10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2024; 20(1):280-295. doi:10.7150/ijbs.87679 This issue Cite
Review
The biological mechanism and emerging therapeutic interventions of liver aging
1. Division of Hepatobiliary and Pancreatic Surgery, Department of Surgery, First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou 310003, China.
2. NHC Key Laboratory of Combined Multi-organ Transplantation, Hangzhou 310003, China.
3. Key Laboratory of the diagnosis and treatment of organ Transplantation, Research Unit of Collaborative Diagnosis and Treatment for Hepatobiliary and Pancreatic Cancer, Chinese Academy of Medical Sciences (2019RU019), Hangzhou 310003, China.
4. Key Laboratory of Organ Transplantation, Research Center for Diagnosis and Treatment of Hepatobiliary Diseases, Zhejiang Province, Hangzhou 310003, China.
5. Shulan International Medical College, Zhejiang Shuren University, Hangzhou 310015, Zhejiang, China.
6. Shulan (Hangzhou) Hospital Affiliated to Zhejiang Shuren University Shulan International Medical College, Hangzhou 310000, China.
#These authors contributed equally to this work.
Received 2023-7-1; Accepted 2023-10-22; Published 2024-1-1
Abstract
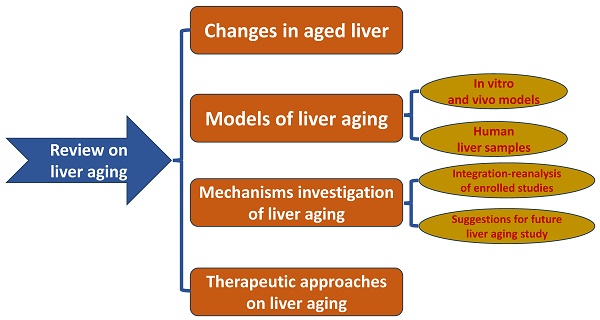
Research on liver aging has become prominent and has attracted considerable interest in uncovering the mechanism and therapeutic targets of aging to expand lifespan. In addition, multi-omics studies are widely used to perform further mechanistic investigations on liver aging. In this review, we illustrate the changes that occur with aging in the liver, present the current models of liver aging, and emphasize existing multi-omics studies on liver aging. We integrated the multi-omics data of enrolled studies and reanalyzed them to identify key pathways and targets of liver aging. The results indicated that C-X-C motif chemokine ligand 9 (Cxcl9) was a regulator of liver aging. In addition, we provide a flowchart for liver aging research using multi-omics analysis and molecular experiments to help researchers conduct further research. Finally, we present emerging therapeutic treatments that prolong lifespan. In summary, using cells and animal models of liver aging, we can apply a multi-omics approach to find key metabolic pathways and target genes to mitigate the adverse effects of liver aging.
Keywords: Liver aging, Multi-omics analysis, Model of liver aging, Therapeutic approach.
Background
Senescent cells experience growth arrest, increased autophagy, metabolic reprogramming, the implementation of a complex proinflammatory secretome, and chromatin remodeling [1]. Hayflick et al. first described cellular senescence 60 years ago when they discovered that human diploid fibroblasts have a limited replicative potential in culture, after which they enter a state of irreversible replicative arrest [2]. This phenomenon was labeled replicative senescence, and it is related to the constant shortening of telomere DNA during cell division. Finally, cell division is precluded. This is known as the “telomere theory of senescence” [3]. In addition, cyclin-dependent kinase inhibitor 2A (P16) and tumor protein p53 (P53) are upregulated, and the cell cycle-promoting genes E2F transcription factor 8 (E2F8) and transcription factor Dp-1 (TFDP1) are downregulated, in senescent cells [4, 5].
The aged liver exhibits many changes in morphology and structure [6]. Macroscopically, the aged liver is reduced in size and blood flow [7, 8]. Among hepatocytes, the smooth endoplasmic reticulum is lost, the number of mitochondria is reduced together with an increase in volume, the volume of the dense body compartment is enlarged, and polyploidy is enhanced [9]. Biological processes are changed in aged livers, leading to the development of age-associated liver diseases. The incidence of high triglyceride levels, nonalcoholic fatty liver diseases, and hepatocellular carcinoma increases with liver aging [10-12]. However, the most dramatic effect of liver aging is its compromised ability to regenerate after loss of mass from surgical or chemical injury.
Various kinds of stress responses, such as the unfolded protein response and responses to reactive oxygen species (ROS), DNA-damaging agents, and radiation, are all contributors to cell senescence [3, 13]. As such, there exist some methods that can induce aging, including D-galactose (D-gal) injection, hydrogen peroxide (H2O2) induction, and X-ray induction, which can commonly be used to induce cell or rat aging [14, 15]. Azman et al. summarized the effects of D-gal on liver aging and its underlying mechanisms, including ROS generation, oxidative stress induction, and an increased inflammatory response, which in turn lead to mitochondrial dysfunction, apoptosis, and hepatic structural damage [16].
Currently, liver transplantation (LT) is considered one of the most important curative treatments for end-stage liver disease. In light of the growing number of patients on the waiting list for LT, the use of marginal grafts has also been maximized to expand the donor pool [17]. Hence, an increasing number of grafts from older donors are being used in LT. However, the antioxidant and regenerative abilities decline with liver aging, livers from old donors suffer more severe ischemia-reperfusion injury (IRI), and the recovery ability of the liver after transplantation is poor, which affects the success of LT. Hence, advanced donor age is a contributor to poor quality of the liver, and there is a high incidence of graft loss and complications, including ischemic-type biliary complications and hepatic artery thrombosis, following LT from older donors [18]. Hence, it is necessary for clinicians to discover new therapeutic interventions to combat liver aging that remove or mitigate the known adverse effects to improve graft survival after LT.
In this manuscript, we discuss liver aging in detail and introduce the present models for mimicking the aged liver. We also summarize the mechanisms and pathways of liver aging and provide insights into the study of liver aging using multi-omics analysis and molecular experiments based on models of liver aging. Finally, we review the therapeutic interventions reported by published articles. The flowchart of the study is in Figure 1. We hope that this review can help researchers design antiaging therapies to improve the quality of the aged liver.
Flow chart of the study design.
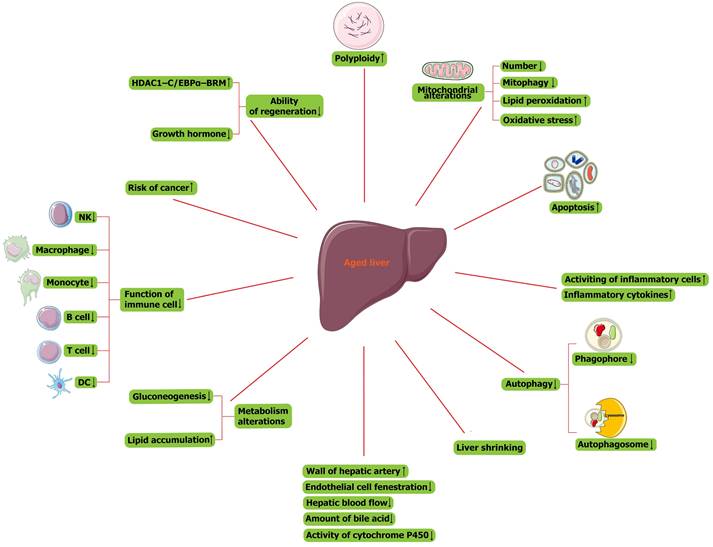
Main changes in the liver with physiological aging. NK cell, natural killer cell; DC, dendritic cell; HDAC1, histone deacetylase 1; C/EBPα, CCAAT/enhancer-binding protein (C/EBP) α; BRM, SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily a, member 2.
Main text
What is liver aging?
The signature of cellular senescence includes the presence of permanent cell cycle arrest, acquisition of major morphological changes, expression of senescence-associated beta-galactosidase (SA-β-gal), accumulation of senescence-associated heterochromatic foci (SAHF), senescence-associated DNA damage foci (SADF), acquisition of a senescence-associated secretory phenotype (SASP), increased ROS production, and autophagy [19-21]. Main changes in the aged liver were presented in Figure 2. At the cellular level, age-related liver changes present as an increase in the size of hepatocytes, which relates to the observed increase in polyploidy (Figure 2). Polyploid cells account for 6%-15% of hepatocytes in 20-year-old adults and 25%-42% of hepatocytes in 80-year-old adults [22, 23]. Therefore, polyploidy is considered a sign of cellular senescence and a stress response to limit the proliferation of damaged cells [24, 25]. In addition, the number of mitochondria and mitochondrial mitophagy are decreased (Figure 2). Mitochondrial dysfunction of senescent cells leads to DNA damage and lipid peroxidation, further causing a vicious cycle of increased levels of oxidative stress and increased cell sensitivity to this stress (Figure 2) [26]. Although apoptosis increases in the aged liver (Figure 2) and studies have reported that the apoptosis of senescent cells is helpful for liver homeostasis [27], senescent cells are generally considered resistant to apoptosis [28]. In addition, the aged liver is more susceptible to fibrosis. A significant number of inflammatory cells are activated, and inflammatory cytokine levels are also increased (Figure 2) [29]. In phagocytes, the number of phagophores and autophagosomes is decreased, so autophagy is decreased (Figure 2). All these factors contribute to the tendency toward liver fibrosis [30].
