10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2024; 20(5):1778-1795. doi:10.7150/ijbs.92897 This issue Cite
Review
Exosomes: The emerging mechanisms and potential clinical applications in dermatology
Department of Plastic and Cosmetic Surgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030, China.
# Co-first author: Both authors contributed equally to this work
Received 2023-12-4; Accepted 2024-2-15; Published 2024-2-25
Abstract
Skin tissue, composed of epidermis, dermis, and subcutaneous tissue, is the largest organ of the human body. It serves as a protective barrier against pathogens and physical trauma and plays a crucial role in maintaining homeostasis. Skin diseases, such as psoriasis, dermatitis, and vitiligo, are prevalent and can seriously impact the quality of patient life. Exosomes are lipid bilayer vesicles derived from multiple cells with conserved biomarkers and are important mediators of intercellular communication. Exosomes from skin cells, blood, and stem cells, are the main types of exosomes that are involved in modulating the skin microenvironment. The dysregulation of exosome occurrence and transmission, as well as alterations in their cargoes, are crucial in the complex pathogenesis of inflammatory and autoimmune skin diseases. Therefore, exosomes are promising diagnostic and therapeutic targets for skin diseases. Importantly, exogenous exosomes, derived from skin cells or stem cells, play a role in improving the skin environment and repairing damaged tissues by carrying various specific active substances and involving a variety of pathways. In the domain of clinical practice, exosomes have garnered attention as diagnostic biomarkers and prospective therapeutic agents for skin diseases, including psoriasis and vitiligo. Furthermore, clinical investigations have substantiated the regenerative efficacy of stem cell-derived exosomes in skin repair. In this review, we mainly summarize the latest studies about the mechanisms and applications of exosomes in dermatology, including psoriasis, atopic dermatitis, vitiligo, systemic lupus erythematosus, systemic sclerosis, diabetic wound healing, hypertrophic scar and keloid, and skin aging. This will provide a novel perspective of exosomes in the diagnosis and treatment of dermatosis.
Keywords: skin, exosomes, inflammatory skin disease, autoimmune skin disease, skin regeneration
1. Introduction
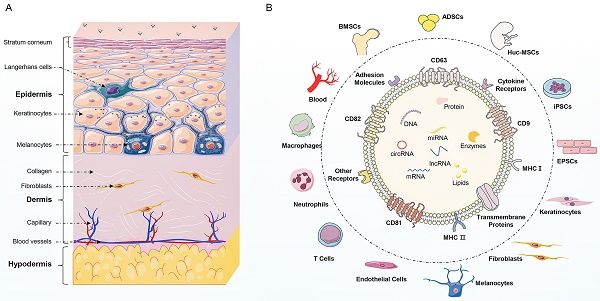
Skin, the largest organ of the human body, serves as a protective barrier against pathogens and physical trauma and plays a crucial role in maintaining homeostasis (1). It is composed of epidermis, dermis, and subcutaneous tissue, each with distinct cell types and functions. The epidermis is the outermost layer of skin and mainly consists of keratinocytes. In addition to keratinocytes and melanocytes, Langerhans cells are also found in the epidermis and are responsible for pigment synthesis and immune response respectively (2). The second layer is the dermis composed of connective tissue. Dermal fibroblasts produce collagen and elastin fibers comprising extracellular matrix (ECM), which provide strength and elasticity to the skin (3). The deepest layer of the skin is the subcutaneous tissue, which encompasses adipocytes, mesenchymal stem cells (MSCs), and blood vessels (4). Skin homeostasis depends on the complex and interconnected relationships among various skin cells, including keratinocytes, macrophages, melanocytes, fibroblasts, MSCs, and endothelial cells. Immune system disorders, inflammation, and wound can disrupt the skin barrier, consequently leading to the instability and dysfunction of skin (5). These skin-related disorders can be broadly categorized as autoimmune skin diseases, inflammatory skin diseases, and skin regeneration. These skin-related disorders are prevalent and can seriously impact the quality of patient life. Current treatment options for these skin diseases often focus on symptom management, but the limited efficacy and side effects are unsatisfactory for patients (6). Therefore, it is intriguing to understand the underlying mechanisms of skin diseases and develop targeted therapies.
Recently, exosomes have emerged as important mediators of intercellular communication (7). Exosomes are lipid bilayer vesicles derived from cells, with a diameter ranging from 30 to 100 nm (8). Regarding the distinctive biomarkers, exosomes are enriched in a group of conserved proteins, including tetraspanins (CD81, CD63, CD9), heat shock proteins (HSP70, HSP90), biogenesis-related proteins (ALIX, TSG101), and major histocompatibility complex (MHC-I, MHC-II) (9). Besides, exosomes contain multiple proteins, lipids, and nucleic acids, including mRNAs, miRNAs, lncRNAs, and circRNAs. These active cargoes in exosomes are involved in complex intercellular communication between different cells and regulate fundamental cellular processes, such as proliferation, differentiation, migration, and apoptosis (10). Therefore, exosomes can regulate the function of neighboring or distant cells to modulate inflammation, angiogenesis, fibrosis, and tissue regeneration.
(Summary Figure). The structural organization of the skin and the compositions of exosomes. (A) Skin is composed of epidermis, dermis, and hypodermis. The epidermis consists of keratinocytes, along with melanocytes and Langerhans cells. The dermis contains fibroblasts, which produce collagen and elastin fibers comprising extracellular matrix, that provide strength and elasticity to the skin. The dermis also contains blood vessels and nerve endings. The hypodermis is composed of adipocytes, mesenchymal stem cells, and blood vessels. (B) The source of exosomes involved in the regulation of skin function mainly include skin cells (keratinocytes, fibroblasts, melanocytes, and endothelial cells), immune cells (T cells, neutrophils, and macrophages), stem cells (ADSCs, BMSCs, hucMSCs, iPSCs, and EPSCs) and blood. Besides, exosomes display similar biomarkers, including tetraspanins (CD81, CD82, CD9), heat shock proteins (HSP70, HSP90), biogenesis-related proteins (ALIX, TSG101), and major histocompatibility complex (MHC-I and MHC-II). Additionally, exosomes enrich in multiple bioactive cargoes, mainly proteins, lipids, and nucleic acid, including DNA, mRNA, miRNA, lncRNA, and circRNA.

In the context of skin diseases, exosomes mediate the communication of skin cells and modulate the skin microenvironment. Exosomes from skin cells, blood, and MSCs, are the main types of exosomes that are involved in modulating the skin microenvironment (11). The dysregulation of skin cell interactions and alterations in exosome cargoes are the participants in the complex pathogenesis of chronic inflammatory and autoimmune skin diseases (12). Therefore, exosomes are promising diagnostic and therapeutic targets for skin diseases. Circulating exosomes of patients with skin diseases have specific nucleic acid protein expression profiles for the diagnosis of skin disease. The cargoes in circulating exosomes can be transferred to skin cells along with blood flow to promote the development of skin diseases (13). For example, circulating exosomal miRNAs may serve as auxiliary indicative biomarkers for the diagnosis and progression of vitiligo (14). In addition, exogenous exosomes, especially MSC-exos, possess huge potential in skin disease treatment and skin regeneration (5). Exogenous exosomes possess the distinctive properties of transferring biologically active molecules and modulating cellular responses, contributing to the restoration of cellular function and the immune microenvironment in skin diseases (15). Compared with traditional dermatological therapy, exosome as a cell-free therapy offers several advantages, including targeted delivery, low toxicity, facilitated tissue repair, personalized treatment, and multifunctionality (16). These advantages endow exosome-based therapy promising frontier for more effective, safe, and personalized treatment options for skin diseases.
This review aims to provide a comprehensive summary of the most recent research findings regarding the mechanisms and applications of exosomes of dermatology, including psoriasis, atopic dermatitis (AD), vitiligo, systemic lupus erythematosus (SLE), systemic sclerosis (SSc), diabetic wound healing, hypertrophic scar and keloid, and skin aging. We hope this will provide further insights into the mechanisms and applications of exosomes in dermatology and evidence supporting the utilizing exosomes as novel diagnostic biomarkers and therapeutic agents in skin disease. To achieve goals, we conducted a literature search on Pubmed using the keywords “exosomes” and the 8 related fields within the past 5 years. The identified studies involved in cell, animal experiments, or clinical trials, especially the original articles were included according to relevance to the topic.
2. Exosomes in inflammatory skin disease
2.1 Psoriasis
Psoriasis is a chronic inflammatory skin disorder characterized by erythematous plaques, scaling, and pruritus. The main pathological manifestations are the abnormal proliferation of keratinocytes and immune dysregulation (17). Immune cells are infiltrated in psoriatic lesions, including macrophages, T cells, dendritic cells (DCs), and neutrophils. The interaction between immune cells and keratinocytes, facilitated by exosomes and inflammatory cytokines, promotes the development of psoriasiform skin inflammation (18).
2.1.1 Exosomes in psoriasis pathogenesis
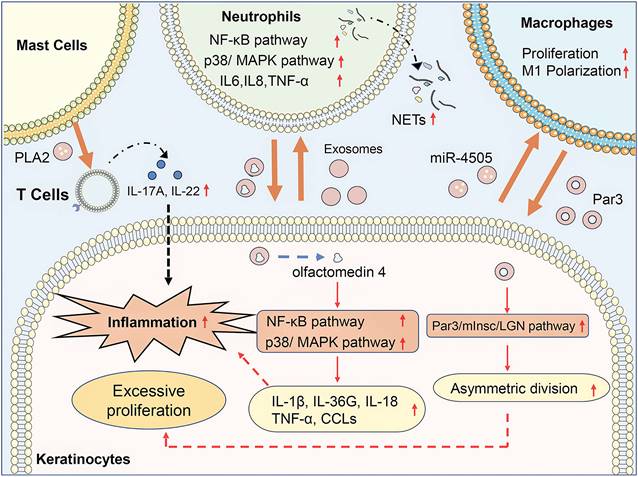
Exosome-mediated communication between activated keratinocytes and infiltrated immune cells contributes to the progression of psoriasis (Figure 1). Jiang et al. found that psoriasis-like keratinocytes, which were induced by IL-17A, IL-22, and TNF-α, promoted neutrophil extracellular trap (NET) formation and pro-inflammatory cytokine expression in neutrophils via activating the NF-κB and p38 MAPK pathway (19). Besides, subcutaneously injection of exosomes from imiquimod (IMQ)-treated epidermis exacerbates the psoriasis-related inflammation and neutrophils infiltration in IMQ-induced psoriasis mouse models. Sun et al. demonstrated that keratinocytes from psoriasis patients had a lower level of vitamin D receptor (VDR) expression, and exosomes from VDR-deficient keratinocytes transferred miR-4505 to macrophages, promoting their proliferation and M1 polarization (20). It indicated that keratinocyte exosomes affected the epidermal microenvironment of psoriasis via regulating the function of neutrophils and macrophages. Moreover, immune cells could also interact with other immune cells and keratinocytes through exosomes, which modulated immune responses and led to the progression of psoriasis. Neutrophils from patients with generalized pustular psoriasis secreted more exosomes than controls and induced higher expressions of inflammatory genes, including IL-36G, TNF-α, IL-1β, IL-18, and C-X-C motif chemokine ligands (CCLs) in keratinocytes via activating MAPK and NF-κB signaling pathways (21). Proteomic profiles of exosomes revealed that olfactomedin 4 might mediate the inflammatory responses of generalized pustular psoriasis. Macrophage-derived exosomes manipulated the asymmetric division of keratinocytes to produce a large number of transit-amplifying cells in psoriasis via activating the Par3/mInsc/LGN signaling pathway (22). IFN-α-treated mast cells released exosomes containing cytoplasmic PLA2, which transferred to CD1a-reactive psoriatic T cells and led to the generation of neo-lipid antigens (23). The neolipid antigens were subsequently recognized by lipid-specific CD1a-reactive T cells and induced the production of IL-22 and IL-17A in psoriasis patients.
Circulating exosomes from patients with psoriasis have specific gene expression profiles and phospholipid profiles, which are correlated with the severity of psoriasis. IL-23 and TNF-α mRNA expression levels in circulating exosomes of psoriasis patients were significantly higher than those in healthy controls, and circulating exosomal IL-23 level was significantly positively correlated with severity index score (24). Moreover, circulating exosomal IL-17A level was significantly higher in patients with moderate-to-severe psoriasis compared with those with mild psoriasis (25). Chen et al. performed miRNA sequencing for serum exosome of psoriasis vulgaris patients and healthy controls. They identified 246 differentially expressed miRNAs between the two groups and found that the predicted target genes of differentially expressed miRNAs were enriched in metabolic processes (26). Paolino et al. demonstrated that the plasma of psoriatic patients had a higher concentration of exosomes in comparison to that of healthy controls (27). The phospholipid profile of exosomes showed that phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylglycerol, and lysoPC levels were increased in psoriatic patients. Besides, the ustekinumab therapy on psoriatic patients reverted the PE and PC lipid composition of circulating exosomes toward that of healthy control. PE and PC levels of circulating exosomes could be used in the diagnosis and therapeutic effect prediction of psoriasis.
