10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2024; 20(8):3236-3256. doi:10.7150/ijbs.93875 This issue Cite
Review
Polyphenols as Therapeutics in Respiratory Diseases: Moving from Preclinical Evidence to Potential Clinical Applications
1. Department of Pathology and Laboratory Medicine, Warren Alpert Medical School, Brown University, Providence, RI 02912, USA.
2. Legorreta Cancer Center, Brown University, Providence, RI 02912, USA.
3. Department of Pharmacy, Faculty of Allied Health Sciences, Daffodil International University, Dhaka 1207, Bangladesh.
4. Department of Pharmacy, Faculty of Biological Sciences, University of Chittagong, Chittagong 4331, Bangladesh.
5. Department of Clinical Pharmacy, College of Dentistry & Pharmacy, Buraydah Private Colleges, Buraydah 51418, Saudi Arabia.
6. Department of Pharmacy, International Islamic University Chittagong, Chittagong 4318, Bangladesh.
7. Department of Pharmacy Practice, M. M. College of Pharmacy (Maharishi Markandeshwar Deemed University), Mullana-Ambala, Haryana 133207, India.
8. Department of Pharmaceutics, School of Pharmaceutical Sciences, Vels Institute of Science, Technology, and Advanced Studies (VISTAS), Tamil Nadu, India.
9. Department of Pharmaceutics, GRT Institute of Pharmaceutical Education and Research, Tiruttani, India.
10. Department of Pharmacognosy, School of Pharmacy, Vishwakarma University, Kondhwa, Pune, India.
11. Department of Pharmacy, Faculty of Pharmacy, Hasanuddin University, Makassar 90245, Indonesia.
12. Department of Pharmacology and Clinical/Community Pharmacy, Faculty of Pharmacy, Universitas Sumatera Utara, Medan, 20155, Indonesia.
13. Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, King Khalid University, Abha, Saudi Arabia.
14. Faculty of Applied Health Science Technology, Misr University for Science and Technology, Giza 12568, Egypt.
15. Department of Clinical Tropical Medicine, Faculty of Tropical Medicine, Mahidol University, Bangkok 10400, Thailand.
16. Department of Pathology, College of Korean Medicine, Kyung Hee University, 26 Kyungheedae-ro, Dongdaemun-gu, Seoul, 02453, Republic of Korea.
* These authors contributed equally.
Received 2024-1-4; Accepted 2024-4-4; Published 2024-6-3
Abstract
Respiratory diseases are the most common and severe health complication and a leading cause of death worldwide. Despite breakthroughs in diagnosis and treatment, few safe and effective therapeutics have been reported. Phytochemicals are gaining popularity due to their beneficial effects and low toxicity. Polyphenols are secondary metabolites with high molecular weights found at high levels in natural food sources such as fruits, vegetables, grains, and citrus seeds. Over recent decades, polyphenols and their beneficial effects on human health have been the subject of intense research, with notable successes in preventing major chronic non-communicable diseases. Many respiratory syndromes can be treated effectively with polyphenolic supplements, including acute lung damage, pulmonary fibrosis, asthma, pulmonary hypertension, and lung cancer. This review summarizes the role of polyphenols in respiratory conditions with sufficient experimental data, highlights polyphenols with beneficial effects for each, and identifies those with therapeutic potential and their underlying mechanisms. Moreover, clinical studies and future research opportunities in this area are discussed.
Keywords: Polyphenols, Respiratory diseases, Lung cancer, Asthma, ARDS, Tuberculosis
Introduction
Respiratory or lung diseases are medical conditions that impede the gas exchange process in air-breathing animals by affecting the lungs and other respiratory tissues. In addition to the lungs, these conditions also affect other respiratory organs, including the trachea, bronchi, bronchioles, alveoli, pleurae, pleural cavity, and respiratory nerves and muscles [1]. Asthma, characterized by recurrent episodes of wheezing, coughing, and breathlessness, arises from chronic airway inflammation and hyperresponsiveness. Triggered by allergens, irritants, or even emotional stress, inflammatory cells and mediators including histamine infiltrate the airways, causing their narrowing and mucus overproduction. This “bronchoconstriction” hinders airflow, leading to the characteristic symptoms [2]. COPD, encompassing emphysema and chronic bronchitis, is a progressive, often irreversible disease. While cigarette smoking is the leading culprit, occupational exposure and genetics also play a role [3]. Emphysema involves the destruction of the air sacs (alveoli), diminishing the surface area for gas exchange. Chronic bronchitis, on the other hand, features chronic inflammation and mucus hypersecretion, obstructing the airways. Both mechanisms culminate in breathlessness, reduced exercise tolerance, and impaired quality of life [4]. According to GOLD report 2023, the global prevalence of COPD oscillates around 12% of the general population. The incidence of COPD is expected to rise over the next 40 years. By 2060, it is estimated that there may be over 5.4 million deaths annually from COPD [5].
