its downstream transcriptional targets Cyr61 and CTGF. Cancer Res. 2011;71(7):2728-38
81. Ma J, Fan Z, Tang Q, Xia H, Zhang T, Bi F. Aspirin attenuates YAP and β-catenin expression by promoting β-TrCP to overcome docetaxel and vinorelbine resistance in triple-negative breast cancer. Cell Death Dis. 2020;11(7):530
82. Xia Y, Zhang YL, Yu C, Chang T, Fan HY. YAP/TEAD co-activator regulated pluripotency and chemoresistance in ovarian cancer initiated cells. PloS one. 2014;9(11):e109575
83. Weiler SME, Pinna F, Wolf T, Lutz T, Geldiyev A, Sticht C. et al. Induction of Chromosome Instability by Activation of Yes-Associated Protein and Forkhead Box M1 in Liver Cancer. Gastroenterology. 2017;152(8):2037-51.e22
84. Pattschull G, Walz S, Gründl M, Schwab M, Rühl E, Baluapuri A. et al. The Myb-MuvB Complex Is Required for YAP-Dependent Transcription of Mitotic Genes. Cell Rep. 2019;27(12):3533-46.e7
85. Yang S, Zhang L, Liu M, Chong R, Ding SJ, Chen Y. et al. CDK1 phosphorylation of YAP promotes mitotic defects and cell motility and is essential for neoplastic transformation. Cancer Res. 2013;73(22):6722-33
86. Pellosi DS, Paula LB, de Melo MT, Tedesco AC. Targeted and Synergic Glioblastoma Treatment: Multifunctional Nanoparticles Delivering Verteporfin as Adjuvant Therapy for Temozolomide Chemotherapy. Mol Pharm. 2019;16(3):1009-24
87. El-Sahli S, Hua K, Sulaiman A, Chambers J, Li L, Farah E. et al. A triple-drug nanotherapy to target breast cancer cells, cancer stem cells, and tumor vasculature. Cell Death Dis. 2021;12(1):8
88. Tsimberidou AM. Targeted therapy in cancer. Cancer Chemother Pharmacol. 2015;76(6):1113-32
89. Dankner M, Rose AAN, Rajkumar S, Siegel PM, Watson IR. Classifying BRAF alterations in cancer: new rational therapeutic strategies for actionable mutations. Oncogene. 2018;37(24):3183-99
90. Sun C, Wang L, Huang S, Heynen GJ, Prahallad A, Robert C. et al. Reversible and adaptive resistance to BRAF(V600E) inhibition in melanoma. Nature. 2014;508(7494):118-22
91. Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelson T. et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343(6166):84-7
92. Kim MH, Kim J, Hong H, Lee SH, Lee JK, Jung E. et al. Actin remodeling confers BRAF inhibitor resistance to melanoma cells through YAP/TAZ activation. EMBO J. 2016;35(5):462-78
93. Kim MH, Kim CG, Kim SK, Shin SJ, Choe EA, Park SH. et al. YAP-Induced PD-L1 Expression Drives Immune Evasion in BRAFi-Resistant Melanoma. Cancer Immunol Res. 2018;6(3):255-66
94. Tomas A, Futter CE, Eden ER. EGF receptor trafficking: consequences for signaling and cancer. Trends Cell Biol. 2014;24(1):26-34
95. Wang H, Lu B, Castillo J, Zhang Y, Yang Z, McAllister G. et al. Tankyrase Inhibitor Sensitizes Lung Cancer Cells to Endothelial Growth Factor Receptor (EGFR) Inhibition via Stabilizing Angiomotins and Inhibiting YAP Signaling. J Biol Chem. 2016;291(29):15256-66
96. Lee JE, Park HS, Lee D, Yoo G, Kim T, Jeon H. et al. Hippo pathway effector YAP inhibition restores the sensitivity of EGFR-TKI in lung adenocarcinoma having primary or acquired EGFR-TKI resistance. Biochem Biophys Res Comm. 2016;474(1):154-60