Exosomes in psoriasis pathogenesis. In psoriasis, keratinocyte exosomes promote neutrophil extracellular trap (NET) formation and pro-inflammatory cytokine expression in neutrophils via activating the NF-κB and p38/MAPK pathway and promote their proliferation and M1 polarization of macrophages via transferring miR-4505. Neutrophil exosomes containing olfactomedin 4 activate NF-κB and MAPK pathway in keratinocytes to promote expression of IL-1β, IL-36G, IL-18, TNF-α, and C-X-C motif chemokine ligands (CCLs). Macrophage exosomes manipulated the asymmetric division of keratinocytes via activating the Par3/mInsc/LGN signaling pathway. Mast cell-released exosomes transferred cytoplasmic PLA2 to CD1a-reactive psoriatic T cells inducing the production of IL-22 and IL-17A in psoriasis patients. In conclusion, exosome-mediated communication between activated keratinocytes and infiltrated immune cells contributes to the progression of psoriasis.
2.1.2 Exosomes in psoriasis application
Exosomes from stem cells, including epidermal stem cells (ESCs), human umbilical cord MSCs (hucMSCs), and umbilical cord blood mononuclear cells (UCB-MNCs), are proven to alleviate psoriasis through immune regulation. Topical application of ESC-exos relieved IMQ-induced psoriasis in mice via reducing IL-17 and terminal complement activation complex C5b-9 in the skin (28). Topically applied exosomes are mainly localized to the stratum corneum of human skin. Besides, subcutaneous injection of hucMSC-exos significantly reduced the IL-23, IL-17, and CCL20 expression of keratinocytes and inhibited the maturation and activation of DCs in IMQ-induced psoriasis mice via down-regulating STAT3 phosphorylation (29). In addition, Rodrigues et al. found that UCB-MNC-exos promoted the differentiation of anti-inflammatory M2 macrophages and inhibited the proliferation and cytokine production of activated T cells. UCB-MNC-exos also reduced psoriasis-related inflammation in vitro 3D Model and an IMQ-induced psoriasis mouse model (30).
2.2 Atopic Dermatitis
AD is a chronic inflammatory skin condition characterized by intense itching, redness, and dryness of the skin (31). The pathogenesis of AD is thought to be related to impaired skin barrier function, immune dysregulation, and skin microbiota dysbiosis (32).
2.2.1 Exosomes in AD pathogenesis
Skin microorganisms have been proven to affect AD-related inflammation by secreting exosomes or changing the contents of skin cell exosomes. S. aureus and C. albicans frequently colonized the atopic skin. Extracellular vesicles (EVs) from S. aureus promoted the secretion of pro-inflammatory cytokines in human dermal fibroblasts (HDFs), and induced AD-like inflammation in SKH-HR1 mice via thickening epidermal increasing mast cell and eosinophil infiltration (33). Besides, Kobiela et al. reported that S. aureus enhanced filaggrin loading into the keratinocyte exosome via a Toll-like receptor 2 (TLR2)‐mediated mechanism. The filaggrin removal system protected the keratinocytes from keratinocyte premature death and epidermal barrier dysfunction and helped safeguard bacterial growth (34). Kobiela et al. also reported that AD cytokines and C. albicans collaboratively changed the surface glycosylation pattern of keratinocyte exosomes to increase their interaction with DCs via inhibitory Siglec receptors (35). In patients with AD, mast cells mediated immediate hypersensitivity and led to the release of various inflammatory mediators, such as histamine, cytokines, and chemokines. Kwon et al. reported that exosomes derived from dinitrochlorobenzene (DNCB)-treated Nc/Nga mouse skin mast cells enhanced the invasion and autophagy of HaCaT cells and dermal fibroblast cells by up-regulating HDAC6/CXCL13/SIRT1 axis (36). Furthermore, circulating exosomes of pediatric AD patients significantly increased the apoptosis rate and the expression levels of the pro-inflammatory cytokines in keratinocytes, including K6, K10, TSLP, and IL-33 (37). Meng et al. reported that over a hundred transfer RNA-derived fragments (tRFs) from plasma exosomes were differentially expressed in pediatric patients with AD than in healthy controls. Among them, tRF-28-QSZ34KRQ590K was predicted to be involved in multiple functions and pathways associated with AD and exhibited significance in the receiver operating characteristic (ROC) curve (38). Thus, exosomes are involved in the pathogenesis of AD and might be potential biomarkers for diagnosis and targeted treatment of AD.
2.2.2 Exosomes in AD application
Adipose-derived stem cell (ADSC)-exos are proven to effectively suppress inflammatory responses and promote skin barrier repair in patients with AD, thereby offering a potential therapeutic approach for treating AD. Cho et al. reported that intravenous or subcutaneous injection of ADSC-Exos ameliorated AD symptoms in NC/Nga mice treated with house dust mite via decreasing serum IgE levels, immune cell infiltration, and the IL-4, IL-23, IL31, and TNF-α expression (39). Besides, subcutaneous injection of ADSC-exos promoted epidermal barrier repair in an oxazolone-induced AD mouse model via enhancing stratum corneum hydration decreasing the levels of inflammatory cytokines, such as IL-4, IL-5, IL-13, IL-17, IFN-γ, TNF-α, and TSLP, and stimulating the production of epidermal ceramide (40). Meanwhile, deep RNA sequencing analysis of skin lesions demonstrated that ADSC-exos normalized the expression of genes altered during AD pathogenesis, which involved skin barrier, lipid metabolism, cell cycle, and inflammatory response. Shi et al.reported that miR-147a-overexpressing ADSC-exos inhibited inflammatory response, cell apoptosis, and angiogenesis in the DNCB-induced AD mouse model via targeting VEGF-A and MEF2A-TSLP axis (41). In addition, exosomes based on skin component cells or other stem cells, such as dermal fibroblasts and iPSC-derived MSCs (iMSCs), possess outstanding advantages in modulating inflammation and promoting tissue regeneration and repair and are expected to be candidates for AD therapy. Yoo et al. reported that exosomes derived from dermal fibroblasts restored expression levels of skin permeability barrier maintenance biomarkers in DNCB-treated keratinocytes, such as FLG, LOR, IVL, and HAS1, thus increasing the recovery rate of skin damage in AD (42). Yoon et al.reported that topical application of exosomes derived from IFN-γ-primed iMSCs significantly improved clinical and histological outcomes of Aspergillus fumigatus-induced AD mouse model, including epidermal thickness, inflammatory cell infiltration, transepidermal water loss (TEWL) level and serum IgE level (43).
3. Exosomes in autoimmune skin disease
3.1 Vitiligo
Vitiligo is a chronic skin disorder characterized by the loss of melanocytes, resulting in depigmented patches on the skin. Genetic predisposition, oxidative stress, and immune dysregulation are thought to contribute to the destruction of melanocytes (44). Treatment options for vitiligo include topical corticosteroids, calcineurin inhibitors, phototherapy, and surgical interventions (45). However, the effectiveness of these treatments varies, and there is still a need for more targeted and personalized therapies for vitiligo.
3.1.1 Exosomes in vitiligo pathogenesis
Circulating exosomal miRNAs contribute to melanocyte dysregulation in vitiligo and are associated with the progression of vitiligo. For instance, circulating exosomal miR-493-3p was significantly increased in segmental vitiligo (SV) patients. MiR-493-3p-overexpressing keratinocyte induced melanocyte apoptosis and decreased melanin synthesis through inhibiting HNRNPU expression and increasing dopamine secretion (46). Circulating exosomes from vitiligo patients, containing miR-21-5p, inhibited melanin content, tyrosinase activity, and melanogenesis-related protein expression in melanocytes via inhibiting SATB1 expression (47). Moreover, Luo et al. reported that exosomal hsa-miR-487b-3p in serum was significantly lower in vitiligo patients and positively correlated with the area of lesions in progressive vitiligo patients (14). Keratinocytes are the main cell type in the epidermis and play an important role in maintaining melanocyte homeostasis. Zhao et al. demonstrated that miR-200c expression was significantly declined in keratinocyte exosomes from vitiligo lesions than that from healthy skin. And miR‐200c from keratinocyte exosomes up-regulated the expression of melanogenesis‐related genes in melanocytes via inhibiting SOX1 and activating β‐catenin (48). Overall, by carrying specific miRNAs, circulating exosomes and keratinocyte exosomes played crucial roles in the pathogenesis of vitiligo (Table 1).
The mechanisms of exosomes in vitiligo pathogenesis
| Exosome Source | Model | Mechanism | Ref. |
|---|---|---|---|
| Blood of segmental vitiligo patients | Human primary keratinocytes and melanocytes | MiR-493-3p-overexpressing keratinocyte induced melanocyte apoptosis and decreased melanin synthesis through miR-493-3p/HNRNPU/COMT/dopamine axis | (46) |
| Peripheral blood of vitiligo patients | Human melanocytes cell | Circulating exosomes inhibited melanin content, tyrosinase activity, and melanogenesis-related protein expression via transferring miR-21-5p and inhibiting SATB1 expression | (47) |
| Serum of progressive vitiligo patients | Progressive vitiligo patients | Hsa-miR-487b-3p was significantly lower and positively correlated with the area of lesions | (14) |
| Keratinocytes in vitiligo lesions | NHEM | MiR‐200c up-regulated the expression of melanogenesis‐related genes via inhibiting SOX1 and activating β‐catenin | (48) |
3.2 Systemic lupus erythematosus
SLE is a complex autoimmune disease characterized by the production of autoantibodies and immune system dysregulation (49). Dysregulation of the immune system leads to the production of autoantibodies that target various organs and tissues, resulting in inflammation and tissue damage (50). Ongoing research aims to develop targeted therapies that modulate the immune response and improve outcomes for SLE patients.
3.2.1 Exosomes in SLE pathogenesis
SLE serum exosomes are immunologically active and can be used as a novel biomarker to predict SLE disease activity (Table 2). Serum exosome levels are significantly higher in SLE patients than in healthy controls and were positively correlated with SLE disease activity. Treatment of SLE serum exosomes up-regulated the expression of IL-1β, IFN-α, TNF-α, and IL-6 in healthy peripheral blood mononuclear cells (PBMCs) in a TLR-dependent manner (51). Song et al. performed RNA-sequencing and proteomic analysis and revealed that the differently expressed exosomal miRNAs and proteins mainly enriched in pathways associated with inflammation and autophagy (52). Li et al. found that exosomal miR-21 and miR-155 were elevated in the serum of SLE patients compared to healthy controls, with even higher levels in SLE patients with lupus nephritis (LN) (53). Salvi et al. indicated that plasma exosomes from SLE patients activated the secretion of IFN-α in human plasmacytoid DCs (54). Besides, exosome-delivered miRNAs containing an IFN induction motif promoted activation, maturation, and survival of human plasmacytoid DCs in SLE patients via stimulating TLR7 expression.
The mechanisms of exosomes in systemic lupus erythematosus pathogenesis
| Exosome Source | Model | Mechanism | Ref. |
|---|---|---|---|
| Serum of SLE patients | PBMCs | Up-regulated the expression of IFN-α, TNF-α, IL-1β, and IL-6 in healthy peripheral blood mononuclear cells (PBMCs) in a TLR-dependent manner | (51) |
| Plasma of SLE patients | Exosome-delivered microRNAs promote IFN-α secretion by human plasmacytoid DCs via TLR7 | (54) | |
| Blood of SLE patients | SLE patients | Differently expressed exosomal miRNAs and proteins mainly enriched in pathways associated with inflammation and autophagy | (52) |
| Serum of SLE patients with or without lupus nephritis | MiR-21 and miR-155 were elevated in the serum of SLE patients compared to healthy controls, with even higher levels in SLE patients with LN | (53) | |
| Serum of SLE patients | MiRNAs containing an IFN induction motif promoted activation, maturation, and survival of human plasmacytoid DCs via stimulating TLR7 expression | (55) | |
| T cells of Lck-BPI Tg mice | C57BL/6J mice | Increased autoantibody levels, tissue IFN-γ levels, and induced hepatitis, nephritis, and arthritis in recipient mice | (56) |
| T cells of T-cell-specific BPI transgenic mice | Stimulated IL-1β production of macrophages and induced liver, kidney, and joint inflammatory responses | (57) |
T-cell exosomes from SLE patients could induce tissue inflammation via transferring specific proteins (Table 2). The higher expression of eosinophil cationic protein (ECP) in T cell-derived exosomes from SLE patients compared to healthy controls. Exosomes derived from T cells of Lck-ECP-transgenic mice increased autoantibody levels, tissue IFN-γ levels, and induced hepatitis, nephritis, and arthritis in recipient mice (55). Proteomics analysis identified that bactericidal/permeability-increasing protein (BPI) was highly expressed in T cell exosomes from SLE patients compared to that from healthy controls (56). Exosomes from BPI-overexpressing T cells stimulated IL-1β production of macrophages and induced liver, kidney, and joint inflammatory responses in recipient mice. These indicated that ECP and BPI in the T cell-derived exosomes might be a key pathogenic factor for SLE-related tissue inflammation. Furthermore, T cell exosome levels were positively correlated with the activation of myeloid cells and lymphocytes in SLE, which might be mediated by type I IFN signaling (57).