Polyphenols are phytochemical compounds present in fruits, vegetables, and grains. These chemicals provide several health advantages, including immune-modulating, vasodilating, and antioxidant properties [6]. Natural food sources contain the most common bioactive molecules, particularly polyphenols that comprise two or more phenolic rings with attached hydroxyl groups in their structures. Polyphenolic compounds include flavonoids, flavanones, and anthocyanins, which are present at high concentrations in various fruits and vegetables. Their pharmacological and therapeutic profiles have been studied extensively, particularly in the context of respiratory diseases [7]. For example, the study of 582 Hawaiian lung cancer patients found a significant inverse relationship between lung cancer and the polyphenols quercetin and naringin, with a 40-50% lower risk of lung cancer in patients who consumed the most polyphenols compared to those who consumed the least [8]. In addition, other studies have reported favorable findings with polyphenols in lung cancer [9]. Resveratrol exerts its potential health benefits through various pathways and cellular components. It acts primarily as an antioxidant, scavenging harmful free radicals and mitigating oxidative stress, which is linked to multiple diseases.
Additionally, it influences several cellular signaling pathways, including those involving SIRT1, AMPK, and Nrf2. These pathways are involved in regulating cellular metabolism, inflammation, and stress responses [10]. Polyphenols exhibit several health advantages, including hypoglycemic, anti-inflammatory, and cancer-preventive properties. Additionally, they play a significant role in improving the flavor of food [11]. The exploration of non-toxic and cost-effective polyphenols, such as epigallocatechin 3-gallate and myricetin, for health improvement and disease treatment has recently attracted substantial research attention. The recent COVID-19 pandemic has provided a unique opportunity for the investigation and identification of polyphenols capable of treating viral infections, as well as gathering the evidence needed to address the significant challenges presented by public health emergencies. Polyphenols hold great potential as a starting point for further drug development for the treatment and prevention of SARS-CoV-2 infection and diseases associated with the respiratory system owing to their excellent safety, broad-spectrum antiviral activities, and multi-organ protective capacity [12,13].
This review provides a comprehensive summary of the pharmacotherapeutic properties of dietary polyphenols in respiratory diseases and identifies promising candidates for novel drug discovery.
Polyphenols: An overview
Polyphenols are a category of bioactive chemicals found in plant-based diets [14,15]. Polyphenols are small organic compounds that have an aromatic ring, such as benzene or phenol, with one or more hydroxyl groups in their structure [16,17]. Polyphenols generated from foods are secondary compounds often present in fruits and vegetables and provide many health advantages to humans [18,19]. Polyphenols are a diverse set of molecules that consist of phenolic acids, flavonoids (such as flavonols, flavanones, flavan-3-ols, flavones, anthocyanins, and isoflavones), lignans, stilbenes and, in some classifications, tannins and coumarins [20-22]. Flavonoids are a primary category of dietary polyphenols known for their potent antioxidant and anti-carcinogenic effects [23]. Flavonoids are present in fruits, vegetables, nuts, seeds, coffee, wine, and tea, and they have notable antioxidant properties linked to conditions including cancer, atherosclerosis, and Alzheimer's disease [24-26]. They are categorized based on their chemical composition, level of unsaturation, and carbon ring oxidation. Flavonoids are divided into many subgroups, including anthoxanthins (flavanone and flavanol), flavanones, flavanonols, flavans, chalchones, anthocyanidins, and isoflavonoids [27,28]. Flavonoids consist of a fundamental 15-carbon flavone structure, C6-C3-C6, with two benzene rings (A and B) connected by a three-carbon pyran ring (C). The location of the catechol B-ring on the pyran C-ring and the quantity and placement of hydroxy groups on the catechol group of the B-ring impact the antioxidant potential of flavonoids [29,30]. Flavonols are similar in structure to flavones and are distinguished by a hydroxyl group (-OH) at the C3 position and a carbonyl function (C=O) at the C4 position on the C ring [31,32]. Quercetin and kaempferol are distinguished by the presence of an extra hydroxyl group at the R1 position in the quercetin molecule [33,34]. Flavonols, particularly quercetin, exhibit a wide variety of biological roles. Due to their impact on cell-signaling pathways related to oxidative stress and inflammation, they may enhance lipid metabolism, vascular function, blood pressure, and glucose metabolism [26,35,36]. The flavanone family is prevalent in fruits and fruit juices of the Citrus species, making up around 95% of flavonoids in this subclass [37,38]. The only chemical structural distinction between flavanones and other flavonoids is the unsaturated double bond located between positions C2′ and C3′ of the C-ring [39,40]. Neohesperidin, hesperidin, and hesperetin (Figure 1) are citrus flavonoids belonging to the flavanones subclass known for their anti-inflammatory and antioxidant properties [41].