There are many morphological and functional changes in the aged liver. As the liver ages, its size shrinks by approximately 20%-40% (Figure 2), and this change is more pronounced in women than in men [26]. In addition, the wall of the hepatic artery becomes thicker, endothelial cell fenestration is reduced, and the hepatic blood flow and amount of bile acid secreted by hepatocytes are decreased (Figure 2). Furthermore, the activity of cytochrome P450 is also reduced in senescent hepatocytes (Figure 2) [31]. Broadly, the metabolism of the liver is altered with aging: the hepatic capacity for gluconeogenesis is decreased, and more lipids accumulate (Figure 2), so steatosis occurs in the aged liver [32-34]. In addition, the immune system becomes abnormal in the aged liver (Figure 2). The function of immune cells, including natural killer cells, macrophages/monocytes, peripheral B cells, and regulatory T cells, is decreased (Figure 2) [35], and the functions of antigen presentation and T-cell activation among aging dendritic cells, are also reduced (Figure 2).
The effect of senescence on carcinogenesis remains rather complex and controversial. As long as epithelial cells are in a state of senescence, their proliferation will be blocked, which prevents them from being regarded as tumor-derived cells. In contrast, they can also be regarded as storage pools for damaged cells, and once they re-enter the cell cycle, there is a risk of cancer-causing mutations (Figure 2). In addition, senescent hepatocytes secrete proinflammatory factors and matrix degradation molecules, which can stimulate the proliferation of cancer cells and epithelial-mesenchymal transformation. While these factors recruit immune-tolerant cells, they also attract immune cells to eliminate cancer cells [36].
The aged liver has a reduced ability to regenerate (Figure 2). Nikolai et al. reported that a polyprotein complex containing histone deacetylase 1 (HDAC1); SWI/SNF related, matrix-associated, actin-dependent regulator of chromatin, subfamily a, member 2 (BRM); and CCAAT/enhancer-binding protein (C/EBP)α was increased in aged hepatocytes (Figure 2), occupying E2F-dependent promoters and affecting the expression of genes about liver regeneration [37]. In addition, a decline in growth hormone is associated with aging (Figure 2), so the reduced regenerative capacity of the liver is associated with the growth hormone axis [38]. The two pathways are related because the decline in regeneration capacity can be attenuated by growth hormone in mice to remove the aforementioned HDAC1-C/EBPα-BRM inhibitory complex from the promoters [39]. In fact, in our recent study, we also found that genes enriched in the cell cycle are downregulated and that E2F8, which is also downregulated in senescent hepatocytes, is the most significant transcription factor (TF) correlated with downregulated genes enriched in the cell cycle. In summary, when the capacity of hepatocyte regeneration declines, fewer cells enter S-phase in the aged liver.
Basic information on the enrolled studies involving liver aging models.
| Name, Publication year | Species, sample | Methods of inducing aging | Result | Clinical implications of aging model |
|---|---|---|---|---|
| Eunhui,2019 | Human, HepG2 cells line, primary hepatocytes | H2O2, 500 umol/L, 48h | ROS contributed to the accumulation of cholesterol in aged liver. | Based on cell models, researchers can study the molecular mechanism of hepatocyte aging in vitro, and it is more convenient to screen antiaging drugs with this model than with others. Research findings can also be mutually validated in these two kinds of hepatocyte aging models induced by D-gal and H2O2, which increases the persuasiveness of the results. |
| Cong,2014 | Human, hepatocytes | D-gal,200mmol/L,120h | ROS and aging markers of P21, P53 and SA-β-gal were increased in hepatocytes. Moreover, senescent hepatocytes had decreased ability of regeneration. | |
| Dalia,2019 | Rat, livers | D-gal, 300mg/kg*d, 30days | Sulforaphane regulated Keap-1, Nrf-2, HO-1 and antioxidant enzyme activities for reducing oxidative stress to against aging. | Based on the D-gal-induced liver aging model, we can perform intervention studies on the liver in vivo to explore new treatments for liver aging and liver aging-related diseases. |
| Khairunnuur,2021 | Rat, livers | D-gal, 60-300mg/kg*d, 6-8weeks | D-gal induced the production of ROS and contributed to oxidative stress. | |
| Beatriz,2019 | Zebrafish, livers | Rag1-/- | Rag1-/- can accelerate the liver aging of zebrafish and ALCAR could be considered a new biomarker and target for aging. | Rag1-/- zebrafish are a very useful model for studying the properties of liver aging in vivo, and researchers can use them to screen antiaging drugs. |
| Madalena,2016 | Zebrafish, livers | Telomerase mutant | Telomerase mutant can construct the aging model for the study of physiological aging. Similar to natural zebrafish aging, terthu3430/hu3430 zebrafish had degenerative phenotypes and dysfunctional liver. It developed aging related diseases more easily and died prematurely | Telomerase mutant aging model can be used to unravel the relationship among telomere shortening, hepatic tissue regeneration, hepatic aging, and disease. |
| Norihisa, 2021 | Human, livers | Nature aging | CHI3L1 played a central role in the increased susceptibility of aged livers to fibrosis progression. | Researchers can find key molecules using aged human liver samples and then perform molecular experiments to study the characteristics of liver aging. Additionally, findings about liver aging from animal and cell models can be validated in aged liver specimens from humans directly or indirectly. |
| Leonardo, 2021 | Human, livers | Nature aging | This study indicated that in aged liver, hepatic sinusoid was significantly dysfunctional and vulnerable to injuries. |
H2O2, hydrogen peroxide; ROS, reactive oxidative species; D-gal, D-galactose; P21, cyclin dependent kinase inhibitor 1A; P53, tumor protein p53; SA-β-gal, senescence-associated beta-galactosidase; Keap-1, Kelch-like ECH-associated protein-1; Nrf-2, NF-E2-related factor-2; HO-1, hemeoxygenase-1; Rag1-/-, recombination activating gene 1 mutants; ALCAR, L‐acetylcarnitine.
The current experimental model for research on liver aging
Presently, aged liver models are commonly used to study aging, and we review the published literature about liver aging models (Table 1). Hepatocyte aging models are induced by D-gal and H2O2, respectively, and for animal models, there are zebrafish models and D-gal-induced liver aging models. Additionally, aged liver samples from humans are also obtained to study the underlying mechanism of liver aging. In this section, we have divided these models into three distinct parts, namely, in vitro models, in vivo models, and human patient samples, and introduced them in detail.
In vitro models
Seo et al. treated HepG2 cells and primary hepatocytes with H2O2 (500 µmol/L) for 48 hours and successfully induced cell aging [14]. They then studied the changes in glucose and lipid metabolism and the accumulation of cholesterol in this model of aged hepatocytes [14]. The results demonstrated that aged cells take up more cholesterol and glucose than younger cells and contributing to the synthesis of cholesterol from glucose, which leads to an accumulation of cholesterol in senescent hepatocytes [14]. Additionally, Cong et al. used D-gal (200 mmol/L) to induce the senescence of hepatocytes for 120 hours [40]. They reported that the levels of the aging markers cyclin-dependent kinase inhibitor 1A (P21) and P53 are increased and that SA-β-gal staining is positive in cells [40]. Moreover, senescent hepatocytes have a decreased regeneration ability [40]. Based on cell models, researchers can study the molecular mechanism of hepatocyte aging in vitro, and it is more convenient to screen antiaging drugs with this model than with others. Research findings can also be mutually validated in these two kinds of hepatocyte aging models induced by D-gal and H2O2, which increases the persuasiveness of the results.
