10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2024; 20(6):2044-2071. doi:10.7150/ijbs.92274 This issue Cite
Review
Cholesterol metabolism in tumor microenvironment: cancer hallmarks and therapeutic opportunities
1. Department of General Surgery, The First Affiliated Hospital of Anhui Medical University, Hefei 230022, P. R. China.
2. Institute of Cancer Neuroscience, Medical Frontier Innovation Research Center, The First Hospital of Lanzhou University, Lanzhou 730000, P. R. China.
3. Anhui Public Health Clinical Center, Hefei 230022, P. R. China.
Received 2023-11-16; Accepted 2024-2-27; Published 2024-3-17
Abstract
Cholesterol is crucial for cell survival and growth, and dysregulation of cholesterol homeostasis has been linked to the development of cancer. The tumor microenvironment (TME) facilitates tumor cell survival and growth, and crosstalk between cholesterol metabolism and the TME contributes to tumorigenesis and tumor progression. Targeting cholesterol metabolism has demonstrated significant antitumor effects in preclinical and clinical studies. In this review, we discuss the regulatory mechanisms of cholesterol homeostasis and the impact of its dysregulation on the hallmarks of cancer. We also describe how cholesterol metabolism reprograms the TME across seven specialized microenvironments. Furthermore, we discuss the potential of targeting cholesterol metabolism as a therapeutic strategy for tumors. This approach not only exerts antitumor effects in monotherapy and combination therapy but also mitigates the adverse effects associated with conventional tumor therapy. Finally, we outline the unresolved questions and suggest potential avenues for future investigations on cholesterol metabolism in relation to cancer.
Keywords: antitumor immunity, cholesterol homeostasis, drug repurposing, metastasis, statin, tumor microenvironment
Introduction
The hallmarks of cancer are the acquired capabilities of cells during the transition from normal to neoplastic growth, facilitating the formation of malignant tumors. Six hallmarks of cancer were originally identified [1], but this number has since increased to fourteen. These include sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative mortality, inducing or accessing vasculature, activating invasion and metastasis, deregulating cellular metabolism, avoiding immune destruction, genome instability and mutation, tumor-promoting inflammation, unlocking phenotypic plasticity, nonmutational epigenetic reprogramming, polymorphic microbiomes, and senescent cells [2]. The tumor microenvironment (TME) contributes to the acquisition and maintenance of these hallmarks to varying degrees. The TME refers to a micro-ecosystem comprising non-cancerous cells and tumor components that provide nutritional support and growth-stimulating signals to tumor cells [3]. The evolving understanding of TME has resulted in a shift in the focus of cancer therapy from a tumor-centric approach to a TME-centric approach. However, specialized microenvironments provide more precise targets for tumor treatment than the whole TME. The complex TME was previously divided into six specialized microenvironments [4]. Recently, this classification has been expanded to include seven specialized microenvironments: hypoxic niche, immune microenvironment, metabolism microenvironment, acidic niche, innervated niche, mechanical microenvironment, and microbial microenvironment [2, 5]. These specialized microenvironments interact with each other to form a dynamic tumor ecosystem.
Cholesterol is an essential constituent of the cell membrane and plays a vital role in cell survival and proliferation. In addition, cholesterol serves as a precursor for bile acids, steroid hormones, and oxysterol, which are crucial for maintaining various physiological processes [6]. Therefore, the maintenance of cholesterol homeostasis is critical for physiological functions. Dysregulation of cholesterol homeostasis not only leads to cardiovascular diseases, but also involved in tumorigenesis and progression of cancer [7]. The involvement of cholesterol in cancer has received increasing attention, with evidence of a dysregulated cholesterol balance in tumors. This imbalance in cholesterol homeostasis affects tumor hallmarks, promoting tumorigenesis, metastasis, and treatment resistance by reprogramming multiple microenvironments.
This review provides an overview of the regulatory mechanisms of cholesterol homeostasis and the effects of dysregulated cholesterol homeostasis on tumor hallmarks and interactions with various microenvironments. Finally, this paper provides a summary and discussion of the recent advances in the use of cholesterol metabolism as a target for cancer treatment.
Brief overview of cholesterol homeostasis
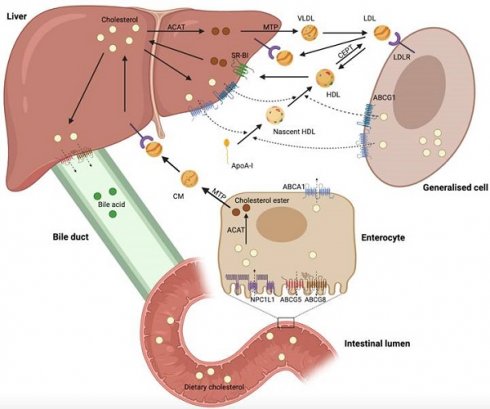
Cholesterol biosynthesis occurs in most mammalian cells, with hepatic cholesterol biosynthesis and dietary cholesterol being the primary sources of human cholesterol (Figure 1) [8]. Generally, cholesterol homeostasis is dynamically maintained through various cellular processes, including biosynthesis, uptake, esterification, efflux, and processing (Figure 2). Cholesterol biosynthesis is accomplished through the mevalonate pathway, which not only provides a metabolic route of cholesterol synthesis, but also provides various metabolites with significant biological functions. The mevalonate pathway involves the conversion of acetyl-CoA, the end product of glycolysis, to 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA), which is further metabolized through a series of enzymatic reactions to produce mevalonate, isopentenyl pyrophosphate (IPP), geranyl pyrophosphate (GPP), farnesyl pyrophosphate (FPP), squalene, lanosterol, and finally cholesterol [6]. Cholesterol homeostasis is regulated by transcription factors, such as sterol regulatory element-binding protein 2 (SREBP2) and liver X receptor (LXR). SREBP2 monitors cholesterol levels in the endoplasmic reticulum (ER) and remains inactive until intracellular cholesterol levels decrease [9]. Low cholesterol levels result in the release of the SREBP cleavage-activating protein (SCAP)-SREBP2 complex from the insulin-induced gene (INSIG) protein in the ER. This complex is then transported to the Golgi apparatus via COPII-coated vesicles, where SREBP2 undergoes proteolytic cleavage by site-1 (S1P) and site-2 (S2P) proteases [10]. Upon cleavage, the N-terminus of SREBP2 enters the nucleus and activates the transcription of target genes, such as HMG‑CoA reductase (HMGCR), low-density lipoprotein (LDL) receptor (LDLR), and Niemann-Pick type C1-like 1 (NPC1L1). This results in elevated cholesterol biosynthesis and uptake [6, 10, 11]. Elevated intracellular cholesterol levels result in an interaction between SCAP and INSIG proteins, preventing SCAP from binding to COPII and retaining the SCAP-SREBP2 complex within the ER, thereby impeding cholesterol biosynthesis. Furthermore, ER retention of the SCAP-SREBP2 complex modulates cholesterol uptake by reducing the expression of LDLR and NPCL1L [6]. In addition to biosynthesis, diet and subsequent uptake of cholesterol from the circulation play a significant role in maintaining cholesterol homeostasis. Cells typically acquire cholesterol from circulation via LDLR-mediated endocytosis. LDLR binds to LDL in the bloodstream, and the resulting LDL-LDLR complex is transported to lysosomes for degradation. This process releases free cholesterol via Niemann-Pick C1 (NPC1) and C2 (NPC2) proteins [12]. Proprotein convertase subtilisin/kexin type 9 (PCSK9) binds to LDLR and promotes its cellular uptake. Finally, the LDLR-PCSK9 complex undergoes lysosomal degradation [13]. Dietary cholesterol is a major source of cholesterol in humans, and its uptake by enterocytes is mediated by the NPC1L1 protein [14]. Following a series of processes, free cholesterol absorbed by NPC1L1 is esterified and transported as chylomicrons into circulation, ultimately being assimilated by the liver [14, 15]. The human NPC1L1 gene comprises two sterol regulatory elements (SRE), the sterol-sensing structural domain, and is activated by SREBP2. A high-cholesterol diet was found to suppress NPC1L1 expression, suggesting negative feedback regulation between cholesterol content and its absorption pathway [16].
Maintaining cholesterol homeostasis requires ensuring sufficient cholesterol biosynthesis and uptake for cell growth and function, while also preventing the overabundance of intracellular cholesterol through esterification, efflux, and processing. When intracellular cholesterol levels exceed demand, excess cholesterol can be esterified to cholesterol esters (CEs) by acyl-CoA: cholesteryl acyltransferase (ACAT) and stored in the cytoplasm as lipid droplets (LDs) [17]. In addition, cholesterol can be further metabolized into bile acids or steroid hormones secreted extracellularly [18, 19]. Cholesterol serves as a vital precursor for oxysterols. Excess cholesterol can be converted into oxysterols, which are more polar and have distinct physiological functions [20]. Activation of LXR by specific oxysterols increases the expression of cholesterol efflux-related genes, such as ATP-binding cassette (ABC) subfamily A member 1 (ABCA1), ABC subfamily G member 1 (ABCG1), ABCG5, and ABCG8 [21]. Excess cholesterol is exported into circulation via ABCA1 and ABCG1 and transported back to the liver as high-density lipoprotein (HDL) complexes [22, 23]. ABCG5 and ABCG8 are expressed on the apical surface of hepatocytes and enterocytes, where they function as heterodimers to transport excess cholesterol to the bile duct and intestinal lumen [6].
The overview reveals that cholesterol homeostasis involves a complex regulatory network comprising various pathways and components, including cholesterol biosynthesis (HMGCR, SREBP2, SCAP, INSIG, S1P, and S2P), uptake (LDLR, PCSK9, and NPC1L1), esterification (ACAT), efflux (LXR, ABCA1, ABCG1, ABCG5, and ABCG8), and processing (bile acids, steroid hormones, and oxysterols). The complexity and accuracy of this regulatory network ensure a dynamic balance in intracellular cholesterol levels.
Dysregulated cholesterol homeostasis as a contributor to the hallmarks of cancer
Dysregulation of cholesterol homeostasis is a characteristic feature of cancer cells. Cancer cells require higher cholesterol levels for membrane formation and signal transduction because of their rapid proliferation compared with normal cells [24, 25]. Lipid rafts, which contain cholesterol, are involved in diverse cellular processes [26]. Lipid rafts are abundant in many cancer cells, and their disruption can inhibit cancer cell growth [27]. Moreover, certain cholesterol precursors and derivatives have been found to affect cancer progression [28, 29]. Recent studies have shown that dysregulated cholesterol homeostasis affects tumor development, progression, metastasis, and therapeutic resistance through various regulatory mechanisms (Figure 3 and Table 1) [30-33].
Regulation of mammalian cholesterol homeostasis. Hepatic cholesterol biosynthesis and dietary cholesterol primary sources of cholesterol in humans. Excess cholesterol in the liver is excreted into the bile and eventually into the intestinal lumen for fecal excretion. Cholesterol in the circulation can be excreted directly into the intestinal lumen via enterocytes. This figure was created using BioRender (https://biorender.com/).
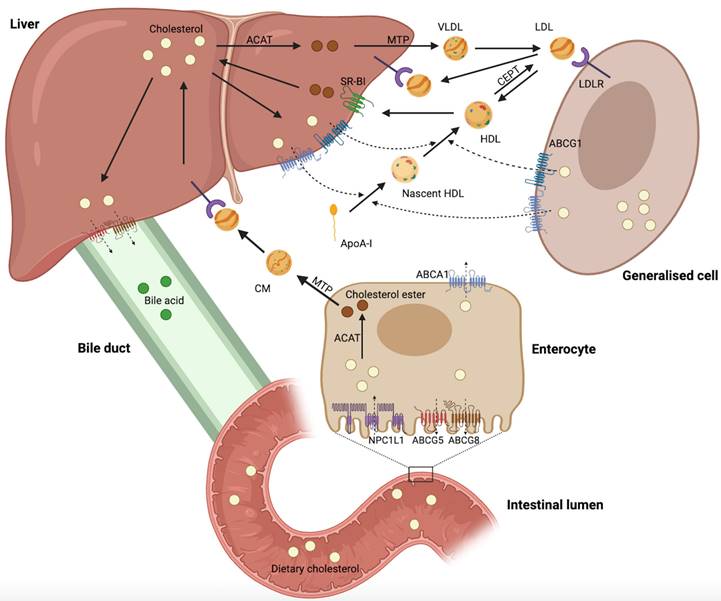
Regulation of cellular cholesterol homeostasis. The maintenance of cholesterol homeostasis is critical for physiological functions. Cellular cholesterol homeostasis is dynamically balanced through various processes, such as biosynthesis, uptake, esterification, efflux, and processing. Intracellular cholesterol levels are precisely regulated by these processes. This figure was created using BioRender (https://biorender.com/).
Dysregulation of cholesterol homeostasis in the development and progression of cancer
The association between cholesterol and tumorigenesis has been explored since the last century. Several prospective cohort studies have indicated a positive correlation between high dietary cholesterol intake and elevated plasma cholesterol levels with cancer incidence [34, 35]. Dysregulated cholesterol homeostasis is prevalent in many types of cancer and contributes to the onset and progression of the disease. Recent studies have reported various regulatory mechanisms by which dysregulation of cholesterol homeostasis affects cancer development and progression.
A recent report suggested that cholesterol and saturated fatty acids synergistically promote prostate cancer progression in mice [36]. Wu et al. found that cholesterol activates the PI3K/AKT pathway to promote colorectal cancer (CRC) progression [37]. In addition, PTEN loss and PI3K/AKT activation-induced cholesterol ester accumulation to promote prostate cancer progression [38]. Cancer cells are characterized by their resistance to ferroptosis, a process closely linked to cholesterol biosynthesis. Liu et al. found that dysregulated cholesterol homeostasis contributes to ferroptosis resistance, promoting cancer tumorigenicity [7]. N1-methyladenosine methylation in tRNA has been shown to drive liver tumorigenesis by inducing cholesterol biosynthesis, which activates Hedgehog signaling [39]. SREBP2, a key transcription factor involved in cholesterol biosynthesis, is frequently upregulated in various cancers. Copy number amplification of gene alpha-endosulfine (ENSA) promotes triple-negative breast cancer (TNBC) progression by increasing SREBP2 expression [32]. Gu et al. found that kinesin-like protein KIF11 promotes the progression of pancreatic ductal adenocarcinoma (PDAC) via SREBP2-dependent activation of mevalonate crosstalk [40]. Furthermore, Wei et al. found that unspliced X-box binding protein 1 (XBP1) colocalizes with SREBP2 and inhibits its degradation, promoting cholesterol biosynthesis and hepatocellular carcinoma (HCC) tumorigenesis [41]. Cellular senescence is associated with suppression of tumorigenesis [42-44], and tumor cells use various strategies to overcome senescence. A recent study found that transcription factor CP2 (TFCP2) interacts with SREBP2 to synergistically activate cholesterol biosynthesis and overcome cellular senescence in pancreatic cancer [45]. Oxysterols, being a derivative of cholesterol, have been extensively studied for their association with cancer due to their beneficial and detrimental effects on the disease [46, 47]. CYP27A1, a cytochrome P450 oxidase, catalyzes the conversion of cholesterol to 27-hydroxycholesterol (27HC) [48]. CYP27A1 has demonstrated renal cell carcinoma (RCC)-inhibiting effects by increasing 27HC concentrations in the body [49]. Similarly, Liang et al. found that CYP27A1 activates LXRs/ABCA1 by upregulating 27HC, promoting intracellular cholesterol efflux, and eventually inhibiting proliferation and migration in clear cell renal cell carcinoma (ccRCC) [50]. Evidence suggests that 27HC may promote the onset and progression of tumors. Luo et al. found that the histone reader zinc finger MYND-type containing 8 (ZMYND8) activates LXR and promotes breast cancer initiation by inhibiting 27HC catabolism and cholesterol efflux [51]. Avena et al. reported that 27HC promotes the progression of estrogen receptor-negative breast cancer (ER-BC) by binding to G protein-coupled estrogen receptor (GPER) [52]. In addition, 22HC induces cell cycle arrest by activating LXR, thereby inhibiting the progression of prostate, breast, and liver cancers [53]. However, Yoon et al. found that 22HC contributes to the development and progression of cholangiocarcinoma by stimulating COX-2 expression via a p38 MAPK-dependent mechanism [54]. Other oxysterols, such as 24HC and 25HC, have been identified as significant contributors to the development and progression of cancer [55-58]. PCSK9 promotes LDLR degradation, leading to increased circulating LDL levels. PCSK9 is a key regulator of cholesterol homeostasis and has been implicated in various aspects of cancer biology [59]. PCSK9 overexpression in CRC promotes cholesterol biosynthesis and accumulation of its intermediate geranylgeranyl diphosphate (GGPP) by inhibiting cholesterol uptake, ultimately inducing tumorigenesis [60]. In addition, squalene epoxidase (SQLE), the rate-limiting enzyme of cholesterol biosynthesis, has also been shown to promote the progression of p53-deficient castration-resistant prostate cancer (CRPC) [61], proliferation of p53 wild-type HCC cells, liver tumorigenesis in p53 knockout mice [62], and colorectal carcinogenesis by promoting gut dysbiosis [63]. Interestingly, Zhang et al. also found that dietary cholesterol drives the development of non-alcoholic fatty liver disease in HCC by altering gut microbiota and metabolites [64]. These findings establish a correlation between cholesterol homeostasis, gut microbiota, and tumorigenesis, providing a novel approach to exploring the involvement of cholesterol homeostasis in tumorigenesis.
Dysregulation of cholesterol homeostasis and hallmarks of cancer. Hanahan and Weinberg originally proposed the concept of cancer hallmarks, which has since been expanded to encompass fourteen hallmarks. We summarize the association between dysregulated cholesterol homeostasis and some of these cancer hallmarks, although further exploration is necessary to fully understand this relationship. This figure was created using BioRender (https://biorender.com/).
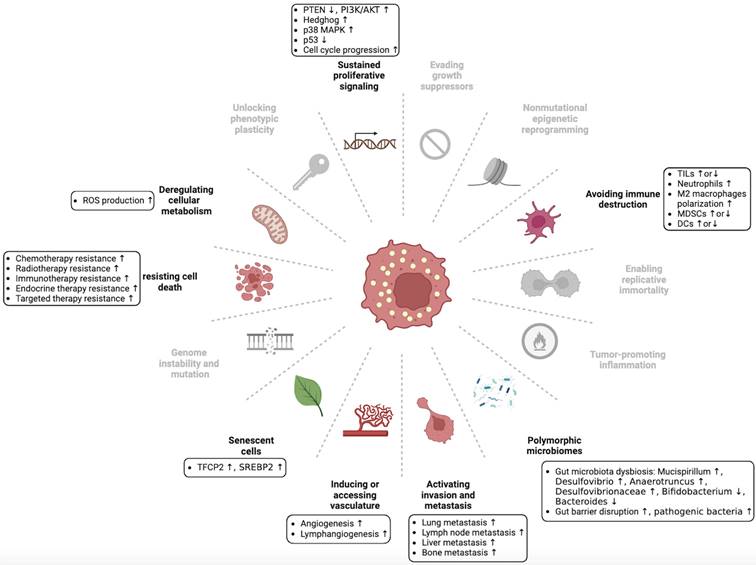
Summary of the dysregulation of cholesterol homeostasis and its functions in different cancer types.
| Cancer types | Mechanism | Phenotype/effect | References |
|---|---|---|---|
| Breast cancer | Activation of LXRs by inhibiting 27HC catabolism and cholesterol efflux | Tumorigenesis | [51] |
| Copy number amplification of ENSA enhances cholesterol biosynthesis | Tumor progression | [32] | |
| Dysregulation of cholesterol homeostasis induces the resistance of metastatic cells to ferroptosis | Enhanced metastatic capacity | [7] | |
| Chemokine regulatory loop induces cholesterol biosynthesis | Enhanced metastatic capacity | [30] | |
| Enhanced cholesterol biosynthesis pathway | Enhanced metastatic capacity | [69] | |
| 27HC activates LXR | Enhanced metastatic capacity | [77] | |
| 27HC promotes an immunosuppressive microenvironment by interacting with immune cells at distal metastatic sites | Enhanced metastatic capacity | [78] | |
| SREBP2 regulates osteoclast formation and function | Enhanced metastatic capacity | [84] | |
| Enhanced cholesterol biosynthesis pathway | Therapeutic resistance | [105] | |
| Liver cancer | Enhanced cholesterol biosynthesis activates Hedgehog signaling | Tumorigenesis | [39] |
| Unspliced XBP1 enhances cholesterol biosynthesis by stabilizing SREBP2 | Tumorigenesis | [41] | |
| Dietary cholesterol induces alterations in gut microbiota and metabolites | Tumorigenesis | [64] | |
| LDLR inhibition enhances cholesterol biosynthesis through the MEK/ERK signaling pathway | Enhanced metastatic capacity | [70] | |
| SCAP regulates autophagy by influencing AMPK signaling | Therapeutic resistance | [110] | |
| Caspase-3-induced SREBP2 activation enhances cholesterol biosynthesis | Therapeutic resistance | [111] | |
| Prostate cancer | PTEN deletion and PI3K/AKT activation induce cholesterol ester accumulation | Tumor progression | [38] |
| PTEN/p53 deficiency enhances cholesterol biosynthesis by upregulating SQLE expression | Tumor progression | [61] | |
| SREBP2 induces transcriptional activation of c-Myc | Enhanced metastatic capacity | [83] | |
| 27HC enhances the transcriptional activity of androgen receptors and expression of prostate-specific antigens | Therapeutic resistance | [92] | |
| HMGCR overexpression | Therapeutic resistance | [107] | |
| Cholesterol-rich macrophages transfer cholesterol to tumor cells | Therapeutic resistance | [108] | |
| Lung cancer | 27HC enhances osteoclast differentiation | Enhanced metastatic capacity | [81] |
| Cholesterol induces ABCG2 expression | Therapeutic resistance | [96] | |
| HOXB13 induces ABCG1 expression | Therapeutic resistance | [97] | |
| Cholesterol promotes ERRα re-expression through the EGFR/Src/Erk/SP1 signaling pathway | Therapeutic resistance | [31] | |
| High cholesterol levels in lipid rafts | Therapeutic resistance | [109] | |
| Gastric cancer | PCSK9 promotes the MAPK signaling pathway by upregulating HSP70 | Enhanced metastatic capacity | [73] |
| 25HC upregulates TLR2/NF‑κB‑mediated MMP expression | Enhanced metastatic capacity | [76] | |
| SOAT1 enhances cholesterol biosynthesis by regulating the expression of SREBP1 and SREBP2 | Enhanced metastatic capacity | [87] | |
| Pancreatic cancer | KIF11 activates mevalonate crosstalk in a SREBP2-dependent manner | Tumor progression | [40] |
| TFCP2 interacts with SREBP2 to synergistically activate cholesterol biosynthesis | Cellular senescence | [45] | |
| Cholesterol accumulation in lipid rafts | Therapeutic resistance | [91] | |
| Colorectal cancer | Overexpression of PSCK9 promotes cholesterol biosynthesis and accumulation of its intermediate GGPP by inhibiting cholesterol uptake | Tumorigenesis | [60] |
| SQLE promotes gut dysbiosis | Tumorigenesis | [63] | |
| Cholesterol activates the PI3K/AKT signaling pathway | Tumor progression | [37] | |
| Melanoma | Ahnak regulates PCSK9 expression | Enhanced metastatic capacity | [72] |
| Ovarian cancer | Cholesterol in malignant ascites activates LXRɑ/β | Therapeutic resistance | [99] |
| Cholangiocarcinoma | 22HC promotes COX-2 expression via a p38 MAPK-dependent mechanism | Tumorigenesis and tumor progression | [54] |
| Cervical cancer | Fatty acid synthase regulates cholesterol reprogramming and induces lymphangiogenesis | Enhanced metastatic capacity | [89] |
| Bladder cancer | Activation of the mevalonate pathway | Therapeutic resistance | [95] |
Clinical and preclinical studies have demonstrated a strong link between cholesterol homeostasis and cancer. However, the mechanism by which dysregulated cholesterol homeostasis contributes to the onset and progression of cancer remains to be fully elucidated.