Polyphenols as therapeutics in respiratory diseases.
Polyphenols, a diverse group of plant-based compounds, can be broadly categorized based on their mechanisms of action against respiratory diseases [42]. Some, like flavonoids and curcumin, act as antioxidants, scavenging harmful free radicals that contribute to lung inflammation [43]. Others, like resveratrol, possess anti-inflammatory properties, dampening the immune response that can worsen respiratory conditions like asthma [44]. Additionally, certain polyphenols, such as quercetin, exhibit bronchodilatory effects, relaxing airway muscles and easing breathing difficulties [45]. It's important to note that research on the effectiveness of individual polyphenols and their optimal dosages for respiratory ailments is ongoing, and seeking professional medical advice is crucial before using any supplements [46].
Role of polyphenols in respiratory diseases
COPD
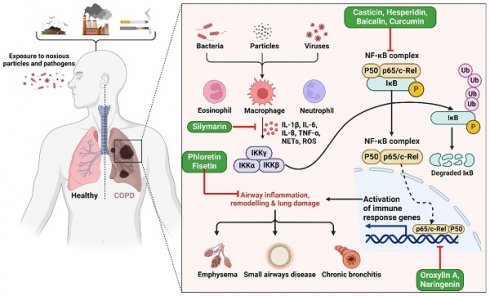
An estimated 300 million new cases of COPD are diagnosed annually worldwide [47]. Individuals suffering from bronchitis, characterized by shortness of breath, are at risk of developing long-term respiratory problems if it is not effectively treated. Shortness of breath is one of the most common symptoms of long-term respiratory issues [48]. Over the last decade, there has been a growing emphasis on the creation of efficient methods and tools for the early identification of COPD [49]. Inflammation caused by COPD has been associated with the risk of lung cancer. While there is currently no effective treatment available for COPD [50], there is evidence that flavonoids may be beneficial in treating this disease [51,52]. The protective functions of quercetin against chronic lung obstruction and pulmonary emphysema progression have been investigated in animal models [53].
Studies have displayed that baicalin has a notable capacity to enhance COPD and inflammation in animal models and cell cultures [54]. Baicalin was administered to six groups in a rat lung cancer model, both with and without exposure to cigarette smoke (CS) [55]. An optical microscope was used to assess the leukocyte count in the bronchoalveolar lavage fluid (BALF). The research revealed that baicalin effectively mitigated inflammation caused by CS. The researchers used CS extract (CSE) to stimulate type II pneumocytes and then examined the impacts of both CSE and pyrrolidine dithiocarbamate (PDTC). The study demonstrates that baicalin has noteworthy anti-inflammatory properties in rat models of COPD caused by CS, as well as in cell models created by CSE. Furthermore, the efficacy of baicalin rises proportionally with higher dosages [55]. Another study found casticin to protect the lungs against COPD by blocking NF-κB, improving pulmonary performance, and lowering oxidative stress and inflammation throughout the body (Figure 2) [50]. When rats were exposed to CSE at doses of 10, 20, and 30 mg/kg, plasma levels of leptin, C-reactive protein, and pro-inflammatory cytokines interleukin 1 (IL-1), IL-6, and TNF-α were restored to near normal levels by casticin (CST) treatment [50]. Activating the nuclear factor E2-related factor 2 (Nrf2) signaling pathway with oroxylin A was found to reduce oxidative stress and inflammation in the lungs, which may represent an effective preventative strategy against CS-induced lung inflammation and COPD [56]. In addition, studies exploring the effects of oroxylin A on RAW264.7 and BEAS-2B bronchial epithelial found it attenuated CS-induced cytokine production and 3-nitrotyrosine and 8-isoprostane levels, as well as histological abnormalities in the lungs of mice given daily intraperitoneal injections of oroxylin A for five days before CS treatment. However, oroxylin A increases glutathione (GSH) levels and glucocorticoid receptor (GR) activity in lung tissues. Moreover, cells treated with oroxylin A and then exposed to CSE showed substantial increases in NRF2 and GSH levels. Furthermore, oroxylin A increases Nrf2 binding to antioxidant response elements (AREs), increasing the expression of genes involved in the antioxidant response, including heme oxygenase 1 (HO-1), glutathione peroxidase (GPx), and GR, in CSE-stimulated cells [56].