97. Lee TF, Tseng YC, Nguyen PA, Li YC, Ho CC, Wu CW. Enhanced YAP expression leads to EGFR TKI resistance in lung adenocarcinomas. Sci Rep. 2018;8(1):271
98. Nilsson MB, Sun H, Robichaux J, Pfeifer M, McDermott U, Travers J. et al. A YAP/FOXM1 axis mediates EMT-associated EGFR inhibitor resistance and increased expression of spindle assembly checkpoint components. Sci Transl Med. 2020;12(559):eaaz4589
99. Ghiso E, Migliore C, Ciciriello V, Morando E, Petrelli A, Corso S. et al. YAP-Dependent AXL Overexpression Mediates Resistance to EGFR Inhibitors in NSCLC. Neoplasia. 2017;19(12):1012-21
100. Park HS, Lee DH, Kang DH, Yeo MK, Bae G, Lee D. et al. Targeting YAP-p62 signaling axis suppresses the EGFR-TKI-resistant lung adenocarcinoma. Cancer Med. 2021;10(4):1405-17
101. Shi J, Li F, Yao X, Mou T, Xu Z, Han Z. et al. The HER4-YAP1 axis promotes trastuzumab resistance in HER2-positive gastric cancer by inducing epithelial and mesenchymal transition. Oncogene. 2018;37(22):3022-38
102. Cheng H, Zhang Z, Rodriguez-Barrueco R, Borczuk A, Liu H, Yu J. et al. Functional genomics screen identifies YAP1 as a key determinant to enhance treatment sensitivity in lung cancer cells. Oncotarget. 2016;7(20):28976-88
103. González-Alonso P, Zazo S, Martín-Aparicio E, Luque M, Chamizo C, Sanz-Álvarez M. et al. The Hippo Pathway Transducers YAP1/TEAD Induce Acquired Resistance to Trastuzumab in HER2-Positive Breast Cancer. Cancers (Basel). 2020;12(5):1108
104. Coggins GE, Farrel A, Rathi KS, Hayes CM, Scolaro L, Rokita JL. et al. YAP1 Mediates Resistance to MEK1/2 Inhibition in Neuroblastomas with Hyperactivated RAS Signaling. Cancer Res. 2019;79(24):6204-14
105. Rao G, Kim IK, Conforti F, Liu J, Zhang YW, Giaccone G. Dasatinib sensitises KRAS-mutant cancer cells to mitogen-activated protein kinase kinase inhibitor via inhibition of TAZ activity. Eur J Cancer. 2018;99:37-48
106. Yun MR, Choi HM, Lee YW, Joo HS, Park CW, Choi JW. et al. Targeting YAP to overcome acquired resistance to ALK inhibitors in ALK-rearranged lung cancer. EMBO Mol Med. 2019;11(12):e10581
107. Gobbi G, Donati B, Do Valle IF, Reggiani F, Torricelli F, Remondini D. et al. The Hippo pathway modulates resistance to BET proteins inhibitors in lung cancer cells. Oncogene. 2019;38(42):6801-17
108. Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018;8(9):1069-86
109. Liu X, Yang L, Tan X. PD-1/PD-L1 pathway: A double-edged sword in periodontitis. Biomed Pharmacother. 2023;159:114215
110. Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest. 2015;125(9):3335-7
111. Rodríguez-Abreu D, Powell SF, Hochmair MJ, Gadgeel S, Esteban E, Felip E. et al. Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: protocol-specified final analysis from KEYNOTE-189. Ann Oncol. 2021;32(7):881-95
112. Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H. et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med. 2018;379(22):2108-21
113. Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E. et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med. 2019;381(21):2020-31