Dysregulation of cholesterol homeostasis in tumor metastasis
Cancer metastasis is a dynamic and multi-step process [65, 66]. The prognosis of patients with metastatic cancer is extremely poor, with over 90% of cancer-related deaths attributable to metastasis [67]. However, the regulatory mechanisms that underlie cancer metastasis remain unclear. The correlation between cholesterol homeostasis and the mechanism of cancer metastasis is currently under investigation.
A clinical study highlighted that pretreatment serum cholesterol levels were higher in patients with metastatic prostate cancer than those with nonmetastatic prostate cancer [68]. Additionally, Liu et al. found that dysregulated cholesterol homeostasis in breast cancer promotes metastasis by inducing resistance to ferroptosis [7]. Han et al. revealed that regulation of the cholesterol synthesis pathway through a chemokine regulatory loop could promote metastatic growth of lung-colonizing TNBC cells [30]. Kim et al. reported that the cholesterol synthesis pathway increases tumor sphere formation and invasion in breast cancer cell metastasis [69]. Chen et al. found that the inhibition of LDLR expression in liver cancer stimulates intracellular cholesterol biosynthesis via the MEK/ERK signaling pathway, thereby promoting lung metastasis of HCC [70]. Interestingly, PCSK9, an inhibitor of LDLR, inhibits the lung metastasis of HCC [71], but stimulates lung metastasis of melanoma cells [72]. Xu et al. reported that PCSK9 could enhance lung and lymph node metastasis in gastric cancer (GC) by upregulating heat shock protein 70 levels and promoting the MAPK signaling pathways [73]. Sun et al. found that PCSK9 deficiency reduced liver metastasis in melanoma by decreasing cholesterol levels [74]. Oxysterols have also been implicated in cancer metastasis. Ortiz et al. found that cholesterol 25-hydroxylase (CH25H) produces 25HC, which inhibits the uptake of tumor-derived extracellular vesicles by normal cells and restricts the development of premetastatic niches in melanoma [75]. 25HC promotes lung metastasis of gastric cancer by upregulating TLR2/NF-κB mediated matrix metalloproteinase (MMP) expression [76]. Additionally, Nelson et al. found that 27HC promotes breast cancer lung metastasis by activating LXR [77]. Baek et al. reported that 27HC promotes the distant metastasis of breast cancer cells to the lungs by interacting with γδ-T cells and polymorphonuclear neutrophils [78]. Recently, Deng et al. reported that anoctamin 1 (ANO1) interacts with JUN to suppress CYP27A1-LXR signaling, leading to intracellular cholesterol accumulation and TME reprogramming, thus enhancing esophageal squamous cell carcinoma metastasis [79]. Late-stage lung cancer is associated with a higher incidence of osteolytic bone metastases [80]. Zhang et al. found that 27HC promotes the colonization of lung adenocarcinoma cells in the bone by stimulating osteoclast differentiation and creating a favorable microenvironment for tumor growth [81]. In addition, elevated cholesterol levels have been observed in bone metastases from prostate cancer [82]. SREBP2 facilitates distant metastasis of prostate cancer to the bone, adrenal gland, and lungs by transcriptionally activating c-Myc [83] and promotes bone metastasis in breast cancer by regulating osteoclast formation and function [84]. When intracellular cholesterol levels are high, sterol O-acyltransferase 1 (SOAT1), also known as ACAT1, converts excess cholesterol to cholesteryl ester (CE) [9]. SOAT1 is overexpressed in cancer and associated with poor patient prognosis [47, 85, 86]. Studies have shown that SOAT1 promotes lymph node metastasis in GC by regulating the expression of SREBP1 and SREBP2 [87], and its inhibition significantly suppresses lymph node and liver metastasis of pancreatic cancer [88]. Furthermore, Du et al. revealed that fatty acid synthase promotes cervical cancer lymph node metastasis by regulating cholesterol reprogramming and inducing lymphangiogenesis in cervical cancer [89].
Therefore, dysregulation of cholesterol homeostasis can contribute to cancer metastasis through multiple mechanisms. Consequently, there has been extensive research on potential drugs that target cholesterol metabolism for cancer therapy. Statins, a well-studied class of repurposed drugs that target cholesterol biosynthesis, have emerged as promising anticancer agents because of their ability to inhibit cancer metastasis via multiple mechanisms [90]. However, further investigation is required to understand the mechanism by which dysregulated cholesterol homeostasis promotes cancer metastasis, and to develop effective anticancer drugs targeting cholesterol metabolism.
Dysregulation of cholesterol homeostasis promotes cancer therapeutic resistance
Advancements in cancer research have led to significant progress in the exploration and implementation of various anticancer strategies. However, therapeutic resistance often results in treatment failure in most cancer patients. Studies have shown that dysregulated cholesterol homeostasis contributes significantly to resistance against cancer therapeutic strategies, including chemotherapy, radiotherapy, immunotherapy, endocrine therapy, and targeted therapy through multiple mechanisms.
Chemotherapy is a common treatment for cancer, and the dysregulation of cholesterol homeostasis has been linked to chemotherapy resistance in various studies. In pancreatic ductal adenocarcinoma, Yu et al. found that overexpression of cellular retinoic acid-binding protein II (CRABP-II) induces cholesterol accumulation in lipid rafts by upregulating the downstream SREBP-1c and eventually promoting resistance to gemcitabine [91]. Similarly, 27HC promotes the proliferation of prostate cancer cells and induces resistance to docetaxel via an androgen receptor (AR)-dependent mechanism [92]. Furthermore, Wang et al. demonstrated that 25HC promotes 5‑fluorouracil (5-FU) resistance in human GC cells [76]. Autophagy plays an important role in cell growth and development and has a biphasic effect on cancer progression, with studies demonstrating its ability to both suppress and promote tumors at different stages [93]. Lipid raft deficiency has been linked to doxorubicin resistance in breast cancer by promoting autophagy [94], whereas activation of the mevalonate pathway has been associated with doxorubicin resistance in bladder cancer cells [95]. Cholesterol and HOXB13 have been found to induce resistance to platinum-based chemotherapy in lung adenocarcinoma by upregulating ABCG2 and ABCG1 expression, respectively [96, 97]. The SREBP2 pathway [98] and malignant ascites cholesterol have been found to contribute to cisplatin resistance in ovarian cancer cells. Malignant ascites cholesterol activates LXRɑ/β, which increases the resistance of ovarian cancer cells to cisplatin and paclitaxel [99]. Research indicates that inhibition of PCSK9 can protect prostate cancer cells from radiation-induced cell damage, suggesting that PCSK9 may be a promising therapeutic target for enhancing radiosensitivity in prostate cancer [100]. Programmed death ligand 1 (PD-L1) is a therapeutic target in cancer immunotherapy, and PD-L1 inhibitors have been successfully used to treat cancer by restoring T-cell tumor-killing activity [101, 102]. However, tumor cell resistance to PD-L1 inhibitors is a significant concern. Studies have shown that statin treatment significantly lowers the expression of PD-L1, suggesting that cholesterol is closely related to PD-L1 [103]. Recently, Wang et al. found that the transmembrane domain of PD-L1 contains two cholesterol-recognition amino acid consensus (CRAC) motifs that can be recognized and bound by cholesterol, resulting in increased stability of PD-L1 in cancer cells and immunoevasion [104]. Furthermore, although endocrine therapy has been proven beneficial for numerous patients, the emergence of resistance to endocrine therapy has become a growing concern in recent years. Research indicates that 25HC may induce resistance to estrogen deprivation in estrogen receptor-positive (ER+) breast cancer by mimicking hormones [105]. Recently, Palma et al. reported that cholesterol depletion sensitizes breast cancer cells to tamoxifen [106]. HMGCR is a crucial enzyme in cholesterol biosynthesis. Kong et al. found that HMGCR overexpression promoted resistance of CRPC to enzalutamide, and statins were effective in overcoming this resistance [107]. Furthermore, El-Kenawi et al. found that cholesterol-rich macrophages induce resistance to endocrine therapy in CRPC by transferring cholesterol to cancer cells [108].
Targeted therapy is a type of precision medicine that provides optimism for cancer treatment. The continuous emergence of targeted drugs has significantly improved the prognosis of cancer patients. However, drug resistance presents a significant challenge for cancer treatment. Therefore, exploring the resistance mechanisms of targeted drugs can offer insight into improving their therapeutic efficacy. Cholesterol induces resistance to epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) in non-small cell lung cancer (NSCLC) via the EGFR/Src/Erk/SP1 signaling pathway [31], while elevated levels in lipid rafts induce gefitinib resistance [109]. SCAP and caspase-3 promote HCC resistance to sorafenib via AMPK-mediated autophagy [110] and SREBP2-induced sonic hedgehog signaling [111], respectively. Interestingly, statins can overcome sorafenib resistance in HCC [111] and delay GC resistance to Trastuzumab [112].
Cancer therapeutic resistance is a major concern that requires further investigation. Studies have demonstrated a correlation between cholesterol homeostasis and therapeutic resistance, with ongoing research aimed at understanding the underlying mechanisms of cholesterol-induced therapeutic resistance. Fortunately, cholesterol biosynthesis-targeting drugs have shown significant efficacy in overcoming therapeutic resistance. In the future, the combination of cholesterol metabolism-targeting drugs with conventional anticancer drugs may serve as a promising novel cancer therapeutic strategy.
Cholesterol metabolism and the TME
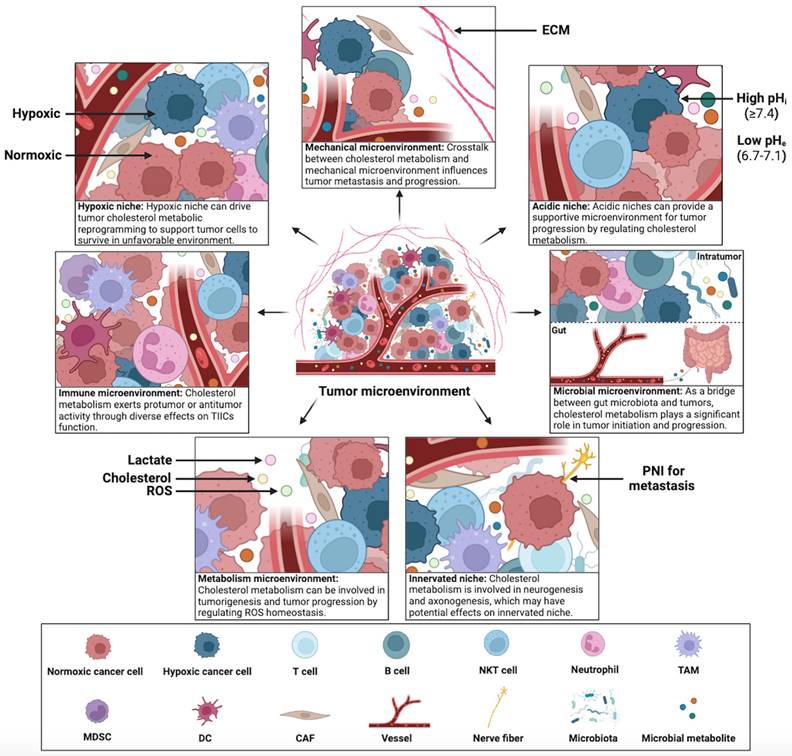
Constant interaction between tumor cells and their microenvironment is a crucial factor in the development, progression, metastasis, and therapeutic outcomes of cancer. Cholesterol metabolism appears to contribute significantly to this interaction, tumor cells in the TME can adapt to the complex microenvironment by reprogramming cholesterol metabolism. Therefore, exploring the mechanisms of cholesterol metabolism in seven specialized microenvironments can provide insights into the crosstalk between tumor cells and the complex TME. This section summarizes the diverse effects of cholesterol metabolism on hypoxic niche, immune microenvironment, metabolism microenvironment, acidic niche, innervated niche, mechanical microenvironment, and microbial microenvironment (Figure 4).
Cholesterol metabolism and immune microenvironment
During all stages of tumor progression, there is a constant interplay between tumor cells and tumor-infiltrating immune cells (TIICs) in the tumor immune microenvironment (TIME). These TIICs comprise immune effector and immunosuppressive cells, including T lymphocytes, B lymphocytes, natural killer (NK) cells, neutrophils, tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), and dendritic cells (DCs), which perform diverse antitumor or protumor functions in the TME [113, 114]. Evidence suggests that cholesterol metabolism influences antitumor immunity by acting on various TIICs [115].
Tumor-infiltrating lymphocytes (TILs) recognize and kill tumor cells, and cytotoxic T lymphocytes (CTLs) play a central role in antitumor immunity. Cholesterol metabolic reprogramming occurs in the TME during the functional maturation and activation of TILs. Cholesterol is required for T cell proliferation and activation. SREBP-mediated upregulation of cholesterol biosynthesis plays an important role in the activation and proliferation of CD8+ T cells [116]. Research has demonstrated that inhibiting ACAT1 activity upregulates plasma membrane cholesterol levels in CD8+ T cells, which results in enhanced T cell receptor clustering and signal transduction, as well as more efficient immunological synapse formation and antitumor response [117]. Wang et al. recently found that SOAT1-targeting compounds reprogrammed cholesterol metabolism in tumor cells and enhanced the antitumor response of CD8+ T cells against liver cancer [118]. Additionally, trogocytosis has been identified as an important process in immune regulation and other biological processes [119]. Trogocytosis refers to the extraction and transfer of biomolecules between adjacent cells, resulting in changes in both donor and acceptor cell functions [119, 120]. Trogocytosis between tumor cells and CTLs promotes the loss of antigens on target cells and destruction of CTLs, enabling tumor cells to evade the immune system [121]. Recent studies have shown that CH25H can enhance antitumor immunity by inhibiting trogocytosis and stimulating CTL activity [122]. Furthermore, another study found that CH25H promotes major histocompatibility complex class I (MHC-I) presentation and increases CD8+ T cell infiltration into tumors, sensitizing PDAC cells to immune checkpoint inhibitors [123]. In contrast, 27HC promotes the development of the breast cancer premetastatic niche by attracting polymorphonuclear neutrophils and γδ-T cells at metastatic sites while depleting CD8+ T cells [78]. Recently, Yan et al. found that 27HC induces cholesterol deficiency in T cells by inhibiting SREBP2 and activating LXR, subsequently leading to autophagy-mediated apoptosis of T cells [124].
Crosstalk between cholesterol metabolism and the TME. The TME has been categorized into seven specialized microenvironments: hypoxic niche, immune microenvironment, metabolism microenvironment, acidic niche, innervated niche, mechanical microenvironment, and microbial microenvironment (including intratumor and gut microbiota). Crosstalk between cholesterol metabolism and specialized microenvironments plays a significant role in tumorigenesis and tumor progression. This figure was created using BioRender (https://biorender.com/).
Interestingly, increasing cholesterol levels of chimeric antigen receptor (CAR)-T cells by blocking LXR can enhance antitumor activity, suggesting that improving CAR-T therapy by regulating cholesterol metabolism is a promising antitumor strategy [124]. Cytotoxic NK cells, which are crucial for antitumor immunity, are also affected by cholesterol metabolism. Qin et al. found that upregulating LDLR expression in NK cells improved their ability to combat HCC by elevating intracellular cholesterol levels, suggesting that increasing cholesterol uptake in NK cells could be a promising therapeutic strategy for HCC [125]. Yuan et al. found that the antitumor activity of CD8+ T cells is enhanced by LDLR via regulation of T-cell receptor (TCR) cycling and signaling through its interaction with the TCR complex [126] and inhibited by PCSK9 via inhibition of the recycling of LDLR and TCR to the CD8+ T cell plasma membrane [126]. Furthermore, Liu et al. revealed that PCSK9 binds to MHC-I in lysosomes and triggers its degradation, thereby preventing CTL infiltration and inhibiting antitumor immunity [127]. These findings suggest that PCSK9 inhibition could be a promising strategy for enhancing the immune response against tumors. However, the effect of cholesterol on T cell function is controversial, and high cholesterol levels in the TME have been linked to CD8+ T cell exhaustion by triggering immune checkpoint activation and ER stress [128]. Therefore, reducing cholesterol levels in the TME can effectively restore the antitumor activity of CD8+ T cells [128]. CD8+ T cell subset interleukin-9 (IL-9)-secreting (Tc9) cells have stronger antitumor activity than Tc1 cells, and IL-9 is indispensable for the antitumor activity of Tc9 cells. Cholesterol suppresses IL-9 expression by activating LXRs, thereby inhibiting the antitumor activity of Tc9 cells [129]. Recent studies have shown that colorectal cancer with microsatellite stability (MSS CRC) cells polarize T cells toward Th17 cells by secreting distal cholesterol precursors, thus promoting tumor progression [130]. SREBP2-mediated hepatic cholesterol accumulation suppresses natural killer T (NKT) cell cytotoxicity and antitumor immunosurveillance in liver cancer [131]. Wang et al. found that reducing serum cholesterol levels inhibited the mammalian target of rapamycin complex 2 (mTORC2) signaling in T lymphocytes and increased CD8+ T cell infiltration in prostate cancer [132]. These findings suggest that intrinsic and extrinsic cholesterol may exert differential effects on the function of TILs. Increasing intrinsic cholesterol biosynthesis and uptake of TILs may enhance their antitumor activity, whereas excessive extrinsic cholesterol in the TME may inhibit it. Moreover, B cells play a crucial role in the regulation of immune responses to tumors. Recent research indicates that SREBP signaling is crucial for B cell maturation, suggesting the significance of cholesterol metabolism in B cell regulation [133]. Bibby et al. found that cholesterol metabolism promotes the production of IL-10 from regulatory B cells via GGPP, thus restraining the immune response [134].
In addition to TILs, other TIICs have also been shown to be regulated by cholesterol metabolism. In the TIME, neutrophils typically exhibit protumor activity, and cholesterol derivatives, oxysterols, have been reported to exert protumor activity by regulating neutrophils. Raccosta et al. found that tumor-derived 22HC can recruit neutrophils via CXC chemokine receptor 2 (CXCR2) and promote tumor growth by stimulating angiogenesis and immunosuppression [135]. Soncini et al. found that hypoxia-inducible factor-1α (HIF-1α) can enhance neoangiogenesis in pancreatic neuroendocrine tumors by attracting neutrophils via 24HC upregulation [55]. Furthermore, 27HC has been reported to promote breast cancer metastasis through the recruitment of polymorphonuclear neutrophils at metastatic sites [78]. Tumor-infiltrating myeloid cells, including TAMs, MDSCs, and DCs, are also important components of the TIME. Cholesterol metabolic reprogramming can shift these myeloid cells towards an antitumor or a protumor phenotype. TAMs exhibit distinct antitumor M1-like and protumor M2-like phenotypes. Studies have suggested that tumors can alter cholesterol metabolism to promote the protumor function of TAMs. For instance, ovarian cancer cells secrete hyaluronic acid to promote cholesterol efflux from TAM membranes and induce a protumor M2-like phenotype [136]. Moreover, glioblastoma multiforme (GBM)-derived 25HC can activate the recruitment of macrophages to tumors through the G-protein-coupled receptor 183 (GPR183) [137]. MDSCs, as immature myeloid cells, typically exhibit immunosuppressive properties in TIME. Recently, Yang et al. reported that the UPR component XBP1 stimulates cholesterol biosynthesis and secretion in tumor cells, activating MDSCs to exhibit immunosuppressive properties [138]. In contrast, Tavazoie et al. found that LXRβ reduces the infiltration of protumor MDSCs into tumors, thus enhancing antitumor immunity [139]. However, Xie et al. recently found that chronic activation of LXRα can have an immunosuppressive effect by increasing the infiltration of MDSCs into tumors [140]. Therefore, future studies must elucidate the contradictory functions of LXRs in the immune response to cancer. The maturation of antigen-presenting DCs is essential for the activation and maintenance of the antitumor activity of TILs. Zhang et al. found that cholesterol-modified antimicrobial peptide DP7 induces DCs maturation and enhances antigen presentation by transporting various antigen peptides into DCs [141]. Xia et al. found that inhibiting geranylgeranylation of Rab5 in DCs resulted in cell surface antigen retention, enhanced antigen presentation, and CD8+ T cell activation [142]. Villablanca et al. found that oxysterol in the TME inhibits CC chemokine receptor-7 (CCR7) expression on the surface of mature DCs by activating LXRα, thereby inhibiting DC migration to lymphoid organs and reducing antitumor immunity [143]. In contrast, another study showed that LXR inverse agonists facilitate DC migration to the lymph nodes and boost antitumor immunity [144]. These findings suggest that the inhibition of LXR expression may be a promising immunotherapeutic approach for tumors.