Illustration representing the probable site of action of bioactive polyphenols. Upon exposure to harmful particles and pathogens, macrophages are activated, triggering the release of pro-inflammatory cytokines (e.g., IL-6, TNF-α) and reactive oxygen species. This cascade activates the NF-κB pathway and immune response genes, resulting in airway inflammation, structural remodeling, and lung damage, ultimately culminating in COPD. The figure was designed by Biorender.com program (https://biorender.com/, accessed on 20 March 2024).
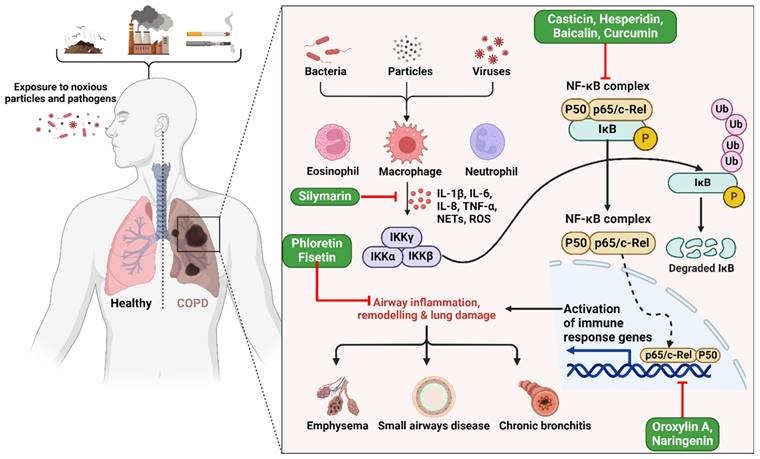
Fisetin has been shown to be an effective drug for treating inflammation-related lung illnesses such as COPD [57], reducing NF-κB binding in the IL-8 promoter region in NCI-H292 lung epithelial cells, resulting in reduced IL-8 production in response to TNF-α [57]. The suppression of NF-κB signaling by fisetin is most likely due to the disruption of its upstream regulators, including IκB kinase (IKK) and IB. Indeed, fisetin may have an inhibitory effect on these regulators, reducing NF-κB activation and IL-8 synthesis [57]. A case-control COPD study aimed to evaluate the effects of resveratrol and genistein on NF-κB, TNF-α, and matrix metallopeptidase 9 (MMP9) expression. The study involved 34 COPD patients and 30 healthy individuals in four groups: untreated control, dexamethasone-treated, resveratrol-treated (12.5 mol/L), and genistein-treated (25 mol/L). NF-κB-positive cell numbers increased with resveratrol concentration, while genistein-positive cell numbers decreased with lower dosages. TNF-α levels in resveratrol and genistein-treated groups correlated positively with the percentage of NF-κB-positive cells. Increased resveratrol concentrations were associated with lower MMP9 levels in a dose-dependent manner. Phytonutrient levels stabilized and increased slightly with resveratrol concentrations. MMP9 levels were inversely correlated with genistein concentration. Resveratrol was found most effective at 12.5 mol/L, while genistein was most effective at 25 µmol/L [58].
Asthma
Flavonoids have been found to decrease airway inflammation and the immunoglobulin E (IgE) response in asthmatic animals, dependent on their in vitro anti-asthmatic properties. Apigenin, fisetin, and luteolin are potent inhibitors of interleukin-4 (IL-4) production (Figure 3) [59,60]. Ovalbumin (OVA) was injected intraperitoneally into BALB/c mice on days 0, 7, and 14 to sensitize them. They then inhaled OVA daily by aerosol on days 19 to 23. Daily oral administration of luteolin at 0.1, 1.0, or 10 mg/kg was provided throughout the sensitization period. After sensitization, oral administration of luteolin at 1 mg/kg was provided on days 26 to 32. Luteolin was found to have a considerable suppressive effect on OVA-induced bronchial hyper-reactivity and airway bronchoconstriction at the dosages investigated, both during and after sensitization [61]. In addition, Luteolin treatment reduced OVA-specific IgE serum levels and increased IFN-γ but decreased IL-4 and interleukin 5 (IL-5) levels in BALF. Moreover, a preventive effect of luteolin and omega-3 polyunsaturated fatty acids (PUFA) supplementation on airway responsiveness was observed in cats exposed to Ascaris suum [62]. Studies have injected mice intraperitoneally with apigenin at doses of 5 and 10 mg/kg before the final OVA challenge to explore whether it can reduce asthmatic symptoms in an OVA-sensitized mouse model. Apigenin treatment prevented mice from developing asthmatic symptoms such as elevated serum IgE levels, eosinophil accumulation, and IL-4, IL-5, and erythropoietin (EPO) activity in blood and lung fluid [63]. Similar effects were also observed at apigenin doses of 2 and 20 mg/kg treatment in an OVA-induced asthmatic model [64]. Intraperitoneal injections of fisetin at 3 mg/kg were found to reduce hyperplasia, inflammation, and hyperresponsiveness of the airways in mice exposed to OVA aerosols [65]. In addition, this treatment decreased the expression of the primary initiators of allergic airway inflammation and T helper 2 (Th2)-associated cytokines IL-4, IL-5, and interleukin 13 (IL-13) in lung tissues. While it had been previously reported that fisetin inhibits NF-κB activity [66], it also impairs NF-κB activation in OVA-induced lung tissues. Moreover, fisetin suppressed OVA-induced increases in eosinophil count, total cell count, and IL-4, IL-5, and IL-13 levels in BALF in a dose-dependent manner when administered intravenously at 0.3, 1, or 3 mg/kg before OVA aerosol challenge on days 22 to 24 [67]. Finally, it also reduced the effects of OVA on lung tissue eosinophilia, airway mucus buildup, airway hyperresponsiveness, and expression of adhesion molecules, chitinase (CHIA), interleukins 17 (IL-17) and 33 (IL-33), major airway glycoprotein mucin 5AC oligomeric mucus/gel-forming (Muc5ac), and inducible nitric oxide synthase (iNOS).