3.2.2 Exosomes in SLE application
Bone marrow mesenchymal stem cells (BMSCs)-exos can promote M2 macrophage polarization and decrease T cell infiltration, leading to the alleviation of clinical symptoms associated with SLE. BMSC-exos increased the radio of M2-like macrophages and reduced the T cell infiltration in the kidney to alleviate SLE nephritis in MRL/lpr mice via transferring miR-16 and miR-21 (58). Dou et al. suggested that tsRNA-21109 was down-regulated in SLE patients compared to healthy control, and BMSC exosomal tsRNA-21109 alleviated SLE by inhibiting macrophage M1 polarization (59). In addition to inducing M2 macrophage polarization like BMSC-exos, hucMSC-exos also regulated the balance of the T cell subset and the activation of B cells in SLE. It was reported that intravenous injection of hucMSC-exos alleviated nephritis, liver, and lung injuries of MRL/lpr mice via increasing the radio of M2 macrophages and the expansion of Tregs (60). The ratio between Th17 and Treg cell subsets was increased in SLE patients compared to healthy controls. HucMSC-exos restored the balance of Th17/Treg and inhibited inflammatory cytokine secretion of PBMCs from SLE patients via delivering miR-19b and inhibiting KLF13 expression (61). Additionally, Zhao et al. detected the effect of hucMSC-exos on B cells selected from PBMC of SLE patients, showing that hucMSC-exos promoted B cell apoptosis, prevented B cell overactivation, and reduced IL-16, IL-10, and TNF-α expression of B cells via transferring miR-155 and activating ERK pathway (62). In conclusion, hucMSC-exos inhibited the overactivation of B cells in SLE patients via the miR-155/SHIP-1/ERK axis. Among the drugs currently used to treat SLE, immunosuppressants are often limited due to their risk of side effects. Compared to traditional therapies, MSC-exos have the potential for targeted delivery with minimal side effects and can regulate the immune response, reduce inflammation, and promote tissue repair to address the underlying pathogenesis of SLE (63). In addition, MSC-exos are easier to preserve and manage with low immunogenicity and easily cross the blood-brain barrier compared to stem cell therapy.
3.3 Systemic sclerosis
SSc, also known as scleroderma, is a chronic autoimmune disease characterized by fibrosis and vascular abnormalities (64). Immune dysregulation leads to the activation of fibroblasts and excessive production of collagen, resulting in tissue fibrosis and vascular damage. Treatment options consist of immunosuppressants, vasodilators, and medications to manage specific organ involvement (65). The development of targeted therapies that modulate the immune response and prevent fibrosis in SSc is a current research concern.
3.3.1 Exosomes in SSc pathogenesis
Fibroblasts are key components of the dermis and their overactivation is a characteristic feature of SSc. Activated fibroblasts lead to the excessive production and deposition of ECM components, which contributes to the characteristic skin thickening and organ fibrosis in SSc patients. Bhandari et al. found that exosomes from human SSc dermal fibroblasts increased pro-inflammatory and pro-fibrotic cytokine secretion of macrophages compared with those incubated with healthy control fibroblasts (66). Besides, co-culture with macrophages pretreated with SSc exosomes promoted the collagen and fibronectin production of fibroblasts. This indicated that macrophages and fibroblasts were cooperative mediators of fibrosis in SSc. In addition, the expressions of exosomal biomarkers CD63, CD81, and CD9 were significantly increased in SSc dermal fibroblasts (67). Exosomes derived from SSc dermal fibroblasts stimulated the expression of type I collagen in normal fibroblasts. Interestingly, SSc patients with vascular involvement had reduced levels of serum exosomes, while exosomes derived from serum accelerated skin ulcer healing in mice. These findings suggested that vascular abnormalities in SSc might disrupt the transfer of exosomes from skin tissue to the bloodstream, leading to delayed wound healing. Exosomes from neutrophils induced inflammation, vasculopathy, and fibrosis in the pathological process of SSc. Li et al. found that the differently expressed miRNAs and lncRNAs between the neutrophil exosomes from diffuse cutaneous systemic sclerosis (dSSc) patients and those from healthy controls were enriched in Wnt, AMPK, IL-23, and NOTCH signaling pathways (68). Besides, dSSc neutrophil exosome treatment up-regulated the fibrosis-related gene expression of human dermal microvascular endothelial cells (HDMECs) and fibroblasts. Moreover, neutrophil exosomes from SSc patients suppressed the proliferation and migration of HDMECs via up-regulating S100A8/A9 (69). Circulating exosomes also participated in the fibrosis prosses in SSc. Wermuth et al. demonstrated that 6 profibrotic miRNAs were increased and 10 antifibrotic miRNAs were decreased in SSc serum exosomes (70). Serum exosomes isolated from SSc patients caused dose-dependent stimulation of fibrosis-related genes and myofibroblast activation in HDFs in vitro.
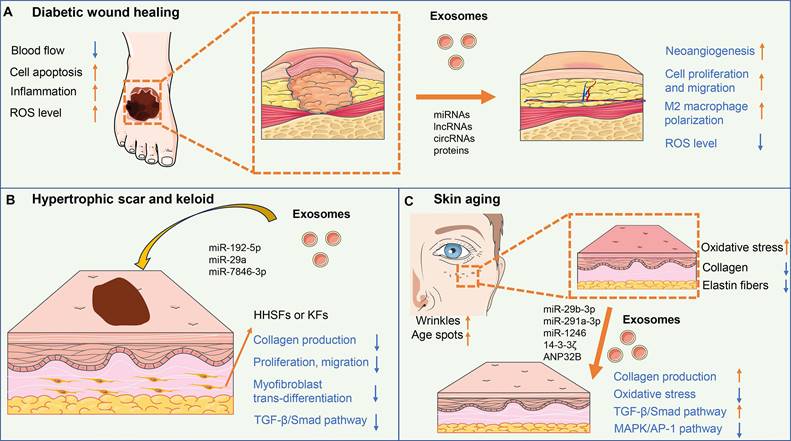
The mechanisms of exosomes in skin regeneration. (A) Exosomes could improve angiogenesis, cell proliferation and migration, and M2 macrophage polarization, and reduce ROS level, thus accelerating diabetic wound healing. (B) ADSC-exos containing miR-29a, miR-192-5p, and miR-7846-3p inhibit collagen production, proliferation, migration, and myofibroblast trans-differentiation of fibroblasts via targeting TGF-β/Smad pathway, resulting in reduced excessive fibrosis and scar formation. (C) Exosomes could up-regulate collagen synthesis and reduced oxidative stress of fibroblasts via activating the TGF-β/Smad pathway and inhibiting the MAPK/AP-1 pathway, thus enhancing skin elasticity and easing the wrinkles for anti-aging.
3.3.2 Exosomes in SSc application
Recently, multiple MSC-exos can reduce fibrosis of fibroblasts and regulate the function of immune cells to ameliorate SSc. HucMSC-exos alleviated skin fibrosis, suppressed the epithelial-mesenchymal transition (EMT) process, and promoted M1 polarization of macrophages in BLM-treated mice (71). In another research, hucMSC-exos reduced dermal thickness, down-regulated fibrosis-related gene expression, and inhibited myofibroblast activation in BLM-treated mice via suppressing TGF-β/Smad signaling pathway (72). In addition, MSC-exos enriched anti-fibrotic and anti-inflammatory miRNAs, which were potential for the treatment of SSc. BMSC-exos attenuated the fibrosis of BLM-treated mice via transferring miR-214 to inhibit the IL-33/ST2 axis (73). Besides, miR-196b-5p from BMSC-exos significantly inhibited BLM-induced dermal fibrosis in mice via inhibiting collagen production, myofibroblast activation, and infiltration of macrophages and CD3+T cells in lesional skin (74). Rozier et al. reported that ADSC-exos attenuated clinical symptoms and down-regulated the expression of several pro-fibrotic, remodeling, and anti-apoptotic factors in HOCl-induced mouse SSc model via delivering miR-29a-3p (75). These findings highlight the potential of MSC-exos and their cargoes as promising therapeutic strategies for SSc.
4. Exosomes in skin regeneration
4.1 Diabetic wound healing
Diabetic wounds are a common complication of diabetes mellitus and are characterized by impaired wound healing and increased risk of infection (76). High blood sugar levels in diabetes can lead to damage to blood vessels, resulting in reduced blood flow to the wound site and delayed wound healing. Additionally, immune dysfunction in diabetes impairs the ability to fight infection and causes persistent inflammation of the wound area. The clinical treatment for diabetic wounds mainly includes wound debridement, infection control, and the use of dressings, growth factors, and negative pressure wound therapy (77) (Figure 2A).
4.1.1 Exosomes in diabetic wound pathogenesis
During the process of diabetic wound healing, exosomes mediate the intercellular interaction among various skin cells, including keratinocytes, fibroblasts, macrophages, endothelial cells, and ESCs. Macrophage-derived exosomes accelerated diabetic wound healing by inducing angiogenesis, enhancing cell bioactivity, and inhibiting inflammatory cytokines (78). Lean adipose tissue macrophage-derived exosomes promoted M2 macrophage polarization to improve diabetic wound healing via transferring miR-222-3p to inhibit Bim expression in mice (79). ESC-exos induced M2 macrophage polarization to promote diabetic wound healing via miR-203a-3p/SOCS3-mediated JAK2/STAT3 signaling activation (80). ESC-exos accelerated diabetic wound healing via promoting cell proliferation, angiogenesis, and M2 macrophage polarization. ESC-exos regulated the phosphatidylinositol-3 kinase/protein kinase B and TGF-β signaling pathways via delivering miRNAs (81). Endothelial progenitor cell-derived exosomes (EPC-exos) promoted proliferation and migration while inhibiting apoptosis of HaCaTs challenged by HG via miR-182-5p/ PPARG axis, thus accelerating diabetic wound healing (82). EPC-exos promoted angiogenesis in diabetic wound healing via delivering miRNA-221-3p (83). Exosomes derived from autologous dermal fibroblasts improved angiogenesis and epithelial regeneration in diabetic wounds via activating the AKT/β-catenin pathway (84). Exosomes from human keratinocytes enhanced the phagocytosis and pro-healing function of macrophages to promote diabetic wound healing via MALAT1/miR-1914-3p/FGE8 axis (85).
Endogenous circulating exosomes are involved in the progression of diabetic wounds. MiR-20b-5p was overexpressed in the circulating exosome of diabetic patients and impaired diabetic wound healing via targeting VEGFA and inhibiting the function of HSFs (86). Circulating exosomes of diabetic patients inhibited angiogenesis in diabetic wound healing via transferring miR-15a-3p and inhibiting NOX5/ROS signaling pathway (87). Circulating exosomal miR-20b-5p inhibited angiogenesis in diabetic wound healing via inhibiting the Wnt9b/β-catenin pathway (88). Circulating exosomal miR-181b-5p promoted cell senescence and inhibited angiogenesis to impair diabetic foot ulcer (DFU) healing via the NRF2/HO-1 pathway (89).
4.1.2 Exosomes in diabetic wound healing application
MSC-exos have emerged as an attractive approach in diabetic wound regeneration due to their excellent pro-regenerative and immunoregulatory functions. Exosomes from ADSCs, hucMSCs, BMSCs, induced pluripotent stem cells (iPSCs), menstrual blood-derived MSCs, and human urine-derived stem cells were proven to accelerate the healing of diabetic wounds. The current evidence on exosomes for the treatment of diabetes is largely based on the results of animal experiments and in vitro experiments. ADSC-exos protected the skin cells against oxidative stress damage and enhanced angiogenesis through activating the eHSP90/LRP1/AKT axis and enhancing SIRT3 and SOD2 expression, ultimately promoting diabetic wound healing (90) (91). Diabetic ADSC-exos promoted diabetic wound healing via stimulating macrophages to secrete more TGF-β1 to activate the TGF-β/Smad3 signaling pathway in fibroblasts (92). HucMSC-exos reduced oxidative stress, and enhanced collagen deposition, angiogenesis, and M2 macrophage polarization to accelerate diabetic wound healing (93) (94). Exosomes from menstrual blood-derived mesenchymal stem cells (MenSC-exos) promoted angiogenesis and M2 macrophage polarization and reduced scar formation in diabetic wound healing, and was mainly mediated by NF-κB pathway (95). Exosomes from human urine-derived stem cells were reported to promote angiogenesis in diabetic wounds via transferring DMBT1 (96).
The ncRNAs, including miRNAs, lncRNAs, and circRNAs are crucial bioactive substances in MSC-exos, which are widely involved in the processes of angiogenesis, collagen synthesis, inflammation regulation, and ECM remodeling. HucMSC-exos improved angiogenesis in the diabetic wound by transferring lncRNAs related to angiogenesis, such as PANTR1, H19, OIP5-AS1, and NR2F1-AS1 (97). Hair follicle MSC exosomal lncRNA H19 promoted proliferation and migration and inhibited NLRP3-related pyroptosis in keratinocytes to promote diabetic wound healing (98). In addition, genetic engineering editing in MSCs can improve the therapeutic effect of MSC-exos on diabetic wounds. Exosomes secreted by multiple stem cells play an active role in repairing diabetic wounds, including miR-132-overexpressing ADSCs (99), linc00511-overexpressing ADSCs (100), lncRNA KLF3-AS1-overexpressing BMSCs (101), HOTAIR-overexpressing BMSCs (102), lncRNA H19-overexpressing BMSCs (103), mmu_circ_0000250-overexpressing ADSCs (104), mmu_circ_0001052-overexpressing ADSCs (105), circ-Astn1-overexpressing ADSCs (106), circHIPK3-overexpressing hucMSCs (107), circ-ITCH-overexpressing BMSCs (108), and Nrf2-overexpressing ADSCs (109). The detailed information can be seen in Table 3.
