10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2024; 20(1):127-136. doi:10.7150/ijbs.89890 This issue Cite
Review
The role of TNC in atherosclerosis and drug development opportunities
1. Cancer Institute, The Affiliated Hospital of Qingdao University, Qingdao University, Qingdao Cancer Institute, Qingdao, Shandong, 266071, China.
2. Department of Pharmacy, Women's and Children's Hospital Afliated to Qingdao University, Qingdao Women's and Children's Hospital, Qingdao, Shandong, 266000, China.
3. Obstetrical Department, The Affiliated Hospital of Qingdao University, Qingdao, Shandong, 266003, China
4. Qingdao Medical College, Qingdao University, Qingdao, Shandong, 266071, China.
5. School of Life Sciences, Tsinghua University, Beijing, 100084, China.
#These authors contributed equally to this work.
Received 2023-9-6; Accepted 2023-10-23; Published 2024-1-1
Abstract
Tenascin C (TNC), a rich glycoprotein of the extracellular matrix, exhibits a pro-atherosclerosis or anti-atherosclerosis effect depending on its location. TNC, especially its C domain/isoform (TNC-C), is strongly overexpressed in atherosclerotic plaque active areas but virtually undetectable in most normal adult tissues, suggesting that TNC is a promising delivery vector target for atherosclerosis-targeted drugs. Many delivery vectors were investigated by recognizing TNC-C, including G11, G11-iRGD, TN11, PL1, and PL3. F16 and FNLM were also investigated by recognizing TNC-A1 and TNC, respectively. Notably, iRGD was undergoing clinical trials. PL1 not only recognizes TNC-C but also the extra domain-B (EDB) of fibronectin (FN), which is also a promising delivery vector for atherosclerosis-targeted drugs, and several conjugate agents are undergoing clinical trials. The F16-conjugate agent F16IL2 is undergoing clinical trials. Therefore, G11-iRGD, PL1, and F16 have great development value. Furthermore, ATN-RNA and IMA950 were investigated in clinical trials as therapeutic drugs and vaccines by targeting TNC, respectively. Therefore, targeting TNC could greatly improve the success rate of atherosclerosis-targeted drugs and/or specific drug development. This review discussed the role of TNC in atherosclerosis, atherosclerosis-targeted drug delivery vectors, and agent development to provide knowledge for drug development targeting TNC.
Keywords: TNC, atherosclerosis, G11-iRGD, PL1, F16, ATN-RNA, drug development
1. Introduction
Coronary heart disease (CHD) is the leading cause of death worldwide and is primarily caused by atherosclerosis. Atherosclerotic plaque growth blocks blood flow, leading to ischemia of the surrounding tissue by promoting narrowing of the vessel lumen. Plaque ruptures lead to life-threatening myocardial infarction (MI), coronary artery disease (CAD), cerebral infarction (ischemic stroke), and acute coronary syndrome (ACS) by promoting platelet aggregation, fluid coagulation, and thrombosis. Atherosclerosis is characterized by the accumulation of lipids in the artery walls, accompanied by infiltration of immune cells and chronic inflammation. The early stage of atherosclerosis is the formation of "fatty streaks" that consist of cholesterol-laden foam cells. As the disease progresses, necrotic cells accumulate in the plaque to form necrotic nuclei that infiltrate numerous inflammatory cells and release proinflammatory cytokines and chemokines. Therefore, lipid-lowering, anti-inflammatory, or immunomodulatory therapy is necessary to delay the progression of atherosclerosis [1-3]. However, atherosclerosis mainly occurs in the heart aorta. Many drugs have many side effects. These include statin-induced liver and neuromuscular toxicity, cramps, myalgia, necrotizing myopathy, and rhabdomyolysis [4, 5]. Ezetimibe has a placebo-like side effect profile. PCSK9 inhibitors induce nasopharyngitis [6, 7]. Therefore, the development of drugs that target aortic plaques without affecting other tissues will greatly improve the efficacy and reduce the side effects of drugs. Conjugative drugs that conjugate the genes highly expressed in pathological tissue but rarely expressed in normal tissue have a specificity and potency that traditional drugs cannot achieve. Current conjugative drugs include antibody‒drug conjugates (ADCs), antibody degraducer conjugates (ADeCs), aptamer-drug conjugates (ApDCs), antibody-oligonucleotide conjugates (AOCs), antibody fragment-drug conjugates (FDCs), immune-stimulating antibody conjugates (ISACs), radionuclide-drug conjugates (RDCs), small molecule-drug conjugates (SMDCs), and virus-like drug conjugates (VDCs). ADCs, RDCs, SMDCs, and ISACs are the most successful coupling drugs because many related drugs have been approved on the market or entered into clinical trials [8-10]. Therefore, the development of these conjugative agents will greatly improve the success rate of drug development.
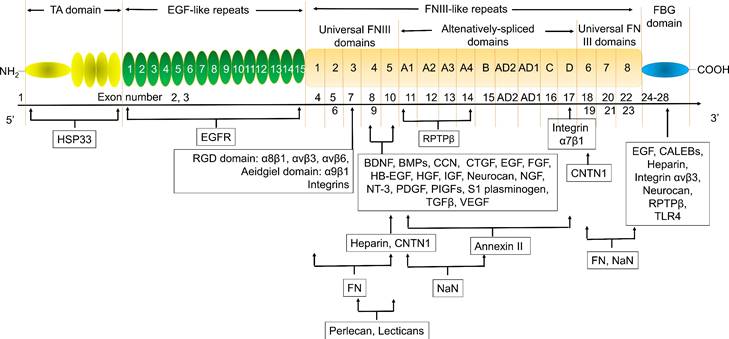
Tenascin C (TNC) is a member of the extracellular matrix (ECM) protein family and plays a key role in wound healing and tissue remodeling. TNC has multiple biological roles by binding to and interacting with multiple genes. Heat shock protein 33 (HSP33) is responsible for TNC accumulation in cells. The epidermal growth factor (EGF)-like repeat domains of TNC regulated cell adhesion and cell motility and were anti-adhesion regions of fibroblasts, neurons, and glial cells. This domain also regulates neuronal migration and axon pathfinding during development. TNC has many isomers with different functions and sizes due to the alternative splicing of fibronectin type III (FNIII)-like repeats (Fig. 1) [11-22]. Many studies have shown that TNC plays a key role in atherosclerosis. TNC expression was increased in the plaque in apoE-/- mice [23, 24]. Serum TNC levels were increased in 307 patients with CHD [25-27] and 170 patients with ACS [28]. The single nucleotide polymorphisms (SNPs) of TNC, such as rs3789875 and rs12347433, were related to atherosclerosis and CAD [29]. TNC was rapidly upregulated after ischemic events, such as MI, suggesting that circulating TNC is a diagnostic or prognostic auxiliary biomarker in patients with cardiovascular disease [30]. However, the friend and foe of TNC in atherosclerosis depended on its location in vivo. Interestingly, TNC was strongly overexpressed in atherosclerotic plaque active areas but was virtually undetectable in most normal adult tissues, especially the TNC C domain/isoform (TNC-C). G11, G11-iRGD, TN11, PL1, PL3, F16, and FNLM are promising delivery vectors for atherosclerosis-targeted drugs. In particular, G11-iRGD, PL1, and F16 have great development value because they contain or recognize components of clinical studies or their conjugate agents being investigated in clinical trials. In addition, to the best of our knowledge, ATN-RNA, a TNC-specific agent, was investigated in clinical trials as a therapeutic drug, while IMA950, a TNC agent, was investigated in clinical trials as a vaccine. Therefore, using TNC could greatly improve the success rate of atherosclerosis-targeted drugs and/or specific drug development. In this review, we focused on the potential of TNC in atherosclerosis, the delivery vector for atherosclerosis-targeted drugs, and TNC agents in the hope of providing knowledge for drug development by targeting TNC.