Lung cancer
It is estimated that 2 million individuals are diagnosed with lung cancer annually, and 1.8 million individuals die from it [68], making it the leading cause of cancer-related death worldwide [69]. Small-cell lung cancer (SCLC) and non-small-cell lung cancer (NSCLC) are two lung cancer subtypes distinguished by their tumor-causing cells. Squamous cell carcinoma (SCC), adenocarcinomas (cancer of glandular cells), and neuroendocrine tumors such as SCLC, large cell neuroendocrine carcinoma (LCNEC), and carcinoids are the most common based on recent reports [70,71]. Polyphenols found abundantly in fruits, vegetables, and beverages like tea hold promise in inhibiting lung cancer development through various mechanisms. They act as antioxidants, scavenging free radicals that damage DNA and contribute to cancer prevention [72]. Additionally, they can block the activation of pro-carcinogens by enzymes, preventing their harmful effects. Polyphenols also interfere with cancer cell signaling pathways, hindering their uncontrolled growth and promoting cell death (apoptosis) [73].
Antioxidants such as flavonoids and proanthocyanidins found in high concentrations in fruits and vegetables can help reduce lung cancer risk. Nutrient polyphenols are involved in regulating cell survival pathways, which, in addition to their antioxidant properties, contribute to their anticancer and antimutagenic effects. There is increasing data from in vitro, in vivo, and epidemiological studies supporting the chemopreventive influence of polyphenols and their role in cancer prevention [74]. Despite recent advances in therapy, less than one-fifth of lung cancer patients live beyond five years [75]. According to a recent study, resveratrol inhibits NSCLC cell growth by causing early necrosis through reactive oxygen species (ROS)-induced DNA damage. Resveratrol may increase ROS production in A549 and H460 lung cancer cells by regulating nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 5 (Nox5) expression in cells [76]. However, trans-resveratrol induces apoptosis in human adenocarcinoma epithelial cells via mitochondrial-dependent pathways [77]. Resveratrol has also been found to have anti-apoptotic and anti-proliferative effects on lung cancer cells. Resveratrol has been found to bind to synthetic or natural promoters of early growth response 1 (Egr-1) and to enhance the expression of growth arrest and DNA damage-inducible (GADD45) in A549 lung cancer cells (Figure 4). Egr-1 mRNA and protein levels were elevated in A549 lung cancer cells grown with 100 M resveratrol within two hours of administration and increased in a dose-dependent manner when the resveratrol was administered for six hours at concentrations of 0, 25, 50, and 100 µM [78].
Illustration representing the sites of action of different polyphenols in asthma pathway. Hormonal fluctuations and airway epithelium damage trigger the secretion of IL-33, IL-25, and TSLP, which, in collaboration with dendritic cells, activate CD4+ T cells. This sequence leads to B cell stimulation of IgE production, activating mast cells and causing eosinophil expansion, resulting in asthma. The involvement of the NF-κB complex activates immune response genes, stimulating IgE and histamine release from mast cells. Histamine induces bronchoconstriction, contributing to asthma-related respiratory distress. The figure was designed by Biorender.com program (https://biorender.com/, accessed on 20 March 2024).