In addition to genetic engineering editing, drug pretreating, and hypoxic culture can also improve the therapeutic effect of MSC-exos. Exosomes from melatonin-stimulated MSCs improved diabetic wound healing and promoted macrophage M2 polarization via inhibiting PTEN/AKT pathway (110). Exosomes from MSCs pretreated with pioglitazone (PGZ-Exos) improved angiogenesis in diabetic wound healing via activating the PI3K/AKT/eNOS pathway (111). Exosomes from atorvastatin-pretreated MSCs improved angiogenesis via delivering miR-221-3p and activating AKT/eNOS pathway (112).
The mechanism of gene-modified MSC-exos in diabetic wound healing
| Gene Modification | Exosome Source | Model | Mechanism | Ref. |
|---|---|---|---|---|
| MiR-132 overexpressing | ADSCs | Backside full-thickness skin wounds and random skin flaps in STZ-induced diabetic mice | Promoted angiogenesis and M2-macrophages polarization via inhibiting NF-κB signaling pathway | (99) |
| Linc00511 overexpressing | Rat DFU models | Accelerated angiogenesis via up-regulating PAQR3 expression and suppressing Twist1 ubiquitin degradation | (100) | |
| Mmu_circ_0000250 overexpressing | Backside full-thickness skin wounds in STZ-induced diabetic mice | Activated autophagy and promoted angiogenesis of EPCs via inhibiting miR-128-3p to up-regulating SIRT1 axis | (104) | |
| Mmu_circ_0001052 overexpressing | Mouse DFU models | Promoted angiogenesis via miR-106a-5p/FGF4/p38MAPK axis | (105) | |
| Circ-Astn1 overexpressing | Backside full-thickness skin wounds in STZ-induced diabetic mice | Suppressed apoptosis and improved angiogenesis via miR-138-5p/SIRT1/FOXO1 axis | (106) | |
| Nrf2 overexpressing | Rat DFU models | Improved glucose-induced EPC senescence and inhibited ROS production and inflammatory cytokine expression | (109) | |
| LncRNA KLF3-AS1 overexpressing | BMSCs | Backside full-thickness skin wounds in STZ-induced diabetic mice | Stimulated angiogenesis via reversing miR-383-mediated VEGFA inhibition | (101) |
| HOTAIR overexpressing | Backside full-thickness skin wounds in db/db mice | Enhanced angiogenesis via up-regulating VEGF | (102) | |
| LncRNA H19 overexpressing | Mouse DFU models | Inhibited the apoptosis and inflammation of fibroblasts via sponging miR-152-3p and activating PTEN expression to reduce AKT phosphorylation | (103) | |
| Circ-ITCH overexpressing | Mouse DFU models | Inhibited ferroptosis and improved angiogenesis by recruiting TAF15 and activating the Nrf2 signaling pathway | (108) | |
| CircHIPK3 overexpressing | HucMSCs | Mouse DFU models | Promoted angiogenesis via inhibiting miR-20b-5p to activate Nrf2 and VEGFA expression | (107) |
Exosomes from hypoxia ADSCs improved the function of fibroblasts, including proliferation, migration, and collagen formation in diabetic wound healing via activating PI3K/Akt pathways (113).
Exosomes from blood can also be used to treat diabetic wounds. Exosomes derived from platelet-rich plasma (PRP) promoted the re-epithelization and angiogenesis in diabetic wounds via activating RhoA/YAP signaling (114). PRP-exos containing sphingosine-1-phosphate (S1P) promoted angiogenesis in diabetic wound healing via the S1PR1/AKT/FN1 pathway (115). PRP-exos inhibited apoptosis and pyroptosis of DFU fibroblasts to accelerate DFU healing via lncRNA MALAT1 /miR-374a-5p/ DNMT3A (116). Serum exosomes from normal healthy mice accelerated diabetic wound healing by enhancing angiogenesis and collagen synthesis (117).
In summary, MSC-exos can regulate diabetic wound healing via multiple mechanisms, including promoting proliferation and angiogenesis, reducing inflammatory response, and combating oxidative stress. Multitude origins of MSC-exos uniformly contribute to the facilitation of wound repair, potentially via distinct underlying mechanisms. Pomatto et al. compared the differential therapeutic effects of BMSC-exos and ADSC-exos on diabetic ulcer wound healing, confirming that BMSC-exos possessed a major effect on cell proliferation, while ADSC-exos were mainly involved in the angiogenesis process (118). iPSC-exos presented similar vascularization bioactivity compared with iPSC-derived MSC-exos, but with superior anti-inflammatory effect (119). In addition, genetic engineering modification and drug preconditioning can improve the efficacy of MSC-exos in the treatment of diabetic wounds.
4.2 Hypertrophic scar and keloid
Hypertrophic scars and keloids are abnormal wound-healing responses characterized by excessive collagen production and fibrosis (120). The pathogenesis of hypertrophic scars and keloids is believed to involve a combination of genetic, mechanical tension, and growth factor dysfunction. Exosome-mediated cellular communication between fibroblasts and immune cells participates in the formation of hypertrophic scar and keloid (121) (Figure 2B).
4.2.1 Exosomes in the pathogenesis of hypertrophic scar and keloid
Exosomes derived from hypertrophic scar fibroblasts have been found to play a significant role in the development of hypertrophic scars. Cui et al. found that exosomes from human hypertrophic scar fibroblasts (HHSFs) induced the EMT, and increased the expression of fibronectin, type Ⅰ and Ⅲ collagen in human normal fibroblasts (HNFs) via activating SMAD and TAK1 signaling (122). This suggested that HSF-derived exosomes can modulate the behavior of normal fibroblasts and promote the production of ECM components. In another research, HHSFs-derived exosomes promoted the proliferation, migration, and EMT and differentiation marker KRT1 and KRT10 expression of normal human keratinocytes (NHK) (123). In the context of keloids, miR-21 expression was significantly higher in exosomes from keloid fibroblasts (KFs) compared to that in normal fibroblasts (124). Exosomal miR-21 promoted the collagen production and proliferation of KFs via targeting Smad7 and activating the TGF-β/Smad pathway. This suggested that miR-21-containing exosomes derived from KFs may contribute to the pathogenesis of keloids.
M2 macrophages play a crucial role in scar formation and tissue remodeling via promoting the differentiation of fibroblasts into myofibroblasts and collagen deposition. Chen et al. found that M2 macrophage exosomes increased the expression of fibronectin, collagen I and III, and α-SMA in HDFs by transferring lncRNA-ASLNCS5088 (125). Exosomal lncRNA-ASLNCS5088 promoted HDF fibrosis by targeting miR-200c-3p and increasing glutaminases (GLAs) expression. In mice, the exosome inhibitor GW4869 reduced GLS, α-SMA, and collagen synthesis after wound healing. Therefore, the inhibition of M2 macrophage exosomal lncRNA-ASLNCS5088 might be a novel therapeutic target for hypertrophic scar therapy. In addition, LINC01605 from M2 macrophage exosomes promoted the initiation and formation of hypertrophic scars via targeting miR-493-3p and activating AKT pathway (126). Hu et al. found that the has-circ-0020792 level was increased and the miR-193a-5p level was reduced in plasma exosomes in keloid patients. Simultaneously, exosomal has-circ-0020792 promoted proliferation, migration, and ECM synthesis of HNFs via inhibiting miR-193a-5p and activated TGF-β1/Smad signaling (127). Furthermore, melanocytes have been implicated in keloid formation and melanocytes in keloids exhibited increased activity compared to those in normal scars. Shen et al. found that melanocyte-derived exosomes activated the TGF-β/Smad pathway and ECM synthesis in fibroblasts, thereby contributing to the occurrence and development of keloids (128). Specifically, melanocyte-derived exosomal miR-7704 was shown to promote collagen synthesis and activate the TGF-β/Smad pathway via targeting Smurf1. Hence, exosome-mediated communication between melanocytes and fibroblasts contributed to the excessive ECM synthesis and scar formation.
4.2.2 Exosomes in the treatment of hypertrophic scar and keloid
ADSC-exos enrich in anti-fibrotic cargos and represent promising effects in inhibiting fibroblast activity and collagen deposition, as well as reducing scar formation. ADSC-exos inhibited the proliferation, migration, and collagen synthesis, and promoted the apoptosis of KFs by inhibiting TGF-β1/Smad pathway (129). For instance, ADSC-exos inhibited the bioactivity, collagen deposition, and myofibroblast trans-differentiation of HHSFs via transferring miR-192-5p and inhibiting IL-17RA/SMAD pathway, and ADSC-exos also decreased collagen deposition during wound healing (130). Thus, ADSC-exos had the potential to modulate fibroblast behavior and reduce scar formation. MiR-29a, another miRNA of interest, has been found to be down-regulated in scar tissues and HHSFs. MiR-29a-overexpressing hADSC-exos inhibited the proliferation, migration, and collagen deposition of HHSFs, and reduced scar hyperplasia after scalding of mice via inhibiting TGF-β2/Smad3 signaling pathway (131). Furthermore, hADSC-exos were found to suppress the expression of ECM-related proteins in KFs, reduced collagen production, and angiogenesis in keloid tissue explants via inhibiting TGF-β2/Smad3 and Notch-1 signaling (132). Additionally, hADSC-exos-derived miR-7846-3p reduced proliferation and pro-angiogenic effects of KFs via targeting NRP2 and inhibiting the Hh signaling (133).
4.3 Skin aging
Skin aging is a natural process characterized by the gradual loss of skin elasticity, and firmness, and the appearance of wrinkles and age spots (134). Sun exposure, pollution, smoking, and poor skincare habits can accelerate the aging process. These factors lead to oxidative stress, inflammation, and the breakdown of collagen and elastin fibers in the skin (135). Stem cells and their paracrine products are proven to restore fibroblast function and reverse skin aging (136) (Figure 2C).
4.3.1 Exosomes in combating skin aging
Recently, exosome-mediated cell communication was shown to restore the senility of fibroblasts and remodel ECM in the skin. HUVEC-exos significantly increased cell proliferation and collagen type Ⅰ expression while decreasing matrix metalloproteinases-1 (MMP-1) expression of UVB-treated fibroblasts (137). Trophoblast-derived exosomes restored the senility of replicative senescent HDFs and UVB-treated HDFs via up-regulating CXCL family gene expression (138). Pretreatment with iPSC-exos reduced the expression level of senescence marker β-galactosidase (SA-β-Gal), MMP-1, and MMP-3, and restored the expression level of collagen type I in UVB-induced photo-aged HDFs and replicative senescence HDFs (139). UVB radiation activated the MAPK/AP-1 pathway in the skin, leading to an increased production of MMPs. These MMPs, in turn, degrade collagen and elastin proteins in the skin. BMSC-exos were reported to protect our skin from UVB-induced skin aging via inhibiting MAPK/AP-1 activation (140). Besides, ADSC-exos also ameliorated the aging phenotype of photo-aged HDFs (141). Mechanically, ADSC-exos reversed UVB-induced DNA damage and ROS production in fibroblasts by activating the Nrf2 pathway, reduced MMP-1 expression via inhibiting MAPK/AP-1 pathway, and promoted collagen synthesis via activating the TGF-β/Smad pathway.
Exosomal miRNAs function as a communication medium, playing a significant role in the exosome-mediated mechanisms underlying anti-aging effects. BMSC-exos promoted collagen production and reduced oxidative stress accumulation in UVB-irradiated HDFs via transferring miR-29b-3p (142). In addition, exosomes from embryonic stem cells were reported to inhibit replicative, adriamycin-induced, and ionizing radiation-induced HDF senescence via transferring miR-291a-3p and inhibiting TGFBR2 expression (143). Gao et al. found that miR-1246-overexpressing ADSC-exos exhibited an excellent improvement effect on wrinkle formation, epidermis thickening, and collagen fiber reduction of the UVB-induced photo-aging mouse model (144). The beneficial effects of miR-1246-overexpressing ADSC-exos were realized by inhibiting the MAPK/AP-1 and NFκB signaling pathways and activating TGF-β/SMAD signaling pathways.
Moreover, exosomal proteins also contributed to the anti-aging effects of exosomes. Wu et al. reported that hucMSC-exos reduced expression of aging makers p-p65, proliferating cell nuclear antigen (PCNA), and DNA damage markers in UV radiation-induced skin models via delivering natural protein 14-3-3ζ. Besides, exosomal 14-3-3ζ protects skin keratinocytes from oxidative stress via up-regulating SIRT1 expression and activating autophagy (145). ANP32B expression was significantly lower in the old skin compared to young skin, and dermal stem/progenitor cell (DSPC) exosomal ANP32B improved collagen synthesis of fibroblasts via activating Akt phosphorylation (146). Recently clinical trials also confirmed the clinical application prospect of ADSC-exos in improving skin aging. A randomized split-face study showed that the side treated with hADSC-exos and microneedling exhibited significant clinical improvements in skin wrinkles, elasticity, hydration, and pigmentation, compared to the control side treated with normal saline solution and microneedling (147).
3D spheroid culture strategy is proven to enhance the production of exosomes and improve cellular regenerative function by enhancing the paracrine effects of parental cells. Exosomes from the 3D culture of HDFs (3D-HDF-exos) increased collagen biosynthesis and ameliorated inflammation in the UVB radiation-treated skin via activating TGF-β and inhibiting TNF-α pathway (148). Besides, 3D-HDF-exos exhibited higher efficacy in reversing wrinkles than 2D-HDF-exos and MSC-exos in a nude photoaging mouse model. Exosomes from hucMSCs grown in a 3D culture system (3D-hucMSC-exos) enhanced the proliferation and migration of normal HaCaT cells (149). And 3D-hucMSC-exos increased the collagen type I expression and reduced MMP1 expression of UVB-induced photo-aged HaCaT cells. In conclusion, exosomes represent a promising cell-free therapeutic approach with potential implications for the treatment of skin aging.