2. The role and mechanism of TNC in atherosclerosis
2.1 Anti-atherosclerotic effect and mechanism of TNC
TNC promoted coronary vessel development to primitive endothelial tubes by promoting the recruitment of α-smooth muscle actin (SMA)-positive mural cells to primitive endothelial tubes by stimulating the integrin αvβ3/PDGF-BB/PDGFRβ signaling pathway [24]. TNC is also an endogenous ligand of TLR-4 and promotes chronic inflammatory and foam cell formation by activating the TLR-4-mediated NF-κB signaling pathway and CD36 expression in macrophages from THP-1 cells [31, 32]. Macrophage TNC enhanced macrophage migration and VEGF release and activated the Akt/NF-κB and ERK pathways by binding to its receptor annexin II in RAW 264.7 macrophages and human primary macrophages [33]. TNC promoted platelet adhesion, activation, and thrombosis by stimulating the integrin α(2)β(1) and α(IIb)β(3) by binding to Von Willebrand factor (VWF) [34]. In addition, the TNC+ SMC subset was increased in plaques from human carotid and dog arteries. The TNC+ SMC subset promoted atherogenesis by enhancing ECM deposition and remodeling by increasing the expression of 11 related genes, including clusterin (CLU), collagen type XIV alpha 1 chain (COL14A1), ENSCAFG00000015206, fibrillin-1 (FBN1), LGALS3, matrix Gla protein (MGP), NADH dehydrogenase (ubiquinone) 1 alpha subcomplex 4-like 2 (NDUFA4L2), periostin (POSTN), serum amyloid A1 (SAA1), secreted modular calcium-binding protein 2 (SMOC2), and versican (VCAN) [35]. Of note, the EGF-like domain of TNC promoted SMC apoptosis by activating caspase-3 [22, 36]. The A1 and A2 domains of TNC were mainly expressed in SMCs and promoted SMC chemotaxis and migration [22]. The A1 and A2 domains of TNC also inhibited T-cell activation. The A2 domain of TNC suppressed the proliferation of human dermal microvascular endothelial cells. The fibrinogen-like globe (FBG) domain of TNC promoted IL-6, IL-8, and TNFα in human macrophages. The TnfnIII 1-5 domains of TNC inhibited aVb1 and a4b1-mediated adhesion to fibronectin [21]. All of these factors were risk factors for atherosclerosis. Therefore, many TNC domains/isoforms promote atherosclerosis development, such as the A1 domain, A2 domain, EGF-like domain, FBG domain, and TnfnIII 1-5 domains. TNC from macrophages, platelets, and SMCs promoted atherosclerosis development.
The exon structure, corresponding domains, and binding factors of TNC. BDNF, brain derived neurotrophic factor; BMPs, Bone morphogenetic proteins; CALEB, chicken acidic leucine-rich EGF-like domain containing brain protein; CNTN1, Contactin-1; CTGF, connective tissue growth factor; EGF, Epidermal growth factor; EGFR, EGF receptor; FBG, Fibrinogen-like globe; FN, Fibronectin; FNIII, Fibronectin type III; HB-EGF, heparin-binding EGF-like growth factor; FGF, fibroblast growth factor; HGF, hepatocyte growth factor; HSP33, heat shock protein 33; IGF, insulin-like growth factor; NaN, sodium channel subunit β2; NGF, Nerve growth factor; NT-3, Neurotrophin-3; PDGF, Platelet-derived growth factor; PIGF, placental growth factor; RPTPβ, receptor protein tyrosine phosphatase β; TA, Tenascin assembly; TGFβ, Transforming growth factor β; TLR4, toll-like receptor-4; VEGF, vascular endothelial growth factor. The information taken and modified from Refs [11-22].
2.2 Proatherogenic effect and mechanism of TNC
TNC not only stimulated pro-inflammatory cytokine expression, such as IL-6, IL-8, and TNFα but also anti-inflammatory cytokine expression, such as IL-4 and IL-13, suggesting that TNC has both pro-inflammatory and anti-inflammatory activity [37]. However, knockout of TNC not only did not change the lipoprotein profile but also the adhesion, migration, and proliferation of SMCs [37], suggesting that the effect of TNC on atherosclerosis was independent of lipid metabolism and SMCs. Knockout of TNC also exacerbated the systemic inflammatory response and atherosclerosis by enhancing eotaxin (also named CCL11) levels in apoE-/- mice [38]. Interestingly, among the 62 inflammatory cytokines (such as Axl, CXCL16, IGFBP-3, IGFBP-6, IL-12 p70, Leptin R, LIX, soluble L-selectin, MIP-1γ, PF-4, soluble P-selectin, TNF-RI, TNFRII, and soluble VCAM-1), eotaxin was the only cytokine that was regulated by TNC, suggesting that eotaxin plays a key role in TNC-mediated atheroprotective activity [38]. In another study, knockout of TNC exacerbated intraplaque hemorrhage and inflammation in endothelial cells (ECs) and macrophages to induce atherosclerosis development by enhancing VCAM-1 expression [37]. Bone marrow-derived TNC reduced cardiac hypertrophy by reducing inflammation in mice [39]. Thus, TNC from bone marrow and the whole body exhibited atheroprotective activity, while TNC from macrophages, platelets, and SMCs promoted atherosclerosis development.
3. The promising delivery vector for atherosclerosis-targeted drugs
3.1 G11
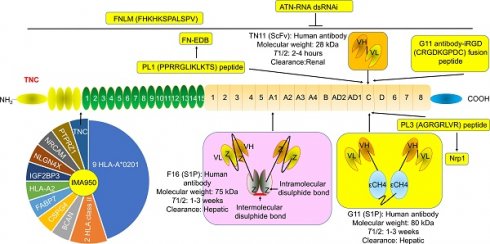
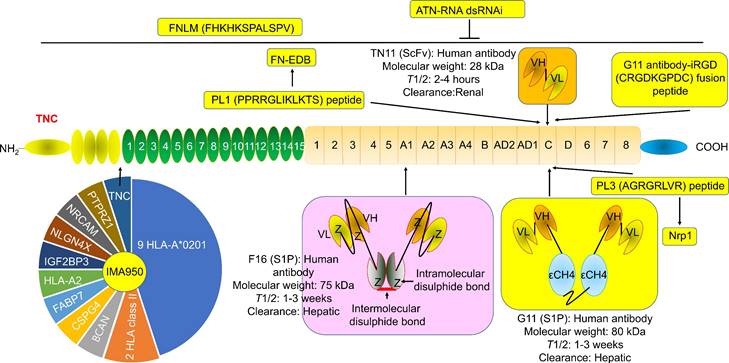
G11 (mini antibody SIP format) was a human antibody that only recognized TNC-C. G11 was used to observe the angiogenesis of advanced atherosclerotic plaques in mice, as well as gliomas and lung tumors [30]. 125I (iodine)-G11 is a G11 with 125I labeling. 125I-G11 (intravenous injection, I.V., 0.3 MBq, 8 μg) was taken up in fat and activated macrophages in aortic plaques in apoE-/- mice. The sensitivity of autoradiography to detect plaque fatty stained areas was 74% and 76% at 4 hours and 24 hours after intravenous administration of 125I-G11. The signal-to-noise ratio (SNR) of vessel walls with plaque and without plaque was 43:1 and 104:1 at 4 hours and 24 hours, respectively. The SNR of activated macrophages in aortic plaques was 50:1 and 90:1 at 4 hours and 24 hours, respectively. The aorta-to-blood ratios of 125I-G11 were 0.45 and 1.53. After 4-24 hours of administration, the clearance of 125I-G11 was 86%, suggesting that the blood half-life of 125I-G11 was less than 20 min [30, 40]. Therefore, G11 may facilitate molecular imaging of advanced atherosclerotic plaques and the delivery of related drugs. No G11 conjugate agents have been investigated in clinical trials. More studies are needed to confirm G11's development value. Of note, TNC-C was undetectable in normal adult tissue and exhibited a more restricted expression pattern than other TNC domains. TNC-C was also strongly abundant in macrophage-rich plaques but was not detected in normal adult tissues [30, 40, 41], suggesting that targeting TNC-C facilitated the development of diagnostic and targeting agents. Importantly, several other antibodies or antibodies can specifically recognize TNC-C, including G11-iRGD, TN11, PL1, and PL3 (Fig. 2).