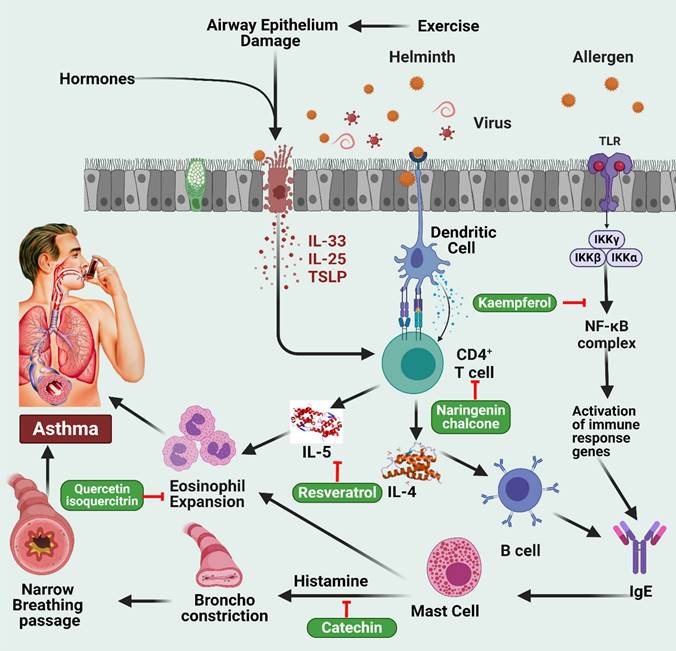
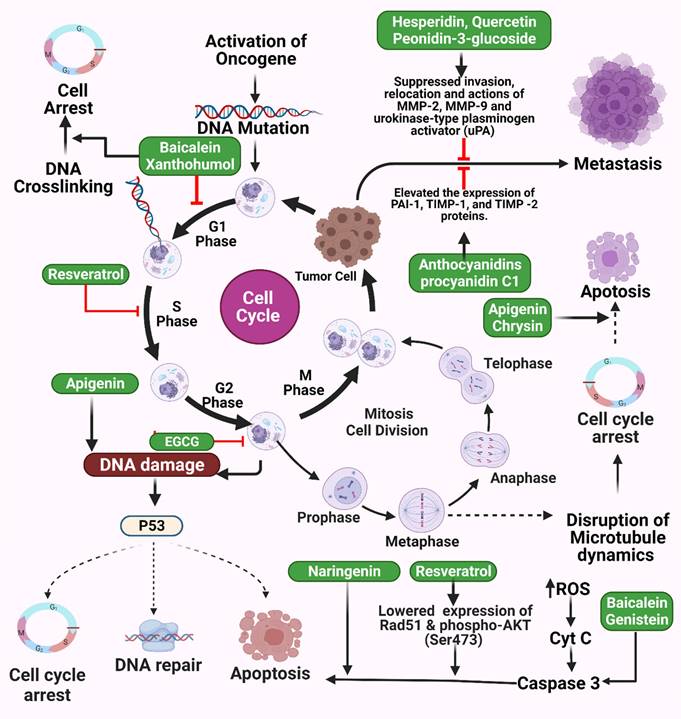
Illustration representing the sites of action of different polyphenols in cancer pathway. Activation of oncogene triggers abnormal cell division and finally results in tumor cells followed by metastasis. DNA crosslinking, DNA damage, abnormal p53 activities, and microtubule disruption collectively result in abnormal apoptosis and cell cycle arrest which cause cancer. Different polyphenols probably work on these pathways to prevent cancer. The figure was designed by Biorender.com program (https://biorender.com/, accessed on 20 March 2024).
While studies of lung carcinogens are limited, those on phytochemicals are becoming more common. Chromium-induced caspase-3 (CASP3) activation, ROS production, and protein crosslinking in DNA decreased in BEAS-2B cells treated with epigallocatechin gallate (EGCG) in a dose-dependent manner [79]. Curcuminoid bisdemethoxycurcumin derived from the turmeric plant Curcuma longa has been shown to prevent premature aging of normal lung fibroblast WI-38 cells, potentially through the sirtuin 1 (Sirt1)/AMP-activated protein kinase (AMPK) signaling pathway [80]. Dieckol was found to reduce the invasive and apoptotic abilities of A549 lung cancer cells in vitro by inhibiting signaling through phosphoinositide 3-kinase (PI3K), protein kinase B (AKT1), and mammalian target of rapamycin (mTOR) and activation of the tumor suppressor protein E cadherin (CDH1) [81]. Therefore, dieckol may represent a natural anticancer drug effective against NSCLC [81,82]. In addition, kaempferol was found to inhibit Akt1-mediated phosphorylation of Thr179 in Smad3, reducing the epithelial-to-mesenchymal transition (EMT) induced by transforming growth factor β 1 (TGF-β1) in lung cancer cell transplants [83]. This was the first demonstration of a potential molecular mechanism for kaempferol's anti-cancer activity that might also mediate camphor's suppression of malignant cell proliferation. Moreover, it showed that phosphorylation of the Smad3 linker region is required for EMT and cell migration and induced by TGF-β1, with lower EMT and cell migration in the presence of kaempferol due to low levels of Thr179 Smad3 phosphorylation. In contrast, phosphorylation of Smad3 did not occur at Ser204, Ser208, or Ser213. Moreover, Akt1 is essential for cell transplantation as well as EMT formation induced by TGF-β1. In addition, it has been shown that Akt1 can directly phosphorylate Smad3 at Thr179, which ultimately inhibits Akt1 phosphorylation induced by TGF-1 with camphor [82].