5. Clinical application of exosomes in dermatology
Exosomes have gained significant attention in recent years. Exosomes have prompted exploration as promising therapeutic agents, diagnostic biomarkers, and potential targets for personalized medicine in dermatology.
For exosomes as therapeutic agents, the current clinical research on exosome-related therapy is still in the early stages. Currently, exosomes are clinically effective in promoting wound healing, resisting skin aging, and treating scars, but research on treating inflammatory skin diseases and autoimmune skin diseases is still in the preclinical stage. In a clinical trial, Johnson et al. suggested that human platelet exosome could be safely administered for wound healing and there were no adverse reactions (150). For skin aging, Park et al. performed prospective, randomized, split-face study, thus confirming that the side treated with hADSC-exos and microneedling exhibited significant clinical improvements in skin wrinkles, elasticity, hydration, and pigmentation, compared to the control side treated with normal saline solution and microneedling (147). Another clinical study confirmed that combination treatment with the ADSC-exos and fractional CO2 laser can significantly improve acne scars compared to only laser therapy (151).
In the aspect of diagnosis application, miRNA and cytokines in exosomes have been shown to assist in the diagnosis of autoimmune diseases and inflammatory skin conditions. In psoriasis, the circulating exosomal IL-23 and TNF-α mRNA in psoriasis patients were significantly higher than those in healthy controls, and the level of circulating exosome IL-23 was significantly positively correlated with the severity index score (24). In SLE, circulating exosome miR-21 and miR-155 in SLE patients were significantly higher than those in healthy controls, and the expression levels were even higher in SLE patients with LN (53).
Although many studies have demonstrated that exosomes are crucial in the pathogenesis of skin diseases, there are currently no clinical studies on exosomes as personalized therapeutic targets for skin diseases.
The mechanism of exosomes in combating skin aging
| Exosome Source | Model | Mechanism | Potential Application | Ref. |
|---|---|---|---|---|
| HUVECs | UVB-treated fibroblasts | Increased cell proliferation, collagen type Ⅰ, and decreased matrix MMP-1expression | Ameliorated the photo-aging of fibroblasts | (137) |
| Trophoblasts | Replicative senescent HDFs and UVB-treated HDFs | Up-regulated CXCL family gene expression | Restored the senility of fibroblasts | (138) |
| iPSCs | Reduced the expression of SA-β-Gal, MMP-1, and MMP-3, and restored the expression of collagen type I | Ameliorated the photo-aging of fibroblasts | (139) | |
| BMSCs | UVB-treated HDFs | Inhibited MAPK/AP-1 pathway activation | Protect skin from aging | (140) |
| ADSCs | Activated Nrf2 pathway, TGF-β/Smad pathway and inhibited MAPK/AP-1 pathway | Ameliorated the aging phenotype of fibroblasts | (141) | |
| BMSCs | Promoted collagen production and reduced oxidative stress accumulation via transferring miR-29b-3p | Ameliorated the photo-aging of fibroblasts | (142) | |
| Embryonic stem cells | Replicative, adriamycin-induced, and ionizing radiation-induced HDFs | Transferred miR-291a-3p and inhibited TGFBR2 | Ameliorated senescence of fibroblasts | (143) |
| MiR-1246-overexpressing ADSCs | UVB-induced photo-aging mice | Inhibited the MAPK/AP-1 , NFκB pathways and activated TGF-β/SMAD pathways | Improved wrinkle formation, and epidermis thickening. | (144) |
| HucMSC | UV radiation-induced SD rats' acute skin photodamage | Transferred14-3-3ζ, up-regulated SIRT1 expression, and activated autophagy | Alleviated UV radiation-induced photodamage | (145) |
| DSPCs | Fibroblasts | Transferred ANP32B and activated Akt phosphorylation | Improved collagen synthesis of fibroblasts | (146) |
| hADSCs | Human with facial skin aging | Promoted collagen synthesis and deposition | Improved skin wrinkles, elasticity, hydration, and pigmentation | (147) |
| 3D-HDFs | UVB radiation-treated skin and nude photoaging mice | Increased collagen biosynthesis and ameliorated inflammation via activating TGF-β and inhibited TNF-α pathway | Ameliorated skin photoaging | (148) |
| 3D-hucMSCs | UVB-induced photo-aged HaCaT cells | Enhanced the proliferation, migration, and collagen type I synthesis and reduced MMP1 | Ameliorated HaCaT Cell photo-aging | (149) |
Exosomes facilitate critical intercellular communication between cutaneous cells and immune cells; consequently, future endeavors might focus on attenuating disease progression in dermatological disorders by impeding exosomal secretion. In addition, under pathological conditions, the contents of exosomes secreted by skin cells or immune cells have changed. Future studies can intervene and treat key aspects of skin diseases by regulating exosome components.
6. Conclusions and expectations
Exosomes are pivotal paracrine mediators in the microenvironment of skin tissue and are involved in the pathophysiological processes of many skin diseases. Here, we reviewed the value of exosomes in the pathogenesis, diagnosis, and treatment of skin diseases, with emphasis on the role of endogenous exosomes in triggering skin diseases and the role of exosomes derived from exogenous stem cells in skin tissue repair.
The mechanisms and clinical applications of exosomes differ in inflammatory skin diseases, autoimmune skin diseases, and skin regeneration. In inflammatory skin diseases, exosomes are mainly involved in the communication between keratinocytes and immune cells. Exosome-related therapy primarily functions by suppressing inflammation and promoting tissue repair to alleviate inflammation and lesions. The bioactive molecules within exosomes can inhibit the production of inflammatory mediators and activation of inflammatory cells, while promoting tissue regeneration and regulating the skin microenvironment. In autoimmune skin diseases, circulating exosomes play an important role in pathogenesis. Exosomes may alleviate inflammation and autoimmune responses by modulating the immune response. In skin regeneration, exosomes can mediate skin regeneration and repair. Exosomes promote cell proliferation, angiogenesis, and tissue regeneration by transporting growth factors, cytokines, and other bioactive molecules.
However, despite the promising findings, there still are some challenges that are worthy of attention. Firstly, there are limitations of exosome isolation and purification. The current methods for isolating exosomes, such as ultracentrifugation and size exclusion chromatography, are time-consuming, labor-intensive, and may result in low yields (152). Moreover, the isolated exosomes are usually accompanied by other extracellular vesicles or contaminants. Therefore, it is needed to seek for novel extraction methods to improve the production and concentration of exosomes. Secondly, exosomes are a heterogeneous group of cell-derived membranous structures (153). Exosomes derived from different cell types or under different physiological or pathological conditions can change their cargo composition and functional properties. The heterogeneity of exosomes poses challenges in deciphering the specific roles of exosomes in skin diseases and developing targeted therapies. Due to the heterogeneity of exosomes and the complexity of the skin microenvironment, it is difficult to repeat the therapeutic effect of exosome therapy in clinical practice. Further research is needed to characterize the molecular and functional diversity of exosomes and identify specific markers or signatures that can be used to distinguish subpopulations with distinct functions. Thirdly, exosomes are vesicles carrying multi-component cargoes. The current application of exosomes in the diagnosis of skin diseases mainly depends on one specific miRNA or protein (154). It is necessary to construct signatures based on multiple disease-specific biomarkers in exosomes for the diagnosis and severity grading of skin diseases. In addition, the current research on exosomes in the mechanism and treatment of dermatosis mainly focuses on cellular and animal levels. But human skin has significant species differences from animal skin. For example, mouse skin is thinner and looser than human skin, and hair follicles in mice are more abundant than in humans (155). Therefore, the results of preclinical studies cannot fully reflect and predict the functional mechanisms and therapeutic effects of exosomes in human skin disease. Clinical trials or animal models closer to human skin to verify the role of exosomes in skin diseases are urgent. Finally, although engineered exosomes and drug-preconditioning exosomes have been extensively investigated in the realm of tissue regeneration and tumors, they have rarely been studied in inflammatory and autoimmune skin diseases. Future studies could consider performing exosome modification to improve the efficacy of exosomes in curing skin diseases.
In light of these limitations above, these directions need to be paid attention to in future research. Efficient, stable, and mass-producible exosomes for the treatment of skin disease are yet to be explored and developed. Fully studying the heterogeneity of skin diseases and the diversity of exosome containings can explore novel therapeutic targets for skin diseases and improve the reproducibility of exosome-related therapy. At present, exosome-related therapy is still mainly in the pre-clinical stage, and the therapeutic effect needs to be proved by multi-center clinical studies. In addition, physical, chemical, or biological methods for preconditioning, genetic engineering, and transfection were proven to enhance the therapeutic potential of exosomes It is a new therapeutic direction to explore the engineering strategy of modifying exosomes to improve the efficacy of exosomes in the treatment of skin diseases.
In conclusion, our review provided novel insight into the involvement of exosomes in the pathological process, diagnosis, and treatment of skin disease. Further studies are needed to explore deeper mechanisms related to exosomes in dermatosis progress and to optimize the function of exosomes for clinical treatment of dermatosis.
Abbreviations
ADSCs: Adipose-derived stem cells; AD: atopic dermatitis; BPI: bactericidal/permeability-increasing protein; BMSCs: bone marrow mesenchymal stem cells; CCLs: C-X-C motif chemokine ligands; DCs: dendritic cells; DSPC: dermal stem/progenitor cell; DFU: diabetic foot ulcer; dSSc: diffuse cutaneous systemic sclerosis; DNCB: dinitrochlorobenzene; EPC-exos: endothelial progenitor cell-derived exosomes; ECP: eosinophil cationic protein; ESCs: epidermal stem cells; 3D-hucMSC-exo: exosomes from hucMSCs grown in a three-dimensional culture system; PGZ-Exos: exosomes from MSCs pretreated with pioglitazone; ECM: extracellular matrix; EVs: extracellular vesicles; GLAs: glutaminases; HDFs: human dermal fibroblasts; HDMECs: human dermal microvascular endothelial cells; HHSFs: human hypertrophic scar fibroblasts; HNFs: human normal fibroblasts; hucMSCs: human umbilical cord MSCs; IMQ: imiquimod; iMSC: induced pluripotent stem cell-derived mesenchymal stem cells; iPSCs: induced pluripotent stem cells; KFs: keloid fibroblasts; LN: lupus nephritis; MMP-1: matrix metalloproteinases-1; MenSC-exos: menstrual blood-derived mesenchymal stem cells; MSCs: mesenchymal stem cells; NET: neutrophil extracellular trap; NHK: normal human keratinocytes; PC: phosphatidylcholine; PE: phosphatidylethanolamine; PRP: platelet-rich plasma; PCNA: proliferating cell nuclear antigen; ROC: receiver operating characteristic; SV: segmental vitiligo; SA-β-Gal: senescence marker β-galactosidase; S1P: sphingosine-1-phosphate; SLE: systemic lupus erythematosus; SSc: systemic sclerosis; TLR2: Toll-like receptor 2; TEWL: transepidermal water loss; tRFs: transfer RNA-derived fragments; UCB-MNC: umbilical cord blood mononuclear cells; VDR: vitamin D receptor.
Acknowledgements
Funding
This work was supported by The Science and Technology Project of Hubei Province [grant number: 2022CFB226], Research Foundation Project of Tongji Hospital [grant number: 2022A16].