G11-iRGD is a fusion peptide that contains the G11 antibody and iRGD peptide (sequence: CRGDKGPDC) [42]. iRGD is also known as CEND-1 and LSTA1 and is a promising delivery vector. Interestingly, iRGD was investigated in clinical trials in cancer [43, 44]. Therefore, G11-iRGD may be a codelivery system than G11 and iRGD. TN11 is a human antibody fragment antibody (scFV) that only recognizes TNC-C [45]. However, to the best of our knowledge, the role of G11-iRGD, iRGD, and TN11 in atherosclerosis has not been investigated. No G11-iRGD or TN11 conjugate agents were investigated in clinical trials. More studies are needed to confirm the development value of G11-iRGD and TN11.
PL1 (sequence: PPRRGLIKLKTS), a peptide, recognizes not only TNC-c but also Extra Domain-B (EDB) of fibronectin (FN), which plays a key role in atherosclerosis development [46]. However, FN is a double-edged sword in atherosclerosis. FN exhibited an anti-atherosclerotic effect by preventing plaque rupture and vascular occlusion by promoting the formation of thick fiber caps, while FN exhibited a proatherogenic effect by promoting lipoprotein retention and inflammatory cell infiltration by expanding the ECM [47-49]. Interestingly, FN was also a promising delivery vector for atherosclerosis-targeted drugs, such as TPTS/C/T. TPTS/C/T is a simvastatin nanoprodrug (TPTS) with ROS-responsive cleavage properties and a fibronectin-targeted system. TPTS/C/T could codeliver the simvastatin prodrug and ticagrelor. TPTS/C/T exhibited stronger anti-inflammatory and antioxidant effects than free simvastatin by decreasing the M1-type polarization of macrophages, intracellular reactive oxygen species (ROS), and proinflammatory cytokines, such as IL-1β, MCP-1, and TNFα levels. In the apoE-/- mouse model of atherosclerosis, TPTS/C/T exhibited good synergistic therapy, anti-atherosclerosis effects, and biosafety by targeting the release of simvastatin and ticagrelor in plaques [50]. In addition, L19 is a human recombinant antibody that specifically recognizes FN-EDB. L19 exhibited similar results with G11 in atherosclerotic plaques in apoE-/- mice [40]. More importantly, many L19 antibody cytokine fusion proteins are currently being investigated in clinical trials, such as L19-IL2 (also named Darleukin) [51, 52], L19-TNF (also named Fibromun) [52, 53], and NidlegyTM (combination of L19-IL2 and L19-TNF) (Table 1) [52, 54]. Several L19 antibody cytokine fusion proteins were also investigated in preclinical trials, such as L19-TNF-IL2 (also named Tripokin) [55], L19-IFNγ (also named L19-IFNγ KRG) [56], L19-IL12 [57], and L19-IL15 [58, 59]. Therefore, PL1 may be a codelivery system other than G11, FN, and L19 because it specifically recognizes TNC-C and FN-EDB. However, the role of PL1 in atherosclerosis has not been investigated. No PL1 conjugate agents have been investigated in clinical trials. More studies are needed to confirm the development value of PL1.
PL3 (sequence: AGRGRLVR), a peptide, recognizes not only the C domain of TNC but also neuropilin-1 (Nrp1) [60]. Nrp1, a type I transmembrane protein, is also a double-edged sword in atherosclerosis. Nrp1 from T cells promoted atherosclerosis development by promoting the recruitment of CD4 T cells into the aorta [61]. Nrp1 suppressed leukocyte rolling and atherosclerotic plaque size by reducing proinflammatory cytokine and adhesion molecule levels by interacting with VE-cadherin and transforming growth factor-β (TGF-β) receptor II (TGFBR2) [62]. In addition, the role of PL3 in atherosclerosis has not been investigated. No PL3 conjugate agents have been investigated in clinical trials. More studies are needed to confirm the development value of PL3.
The structure and location of TNC agents. BCAN, bidirectional correct attention network; CSPG4, chondroitin sulfate proteoglycan 4; FABP7, fatty acid binding protein 7; FN-EDB, Extra Domain-B of fibronectin; IGF2BP3, insulin-like growth factor 2 mRNA binding protein 3; NLGN4X, neuroligin 4 X-linked; NRCAM, neuronal cell adhesion molecule; PTPRZ1, protein tyrosine phosphatase receptor type Z1; TNC, tenascin C; T1/2, half-life. The information was taken and modified from Refs [15, 40, 42, 46, 63, 65, 69, 74].
Clinical trials of iRGD, L19-IL2, L19-TNF, the combination of L19-IL2 and L19-TNF, and F16IL2.