In vivo models
Many studies have reported the induction of liver aging by D-gal in rats [16, 41]. Research on the mechanisms of D-gal-induced senescence, which include ROS production, oxidative stress induction, and increased inflammation, has been reported [16]. These mechanisms all cause hepatocellular apoptosis, liver structural damage, and mitochondrial dysfunction [16]. Dalia et al. evaluated the antiaging properties of sulforaphane on liver aging induced by D-gal (300 mg/kg*d, for 5 days a week) in rats [41]. The results indicated that sulforaphane decreased serum levels of aspartate transaminase (AST), alanine transaminase (ALT), and total and direct bilirubin. It promoted hepatic antioxidant ability by regulating Kelch-like ECH-associated protein 1 (Keap-1), NF-E2-related factor 2 (Nrf-2), heme oxygenase-1 (HO-1), and antioxidant enzyme activity [41]. In addition, sulforaphane can also decrease the levels of the inflammatory cytokines tumor necrosis factor-alpha (TNF-ɑ) and transforming growth factor-β (TGF-β) and inhibit hepatic fibrosis [41]. Together, the data indicate that sulforaphane can inhibit D-gal-induced liver aging [41]. Based on the D-gal-induced liver aging model, we can perform intervention studies on the liver in vivo to explore new treatments for liver aging and liver aging-related diseases.
In addition, Beatriz et al. demonstrated that recombination activating gene 1 mutation (rag1-/-) can accelerate the aging of zebrafish. Specifically, the senescence markers P21, P53, MDM2 proto-oncogene (MDM2), cyclin-dependent kinase inhibitor 1C (P57), and the activity of SA‐β‐gal are all increased in the livers of rag1-/- zebrafish. Moreover, they also found that in the livers of rag1-/- zebrafish, telomere length is reduced, and the ability to self-renew and repair is abnormal. These investigators suggested that L‐acetylcarnitine (ALCAR) could be considered a new biomarker for aging and is an essential metabolite for avoiding premature aging [42]. In addition, ABT-263 (navitoclax) has a significant senolytic effect in Rag1-/- zebrafish [42]. Hence, Rag1-/- zebrafish are a very useful model for studying the properties of liver aging in vivo, and researchers can use them to screen antiaging drugs. In addition, telomerase mutants can also accelerate the aging of zebrafish, and there are shorter telomeres in the telomerase-deficient zebrafish strain terthu3430/hu3430 than in wild-type zebrafish [44]. Similar to naturally aging zebrafish, terthu3430/hu3430 zebrafish have degenerative phenotypes and liver dysfunction [44]. Terthu3430/hu3430 zebrafish more easily develop aging-related diseases than wild-type zebrafish and die prematurely [44]. This telomerase mutant aging model can be used to unravel the relationship among telomere shortening, hepatic tissue regeneration, hepatic aging, and disease. Collectively, the evidence indicates that D-gal and zebrafish can be used to build an aging model and that animal models can be used to conduct further molecular studies on liver aging and screen effective antiaging drugs.
Human patient samples
Currently, liver samples from humans are also used to study liver aging and liver aging-related diseases. The risk of hepatic fibrosis progression is highly increased when patients are infected with hepatitis viruses at an older age [45, 46]. To understand the underlying mechanism, Norihisa et al. obtained liver samples from donors with normal livers and divided them into younger and older groups [46]. They found that chitinase 3-like 1 (CHI3L1) was overexpressed in the aging liver [46]. In addition, they obtained liver tissues from patients with liver cirrhosis and found that the expression level of CHI3L1 was increased in these cirrhotic livers [46]. Next, they conducted a series of experiments and concluded that CHI3L1 played a central role in the increased susceptibility of aged livers to fibrosis progression [46]. In addition, Raquel et al. first studied the hepatic function and microcirculatory status in aged livers from rats, and then they validated their research results in aged livers from humans [47]. The results indicated that aged livers had dysfunctional hepatic sinusoids with increased vulnerability to injuries [47]. The resistance of the hepatic vasculature and portal pressure were increased in the aged liver [47]. Typical molecules from hepatic sinusoid cells were changed, and the function of hepatocytes also deteriorated in the aged liver [47]. Moreover, hepatic macrophages were in a proinflammatory state, and hepatic stellate cells were activated spontaneously [47]. Most importantly, these results were verified in aged liver samples from humans. Overall, these studies all used human liver specimens, increasing the reliability of the results. Researchers can find key molecules using aged human liver samples and then perform molecular experiments to study the characteristics of liver aging. Additionally, findings about liver aging from animal and cell models can be validated in aged liver specimens from humans directly or indirectly.
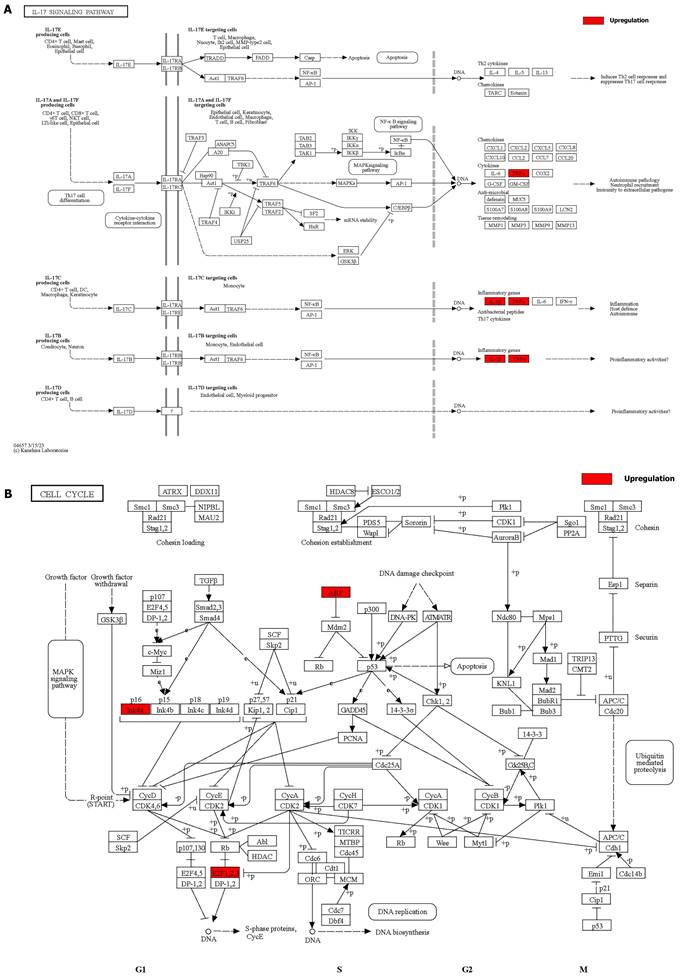
Indeed, patients older than 70 years old undergoing hepatectomy often show increased prevalence rates of comorbidities, and liver aging increases the incidence of graft failure after LT [48, 49]. Thus, the above models provide opportunities for us to conduct deep data mining regarding liver aging to find the underlying targets. In doing so, aging models can be induced, and senescence markers (P16, P21, P53, SA‐β‐gal, and SASP) can be used to determine whether a model has been successfully constructed. Based on these models, we can perform basic and clinical studies to find key targets so that the quality of the aged liver can be improved.
Omics analysis of the aged liver and potential mechanisms
Recently, omics analysis has gained momentum in liver aging. “Omics” refers to the acquisition of systematic knowledge of collective molecular features across the genome, transcriptome, proteome, metabolome, and microbiome. Omics data offer a potential way to depict the working environment of the liver in the body and are thus very helpful for mechanistic studies [50]. In addition, comprehensive multi-omics studies have shown the advantages of improving the understanding of molecular functions and studying disease prediction, and they mutually validate each other at various levels [50]. In Table 2, we provide a comprehensive review of omics studies related to liver aging. The search strategy for these studies is presented in Table S1 and the process of literature extraction is shown in Figure S1. Tabula Muris Senis data report differentially expressed genes in the aged livers of mice as determined by single-cell RNA sequencing [51]. Ten senescence genes, bone marrow stromal cell antigen 1 (Bst1), cyclin-dependent kinase inhibitor 2A (Cdkn2a), E2F transcription factor 2 (E2f2), interleukin 10 (Il-10), interleukin 1 beta (Il-1b), integrin alpha M (Itgam), integrin alpha X (Itgax), lamin B1 (Lmnb1), poly (ADP-ribose) polymerase family, member 14 (Parp14), and tumor necrosis factor (Tnf), are overexpressed in the aged liver, especially in Kupffer cells [52]. In addition, Han Qunhua et al. reported that the levels of 70 metabolites were decreased and that the levels of another 83 metabolites were increased in aged livers compared with young livers. Organic acids and derivatives, lipids and lipid-like molecules, organic oxygen compounds, and organoheterocyclic compounds were the top four ranked altered metabolites. Most of the metabolites with increased levels were glycerophospholipid metabolites and fatty acyl group members, especially lysoglycerophospholipids. Meanwhile, the levels of metabolites such as keto acids, carboxylic acids, and their derivatives were mostly decreased. Moreover, the differentially abundant metabolites were enriched mainly in the glycerophospholipid metabolic pathway [53]. Regarding transcriptomic data, there are significant differences between young and old livers. Differential expression of genes in glycerophospholipid metabolism, arachidonic acid metabolism, histidine metabolism, and linoleic acid metabolism leads to changes in metabolites; for example, overproduction of phospholipase A2 (Pla2) catalyzes the formation of arachidonic acid and lysophospholipids from glycerophospholipids, which can cause hepatic disease and inflammation [54]. In addition, histidine decarboxylase is overexpressed, which leads to increased production of histamine [55].