Author contributions
HY, HF, and HZ performed literature searches and wrote the manuscript. MW, KH, and JY conceived the project and revised the manuscript. YW and QZ edited the manuscript. All the authors reviewed the manuscript and all approved of the final version.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Qu F, Geng R, Liu Y, Zhu J. Advanced nanocarrier- and microneedle-based transdermal drug delivery strategies for skin diseases treatment. Theranostics. 2022;12(7):3372-406
2. de Szalay S, Wertz PW. Protective Barriers Provided by the Epidermis. Int J Mol Sci. 2023Feb;24(4):3145
3. Ganier C, Rognoni E, Goss G, Lynch M, Watt FM. Fibroblast Heterogeneity in Healthy and Wounded Skin. Cold Spring Harb Perspect Biol. 2022Jun;14(6):a041238
4. Kruglikov IL, Zhang Z, Scherer PE. Phenotypical Conversions of Dermal Adipocytes as Pathophysiological Steps in Inflammatory Cutaneous Disorders. Int J Mol Sci. 2022Mar;23(7):3828
5. Tienda-Vázquez MA, Hanel JM, Márquez-Arteaga EM, Salgado-Álvarez AP, Scheckhuber CQ, Alanis-Gómez JR. et al. Exosomes: A Promising Strategy for Repair, Regeneration and Treatment of Skin Disorders. Cells. 2023Jun;12(12):1625
6. Dissemond J, Romanelli M. Inflammatory skin diseases and wounds. Br J Dermatol. 2022;187(2):167-77
7. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science (80- ) [Internet]. 2020Feb7;367(6478):eaau6977 Available from: http://science.sciencemag.org/content/367/6478/eaau6977.abstract
8. Xiong M, Zhang Q, Hu W, Zhao C, Lv W, Yi Y. et al. Exosomes From Adipose-Derived Stem Cells: The Emerging Roles and Applications in Tissue Regeneration of Plastic and Cosmetic Surgery. Front Cell Dev Biol. 2020;8(September):1-17
9. Gurung S, Perocheau D, Touramanidou L, Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal. 2021Apr;19(1):47
10. Wei H, Chen Q, Lin L, Sha C, Li T, Liu Y. et al. Regulation of exosome production and cargo sorting. Int J Biol Sci. 2021;17(1):163-77
11. Wang W, Wu C, Jin H. Exosomes in chronic inflammatory skin diseases and skin tumors. Exp Dermatol. 2019Mar;28(3):213-8
12. Xiong M, Zhang Q, Hu W, Zhao C, Lv W, Yi Y. et al. The novel mechanisms and applications of exosomes in dermatology and cutaneous medical aesthetics. Pharmacol Res [Internet]. 2021Apr;166:105490 Available from: https://linkinghub.elsevier.com/retrieve/pii/S1043661821000748
13. McBride JD, Rodriguez-Menocal L, Badiavas E V. Extracellular Vesicles as Biomarkers and Therapeutics in Dermatology: A Focus on Exosomes. J Invest Dermatol. 2017Aug;137(8):1622-9
14. Luo H, Xie B, Xu J, Zhu Y, Sun J, Shen Y. et al. Differential Expression of Serum Exosomal Hsa-miR-487b-3p in Progressive Vitiligo Before and After Systemic Corticosteroid Treatment. Clin Cosmet Investig Dermatol. 2022Jul;15:1377-86
15. Zhuang X, Hu X, Zhang S, Li X, Yuan X, Wu Y. Mesenchymal Stem Cell-Based Therapy as a New Approach for the Treatment of Systemic Sclerosis. Clin Rev Allergy Immunol. 2022Jan;64(3):284-320
16. Kose O, Botsali A, Caliskan E. Role of exosomes in skin diseases. J Cosmet Dermatol. 2022;21(8):3219-25
17. Schön MP, Wilsmann-Theis D. Current developments and perspectives in psoriasis. JDDG J der Dtsch Dermatologischen Gesellschaft. 2023Apr;21(4):363-72
18. Rendon A, Schäkel K. Psoriasis Pathogenesis and Treatment. Int J Mol Sci. 2019Mar;20(6):1475
19. Jiang M, Fang H, Shao S, Dang E, Zhang J, Qiao P. et al. Keratinocyte exosomes activate neutrophils and enhance skin inflammation in psoriasis. FASEB J. 2019Dec;33(12):13241-53
20. Sun W, Chen J, Li J, She X, Ma H, Wang S. et al. Vitamin D receptor-deficient keratinocytes-derived exosomal miR-4505 promotes the macrophage polarization towards the M1 phenotype. PeerJ. 2023Aug;11:e15798
21. Shao S, Fang H, Zhang J, Jiang M, Xue K, Ma J. et al. Neutrophil exosomes enhance the skin autoinflammation in generalized pustular psoriasis via activating keratinocytes. FASEB J. 2019Jun;33(6):6813-28
22. Yan J, Zhang K, Guo T, Yang S, Jia H. Par3 regulates the asymmetric division of basal stem cells in psoriasis via the Par3/mInsc/LGN signaling axis. Cell Immunol. 2022Mar;373:104496
23. Cheung KL, Jarrett R, Subramaniam S, Salimi M, Gutowska-Owsiak D, Chen Y-L. et al. Psoriatic T cells recognize neolipid antigens generated by mast cell phospholipase delivered by exosomes and presented by CD1a. J Exp Med. 2016Oct;213(11):2399-412
24. Sawamura S, Tselmeg Mijiddorj M, Kajihara I, Shimada S, Kanemaru H, Yamada-Kanazawa S. et al. Clinical significance of circulating exosomal interleukin-23 and tumour necrosis factor-α messenger RNA in psoriasis. J Eur Acad Dermatol Venereol. 2023Jun;37(6):e815-7
25. Jacquin-Porretaz C, Cordonnier M, Nardin C, Boullerot L, Chanteloup G, Vautrot V. et al. Increased Levels of Interleukin-17A Exosomes in Psoriasis. Acta Derm Venereol. 2019;99(12):1143-7
26. Chen X-M, Yao D-N, Wang M-J, Wu X-D, Deng J-W, Deng H. et al. Deep Sequencing of Plasma Exosomal microRNA Level in Psoriasis Vulgaris Patients. Front Med. 2022 May;9
27. Paolino G, Buratta S, Mercuri SR, Pellegrino RM, Urbanelli L, Emiliani C. et al. Lipidic Profile Changes in Exosomes and Microvesicles Derived From Plasma of Monoclonal Antibody-Treated Psoriatic Patients. Front Cell Dev Biol. 2022 Jun;10
28. Zhang B, Lai RC, Sim WK, Choo ABH, Lane EB, Lim SK. Topical Application of Mesenchymal Stem Cell Exosomes Alleviates the Imiquimod Induced Psoriasis-Like Inflammation. Int J Mol Sci. 2021Jan;22(2):720
29. Zhang Y, Yan J, Li Z, Zheng J, Sun Q. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Alleviate Psoriasis-like Skin Inflammation. J Interf Cytokine Res. 2022Jan;42(1):8-18
30. Rodrigues SC, Cardoso RMS, Freire PC, Gomes CF, Duarte F V, Neves RP Das. et al. Immunomodulatory Properties of Umbilical Cord Blood-Derived Small Extracellular Vesicles and Their Therapeutic Potential for Inflammatory Skin Disorders. Int J Mol Sci. 2021Sep;22(18):9797
31. Schuler CF, Billi AC, Maverakis E, Tsoi LC, Gudjonsson JE. Novel insights into atopic dermatitis. J Allergy Clin Immunol. 2023May;151(5):1145-54
32. Bakker D, de Bruin-Weller M, Drylewicz J, van Wijk F, Thijs J. Biomarkers in atopic dermatitis. J Allergy Clin Immunol. 2023May;151(5):1163-8
33. Hong S-W, Kim M-R, Lee E-Y, Kim JH, Kim Y-S, Jeon SG. et al. Extracellular vesicles derived from Staphylococcus aureus induce atopic dermatitis-like skin inflammation. Allergy. 2011Mar;66(3):351-9
34. Kobiela A, Hovhannisyan L, Jurkowska P, de la Serna JB, Bogucka A, Deptuła M. et al. Excess filaggrin in keratinocytes is removed by extracellular vesicles to prevent premature death and this mechanism can be hijacked by Staphylococcus aureus in a TLR2-dependent fashion. J Extracell Vesicles. 2023 Jun;12(6)
35. Kobiela A, Frackowiak JE, Biernacka A, Hovhannisyan L, Bogucka AE, Panek K. et al. Exposure of Keratinocytes to Candida Albicans in the Context of Atopic Milieu Induces Changes in the Surface Glycosylation Pattern of Small Extracellular Vesicles to Enhance Their Propensity to Interact With Inhibitory Siglec Receptors. Front Immunol. 2022 Jun;13
36. Kwon Y, Choi Y, Kim M, Jeong MS, Jung HS, Jeoung D. HDAC6 and CXCL13 Mediate Atopic Dermatitis by Regulating Cellular Interactions and Expression Levels of miR-9 and SIRT1. Front Pharmacol. 2021 Sep;12
37. Zhu T, Sun J, Ma L, Tian J. Plasma Exosomes from Children with Atopic Dermatitis May Promote Apoptosis of Keratinocytes and Secretion of Inflammatory Factors in vitro. Clin Cosmet Investig Dermatol. 2022 Sep;Volume 15:1909-17
38. Meng L, Jiang L, Chen J, Ren H, Gao Z, Wu F. et al. Transfer RNA-derived fragment tRF-28-QSZ34KRQ590K in plasma exosomes may be a potential biomarker for atopic dermatitis in pediatric patients. Exp Ther Med. 2021Mar;21(5):489
39. Cho BS, Kim JO, Ha DH, Yi YW. Exosomes derived from human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis. Stem Cell Res Ther. 2018Dec;9(1):187
40. Shin K-O, Ha DH, Kim JO, Crumrine DA, Meyer JM, Wakefield JS. et al. Exosomes from Human Adipose Tissue-Derived Mesenchymal Stem Cells Promote Epidermal Barrier Repair by Inducing de Novo Synthesis of Ceramides in Atopic Dermatitis. Cells. 2020Mar;9(3):680
41. Shi C, Pei S, Ding Y, Tao C, Zhu Y, Peng Y. et al. Exosomes with overexpressed miR 147a suppress angiogenesis and infammatory injury in an experimental model of atopic dermatitis. Sci Rep. 2023Jun;13(1):8904
42. Yoo K, Thapa N, Lee J, Jang Y, Lee JO, Kim J. Dermal fibroblast cell-derived exosomes for atopic dermatitis: In-vitro test. Ski Res Technol. 2023 Jul;29(7)
43. Yoon J, Lee SK, Park A, Lee J, Jung I, Song KB. et al. Exosome from IFN-γ-Primed Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Improved Skin Inflammation and Barrier Function. Int J Mol Sci. 2023;24(14):1-15
44. Bergqvist C, Ezzedine K. Vitiligo: A focus on pathogenesis and its therapeutic implications. J Dermatol. 2021Mar;48(3):252-70
45. Frisoli ML, Essien K, Harris JE. Vitiligo: Mechanisms of Pathogenesis and Treatment. Annu Rev Immunol. 2020Apr;38(1):621-48
46. Li D, Zhou T, She Q, Nie X, Liu Z, Pan R. et al. Circulating Exosomal miR-493-3p Affects Melanocyte Survival and Function by Regulating Epidermal Dopamine Concentration in Segmental Vitiligo. J Invest Dermatol. 2022;142(12):3262-3273.e11
47. Zhang C, Guo W, Wang S, Di Y, Wu D. Peripheral Blood of Vitiligo Patients-Derived Exosomal MiR-21-5p Inhibits Melanocytes Melanogenesis via Targeting SATB1. Iran J Public Health. 2022;51(12):2706-16
48. Zhao C, Wang D, Wang X, Mao Y, Xu Z, Sun Y. et al. Down-regulation of exosomal miR-200c derived from keratinocytes in vitiligo lesions suppresses melanogenesis. J Cell Mol Med. 2020;24(20):12164-75
49. Crow MK. Pathogenesis of systemic lupus erythematosus: risks, mechanisms and therapeutic targets. Ann Rheum Dis. 2023Aug;82(8):999-1014
50. Lazar S, Kahlenberg JM. Systemic Lupus Erythematosus: New Diagnostic and Therapeutic Approaches. Annu Rev Med. 2023Jan;74(1):339-52
51. Lee JY, Park JK, Lee EY, Lee EB, Song YW. Circulating exosomes from patients with systemic lupus erythematosus induce a proinflammatory immune response. Arthritis Res Ther. 2016Dec;18(1):264
52. Song W, Li C, Qiu J, Dong J, Liu D, Dai Y. Differential expression of exosomal miRNAs and proteins in the plasma of systemic lupus erythematous patients. Heliyon. 2023Feb;9(2):e13345
53. Li W, Liu S, Chen Y, Weng R, Zhang K, He X. et al. Circulating Exosomal microRNAs as Biomarkers of Systemic Lupus Erythematosus. Clinics. 2020;75:e1528
54. Salvi V, Gianello V, Busatto S, Bergese P, Andreoli L, D'Oro U. et al. Exosome-delivered microRNAs promote IFN-α secretion by human plasmacytoid DCs via TLR7. JCI Insight. 2018May;3(10):1-12
55. Chuang HC, Chen MH, Chen YM, Ciou YR, Hsueh CH, Tsai CY. et al. Induction of Interferon-γ and Tissue Inflammation by Overexpression of Eosinophil Cationic Protein in T Cells and Exosomes. Arthritis Rheumatol. 2022;74(1):92-104
56. Chuang HC, Chen MH, Chen YM, Yang HY, Ciou YR, Hsueh CH. et al. BPI overexpression suppresses Treg differentiation and induces exosome-mediated inflammation in systemic lupus erythematosus. Theranostics. 2021;11(20):9953-66
