10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2024; 20(1):367-386. doi:10.7150/ijbs.87867 This issue Cite
Research Paper
Rebalancing TGF-β/PGE2 breaks RT-induced immunosuppressive barriers by enhancing tumor-infiltrated dendritic cell homing
1. Department of radiation oncology, Peking Union Medical College Hospital. Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People's Republic of China.
2. Institute of Health Service and Transfusion Medicine, Tai Ping Road, Beijing 100850, People's Republic of China.
3. Department of Oncology, Beijing Shijitan Hospital, Capital Medical University, Beijing, People's Republic of China.
4. State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, People's Republic of China.
#: These authors contributed equally to this work.
Abstract
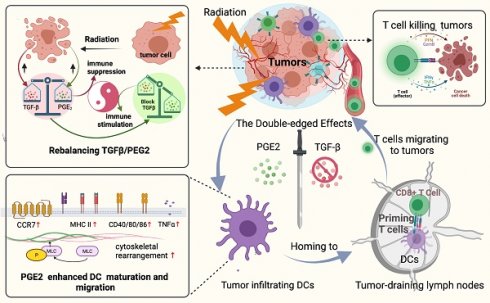
A better understanding of how tumor microenvironments shape immune responses after radiotherapy (RT) is required to improve patient outcomes. This study focuses on the observation that dendritic cells (DCs) infiltrating irradiated cervical tumors are retained in transforming growth factor (TGF)-β-abundant regions. We report that TGF-β secretion from cervical cancer cells was increased by irradiation in a dose-dependent manner and that this significantly suppressed the expression of allostimulatory markers and Th1 cytokines in DCs. To investigate further, we blocked the TGF-β signal in DCs and observed that RT had a dose-dependent immune-promoting effect, improving DC maturation. This suggested that proinflammatory mediators may also be induced by RT, but their effects were being counteracted by the simultaneously increased levels of TGF-β. Prostaglandin E2 (PGE2), a proinflammatory molecule, was shown to be one such mediator. Adjusting the TGF-β/PGE2 ratio by inhibiting TGF-β rebooted RT-induced DC cytoskeletal organization by stimulating myosin light chain (MLC) phosphorylation. Consequently, the homing of intra-tumorally infiltrated DCs to tumor-draining lymph nodes was enhanced, leading to the induction of more robust cytotoxic T cells. Ultimately, rebalancing the TGF-β/PGE2 ratio amplified the therapeutic effects of RT, resulting in increased intra-tumoral infiltration and activation of CD8+ T cells, and improved tumor control and overall survival rate in mice. DC depletion experiments verified that the improvement in tumor control is directly correlated with the involvement of DCs via the PGE2-MLC pathway. This study emphasizes the importance of maintaining a balanced cytokine environment during RT, particularly hypofractionated RT; and it is advisable to block TGF-β while preserving PGE2 in the tumor microenvironment in order to better stimulate DC homing and DC -T priming.
Keywords: dendritic cell, radiotherapy, cervical cancer, TGF-β

 Global reach, higher impact
Global reach, higher impact