114. Lee BS, Park DI, Lee DH, Lee JE, Yeo MK, Park YH. et al. Hippo effector YAP directly regulates the expression of PD-L1 transcripts in EGFR-TKI-resistant lung adenocarcinoma. Biochem Biophys Res Commun. 2017;491(2):493-9
115. Janse van Rensburg HJ, Azad T, Ling M, Hao Y, Snetsinger B, Khanal P. et al. The Hippo Pathway Component TAZ Promotes Immune Evasion in Human Cancer through PD-L1. Cancer Res. 2018;78(6):1457-70
116. Ni X, Tao J, Barbi J, Chen Q, Park BV, Li Z. et al. YAP Is Essential for Treg-Mediated Suppression of Antitumor Immunity. Cancer Discov. 2018;8(8):1026-43
117. Wang G, Lu X, Dey P, Deng P, Wu CC, Jiang S. et al. Targeting YAP-Dependent MDSC Infiltration Impairs Tumor Progression. Cancer Discov. 2016;6(1):80-95
118. Murakami S, Shahbazian D, Surana R, Zhang W, Chen H, Graham GT. et al. Yes-associated protein mediates immune reprogramming in pancreatic ductal adenocarcinoma. Oncogene. 2017;36(9):1232-44
119. Carneiro BA, El-Deiry WS. Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol. 2020;17(7):395-417
120. Zhang C, Liu X, Jin S, Chen Y, Guo R. Ferroptosis in cancer therapy: a novel approach to reversing drug resistance. Mol Cancer. 2022;21(1):47
121. Cheng Y, Mao M, Lu Y. The biology of YAP in programmed cell death. Biomark Res. 2022;10(1):34
122. Yan B, Li T, Shen L, Zhou Z, Liu X, Wang X. et al. Simultaneous knockdown of YAP and TAZ increases apoptosis of hepatocellular carcinoma cells under hypoxic condition. Biochem Biophys Res Commun. 2019;515(2):275-81
123. Wang J, Chen M, Wang M, Zhao W, Zhang C, Liu X. et al. The novel ER stress inducer Sec C triggers apoptosis by sulfating ER cysteine residues and degrading YAP via ER stress in pancreatic cancer cells. Acta Pharm Sinica B. 2022;12(1):210-27
124. Su M, Zhan L, Zhang Y, Zhang J. Yes-activated protein promotes primary resistance of BRAF V600E mutant metastatic colorectal cancer cells to mitogen-activated protein kinase pathway inhibitors. J Gastrointest Oncol. 2021;12(3):953-63
125. Ma L, Yang F, Wu X, Mao C, Guo L, Miao T. et al. Structural basis and molecular mechanism of biased GPBAR signaling in regulating NSCLC cell growth via YAP activity. Proc Natl Acad Sci U S A. 2022;119(29):e2117054119
126. Song Y, Sun Y, Lei Y, Yang K, Tang R. YAP1 promotes multidrug resistance of small cell lung cancer by CD74-related signaling pathways. Cancer Med. 2020;9(1):259-68
127. Patrick S, Gowda P, Lathoria K, Suri V, Sen E. YAP1-mediated regulation of mitochondrial dynamics in IDH1 mutant gliomas. J Cell Sci. 2021;134(22):jcs259188
128. Li H, He F, Zhao X, Zhang Y, Chu X, Hua C. et al. YAP Inhibits the Apoptosis and Migration of Human Rectal Cancer Cells via Suppression of JNK-Drp1-Mitochondrial Fission-HtrA2/Omi Pathways. Cell Physiol Biochem. 2017 44(5) 2073-89
129. He L, Yuan L, Yu W, Sun Y, Jiang D, Wang X. et al. A Regulation Loop between YAP and NR4A1 Balances Cell Proliferation and Apoptosis. Cell Rep. 2020;33(3):108284
130. Han Y, Zhang X, Guan M, Huo C, Yu C, Hu B. et al. RASSF4 inhibits cell proliferation and increases drug sensitivity in colorectal cancer through YAP/Bcl-2 pathway. J Cell Mol Med. 2022;26(12):3538-47