| Name | Combination | Disease | Status | Refs/ClinicalTrials |
|---|---|---|---|---|
| iRGD | Paclitaxel and gemcitabine | Advanced metastatic pancreatic ductal adenocarcinoma | Phase 1/2 (Recruiting on 13 January 2023) | NCT05052567 |
| FOLFIRINOX and panitumumab | Pancreatic, colon, and appendiceal cancers | Phase 1/2 (Recruiting on 24 November 2021) | NCT05121038 | |
| Gemcitabine and nab-paclitaxel | Metastatic pancreatic cancer | Phase 1 (Completed on 6 July 2022) | [43, 44] | |
| Untreated metastatic pancreatic cancer | Phase 2 (Recruiting on 26 January 2023) | NCT05042128 | ||
| Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) | Advanced breast or pancreatic cancer with metastases to the liver or lung | Phase 1 (Withdrawn on 30 May 2014) | NCT01741597 | |
| L19-IL2 | Radiation | Non-Small Cell Lung Cancer | Phase 2 (Recruiting on 20 October 2020) | NCT03705403 |
| L19-TNF | Doxorubicin | 1st line Soft-Tissue Sarcoma | Phase 3 (Recruiting on 23 December 2022) | NCT04650984 |
| Doxorubicin | 1st line Leiomyosarcoma | Phase 2 (Recruiting on 14 April 2022) | NCT03420014 | |
| Dacarbazine | Pretreated Soft-Tissue Sarcoma | Phase 2 (Recruiting on 23 December 2022) | NCT04733183 | |
| Lomustine | Glioblastoma | Phase 1 (Not yet recruiting on 20 April 2022) | NCT05304663 | |
| Glioblastoma | Phase 1/2 (Recruiting on 22 June 2023) | NCT04573192 | ||
| Radiation and Temozolomide | Glioblastoma | Phase 1/2 (Recruiting on 14 April 2022) | NCT04443010 | |
| Alone | Grade III/IV Glioma | Phase 1/2 (Completed on 29 June 2023) | NCT03779230 | |
| Combination of L19-IL2 and L19-TNF | Surgery | Stage III B/C Melanoma | Phase 3 (Recruiting on 11 April 2022) | NCT02938299 |
| Surgery and adjuvant therapy | Stage III B/C Melanoma | Phase 3 (Recruiting on 22 June 2023) | NCT03567889 | |
| Alone | Basket of Non-Melanoma Skin Cancers | Phase 2 (Recruiting on 20 April 2022) | NCT04362722 | |
| Alone | Skin Cancer | Phase 2 (Recruiting on 22 June 2023) | NCT05329792 | |
| F16IL2 | Nivolumab | Non-small Cell Lung Cancer (NSCLC) | Phase 1/2 (Active, not recruiting on 21 July 2022) | NCT05468294 |
| Cytarabine | Acute Myeloid Leukemia (AML) | Phase 1/2 (Active, not recruiting on 20 April 2022) | NCT02957032 | |
| Paclitaxel | Merkel Cell Carcinoma | Phase 1/2 (Terminated due to lack of enrollment on 18 May 2018) | NCT02054884 | |
| BI 836858 | AML Relapse | Phase 1 (Completed on 20 April 2022) | NCT03207191 | |
| Doxorubicin | Solid Tumor | Phase 1/2 (Terminated on 25 February 2014) | NCT01131364 | |
| Paclitaxel | Solid Tumor | Phase 1/2 (Completed on 15 April 2022) | NCT01134250 |
3.2 F16
F16 is a fully human monoclonal antibody that only recognizes TNC A1 domain//isoform (TNC-A1) [63]. F16 was investigated in atherosclerosis in clinical trials [64]. F16 could bind to macrophages, blood vessels, and proliferating cells in human atherosclerotic plaque active areas but not to normal arteries and resting plaque areas (28 atherosclerotic plaques and 11 normal arteries), suggesting that the F16 antibody has strong specificity and selectivity for active plaques and may be a strong candidate for plaque imaging radiopharmaceuticals [64]. Interestingly, the F16 antibody cytokine fusion protein F16IL2 (also named Teleukin) was investigated in clinical trials (Table 1). Therefore, F16 is a promising delivery vector for atherosclerosis-targeted drugs. However, in our capacity, we did not find any information about the F16 conjugate agents in atherosclerosis.
3.3 FNLM
FH (sequences, FHKHKSPALSPV) is a peptide that recognizes the large isoform of TNC. TNC is highly expressed in some inflammatory conditions, such as MI injury, but is sparsely expressed in normal adult myocardium. FNLM-miR consists of a hybrid membrane shell and MSNs-miR. The hybrid membrane shell is an artificial lipid membrane modified with FH peptide and fused with neutrophil membrane proteins (NMPs). MSNs-miR are mesoporous silica nanoparticles (MSNs) loaded with miR-1, miR-133a, miR-208, and miR-499 (miRCombo). FNLM-miR delivered more miRCombo into cardiac fibroblasts (CFs) in the injured heart to induce reprogramming into induced cardiomyocyte-like cells (iCMs). FNLM-miR reduced the expression of multiple cytokines, including CCR2, CXCR1, CXCR2, LFA-1, IL-1β, and IL-6. FNLM-miR also increased the expression of sarcomere-related genes, such as Myh6 and Tnnis, transcription factor-related genes, such as Gata4, Mef2c, and Tbx5, and ion channel-related genes, such as Kcnj2 and Scn5a. FNLM-miR (I.V.) induced reprogramming to improve cardiac function and alleviate fibrosis by delivering miRCombo into fibroblasts in a myocardial ischemia/reperfusion (MIR) injury mouse model. FNLM-miR has good safety, long blood circulation, and a fine fibroblast targeting profile in this model [65]. These results suggest that FNLM-miR could deliver miRCombo into the cardiovascular system. FNLM is a promising delivery vector for atherosclerosis-targeted drugs. No FNLM conjugate agents have been investigated in clinical trials. More studies are needed to confirm the development value of FNLM.
4. Clinical advances in TNC-specific agents
4.1 ATN-RNA
ATN-RNA, an RNA interference (RNAi), is a double-stranded RNA (dsRNA) that targets TNC. ATN-RNA inhibited TNC synthesis in vivo [66, 67]. ATN-RNA (injection into the brain after resection) for the treatment of brain tumors (N=46) inhibited tumor growth and disease recurrence. ATN-RNA prolonged the survival period of patients and improved the quality of life [67-69]. The median overall survival (OS) of patients treated with ATN-RNA was 106.6 weeks and was higher than that of the control group (48.2 weeks). The longest survival was 180.9 weeks (Table 2) [69]. These results support the continued development of ATN-RNA. However, the role of ATN-RNA in atherosclerosis was not investigated. Of note, the development of ATN-RNA has obtained patent protection, such as US8946400 (B2), EP2121927 (B1), US2010076053 (A1), EP2121927 (A2), WO2008016317 (A3), WO2008016317 (A2), and WO2008016317. However, since the last report of clinical results (2010), we have not found any clinical reports or clinical trials.
4.2 IMA950
IMA950, a multipeptide glioblastoma vaccine, contains 20 antigens from multiple protein-derived peptides, such as TNC, 9 HLA-A*0201-restricted peptides, 2 HLA class II (DR)-binding peptides (stimulated CD8+ and CD4+ T-cell response), HLA-A2-restricted peptides (immunopotency marker), bidirectional correct attention network (BCAN), chondroitin sulfate proteoglycan 4 (CSPG4), fatty acid binding protein 7 (FABP7), insulin-like growth factor 2 mRNA binding protein 3 (IGF2BP3), neuroligin 4 X-linked (NLGN4X), neuronal cell adhesion molecule (NRCAM), and protein tyrosine phosphatase receptor type Z1 (PTPRZ1) [70-75]. IMA950 promoted the expression of BCAN, CSPG4, IGF2BP3, PTPRZ1, and TNC mRNA and protein in tumor samples from grade II and III glioma (49 grade II and 41 grade III astrocytoma, 30 grade II and 27 grade III oligodendroglioma, and 12 ependymoma). After vaccination, 100% of Grade II and 71% of Grade III patients had spontaneous antigen-specific T-cell responses. These patients showed a better T-cell response, suggesting that IMA950 improved the response to T-cell therapy [72]. IMA950 in combination with granulocyte-macrophage colony-stimulating factor (GM-CSF) exhibited safety, tolerance, and immunogenicity in HLA-A*02-positive glioblastoma patients (N=45) in phase 1 clinical trials (intradermally). Ninety percent of patients (36/40) had tumor-associated peptides (TUMAP). Multi-TUMAP responders were 50%. The progression-free survival (PFS) at 6 months and 9 months was 74.4% and 30.8%, respectively. The disease stabilization rate at 40 weeks was 28.2% (11/39). The median OS was 15.3 months [73]. IMA950 in combination with poly-ICLC (a synthetic TLR3 ligand) exhibited safety, tolerance, and immunogenicity in malignant astrocytoma patients (16 glioblastomas and 3 grade III astrocytoma) in phase 1/2 clinical trials (intradermally, intramuscularly, or subcutaneously). The median OS and disease control rates for all patients were 19 months (21 months after surgery) and 42%, respectively, including 17 months (19 months after surgery) and 31.2% of glioblastoma patients. The PFS for all patients and glioblastoma patients was 9 months and 9 months, respectively, while it was 10 months and 9.5 months after surgery, respectively. The mode of administration had no significant effect on OS and PFS [74]. Bevacizumab (BEV), a monoclonal anti-VEGF-A IgG1 antibody, was approved for the treatment of multiple cancers, such as cervical cancer, colorectal cancer, glioblastoma, glioma, liver cancer, NSCLC, ovarian cancer, and renal cell carcinoma. However, IMA950/poly-ICLC did not improve the response rate, median OS, or median PFS of BEV for the treatment of relapsing high-grade glioma patients (vaccinated=16, nonvaccinated=40) [75]. These novel results support the continued development of IMA950, except in combination with BEV. Indeed, many clinical trials on IMA950 are ongoing (Table 2). However, the further development of IMA950 in glioblastoma was discontinued on 23 September 2020 [76].