In summary, cholesterol metabolism is critical for regulating the crosstalk between tumor cells and TIME. Cholesterol metabolism affects TIICs in the TIME through multiple mechanisms, resulting in either protumor or antitumor effects on the function of TIICs. Therefore, a deeper understanding of the regulation of cholesterol metabolism in the interaction between tumor cells and TIME will facilitate the development of novel immunotherapeutic strategies for cancer treatment. Targeting cholesterol metabolism in TIME has the potential as an effective antitumor approach by augmenting immunity against tumors.
Cholesterol metabolism and metabolism microenvironment
Metabolic reprogramming is a fundamental characteristic of cancer cells. Aerobic glycolysis (Warburg effect) is an early indication of metabolic reprogramming in cancer, that is, cancer cells prefer glycolysis to oxidative phosphorylation even under normoxic conditions [145]. Aerobic glycolysis allows cancer cells to compete with surrounding normal cells for glucose uptake, thereby sustaining their growth [146]. Lactate, produced through aerobic glycolysis, has significant effects on TME and tumor-associated cells [147]. Aerobic glycolysis can mitigate excessive accumulation of reactive oxygen species (ROS) by avoiding oxidative phosphorylation [148]. ROS plays a dual role in tumorigenesis by promoting tumor onset and progression via activation of various redox reactions and signaling pathways while also causing tumor cell damage and eventual death through oxidative stress [149]. Tumor cells exhibit higher levels of ROS than normal cells, and continuous oxidative stress causes them to tolerate slight accumulation of ROS (known as ROS addiction) [149]. Increasing evidence suggests that ROS addiction contributes to tumorigenesis and tumor progression. Cholesterol metabolism might play a role in this process by regulating ROS homeostasis.
Cholesterol accumulation in the tumor cell mitochondria triggers a cascade of chain reactions, leading to ROS production [150]. Interestingly, in ovarian cancer, increased mitochondrial ROS generation and activation of the AKT/mTOR signaling pathway upregulate SREBP2 expression, thereby promoting cholesterol biosynthesis [151]. Furthermore, increased cholesterol uptake protects against ROS-induced damage in ccRCC [152]. Shapira et al. found that cholesterol depletion decreased autophagic flux in an ROS- and JNK-dependent manner [153]. Wang et al. found that cholesterol promotes CRC progression by activating ROS and MAPK signaling pathway [154]. Liu et al. demonstrated that SQLE contributes to HCC tumorigenesis by silencing PTEN via ROS-induced DNA methyltransferase 3A (DNMT3A) expression [155]. In addition, the interaction between 27HC and ROS has been linked to both drug resistance and tumor progression. 27HC activates glucose-regulated protein 75 (GRP75) via elevated oxidative stress signaling in HCC, which regulates redox homeostasis by regulating ROS production and the antioxidant defense system, thereby inducing multidrug resistance [156]. Furthermore, evidence suggests that ROS contributes to 27HC-induced breast cancer cell invasion and angiogenesis by regulating reversion-inducing-cysteine-rich protein with Kazal motifs (RECK)/STAT-3 signaling via DNA methylation [157] and STAT-3/VEGF signaling [158], respectively.
Cholesterol metabolism and hypoxic niche
Tumors exhibit high adaptability, which enables them to thrive under adverse conditions. Intratumoral hypoxia is induced by rapid tumor growth and inadequate angiogenesis. Hypoxia-inducible factors (HIFs) are significant regulators of hypoxic response [159]. Hypoxia activates vascular endothelial cells, stimulates tumor angiogenesis, and promotes tumor growth and metastasis [160, 161]. More importantly, the hypoxic response triggers the “angiogenic switch” and promotes metabolic reprogramming in tumors [66, 162]. Evidence suggests that tumors can adapt to these hypoxic environments by reprogramming cholesterol metabolism to enhance tumor stemness and angiogenesis, which promotes their survival and growth. HIF-1α facilitates SREBP maturation in the hypoxic niche by upregulating the Ephrin-A3/EphA2 axis expression, thus enhancing cancer stemness in HCC [163]. Hypoxia activates HIF-1 by inducing protein kinase B (PKB) phosphorylation, thereby upregulating SREBP expression in breast cancer cells [164]. In addition, HIF-1α upregulates 24HC to attract neutrophils to hypoxic areas and induce the occurrence of “angiogenic switch” in pancreatic neuroendocrine tumor [55].
In summary, the hypoxic niche drives cholesterol metabolic reprogramming in tumors and interacts with other specialized microenvironments, promoting the survival of tumor cells under unfavorable conditions.
Cholesterol metabolism and acidic niche
Cancer is characterized by dysregulated or reversed pH, with a higher intracellular pH (pHi ≥ 7.4) and a lower extracellular pH (pHe = 6.7-7.1) [165]. Higher pHi facilitates cancer cell proliferation, migration, metabolic adaptation, and anti-apoptosis, whereas lower pHe facilitates cancer cell invasion and metastasis [165]. The acidic niche is closely linked to the hypoxic niche and metabolism microenvironment because the acidic niche is primarily caused by lactate secretion and CO2 production from anaerobic glycolysis and the pentose phosphate pathway, respectively [166]. Evidence suggests that an acidic niche provides a supportive microenvironment for tumor progression by regulating cholesterol metabolism.
Acidic extracellular environments can promote cholesterol biosynthesis in tumor cells by activating SREBP2, thereby promoting tumor growth [167]. Importantly, acidic pH-regulated SREBP2 target genes are inversely correlated with the overall survival of cancer patients [167]. Fukamachi et al. found that reducing GGPP synthesis can inhibit the proliferation of synovial sarcoma cells in acidic environments [168]. Additionally, John et al. found that activation of the IRE1-sXBP1-SREBP2-ACSS2 response axis at low pHe promotes cholesterol biosynthesis and cell membrane surface trafficking in astrocytic tumors, thereby enhancing cell membrane surface mechanical tenacity, preventing acid-mediated cell membrane hydrolysis, and supporting tumor cell survival [169]. In addition, Corbet et al. found that acidosis-induced TGF-β2 activation facilitated LD accumulation, enhancing the distant metastatic potential of cancer cells [170].
Cholesterol metabolism and mechanical microenvironment
The mechanical microenvironment, which is comprised of intracellular components (neurofilaments, vimentin, and actin), extracellular components (fibrin and collagen), stromal cells (fibroblasts), and intercellular signaling (integrin and focal adhesion) has been shown to affect intracellular signal transduction and, consequently, the biological behavior of tumor cells [171, 172]. Cholesterol metabolism appears to play a significant role in the mechanical microenvironment.
Cancer-associated fibroblasts (CAFs) are primary stromal cells in the mechanical microenvironment of tumors and can contribute to metastasis by secreting MMPs or activating yes-associated protein (YAP), leading to extracellular matrix (ECM) remodeling and epithelial-mesenchymal transition (EMT) [173-175]. Han et al. observed that TNBC-derived C-X-C motif chemokines 1/2/8 (CXCL1/2/8) stimulate CAFs and other lung-resident fibroblasts to secrete C-C motif chemokines 2/7 (CCL2/7), thereby activating cholesterol synthesis in TNBC cells to support metastatic tumor growth [30]. Moreover, 25HC was found to promote lung metastasis of GC by upregulating MMP expression [76]. Enhanced mevalonate pathway signaling by mutant p53 promotes the activity of YAP and PDZ-binding motif (TAZ) proto-oncogenes [176], whereas the oncogenic activity of YAP is dependent on ZMYND8-mediated cholesterol biosynthesis [177]. Cholesterol is essential for receptor signaling (e.g., integrin) by maintaining the stability of lipid rafts. Ramprasad et al. reported that depletion of cholesterol in the plasma membrane decreases α5β1 integrin-mediated adhesion and motility of lung adenocarcinoma cells to fibronectin [178]. In addition, Hoque et al. found that LDL-cholesterol promotes the motility and spread of tumor cells by participating in integrin trafficking, focal adhesion assembly, and ECM secretion [179]. Recently, transcription factor EB (TFEB) was shown to facilitate integrin-mediated endothelial cell adhesion to the ECM by upregulating cholesterol synthesis-associated genes in response to integrin signaling [180]. Maja et al. found that cholesterol-enriched cell surface domains are involved in ECM degradation, which promotes breast cancer cell invasion [181]. In addition, Shen et al. found that 27HC increases MMP9 expression and EMT through STAT-3 activation, thus promoting breast cancer migration and invasion [182]. This effect has also been validated by Avena et al. and Torres et al., who demonstrated that 27HC promotes EMT and migration of breast cancer cells [52, 183]. High cholesterol-mediated upregulation of adipocyte plasma membrane-associated protein (APMAP) in cholesterol-induced lipid rafts inhibits EGFR degradation, thereby activating the extracellular-regulated protein kinase 1/2 (ERK1/2) pathway and inducing EMT in prostate cancer cells [184]. Therefore, crosstalk between cholesterol metabolism and the mechanical microenvironment influences tumor metastasis and progression.
Cholesterol metabolism and microbial microenvironment
The microbial microenvironment is an emerging specialized microenvironment that delineates a landscape composed of intratumor microbiota, intestinal microbiota, and their metabolites [5, 185]. Intestinal microbiota plays an indispensable role in cholesterol metabolism. In this section, we discuss the interplay between cholesterol metabolism and the microbiota in the initiation and progression of tumors.
Zhang et al. found that dietary cholesterol contributes to non-alcoholic fatty liver disease (NAFLD)-associated HCC formation by inducing gut microbial dysbiosis [64], which could be effectively prevented by statin treatment [64]. This suggests a potential association between changes in the gut microbiota and tumorigenesis. Additionally, Li et al. proposed that SQLE promotes CRC carcinogenesis by modulating the gut microbiota-metabolite axis [63]. Cholesterol is converted to primary bile acids in the liver and subsequently processed into secondary bile acids by the gut microbiota [186]. Accumulating evidence suggests that secondary bile acids can promote tumorigenesis [187-190]. Yamada et al. found that the accumulation of secondary bile acids produced by the gut microbiota can activate mTOR signaling pathways in hepatocytes, thus promoting HCC carcinogenesis [191]. Primary bile acids have a positive effect on antitumor immunity by increasing CXCL16 expression in liver sinusoidal endothelial cells, consequently promoting the accumulation of CXCR6+ hepatic NKT cells [192]. In contrast, gut microbiome-mediated secondary bile acids suppress antitumor immunity by inhibiting the accumulation of hepatic NKT cells [192]. This suggests that cholesterol metabolism plays a significant role in the microbial microenvironment-TIME crosstalk.
In summary, cholesterol metabolism serves as a bridge between the intestinal microbiota and tumors and plays a significant role in the initiation and progression of tumors by regulating intestinal homeostasis and the immune system.
Cholesterol metabolism and innervated niche
With increasing awareness of the interaction between neurology and cancer science, Monje et al. have proposed a new field of study, “cancer neuroscience,” to explore bidirectional interactions between the nervous system and cancer [193]. We propose that the crosstalk between nerves and cancer is mediated by acellular components such as nerve-derived neurotransmitters or neuropeptides, resulting in a specialized microenvironment called “innervated niche” [4]. Some previous studies have also used the terms “perineural niche,” “neural regulation in TME,” or “neural microenvironment” [194-196].
Currently, there is a lack of evidence to support the effect of cholesterol metabolism on innervated niches despite the significant role of cholesterol in the nervous system [197]. Cholesterol is a vital structural component of neuronal plasma membranes and is necessary for maintaining fluidity and proper functioning of neurons [198]. Myelin is the continuous extension of the neuronal plasma membranes, with a higher lipid content, including 25-30% cholesterol [199]. Cholesterol is also involved in synapse and dendrite formation [200], and axon elongation [201]. In addition, cholesterol is responsible for the bilayer curvature required for synaptic vesicle fusion and fission, which is the basis of neurotransmission [202]. Cholesterol plays a crucial role in the production and maintenance of many neurotransmitter receptors [203-205] and can specifically interact with these receptors, suggesting another mechanism for regulating neurotransmission [203, 206]. Neurogenesis and axonogenesis may be considered as novel hallmarks of cancer associated with cancer progression [207, 208], making them potential targets for tumor therapy. Notably, several studies have demonstrated a strong correlation between cholesterol metabolism and neurogenesis [209-212]. Furthermore, Petro et al. found that sequestering cholesterol-induced membrane raft disruption promotes axonogenesis in murine neuroblastoma cells [213].
In summary, cholesterol is abundant in neurons and contributes to the regulation of vital membrane-associated functions of the nervous system. Additionally, cholesterol metabolism is implicated in neurogenesis and axonogenesis, suggesting its potential effects on the innervated niche. However, currently there is no direct evidence to elucidate the correlation between cholesterol metabolism and the innervated niche. Further research is required to address this gap in the field.
Targeting cholesterol metabolism as a promising antitumor strategy
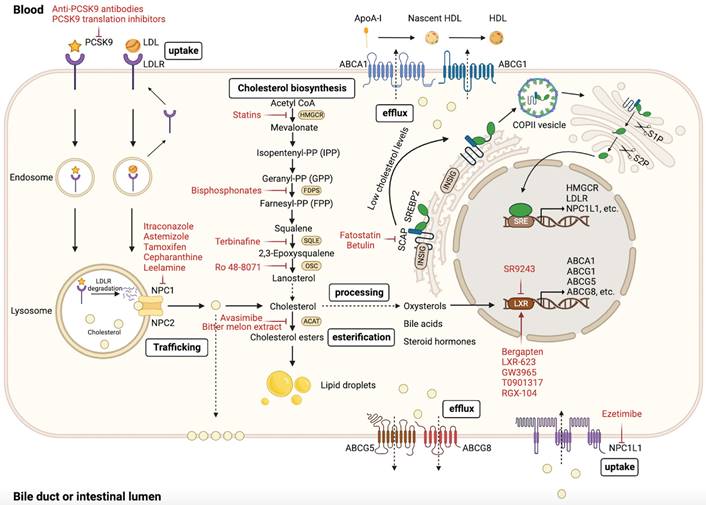
Ample evidence suggests that cholesterol metabolism is critical for tumor progression. Several preclinical and clinical studies have demonstrated that certain drugs can target cholesterol metabolism and exhibit antitumor effects. Ongoing research continues to explore new drugs that can target cholesterol metabolism in the treatment of cancer (Figure 1 and Table 2).
Summary of antitumor drugs that target cholesterol metabolism.
| Target | Drugs | Stage of clinical development | Mechanism | Cancer types | References | |
|---|---|---|---|---|---|---|
| Targeting cholesterol biosynthesis | HMGCR | Statins | Clinical trial | Atorvastatin inhibits breast cancer proliferation by influencing the expression of cyclin D1 and p27 | Breast cancer | [222] |
| Clinical trial | Fluvastatin promotes tumor cell apoptosis | Prostate cancer | [223] | |||
| SREBP | Fatostatin | Preclinical study | Blocking SREBP-regulated metabolic pathways and androgen receptor signaling networks | Prostate cancer | [233] | |
| Preclinical study | Induces tumor cell apoptosis | Endometrial cancer | [234] | |||
| Betulin | Preclinical study | Induces tumor cell apoptosis | Breast cancer | [342] | ||
| FDPS | Bisphosphonates | Preclinical study | Suppress tumor angiogenesis through the HIF-1α/VEGF signaling pathway | Breast cancer | [343] | |
| SQLE | Terbinafine | Preclinical study | Inhibits cancer cell proliferation and angiogenesis | Oral cancer | [248] | |
| OSC | Ro 48-8071 | Preclinical study | Suppresses tumor angiogenesis and metastasis | Colorectal cancer, pancreatic cancer | [249] | |
| Targeting cholesterol uptake and efflux | LXR | Bergapten (LXR agonist) | Preclinical study | Inhibits HCC carcinogenesis and progression by regulating the LXR/PI3K/Akt and LXR/IDOL/LDLR pathways and reducing LDLR expression in a dose-dependent manner | Liver cancer | [254] |
| LXR-623 (LXR agonist) | Preclinical study | Promotes LDLR degradation and induces ABCA1 expression | Glioblastoma | [252] | ||
| Preclinical study | Upregulates ABCA1 expression and downregulates LDLR expression | Kidney cancer | [259] | |||
| GW3965 (LXR agonist) | Preclinical study | Promotes LDLR degradation and induces ABCA1 expression | Glioblastoma | [255] | ||
| Preclinical study | GW3965 induces ApoE expression | Melanoma | [256] | |||
| T0901317 (LXR agonist) | Preclinical study | Upregulates ABCG1 expression | Prostate cancer | [257] | ||
| RGX-104 (LXR agonist) | Preclinical study and clinical trial | Induces MDSCs depletion and increases CTLs activation by upregulating ApoE expression | Multiple cancer types | [139] | ||
| SR9243 (LXR inverse agonist) | Preclinical study | Induces tumor cell apoptosis by inhibiting the Warburg effect and lipogenesis | Multiple cancer types | [258] | ||
| NPC1L1 | Ezetimibe | Preclinical study | Inhibits intracellular lipogenesis | Kidney cancer | [259] | |
| Preclinical study | Suppresses tumor angiogenesis | Prostate cancer | [268] | |||
| Preclinical study and clinical trial | Enhances antitumor immunity in a CD8+ T cell-dependent manner by inhibiting mTORC2 signaling | Multiple cancer types | [132] | |||
| Preclinical study | Blocks dietary cholesterol absorption | Liver cancer | [270] | |||
| Preclinical study | Suppresses tumor angiogenesis | Liver cancer | [269] | |||
| Targeting intracellular cholesterol trafficking | NPC1 | Itraconazole | Preclinical study | Inhibits angiogenesis and tumor growth | Lung cancer | [275] |
| Preclinical study | Inhibits tumor cell proliferation by inducing autophagy | Glioblastoma | [276] | |||
| Clinical trial | High-dose has antitumor activity | Prostate cancer | [272] | |||
| Clinical trial | Antitumor activity | Skin cancer | [271] | |||
| Astemizole | Preclinical study | Inhibits tumor cell proliferation | Cervical cancer | [344] | ||
| Tamoxifen | Preclinical study | Inhibits tumor angiogenesis by blocking intracellular cholesterol trafficking | Breast cancer | [279] | ||
| Cepharanthine | Preclinical study | Inhibits tumor angiogenesis | Multiple cancer types | [280] | ||
| Leelamine | Preclinical study | Inhibits the signaling cascades that drive tumor cell survival by blocking intracellular cholesterol trafficking | Melanoma | [281] | ||
| Targeting cholesterol esterification | ACAT1 | Avasimibe | Preclinical study | Inhibits tumor cell proliferation | Liver cancer | [86] |
| Preclinical study | Promotes tumor cell apoptosis by inhibiting cholesterol esterification | Pancreatic cancer | [88] | |||
| Preclinical study | Induces tumor cell apoptosis | Glioblastoma | [283] | |||
| Preclinical study | Impairs the Wnt/β-catenin pathway | Prostate cancer | [284] | |||
| Preclinical study | Enhances antitumor immunity mediated by CD8+ T cells | Melanoma | [117] | |||
| Preclinical study | Enhances antitumor immunity by increasing CD8+ T cell tumor infiltration | Lung cancer | [286] | |||
| Preclinical study | Enhances chimeric antigen receptor-modified T cell-mediated antitumor immunity | Leukemia | [287] | |||
| Bitter melon extract | Preclinical study | Inhibits tumor cell growth | Breast cancer | [285] | ||
| Combination therapy | Chemotherapy + HMGCR | Doxorubicin + simvastatin | Preclinical study | Synergistically promote cancer cell apoptosis | Breast cancer | [288] |
| Anthracycline + statins | Observational clinical cohort study | Associated with a lower incidence of heart failure | Breast cancer | [332] | ||
| Irinotecan + simvastatin | Preclinical study | Simvastatin enhances irinotecan-induced growth inhibition and apoptosis of cancer cells | Prostate cancer | [289] | ||
| Gemcitabine + pitavastatin | Preclinical study | Synergistically suppress tumor growth | Pancreatic cancer | [291] | ||
| Dacarbazine + pitavastatin | Preclinical study | Synergistically promote autophagy and apoptosis in tumor cells | Melanoma | [293] | ||
| Cisplatin + lovastatin | Preclinical study | Lovastatin sensitizes cancer cells to cisplatin | Gallbladder cancer | [294] | ||
| Cisplatin + atorvastatin | Clinical trial | Atorvastatin significantly reduces the incidence of cisplatin-induced hearing loss without reducing the efficacy of cisplatin | Head and neck cancer | [326] | ||
| 5-FU + pravastatin | Clinical trial | Prolongs the survival of patients with advanced HCC | Liver cancer | [221] | ||
| 5-FU + atorvastatin | Preclinical study | Atorvastatin effectively prevents leukopenia secondary to experimental 5-FU chemotherapy | Not reported | [320] | ||
| Radiotherapy + HMGCR | Radiotherapy + atorvastatin | Preclinical study | Atorvastatin enhances the radiosensitivity of prostate cancer cells by inhibiting hypoxia-induced HIF-1α protein expression | Prostate cancer | [290] | |
| SREBP + HMGCR | Dipyridamole + statins (Atorvastatin, simvastatin, and rosuvastatin) | Preclinical study | Dipyridamole enhances the antitumor activity of statins and prevents their resistance | Breast cancer | [226] | |
| Dipyridamole + fluvastatin | Preclinical study | Dipyridamole enhances apoptosis in statin-insensitive tumor cells | Prostate cancer | [229] | ||
| Targeted therapy + HMGCR | Erlotinib + pitavastatin | Preclinical study | Synergistcally enhances cytotoxicity and overcome erlotinib resistance | Lung cancer | [292] | |
| Gefitinib + simvastatin | Clinical trial | Increases PFS in patients | Lung cancer | [307] | ||
| Trastuzumab+ statins (Atorvastatin, simvastatin, rosuvastatin, and pravastatin) | Randomized Controlled Trial | Statins effectively reduce trastuzumab- induced cardiotoxicity | Breast cancer | [331] | ||
| Immunotherapy + HMGCR | PD-1 inhibitors+ statins (Atorvastatin, simvastatin, rosuvastatin, and others) | Clinical trial | High-intensity statins can enhance the clinical activity of PD-1 inhibitors | Lung cancer | [308] | |
| FDPS + HMGCR | Zoledronic acid + statins (Atorvastatin, simvastatin, and rosuvastatin) | Preclinical study | Synergistically exert antitumor effects | Breast cancer | [295] | |
| Chemotherapy + FDPS | Paclitaxel + Zoledronic acid | Preclinical study | Synergistically promote tumor cell apoptosis | Breast cancer | [296] | |
| Epirubicin + docetaxel + bisphosphonates | Clinical trial | Significantly enhances the clearance of disseminated tumor cells in patients with locally advanced breast cancer compared with chemotherapy alone | Breast cancer | [313] | ||
| Targeted therapy + LXR | Afatinib + GW3965 | Preclinical study | Synergistically inhibit tumor progression | Prostate cancer | [297] | |
| Gefitinib + GW3965 | Preclinical study | GW3965 enhances the sensitivity of NSCLC cells to gefitinib by inhibiting activation of the Akt‑NF‑κB signaling pathway | Lung cancer | [298] | ||
| Gefitinib + GW3965 | Preclinical study | GW3965 reverses gefitinib resistance in NSCLC by inhibiting vimentin expression | Lung cancer | [299] | ||
| Gefitinib + T0901317 | Preclinical study | Suppresses the migration and invasion of tumor cells by inhibiting the ERK/MAPK signaling | Lung cancer | [300] | ||
| Immunotherapy + PCSK9 | PD-1 inhibitors + PCSK9 antibodies | Preclinical study | PCSK9 antibodies synergistically inhibit tumor growth with PD-1 inhibitors by promoting the intratumoral infiltration of cytotoxic T cells | Multiple cancer types | [127] | |
| Chemotherapy + NPC1 | 5-FU + itraconazole | Preclinical study | Synergistically inhibit tumor cell growth | Gastric cancer | [302] | |
| Pemetrexed + itraconazole | Clinical trial | Improved OS in patients with advanced lung cancer compared with pemetrexed alone | Lung cancer | [319] | ||
| 5-FU + cepharanthine | Preclinical study | Synergistically inhibit the growth of colorectal cancer cells containing p53 mutations | Colorectal cancer | [303] | ||
| Chemotherapy + ACAT1 | Gemcitabine + avasimibe | Preclinical study | Synergistically exert antitumor effects | Pancreatic cancer | [304] | |
| Chemoimmunotherapy + ACAT1 | Paclitaxel + immunoadjuvant αGC + avasimibe | Preclinical study | Avasimibe enhances the antitumor activity of chemo-immunotherapy by relieving CD8+ T cell suppression | Melanoma | [305] | |
| Anticancer vaccines + ACAT1 | CSCs-DC vaccine + avasimibe | Preclinical study | Synergistically inhibit tumor cell growth | Head and neck cancer | [306] |
Targeting cholesterol biosynthesis
Evidence suggests that dysregulation of the mevalonate pathway drives oncogenic lesions, and targeting the mevalonate pathway to block cholesterol biosynthesis is a feasible antitumor strategy [214, 215]. Statins (HMGCR inhibitors) have been extensively studied as repurposed drugs for their antitumor activity. Interestingly, lipophilic statins exhibit higher antitumor activity than hydrophilic statins, indicating their potential superiority in antitumor therapy [216-218]. Many epidemiological and clinical studies have shown that statins can reduce the incidence and mortality of tumors [219, 220]. We previously summarized that statins can exert antitumor properties in vivo and in vitro through multiple mechanisms [90], and have emerged as promising antitumor agents in various clinical trials [221-223]. A randomized controlled trial has shown that fluvastatin suppresses proliferation and increases apoptosis in high grade breast cancer [224]. Interestingly, in addition to having direct effects on tumor cells, statins have also been demonstrated to enhance antitumor immunity. Recent studies have shown that simvastatin suppresses lncRNA SNHG29-mediated YAP activation and inhibits PD-L1 expression in CRC [225]. Furthermore, statins may maximize efficacy and overcome the shortcomings of conventional cancer therapy [90]. However, statin-induced inhibition of the mevalonate pathway drives SREBP activation and the mevalonate pathway-related gene transcription, thereby restoring the mevalonate pathway activity [226, 227]. This mechanism may contribute to resistance to statins, potentially accounting for the lack of response to statin therapy in certain patients with cancer [226, 227]. Therefore, targeting SREBP is a viable strategy to improve the anticancer effects of statins and overcome drug resistance. Combining statin therapy with SREBP targeting can optimize the antitumor activity. Dipyridamole, which inhibits SREBP cleavage, has been found to enhance the antitumor effects of statins and prevent their resistance by blocking the restorative feedback response of the mevalonate pathway [226, 228-232]. Fatostatin and betulin, which are SCAP inhibitors that specifically target SREBP activation, have also been shown to exhibit high antitumor activity [233-238]. Furthermore, inhibiting PCSK9 can also inhibit the expression of SREBP. Studies have shown that PCSK9 inhibitors (anti-PCSK9 antibodies and PCSK9 translation inhibitors) combined with simvastatin exert a synergistic antitumor effect in APC/KRAS-mutant CRC [60]. Further well-designed clinical trials are required to evaluate the efficacy of combining statins and SREBP inhibitors in cancer therapy.