White et al. reported that aged mice show greater variability in the transcriptome of the liver than younger mice [56]. Their study found that lymphocyte antigen 6 family member A (Ly6a), matrix metallopeptidase 12 (Mmp12), Cxcl9, guanylate binding protein 2 (Gbp2), interleukin 7 (Il7), Rac family small GTPase 2 (Rac2), fibroblast growth factor receptor 3 (Fgfr3), cathepsin S (Ctss), and telomerase RNA component (Terc) (noncoding RNA (ncRNA)) were overexpressed but that metallothionein 1 (Mt1), E2F transcription factor 7 (E2f7), heat shock protein 1B (Hspa1b), and nuclear paraspeckle assembly transcript 1 (Neat1) (ncRNA) were downregulated. In addition, these ncRNAs, Neat1, and Pvt1 oncogene (Pvt1) play important roles in the process of aging and maternally expressed gene 3 (Meg3), RNA imprinted and accumulated in nucleus (Rian), and miRNA containing gene (Mirg) all work together to regulate cell proliferation. They also reported that there were three main networks of liver aging: lipid metabolism, proliferative homeostasis, and inflammation. The network of inflammation is relevant to cancer because inflammatory cytokines secreted by aged cells can promote the hyperplastic growth of hepatocytes, which can cause an increase in aging-related tumors. Another network associated with aging involves the proliferation and death of hepatocytes. Proliferative homeostasis of the aged liver is lost, and the regeneration rate of hepatocytes is decreased [57]. The third interaction network correlated with aging was the synthesis and oxidation of lipids. Cytochrome P450, family 8, subfamily b, polypeptide 1 (Cyp8b1); cytochrome P450, family 1, subfamily b, polypeptide 1 (Cyp1b1); cytochrome P450, family 4, subfamily a, polypeptide 14 (Cyp4a14); and cytochrome P450, family 26, subfamily a, polypeptide 1 (Cyp26a1) are the most abundant families of enzymes in the network. The failure of these pathways results in lipofuscin accumulation, which reflects a reduced ability of cells to metabolize their waste products [58].
Son et al. analyzed hepatic metabolites in aged rats. They found three kinds of metabolism associated with aging, including lipid energy metabolism through a betaine-methionine-carnitine system, degradation of nucleic acid metabolism, and nicotinamide adenine dinucleotide (NAD) metabolism. Although the levels of metabolites related to energy metabolism (betaine, methionine, carnitine, and fatty acylcarnitines) were increased, those of NAD and NAD/NADH were decreased. As a result, the production of energy did not increase by β-oxidation with aging because the activity of metabolism related to NAD declined with age. In addition, the production of nucleic acid degradation products (hypoxanthine and xanthine) increased with aging, which could have caused the accumulation of ROS. Therefore, these results strongly demonstrate that aging leads to the dysregulation of hepatic energy metabolism and the accumulation of ROS. The above results are helpful for us to better understand aging-related diseases [59].
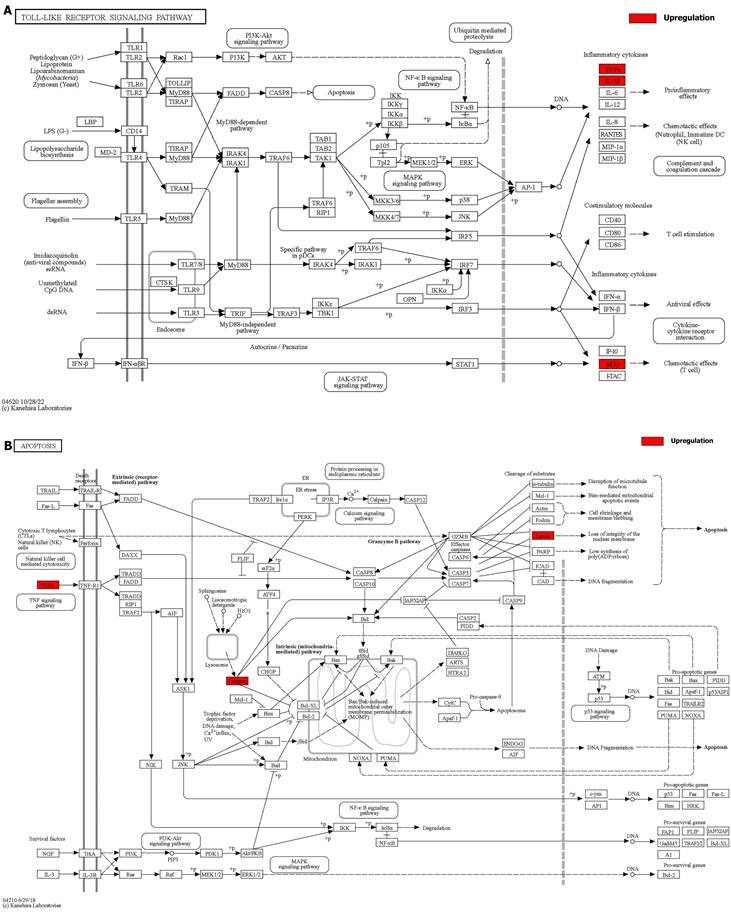
We integrated the different genes above and reanalyzed them using omics methods (Figure 3, Table 2). These results showed that the mitogen-activated protein kinase (MAPK) signaling pathway, cytokine-cytokine receptor interaction, toll-like receptor signaling pathway, apoptosis, interleukin 17 (IL-17) signaling pathway, and cell cycle pathway exhibited significant deviations in the aged liver (Figure 4-6). The MAPK signaling pathway plays an important role in liver aging and can regulate the expression of the senescence markers BCL2 apoptosis regulator (Bcl2), BCL2-associated X apoptosis regulator (Bax), and P21 [60]. In addition, the MAPK signaling pathway plays an important role in promoting the development of nonalcoholic fatty liver disease (NAFLD) [61], and aged livers are more susceptible than young livers to NAFLD. Cytokine‒cytokine receptor interactions, the toll-like receptor signaling pathway, and the IL-17 signaling pathway are all inflammation-related pathways and senescent hepatocytes secrete inflammatory factors contributing to liver aging-related diseases. For instance, Cxcl9 is overexpressed in the cytokine-cytokine receptor interaction pathway (Figure 4B). Cxcl9 promotes the senescence of cells, and silencing Cxcl9 can effectively reverse cellular senescence [62]. The toll-like receptor signaling pathway also plays an important role in promoting liver fibrosis, and aged livers are generally susceptible to fibrosis [63]. Hence, it is worth conducting further study on the toll-like receptor signaling pathway in the process of liver aging. In addition, senescent hepatocytes have compromised regenerative ability, and as shown in Figure 6B, the expression of cyclin-dependent kinase inhibitor P16 was increased. Overall, these pathways are all important in liver aging and worthy of further study.
There are some limitations in current liver aging-related omics studies. Overall, available omics data concerning liver aging are fragmented and lack systematic frameworks and validation tests. Moreover, most omics studies have been conducted using animal models; rarely have they used aged liver specimens from humans. Moreover, there is a lack of studies about whether there are any differences between commonly used animal models and humans. Next-generation sequencing and multi-omics studies with mutual validation in a fixed cohort are urgently needed in future liver aging-related omics investigations. In addition, more attention should be given to omics studies of blood samples, as blood can closely reflect liver aging. However, few studies including multidimensional samples (tissues and sera) have been conducted with liver aging models. These limitations may affect the translational value of omics data in liver aging research. However, there is an urgent need to use multi-omics approaches to study liver aging to discover key intervention targets. Thus, it is necessary to solve the above limitations in omics studies.
Reanalysis of positive genes associated with liver aging in prior omics studies. (A) Bubble diagram of enrichment analysis based on positive genes associated with the aged liver. (B) Circle diagram of enrichment analysis based on positive genes associated with the aged liver.