5. Several issues of concern
TNC, especially TNC-C, is a promising target and delivery vector for drug development and atherosclerosis-targeted drugs. However, several issues of concern need to be noted. (1) Anti-TNC-USPIO, ultrasmall superparamagnetic iron oxide (USPIO) nanoparticle-labeled mouse anti-TNC monoclonal antibody, is a promising molecular imaging tool for detecting and monitoring atherosclerotic plaques by magnetic resonance imaging (MRI) in preclinical trials [23, 77]. However, the role of anti-TNC-USPIO in clinical trials has not been investigated. (2) Many TNC domains/isoforms were investigated in atherosclerosis, such as EGF-like, A1, A2, FBG, C, and TnfnIII 1-5 domains. However, there are many domains/isoforms that have not been investigated. (3) Targeting TNC-C, such as G11, G11-iRGD, TN11, PL1, and PL3, is a promising delivery vector for atherosclerosis-targeted agents. However, no conjugate agents have been investigated in clinical trials. The role of G11-iRGD, TN11, PL1, and PL3 in atherosclerosis is also not unclear. (4) TTA1, a 13-kDa oligonucleotide (39-mer) aptamer, could recognize the TNC fibrinogen-like domain. TTA1 could target delivery to the ECM of atherosclerotic lesions and tumors [78]. Therefore, TTA1 is a promising delivery vector for atherosclerosis-targeted agents.
The clinical trials and results of TNC agents ATN-RNA and IMA950. GM-CSF, granulocyte-macrophage colony-stimulating factor; OS, overall survival; PFS, progression-free survival; TUMAP, tumor-associated peptide.
| Name | Instructions | Diseases | Phase | Results | Developers | Refs | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| ATN-RNA | RNA interference | Brain tumors | Unknown | Toxicity | Safety | Institute of Bioorganic Chemistry of the Polish Academy of Sciences | [69] | |||
| Efficacy | Median OS | 106.6 weeks | ||||||||
| IMA950 | Multipeptide vaccine | Glioblastoma | Unknown | Efficacy | Antigen-specific T-cell responses | Grade II glioma | 100% (79/79) | Immatics Biotechnologies GmbH, Geneva University Hospital | [72] | |
| Grade III glioma | 71% (48/68) | |||||||||
| HLA-A*02-positive glioblastoma | Phase 1 (combination GM-CSF) | Toxicity | Safety | Cancer Research UK, Immatics Biotechnologies GmbH | [73] | |||||
| Efficacy | TUMAP | 90% (36/40) | ||||||||
| Median OS | 15.3 months | |||||||||
| Malignant astrocytoma | Phase 1 (combination poly-ICLC) | Toxicity | Safety | Immatics Biotechnologies GmbH, Geneva University Hospital | [74] | |||||
| Efficacy | Median OS (after surgery) | 21 months | ||||||||
| Disease control rate | 42% | |||||||||
| PFS (after surgery) | 10 months | |||||||||
| Relapsing high-grade glioma | Phase 1 (in combination with Bevacizumab and poly-ICLC) | IMA950/poly-ICLC did not improve the therapeutic effect of Bevacizumab (vaccinated=16, nonvaccinated=40) | Immatics Biotechnologies GmbH, Geneva University Hospital | [75] | ||||||
| Pembrolizumab and Poly-ICLC | Relapsing Glioblastoma | Phase 1/2 (Active, not recruiting on 27 December 2022) | Geneva University Hospital | NCT03665545 | ||||||
| Varlilumab (also named CDX-1127) and Poly-ICLC | WHO Grade II Low-Grade Glioma (LGG) | Phase 1/2 (Active, not recruiting on 27 March 2023) | Nicholas Butowski, University of California, San Francisco, Celldex Therapeutics | NCT02924038 | ||||||
| Cyclophosphamide, Imiquimod, and GM-CSF | Glioblastoma | Phase 1 (Terminated due to poor accrual. on 19 May 2014) | Immatics Biotechnologies GmbH, National Cancer Institute | NCT01403285 | ||||||
| Temozolomide and Radiation Therapy | Newly Diagnosed Glioblastoma Multiforme | Phase 1 (Completed on 14 October 2015) | Unknown | Cancer Research UK, Immatics Biotechnologies GmbH | NCT01222221 | |||||
However, no TTA1 conjugate agents have been investigated. The role of TTA1 in atherosclerosis is also not unclear. (5) IL-2 not only plays a key role in cancer but also in atherosclerosis [79-83]. F16IL2 can deliver IL-2 to tumor tissue very well. F16 is a promising delivery vector for atherosclerosis-targeted agents. However, the role of F16IL2 in atherosclerosis has not been investigated. (6) Knockout and knockdown of TNC in vivo may suppress atherosclerosis development, suggesting that ATN-RNA may suppress atherosclerosis development by reducing TNC expression in vivo. However, the role of ATN-RNA in atherosclerosis has not been investigated. (7) The low uptake of ATN-RNA in cells weakens its anticancer ability and requires delivery vectors to enhance its uptake [84]. Magnetic nanoparticles coated with polyethyleneimine (PEI) could improve the delivery of ATN-RNA in human U-118 MG cell lines [84, 85]. However, the role of this nanomediated delivery of ATN-RNA in vivo is unclear. (8) TNC monoclonal antibodies may improve the delivery of ATN-RNA. However, attention should be given to cross-reactions within the TNC gene. (9) ATN-RNA also suppressed MDA-MB-231 breast cancer cell proliferation, migration, and adhesion in vitro [86]. However, the role of ATN-RNA in suppressing breast cancer in vivo is unclear. (10) TNC is one of the antigens of the IMA950 vaccine. However, to the best of our knowledge, the role of TNC in IMA950 has not been investigated. (11) The investigation of the reasons for stopping the development of IMA950 is of great significance for restarting its development. Of note, GM-CSF and poly-ICLC were used as adjuvants for the IMA950 vaccine. However, these adjuvants are only ongoing in clinical research and are not found in marketed vaccines. Many adjuvants are approved for use in marketed vaccines, such as AS01 (TLR4 and NLRP3 agonist), AS03 (apoptosis-associated speck-like protein (ASC) agonist), AS04 (TLR4 and NLRP3 agonist), CpG ODN 1018 (TLR9 agonist), and MF59 (apoptosis-associated speck-like protein (ASC) agonist) [87-90]. These adjuvants may be more suitable for the development of the IMA950 vaccine.