57. López P, Rodríguez-Carrio J, Caminal-Montero L, Suárez A. Relationship Between T-Cell Exosomes and Cellular Subsets in SLE According to Type I IFN-Signaling. Front Med. 2020Nov;7(November):1-10
58. Zhang M, Johnson-Stephenson TK, Wang W, Wang Y, Li J, Li L. et al. Mesenchymal stem cell-derived exosome-educated macrophages alleviate systemic lupus erythematosus by promoting efferocytosis and recruitment of IL-17+ regulatory T cell. Stem Cell Res Ther. 2022Sep;13(1):484
59. Dou R, Zhang X, Xu X, Wang P, Yan B. Mesenchymal stem cell exosomal tsRNA-21109 alleviate systemic lupus erythematosus by inhibiting macrophage M1 polarization. Mol Immunol. 2021Nov;139:106-14
60. Sun W, Yan S, Yang C, Yang J, Wang H, Li C. et al. Mesenchymal Stem Cells-derived Exosomes Ameliorate Lupus by Inducing M2 Macrophage Polarization and Regulatory T Cell Expansion in MRL/lpr Mice. Immunol Invest. 2022;51(6):1785-803
61. Tu J, Zheng N, Mao C, Liu S, Zhang H, Sun L. UC-BSCs Exosomes Regulate Th17/Treg Balance in Patients with Systemic Lupus Erythematosus via miR-19b/KLF13. Cells. 2022Dec;11(24):4123
62. Zhao Y, Song W, Yuan Z, Li M, Wang G, Wang L. et al. Exosome Derived from Human Umbilical Cord Mesenchymal Cell Exerts Immunomodulatory Effects on B Cells from SLE Patients. Niedźwiedzka-Rystwej P, editor. J Immunol Res. 2023May;2023:1-15
63. Liu YJ, Miao HB, Lin S, Chen Z. Current Progress in Treating Systemic Lupus Erythematosus Using Exosomes/MicroRNAs. Cell Transplant. 2023 32
64. Volkmann ER, Andréasson K, Smith V. Systemic sclerosis. Lancet. 2023Jan;401(10373):304-18
65. Lescoat A, Roofeh D, Kuwana M, Lafyatis R, Allanore Y, Khanna D. Therapeutic Approaches to Systemic Sclerosis: Recent Approvals and Future Candidate Therapies. Clin Rev Allergy Immunol. 2021Sep;64(3):239-61
66. Bhandari R, Yang H, Kosarek NN, Smith AE, Garlick JA, Hinchcliff M. et al. Human dermal fibroblast-derived exosomes induce macrophage activation in systemic sclerosis. Rheumatology. 2023;62(SI):SI114-24
67. Nakamura K, Jinnin M, Harada M, Kudo H, Nakayama W, Inoue K. et al. Altered expression of CD63 and exosomes in scleroderma dermal fibroblasts. J Dermatol Sci. 2016;84(1):30-9
68. Li L, Zuo X, Liu D, Luo H, Zhu H. The profiles of miRNAs and lncRNAs in peripheral blood neutrophils exosomes of diffuse cutaneous systemic sclerosis. J Dermatol Sci. 2020;98(2):88-97
69. Li L, Zuo X, Xiao Y, Liu D, Luo H, Zhu H. Neutrophil-derived exosome from systemic sclerosis inhibits the proliferation and migration of endothelial cells. Biochem Biophys Res Commun. 2020;526(2):334-40
70. Wermuth PJ, Piera-Velazquez S, Jimenez SA. Exosomes isolated from serum of systemic sclerosis patients display alterations in their content of profibrotic and antifibrotic microRNA and induce a profibrotic phenotype in cultured normal dermal fibroblasts. Clin Exp Rheumatol. 2017 35 Suppl 1(4):21-30
71. Yu Y, Shen L, Xie X, Zhao J, Jiang M. The Therapeutic Effects of Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells on Scleroderma. Tissue Eng Regen Med. 2022;19(1):141-50
72. Li M, Zhang HP, Wang XY, Chen ZG, Lin XF, Zhu W. Mesenchymal Stem Cell-Derived Exosomes Ameliorate Dermal Fibrosis in a Murine Model of Bleomycin-Induced Scleroderma. Stem Cells Dev. 2021;30(19):981-90
73. Xie L, Long X, Mo M, Jiang J, Zhang Q, Long M. et al. Bone marrow mesenchymal stem cell-derived exosomes alleviate skin fibrosis in systemic sclerosis by inhibiting the IL-33/ST2 axis via the delivery of microRNA-214. Mol Immunol. 2023May;157:146-57
74. Baral H, Uchiyama A, Yokoyama Y, Sekiguchi A, Yamazaki S, Amalia SN. et al. Antifibrotic effects and mechanisms of mesenchymal stem cell-derived exosomes in a systemic sclerosis mouse model: Possible contribution of miR-196b-5p. J Dermatol Sci. 2021;104(1):39-47
75. Rozier P, Maumus M, Maria ATJ, Toupet K, Lai-Kee-Him J, Jorgensen C. et al. Mesenchymal stromal cells-derived extracellular vesicles alleviate systemic sclerosis via miR-29a-3p. J Autoimmun. 2021Jul;121:102660
76. Powers JG, Higham C, Broussard K, Phillips TJ. Wound healing and treating wounds. J Am Acad Dermatol. 2016Apr;74(4):607-25
77. Kumari P, Sharma S, Sharma PK, Alam A. Treatment Management of Diabetic Wounds Utilizing Herbalism: An Overview. Curr Diabetes Rev. 2023 Jan;19(1)
78. Li M, Wang T, Tian H, Wei G, Zhao L, Shi Y. Macrophage-derived exosomes accelerate wound healing through their anti-inflammation effects in a diabetic rat model. Artif Cells, Nanomedicine Biotechnol. 2019;47(1):3793-803
79. Xia W, Liu Y, Jiang X, Li M, Zheng S, Zhang Z. et al. Lean adipose tissue macrophage derived exosome confers immunoregulation to improve wound healing in diabetes. J Nanobiotechnology. 2023Apr;21(1):128
80. Yang H, Xu H, Wang Z, Li X, Wang P, Cao X. et al. Analysis of miR-203a-3p/SOCS3-mediated induction of M2 macrophage polarization to promote diabetic wound healing based on epidermal stem cell-derived exosomes. Diabetes Res Clin Pract. 2023Mar;197:110573
81. Wang P, Theocharidis G, Vlachos IS, Kounas K, Lobao A, Shu B. et al. Exosomes Derived from Epidermal Stem Cells Improve Diabetic Wound Healing. J Invest Dermatol. 2022;142(9):2508-2517.e13
82. Li P, Hong G, Zhan W, Deng M, Tu C, Wei J. et al. Endothelial progenitor cell derived exosomes mediated miR-182-5p delivery accelerate diabetic wound healing via down-regulating PPARG. Int J Med Sci. 2023;20(4):468-81
83. Xu J, Bai S, Cao Y, Liu L, Fang Y, Du J. et al. MiRNA-221-3p in endothelial progenitor cell-derived exosomes accelerates skin wound healing in diabetic mice. Diabetes, Metab Syndr Obes. 2020;13:1259-70
84. Han X, Wu P, Li L, Sahal HM, Ji C, Zhang J. et al. Exosomes derived from autologous dermal fibroblasts promote diabetic cutaneous wound healing through the Akt/β-catenin pathway. Cell Cycle. 2021;20(5-6):616-29
85. Kuang L, Zhang C, Li B, Deng H, Chen R, Li G. Human Keratinocyte-Derived Exosomal MALAT1 Promotes Diabetic Wound Healing by Upregulating MFGE8 via microRNA-1914-3p. Int J Nanomedicine. 2023;18:949-70
86. Chen K, Yu T, Wang X. Inhibition of Circulating Exosomal miRNA-20b-5p Accelerates Diabetic Wound Repair. Int J Nanomedicine [Internet]. 2021;16(17):371-81 Available from: http://www.ncbi.nlm.nih.gov/pubmed/33469291
87. Xiong Y, Chen L, Yu T, Yan C, Zhou W, Cao F. et al. Inhibition of circulating exosomal microRNA-15a-3p accelerates diabetic wound repair. Aging (Albany NY). 2020;12(10):8968-86
88. Xiong Y, Chen L, Yan C, Zhou W, Endo Y, Liu J. et al. Circulating Exosomal miR-20b-5p Inhibition Restores Wnt9b Signaling and Reverses Diabetes-Associated Impaired Wound Healing. Small. 2020 Jan;16(3)
89. Wang S, Shi M, Zhou J, Wang W, Zhang Y, Li Y. Circulating Exosomal miR-181b-5p Promoted Cell Senescence and Inhibited Angiogenesis to Impair Diabetic Foot Ulcer via the Nuclear Factor Erythroid 2-Related Factor 2/Heme Oxygenase-1 Pathway. Front Cardiovasc Med. 2022Apr;9(April):1-11
90. Ren S, Chen J, Guo J, Liu Y, Xiong H, Jing B. et al. Exosomes from Adipose Stem Cells Promote Diabetic Wound Healing through the eHSP90/LRP1/AKT Axis. Cells. 2022Oct;11(20):3229
91. Zhang Y, Bai X, Shen K, Luo L, Zhao M, Xu C. et al. Exosomes Derived from Adipose Mesenchymal Stem Cells Promote Diabetic Chronic Wound Healing through SIRT3/SOD2. Cells. 2022Aug;11(16):2568
92. Hsu H-H, Wang AYL, Loh CYY, Pai AA, Kao H-K. Therapeutic Potential of Exosomes Derived from Diabetic Adipose Stem Cells in Cutaneous Wound Healing of db/db Mice. Pharmaceutics. 2022Jun;14(6):1206
93. Teng L, Maqsood M, Zhu M, Zhou Y, Kang M, Zhou J. et al. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Accelerate Diabetic Wound Healing via Promoting M2 Macrophage Polarization, Angiogenesis, and Collagen Deposition. Int J Mol Sci. 2022Sep;23(18):10421
94. Yan C, Xv Y, Lin Z, Endo Y, Xue H, Hu Y. et al. Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes Accelerate Diabetic Wound Healing via Ameliorating Oxidative Stress and Promoting Angiogenesis. Front Bioeng Biotechnol. 2022 Jan;10
95. Dalirfardouei R, Jamialahmadi K, Jafarian AH, Mahdipour E. Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. J Tissue Eng Regen Med. 2019;13(4):555-68
96. Chen CY, Rao SS, Ren L, Hu XK, Tan YJ, Hu Y. et al. Exosomal DMBT1 from human urine-derived stem cells facilitates diabetic wound repair by promoting angiogenesis. Theranostics. 2018;8(6):1607-23
97. Fu S, Zhang H, Li X, Zhang Q, Guo C, Qiu K. et al. Exosomes Derived from Human Amniotic Mesenchymal Stem Cells Facilitate Diabetic Wound Healing by Angiogenesis and Enrich Multiple lncRNAs. Tissue Eng Regen Med. 2023;20(2):295-308
98. Yang H, Zhang Y, Du Z, Wu T, Yang C. Hair follicle mesenchymal stem cell exosomal lncRNA H19 inhibited NLRP3 pyroptosis to promote diabetic mouse skin wound healing. Aging (Albany NY). 2023;15(3):791-809
99. Ge L, Wang K, Lin H, Tao E, Xia W, Wang F. et al. Engineered exosomes derived from miR-132-overexpresssing adipose stem cells promoted diabetic wound healing and skin reconstruction. Front Bioeng Biotechnol. 2023;11(March):1-18
100. Qiu J, Shu C, Li X, Ye C, Zhang W-C. Exosomes from linc00511-overexpressing ADSCs accelerates angiogenesis in diabetic foot ulcers healing by suppressing PAQR3-induced Twist1 degradation. Diabetes Res Clin Pract. 2021Oct;180:109032
101. Han Z-F, Cao J-H, Liu Z-Y, Yang Z, Qi R-X, Xu H-L. Exosomal lncRNA KLF3-AS1 derived from bone marrow mesenchymal stem cells stimulates angiogenesis to promote diabetic cutaneous wound healing. Diabetes Res Clin Pract. 2022Jan;183:109126
102. Born LJ, Chang K, Shoureshi P, Lay F, Bengali S, Hsu ATW. et al. HOTAIR-Loaded Mesenchymal Stem/Stromal Cell Extracellular Vesicles Enhance Angiogenesis and Wound Healing. Adv Healthc Mater. 2022 Mar;11(5)
103. Li B, Luan S, Chen J, Zhou Y, Wang T, Li Z. et al. The MSC-Derived Exosomal lncRNA H19 Promotes Wound Healing in Diabetic Foot Ulcers by Upregulating PTEN via MicroRNA-152-3p. Mol Ther - Nucleic Acids. 2020;19(157):814-26
104. Shi R, Jin Y, Hu W, Lian W, Cao C, Han S. et al. Exosomes derived from mmu_circ_0000250-modified adipose-derived mesenchymal stem cells promote wound healing in diabetic mice by inducing miR-128-3p/SIRT1-mediated autophagy. Am J Physiol - Cell Physiol. 2020;318(5):C848-56
105. Liang Z-H, Pan N-F, Lin S-S, Qiu Z-Y, Liang P, Wang J. et al. Exosomes from mmu_circ_0001052-modified adipose-derived stem cells promote angiogenesis of DFU via miR-106a-5p and FGF4/p38MAPK pathway. Stem Cell Res Ther. 2022Dec;13(1):336
106. Wang Z, Feng C, Liu H, Meng T, Huang WQ, Song KX. et al. Exosomes from circ-Astn1-modified adipose-derived mesenchymal stem cells enhance wound healing through miR-138-5p/SIRT1/FOXO1 axis regulation. World J Stem Cells. 2023;15(5):476-89
107. Liang ZH, Lin SS, Pan NF, Zhong GY, Qiu ZY, Kuang SJ. et al. UCMSCs-derived exosomal circHIPK3 promotes ulcer wound angiogenesis of diabetes mellitus via miR-20b-5p/Nrf2/VEGFA axis. Diabet Med. 2023 40(2)