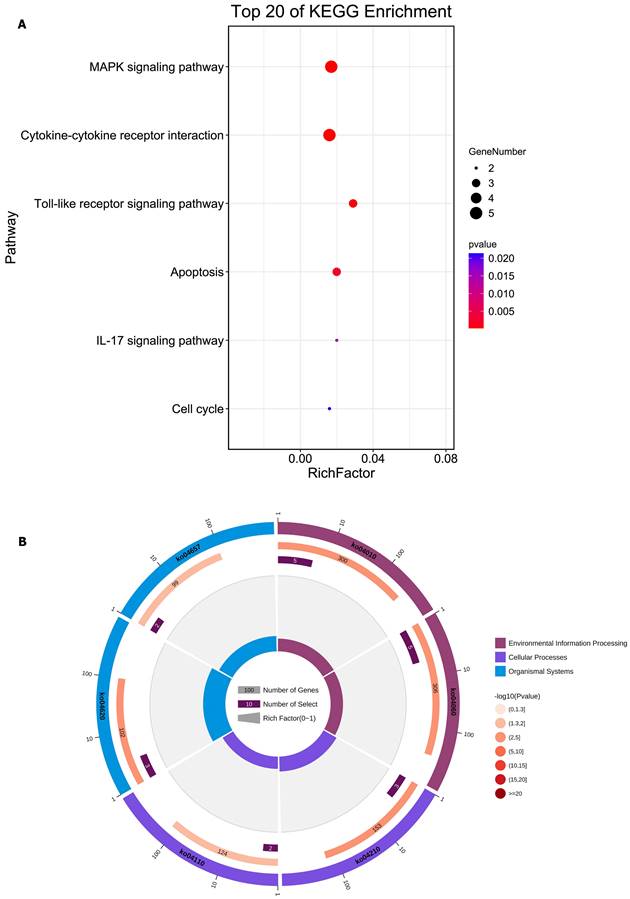
Details of positive signaling pathways and genes associated with liver aging. (A) Details of the MAPK signaling pathway and positive genes associated with liver aging. (B) Details of the cytokine-cytokine receptor interaction pathway and positive genes associated with liver aging.
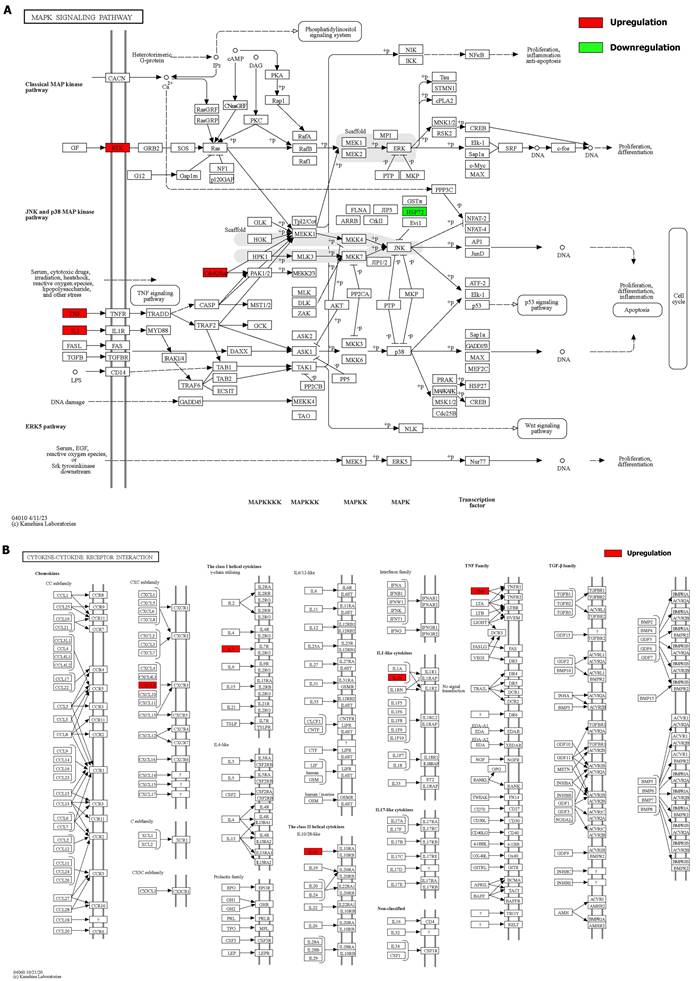
Details of positive signaling pathways and genes associated with liver aging. (A) Details of the toll-like receptor signaling pathway and positive genes associated with liver aging. (B) Details of the apoptosis pathway and positive genes associated with liver aging.
Details of positive signaling pathways and genes associated with liver aging. (A) Details of the IL-17 signaling pathway and positive genes associated with liver aging. (B) Details of the cell cycle pathway and positive genes associated with liver aging.
Basic information on the enrolled omics studies on liver aging.
| Name, Publication year | Species, sample, assay | Key molecule | Key pathway |
|---|---|---|---|
| Tabula, 2019 | Mice, liver, single-cell transcriptomics | UP: Bst1, Cdkn2a, E2f2, Il-10, Il-1b, Itgam, Itgax, Lmnb1, Parp14, Tnf | Na |
| Qunhua, 2021 | Rat, liver, metabolomics, transcriptomics | Metabolites-UP: glycero-phospholipid metabolites, fatty acyl, lysoglycerophospholipids; Metabolites-DN: keto acids, carboxylic acids; transcriptomics-up: pla2, arachidonic acid, lysophospholipids; histidine decarboxylase, histamine | Glycerophospholipid metabolic pathway, glycerophospholipid metabolism, arachidonic acid metabolism, histidine metabolism and linoleic acid metabolism |
| Ryan, 2015 | Mouse, liver, transcriptomics | UP: Ly6a, Mmp12, Cxcl9, Gbp2, Il7, Rac2, Fgfr3, Ctss, and Terc(ncRNA); DN: Mt1, E2f7, Hspa1b, and Neat1(ncRNA); | Lipid metabolism, proliferative homeostasis, inflammation |
| Nari, 2012 | Rat, liver, metabolomics | UP: betaine, methionine, carnitine, fatty acylcarnitines, hypoxanthine and xanthine, ROS; DN: NAD, NAD/NADH | lipid energy metabolism, degradation of nucleic acid metabolism, NAD metabolism |
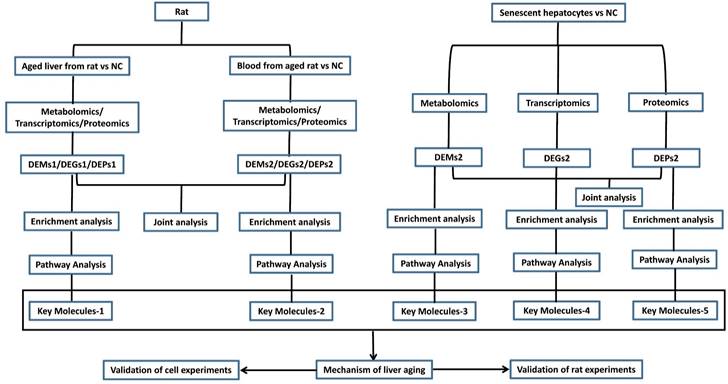
Hence, we formulated a flowchart of research on liver aging using multi-omics analysis and molecular experiments (Figure 7). The aged liver model can be natural or induced by D-gal, and senescence in hepatocytes can be induced by treatment of the HepG2 cell line with D-gal and H2O2, as reported in many studies [14, 64-66]. Integrative transcriptomics, metabolomics, and proteomics analyses were performed on these blood, liver, and hepatocyte specimens. These joint multi-omics analyses depicted the panorama of the working environment for the liver in the body. The differentially expressed genes (DEGs), differentially expressed metabolites (DEMs), and differentially expressed proteins (DEPs) were used for enrichment pathway analysis, and we identified the most important molecules. According to reported studies on liver aging, we can speculate on the underlying mechanism and validate it with cell experiments and rat experiments.
Potential therapeutic approaches and their application to liver aging
Antiaging is a promising and challenging field of research due to the complexity of aging mechanisms. Scientists have performed many anti-aging studies, including studies testing caloric restriction (CR) through diet control, clearance of senescent cells, and the treatment of liver aging with matrine [67, 68].
Diet control
CR is effective against aging-related diseases and for improving physical condition. For instance, many research findings have demonstrated that CR can reduce the occurrence of obesity-induced aging-related diseases [69]. In addition, moderate reductions in caloric intake help to improve the viability of organisms [70]. Long-term CR without malnutrition can improve the efficiency of resting energy expenditure, which reduces oxidative damage to the liver and suppresses liver inflammation [71, 72]. Miguel et al. reported that increasing the fasting time and reducing the total caloric intake are the determinants of prolonged longevity. Their multi-omics analysis indicated that the glycine-serine-threonine metabolic axis correlates with longevity and that short-chain fatty acid metabolism and polyunsaturated fatty acid metabolism is the key pathways for improving health. In addition, protein restriction in aging male mice is also beneficial for expanding lifespan and metabolic health, and fibroblast growth factor 21 (FGF21) is necessary for the antiaging effect of protein restriction [73]. Dietary intervention and exercise can increase lipophagy to ameliorate high-fat diet-induced NAFLD and liver aging [74]. The purposes of CR and exercise are to increase energy expenditure and reduce the burden on the body. In particular, a diet of low protein and low lipids may also be beneficial to liver metabolism and can expand longevity. In our opinion, lipid metabolism dysfunction is an important contributor to liver aging, and our recent study found that the pathways of fatty acid elongation, fatty acid degradation, and biosynthesis of unsaturated fatty acids are all downregulated in aged livers. Hence, the role of lipid metabolism in the liver needs to be further investigated. Overall, these findings provide us with new insight for understanding the remodeling of the genome/metabolome in the aged liver and will help design therapeutic interventions against aging-related metabolic alterations [75].