131. Yan H, Qiu C, Sun W, Gu M, Xiao F, Zou J. et al. Yap regulates gastric cancer survival and migration via SIRT1/Mfn2/mitophagy. Oncol Rep. 2018;39(4):1671-81
132. Li Y, Zhu X, Yang M, Wang Y, Li J, Fang J. et al. YAP/TEAD4-induced KIF4A contributes to the progression and worse prognosis of esophageal squamous cell carcinoma. Mol Carcinog. 2021;60(7):440-54
133. Zhao Q, Jia X, Zhang Y, Dong Y, Lei Y, Tan X. et al. Tetrandrine induces apoptosis in human neuroblastoma through regulating the Hippo/YAP signaling pathway. Biochem Biophys Res Commun. 2019;513(4):846-51
134. Ciamporcero E, Shen H, Ramakrishnan S, Yu Ku S, Chintala S, Shen L. et al. YAP activation protects urothelial cell carcinoma from treatment-induced DNA damage. Oncogene. 2016;35(12):1541-53
135. Kurppa KJ, Liu Y, To C, Zhang T, Fan M, Vajdi A. et al. Treatment-Induced Tumor Dormancy through YAP-Mediated Transcriptional Reprogramming of the Apoptotic Pathway. Cancer Cell. 2020;37(1):104-22.e12
136. Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060-72
137. Vucetic M, Daher B, Cassim S, Meira W, Pouyssegur J. Together we stand, apart we fall: how cell-to-cell contact/interplay provides resistance to ferroptosis. Cell Death Dis. 2020;11(9):789
138. Battaglia AM, Chirillo R, Aversa I, Sacco A, Costanzo F, Biamonte F. Ferroptosis and Cancer: Mitochondria Meet the "Iron Maiden" Cell Death. Cells. 2020;9(6):1505
139. Chen X, Kang R, Kroemer G, Tang D. Ferroptosis in infection, inflammation, and immunity. J Exp Med. 2021;218(6):e20210518
140. Wu X, Li Y, Zhang S, Zhou X. Ferroptosis as a novel therapeutic target for cardiovascular disease. Theranostics. 2021;11(7):3052-9
141. Xl L, Gy Z, R G, N C. Ferroptosis in sepsis: The mechanism, the role and the therapeutic potential. Front Immunol. 2022;13:956361
142. Zhu G, Murshed A, Li H, Ma J, Zhen N, Ding M. et al. O-GlcNAcylation enhances sensitivity to RSL3-induced ferroptosis via the YAP/TFRC pathway in liver cancer. Cell Death Discov. 2021;7(1):83
143. Qin Y, Pei Z, Feng Z, Lin P, Wang S, Li Y. et al. Oncogenic Activation of YAP Signaling Sensitizes Ferroptosis of Hepatocellular Carcinoma via ALOXE3-Mediated Lipid Peroxidation Accumulation. Front Cell Dev Biol. 2021;9:751593
144. Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A. 2016;113(34):E4966-75
145. Wu J, Minikes AM, Gao M, Bian H, Li Y, Stockwell BR. et al. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature. 2019;572(7769):402-6
146. Yang WH, Ding CC, Sun T, Rupprecht G, Lin CC, Hsu D. et al. The Hippo Pathway Effector TAZ Regulates Ferroptosis in Renal Cell Carcinoma. Cell Rep. 2019;28(10):2501-8.e4
147. Yang WH, Huang Z, Wu J, Ding CC, Murphy SK, Chi JT. A TAZ-ANGPTL4-NOX2 Axis Regulates Ferroptotic Cell Death and Chemoresistance in Epithelial Ovarian Cancer. Mol Cancer Res. 2020;18(1):79-90
148. Ye S, Xu M, Zhu T, Chen J, Shi S, Jiang H. et al. Cytoglobin promotes sensitivity to ferroptosis by regulating p53-YAP1 axis in colon cancer cells. J Cell Mol Med. 2021;25(7):3300-11
149. Gao R, Kalathur RKR, Coto-Llerena M, Ercan C, Buechel D, Shuang S. et al. YAP/TAZ and ATF4 drive resistance to Sorafenib in hepatocellular carcinoma by preventing ferroptosis. EMBO Mol Med. 2021;13(12):e14351