6. Conclusions
The pro-atherosclerosis or anti-atherosclerosis effect of TNC depended on its location. TNC was overexpressed in blood and atherosclerotic plaque active areas from patients with CHD. However, its expression is virtually undetectable in most normal adult tissues, especially TNC-C. Targeting TNC-C with G11, G11-iRGD, TN11, PL1, and PL3 is a promising delivery vector for atherosclerosis-targeted drugs. In particular, G11-iRGD and PL1 have great development value because they contain or recognize components of clinical studies. F16 and FNLM are also targeted drug delivery vectors with great development value due to their conjugate agents being investigated in preclinical and clinical trials, especially F16 (clinical trials). Targeting TNC is a promising target for drug development due to the specific agents ATN-RNA and IMA950 being investigated in clinical trials. With the deepening of research and cooperation in scientific research, it is believed that more scientists will develop more therapeutic (conjugate and/or specific agents), vaccine, and diagnostic agents using TNC as a delivery vector or target.
Acknowledgements
We thank Dr. Dongming Xing's laboratory for technical support and stimulating discussions.
Funding
The authors are grateful for financial support from the Qingdao Major Scientific and Technological Project for Distinguished Scholars (20170103), the Laoshan Major Scientific and Technological Project for Distinguished Scholars (20181030), and the Natural Science Foundation of Shandong Province (ZR2020MH369).
Author contributions
Yanhong Wang, Chunling Ren, Chao Wang, Jiyao Xing, and Jiazhen Xu participated in the literature search, outline design, and manuscript writing. Sha Yu, Saisai Yan, Tingting Zhang, and Qian Li contributed substantially to the discussion of its content. Dongming Xing introduced the central concept of this review and elaborated on the overall framework of the review. Wujun Chen, Daijun Zhang, and Dongming Xing provided guidance and supervision throughout the writing process, reviewed and revised the manuscript, and provided critical input and expertise. Wujun Chen, Yingchun Shao, Xiaojin Peng, and Renshuai Zhang reviewed and edited the manuscript before submission.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Doran AC. Inflammation Resolution: Implications for Atherosclerosis. Circ Res. 2022;130:130-48
2. Fan J, Watanabe T. Atherosclerosis: Known and unknown. Pathol Int. 2022;72:151-60
3. Kong P, Cui ZY, Huang XF, Zhang DD, Guo RJ, Han M. Inflammation and atherosclerosis: signaling pathways and therapeutic intervention. Signal Transduct Target Ther. 2022;7:131
4. Attardo S, Musumeci O, Velardo D, Toscano A. Statins Neuromuscular Adverse Effects. Int J Mol Sci. 2022 23
5. Ward NC, Watts GF, Eckel RH. Statin Toxicity. Circ Res. 2019;124:328-50
6. Gunta SP, O'Keefe JH, O'Keefe EL, Lavie CJ. PCSK9 inhibitor, ezetimibe, and bempedoic acid: Evidence-based therapies for statin-intolerant patients. Prog Cardiovasc Dis. 2023
7. Paraskevas KI, Gloviczki P, Antignani PL, Comerota AJ, Dardik A, Davies AH. et al. Benefits and drawbacks of statins and non-statin lipid lowering agents in carotid artery disease. Prog Cardiovasc Dis. 2022;73:41-7
8. Ekladious I, Colson YL, Grinstaff MW. Polymer-drug conjugate therapeutics: advances, insights and prospects. Nat Rev Drug Discov. 2019;18:273-94
9. Fu Z, Li S, Han S, Shi C, Zhang Y. Antibody drug conjugate: the "biological missile" for targeted cancer therapy. Signal Transduct Target Ther. 2022;7:93
10. Yang Y, Wang S, Ma P, Jiang Y, Cheng K, Yu Y. et al. Drug conjugate-based anticancer therapy - Current status and perspectives. Cancer Lett. 2023;552:215969
11. Berdel AF, Ruhnke L, Angenendt L, Wermke M, Rollig C, Mikesch JH. et al. Using stroma-anchoring cytokines to augment ADCC: a phase 1 trial of F16IL2 and BI 836858 for posttransplant AML relapse. Blood Adv. 2022;6:3684-96
12. Catania C, Maur M, Berardi R, Rocca A, Giacomo AM, Spitaleri G. et al. The tumor-targeting immunocytokine F16-IL2 in combination with doxorubicin: dose escalation in patients with advanced solid tumors and expansion into patients with metastatic breast cancer. Cell Adh Migr. 2015;9:14-21
13. Schliemann C, Kessler T, Berdel AF, Hemmerle T, Angenendt L, Altvater B. et al. Phase I study of F16IL2 antibody-cytokine fusion with very low-dose araC in acute myeloid leukaemia relapse after allogeneic stem cell transplantation. Br J Haematol. 2021;192:e148-e51
14. Marlind J, Kaspar M, Trachsel E, Sommavilla R, Hindle S, Bacci C. et al. Antibody-mediated delivery of interleukin-2 to the stroma of breast cancer strongly enhances the potency of chemotherapy. Clin Cancer Res. 2008;14:6515-24
15. Brack SS, Silacci M, Birchler M, Neri D. Tumor-targeting properties of novel antibodies specific to the large isoform of tenascin-C. Clin Cancer Res. 2006;12:3200-8
16. De Laporte L, Rice JJ, Tortelli F, Hubbell JA. Tenascin C promiscuously binds growth factors via its fifth fibronectin type III-like domain. PLoS One. 2013;8:e62076
17. Giblin SP, Midwood KS. Tenascin-C: Form versus function. Cell Adh Migr. 2015;9:48-82
18. Shao H, Kirkwood JM, Wells A. Tenascin-C Signaling in melanoma. Cell Adh Migr. 2015;9:125-30
19. Wang Y, Wang G, Liu H. Tenascin-C: A Key Regulator in Angiogenesis during Wound Healing. Biomolecules. 2022 12
20. Petronzelli F, Pelliccia A, Anastasi AM, D'Alessio V, Albertoni C, Rosi A. et al. Improved tumor targeting by combined use of two antitenascin antibodies. Clin Cancer Res. 2005;11:7137s-45s
21. Golledge J, Clancy P, Maguire J, Lincz L, Koblar S. The role of tenascin C in cardiovascular disease. Cardiovasc Res. 2011;92:19-28
22. Wallner K, Shah PK, Sharifi BG. Balloon catheterization induces arterial expression of new Tenascin-C isoform. Atherosclerosis. 2002;161:75-83
23. Meng H, Ma ZL, Yan HL, Chen XX, Shang SA, Yu J. et al. [Feasibility of targeted magnetic resonance imaging on visualizing tenascin-C expression in atherosclerosis plaque in high-fat diet fed ApoE(-/-) mice]. Zhonghua Xin Xue Guan Bing Za Zhi. 2016;44:342-7
24. Imanaka-Yoshida K. Tenascin-C in cardiovascular tissue remodeling: from development to inflammation and repair. Circ J. 2012;76:2513-20
25. Gao W, Li J, Ni H, Shi H, Qi Z, Zhu S. et al. Tenascin C: A Potential Biomarker for Predicting the Severity of Coronary Atherosclerosis. J Atheroscler Thromb. 2019;26:31-8