Clearance of senescent cells
Presently, clearing senescent hepatocytes is a new strategy to protect against liver aging. For example, a newly developed drug type, known as a senolytic, can selectively clear senescent hepatocytes and maybe a new strategy to attenuate liver aging. Dasatinib (D) and quercetin (Q) can induce apoptosis in senescent hepatic progenitor cells and ameliorate aging-related phenotypes [76]. Other senolytics, including A-1155463, A-1331852, ABT-737, and ABT-263, can also inhibit the BCL-2 family (BCL-W, BCL-XL, and BCL-2) and therapeutically target senescent hepatocytes [77]. In addition, ATB263 can remove senescent cancer cells and hepatocytes with chemotherapy-induced senescence to inhibit the metastasis and recurrence of tumors [78, 79]. Senolytics (D+Q) can also effectively remove P16-positive cells, reduce the activity of SA‐β‐gal, and limit the release of inflammatory factors [80, 81]. Furthermore, Zhu et al. studied whether the effect of clearing senescent cells persists for many weeks after senolytics are no longer present, and the results demonstrated that D+Q treatment has a long-lasting effect after the drug is no longer present [76].
Although the proliferation of senescent cells is decreased, these cells are resistant to apoptosis. Thus, the prosurvival pathways can be regarded as targets to eliminate senescent cells by RNA interference. The survival viability of senescent hepatocytes can be decreased by interfering with the expression of ephrin B1 (EFNB1), ephrin B3 (EFNB3), phosphatidylinositol-4,5-bisphosphate 3-kinase delta catalytic subunit (PI3KCD), P21, BCL-xL or plasminogen-activated inhibitor-2 (PAI-2). In addition, forkhead box O4 (FOXO4) is pivotal in the viability of senescent hepatocytes because it can inhibit the apoptosis of senescent hepatocytes to maintain their activity. Hence, the FOXO4 peptide is a D-retro-inverso isoform induced by the modification of peptides, and it can disrupt the interaction between FOXO4 and P53 to induce the targeted apoptosis of senescent hepatocytes. Moreover, the FOXO4 peptide can cause nuclear exclusion of senescent cells. Hence, doxorubicin-induced senescence can be effectively reduced in vitro and in vivo by the FOXO4 peptide. In doing so, the doxorubicin-induced loss in body weight and liver toxicity can also be neutralized. The body can tolerate the FOXO4 peptide well, so it is a newly available option for clinicians to attenuate liver aging [27]. Overall, clearing senescent hepatocytes can help to protect against liver aging and eliminate the adverse effects of senescent hepatocytes on peripheral normal hepatocytes.
Flow diagram of the study design on liver aging. DEGs, differentially expressed genes; DEMs, differentially expressed metabolites; DEPs, differentially expressed proteins.
Matrine administration against liver aging
Matrine is an alkaloid extracted from Sophora flavescens that has a significant antiaging effect. It mainly inhibits cellular senescence and oxidative stress. First, matrine significantly inhibits the increase in aging-induced nuclear size in hepatocytes and inhibits the increase in SA-β-gal--positive hepatocytes. In fact, in our recent study, we also found that peroxisome protein expression was downregulated in aged livers and that catalase and malondialdehyde (MDA) levels also declined, which indicated that aged livers had decreased antioxidant ability. However, matrine can also inhibit the decline in the antioxidative ability of the aged liver; for instance, it can attenuate the decreases in total superoxide dismutase, catalase, and MDA. The expression of aging-related genes is also downregulated by matrine. P16, CDKN2D (P19), and P21 are all overexpressed in the aged liver, but matrine can significantly reverse their increases. Moreover, the expression levels of interleukin 1 beta (IL-1β) and interleukin 6 (IL-6) are reduced in senescent hepatocytes by matrine. Overall, the data indicate that matrine can be used to prevent liver aging and treat liver aging-related diseases [82].
Conclusions
In summary, this review retrospectively reports the changes in the aged liver, cellular and animal models, and presents omics studies on liver aging. In addition, we discuss current treatments to prolong lifespan. Based on cell and animal models of liver aging, a multi-omics approach can be applied to find key metabolic pathways and target genes, and these pathways and targets can be validated through molecular experiments. Thus, in doing so, we can eliminate the adverse effects of liver aging to expand longevity and aged donor livers can be transplanted into recipients safely with the maintenance of homeostasis.
Abbreviations
Cxcl9: C-X-C motif chemokine ligand 9; P16: cyclin dependent kinase inhibitor 2A; P53: tumor protein p53; E2F8: E2F transcription factor 8; TFDP1: transcription factor Dp-1; ROS: reactive oxygen species; H2O2: hydrogen peroxide; D-gal: D-galactose; LT: liver transplantation; IRI: ischemia-reperfusion injury; SA-β-gal: senescence-associated beta-galactosidase; SAHF: senescence associated heterochromatic foci; SADF: senescence-associated DNA damage foci; SASP: senescence-associated secretory phenotype; HDAC1: histone deacetylase 1; BRM: SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily a, member 2; C/EBP: CCAAT/enhancer-binding protein; TF: transcription factor; Rag1-/-: recombination activating gene 1 mutants; P21: cyclin dependent kinase inhibitor 1A; MDM2: MDM2 proto-oncogene; P57: cyclin dependent kinase inhibitor 1C; ALCAR: L‐acetylcarnitine; Bst1: bone marrow stromal cell antigen 1; Cdkn2a: cyclin dependent kinase inhibitor 2A; E2f2: E2F transcription factor 2; Il-10: interleukin 10; Il-1b: interleukin 1 beta; Itgam: integrin alpha M; Itgax: integrin alpha X; Lmnb1: lamin B1; Parp14: poly (ADP-ribose) polymerase family, member 14; Tnf: tumor necrosis factor; Pla2: phospholipase A2; Ly6a: lymphocyte antigen 6 family member A; Mmp12: matrix metallopeptidase 12; Gbp2: guanylate binding protein 2; Il7: interleukin 7; Rac2: Rac family small GTPase 2; Fgfr3: fibroblast growth factor receptor 3; Ctss: cathepsin S; Terc: telomerase RNA component; DN: downregulation; Mt1: metallothionein 1; E2f7: E2F transcription factor 7; Hspa1b: heat shock protein 1B; Neat1: nuclear paraspeckle assembly transcript 1; Pvt1: Pvt1 oncogene; Meg3: maternally expressed gene 3; Rian: RNA imprinted and accumulated in nucleus; Mirg: miRNA containing gene; Cyp8b1: cytochrome P450, family 8, subfamily b, polypeptide 1; Cyp1b1: cytochrome P450, family 1, subfamily b, polypeptide 1; Cyp4a14: cytochrome P450, family 4, subfamily a, polypeptide 14; Cyp26a1: cytochrome P450, family 26, subfamily a, polypeptide 1; NAD: nicotinamide adenine dinucleotide; MAPK: mitogen-activated protein kinase; IL-17: interleukin 17; Bcl2: BCL2 apoptosis regulator; Bax: BCL2 associated X, apoptosis regulator; NAFLD: nonalcoholic fatty liver disease; DEGs: differentially expressed genes; DEMs: differentially expressed metabolites; DEPs: differentially expressed proteins; CR: calorie restriction; FGF21: fibroblast growth factor 21; D: Dasatinib; Q: Quercetin; EFNB1: ephrin B1; EFNB3: ephrin B3; PI3KCD: phosphatidylinositol-4,5-bisphosphate 3-kinase delta catalytic subunit; PAI-2: plasminogen-activated inhibitor-2; FOXO4: forkhead box O4; MDA: malondialdehyde; P19: CDKN2D; IL-1β: interleukin 1 beta; IL-6: interleukin 6.
Supplementary Material
Supplementary figure and table.
Acknowledgements
Funding
This study is supported by the Research Unit Project of the Chinese Academy of Medical Sciences (2019-I2M-5-030) and the Research Project of Jinan Microecological Biomedicine Shandong Laboratory (JNL-2022002A).