108. Chen J, Li X, Liu H, Zhong D, Yin K, Li Y. et al. Bone marrow stromal cell-derived exosomal circular RNA improves diabetic foot ulcer wound healing by activating the nuclear factor erythroid 2-related factor 2 pathway and inhibiting ferroptosis. Diabet Med. 2023 40(7)
109. Li X, Xie X, Lian W, Shi R, Han S, Zhang H. et al. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med. 2018Apr;50(4):1-14
110. Liu W, Yu M, Xie D, Wang L, Ye C, Zhu Q. et al. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res Ther. 2020Dec;11(1):259
111. Hu Y, Tao R, Chen L, Xiong Y, Xue H, Hu L. et al. Exosomes derived from pioglitazone-pretreated MSCs accelerate diabetic wound healing through enhancing angiogenesis. J Nanobiotechnology. 2021Dec;19(1):150
112. Yu M, Liu W, Li J, Lu J, Lu H, Jia W. et al. Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res Ther. 2020Dec;11(1):350
113. Wang J, Wu H, Peng Y, Zhao Y, Qin Y, Zhang Y. et al. Hypoxia adipose stem cell-derived exosomes promote high-quality healing of diabetic wound involves activation of PI3K/Akt pathways. J Nanobiotechnology. 2021Dec;19(1):202
114. Guo SC, Tao SC, Yin WJ, Qi X, Yuan T, Zhang CQ. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics. 2017;7(1):81-96
115. Chen T, Song P, He M, Rui S, Duan X, Ma Y. et al. Sphingosine-1-phosphate derived from PRP-Exos promotes angiogenesis in diabetic wound healing via the S1PR1/AKT/FN1 signalling pathway. Burn Trauma. 2023 Jan;11
116. Chen C, Wang Q, Li D, Qi Z, Chen Y, Wang S. MALAT1 participates in the role of platelet-rich plasma exosomes in promoting wound healing of diabetic foot ulcer. Int J Biol Macromol. 2023May;238:124170
117. Chen L, Qin L, Chen C, Hu Q, Wang J, Shen J. Serum exosomes accelerate diabetic wound healing by promoting angiogenesis and ECM formation. Cell Biol Int. 2021;45(9):1976-85
118. Pomatto M, Gai C, Negro F, Cedrino M, Grange C, Ceccotti E. et al. Differential Therapeutic Effect of Extracellular Vesicles Derived by Bone Marrow and Adipose Mesenchymal Stem Cells on Wound Healing of Diabetic Ulcers and Correlation to Their Cargoes. Int J Mol Sci. 2021Apr;22(8):3851
119. Levy D, Abadchi SN, Shababi N, Ravari MR, Pirolli NH, Bergeron C. et al. Induced Pluripotent Stem Cell-Derived Extracellular Vesicles Promote Wound Repair in a Diabetic Mouse Model via an Anti-Inflammatory Immunomodulatory Mechanism. Adv Healthc Mater. 2023 Jun;2300879:1-15
120. Lee H, Jang Y. Recent Understandings of Biology, Prophylaxis and Treatment Strategies for Hypertrophic Scars and Keloids. Int J Mol Sci. 2018Mar;19(3):711
121. Wang ZC, Zhao WY, Cao Y, Liu YQ, Sun Q, Shi P. et al. The Roles of Inflammation in Keloid and Hypertrophic Scars. Front Immunol. 2020;11(December):1-10
122. Cui HS, Kim DH, Joo SY, Cho YS, Kim J-B, Seo CH. Exosomes derived from human hypertrophic scar fibroblasts induces smad and TAK1 signaling in normal dermal fibroblasts. Arch Biochem Biophys. 2022Jun;722:109215
123. Cui HS, Joo SY, Lee SY, Cho YS, Kim DH, Seo CH. Effect of Hypertrophic Scar Fibroblast-Derived Exosomes on Keratinocytes of Normal Human Skin. Int J Mol Sci. 2023Mar;24(7):6132
124. Li Q, Fang L, Chen J, Zhou S, Zhou K, Cheng F. et al. Exosomal MicroRNA-21 Promotes Keloid Fibroblast Proliferation and Collagen Production by Inhibiting Smad7. J Burn Care Res. 2021;42(6):1266-74
125. Chen J, Zhou R, Liang Y, Fu X, Wang D, Wang C. Blockade of lncRNA-ASLNCS5088-enriched exosome generation in M2 macrophages by GW4869 dampens the effect of M2 macrophages on orchestrating fibroblast activation. FASEB J. 2019;33(11):12200-12
126. Zhu Z, Chen B, Peng L, Gao S, Guo J, Zhu X. Blockade of LINC01605-enriched exosome generation in M2 macrophages impairs M2 macrophage-induced proliferation, migration, and invasion of human dermal fibroblasts. Int J Immunopathol Pharmacol. 2021Jan;35:205873842110167
127. Hu H, Mao G, Zheng J, Guo F. Keloid Patient Plasma-Derived Exosomal hsa_circ_0020792 Promotes Normal Skin Fibroblasts Proliferation, Migration, and Fibrogenesis via Modulating miR-193a-5p and Activating TGF-β1/Smad2/3 Signaling. Drug Des Devel Ther. 2022;16:4223-34
128. Shen Z, Shao J, Sun J, Xu J. Exosomes released by melanocytes modulate fibroblasts to promote keloid formation: a pilot study. J Zhejiang Univ B. 2022Aug;23(8):699-704
129. Wu ZY, Zhang HJ, Zhou ZH, Li ZP, Liao SM, Wu ZY. et al. The effect of inhibiting exosomes derived from adipose-derived stem cells via the TGF-β1/Smad pathway on the fibrosis of keloid fibroblasts. Gland Surg. 2021;10(3):1046-56
130. Li Y, Zhang J, Shi J, Liu K, Wang X, Jia Y. et al. Exosomes derived from human adipose mesenchymal stem cells attenuate hypertrophic scar fibrosis by miR-192-5p/IL-17RA/Smad axis. Stem Cell Res Ther. 2021Dec;12(1):221
131. Yuan R, Dai X, Li Y, Li C, Liu L. Exosomes from miR-29a-modified adipose-derived mesenchymal stem cells reduce excessive scar formation by inhibiting TGF-β2/Smad3 signaling. Mol Med Rep. 2021Sep;24(5):758
132. Li J, Li Z, Wang S, Bi J, Huo R. Exosomes from human adipose-derived mesenchymal stem cells inhibit production of extracellular matrix in keloid fibroblasts via downregulating transforming growth factor-β2 and Notch-1 expression. Bioengineered. 2022;13(4):8515-25
133. Wu D, Liu X, Jin Z. Adipose-derived mesenchymal stem cells-sourced exosomal micro <scp>RNA</scp> -7846-3p suppresses proliferation and pro-angiogenic role of keloid fibroblasts by suppressing neuropilin 2. J Cosmet Dermatol. 2023Aug;22(8):2333-42
134. Zhang C, Gao X, Li M, Yu X, Huang F, Wang Y. et al. The role of mitochondrial quality surveillance in skin aging: Focus on mitochondrial dynamics, biogenesis and mitophagy. Ageing Res Rev. 2023Jun;87:101917
135. Fernandes A, Rodrigues PM, Pintado M, Tavaria FK. A systematic review of natural products for skin applications: Targeting inflammation, wound healing, and photo-aging. Phytomedicine. 2023Jul;115:154824
136. Tran D-K, Phuong TNT, Bui N-L, Singh V, Looi QH, Koh B. et al. Exploring the Potential of Stem Cell-Based Therapy for Aesthetic and Plastic Surgery. IEEE Rev Biomed Eng. 2023;16:386-402
137. Ellistasari EY, Kariosentono H, Purwanto B, Wasita B, Riswiyant RCA, Pamungkasari EP. et al. Exosomes Derived from Secretome Human Umbilical Vein Endothelial Cells (Exo-HUVEC) Ameliorate the Photo-Aging of Skin Fibroblast. Clin Cosmet Investig Dermatol. 2022;15:1583-91
138. Go YY, Lee CM, Ju WM, Chae S-W, Song J-J. Extracellular Vesicles (Secretomes) from Human Trophoblasts Promote the Regeneration of Skin Fibroblasts. Int J Mol Sci. 2021Jun;22(13):6959
139. Oh M, Lee J, Kim Y, Rhee W, Park J. Exosomes Derived from Human Induced Pluripotent Stem Cells Ameliorate the Aging of Skin Fibroblasts. Int J Mol Sci. 2018Jun;19(6):1715
140. Yan T, Huang L, Yan Y, Zhong Y, Xie H, Wang X. MAPK/AP-1 Signaling Pathway Is Involved in the Protection Mechanism of Bone Marrow Mesenchymal Stem Cells-Derived Exosomes against Ultraviolet-Induced Photoaging in Human Dermal Fibroblasts. Skin Pharmacol Physiol. 2023;36(2):98-106
141. Gao W, Wang X, Si Y, Pang J, Liu H, Li S. et al. Exosome Derived from ADSCs Attenuates Ultraviolet B-mediated Photoaging in Human Dermal Fibroblasts. Photochem Photobiol. 2021;97(4):795-804
142. Yan T, Huang L, Yan Y, Zhong Y, Xie H, Wang X. Bone marrow mesenchymal stem cell-derived exosome miR-29b-3p alleviates UV irradiation-induced photoaging in skin fibroblast. Photodermatol Photoimmunol Photomed. 2023;39(3):235-45
143. Bae YU, Son Y, Kim CH, Kim KS, Hyun SH, Woo HG. et al. Embryonic Stem Cell-Derived mmu-miR-291a-3p Inhibits Cellular Senescence in Human Dermal Fibroblasts Through the TGF-β Receptor 2 Pathway. Journals Gerontol - Ser A Biol Sci Med Sci. 2019;74(9):1359-67
144. Gao W, Yuan L min, Zhang Y, Huang F zhou, Gao F, Li J. et al. miR-1246-overexpressing exosomes suppress UVB-induced photoaging via regulation of TGF-β/Smad and attenuation of MAPK/AP-1 pathway. Photochem Photobiol Sci. 2023;22(1):135-46
145. Wu P, Zhang B, Han X, Sun Y, Sun Z, Li L. et al. HucMSC exosome-delivered 14-3-3ζ alleviates ultraviolet radiation-induced photodamage via SIRT1 pathway modulation. Aging (Albany NY). 2021Apr;13(8):11542-63
146. Sanada A, Yamada T, Hasegawa S, Ishii Y, Hasebe Y, Iwata Y. et al. Enhanced Type I Collagen Synthesis in Fibroblasts by Dermal Stem/Progenitor Cell-Derived Exosomes. Biol Pharm Bull. 2022;45(7):872-80
147. Park G, Kwon HH, Seok J, Yang SH, Lee J, Park BC. et al. Efficacy of combined treatment with human adipose tissue stem cell-derived exosome-containing solution and microneedling for facial skin aging: A 12-week prospective, randomized, split-face study. J Cosmet Dermatol [Internet]. 2023Dec28;22(12):3418-26 Available from: https://onlinelibrary.wiley.com/doi/10.1111/jocd.15872
148. Hu S, Li Z, Cores J, Huang K, Su T, Dinh PU. et al. Needle-Free Injection of Exosomes Derived from Human Dermal Fibroblast Spheroids Ameliorates Skin Photoaging. ACS Nano. 2019;13(10):11273-82
149. Liu SJ, Meng MY, Han S, Gao H, Zhao YY, Yang Y. et al. Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes Ameliorate HaCaT Cell Photo-Aging. Rejuvenation Res. 2021;24(4):283-93
150. Johnson J, Law SQK, Shojaee M, Hall AS, Bhuiyan S, Lim MBL. et al. First-in-human clinical trial of allogeneic, platelet-derived extracellular vesicles as a potential therapeutic for delayed wound healing. J Extracell Vesicles. 2023 12(7)
151. Kwon H, Yang S, Lee J, Park B, Park K, Jung J. et al. Combination Treatment with Human Adipose Tissue Stem Cell-derived Exosomes and Fractional CO2 Laser for Acne Scars: A 12-week Prospective, Double-blind, Randomized, Split-face Study. Acta Derm Venereol [Internet]. 2020;100(18):adv00310 Available from: https://medicaljournalssweden.se/actadv/article/view/1834
152. Zou J, Yang W, Cui W, Li C, Ma C, Ji X. et al. Therapeutic potential and mechanisms of mesenchymal stem cell-derived exosomes as bioactive materials in tendon-bone healing. J Nanobiotechnology. 2023Jan;21(1):14
153. Van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Vol. 19, Nature Reviews Molecular Cell Biology. 2018 p. 213-28
154. Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: A review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Vol. 15, International Journal of Nanomedicine. 2020 p. 6917-34
155. Zomer HD, Trentin AG. Skin wound healing in humans and mice: Challenges in translational research. Vol. 90, Journal of Dermatological Science. 2018 p. 3-12
Author contact
![]() Corresponding authors: Jing Yu, Ph.D., 1095 Jiefang Avenue, Wuhan, Hubei, China; E-mail address: daisy_yujingcom. Kai Hou, Ph.D., 1095 Jiefang Avenue, Wuhan, Hubei, China; E-mail address: kenhaucom. Min Wu, Ph.D., 1095 Jiefang Avenue, Wuhan, Hubei, China; E-mail address: wuminedu.cn; Tel.: +86 13554254796.
Corresponding authors: Jing Yu, Ph.D., 1095 Jiefang Avenue, Wuhan, Hubei, China; E-mail address: daisy_yujingcom. Kai Hou, Ph.D., 1095 Jiefang Avenue, Wuhan, Hubei, China; E-mail address: kenhaucom. Min Wu, Ph.D., 1095 Jiefang Avenue, Wuhan, Hubei, China; E-mail address: wuminedu.cn; Tel.: +86 13554254796.

 Global reach, higher impact
Global reach, higher impact