150. Zhang Y, Li Y, Qiu Q, Chen Z, Du Y, Liu X. MITD1 Deficiency Suppresses Clear Cell Renal Cell Carcinoma Growth and Migration by Inducing Ferroptosis through the TAZ/SLC7A11 Pathway. Oxid Med Cell longev. 2022;2022:7560569
151. Zhang X, Yu K, Ma L, Qian Z, Tian X, Miao Y. et al. Endogenous glutamate determines ferroptosis sensitivity via ADCY10-dependent YAP suppression in lung adenocarcinoma. Theranostics. 2021;11(12):5650-74
152. Wang Y, Qiu S, Wang H, Cui J, Tian X, Miao Y. et al. Transcriptional Repression of Ferritin Light Chain Increases Ferroptosis Sensitivity in Lung Adenocarcinoma. Front Cell Dev Biol. 2021;9:719187
153. Mizushima N, Levine B. Autophagy in Human Diseases. New Engl J Med. 2020;383(16):1564-76
154. Klionsky DJ, Petroni G, Amaravadi RK, Baehrecke EH, Ballabio A, Boya P. et al. Autophagy in major human diseases. EMBO J. 2021;40(19):e108863
155. Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17(9):528-42
156. White E, Mehnert JM, Chan CS. Autophagy, Metabolism, and Cancer. Clin Cancer Res. 2015;21(22):5037-46
157. White E. The role for autophagy in cancer. J Clin Invest. 2015;125(1):42-6
158. Majeed ST, Majeed R, Andrabi KI. Expanding the view of the molecular mechanisms of autophagy pathway. J Cell Physiol. 2022;237(8):3257-77
159. Marsh T, Debnath J. Autophagy suppresses breast cancer metastasis by degrading NBR1. Autophagy. 2020;16(6):1164-5
160. Kudo Y, Sugimoto M, Arias E, Kasashima H, Cordes T, Linares JF. et al. PKCλ/ι Loss Induces Autophagy, Oxidative Phosphorylation, and NRF2 to Promote Liver Cancer Progression. Cancer Cell. 2020;38(2):247-62.e11
161. Miao CC, Hwang W, Chu LY, Yang LH, Ha CT, Chen PY. et al. LC3A-mediated autophagy regulates lung cancer cell plasticity. Autophagy. 2022;18(4):921-34
162. Yamamoto K, Venida A, Yano J, Biancur DE, Kakiuchi M, Gupta S. et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature. 2020;581(7806):100-5
163. Wang D, He J, Huang B, Liu S, Zhu H, Xu T. Emerging role of the Hippo pathway in autophagy. Cell Death Dis. 2020;11(10):880
164. Wang CZ, Yan GX, Dong DS, Xin H, Liu ZY. LncRNA-ATB promotes autophagy by activating Yes-associated protein and inducing autophagy-related protein 5 expression in hepatocellular carcinoma. World J Gastroenterol. 2019;25(35):5310-22
165. Sun T, Peng H, Mao W, Ma L, Liu H, Mai J. et al. Autophagy-mediated negative feedback attenuates the oncogenic activity of YAP in pancreatic cancer. Int J Biol Sci. 2021;17(13):3634-45
166. Lee YA, Noon LA, Akat KM, Ybanez MD, Lee TF, Berres ML. et al. Autophagy is a gatekeeper of hepatic differentiation and carcinogenesis by controlling the degradation of Yap. Nature Commun. 2018;9(1):4962
167. Ma F, Ding MG, Lei YY, Luo LH, Jiang S, Feng YH. et al. SKIL facilitates tumorigenesis and immune escape of NSCLC via upregulating TAZ/autophagy axis. Cell Death Dis. 2020;11(12):1028
168. Jiang Y, Ji F, Liu Y, He M, Zhang Z, Yang J. et al. Cisplatin-induced autophagy protects breast cancer cells from apoptosis by regulating yes-associated protein. Oncol Rep. 2017;38(6):3668-76