Availability of data and materials
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
Author contributions
(I) Conception and design: Z. Liu, L. Zhou, and S. Zheng; (II) Administrative support: None; (III) Provision of study materials or patients: None; (IV) Collection and assembly of data: Z. Liu, W. Wang, K. Xu, and M. Shang; (V) Data analysis and interpretation: Z. Liu and W. Wang; (VI) Manuscript writing: All authors; and (VII) Final approval of manuscript: All authors.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010;24:2463-79
2. Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Experimental Cell Research. 1961;25(3):585-621
3. Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685-705
4. Chatsirisupachai K, Palmer D, Ferreira S, de Magalhaes JP. A human tissue-specific transcriptomic analysis reveals a complex relationship between aging, cancer, and cellular senescence. Aging Cell. 2019;18:e13041
5. Wiley CD, Flynn JM, Morrissey C, Lebofsky R, Shuga J, Dong X. et al. Analysis of individual cells identifies cell-to-cell variability following induction of cellular senescence. Aging Cell. 2017;16:1043-50
6. Lue A, Solanas E, Baptista P, Lorente S, Araiz JJ, Garcia-Gil A. et al. How important is donor age in liver transplantation? World J Gastroenterol. 2016;22:4966-76
7. Wynne HA CL, Mutch E, Rawlins MD, Woodhouse KW, James OF. The effect of age upon liver volume and apparent liver blood flow in healthy man. Hepatology. 1989;9(2):297-301
8. Marchesini G BV, Brunori A, Bianchi G, Pisi P, Fabbri A, Zoli M, Pisi E. Marchesini - Galactose elimination capacity and liver volume in aging man. Hepatology. 1988;8(5):1079-83
9. Schmucker DL. HEPATOCYTE FINE-STRUCTURE DURING MATURATION AND SENESCENCE. Journal of Electron Microscopy Technique. 1990;14:106-25
10. Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nature reviews Molecular cell biology. 2007;8:729-40
11. Serrano M, Blasco MA. Putting the stress on senescence. Current opinion in cell biology. 2001;13:748-53
12. Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223-33
13. Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99-118
14. Seo E, Kang H, Choi H, Choi W, Jun HS. Reactive oxygen species-induced changes in glucose and lipid metabolism contribute to the accumulation of cholesterol in the liver during aging. Aging Cell. 2019;18:e12895
15. He ZH, Li M, Fang QJ, Liao FL, Zou SY, Wu X. et al. FOXG1 promotes aging inner ear hair cell survival through activation of the autophagy pathway. Autophagy. 2021;17:4341-62
16. Azman KF, Safdar A, Zakaria R. D-galactose-induced liver aging model: Its underlying mechanisms and potential therapeutic interventions. Exp Gerontol. 2021;150:111372
17. Liu Z, Wang W, Zhuang L, Liu J, Que S, Zhu D. et al. Clear mortality gap caused by graft macrosteatosis in Chinese patients after cadaveric liver transplantation. Hepatobiliary Surg Nutr. 2020;9:739-58
18. Wang W, Liu Z, Qian J, Xu J, Que S, Zhuang L. et al. Systematic Assessment of Safety Threshold for Donor Age in Cadaveric Liver Transplantation. Front Med (Lausanne). 2021;8:596552
19. Sikora E, Arendt T, Bennett M, Narita M. Impact of cellular senescence signature on ageing research. Ageing Res Rev. 2011;10:146-52
20. Sharpless NE, Sherr CJ. Forging a signature of in vivo senescence. Nature reviews Cancer. 2015;15:397-408
21. Aravinthan A. Cellular senescence: a hitchhiker's guide. Human cell. 2015;28:51-64
22. Hoare M, Das T, Alexander G. Ageing, telomeres, senescence, and liver injury. J Hepatol. 2010;53:950-61
23. Wang M-J, Chen F, Lau JTY, Hu Y-P. Hepatocyte polyploidization and its association with pathophysiological processes. Cell Death & Disease. 2017;8:e2805-e
24. He S, Sharpless NE. Senescence in Health and Disease. Cell. 2017;169:1000-11
25. Wang MJ, Chen F, Li JX, Liu CC, Zhang HB, Xia Y. et al. Reversal of hepatocyte senescence after continuous in vivo cell proliferation. Hepatology. 2014;60:349-61
26. Durand F, Levitsky J, Cauchy F, Gilgenkrantz H, Soubrane O, Francoz C. Age and liver transplantation. J Hepatol. 2019;70:745-58
27. Baar MP, Brandt RMC, Putavet DA, Klein JDD, Derks KWJ, Bourgeois BRM. et al. Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging. Cell. 2017;169(1):132-147
28. Salminen A, Ojala J, Kaarniranta K. Apoptosis and aging: increased resistance to apoptosis enhances the aging process. Cell Mol Life Sci. 2011;68:1021-31
29. Mahrouf-Yorgov M, de l'Hortet AC, Cosson C, Slama A, Abdoun E, Guidotti J-E. et al. Increased Susceptibility to Liver Fibrosis with Age Is Correlated with an Altered Inflammatory Response. Rejuvenation Research. 2011;14:353-63
30. Ilyas G, Zhao E, Liu K, Lin Y, Tesfa L, Tanaka KE. et al. Macrophage autophagy limits acute toxic liver injury in mice through down regulation of interleukin-1beta. J Hepatol. 2016;64:118-27
31. Sotaniemi EA, Arranto AJ, Pelkonen O, Pasanen M. Age and cytochrome P450-linked drug metabolism in humans: An analysis of 226 subjects with equal histopathologic conditions. Clin Pharmacol Ther. 1997;61:331-9
32. Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents - Findings from the Third National Health and Nutrition Examination Survey, 1988-1994. Arch Pediatr Adolesc Med. 2003;157:821-7
33. Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL. et al. Mitochondrial dysfunction in the elderly: Possible role in insulin resistance. Science. 2003;300:1140-2
34. Slawik M, Vidal-Puig AJ. Lipotoxicity, overnutrition and energy metabolism in aging. Ageing Res Rev. 2006;5:144-64
35. Hakim FT, Flomerfelt FA, Boyiadzis M, Gress RE. Aging, immunity and cancer. Curr Opin Immunol. 2004;16:151-6
36. Aravinthan AD, Alexander GJM. Senescence in chronic liver disease: Is the future in aging? Journal of Hepatology. 2016;65:825-34
37. Timchenko NA. Aging and liver regeneration. Trends Endocrinol Metab. 2009;20:171-6
38. Zerrad-Saadi A, Lambert-Blot M, Mitchell C, Bretes H, Collin de l'Hortet A, Baud V. et al. GH receptor plays a major role in liver regeneration through the control of EGFR and ERK1/2 activation. Endocrinology. 2011;152:2731-41
39. Wang GL, Salisbury E, Shi X, Timchenko L, Medrano EE, Timchenko NA. HDAC1 cooperates with C/EBPalpha in the inhibition of liver proliferation in old mice. The Journal of biological chemistry. 2008;283:26169-78
40. SHEN Cong CG, ZHANG Xiu-min, HE ai-yang. Mechanism of cellular senescence induced by D-galactose in normal diploid human cells. Geriatric Medicine And Health Care. 2014;20:372-5
41. Saleh DO, Mansour DF, Hashad IM, Bakeer RM. Effects of sulforaphane on D-galactose-induced liver aging in rats: Role of keap-1/nrf-2 pathway. Eur J Pharmacol. 2019;855:40-9
42. Novoa B, Pereiro P, Lopez-Munoz A, Varela M, Forn-Cuni G, Anchelin M. et al. Rag1 immunodeficiency-induced early aging and senescence in zebrafish are dependent on chronic inflammation and oxidative stress. Aging Cell. 2019;18:e13020
43. Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J. et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2016;22:78-83
44. Carneiro MC, de Castro IP, Ferreira MG. Telomeres in aging and disease: lessons from zebrafish. Dis Model Mech. 2016;9:737-48
45. Schmucker DL. Age-related changes in liver structure and function: Implications for disease ? Exp Gerontol. 2005;40:650-9
46. Nishimura N, De Battista D, McGivern DR, Engle RE, Tice A, Fares-Gusmao R. et al. Chitinase 3-like 1 is a profibrogenic factor overexpressed in the aging liver and in patients with liver cirrhosis. Proceedings of the National Academy of Sciences of the United States of America. 2021;118(17):e2019633118