TB
Tuberculosis (TB) is a leading cause of death caused by the bacteria Mycobacterium tuberculosis. According to recent data, there were 10.4 million new TB infections in the United States in 2017 [84], of which 64% were male, and most were over 15 years old. Co-infection with the human immunodeficiency virus (HIV) is found in 1 in 10 TB sufferers, 72% of whom live in Africa. Isoniazid can cause neuritis, but other side effects, such as ethambutol and rifampin, can also occur. Therefore, there is an urgent need for new TB drugs with minimal side effects, even when used in combination [85].
Epigallocatechin gallate, a green tea catechin with antimicrobial properties, and its derivatives have been studied for their inhibitory effects on Mycobacterium smegmatis. Enoyl reductase (Inh A), a drug target inhibited by isoniazid, was docked with the geometrically optimized conformation of epigallocatechin gallate and two of its derivatives. The Ames test was performed to assess the mutagenic potential of epigallocatechin gallate. Inhibin subunit alpha (InhA) has been successfully docked with a docking capacity of -9.38 kcal mol-1. The minimum inhibitory concentrations for epigallocatechin gallate, per-propionate, and permethyl were 128 (58.2% inhibition), 8 (32.9%), and 4 (12.5%) µg/ml, respectively. Epigallocatechin gallate showed a cytotoxicity of 18.6% at eight µg/ml, while the two derivatives did not show any toxicity. The non-mutagenic epigallocatechin gallate inhibits M. smegmatis growth and warrants further investigation as an adjunct therapy for pathogenic mycobacteria [86].
According to a recent study, TB may alter the effect of curcumin, the primary active ingredient in turmeric, on intracellular clearance. They found curcumin reduces the intracellular concentration of M. tuberculosis in THP-1 cells by 10, 30, or 50 mg/mL (11, 47, and 67%, respectively) after infection. In addition, there was a statistically significant increase in the apoptotic rate of THP-1 cells after treatment with curcumin. In addition, LC3-I and LC3-II autophagy markers have been shown to be strongly induced by curcumin during TB infection. Moreover, intracellular TB load was reduced by 73% in primary human alveolar macrophages when curcumin was given four days after infection (Figure 5) [87].
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS)
Septic shock, trauma, pneumonia, and frequent blood transfusions are among the conditions that lead to death in patients with pancreatitis, multiple organ failure, lung injury, and acute respiratory syndrome (ARDS), respectively [88]. Acute lung injury (ALI) characterized by inflammation and blood clot formation, is often seen as a consequence of SARS-COV-2 infection. Particularly in intensive care, ARDS is a significant problem and can be triggered by ventilation with too high pressure [89]. Proinflammatory cytokines, including TNF-α, IL-1, and IL-6, are critical components of ALI/ARDS due to rapid lung tissue loss, neutrophil infiltration, and parenchymal inflammatory reactions [90]. Despite advances in ALI/ ARDS pathobiology over recent decades, the significant mortality rate (approximately 40%) associated with ALI/ARDS is poorly understood [88,91]. The incidence of severe ARDS had a significant surge on a global scale during the COVID-19 epidemic, resulting in a substantial fatality rate [92]. ARDS is still recoverable in the absence of treatment. Individuals are often treated with N-acetylcysteine (NAC) and other antioxidants, such as low tide breathing and water restriction. However, their survival rates did not improve as a result of these therapeutic approaches [93]. Studies on rats have shown that the polyphenolic compound curcumin regulates IL-10 immunomodulation against lung injury. In the cecal ligation and puncture (CLP) mouse model, lung damage was improved with 20 mg/ml of curcumin administered intraperitoneally [94]. Curcumin can reduce ALI severity and uncontrolled inflammation by promoting the differentiation of naïve CD4+ T cells to CD4+ CD25+ FOXP3+ Tregs and convert macrophages from M1 to M2, potentially influencing IL-10 immune modulation through Treg differentiation [94].