Other enzymes in the cholesterol biosynthesis pathway may serve as viable targets for pharmacological intervention. Bisphosphonates are inhibitors of the cholesterol biosynthesis pathway that target farnesyl diphosphate synthase (FDPS) to block the production of FPP [239]. Preclinical and clinical studies have reported the direct antitumor effects of bisphosphonates [240-243]. Furthermore, alendronate, a bisphosphonate used to treat osteoporosis, has been reported to suppress glioblastoma spheres maintenance and activate necrosis-related pathways by inhibiting FDPS [244]. SQLE is an oncogene that is frequently overexpressed in various tumors [245]. Accumulating evidence indicates that SQLE promotes tumor progression through multiple mechanisms; therefore it is a promising antitumor target [63, 155, 246]. Terbinafine, a US Food and Drug Administration (FDA)-approved antifungal drug, has also been investigated for its potential as an antitumor drug by targeting SQLE in multiple studies [62, 155, 247, 248]. In addition, Ro 48-8071 can suppress cancer cell proliferation and metastasis by targeting oxidosqualene cyclase (OSC) and preventing the conversion of 2,3-oxidosqualene to lanosterol [249].
Targeting cholesterol uptake and efflux
LXR is a key transcriptional regulator that maintains cholesterol homeostasis by regulating the function of target genes involved in cholesterol uptake and efflux [250]. Several studies have evaluated the potential of targeting cholesterol uptake and efflux as an antitumor strategy, yielding promising outcomes.
Tumor cells upregulate LDLR expression to acquire extrinsic cholesterol and reduce energy consumption during cholesterol biosynthesis [251-253]. Bergapten, an LXR agonist, impedes the onset and progression of HCC by regulating the LXR/PI3K/Akt and LXR/IDOL/LDLR pathways and decreasing LDLR expression in a dose-dependent manner [254]. Additionally, other LXR agonists, such as LXR-623 and GW3965, induce tumor cell death in GBM via LDLR degradation and ABCA1 cholesterol efflux transporter upregulation [252, 255]. Furthermore, GW3965 inhibits melanoma invasion, angiogenesis, and metastasis by inducing apolipoprotein-E (ApoE) expression transcriptionally [256]. The LXR agonist T0901317 has shown promise in prostate cancer treatment by inducing apoptosis through ABCG1 expression-mediated cholesterol efflux and subsequent lipid raft disruption [257]. In addition to the effects on cancer cells, LXR agonists exert regulatory effects on immune cells. RGX-104, an LXR agonist, has been reported to enhance antitumor immunity by inducing depletion of MDSCs and upregulating ApoE expression, leading to increased activation of CTLs [139]. This finding was validated in a phase I clinical trial [139]. Interestingly, in addition to LXR activation, LXR repression also affects tumor progression. SR9243, an LXR inverse agonist, induces tumor cell apoptosis by inhibiting the Warburg effect and lipogenesis without causing toxic effects on non-malignant cells [258]. Furthermore, in ccRCC, Wu et al. found that both LXR agonist LXR-623 and LXR inverse agonist SR9243 could inhibit tumor cell growth [259]. Mechanistically, LXR-623 and SR9243 induce tumor cell apoptosis through distinct mechanisms. LXR-623 reduces intracellular cholesterol levels by upregulating ABCA1 expression and downregulating LDLR expression, whereas SR9243 inhibits intracellular lipogenesis [259]. These findings suggest that both LXR activation and repression can influence tumor progression.
PCSK9 regulates cholesterol homeostasis by facilitating LDLR degradation. Recent studies have demonstrated a close association between PCSK9 expression and tumor progression. Clinical evidence suggests that higher PCSK9 expression levels in tumors are negatively correlated with clinical prognosis [260-262], although contradictory findings have been reported [263]. PCSK9 has been found to promote tumor progression through multiple mechanisms [59, 72, 73], while also exerting antitumor effects [71, 263]. Recently, PCSK9 inhibitors (anti-PCSK9 antibodies and PCSK9 translation inhibitors) have been reported to suppress the growth of APC/KRAS-mutant CRC and exert synergistic antitumor effects with simvastatin [60]. Liu et al. reported that PCSK9 inhibition showed promising synergistic antitumor effects in combination with immune checkpoint therapy for tumors [127]. Consequently, PCSK9-targeting agents, such as anti-PCSK9 antibodies, vaccines, antisense RNAi, and some drugs (acRoots, lupin peptides, and pseurotin A) have been developed to target the regulation of PCSK9 for potential cancer treatment strategies. [264, 265]. Preclinical and clinical investigations are required to evaluate the efficacy and safety of PCSK9 inhibition as a potential tumor therapy. NPC1L1, a cholesterol uptake mediator on the apical surface of enterocytes, facilitates dietary cholesterol absorption [6] and has been linked to colitis-associated tumorigenesis [266]. The FDA-approved drug ezetimibe, which inhibits intestinal cholesterol absorption, has demonstrated significant antitumor effects by targeting NPC1L1. Multi-omics analysis revealed that NPC1L1 is an effective therapeutic target for the treatment of PDAC, and ezetimibe inhibits tumor growth without affecting the cytotoxicity of gemcitabine [267]. Furthermore, Ezetimibe inhibits tumor angiogenesis in prostate cancer [268], suppresses HCC progression [269], blocks dietary cholesterol absorption in nonalcoholic steatohepatitis (NASH)-driven HCC [270], and enhances antitumor immunity by inhibiting mTORC2 signaling in a CD8+ T cell-dependent manner [132]. Therefore, targeting intestinal cholesterol absorption is another promising strategy for treating tumors.
Targeting intracellular cholesterol trafficking
Cholesterol homeostasis depends on intracellular cholesterol trafficking. Studies have shown that inhibition of lysosomal cholesterol release can exert significant antitumor effects. Certain azoles, such as itraconazole, function as intracellular inhibitors of cholesterol trafficking and induce lysosomal cholesterol accumulation by targeting NPC1 and blocking its function [271-274]. Thus, itraconazole suppresses tumor growth and angiogenesis by downregulating mTORC1 signaling [273-276]. Importantly, the antitumor properties of itraconazole have been further validated in clinical trials [271, 272, 277]. Furthermore, astemizole has also been reported to exert antitumor activity by blocking intracellular cholesterol trafficking [278].
In addition to itraconazole and astemizole, other repurposed drugs have demonstrated antineoplastic effects by targeting intracellular cholesterol trafficking, such as selective estrogen receptor (ER) modulators (SERMs), cepharanthine, and leelamine. Tamoxifen, an estrogen receptor modulator, inhibits tumor angiogenesis by blocking intracellular cholesterol trafficking [279]. Additionally, cepharanthine, an anti-inflammatory drug, targets NPC1 to inhibit angiogenesis and tumor growth [280]. Furthermore, leelamine, a lipophilic diterpene amine, inhibits the tumor cell survival signaling cascade by blocking intracellular cholesterol trafficking [281]. More well-designed clinical trials are required to validate the efficacy of these repurposed drugs in cancer treatment.
Targeting cholesterol esterification
Tumor cells typically exhibit elevated CE levels, suggesting a correlation between cholesterol esterification and tumor progression [282]. Evidence suggests that targeting cholesterol esterification is a viable approach for tumor therapy. Avasimibe, a well-studied inhibitor of cholesterol esterification, has been found to effectively suppress tumor cell proliferation by targeting ACAT1 (SOAT1) to inhibit CE production. ACAT1 overexpression in HCC is associated with a poor prognosis [86]. Avasimibe has been reported to effectively suppress pancreatic cancer cell proliferation and metastasis by inhibiting cholesterol esterification [88]. Bemlih et al. found that avasimibe targets ACAT1 to inhibit tumor cell growth and induce apoptosis in glioblastoma [283], as well as depleting CEs in prostate cancer and impairing the Wnt/β-catenin pathway, which inhibits metastasis [284]. Bitter melon extract has been reported to inhibit tumor cell growth in TNBC by downregulating ACAT1 expression [285].
In addition to having direct effects on tumor cells, cholesterol esterification inhibition has also been demonstrated to exert antitumor activity by affecting immune cells. Yang et al. found that avasimibe improves CD8+ T cell receptor clustering and immune synapse formation in melanoma by targeting ACAT1, which increases CD8+ T cell plasma membrane cholesterol levels, thereby enhancing CD8+ T cell-mediated antitumor immunity [117]. Avasimibe has also been reported to increase CD8+ T cell infiltration in lung cancer [286] and CAR-T cell-mediated antitumor immunity by inhibiting cholesterol esterification [287]. Therefore, targeting cholesterol esterification can directly inhibit tumor cells and enhance antitumor immunity, suggesting that targeting cholesterol esterification may be a promising avenue for developing novel therapeutic approaches against cancer.
Combination therapy
Previously, we provided a summary of the evidence supporting the effectiveness of targeting cholesterol metabolism as monotherapy for tumors. However, in the context of precision medicine, the investigation of drug combination strategies is a crucial area of research. Accumulating evidence suggests that combining a cholesterol metabolism-targeting strategy with other antitumor therapies improves the effectiveness of tumor therapy. This review article explores the potential of such combination strategies targeting cholesterol metabolism for tumor therapy and highlights the advantages of such strategies in alleviating the adverse effects associated with conventional tumor therapy.
Targeting cholesterol metabolism in preclinical studies has demonstrated significant advantages in combination therapies and potential synergies with conventional tumor therapy. Statins, which target cholesterol biosynthesis, have been reported to act synergistically with conventional tumor therapy. For instance, simvastatin has been shown to enhance doxorubicin-induced apoptosis in breast cancer cells [288] and irinotecan-induced growth inhibition and apoptosis in prostate cancer cells [289]. Furthermore, atorvastatin increases the radiosensitivity of prostate cancer cells by inhibiting hypoxia-induced HIF-1α protein expression [290]. Chen et al. reported that pitavastatin and gemcitabine synergistically inhibited pancreatic cancer cell proliferation by inducing cell cycle arrest, leading to the effective inhibition of tumor growth in nude mouse xenograft models [291]. Pitavastatin has been found to increase cytotoxicity and overcome erlotinib resistance in non-small cell lung cancer (NSCLC) when used in combination with erlotinib [292], as well as enhance melanoma cell autophagy and apoptosis when combined with dacarbazine [293]. Additionally, Zhang et al. found that lovastatin sensitized gallbladder cancer cells to cisplatin and significantly prolonged the survival of subcutaneous xenograft mice [294]. However, statins inhibit the mevalonate pathway, leading to the activation of SREBP and affecting the expression of mevalonate pathway-related genes. This feedback response is a contributing factor to statin resistance [227]. Dipyridamole effectively targets SREBP to prevent statin resistance and enhance statin-mediated antitumor activity [226, 228-232]. Bisphosphonates (FDPS inhibitors) have also been demonstrated to enhance antitumor effects in combination with statins [295]. Jagdev et al. found that the combined use of bisphosphonates and paclitaxel induces apoptosis in breast cancer cells [296]. In addition, targeting cholesterol uptake and efflux has shown synergistic effects with conventional antitumor drugs. Chen et al. found that a combination of the LXR agonist GW3965 and EGFR inhibitor afatinib effectively suppressed prostate cancer progression [297]. Further research has revealed that GW3965 can increase the sensitivity of NSCLC cells to gefitinib, primarily by inhibiting activation of the Akt-nuclear factor (NF)-κB signaling pathway [298]. Additionally, Hu et al. observed that GW3965 could reverse gefitinib resistance in NSCLC by inhibiting vimentin expression [299]. Furthermore, the combination of T0901317, an LXR agonist, and gefitinib reduced the migration and invasion of lung cancer cells by inhibiting ERK/MAPK signaling [300]. PCSK9 antibodies have been reported to synergistically inhibit tumor growth with programmed cell death protein 1 (PD-1) inhibitors by promoting intratumoral infiltration of cytotoxic T cells, suggesting that PCSK9 inhibition is a feasible strategy for enhancing immune checkpoint therapy [127]. In addition, inhibiting drug efflux is also a feasible antitumor strategy. Nelfinavir exhibits synergistic antitumor effects with ezetimibe in multiple myeloma by inhibiting drug efflux transporter ABC subfamily B member 1 (ABCB1) [301]. Combining itraconazole, a cholesterol trafficking inhibitor, with 5-FU has shown a synergistic effect in inhibiting the growth of gastric cancer cells [302]. Cepharanthine, another cholesterol trafficking inhibitor, has been reported to act synergistically with 5-FU to suppress p53 mutated colorectal cancer cell growth [303]. In addition, targeting cholesterol esterification in combination with conventional tumor therapy is a promising strategy. Avasimibe, an ACAT1 inhibitor, exhibits synergistic antitumor effects when used together with gemcitabine in pancreatic cancer [304], enhances the efficacy of chemo-immunotherapy by relieving CD8+ T cell suppression [305], and potentially improves anticancer vaccine responses. Avasimibe has been reported to synergize with the KRAS vaccine to enhance antitumor immunity [286]. In particular, its combination with a cancer stem cell (CSC)-DC vaccine synergistically inhibited head and neck cancer growth in nude mouse xenograft models [306].
Clinical studies have demonstrated the potential of targeting cholesterol metabolism as adjuvant tumor therapy. In a randomized phase II study, combining simvastatin with gefitinib resulted in increased progression-free survival (PFS) in patients with gefitinib-resistant NSCLC [307]. Furthermore, Cantini et al. found that high-intensity statins combined with PD-1 inhibitors improved the prognosis of patients with malignant pleural mesothelioma and advanced NSCLC [308]. A randomized controlled trial revealed that pravastatin combined with 5-FU prolonged the survival of patients with advanced HCC, suggesting its potential as an adjuvant therapy for tumors [221]. Another randomized controlled trial revealed that atorvastatin is associated with decreased adrenal androgens in the serum and possibly in the prostate, suggesting that atorvastatin may improve the efficacy of androgen deprivation therapy [309]. In a randomized, double-blind, placebo-controlled trial, simvastatin improved sensitivity to fluorouracil, adriamycin, and cyclophosphamide therapy in HER2 overexpression locally advanced breast cancer patients [310]. Additionally, a cohort study demonstrated that administering statins to lung cancer patients undergoing EGFR-TKI treatment is linked to increased survival rates and has a synergistic effect on antitumor activity [311]. Bisphosphonates have shown potential in combination strategies. Epidemiological data indicate that combining bisphosphonates with statins significantly improves survival in patients with cancer [312]. A phase 2 clinical trial revealed that combining bisphosphonates with neoadjuvant chemotherapy eliminates disseminated tumor cells more effectively than chemotherapy alone in patients with locally advanced breast cancer [313]. Furthermore, the combination of bisphosphonate and chemotherapy significantly reduces the residual invasive tumor size in patients with breast cancer undergoing surgery [314]. Additionally, adjuvant bisphosphonates can improve disease-free survival (DFS) in postmenopausal patients with early-stage breast cancer [315]. Targeting intracellular cholesterol trafficking in combination with conventional tumor therapy is a promising strategy. Itraconazole combined with conventional tumor therapy has been found to significantly improve the clinical prognosis of cancer patients [316, 317]. A retrospective study has shown that the administration of itraconazole in combination with chemotherapy prolongs PFS and overall survival (OS) in patients with refractory ovarian cancer compared to chemotherapy alone [318]. In a phase 2 clinical study, itraconazole combined with pemetrexed improved OS in patients with advanced lung cancer compared with pemetrexed alone [319]. Therefore, numerous preclinical and clinical studies suggest that cholesterol metabolism-targeting in combination with other standard treatment modalities is a promising antitumor strategy. These evidences may provide a reference for the inclusion of targeting cholesterol metabolism in future tumor therapies.