169. Rivera-Reyes A, Ye S, G EM, Egolf S, G EC, Chor S. et al. YAP1 enhances NF-κB-dependent and independent effects on clock-mediated unfolded protein responses and autophagy in sarcoma. Cell Death Dis. 2018;9(11):1108
170. Zhou W, Weng J, Wu K, Xu X, Wang H, Zhang J. et al. Silencing of TAZ inhibits the motility of hepatocellular carcinoma cells through autophagy induction. Cancer Manag Res. 2019;11:8743-53
171. Xu W, Zhang M, Li Y, Wang Y, Wang K, Chen Q. et al. YAP manipulates proliferation via PTEN/AKT/mTOR-mediated autophagy in lung adenocarcinomas. Cancer Cell Int. 2021;21(1):30
172. Jin L, Chen Y, Cheng D, He Z, Shi X, Du B. et al. YAP inhibits autophagy and promotes progression of colorectal cancer via upregulating Bcl-2 expression. Cell Death Dis. 2021;12(5):457
173. Hou J, Hsu JM, Hung MC. Molecular mechanisms and functions of pyroptosis in inflammation and antitumor immunity. Mol Cell. 2021;81(22):4579-90
174. Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660-5
175. Jia Y, Wang X, Deng Y, Li S, Xu X, Qin Y. et al. Pyroptosis Provides New Strategies for the Treatment of Cancer. J Cancer. 2023;14(1):140-51
176. Hu B, Elinav E, Huber S, Booth CJ, Strowig T, Jin C. et al. Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc Natl Acad Sci U S A. 2010;107(50):21635-40
177. Van Gorp H, Lamkanfi M. The emerging roles of inflammasome-dependent cytokines in cancer development. EMBO Rep. 2019;20(6):e47575
178. Wang WJ, Chen D, Jiang MZ, Xu B, Li XW, Chu Y. et al. Downregulation of gasdermin D promotes gastric cancer proliferation by regulating cell cycle-related proteins. J Dig Dis. 2018;19(2):74-83
179. Li F, Xia Q, Ren L, Nie Y, Ren H, Guo X. et al. GSDME Increases Chemotherapeutic Drug Sensitivity by Inducing Pyroptosis in Retinoblastoma Cells. Oxid Med Cell Longev. 2022;2022:2371807
180. Liang L, Yan B, Liu Y, Jiang S, He H, Huang J. et al. FOXP3 Contributes to TMZ Resistance, Prognosis, and Immune Infiltration in GBM from a Novel Pyroptosis-Associated Risk Signature. Dis Markers. 2022;2022:4534080
181. Cheng Z, Li Z, Gu L, Li L, Gao Q, Zhang X. et al. Ophiopogonin B alleviates cisplatin resistance of lung cancer cells by inducing Caspase-1/GSDMD dependent pyroptosis. J Cancer. 2022;13(2):715-27
182. Zheng ZY, Yang PL, Li RY, Liu LX, Xu XE, Liao LD. et al. STAT3β disrupted mitochondrial electron transport chain enhances chemosensitivity by inducing pyroptosis in esophageal squamous cell carcinoma. Cancer Lett. 2021;522:171-83
183. Li S, Chen P, Cheng B, Liu Y, Zhang X, Xu Q. et al. Pyroptosis predicts immunotherapy outcomes across multiple cancer types. Clin Immunol. 2022;245:109163
184. Wang M, Wu M, Liu X, Shao S, Huang J, Liu B. et al. Pyroptosis Remodeling Tumor Microenvironment to Enhance Pancreatic Cancer Immunotherapy Driven by Membrane Anchoring Photosensitizer. Adv Sci (Weinh). 2022;9(29):e2202914
185. Zhang T, Lu L, Li M, Zhang D, Yu P, Zhang X. et al. Exosome from BMMSC attenuates cardiopulmonary bypass-induced acute lung injury via YAP/β-catenin pathway: down-regulation of pyroptosis. Stem Cells. 2022;40(12):1122-1133