47. Maeso-Díaz R, Ortega-Ribera M, Fernández-Iglesias A, Hide D, Muñoz L, Hessheimer AJ. et al. Effects of aging on liver microcirculatory function and sinusoidal phenotype. Aging Cell. 2018;17:e12829
48. Fernandes AI, Tralhao JG, Abrantes A, Hoti E, Alexandrino H, Oliveiros B. et al. Functional hepatocellular regeneration in elderly patients undergoing hepatectomy. Liver International. 2015;35:1116-23
49. Wang W, Liu Z, Qian J, Xu J, Que S, Zhuang L. et al. Systematic Assessment of Safety Threshold for Donor Age in Cadaveric Liver Transplantation. Frontiers in Medicine. 2021;8:596552
50. Yoo BC, Kim K-H, Woo SM, Myung JK. Clinical multi-omics strategies for the effective cancer management. Journal of Proteomics. 2018;188:97-106
51. Tabula Muris C. A single-cell transcriptomic atlas characterizes ageing tissues in the mouse. Nature. 2020;583:590-595
52. Uyar B, Palmer D, Kowald A, Murua Escobar H, Barrantes I, Moller S. et al. Single-cell analyses of aging, inflammation and senescence. Ageing Res Rev. 2020;64:101156
53. Han Q, Li H, Jia M, Wang L, Zhao Y, Zhang M. et al. Age-related changes in metabolites in young donor livers and old recipient sera after liver transplantation from young to old rats. Aging Cell. 2021;20:e13425
54. Murakami M, Nakatani Y, Atsumi G, Inoue K, Kudo L. Regulatory Functions of Phospholipase A (2). Crit Rev Immunol. 2017;37:121-79
55. Hirasawa N. Expression of Histidine Decarboxylase and Its Roles in Inflammation. Int J Mol Sci. 2019;20(2):376
56. White RR, Milholland B, MacRae SL, Lin M, Zheng D, Vijg J. Comprehensive transcriptional landscape of aging mouse liver. BMC genomics. 2015;16:899
57. Schmucker DL, Sanchez H. Liver regeneration and aging: a current perspective. Curr Gerontol Geriatr Res. 2011;2011:526379
58. Jung T, Bader N, Grune T. Lipofuscin: formation, distribution, and metabolic consequences. Ann N Y Acad Sci. 2007;1119:97-111
59. Son N, Hur HJ, Sung MJ, Kim MS, Hwang JT, Park JH. et al. Liquid chromatography-mass spectrometry-based metabolomic analysis of livers from aged rats. J Proteome Res. 2012;11:2551-8
60. Zhou H, Yang D, Cheng HS, McCoy MG, Pérez-Cremades D, Haemmig S. et al. miR-181b regulates vascular endothelial aging by modulating an MAP3K3 signaling pathway. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2022;36:e22353
61. Bonnet L, Alexandersson I, Baboota RK, Kroon T, Oscarsson J, Smith U. et al. Cellular senescence in hepatocytes contributes to metabolic disturbances in NASH. Front Endocrinol (Lausanne). 2022;13:957616
62. Sayed N, Huang Y, Nguyen K, Krejciova-Rajaniemi Z, Grawe AP, Gao T. et al. An inflammatory aging clock (iAge) based on deep learning tracks multimorbidity, immunosenescence, frailty and cardiovascular aging. Nat Aging. 2021;1:598-615
63. Dong F, Jiang S, Li J, Wang Y, Zhu L, Huang Y. et al. The histone demethylase KDM4D promotes hepatic fibrogenesis by modulating Toll-like receptor 4 signaling pathway. EBioMedicine. 2019;39:472-83
64. Xu X, Hueckstaedt LK, Ren J. Deficiency of insulin-like growth factor 1 attenuates aging-induced changes in hepatic function: role of autophagy. J Hepatol. 2013;59:308-17
65. Seo E, Nam H, Jun HS. Reactive oxygen species induce HNF-4α expression via the ASK1-CREB pathway, promoting ChREBP expression and lipogenesis in hepatocytes. Life sciences. 2022;310:121042
66. Sen B, Rastogi A, Nath R, Shasthry SM, Pamecha V, Pandey S. et al. Senescent Hepatocytes in Decompensated Liver Show Reduced UPR(MT) and Its Key Player, CLPP, Attenuates Senescence In Vitro. Cellular and molecular gastroenterology and hepatology. 2019;8:73-94
67. Di Daniele N, Noce A, Vidiri MF, Moriconi E, Marrone G, Annicchiarico-Petruzzelli M. et al. Impact of Mediterranean diet on metabolic syndrome, cancer and longevity. Oncotarget. 2017;8:8947-8979
68. McCabe LR, Irwin R, Tekalur A, Evans C, Schepper JD, Parameswaran N. et al. Exercise prevents high fat diet-induced bone loss, marrow adiposity and dysbiosis in male mice. Bone. 2019;118:20-31
69. Salvestrini V, Sell C, Lorenzini A. Obesity May Accelerate the Aging Process. Front Endocrinol (Lausanne). 2019;10:266
70. Broskey NT, Marlatt KL, Most J, Erickson ML, Irving BA, Redman LM. The Panacea of Human Aging: Calorie Restriction Versus Exercise. Exerc Sport Sci Rev. 2019;47:169-75
71. Redman LM, Smith SR, Burton JH, Martin CK, Il'yasova D, Ravussin E. Metabolic Slowing and Reduced Oxidative Damage with Sustained Caloric Restriction Support the Rate of Living and Oxidative Damage Theories of Aging. Cell metabolism. 2018;27:805-815
72. Meydani SN, Das SK, Pieper CF, Lewis MR, Klein S, Dixit VD. et al. Long-term moderate calorie restriction inhibits inflammation without impairing cell-mediated immunity: a randomized controlled trial in non-obese humans. Aging-US. 2016;8:1416-31
73. Hill CM, Albarado DC, Coco LG, Spann RA, Khan MS, Qualls-Creekmore E. et al. FGF21 is required for protein restriction to extend lifespan and improve metabolic health in male mice. Nature communications. 2022;13:1897
74. Gao Y, Zhang W, Zeng LQ, Bai H, Li J, Zhou J. et al. Exercise and dietary intervention ameliorate high-fat diet-induced NAFLD and liver aging by inducing lipophagy. Redox biology. 2020;36:101635
75. Aon MA, Bernier M, Mitchell SJ, Di Germanio C, Mattison JA, Ehrlich MR. et al. Untangling Determinants of Enhanced Health and Lifespan through a Multi-omics Approach in Mice. Cell metabolism. 2020;32(1):100-116
76. Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding HS, Giorgadze N. et al. The Achilles' heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14:644-58
77. Di Micco R, Krizhanovsky V, Baker D, di Fagagna FdA. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nature Reviews Molecular Cell Biology. 2021;22:75-95
78. Demaria M, O'Leary MN, Chang J, Shao L, Liu S, Alimirah F. et al. Cellular Senescence Promotes Adverse Effects of Chemotherapy and Cancer Relapse. Cancer Discov. 2017;7(2):165-176
79. Wang L, Leite de Oliveira R, Wang C, Fernandes Neto JM, Mainardi S, Evers B. et al. High-Throughput Functional Genetic and Compound Screens Identify Targets for Senescence Induction in Cancer. Cell Rep. 2017;21(3):773-783
80. Hickson LJ, Langhi Prata LGP, Bobart SA, Evans TK, Giorgadze N, Hashmi SK. et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine. 2019;47:446-456
81. Rossman MJ, Santos-Parker JR, Steward CAC, Bispham NZ, Cuevas LM, Rosenberg HL. et al. Chronic Supplementation With a Mitochondrial Antioxidant (MitoQ) Improves Vascular Function in Healthy Older Adults. Hypertension (Dallas, Tex: 1979). 2018;71:1056-1063
82. Sun K, Yang P, Zhao R, Bai Y, Guo Z. Matrine Attenuates D-Galactose-Induced Aging-Related Behavior in Mice via Inhibition of Cellular Senescence and Oxidative Stress. Oxid Med Cell Longev. 2018;2018:7108604
Author contact
![]() Corresponding authors: Shusen Zheng and Lin Zhou. Division of Hepatobiliary and Pancreatic Surgery, Department of Surgery, First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou 310003, China. E-mail: shusenzhengedu.cn (SS. Z), linzhou19com (L. Zhou). Zhengtao Liu. Shulan International Medical College, Zhejiang Shuren University, Hangzhou, Zhejiang, China. E-mail: liuzhengtaocncom (ZT. L).
Corresponding authors: Shusen Zheng and Lin Zhou. Division of Hepatobiliary and Pancreatic Surgery, Department of Surgery, First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou 310003, China. E-mail: shusenzhengedu.cn (SS. Z), linzhou19com (L. Zhou). Zhengtao Liu. Shulan International Medical College, Zhejiang Shuren University, Hangzhou, Zhejiang, China. E-mail: liuzhengtaocncom (ZT. L).

 Global reach, higher impact
Global reach, higher impact