Importantly, targeting cholesterol metabolism improves the efficacy and mitigates the adverse effects of conventional tumor therapy. Leukopenia is a significant adverse effect of chemotherapy. Atorvastatin was found to be effective in preventing 5-FU chemotherapy-induced leukopenia in an experimental model [320]. Cisplatin is widely used in the treatment of various malignant tumors. Despite its therapeutic advantages, this drug has a high level of ototoxicity, causing permanent hearing loss in over 50% of the treated patients [321-325]. A combined retrospective and prospective observational study reported that atorvastatin significantly reduced the incidence of cisplatin-induced hearing loss, while maintaining the efficacy of cisplatin [326]. Anthracyclines and trastuzumab are frequently used for breast cancer treatment. However, cardiotoxicity associated with these drugs increases the risk of heart failure [327, 328]. Based on the findings of multiple clinical studies, statins are considered promising candidates for attenuating anthracycline- and trastuzumab-induced cardiac injury [329-333]. These evidences validate the potential of statins in preventing chemotherapy-induced cardiac injury and warrants further clinical trials to investigate their efficacy in preventing cardiotoxicity. Tamoxifen is the primary treatment for ER-positive breast cancers. However, prolonged use of tamoxifen can result in NAFLD [334]. When used in combination with tamoxifen, fatostatin, a SREBP inhibitor, has been reported to reduce tamoxifen-induced hepatic lipid accumulation [335]. Further research into the molecular mechanisms that target cholesterol metabolism is required to prevent these adverse effects. This could provide a basis for rational combination strategies involving cholesterol metabolism-targeting and antitumor drugs to optimize tumor therapy. In summary, in addition to improving the survival rate of cancer patients, mitigating the adverse effects of conventional tumor therapy is imperative for improving the quality of life of cancer patients.
Conclusions and perspectives
The role of cholesterol metabolism in tumors has received increasing attention. Therefore, this article provides a summary of the molecular mechanisms by which the dysregulation of cholesterol homeostasis contributes to the hallmarks of cancer. In addition, cholesterol metabolism plays a significant role in the constant communication between tumor cells and their microenvironment. Therefore, this study highlights the diverse effects of cholesterol metabolism on seven specialized microenvironments as well as its involvement in tumor cell-to-TME communication via distinct molecular pathways. Targeting cholesterol metabolism is emerging as a promising antitumor strategy, with drugs capable of exerting antitumor effects by targeting this metabolic pathway. The evidence supporting the use of various antitumor strategies, including targeting cholesterol biosynthesis, uptake and efflux, intracellular trafficking, and esterification, is summarized. Numerous preclinical and clinical studies have suggested that combining conventional tumor therapy with cholesterol-targeting therapies can exert synergistic antitumor effects. Of note, targeting cholesterol enhances the efficacy of conventional tumor therapy and mitigates its adverse effects. These findings suggest that targeting cholesterol metabolism could enhance both the survival and quality of life of patients with cancer.
Despite the initiation of clinical trials for various repurposed drugs that target cholesterol metabolism, certain issues remain unresolved. First, given the low specificity of targeting cholesterol metabolism, which affects both tumor cells and immune cells, improving the specificity of targeting cholesterol metabolism to enhance both direct antitumor effects and antitumor immunity is an urgent problem to be solved. Cholesterol is required for the proliferation and activation of cancer and immune cells [32, 116]. In this context, it is particularly important to improve the specificity of targeting cholesterol metabolism. The advancements in nanotechnology enable controlled release of drugs at target sites [336]. Targeting cholesterol metabolism combined with nanoparticles may be a potential solution. Second, assessing the suitability of these repurposed cholesterol-targeting drugs in patients with different tumor types. A meta-analysis has shown that statin use reduces mortality in breast cancer patients [218]. However, a prospective population-based cohort study suggests that statin use is not associated with prostate cancer recurrence or progression [337]. Therefore, further well-designed clinical trials are required to assess the suitability of these repurposed cholesterol-targeting drugs in patients with different tumor types. Third, elucidating the potential adverse effects of these repurposed drugs in cancer patients. For instance, high doses of statins can lead to some adverse effects, with the most common ones being hepatic dysfunction and statin-associated muscle symptoms (SAMS) [338, 339]. Nevertheless, statins are generally well tolerated, and adverse effects vary depending on the exact statin used, as well as the dosage and combination with other drugs [340]. Therefore, when using repurposed drugs targeting cholesterol metabolism for cancer treatment, adverse effects and other drug interactions should be fully understood, drug dosages should be controlled, and adverse reactions should be monitored. Fourth, evaluating the optimal combination strategy for patients with different tumor types. In a preclinical study, the LXR agonist GW3965 has been reported to increase the sensitivity of NSCLC cells to gefitinib [298]. Furthermore, a clinical trial reported that simvastatin improved the efficacy of gefitinib in patients with gefitinib-resistant NSCLC [307]. Similarly, several studies have reported that avasimibe and pitavastatin combined with gemcitabine have synergistic antitumor effects in pancreatic cancer [291, 304]. A clinical trial reported that pravastatin prolongs survival in patients with advanced HCC treated with 5-FU after catheter arterial embolization [221]. However, another clinical trial reported that pravastatin did not improve survival in advanced HCC patients treated with sorafenib [341]. Therefore, the optimal combination strategy for patients with different tumor types requires further detailed evaluation. Further well-designed preclinical studies and clinical trials are required to address these unresolved issues.
Abbreviations
ANO1: Anoctamin 1
TME: Tumor microenvironment
HMG-CoA: 3-hydroxy-3-methyl-glutaryl-CoA
IPP: Isopentenyl pyrophosphate
GPP: Geranyl pyrophosphate
FPP: Farnesyl pyrophosphate
SREBP2: Sterol regulatory element-binding protein 2
LXR: Liver X receptor
ER: Endoplasmic reticulum
SCAP: SREBP cleavage-activating protein
INSIG: Insulin-induced gene
S1P: Site-1 protease
S2P: Site-2 protease
HMGCR: HMG‑CoA reductase
LDL: Low-density lipoprotein
LDLR: Low-density lipoprotein receptor
NPC1L1: Niemann-Pick type C1-like 1
NPC: Niemann-Pick C
PCSK9: Proprotein convertase subtilisin/kexin type 9
SRE: Sterol regulatory elements
CEs: Cholesterol esters
ACAT: Acyl-CoA: cholesteryl acyltransferase
LDs: Lipid droplets
ABC: ATP-binding cassette
ABCA: ATP-binding cassette subfamily A member
ABCB: ATP-binding cassette subfamily B member
ABCG: ABC subfamily G member
HDL: High-density lipoprotein
CRC: Colorectal cancer
ENSA: Gene alpha-endosulfine
TNBC: Triple-negative breast cancer
PDAC: Pancreatic ductal adenocarcinoma
XBP1: X-box binding protein 1
HCC: Hepatocellular carcinoma
TFCP2: Transcription factor CP2
27HC: 27-hydroxycholesterol
RCC: Renal cell carcinoma
ccRCC: Clear cell renal cell carcinoma
ZMYND8: Zinc finger MYND-type containing 8
ER-BC: Estrogen receptor-negative breast cancer
GPER: G protein-coupled estrogen receptor
GGPP: Geranylgeranyl diphosphate
SQLE: Squalene epoxidase
CRPC: Castration-resistant prostate cancer
GC: Gastric cancer
CH25H: Cholesterol 25-hydroxylase
MMP: Matrix metalloproteinase
SOAT1: Sterol O-acyltransferase 1
CRABP-II: Cellular retinoic acid-binding protein II
AR: Androgen receptor
5-FU: 5‑fluorouracil
PD-L1: Programmed death ligand 1
CRAC: Cholesterol-recognition amino acid consensus
ER+: Estrogen receptor-positive
EGFR-TKIs: Epidermal growth factor receptor tyrosine kinase inhibitors
NSCLC: Non-small cell lung cancer
TIICs: Tumor-infiltrating immune cells
TIME: Tumor immune microenvironment
NK: Natural killer
TAMs: Tumor-associated macrophages
MDSCs: Myeloid-derived suppressor cells
DCs: Dendritic cells
TILs: Tumor-infiltrating lymphocytes
CTLs: Cytotoxic T lymphocytes
MHC-I: Major histocompatibility complex class I
CAR: Chimeric antigen receptor
TCR: T-cell receptor
IL-9: Interleukin-9
NKT: Natural killer T
mTORC2: Mammalian target of rapamycin complex 2
CXCR2: CXC chemokine receptor 2
HIF-1α: Hypoxia-inducible factor-1α
GBM: Glioblastoma multiforme
GPR: G-protein-coupled receptor
CCR7: CC chemokine receptor-7
ROS: Reactive oxygen species
DNMT3A: DNA methyltransferase 3A
RECK: Reversion-inducing-cysteine-rich protein with Kazal motifs
HIFs: Hypoxia-inducible factors
PKB: Protein kinase B
CAFs: Cancer-associated fibroblasts
YAP: Yes-associated protein
ECM: Extracellular matrix
EMT: Epithelial-mesenchymal transition
CXCL1/2/8: C-X-C motif chemokines 1/2/8
CCL2/7: C-C motif chemokines 2/7
TAZ: PDZ-binding motif
TFEB: Transcription factor EB
APMAP: Adipocyte plasma membrane-associated protein
ERK1/2: Extracellular-regulated protein kinase 1/2
NAFLD: Non-alcoholic fatty liver disease
FDPS: Farnesyl diphosphate synthase
FDA: Food and Drug Administration
OSC: Oxidosqualene cyclase
ApoE: Apolipoprotein-E
NASH: Nonalcoholic steatohepatitis
SERMs: Selective estrogen receptor modulators
NF: Nuclear factor
PD-1: Programmed cell death protein 1
CSC: Cancer stem cell
PFS: Progression-free survival
DFS: Disease-free survival
OS: Overall survival
SAMS: Statin-associated muscle symptoms
Acknowledgements
We apologize to those colleagues whose important work could not be cited due to space constraints.
Funding
This work was supported by funding from the Key research and Development Plan of Anhui Province (no. 202104j07020029).
Author contributions
WJ, WLJ and AMX conceptualized the study. WJ and WLJ collected the relevant literature. WJ wrote the original draft and prepared the figure and the tables. WJ, WLJ and AMX significantly edited the manuscript text, figure and tables and supervised the project. All authors reviewed and approved the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57-70
2. Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12:31-46
3. Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904-12
4. Jin MZ, Jin WL. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct Target Ther. 2020;5:166
5. Li WN, Zhang SJ, Feng JQ, Jin WL. Repurposing Vitamin C for Cancer Treatment: Focus on Targeting the Tumor Microenvironment. Cancers (Basel). 2022 14
6. Luo J, Yang H, Song BL. Mechanisms and regulation of cholesterol homeostasis. Nat Rev Mol Cell Biol. 2020;21:225-45
7. Liu W, Chakraborty B, Safi R, Kazmin D, Chang CY, McDonnell DP. Dysregulated cholesterol homeostasis results in resistance to ferroptosis increasing tumorigenicity and metastasis in cancer. Nat Commun. 2021;12:5103
8. Repa JJ, Mangelsdorf DJ. The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Annu Rev Cell Dev Biol. 2000;16:459-81
9. Gong X, Qian H, Shao W, Li J, Wu J, Liu JJ. et al. Complex structure of the fission yeast SREBP-SCAP binding domains reveals an oligomeric organization. Cell Res. 2016;26:1197-211
10. Brown MS, Radhakrishnan A, Goldstein JL. Retrospective on Cholesterol Homeostasis: The Central Role of Scap. Annu Rev Biochem. 2018;87:783-807
11. Miserez AR, Muller PY, Barella L, Barella S, Staehelin HB, Leitersdorf E. et al. Sterol-regulatory element-binding protein (SREBP)-2 contributes to polygenic hypercholesterolaemia. Atherosclerosis. 2002;164:15-26
12. Goldstein JL, Brown MS. The LDL receptor. Arterioscler Thromb Vasc Biol. 2009;29:431-8
13. Lagace TA. PCSK9 and LDLR degradation: regulatory mechanisms in circulation and in cells. Curr Opin Lipidol. 2014;25:387-93
14. Altmann SW, Davis HR Jr, Zhu LJ, Yao X, Hoos LM, Tetzloff G. et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201-4
15. Iqbal J, Hussain MM. Intestinal lipid absorption. Am J Physiol Endocrinol Metab. 2009;296:E1183-94
16. Huff MW, Pollex RL, Hegele RA. NPC1L1: evolution from pharmacological target to physiological sterol transporter. Arterioscler Thromb Vasc Biol. 2006;26:2433-8
17. Chang TY, Li BL, Chang CC, Urano Y. Acyl-coenzyme A:cholesterol acyltransferases. Am J Physiol Endocrinol Metab. 2009;297:E1-9
18. Chang TY, Chang CC, Ohgami N, Yamauchi Y. Cholesterol sensing, trafficking, and esterification. Annu Rev Cell Dev Biol. 2006;22:129-57
19. Russell DW. Fifty years of advances in bile acid synthesis and metabolism. J Lipid Res. 2009;50(Suppl):S120-5
20. Yang C, McDonald JG, Patel A, Zhang Y, Umetani M, Xu F. et al. Sterol intermediates from cholesterol biosynthetic pathway as liver X receptor ligands. J Biol Chem. 2006;281:27816-26
21. Zhao C, Dahlman-Wright K. Liver X receptor in cholesterol metabolism. J Endocrinol. 2010;204:233-40
22. Gelissen IC, Harris M, Rye KA, Quinn C, Brown AJ, Kockx M. et al. ABCA1 and ABCG1 synergize to mediate cholesterol export to apoA-I. Arterioscler Thromb Vasc Biol. 2006;26:534-40
23. Lorenzi I, von Eckardstein A, Radosavljevic S, Rohrer L. Lipidation of apolipoprotein A-I by ATP-binding cassette transporter (ABC) A1 generates an interaction partner for ABCG1 but not for scavenger receptor BI. Biochim Biophys Acta. 2008;1781:306-13
24. Huang B, Song BL, Xu C. Cholesterol metabolism in cancer: mechanisms and therapeutic opportunities. Nat Metab. 2020;2:132-41
25. Kopecka J, Godel M, Riganti C. Cholesterol metabolism: At the cross road between cancer cells and immune environment. Int J Biochem Cell Biol. 2020;129:105876
26. Codini M, Garcia-Gil M, Albi E. Cholesterol and Sphingolipid Enriched Lipid Rafts as Therapeutic Targets in Cancer. Int J Mol Sci. 2021 22
27. Li YC, Park MJ, Ye SK, Kim CW, Kim YN. Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am J Pathol. 2006;168:1107-18 quiz 404-5
28. Stuchbery R, McCoy PJ, Hovens CM, Corcoran NM. Androgen synthesis in prostate cancer: do all roads lead to Rome? Nat Rev Urol. 2017;14:49-58
29. Ortiz N, Díaz C. Mevalonate pathway as a novel target for the treatment of metastatic gastric cancer. Oncol Lett. 2020;20:320
30. Han B, Alonso-Valenteen F, Wang Z, Deng N, Lee TY, Gao B. et al. A chemokine regulatory loop induces cholesterol synthesis in lung-colonizing triple-negative breast cancer cells to fuel metastatic growth. Mol Ther. 2022;30:672-87
31. Pan Z, Wang K, Wang X, Jia Z, Yang Y, Duan Y. et al. Cholesterol promotes EGFR-TKIs resistance in NSCLC by inducing EGFR/Src/Erk/SP1 signaling-mediated ERRα re-expression. Mol Cancer. 2022;21:77
32. Chen YY, Ge JY, Zhu SY, Shao ZM, Yu KD. Copy number amplification of ENSA promotes the progression of triple-negative breast cancer via cholesterol biosynthesis. Nat Commun. 2022;13:791
33. Gu J, Zhu N, Li HF, Zhao TJ, Zhang CJ, Liao DF. et al. Cholesterol homeostasis and cancer: a new perspective on the low-density lipoprotein receptor. Cell Oncol (Dordr). 2022;45:709-28
34. Järvinen R, Knekt P, Hakulinen T, Rissanen H, Heliövaara M. Dietary fat, cholesterol and colorectal cancer in a prospective study. Br J Cancer. 2001;85:357-61
35. Shafique K, McLoone P, Qureshi K, Leung H, Hart C, Morrison DS. Cholesterol and the risk of grade-specific prostate cancer incidence: evidence from two large prospective cohort studies with up to 37 years' follow up. BMC Cancer. 2012;12:25
36. Wang X, Sun B, Wei L, Jian X, Shan K, He Q. et al. Cholesterol and saturated fatty acids synergistically promote the malignant progression of prostate cancer. Neoplasia. 2022;24:86-97
37. Wu C, Wang M, Shi H. Cholesterol Promotes Colorectal Cancer Growth by Activating the PI3K/AKT Pathway. J Oncol. 2022;2022:1515416
38. Yue S, Li J, Lee SY, Lee HJ, Shao T, Song B. et al. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab. 2014;19:393-406
39. Wang Y, Wang J, Li X, Xiong X, Wang J, Zhou Z. et al. N(1)-methyladenosine methylation in tRNA drives liver tumourigenesis by regulating cholesterol metabolism. Nat Commun. 2021;12:6314
40. Gu X, Zhu Q, Tian G, Song W, Wang T, Wang A. et al. KIF11 manipulates SREBP2-dependent mevalonate cross talk to promote tumor progression in pancreatic ductal adenocarcinoma. Cancer Med. 2022;11:3282-95
41. Wei M, Nurjanah U, Herkilini A, Huang C, Li Y, Miyagishi M. et al. Unspliced XBP1 contributes to cholesterol biosynthesis and tumorigenesis by stabilizing SREBP2 in hepatocellular carcinoma. Cell Mol Life Sci. 2022;79:472
42. Hindson J. Inducing senescence sensitizes pancreatic tumours to therapies. Nat Rev Gastroenterol Hepatol. 2020;17:316
43. Lewis S. Targeting senescence. Nat Rev Neurosci. 2019;20:317
44. Gil J. Cellular senescence causes ageing. Nat Rev Mol Cell Biol. 2019;20:388
45. Zhang D, Lu P, Zhu K, Wu H, Dai Y. TFCP2 Overcomes Senescence by Cooperating With SREBP2 to Activate Cholesterol Synthesis in Pancreatic Cancer. Front Oncol. 2021;11:724437
46. Abdalkareem Jasim S, Kzar HH, Haider Hamad M, Ahmad I, Al-Gazally ME, Ziyadullaev S. et al. The emerging role of 27-hydroxycholesterol in cancer development and progression: An update. Int Immunopharmacol. 2022;110:109074
47. Xu H, Zhou S, Tang Q, Xia H, Bi F. Cholesterol metabolism: New functions and therapeutic approaches in cancer. Biochim Biophys Acta Rev Cancer. 2020;1874:188394