186. Hong L, Zha Y, Wang C, Qiao S, An J. Folic Acid Alleviates High Glucose and Fat-Induced Pyroptosis via Inhibition of the Hippo Signal Pathway on H9C2 Cells. Front Mol Biosci. 2021;8:698698
187. García-Gil A, Galán-Enríquez CS, Pérez-López A, Nava P, Alpuche-Aranda C, Ortiz-Navarrete V. SopB activates the Akt-YAP pathway to promote Salmonella survival within B cells. Virulence. 2018;9(1):1390-402
188. Cui J, Zhou Z, Yang H, Jiao F, Li N, Gao Y. et al. MST1 Suppresses Pancreatic Cancer Progression via ROS-Induced Pyroptosis. Mol Cancer Res. 2019;17(6):1316-25
189. Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee SJ, Anders RA. et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26(12):1300-5
190. Zhao X, Wang X, Fang L, Lan C, Zheng X, Wang Y. et al. A combinatorial strategy using YAP and pan-RAF inhibitors for treating KRAS-mutant pancreatic cancer. Cancer Lett. 2017;402:61-70
191. Guo L, Zheng J, Zhang J, Wang H, Shao G, Teng L. Knockdown of TAZ modifies triple-negative breast cancer cell sensitivity to EGFR inhibitors by regulating YAP expression. Oncol Rep. 2016;36(2):729-36
192. Song S, Honjo S, Jin J, Chang SS, Scott AW, Chen Q. et al. The Hippo Coactivator YAP1 Mediates EGFR Overexpression and Confers Chemoresistance in Esophageal Cancer. Clin Cancer Res. 2015;21(11):2580-90
193. Ma K, Xu Q, Wang S, Zhang W, Liu M, Liang S. et al. Nuclear accumulation of Yes-Associated Protein (YAP) maintains the survival of doxorubicin-induced senescent cells by promoting survivin expression. Cancer Lett. 2016;375(1):84-91
194. Song J, Xie LX, Zhang XY, Hu P, Long MF, Xiong F. et al. Role of YAP in lung cancer resistance to cisplatin. Oncol Lett. 2018;16(3):3949-54
195. Huggett MT, Jermyn M, Gillams A, Illing R, Mosse S, Novelli M. et al. Phase I/II study of verteporfin photodynamic therapy in locally advanced pancreatic cancer. Br J Cancer. 2014;110(7):1698-704
196. Hagenbeek TJ, Zbieg JR, Hafner M, Mroue R, Lacap JA, Sodir NM. et al. An allosteric pan-TEAD inhibitor blocks oncogenic YAP/TAZ signaling and overcomes KRAS G12C inhibitor resistance. Nat Cancer. 2023;4(6):812-28
197. Tang TT, Konradi AW, Feng Y, Peng X, Ma M, Li J. et al. Small Molecule Inhibitors of TEAD Auto-palmitoylation Selectively Inhibit Proliferation and Tumor Growth of NF2-deficient Mesothelioma. Mol Cancer Ther. 2021;20(6):986-98
198. Kaneda A, Seike T, Danjo T, Nakajima T, Otsubo N, Yamaguchi D. et al. The novel potent TEAD inhibitor, K-975, inhibits YAP1/TAZ-TEAD protein-protein interactions and exerts an anti-tumor effect on malignant pleural mesothelioma. Am J Cancer Research. 2020;10(12):4399-415
199. Zagiel B, Melnyk P, Cotelle P. Progress with YAP/TAZ-TEAD inhibitors: a patent review (2018-present). Expert Opin Ther Pat. 2022;32(8):899-912
Author contact
![]() Corresponding authors: Dr. Stephen Pandol (e-mail: Stephen.Pandolorg), Dr. Heshui Wu (e-mail: heshuiwuedu.cn) and Dr. Qiang Wang (e-mail: Qiang.Wangorg).
Corresponding authors: Dr. Stephen Pandol (e-mail: Stephen.Pandolorg), Dr. Heshui Wu (e-mail: heshuiwuedu.cn) and Dr. Qiang Wang (e-mail: Qiang.Wangorg).

 Global reach, higher impact
Global reach, higher impact