26. Mehri H, Aslanabadi N, Nourazarian A, Shademan B, Khaki-Khatibi F. Evaluation of the serum levels of Mannose binding lectin-2, tenascin-C, and total antioxidant capacity in patients with coronary artery disease. J Clin Lab Anal. 2021;35:e23967
27. Ozmen Yildiz P, Yildiz I, Ozmen C, Karabacak M, Doven O. Relation between coronary artery calcium score and serum tenascin-C level in patients without known coronary artery disease. Acta Cardiol. 2015;70:633-9
28. Yang JH, Ren F. Clinical implications of tenascin-C and OX40 ligand in patients with acute coronary syndrome. Biomed Rep. 2014;2:132-6
29. Minear MA, Crosslin DR, Sutton BS, Connelly JJ, Nelson SC, Gadson-Watson S. et al. Polymorphic variants in tenascin-C (TNC) are associated with atherosclerosis and coronary artery disease. Hum Genet. 2011;129:641-54
30. Pawitan JA. Weighting the potential of using tenascin C in diagnosis and therapy of atherosclerosis. Acta Med Indones. 2010;42:104-7
31. Liu R, He Y, Li B, Liu J, Ren Y, Han W. et al. Tenascin-C produced by oxidized LDL-stimulated macrophages increases foam cell formation through Toll-like receptor-4. Mol Cells. 2012;34:35-41
32. Luo H, Wang J, Qiao C, Zhang X, Zhang W, Ma N. ATF3 Inhibits Tenascin-C-induced Foam Cell Formation in LPS-Stimulated THP-1 Macrophages by Suppressing TLR-4. J Atheroscler Thromb. 2015;22:1214-23
33. Wang Z, Wei Q, Han L, Cao K, Lan T, Xu Z. et al. Tenascin-c renders a proangiogenic phenotype in macrophage via annexin II. J Cell Mol Med. 2018;22:429-38
34. Schaff M, Receveur N, Bourdon C, Wurtz V, Denis CV, Orend G. et al. Novel function of tenascin-C, a matrix protein relevant to atherosclerosis, in platelet recruitment and activation under flow. Arterioscler Thromb Vasc Biol. 2011;31:117-24
35. Shi X, Zhu S, Liu M, Stone SS, Rong Y, Mao K. et al. Single-Cell RNA-Seq Reveals a Population of Smooth Muscle Cells Responsible for Atherogenesis. Aging Dis. 2022;13:1939-53
36. Wallner K, Li C, Shah PK, Wu KJ, Schwartz SM, Sharifi BG. EGF-Like domain of tenascin-C is proapoptotic for cultured smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:1416-21
37. Wang L, Wang W, Shah PK, Song L, Yang M, Sharifi BG. Deletion of tenascin-C gene exacerbates atherosclerosis and induces intraplaque hemorrhage in Apo-E-deficient mice. Cardiovasc Pathol. 2012;21:398-413
38. Wang L, Shah PK, Wang W, Song L, Yang M, Sharifi BG. Tenascin-C deficiency in apo E-/- mouse increases eotaxin levels: implications for atherosclerosis. Atherosclerosis. 2013;227:267-74
39. Song L, Wang L, Li F, Yukht A, Qin M, Ruther H. et al. Bone Marrow-Derived Tenascin-C Attenuates Cardiac Hypertrophy by Controlling Inflammation. J Am Coll Cardiol. 2017;70:1601-15
40. von Lukowicz T, Silacci M, Wyss MT, Trachsel E, Lohmann C, Buck A. et al. Human antibody against C domain of tenascin-C visualizes murine atherosclerotic plaques ex vivo. J Nucl Med. 2007;48:582-7
41. Wallner K, Li C, Shah PK, Fishbein MC, Forrester JS, Kaul S. et al. Tenascin-C is expressed in macrophage-rich human coronary atherosclerotic plaque. Circulation. 1999;99:1284-9
42. Lingasamy P, Laarmann AH, Teesalu T. Tumor Penetrating Peptide-Functionalized Tenascin-C Antibody for Glioblastoma Targeting. Curr Cancer Drug Targets. 2021;21:70-9
43. Dean A, Gill S, McGregor M, Broadbridge V, Jarvelainen HA, Price T. Dual alphaV-integrin and neuropilin-1 targeting peptide CEND-1 plus nab-paclitaxel and gemcitabine for the treatment of metastatic pancreatic ductal adenocarcinoma: a first-in-human, open-label, multicentre, phase 1 study. Lancet Gastroenterol Hepatol. 2022;7:943-51
44. Springfeld C, Neoptolemos JP. CEND-1: a game changer for pancreatic cancer chemotherapy? Lancet Gastroenterol Hepatol. 2022;7:900-2
45. Viale GL, Castellani P, Dorcaratto A, Pau A, Sehrbundt E, Siri A. et al. Occurrence of a glioblastoma-associated tenascin-C isoform in cerebral cavernomas and neighboring vessels. Neurosurgery. 2002;50:838-42 discussion 42
46. Lingasamy P, Posnograjeva K, Kopanchuk S, Tobi A, Rinken A, General IJ. et al. PL1 Peptide Engages Acidic Surfaces on Tumor-Associated Fibronectin and Tenascin Isoforms to Trigger Cellular Uptake. Pharmaceutics. 2021 13
47. Moore KJ, Fisher EA. The double-edged sword of fibronectin in atherosclerosis. EMBO Mol Med. 2012;4:561-3
48. Park JE, JebaMercy G, Pazhanchamy K, Guo X, Ngan SC, Liou KCK. et al. Aging-induced isoDGR-modified fibronectin activates monocytic and endothelial cells to promote atherosclerosis. Atherosclerosis. 2021;324:58-68
49. Rohwedder I, Montanez E, Beckmann K, Bengtsson E, Duner P, Nilsson J. et al. Plasma fibronectin deficiency impedes atherosclerosis progression and fibrous cap formation. EMBO Mol Med. 2012;4:564-76
50. Zhao R, Ning X, Wang M, Wang H, Xing G, Wang L. et al. A ROS-Responsive Simvastatin Nano-Prodrug and its Fibronectin-Targeted Co-Delivery System for Atherosclerosis Treatment. ACS Appl Mater Interfaces. 2022;14:25080-92
51. Philogen S.p.A. Darleukin, 2023. https://www.philogen.com/pipeline/darleukin/
52. Philogen S.p.A. Pipeline, 2023. https://www.philogen.com/pipeline/
53. Philogen S.p.A. Fibromun, 2023. https://www.philogen.com/pipeline/fibromun/
54. Philogen S.p.A. NidlegyTM, 2023. https://www.philogen.com/pipeline/nidlegytm/
55. Philogen S.p.A. Tripokin, 2023. https://www.philogen.com/pipeline/tripokin/
56. Di Nitto C, Gilardoni E, Mock J, Nadal L, Weiss T, Weller M. et al. An Engineered IFNgamma-Antibody Fusion Protein with Improved Tumor-Homing Properties. Pharmaceutics. 2023 15
57. Millul J, Krudewig C, Zana A, Dakhel Plaza S, Puca E, Villa A. et al. Immunotherapy with Immunocytokines and PD-1 Blockade Enhances the Anticancer Activity of Small Molecule-Drug Conjugates Targeting Carbonic Anhydrase IX. Mol Cancer Ther. 2021;20:512-22
58. Kaspar M, Trachsel E, Neri D. The antibody-mediated targeted delivery of interleukin-15 and GM-CSF to the tumor neovasculature inhibits tumor growth and metastasis. Cancer Res. 2007;67:4940-8
59. Mortensen MR, Mock J, Bertolini M, Stringhini M, Catalano M, Neri D. Targeting an engineered cytokine with interleukin-2 and interleukin-15 activity to the neovasculature of solid tumors. Oncotarget. 2020;11:3972-83