48. Shi SZ, Lee EJ, Lin YJ, Chen L, Zheng HY, He XQ. et al. Recruitment of monocytes and epigenetic silencing of intratumoral CYP7B1 primarily contribute to the accumulation of 27-hydroxycholesterol in breast cancer. Am J Cancer Res. 2019;9:2194-208
49. Zhang X, Yin X, Dai J, Sun G, Zhang H, Liang J. et al. The tumor-repressing effect of CYP27A1 on renal cell carcinoma by 27-HC arising from cholesterol metabolism. Faseb j. 2022;36:e22499
50. Liang Z, Jiao W, Wang L, Chen Y, Li D, Zhang Z. et al. CYP27A1 inhibits proliferation and migration of clear cell renal cell carcinoma via activation of LXRs/ABCA1. Exp Cell Res. 2022;419:113279
51. Luo M, Bao L, Chen Y, Xue Y, Wang Y, Zhang B. et al. ZMYND8 is a master regulator of 27-hydroxycholesterol that promotes tumorigenicity of breast cancer stem cells. Sci Adv. 2022;8:eabn5295
52. Avena P, Casaburi I, Zavaglia L, Nocito MC, La Padula D, Rago V. et al. 27-Hydroxycholesterol Binds GPER and Induces Progression of Estrogen Receptor-Negative Breast Cancer. Cancers (Basel). 2022 14
53. Chuu CP, Lin HP. Antiproliferative effect of LXR agonists T0901317 and 22(R)-hydroxycholesterol on multiple human cancer cell lines. Anticancer Res. 2010;30:3643-8
54. Yoon JH, Canbay AE, Werneburg NW, Lee SP, Gores GJ. Oxysterols induce cyclooxygenase-2 expression in cholangiocytes: implications for biliary tract carcinogenesis. Hepatology. 2004;39:732-8
55. Soncini M, Corna G, Moresco M, Coltella N, Restuccia U, Maggioni D. et al. 24-Hydroxycholesterol participates in pancreatic neuroendocrine tumor development. Proc Natl Acad Sci U S A. 2016;113:E6219-e27
56. Chen L, Zhang L, Xian G, Lv Y, Lin Y, Wang Y. 25-Hydroxycholesterol promotes migration and invasion of lung adenocarcinoma cells. Biochem Biophys Res Commun. 2017;484:857-63
57. Riscal R, Skuli N, Simon MC. Even Cancer Cells Watch Their Cholesterol!. Mol Cell. 2019;76:220-31
58. Zheng S, Lin J, Pang Z, Zhang H, Wang Y, Ma L. et al. Aberrant Cholesterol Metabolism and Wnt/β-Catenin Signaling Coalesce via Frizzled5 in Supporting Cancer Growth. Adv Sci (Weinh). 2022;9:e2200750
59. Bonaventura A, Vecchié A, Ruscica M, Grossi F, Dentali F. PCSK9 as a New Player in Cancer: New Opportunity or Red Herring? Curr Med Chem. 2022;29:960-9
60. Wong CC, Wu JL, Ji F, Kang W, Bian X, Chen H. et al. The cholesterol uptake regulator PCSK9 promotes and is a therapeutic target in APC/KRAS-mutant colorectal cancer. Nat Commun. 2022;13:3971
61. Shangguan X, Ma Z, Yu M, Ding J, Xue W, Qi J. Squalene Epoxidase Metabolic Dependency Is a Targetable Vulnerability in Castration-Resistant Prostate Cancer. Cancer Res. 2022;82:3032-44
62. Sun H, Li L, Li W, Yang F, Zhang Z, Liu Z. et al. p53 transcriptionally regulates SQLE to repress cholesterol synthesis and tumor growth. EMBO Rep. 2021;22:e52537
63. Li C, Wang Y, Liu D, Wong CC, Coker OO, Zhang X. et al. Squalene epoxidase drives cancer cell proliferation and promotes gut dysbiosis to accelerate colorectal carcinogenesis. Gut. 2022;71:2253-65
64. Zhang X, Coker OO, Chu ES, Fu K, Lau HCH, Wang YX. et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut. 2021;70:761-74
65. Welch DR, Hurst DR. Defining the Hallmarks of Metastasis. Cancer Res. 2019;79:3011-27
66. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-74
67. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30
68. Hirano H, Ide H, Lu Y, Inoue Y, Okada H, Horie S. Impact of Pretreatment Total Cholesterol Level Is Associated With Metastasis of Prostate Cancer. Am J Mens Health. 2020;14:1557988320918788
69. Kim HY, Bae SJ, Choi JW, Han S, Bae SH, Cheong JH. et al. Cholesterol Synthesis Is Important for Breast Cancer Cell Tumor Sphere Formation and Invasion. Biomedicines. 2022 10
70. Chen Z, Chen L, Sun B, Liu D, He Y, Qi L. et al. LDLR inhibition promotes hepatocellular carcinoma proliferation and metastasis by elevating intracellular cholesterol synthesis through the MEK/ERK signaling pathway. Mol Metab. 2021;51:101230
71. He M, Hu J, Fang T, Tang W, Lv B, Yang B. et al. Protein convertase subtilisin/Kexin type 9 inhibits hepatocellular carcinoma growth by interacting with GSTP1 and suppressing the JNK signaling pathway. Cancer Biol Med. 2021;19:90-103
72. Suh JM, Son Y, Yoo JY, Goh Y, Seidah NG, Lee S. et al. Proprotein convertase subtilisin/kexin Type 9 is required for Ahnak-mediated metastasis of melanoma into lung epithelial cells. Neoplasia. 2021;23:993-1001
73. Xu B, Li S, Fang Y, Zou Y, Song D, Zhang S. et al. Proprotein Convertase Subtilisin/Kexin Type 9 Promotes Gastric Cancer Metastasis and Suppresses Apoptosis by Facilitating MAPK Signaling Pathway Through HSP70 Up-Regulation. Front Oncol. 2020;10:609663
74. Sun X, Essalmani R, Day R, Khatib AM, Seidah NG, Prat A. Proprotein convertase subtilisin/kexin type 9 deficiency reduces melanoma metastasis in liver. Neoplasia. 2012;14:1122-31
75. Ortiz A, Gui J, Zahedi F, Yu P, Cho C, Bhattacharya S. et al. An Interferon-Driven Oxysterol-Based Defense against Tumor-Derived Extracellular Vesicles. Cancer Cell. 2019;35:33-45.e6
76. Wang S, Yao Y, Rao C, Zheng G, Chen W. 25-HC decreases the sensitivity of human gastric cancer cells to 5-fluorouracil and promotes cells invasion via the TLR2/NF-κB signaling pathway. Int J Oncol. 2019;54:966-80
77. Nelson ER, Wardell SE, Jasper JS, Park S, Suchindran S, Howe MK. et al. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science. 2013;342:1094-8
78. Baek AE, Yu YA, He S, Wardell SE, Chang CY, Kwon S. et al. The cholesterol metabolite 27 hydroxycholesterol facilitates breast cancer metastasis through its actions on immune cells. Nat Commun. 2017;8:864
79. Deng CM, Zhang GG, Liu QW, Xu JJ, Liu ZC, Yang J. et al. ANO1 Reprograms Cholesterol Metabolism and the Tumor Microenvironment to Promote Cancer Metastasis. Cancer Res. 2023;83:1851-65
80. Luis-Ravelo D, Antón I, Zandueta C, Valencia K, Ormazábal C, Martínez-Canarias S. et al. A gene signature of bone metastatic colonization sensitizes for tumor-induced osteolysis and predicts survival in lung cancer. Oncogene. 2014;33:5090-9
81. Zhang L, Liu M, Liu J, Li X, Yang M, Su B. et al. 27-Hydroxycholesterol enhanced osteoclastogenesis in lung adenocarcinoma microenvironment. J Cell Physiol. 2019;234:12692-700
82. Thysell E, Surowiec I, Hörnberg E, Crnalic S, Widmark A, Johansson AI. et al. Metabolomic characterization of human prostate cancer bone metastases reveals increased levels of cholesterol. PLoS One. 2010;5:e14175
83. Li X, Wu JB, Li Q, Shigemura K, Chung LW, Huang WC. SREBP-2 promotes stem cell-like properties and metastasis by transcriptional activation of c-Myc in prostate cancer. Oncotarget. 2016;7:12869-84
84. Jie Z, Xie Z, Xu W, Zhao X, Jin G, Sun X. et al. SREBP-2 aggravates breast cancer associated osteolysis by promoting osteoclastogenesis and breast cancer metastasis. Biochim Biophys Acta Mol Basis Dis. 2019;1865:115-25
85. Geng F, Cheng X, Wu X, Yoo JY, Cheng C, Guo JY. et al. Inhibition of SOAT1 Suppresses Glioblastoma Growth via Blocking SREBP-1-Mediated Lipogenesis. Clin Cancer Res. 2016;22:5337-48
86. Jiang Y, Sun A, Zhao Y, Ying W, Sun H, Yang X. et al. Proteomics identifies new therapeutic targets of early-stage hepatocellular carcinoma. Nature. 2019;567:257-61
87. Zhu T, Wang Z, Zou T, Xu L, Zhang S, Chen Y. et al. SOAT1 Promotes Gastric Cancer Lymph Node Metastasis Through Lipid Synthesis. Front Pharmacol. 2021;12:769647
88. Li J, Gu D, Lee SS, Song B, Bandyopadhyay S, Chen S. et al. Abrogating cholesterol esterification suppresses growth and metastasis of pancreatic cancer. Oncogene. 2016;35:6378-88
89. Du Q, Liu P, Zhang C, Liu T, Wang W, Shang C. et al. FASN promotes lymph node metastasis in cervical cancer via cholesterol reprogramming and lymphangiogenesis. Cell Death Dis. 2022;13:488
90. Jiang W, Hu JW, He XR, Jin WL, He XY. Statins: a repurposed drug to fight cancer. J Exp Clin Cancer Res. 2021;40:241
91. Yu S, Wang L, Che D, Zhang M, Li M, Naito M. et al. Targeting CRABP-II overcomes pancreatic cancer drug resistance by reversing lipid raft cholesterol accumulation and AKT survival signaling. J Exp Clin Cancer Res. 2022;41:88
92. Raza S, Meyer M, Schommer J, Hammer KD, Guo B, Ghribi O. 27-Hydroxycholesterol stimulates cell proliferation and resistance to docetaxel-induced apoptosis in prostate epithelial cells. Med Oncol. 2016;33:12
93. White E. Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer. 2012;12:401-10
94. Shi Y, Ye Z, Lu G, Yang N, Zhang J, Wang L. et al. Cholesterol-enriched membrane micro-domaindeficiency induces doxorubicin resistancevia promoting autophagy in breast cancer. Mol Ther Oncolytics. 2021;23:311-29
95. Greife A, Tukova J, Steinhoff C, Scott SD, Schulz WA, Hatina J. Establishment and characterization of a bladder cancer cell line with enhanced doxorubicin resistance by mevalonate pathway activation. Tumour Biol. 2015;36:3293-300
96. Wu Y, Si R, Tang H, He Z, Zhu H, Wang L. et al. Cholesterol reduces the sensitivity to platinum-based chemotherapy via upregulating ABCG2 in lung adenocarcinoma. Biochem Biophys Res Commun. 2015;457:614-20
97. Zhan J, Wang P, Li S, Song J, He H, Wang Y. et al. HOXB13 networking with ABCG1/EZH2/Slug mediates metastasis and confers resistance to cisplatin in lung adenocarcinoma patients. Theranostics. 2019;9:2084-99
98. Zheng L, Li L, Lu Y, Jiang F, Yang XA. SREBP2 contributes to cisplatin resistance in ovarian cancer cells. Exp Biol Med (Maywood). 2018;243:655-62
99. Kim S, Lee M, Dhanasekaran DN, Song YS. Activation of LXRɑ/β by cholesterol in malignant ascites promotes chemoresistance in ovarian cancer. BMC Cancer. 2018;18:1232
100. Gan SS, Ye JQ, Wang L, Qu FJ, Chu CM, Tian YJ. et al. Inhibition of PCSK9 protects against radiation-induced damage of prostate cancer cells. Onco Targets Ther. 2017;10:2139-46
101. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP. et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018-28
102. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E. et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:123-35
103. Di Bello E, Zwergel C, Mai A, Valente S. The Innovative Potential of Statins in Cancer: New Targets for New Therapies. Front Chem. 2020;8:516
104. Wang Q, Cao Y, Shen L, Xiao T, Cao R, Wei S. et al. Regulation of PD-L1 through direct binding of cholesterol to CRAC motifs. Sci Adv. 2022;8:eabq4722
105. Simigdala N, Gao Q, Pancholi S, Roberg-Larsen H, Zvelebil M, Ribas R. et al. Cholesterol biosynthesis pathway as a novel mechanism of resistance to estrogen deprivation in estrogen receptor-positive breast cancer. Breast Cancer Res. 2016;18:58
106. Henriques Palma GB, Kaur M. Cholesterol Depletion Modulates Drug Resistance Pathways to Sensitize Resistant Breast Cancer Cells to Tamoxifen. Anticancer Res. 2022;42:565-79
107. Kong Y, Cheng L, Mao F, Zhang Z, Zhang Y, Farah E. et al. Inhibition of cholesterol biosynthesis overcomes enzalutamide resistance in castration-resistant prostate cancer (CRPC). J Biol Chem. 2018;293:14328-41
108. El-Kenawi A, Dominguez-Viqueira W, Liu M, Awasthi S, Abraham-Miranda J, Keske A. et al. Macrophage-Derived Cholesterol Contributes to Therapeutic Resistance in Prostate Cancer. Cancer Res. 2021;81:5477-90
109. Chen Q, Pan Z, Zhao M, Wang Q, Qiao C, Miao L. et al. High cholesterol in lipid rafts reduces the sensitivity to EGFR-TKI therapy in non-small cell lung cancer. J Cell Physiol. 2018;233:6722-32
110. Li D, Yao Y, Rao Y, Huang X, Wei L, You Z. et al. Cholesterol sensor SCAP contributes to sorafenib resistance by regulating autophagy in hepatocellular carcinoma. J Exp Clin Cancer Res. 2022;41:116
111. Mok EHK, Leung CON, Zhou L, Lei MML, Leung HW, Tong M. et al. Caspase-3-Induced Activation of SREBP2 Drives Drug Resistance via Promotion of Cholesterol Biosynthesis in Hepatocellular Carcinoma. Cancer Res. 2022;82:3102-15
112. Pereira PMR, Mandleywala K, Monette S, Lumish M, Tully KM, Panikar SS. et al. Caveolin-1 temporal modulation enhances antibody drug efficacy in heterogeneous gastric cancer. Nat Commun. 2022;13:2526
113. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436-44
114. Turley SJ, Cremasco V, Astarita JL. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat Rev Immunol. 2015;15:669-82
115. King RJ, Singh PK, Mehla K. The cholesterol pathway: impact on immunity and cancer. Trends Immunol. 2022;43:78-92
116. Kidani Y, Elsaesser H, Hock MB, Vergnes L, Williams KJ, Argus JP. et al. Sterol regulatory element-binding proteins are essential for the metabolic programming of effector T cells and adaptive immunity. Nat Immunol. 2013;14:489-99
117. Yang W, Bai Y, Xiong Y, Zhang J, Chen S, Zheng X. et al. Potentiating the antitumour response of CD8(+) T cells by modulating cholesterol metabolism. Nature. 2016;531:651-5
118. Wang Z, Wang M, Zhang M, Xu K, Zhang X, Xie Y. et al. High-affinity SOAT1 ligands remodeled cholesterol metabolism program to inhibit tumor growth. BMC Med. 2022;20:292
119. Rechavi O, Goldstein I, Kloog Y. Intercellular exchange of proteins: the immune cell habit of sharing. FEBS Lett. 2009;583:1792-9
120. Dance A. Core Concept: Cells nibble one another via the under-appreciated process of trogocytosis. Proc Natl Acad Sci U S A. 2019;116:17608-10
121. Hamieh M, Dobrin A, Cabriolu A, van der Stegen SJC, Giavridis T, Mansilla-Soto J. et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature. 2019;568:112-6
122. Lu Z, McBrearty N, Chen J, Tomar VS, Zhang H, De Rosa G. et al. ATF3 and CH25H regulate effector trogocytosis and anti-tumor activities of endogenous and immunotherapeutic cytotoxic T lymphocytes. Cell Metab. 2022;34:1342-58.e7
123. McBrearty N, Cho C, Chen J, Zahedi F, Peck AR, Radaelli E. et al. Tumor suppressive and immune-stimulating roles of cholesterol 25-hydroxylase in pancreatic cancer cells. Mol Cancer Res. 2022
124. Yan C, Zheng L, Jiang S, Yang H, Guo J, Jiang LY. et al. Exhaustion-associated cholesterol deficiency dampens the cytotoxic arm of antitumor immunity. Cancer Cell. 2023
125. Qin WH, Yang ZS, Li M, Chen Y, Zhao XF, Qin YY. et al. High Serum Levels of Cholesterol Increase Antitumor Functions of Nature Killer Cells and Reduce Growth of Liver Tumors in Mice. Gastroenterology. 2020;158:1713-27
126. Yuan J, Cai T, Zheng X, Ren Y, Qi J, Lu X. et al. Potentiating CD8(+) T cell antitumor activity by inhibiting PCSK9 to promote LDLR-mediated TCR recycling and signaling. Protein Cell. 2021;12:240-60
127. Liu X, Bao X, Hu M, Chang H, Jiao M, Cheng J. et al. Inhibition of PCSK9 potentiates immune checkpoint therapy for cancer. Nature. 2020;588:693-8
128. Ma X, Bi E, Lu Y, Su P, Huang C, Liu L. et al. Cholesterol Induces CD8(+) T Cell Exhaustion in the Tumor Microenvironment. Cell Metab. 2019;30:143-56.e5
129. Ma X, Bi E, Huang C, Lu Y, Xue G, Guo X. et al. Cholesterol negatively regulates IL-9-producing CD8(+) T cell differentiation and antitumor activity. J Exp Med. 2018;215:1555-69
130. Bai Y, Li T, Wang Q, You W, Yang H, Xu X. et al. Shaping immune landscape of colorectal cancer by cholesterol metabolites. EMBO Mol Med. 2024
131. Tang W, Zhou J, Yang W, Feng Y, Wu H, Mok MTS. et al. Aberrant cholesterol metabolic signaling impairs antitumor immunosurveillance through natural killer T cell dysfunction in obese liver. Cell Mol Immunol. 2022;19:834-47
132. Wang Y, You S, Su S, Yeon A, Lo EM, Kim S. et al. Cholesterol-Lowering Intervention Decreases mTOR Complex 2 Signaling and Enhances Antitumor Immunity. Clin Cancer Res. 2022;28:414-24
133. Luo W, Adamska JZ, Li C, Verma R, Liu Q, Hagan T. et al. SREBP signaling is essential for effective B cell responses. Nat Immunol. 2022
134. Bibby JA, Purvis HA, Hayday T, Chandra A, Okkenhaug K, Rosenzweig S. et al. Cholesterol metabolism drives regulatory B cell IL-10 through provision of geranylgeranyl pyrophosphate. Nat Commun. 2020;11:3412
135. Raccosta L, Fontana R, Maggioni D, Lanterna C, Villablanca EJ, Paniccia A. et al. The oxysterol-CXCR2 axis plays a key role in the recruitment of tumor-promoting neutrophils. J Exp Med. 2013;210:1711-28
136. Goossens P, Rodriguez-Vita J, Etzerodt A, Masse M, Rastoin O, Gouirand V. et al. Membrane Cholesterol Efflux Drives Tumor-Associated Macrophage Reprogramming and Tumor Progression. Cell Metab. 2019;29:1376-89.e4
137. Eibinger G, Fauler G, Bernhart E, Frank S, Hammer A, Wintersperger A. et al. On the role of 25-hydroxycholesterol synthesis by glioblastoma cell lines. Implications for chemotactic monocyte recruitment. Exp Cell Res. 2013;319:1828-38
138. Yang Z, Huo Y, Zhou S, Guo J, Ma X, Li T. et al. Cancer cell-intrinsic XBP1 drives immunosuppressive reprogramming of intratumoral myeloid cells by promoting cholesterol production. Cell Metab. 2022;34:2018-35.e8
139. Tavazoie MF, Pollack I, Tanqueco R, Ostendorf BN, Reis BS, Gonsalves FC. et al. LXR/ApoE Activation Restricts Innate Immune Suppression in Cancer. Cell. 2018;172:825-40.e18
140. Xie Y, Sun R, Gao L, Guan J, Wang J, Bell A. et al. Chronic Activation of LXRα Sensitizes Mice to Hepatocellular Carcinoma. Hepatol Commun. 2022;6:1123-39
141. Zhang R, Tang L, Tian Y, Ji X, Hu Q, Zhou B. et al. Cholesterol-modified DP7 enhances the effect of individualized cancer immunotherapy based on neoantigens. Biomaterials. 2020;241:119852
142. Xia Y, Xie Y, Yu Z, Xiao H, Jiang G, Zhou X. et al. The Mevalonate Pathway Is a Druggable Target for Vaccine Adjuvant Discovery. Cell. 2018;175:1059-73.e21
143. Villablanca EJ, Raccosta L, Zhou D, Fontana R, Maggioni D, Negro A. et al. Tumor-mediated liver X receptor-alpha activation inhibits CC chemokine receptor-7 expression on dendritic cells and dampens antitumor responses. Nat Med. 2010;16:98-105
144. Carpenter KJ, Valfort AC, Steinauer N, Chatterjee A, Abuirqeba S, Majidi S. et al. LXR-inverse agonism stimulates immune-mediated tumor destruction by enhancing CD8 T-cell activity in triple negative breast cancer. Sci Rep. 2019;9:19530
145. Warburg O, Wind F, Negelein E. THE METABOLISM OF TUMORS IN THE BODY. J Gen Physiol. 1927;8:519-30
146. Yang DQ, Freund DM, Harris BR, Wang D, Cleary MP, Hegeman AD. Measuring relative utilization of aerobic glycolysis in breast cancer cells by positional isotopic discrimination. FEBS Lett. 2016;590:3179-87
147. Ganapathy-Kanniappan S. Molecular intricacies of aerobic glycolysis in cancer: current insights into the classic metabolic phenotype. Crit Rev Biochem Mol Biol. 2018;53:667-82
148. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029-33
149. Mao XY, Jin MZ, Chen JF, Zhou HH, Jin WL. Live or let die: Neuroprotective and anti-cancer effects of nutraceutical antioxidants. Pharmacol Ther. 2018;183:137-51
150. Baulies A, Montero J, Matías N, Insausti N, Terrones O, Basañez G. et al. The 2-oxoglutarate carrier promotes liver cancer by sustaining mitochondrial GSH despite cholesterol loading. Redox Biol. 2018;14:164-77
151. Zhao S, Cheng L, Shi Y, Li J, Yun Q, Yang H. MIEF2 reprograms lipid metabolism to drive progression of ovarian cancer through ROS/AKT/mTOR signaling pathway. Cell Death Dis. 2021;12:18
152. Riscal R, Bull CJ, Mesaros C, Finan JM, Carens M, Ho ES. et al. Cholesterol Auxotrophy as a Targetable Vulnerability in Clear Cell Renal Cell Carcinoma. Cancer Discov. 2021;11:3106-25
153. Shapira KE, Shapira G, Schmukler E, Pasmanik-Chor M, Shomron N, Pinkas-Kramarski R. et al. Autophagy is induced and modulated by cholesterol depletion through transcription of autophagy-related genes and attenuation of flux. Cell Death Discov. 2021;7:320
154. Wang C, Li P, Xuan J, Zhu C, Liu J, Shan L. et al. Cholesterol Enhances Colorectal Cancer Progression via ROS Elevation and MAPK Signaling Pathway Activation. Cell Physiol Biochem. 2017;42:729-42
155. Liu D, Wong CC, Fu L, Chen H, Zhao L, Li C. et al. Squalene epoxidase drives NAFLD-induced hepatocellular carcinoma and is a pharmaceutical target. Sci Transl Med. 2018 10
156. Jin M, Yang Y, Dai Y, Cai R, Wu L, Jiao Y. et al. 27-Hydroxycholesterol is a specific factor in the neoplastic microenvironment of HCC that causes MDR via GRP75 regulation of the redox balance and metabolic reprogramming. Cell Biol Toxicol. 2022;38:311-24
157. Zhu D, Shen Z, Liu J, Chen J, Liu Y, Hu C. et al. The ROS-mediated activation of STAT-3/VEGF signaling is involved in the 27-hydroxycholesterol-induced angiogenesis in human breast cancer cells. Toxicol Lett. 2016;264:79-86
158. Shen Z, Jiao K, Teng M, Li Z. Activation of STAT-3 signalling by RECK downregulation via ROS is involved in the 27-hydroxycholesterol-induced invasion in breast cancer cells. Free Radic Res. 2020;54:126-36
159. Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294-309
160. Palazon A, Tyrakis PA, Macias D, Veliça P, Rundqvist H, Fitzpatrick S. et al. An HIF-1α/VEGF-A Axis in Cytotoxic T Cells Regulates Tumor Progression. Cancer Cell. 2017;32:669-83.e5
161. Fukumura D, Xu L, Chen Y, Gohongi T, Seed B, Jain RK. Hypoxia and acidosis independently up-regulate vascular endothelial growth factor transcription in brain tumors in vivo. Cancer Res. 2001;61:6020-4
162. Huang D, Li C, Zhang H. Hypoxia and cancer cell metabolism. Acta Biochim Biophys Sin (Shanghai). 2014;46:214-9
163. Husain A, Chiu YT, Sze KM, Ho DW, Tsui YM, Suarez EMS. et al. Ephrin-A3/EphA2 axis regulates cellular metabolic plasticity to enhance cancer stemness in hypoxic hepatocellular carcinoma. J Hepatol. 2022;77:383-96
164. Furuta E, Pai SK, Zhan R, Bandyopadhyay S, Watabe M, Mo YY. et al. Fatty acid synthase gene is up-regulated by hypoxia via activation of Akt and sterol regulatory element binding protein-1. Cancer Res. 2008;68:1003-11
165. Webb BA, Chimenti M, Jacobson MP, Barber DL. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer. 2011;11:671-7
166. Kato Y, Ozawa S, Miyamoto C, Maehata Y, Suzuki A, Maeda T. et al. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013;13:89
167. Kondo A, Yamamoto S, Nakaki R, Shimamura T, Hamakubo T, Sakai J. et al. Extracellular Acidic pH Activates the Sterol Regulatory Element-Binding Protein 2 to Promote Tumor Progression. Cell Rep. 2017;18:2228-42
168. Fukamachi T, Wang X, Mochizuki Y, Maruyama C, Saito H, Kobayashi H. Acidic environments enhance the inhibitory effect of statins on proliferation of synovial cells. Int Immunopharmacol. 2013;17:148-53
169. John S, K GG, Krishna AP, Mishra R. Neurotherapeutic implications of sense and respond strategies generated by astrocytes and astrocytic tumours to combat pH mechanical stress. Neuropathol Appl Neurobiol. 2022;48:e12774
170. Corbet C, Bastien E, Santiago de Jesus JP, Dierge E, Martherus R, Vander Linden C. et al. TGFβ2-induced formation of lipid droplets supports acidosis-driven EMT and the metastatic spreading of cancer cells. Nat Commun. 2020;11:454
171. Liu Q, Luo Q, Ju Y, Song G. Role of the mechanical microenvironment in cancer development and progression. Cancer Biol Med. 2020;17:282-92
172. Nagelkerke A, Bussink J, Rowan AE, Span PN. The mechanical microenvironment in cancer: How physics affects tumours. Semin Cancer Biol. 2015;35:62-70
173. Panciera T, Azzolin L, Cordenonsi M, Piccolo S. Mechanobiology of YAP and TAZ in physiology and disease. Nat Rev Mol Cell Biol. 2017;18:758-70
174. Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI. et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol. 2013;15:637-46
175. Guo S, Deng CX. Effect of Stromal Cells in Tumor Microenvironment on Metastasis Initiation. Int J Biol Sci. 2018;14:2083-93
176. Ingallina E, Sorrentino G, Bertolio R, Lisek K, Zannini A, Azzolin L. et al. Mechanical cues control mutant p53 stability through a mevalonate-RhoA axis. Nat Cell Biol. 2018;20:28-35
177. Pan Q, Zhong S, Wang H, Wang X, Li N, Li Y. et al. The ZMYND8-regulated mevalonate pathway endows YAP-high intestinal cancer with metabolic vulnerability. Mol Cell. 2021;81:2736-51.e8
178. Ramprasad OG, Srinivas G, Rao KS, Joshi P, Thiery JP, Dufour S. et al. Changes in cholesterol levels in the plasma membrane modulate cell signaling and regulate cell adhesion and migration on fibronectin. Cell Motil Cytoskeleton. 2007;64:199-216
179. Hoque M, Rentero C, Conway JR, Murray RZ, Timpson P, Enrich C. et al. The cross-talk of LDL-cholesterol with cell motility: insights from the Niemann Pick Type C1 mutation and altered integrin trafficking. Cell Adh Migr. 2015;9:384-91
180. Ariano C, Riganti C, Corà D, Valdembri D, Mana G, Astanina E. et al. TFEB controls integrin-mediated endothelial cell adhesion by the regulation of cholesterol metabolism. Angiogenesis. 2022;25:471-92
181. Maja M, Mohammed D, Dumitru AC, Verstraeten S, Lingurski M, Mingeot-Leclercq MP. et al. Surface cholesterol-enriched domains specifically promote invasion of breast cancer cell lines by controlling invadopodia and extracellular matrix degradation. Cell Mol Life Sci. 2022;79:417
182. Shen Z, Zhu D, Liu J, Chen J, Liu Y, Hu C. et al. 27-Hydroxycholesterol induces invasion and migration of breast cancer cells by increasing MMP9 and generating EMT through activation of STAT-3. Environ Toxicol Pharmacol. 2017;51:1-8
183. Torres CG, Ramírez ME, Cruz P, Epuñan MJ, Valladares LE, Sierralta WD. 27-hydroxycholesterol induces the transition of MCF7 cells into a mesenchymal phenotype. Oncol Rep. 2011;26:389-97
184. Jiang S, Wang X, Song D, Liu X, Gu Y, Xu Z. et al. Cholesterol Induces Epithelial-to-Mesenchymal Transition of Prostate Cancer Cells by Suppressing Degradation of EGFR through APMAP. Cancer Res. 2019;79:3063-75
185. Ma J, Huang L, Hu D, Zeng S, Han Y, Shen H. The role of the tumor microbe microenvironment in the tumor immune microenvironment: bystander, activator, or inhibitor? J Exp Clin Cancer Res. 2021;40:327
186. Sipe LM, Chaib M, Pingili AK, Pierre JF, Makowski L. Microbiome, bile acids, and obesity: How microbially modified metabolites shape anti-tumor immunity. Immunol Rev. 2020;295:220-39
187. Jia W, Xie G, Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol. 2018;15:111-28
188. Zeng H, Umar S, Rust B, Lazarova D, Bordonaro M. Secondary Bile Acids and Short Chain Fatty Acids in the Colon: A Focus on Colonic Microbiome, Cell Proliferation, Inflammation, and Cancer. Int J Mol Sci. 2019 20
189. Sánchez B. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis: a role for bifidobacteria and lactobacilli? Nat Rev Gastroenterol Hepatol. 2018;15:205
190. Jia B, Jeon CO. Promotion and induction of liver cancer by gut microbiome-mediated modulation of bile acids. PLoS Pathog. 2019;15:e1007954
191. Yamada S, Takashina Y, Watanabe M, Nagamine R, Saito Y, Kamada N. et al. Bile acid metabolism regulated by the gut microbiota promotes non-alcoholic steatohepatitis-associated hepatocellular carcinoma in mice. Oncotarget. 2018;9:9925-39
192. Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M. et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018 360
193. Monje M, Borniger JC, D'Silva NJ, Deneen B, Dirks PB, Fattahi F. et al. Roadmap for the Emerging Field of Cancer Neuroscience. Cell. 2020;181:219-22
194. Hanoun M, Maryanovich M, Arnal-Estapé A, Frenette PS. Neural regulation of hematopoiesis, inflammation, and cancer. Neuron. 2015;86:360-73
195. Amit M, Na'ara S, Gil Z. Mechanisms of cancer dissemination along nerves. Nat Rev Cancer. 2016;16:399-408
196. Coarfa C, Florentin D, Putluri N, Ding Y, Au J, He D. et al. Influence of the neural microenvironment on prostate cancer. Prostate. 2018;78:128-39
197. Ho WY, Hartmann H, Ling SC. Central nervous system cholesterol metabolism in health and disease. IUBMB Life. 2022;74:826-41
198. Barres BA, Smith SJ. Neurobiology. Cholesterol-making or breaking the synapse. Science. 2001;294:1296-7
199. Dietschy JM, Turley SD. Thematic review series: brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res. 2004;45:1375-97
200. Goritz C, Mauch DH, Pfrieger FW. Multiple mechanisms mediate cholesterol-induced synaptogenesis in a CNS neuron. Mol Cell Neurosci. 2005;29:190-201
201. de Chaves EI, Rusiñol AE, Vance DE, Campenot RB, Vance JE. Role of lipoproteins in the delivery of lipids to axons during axonal regeneration. J Biol Chem. 1997;272:30766-73
202. DeBose-Boyd RA, Ye J. SREBPs in Lipid Metabolism, Insulin Signaling, and Beyond. Trends Biochem Sci. 2018;43:358-68
203. Fantini J, Barrantes FJ. Sphingolipid/cholesterol regulation of neurotransmitter receptor conformation and function. Biochim Biophys Acta. 2009;1788:2345-61
204. Baier CJ, Barrantes FJ. Sphingolipids are necessary for nicotinic acetylcholine receptor export in the early secretory pathway. J Neurochem. 2007;101:1072-84
205. Barrantes FJ. Cholesterol effects on nicotinic acetylcholine receptor: cellular aspects. Subcell Biochem. 2010;51:467-87
206. Fantini J, Barrantes FJ. How cholesterol interacts with membrane proteins: an exploration of cholesterol-binding sites including CRAC, CARC, and tilted domains. Front Physiol. 2013;4:31
207. Jobling P, Pundavela J, Oliveira SM, Roselli S, Walker MM, Hondermarck H. Nerve-Cancer Cell Cross-talk: A Novel Promoter of Tumor Progression. Cancer Res. 2015;75:1777-81
208. Silverman DA, Martinez VK, Dougherty PM, Myers JN, Calin GA, Amit M. Cancer-Associated Neurogenesis and Nerve-Cancer Cross-talk. Cancer Res. 2021;81:1431-40
209. Engel DF, Grzyb AN, de Oliveira J, Pötzsch A, Walker TL, Brocardo PS. et al. Impaired adult hippocampal neurogenesis in a mouse model of familial hypercholesterolemia: A role for the LDL receptor and cholesterol metabolism in adult neural precursor cells. Mol Metab. 2019;30:1-15
210. Theofilopoulos S, Abreu de Oliveira WA, Yang S, Yutuc E, Saeed A, Abdel-Khalik J. et al. 24(S),25-Epoxycholesterol and cholesterol 24S-hydroxylase (CYP46A1) overexpression promote midbrain dopaminergic neurogenesis in vivo. J Biol Chem. 2019;294:4169-76
211. Driver AM, Kratz LE, Kelley RI, Stottmann RW. Altered cholesterol biosynthesis causes precocious neurogenesis in the developing mouse forebrain. Neurobiol Dis. 2016;91:69-82
212. Seo Y, Yang SR, Kim HS, Yu KR, Shin Y, Kang SK. et al. JNK Activation by Up-Regulation of iNOS on Cholesterol Accumulation Limits Neurogenesis and Induces Region-Specific DNA Damage Responses in the Subventricular Zone of NPC Mice. Antioxid Redox Signal. 2012
213. Petro KA, Schengrund CL. Membrane raft disruption promotes axonogenesis in n2a neuroblastoma cells. Neurochem Res. 2009;34:29-37
214. Göbel A, Rauner M, Hofbauer LC, Rachner TD. Cholesterol and beyond - The role of the mevalonate pathway in cancer biology. Biochim Biophys Acta Rev Cancer. 2020;1873:188351
215. Juarez D, Fruman DA. Targeting the Mevalonate Pathway in Cancer. Trends Cancer. 2021;7:525-40
216. Kato S, Smalley S, Sadarangani A, Chen-Lin K, Oliva B, Brañes J. et al. Lipophilic but not hydrophilic statins selectively induce cell death in gynaecological cancers expressing high levels of HMGCoA reductase. J Cell Mol Med. 2010;14:1180-93
217. Menter DG, Ramsauer VP, Harirforoosh S, Chakraborty K, Yang P, Hsi L. et al. Differential effects of pravastatin and simvastatin on the growth of tumor cells from different organ sites. PLoS One. 2011;6:e28813
218. Liu B, Yi Z, Guan X, Zeng YX, Ma F. The relationship between statins and breast cancer prognosis varies by statin type and exposure time: a meta-analysis. Breast Cancer Res Treat. 2017;164:1-11
219. Rosch PJ, McCully K. Statin use and reduced cancer-related mortality. N Engl J Med. 2013;368:576
220. Tan N, Klein EA, Li J, Moussa AS, Jones JS. Statin use and risk of prostate cancer in a population of men who underwent biopsy. J Urol. 2011;186:86-90
221. Kawata S, Yamasaki E, Nagase T, Inui Y, Ito N, Matsuda Y. et al. Effect of pravastatin on survival in patients with advanced hepatocellular carcinoma. A randomized controlled trial. Br J Cancer. 2001;84:886-91
222. Feldt M, Bjarnadottir O, Kimbung S, Jirström K, Bendahl PO, Veerla S. et al. Statin-induced anti-proliferative effects via cyclin D1 and p27 in a window-of-opportunity breast cancer trial. J Transl Med. 2015;13:133
223. Longo J, Hamilton RJ, Masoomian M, Khurram N, Branchard E, Mullen PJ. et al. A pilot window-of-opportunity study of preoperative fluvastatin in localized prostate cancer. Prostate Cancer Prostatic Dis. 2020;23:630-7
224. Garwood ER, Kumar AS, Baehner FL, Moore DH, Au A, Hylton N. et al. Fluvastatin reduces proliferation and increases apoptosis in women with high grade breast cancer. Breast Cancer Res Treat. 2010;119:137-44
225. Ni W, Mo H, Liu Y, Xu Y, Qin C, Zhou Y. et al. Targeting cholesterol biosynthesis promotes anti-tumor immunity by inhibiting long noncoding RNA SNHG29-mediated YAP activation. Mol Ther. 2021;29:2995-3010
226. Göbel A, Breining D, Rauner M, Hofbauer LC, Rachner TD. Induction of 3-hydroxy-3-methylglutaryl-CoA reductase mediates statin resistance in breast cancer cells. Cell Death Dis. 2019;10:91
227. Mullen PJ, Yu R, Longo J, Archer MC, Penn LZ. The interplay between cell signalling and the mevalonate pathway in cancer. Nat Rev Cancer. 2016;16:718-31
228. van Leeuwen JE, Ba-Alawi W, Branchard E, Cruickshank J, Schormann W, Longo J. et al. Computational pharmacogenomic screen identifies drugs that potentiate the anti-breast cancer activity of statins. Nat Commun. 2022;13:6323
229. Longo J, Mullen PJ, Yu R, van Leeuwen JE, Masoomian M, Woon DTS. et al. An actionable sterol-regulated feedback loop modulates statin sensitivity in prostate cancer. Mol Metab. 2019;25:119-30
230. Pandyra A, Mullen PJ, Kalkat M, Yu R, Pong JT, Li Z. et al. Immediate utility of two approved agents to target both the metabolic mevalonate pathway and its restorative feedback loop. Cancer Res. 2014;74:4772-82
231. Pandyra AA, Mullen PJ, Goard CA, Ericson E, Sharma P, Kalkat M. et al. Genome-wide RNAi analysis reveals that simultaneous inhibition of specific mevalonate pathway genes potentiates tumor cell death. Oncotarget. 2015;6:26909-21
232. Longo J, Pandyra AA, Stachura P, Minden MD, Schimmer AD, Penn LZ. Cyclic AMP-hydrolyzing phosphodiesterase inhibitors potentiate statin-induced cancer cell death. Mol Oncol. 2020;14:2533-45
233. Li X, Chen YT, Hu P, Huang WC. Fatostatin displays high antitumor activity in prostate cancer by blocking SREBP-regulated metabolic pathways and androgen receptor signaling. Mol Cancer Ther. 2014;13:855-66
234. Yao L, Chen S, Li W. Fatostatin inhibits the development of endometrial carcinoma in endometrial carcinoma cells and a xenograft model by targeting lipid metabolism. Arch Biochem Biophys. 2020;684:108327
235. Siqingaowa Sekar S, Gopalakrishnan V Taghibiglou C. Sterol regulatory element-binding protein 1 inhibitors decrease pancreatic cancer cell viability and proliferation. Biochem Biophys Res Commun. 2017;488:136-40
236. Li X, Wu JB, Chung LW, Huang WC. Anti-cancer efficacy of SREBP inhibitor, alone or in combination with docetaxel, in prostate cancer harboring p53 mutations. Oncotarget. 2015;6:41018-32
237. Król SK, Kiełbus M, Rivero-Müller A, Stepulak A. Comprehensive review on betulin as a potent anticancer agent. Biomed Res Int. 2015;2015:584189
238. Williams KJ, Argus JP, Zhu Y, Wilks MQ, Marbois BN, York AG. et al. An essential requirement for the SCAP/SREBP signaling axis to protect cancer cells from lipotoxicity. Cancer Res. 2013;73:2850-62
239. van Beek E, Pieterman E, Cohen L, Löwik C, Papapoulos S. Farnesyl pyrophosphate synthase is the molecular target of nitrogen-containing bisphosphonates. Biochem Biophys Res Commun. 1999;264:108-11
240. Göbel A, Riffel RM, Hofbauer LC, Rachner TD. The mevalonate pathway in breast cancer biology. Cancer Lett. 2022;542:215761