60. Puranen J, Korhonen S, Haugas M, Lingasamy P, Teesalu T, Subrizi A. et al. Intravitreal CendR peptides target laser-induced choroidal neovascularization sites in mice. J Control Release. 2023;360:810-7
61. Gaddis DE, Padgett LE, Wu R, Hedrick CC. Neuropilin-1 Expression on CD4 T Cells Is Atherogenic and Facilitates T Cell Migration to the Aorta in Atherosclerosis. J Immunol. 2019;203:3237-46
62. Bosseboeuf E, Chikh A, Chaker AB, Mitchell TP, Vignaraja D, Rajendrakumar R. et al. Neuropilin-1 interacts with VE-cadherin and TGFBR2 to stabilize adherens junctions and prevent activation of endothelium under flow. Sci Signal. 2023;16:eabo4863
63. Rondon A, Rouanet J, Degoul F. Radioimmunotherapy in Oncology: Overview of the Last Decade Clinical Trials. Cancers (Basel). 2021 13
64. Pedretti M, Rancic Z, Soltermann A, Herzog BA, Schliemann C, Lachat M. et al. Comparative immunohistochemical staining of atherosclerotic plaques using F16, F8 and L19: Three clinical-grade fully human antibodies. Atherosclerosis. 2010;208:382-9
65. Wang Q, Song Y, Chen J, Li Q, Gao J, Tan H. et al. Direct in vivo reprogramming with non-viral sequential targeting nanoparticles promotes cardiac regeneration. Biomaterials. 2021;276:121028
66. Zukiel R, Nowak S, Wyszko E, Rolle K, Gawronska I, Barciszewska MZ. et al. Suppression of human brain tumor with interference RNA specific for tenascin-C. Cancer Biol Ther. 2006;5:1002-7
67. Piwecka M, Rolle K, Wyszko E, Zukiel R, Nowak S, Barciszewska MZ. et al. Nucleic acid-based technologies in therapy of malignant gliomas. Curr Pharm Biotechnol. 2011;12:1805-22
68. Wyszko E, Rolle K, Nowak S, Zukiel R, Nowak M, Piestrzeniewicz R. et al. A multivariate analysis of patients with brain tumors treated with ATN-RNA. Acta Pol Pharm. 2008;65:677-84
69. Rolle K, Nowak S, Wyszko E, Nowak M, Zukiel R, Piestrzeniewicz R. et al. Promising human brain tumors therapy with interference RNA intervention (iRNAi). Cancer Biol Ther. 2010;9:396-406
70. Weller M, Roth P, Preusser M, Wick W, Reardon DA, Platten M. et al. Vaccine-based immunotherapeutic approaches to gliomas and beyond. Nat Rev Neurol. 2017;13:363-74
71. Dutoit V, Herold-Mende C, Hilf N, Schoor O, Beckhove P, Bucher J. et al. Exploiting the glioblastoma peptidome to discover novel tumour-associated antigens for immunotherapy. Brain. 2012;135:1042-54
72. Dutoit V, Migliorini D, Ranzanici G, Marinari E, Widmer V, Lobrinus JA. et al. Antigenic expression and spontaneous immune responses support the use of a selected peptide set from the IMA950 glioblastoma vaccine for immunotherapy of grade II and III glioma. Oncoimmunology. 2018;7:e1391972
73. Rampling R, Peoples S, Mulholland PJ, James A, Al-Salihi O, Twelves CJ. et al. A Cancer Research UK First Time in Human Phase I Trial of IMA950 (Novel Multipeptide Therapeutic Vaccine) in Patients with Newly Diagnosed Glioblastoma. Clin Cancer Res. 2016;22:4776-85
74. Migliorini D, Dutoit V, Allard M, Grandjean Hallez N, Marinari E, Widmer V. et al. Phase I/II trial testing safety and immunogenicity of the multipeptide IMA950/poly-ICLC vaccine in newly diagnosed adult malignant astrocytoma patients. Neuro Oncol. 2019;21:923-33
75. Boydell E, Marinari E, Migliorini D, Dietrich PY, Patrikidou A, Dutoit V. Exploratory Study of the Effect of IMA950/Poly-ICLC Vaccination on Response to Bevacizumab in Relapsing High-Grade Glioma Patients. Cancers (Basel). 2019 11
76. AdisInsight. IMA 950. 2021.
77. Li Y, Liu J, Huang JW, Song JC, Ma ZL, Shi HB. In vivo MRI detection of atherosclerosis in ApoE-deficient mice by using tenascin-C-targeted USPIO. Acta Radiol. 2018;59:1431-7
78. Hicke BJ, Marion C, Chang YF, Gould T, Lynott CK, Parma D. et al. Tenascin-C aptamers are generated using tumor cells and purified protein. J Biol Chem. 2001;276:48644-54
79. Li Y, Li X, Geng X, Zhao H. The IL-2A receptor pathway and its role in lymphocyte differentiation and function. Cytokine Growth Factor Rev. 2022;67:66-79
80. Munjal A, Khandia R. Atherosclerosis: orchestrating cells and biomolecules involved in its activation and inhibition. Adv Protein Chem Struct Biol. 2020;120:85-122
81. Yao X, Matosevic S. Chemokine networks modulating natural killer cell trafficking to solid tumors. Cytokine Growth Factor Rev. 2021;59:36-45
82. Zhao TX, Newland SA, Mallat Z. 2019 ATVB Plenary Lecture: Interleukin-2 Therapy in Cardiovascular Disease: The Potential to Regulate Innate and Adaptive Immunity. Arterioscler Thromb Vasc Biol. 2020;40:853-64
83. Zhou P. Emerging mechanisms and applications of low-dose IL-2 therapy in autoimmunity. Cytokine Growth Factor Rev. 2022;67:80-8
84. Grabowska M, Grzeskowiak BF, Rolle K, Mrowczynski R. Magnetic Nanoparticles as a Carrier of dsRNA for Gene Therapy. Methods Mol Biol. 2021;2211:69-81
85. Grabowska M, Grzeskowiak BF, Szutkowski K, Wawrzyniak D, Glodowicz P, Barciszewski J. et al. Nano-mediated delivery of double-stranded RNA for gene therapy of glioblastoma multiforme. PLoS One. 2019;14:e0213852
86. Wawrzyniak D, Grabowska M, Glodowicz P, Kuczynski K, Kuczynska B, Fedoruk-Wyszomirska A. et al. Down-regulation of tenascin-C inhibits breast cancer cells development by cell growth, migration, and adhesion impairment. PLoS One. 2020;15:e0237889
87. Zhao T, Cai Y, Jiang Y, He X, Wei Y, Yu Y. et al. Vaccine adjuvants: mechanisms and platforms. Signal Transduct Target Ther. 2023;8:283
88. Firdaus FZ, Skwarczynski M, Toth I. Developments in Vaccine Adjuvants. Methods Mol Biol. 2022;2412:145-78
89. Lee W, Suresh M. Vaccine adjuvants to engage the cross-presentation pathway. Front Immunol. 2022;13:940047
90. Reyes C, Patarroyo MA. Adjuvants approved for human use: What do we know and what do we need to know for designing good adjuvants? Eur J Pharmacol. 2023;945:175632
Author contact
![]() Corresponding authors: Cancer Institute, The Affiliated Hospital of Qingdao University, Qingdao University, Qingdao Cancer Institute, Qingdao, Shandong, 266071. E-mail addresses: 1. zhdj09edu.cn (Daijun Zhang), 2. xdm_tsinghuacom (Dongming Xing). Tel: 86-532- 82991017, Fax: 86-532- 82991017.
Corresponding authors: Cancer Institute, The Affiliated Hospital of Qingdao University, Qingdao University, Qingdao Cancer Institute, Qingdao, Shandong, 266071. E-mail addresses: 1. zhdj09edu.cn (Daijun Zhang), 2. xdm_tsinghuacom (Dongming Xing). Tel: 86-532- 82991017, Fax: 86-532- 82991017.

 Global reach, higher impact
Global reach, higher impact