241. Pavlakis N, Stockler M. Bisphosphonates for breast cancer. Cochrane Database Syst Rev. 2002: Cd003474.
242. Van Acker HH, Anguille S, Willemen Y, Smits EL, Van Tendeloo VF. Bisphosphonates for cancer treatment: Mechanisms of action and lessons from clinical trials. Pharmacol Ther. 2016;158:24-40
243. Gralow JR, Barlow WE, Paterson AHG, M'Iao J L, Lew DL, Stopeck AT. et al. Phase III Randomized Trial of Bisphosphonates as Adjuvant Therapy in Breast Cancer: S0307. J Natl Cancer Inst. 2020;112:698-707
244. Kim HY, Kim DK, Bae SH, Gwak H, Jeon JH, Kim JK. et al. Farnesyl diphosphate synthase is important for the maintenance of glioblastoma stemness. Exp Mol Med. 2018;50:1-12
245. Cirmena G, Franceschelli P, Isnaldi E, Ferrando L, De Mariano M, Ballestrero A. et al. Squalene epoxidase as a promising metabolic target in cancer treatment. Cancer Lett. 2018;425:13-20
246. Brown DN, Caffa I, Cirmena G, Piras D, Garuti A, Gallo M. et al. Squalene epoxidase is a bona fide oncogene by amplification with clinical relevance in breast cancer. Sci Rep. 2016;6:19435
247. Kalogirou C, Linxweiler J, Schmucker P, Snaebjornsson MT, Schmitz W, Wach S. et al. MiR-205-driven downregulation of cholesterol biosynthesis through SQLE-inhibition identifies therapeutic vulnerability in aggressive prostate cancer. Nat Commun. 2021;12:5066
248. Chien MH, Lee TS, Kao C, Yang SF, Lee WS. Terbinafine inhibits oral squamous cell carcinoma growth through anti-cancer cell proliferation and anti-angiogenesis. Mol Carcinog. 2012;51:389-99
249. Maione F, Oliaro-Bosso S, Meda C, Di Nicolantonio F, Bussolino F, Balliano G. et al. The cholesterol biosynthesis enzyme oxidosqualene cyclase is a new target to impair tumour angiogenesis and metastasis dissemination. Sci Rep. 2015;5:9054
250. Lin CY, Gustafsson J. Targeting liver X receptors in cancer therapeutics. Nat Rev Cancer. 2015;15:216-24
251. Traughber CA, Opoku E, Brubaker G, Major J, Lu H, Lorkowski SW. et al. Uptake of high-density lipoprotein by scavenger receptor class B type 1 is associated with prostate cancer proliferation and tumor progression in mice. J Biol Chem. 2020;295:8252-61
252. Villa GR, Hulce JJ, Zanca C, Bi J, Ikegami S, Cahill GL. et al. An LXR-Cholesterol Axis Creates a Metabolic Co-Dependency for Brain Cancers. Cancer Cell. 2016;30:683-93
253. Guillaumond F, Bidaut G, Ouaissi M, Servais S, Gouirand V, Olivares O. et al. Cholesterol uptake disruption, in association with chemotherapy, is a promising combined metabolic therapy for pancreatic adenocarcinoma. Proc Natl Acad Sci U S A. 2015;112:2473-8
254. Pattanayak SP, Bose P, Sunita P, Siddique MUM, Lapenna A. Bergapten inhibits liver carcinogenesis by modulating LXR/PI3K/Akt and IDOL/LDLR pathways. Biomed Pharmacother. 2018;108:297-308
255. Guo D, Reinitz F, Youssef M, Hong C, Nathanson D, Akhavan D. et al. An LXR agonist promotes glioblastoma cell death through inhibition of an EGFR/AKT/SREBP-1/LDLR-dependent pathway. Cancer Discov. 2011;1:442-56
256. Pencheva N, Buss CG, Posada J, Merghoub T, Tavazoie SF. Broad-spectrum therapeutic suppression of metastatic melanoma through nuclear hormone receptor activation. Cell. 2014;156:986-1001
257. Pommier AJ, Alves G, Viennois E, Bernard S, Communal Y, Sion B. et al. Liver X Receptor activation downregulates AKT survival signaling in lipid rafts and induces apoptosis of prostate cancer cells. Oncogene. 2010;29:2712-23
258. Flaveny CA, Griffett K, El-Gendy Bel D, Kazantzis M, Sengupta M, Amelio AL. et al. Broad Anti-tumor Activity of a Small Molecule that Selectively Targets the Warburg Effect and Lipogenesis. Cancer Cell. 2015;28:42-56
259. Wu G, Wang Q, Xu Y, Li J, Zhang H, Qi G. et al. Targeting the transcription factor receptor LXR to treat clear cell renal cell carcinoma: agonist or inverse agonist? Cell Death Dis. 2019;10:416
260. Marimuthu A, Subbannayya Y, Sahasrabuddhe NA, Balakrishnan L, Syed N, Sekhar NR. et al. SILAC-based quantitative proteomic analysis of gastric cancer secretome. Proteomics Clin Appl. 2013;7:355-66
261. Zhang SZ, Zhu XD, Feng LH, Li XL, Liu XF, Sun HC. et al. PCSK9 promotes tumor growth by inhibiting tumor cell apoptosis in hepatocellular carcinoma. Exp Hematol Oncol. 2021;10:25
262. Wu Y, Liu Y, He A, Guan B, He S, Zhang C. et al. Identification of the Six-RNA-Binding Protein Signature for Prognosis Prediction in Bladder Cancer. Front Genet. 2020;11:992
263. Bhat M, Skill N, Marcus V, Deschenes M, Tan X, Bouteaud J. et al. Decreased PCSK9 expression in human hepatocellular carcinoma. BMC Gastroenterol. 2015;15:176
264. Bhattacharya A, Chowdhury A, Chaudhury K, Shukla PC. Proprotein convertase subtilisin/kexin type 9 (PCSK9): A potential multifaceted player in cancer. Biochim Biophys Acta Rev Cancer. 2021;1876:188581
265. Sun H, Meng W, Zhu J, Wang L. Antitumor activity and molecular mechanism of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibition. Naunyn Schmiedebergs Arch Pharmacol. 2022;395:643-58
266. He J, Shin H, Wei X, Kadegowda AK, Chen R, Xie SK. NPC1L1 knockout protects against colitis-associated tumorigenesis in mice. BMC Cancer. 2015;15:189
267. Nicolle R, Blum Y, Marisa L, Loncle C, Gayet O, Moutardier V. et al. Pancreatic Adenocarcinoma Therapeutic Targets Revealed by Tumor-Stroma Cross-Talk Analyses in Patient-Derived Xenografts. Cell Rep. 2017;21:2458-70
268. Solomon KR, Pelton K, Boucher K, Joo J, Tully C, Zurakowski D. et al. Ezetimibe is an inhibitor of tumor angiogenesis. Am J Pathol. 2009;174:1017-26
269. Miura K, Ohnishi H, Morimoto N, Minami S, Ishioka M, Watanabe S. et al. Ezetimibe suppresses development of liver tumors by inhibiting angiogenesis in mice fed a high-fat diet. Cancer Sci. 2019;110:771-83
270. Ribas V, de la Rosa LC, Robles D, Núñez S, Segalés P, Insausti-Urkia N. et al. Dietary and Genetic Cholesterol Loading Rather Than Steatosis Promotes Liver Tumorigenesis and NASH-Driven HCC. Cancers (Basel). 2021 13
271. Kim DJ, Kim J, Spaunhurst K, Montoya J, Khodosh R, Chandra K. et al. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32:745-51
272. Antonarakis ES, Heath EI, Smith DC, Rathkopf D, Blackford AL, Danila DC. et al. Repurposing itraconazole as a treatment for advanced prostate cancer: a noncomparative randomized phase II trial in men with metastatic castration-resistant prostate cancer. Oncologist. 2013;18:163-73
273. Head SA, Shi WQ, Yang EJ, Nacev BA, Hong SY, Pasunooti KK. et al. Simultaneous Targeting of NPC1 and VDAC1 by Itraconazole Leads to Synergistic Inhibition of mTOR Signaling and Angiogenesis. ACS Chem Biol. 2017;12:174-82
274. Xu J, Dang Y, Ren YR, Liu JO. Cholesterol trafficking is required for mTOR activation in endothelial cells. Proc Natl Acad Sci U S A. 2010;107:4764-9
275. Aftab BT, Dobromilskaya I, Liu JO, Rudin CM. Itraconazole inhibits angiogenesis and tumor growth in non-small cell lung cancer. Cancer Res. 2011;71:6764-72
276. Liu R, Li J, Zhang T, Zou L, Chen Y, Wang K. et al. Itraconazole suppresses the growth of glioblastoma through induction of autophagy: involvement of abnormal cholesterol trafficking. Autophagy. 2014;10:1241-55
277. Tsubamoto H, Sonoda T, Ikuta S, Tani S, Inoue K, Yamanaka N. Combination Chemotherapy with Itraconazole for Treating Metastatic Pancreatic Cancer in the Second-line or Additional Setting. Anticancer Res. 2015;35:4191-6
278. Lyu J, Yang EJ, Head SA, Ai N, Zhang B, Wu C. et al. Astemizole Inhibits mTOR Signaling and Angiogenesis by Blocking Cholesterol Trafficking. Int J Biol Sci. 2018;14:1175-85
279. Shim JS, Li RJ, Lv J, Head SA, Yang EJ, Liu JO. Inhibition of angiogenesis by selective estrogen receptor modulators through blockade of cholesterol trafficking rather than estrogen receptor antagonism. Cancer Lett. 2015;362:106-15
280. Lyu J, Yang EJ, Head SA, Ai N, Zhang B, Wu C. et al. Pharmacological blockade of cholesterol trafficking by cepharanthine in endothelial cells suppresses angiogenesis and tumor growth. Cancer Lett. 2017;409:91-103
281. Kuzu OF, Gowda R, Sharma A, Robertson GP. Leelamine mediates cancer cell death through inhibition of intracellular cholesterol transport. Mol Cancer Ther. 2014;13:1690-703
282. Goudarzi A. The recent insights into the function of ACAT1: A possible anti-cancer therapeutic target. Life Sci. 2019;232:116592
283. Bemlih S, Poirier MD, El Andaloussi A. Acyl-coenzyme A: cholesterol acyltransferase inhibitor Avasimibe affect survival and proliferation of glioma tumor cell lines. Cancer Biol Ther. 2010;9:1025-32
284. Lee HJ, Li J, Vickman RE, Li J, Liu R, Durkes AC. et al. Cholesterol Esterification Inhibition Suppresses Prostate Cancer Metastasis by Impairing the Wnt/β-catenin Pathway. Mol Cancer Res. 2018;16:974-85
285. Shim SH, Sur S, Steele R, Albert CJ, Huang C, Ford DA. et al. Disrupting cholesterol esterification by bitter melon suppresses triple-negative breast cancer cell growth. Mol Carcinog. 2018;57:1599-607
286. Pan J, Zhang Q, Palen K, Wang L, Qiao L, Johnson B. et al. Potentiation of Kras peptide cancer vaccine by avasimibe, a cholesterol modulator. EBioMedicine. 2019;49:72-81
287. Zhao L, Li J, Liu Y, Kang L, Chen H, Jin Y. et al. Cholesterol Esterification Enzyme Inhibition Enhances Antitumor Effects of Human Chimeric Antigen Receptors Modified T Cells. J Immunother. 2018;41:45-52
288. Buranrat B, Suwannaloet W, Naowaboot J. Simvastatin potentiates doxorubicin activity against MCF-7 breast cancer cells. Oncol Lett. 2017;14:6243-50
289. Alqudah MAY, Mansour HT, Mhaidat N. Simvastatin enhances irinotecan-induced apoptosis in prostate cancer via inhibition of MCL-1. Saudi Pharm J. 2018;26:191-7
290. Chen B, Zhang M, Xing D, Feng Y. Atorvastatin enhances radiosensitivity in hypoxia-induced prostate cancer cells related with HIF-1α inhibition. Biosci Rep. 2017 37
291. Chen YH, Chen YC, Lin CC, Hsieh YP, Hsu CS, Hsieh MC. Synergistic Anticancer Effects of Gemcitabine with Pitavastatin on Pancreatic Cancer Cell Line MIA PaCa-2 in vitro and in vivo. Cancer Manag Res. 2020;12:4645-65
292. Otahal A, Aydemir D, Tomasich E, Minichsdorfer C. Delineation of cell death mechanisms induced by synergistic effects of statins and erlotinib in non-small cell lung cancer cell (NSCLC) lines. Sci Rep. 2020;10:959
293. Al-Qatati A, Aliwaini S. Combined pitavastatin and dacarbazine treatment activates apoptosis and autophagy resulting in synergistic cytotoxicity in melanoma cells. Oncol Lett. 2017;14:7993-9
294. Zhang Y, Liu Y, Duan J, Wang H, Zhang Y, Qiao K. et al. Cholesterol depletion sensitizes gallbladder cancer to cisplatin by impairing DNA damage response. Cell Cycle. 2019;18:3337-50
295. Göbel A, Thiele S, Browne AJ, Rauner M, Zinna VM, Hofbauer LC. et al. Combined inhibition of the mevalonate pathway with statins and zoledronic acid potentiates their anti-tumor effects in human breast cancer cells. Cancer Lett. 2016;375:162-71
296. Jagdev SP, Coleman RE, Shipman CM, Rostami HA, Croucher PI. The bisphosphonate, zoledronic acid, induces apoptosis of breast cancer cells: evidence for synergy with paclitaxel. Br J Cancer. 2001;84:1126-34
297. Chen T, Xu J, Fu W. EGFR/FOXO3A/LXR-α Axis Promotes Prostate Cancer Proliferation and Metastasis and Dual-Targeting LXR-α/EGFR Shows Synthetic Lethality. Front Oncol. 2020;10:1688
298. Liu S, Cao H, Chen D, Yu S, Sha H, Wu J. et al. LXR ligands induce apoptosis of EGFR-TKI-resistant human lung cancer cells in vitro by inhibiting Akt-NF-κB activation. Oncol Lett. 2018;15:7168-74
299. Hu Y, Zang J, Qin X, Yan D, Cao H, Zhou L. et al. Epithelial-to-mesenchymal transition correlates with gefitinib resistance in NSCLC cells and the liver X receptor ligand GW3965 reverses gefitinib resistance through inhibition of vimentin. Onco Targets Ther. 2017;10:2341-8
300. Lou R, Cao H, Dong S, Shi C, Xu X, Ma R. et al. Liver X receptor agonist T0901317 inhibits the migration and invasion of non-small-cell lung cancer cells in vivo and in vitro. Anticancer Drugs. 2019;30:495-500
301. Besse L, Besse A, Stolze SC, Sobh A, Zaal EA, van der Ham AJ. et al. Treatment with HIV-Protease Inhibitor Nelfinavir Identifies Membrane Lipid Composition and Fluidity as a Therapeutic Target in Advanced Multiple Myeloma. Cancer Res. 2021;81:4581-93
302. Lan K, Yan R, Zhu K, Li W, Xu Z, Dang C. et al. Itraconazole inhibits the proliferation of gastric cancer cells in vitro and improves patient survival. Oncol Lett. 2018;16:3651-7
303. Unson S, Kongsaden C, Wonganan P. Cepharanthine combined with 5-fluorouracil inhibits the growth of p53-mutant human colorectal cancer cells. J Asian Nat Prod Res. 2020;22:370-85
304. Li J, Qu X, Tian J, Zhang JT, Cheng JX. Cholesterol esterification inhibition and gemcitabine synergistically suppress pancreatic ductal adenocarcinoma proliferation. PLoS One. 2018;13:e0193318
305. Li M, Yang Y, Wei J, Cun X, Lu Z, Qiu Y. et al. Enhanced chemo-immunotherapy against melanoma by inhibition of cholesterol esterification in CD8(+) T cells. Nanomedicine. 2018;14:2541-50
306. Chen X, Song Q, Xia L, Xu X. Synergy of Dendritic Cell Vaccines and Avasimibe in Treatment of Head and Neck Cancer in Mice. Med Sci Monit. 2017;23:4471-6
307. Han JY, Lee SH, Yoo NJ, Hyung LS, Moon YJ, Yun T. et al. A randomized phase II study of gefitinib plus simvastatin versus gefitinib alone in previously treated patients with advanced non-small cell lung cancer. Clin Cancer Res. 2011;17:1553-60
308. Cantini L, Pecci F, Hurkmans DP, Belderbos RA, Lanese A, Copparoni C. et al. High-intensity statins are associated with improved clinical activity of PD-1 inhibitors in malignant pleural mesothelioma and advanced non-small cell lung cancer patients. Eur J Cancer. 2021;144:41-8
309. Raittinen PVH, Syvälä H, Tammela TLJ, Häkkinen MR, Ilmonen P, Auriola S. et al. Atorvastatin induces adrenal androgen downshift in men with prostate cancer: A post Hoc analysis of a pilot adaptive Randomised clinical trial. EBioMedicine. 2021;68:103432
310. Yulian ED, Siregar NC, Bajuadji. Combination of Simvastatin and FAC Improves Response to Neoadjuvant Chemotherapy in Locally Advanced Breast Cancer. Cancer Res Treat. 2021;53:1072-83
311. Nguyen PA, Chang CC, Galvin CJ, Wang YC, An SY, Huang CW. et al. Statins use and its impact in EGFR-TKIs resistance to prolong the survival of lung cancer patients: A Cancer registry cohort study in Taiwan. Cancer Sci. 2020;111:2965-73
312. El-Refai SM, Brown JD, Arnold SM, Black EP, Leggas M, Talbert JC. Epidemiologic Analysis Along the Mevalonate Pathway Reveals Improved Cancer Survival in Patients Who Receive Statins Alone and in Combination With Bisphosphonates. JCO Clin Cancer Inform. 2017;1:1-12
313. Aft R, Naughton M, Trinkaus K, Watson M, Ylagan L, Chavez-MacGregor M. et al. Effect of zoledronic acid on disseminated tumour cells in women with locally advanced breast cancer: an open label, randomised, phase 2 trial. Lancet Oncol. 2010;11:421-8
314. Coleman RE, Winter MC, Cameron D, Bell R, Dodwell D, Keane MM. et al. The effects of adding zoledronic acid to neoadjuvant chemotherapy on tumour response: exploratory evidence for direct anti-tumour activity in breast cancer. Br J Cancer. 2010;102:1099-105
315. Coleman RE, Collinson M, Gregory W, Marshall H, Bell R, Dodwell D. et al. Benefits and risks of adjuvant treatment with zoledronic acid in stage II/III breast cancer. 10 years follow-up of the AZURE randomized clinical trial (BIG 01/04). J Bone Oncol. 2018;13:123-35
316. Li CL, Fang ZX, Wu Z, Hou YY, Wu HT, Liu J. Repurposed itraconazole for use in the treatment of malignancies as a promising therapeutic strategy. Biomed Pharmacother. 2022;154:113616
317. Tsubamoto H, Ueda T, Inoue K, Sakata K, Shibahara H, Sonoda T. Repurposing itraconazole as an anticancer agent. Oncol Lett. 2017;14:1240-6
318. Tsubamoto H, Sonoda T, Yamasaki M, Inoue K. Impact of combination chemotherapy with itraconazole on survival of patients with refractory ovarian cancer. Anticancer Res. 2014;34:2481-7
319. Rudin CM, Brahmer JR, Juergens RA, Hann CL, Ettinger DS, Sebree R. et al. Phase 2 study of pemetrexed and itraconazole as second-line therapy for metastatic nonsquamous non-small-cell lung cancer. J Thorac Oncol. 2013;8:619-23
320. Campos MI, Vieira WD, Campos CN, Aarestrup FM, Aarestrup BJ. Atorvastatin and trans-caryophyllene for the prevention of leukopenia in an experimental chemotherapy model in Wistar rats. Mol Clin Oncol. 2015;3:825-8
321. Bertolini P, Lassalle M, Mercier G, Raquin MA, Izzi G, Corradini N. et al. Platinum compound-related ototoxicity in children: long-term follow-up reveals continuous worsening of hearing loss. J Pediatr Hematol Oncol. 2004;26:649-55
322. Coradini PP, Cigana L, Selistre SG, Rosito LS, Brunetto AL. Ototoxicity from cisplatin therapy in childhood cancer. J Pediatr Hematol Oncol. 2007;29:355-60
323. Frisina RD, Wheeler HE, Fossa SD, Kerns SL, Fung C, Sesso HD. et al. Comprehensive Audiometric Analysis of Hearing Impairment and Tinnitus After Cisplatin-Based Chemotherapy in Survivors of Adult-Onset Cancer. J Clin Oncol. 2016;34:2712-20
324. Knight KR, Chen L, Freyer D, Aplenc R, Bancroft M, Bliss B. et al. Group-Wide, Prospective Study of Ototoxicity Assessment in Children Receiving Cisplatin Chemotherapy (ACCL05C1): A Report From the Children's Oncology Group. J Clin Oncol. 2017;35:440-5
325. Marnitz S, Schermeyer L, Dommerich S, Köhler C, Olze H, Budach V. et al. Age-corrected hearing loss after chemoradiation in cervical cancer patients. Strahlenther Onkol. 2018;194:1039-48
326. Fernandez KA, Allen P, Campbell M, Page B, Townes T, Li CM. et al. Atorvastatin is associated with reduced cisplatin-induced hearing loss. J Clin Invest. 2021 131
327. Abdel-Qadir H, Thavendiranathan P, Austin PC, Lee DS, Amir E, Tu JV. et al. The Risk of Heart Failure and Other Cardiovascular Hospitalizations After Early Stage Breast Cancer: A Matched Cohort Study. J Natl Cancer Inst. 2019;111:854-62
328. Thavendiranathan P, Abdel-Qadir H, Fischer HD, Camacho X, Amir E, Austin PC. et al. Breast Cancer Therapy-Related Cardiac Dysfunction in Adult Women Treated in Routine Clinical Practice: A Population-Based Cohort Study. J Clin Oncol. 2016;34:2239-46
329. Chotenimitkhun R, D'Agostino R Jr, Lawrence JA, Hamilton CA, Jordan JH, Vasu S. et al. Chronic statin administration may attenuate early anthracycline-associated declines in left ventricular ejection function. Can J Cardiol. 2015;31:302-7
330. Acar Z, Kale A, Turgut M, Demircan S, Durna K, Demir S. et al. Efficiency of atorvastatin in the protection of anthracycline-induced cardiomyopathy. J Am Coll Cardiol. 2011;58:988-9
331. Calvillo-Argüelles O, Abdel-Qadir H, Michalowska M, Billia F, Suntheralingam S, Amir E. et al. Cardioprotective Effect of Statins in Patients With HER2-Positive Breast Cancer Receiving Trastuzumab Therapy. Can J Cardiol. 2019;35:153-9
332. Seicean S, Seicean A, Plana JC, Budd GT, Marwick TH. Effect of statin therapy on the risk for incident heart failure in patients with breast cancer receiving anthracycline chemotherapy: an observational clinical cohort study. J Am Coll Cardiol. 2012;60:2384-90
333. Abdel-Qadir H, Bobrowski D, Zhou L, Austin PC, Calvillo-Argüelles O, Amir E. et al. Statin Exposure and Risk of Heart Failure After Anthracycline- or Trastuzumab-Based Chemotherapy for Early Breast Cancer: A Propensity Score-Matched Cohort Study. J Am Heart Assoc. 2021;10:e018393
334. Lee B, Jung EA, Yoo JJ, Kim SG, Lee CB, Kim YS. et al. Prevalence, incidence and risk factors of tamoxifen-related non-alcoholic fatty liver disease: A systematic review and meta-analysis. Liver Int. 2020;40:1344-55
335. Li M, Lu Q, Zhu Y, Fan X, Zhao W, Zhang L. et al. Fatostatin inhibits SREBP2-mediated cholesterol uptake via LDLR against selective estrogen receptor α modulator-induced hepatic lipid accumulation. Chem Biol Interact. 2022;365:110091
336. Korani S, Korani M, Bahrami S, Johnston TP, Butler AE, Banach M. et al. Application of nanotechnology to improve the therapeutic benefits of statins. Drug Discov Today. 2019;24:567-74
337. Geybels MS, Wright JL, Holt SK, Kolb S, Feng Z, Stanford JL. Statin use in relation to prostate cancer outcomes in a population-based patient cohort study. Prostate. 2013;73:1214-22
338. Thompson PD, Panza G, Zaleski A, Taylor B. Statin-Associated Side Effects. J Am Coll Cardiol. 2016;67:2395-410
339. Stroes ES, Thompson PD, Corsini A, Vladutiu GD, Raal FJ, Ray KK. et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36:1012-22
340. Armitage J. The safety of statins in clinical practice. Lancet. 2007;370:1781-90
341. Jouve JL, Lecomte T, Bouché O, Barbier E, Khemissa Akouz F, Riachi G. et al. Pravastatin combination with sorafenib does not improve survival in advanced hepatocellular carcinoma. J Hepatol. 2019;71:516-22
342. Rzeski W, Stepulak A, Szymański M, Juszczak M, Grabarska A, Sifringer M. et al. Betulin elicits anti-cancer effects in tumour primary cultures and cell lines in vitro. Basic Clin Pharmacol Toxicol. 2009;105:425-32
343. Tang X, Zhang Q, Shi S, Yen Y, Li X, Zhang Y. et al. Bisphosphonates suppress insulin-like growth factor 1-induced angiogenesis via the HIF-1alpha/VEGF signaling pathways in human breast cancer cells. Int J Cancer. 2010;126:90-103
344. de Guadalupe Chávez-López M, Hernández-Gallegos E, Vázquez-Sánchez AY, Gariglio P, Camacho J. Antiproliferative and proapoptotic effects of astemizole on cervical cancer cells. Int J Gynecol Cancer. 2014;24:824-8
Author contact
![]() Corresponding authors: xuamanedu.cn (A-Man Xu); ldyy_jinwledu.cn (Wei-Lin Jin).
Corresponding authors: xuamanedu.cn (A-Man Xu); ldyy_jinwledu.cn (Wei-Lin Jin).

 Global reach, higher impact
Global reach, higher impact