Flavonoid compounds have been shown to reduce the acute lung damage caused by lipopolysaccharide (LPS). Chen et al. showed that 100 mg/kg kaempferol administered intragastrically to male BALB/c rats after intranasal LPS treatment led to a decrease in the number of inflammatory cells in the BALF. Levels of proinflammatory cytokines, including TNF-α, IL-1, and IL-6, have also been shown to be significantly reduced in the BALF of rats after kaempferol therapy. One study has suggested that the anti-inflammatory properties of kaempferol may be associated with its ability to inhibit the activation of the mitogen-activated protein kinase (MAPK) and NF-κB signaling pathways, reducing tissue damage and pneumonia [95]. Wasi et al. found that flavonoids protect against pinocembrin and 5,7-dihydroxyflavone in LPS-induced inflammatory reactions in vitro and in vivo [96]. Dosage-dependent reduction in phosphorylation of IB, extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun N-terminal kinase (JNK), and p38 MAPK significantly inhibits the synthesis of TNF-α, IL-1, IL-6, and IL-10 in vitro in the presence of 0-300 g/ml pinocembrin. LPS-induced pulmonary edema and infiltration of neutrophils, lymphocytes, and macrophages were significantly reduced in rats given the anti-inflammatory drug pinocembrin at 20 or 50 mg/kg intraperitoneally. Moreover, pretreatment with pinocembrin reduced considerably levels of TNF-α, IL-1, and IL-6 but increased IL-10 levels. This study found a decrease in LPS-induced lung damage, with pinocembrin found to inhibit IB, JNK, and p38 MAPK activity (Table 1) [96].
Studies have examined the development of paraquat (PQ)-induced ALI and pulmonary fibrosis using naringin (Nar) as a protective agent, exploring the probability of survival after PQ poisoning at a 50 mg/kg dose in 10 km male and female rats randomly assigned to one of five groups: PQ, N-acetylcysteine (NAC), Nar1, and Nar3. The PQ group was injected intraperitoneally with PQ at 50 mg/kg. The NAC group was administered NAC intragastrically at 1 g/kg/d for three days. The Nar1 and Nar3 groups were administered naringin at 30 mg/kg/d and 120 mg/kg/d, respectively, for three days, then PQ at 50 mg/kg for three further days. The rat died of PQ poisoning after just seven days. It was found that mortality rates in the Nar1 and Nar3 groups were 20-60% of that of the PQ and NAC groups. Moreover, levels of TNF-a, TGF-β1, MMP9, TIMP metallopeptidase inhibitor 1 (TIMP1), pulmonary malonaldehyde, and pulmonary fibrosis deposition after PQ-inducement were attenuated significantly by naringin at 60 or 120 mg/kg [97].
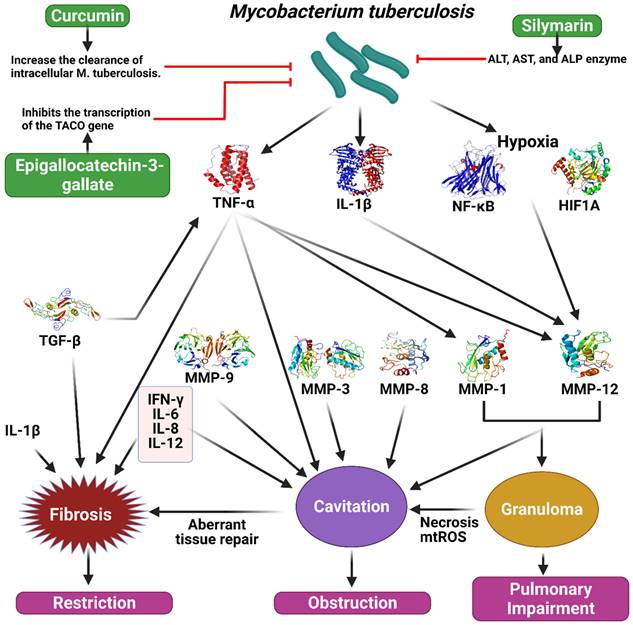
Illustration representing the sites of action of different polyphenols in tuberculosis pathway. Mycobacterium tuberculosis stimulate the secretion of TNF-alpha, IL-1B, NF-kB, HIF1A, which cause the activation of different MMPs and TGF-B. As a result, fibrosis, granuloma, and cavitation occur which ultimately create restriction, obstruction and pulmonary impairment in tuberculosis. Polyphenols could work on this pathway to prevent tuberculosis. The figure was designed by Biorender.com program (https://biorender.com/, accessed on 20 March 2024).
Experimental evidence on the use of polyphenols against respiratory diseases.
| Respiratory diseases | Polyphenolic compounds | Study model | Doses/Conc. | Results | Ref. |
|---|---|---|---|---|---|
| Asthma | Naringenin chalcone | BALB/c mice | 0.8 mg/kg/day | ↓ Th-2 cytokine secretion | [99] |
| Resveratrol | Female BALB/c mice | 30 mg/kg | Resveratrol lowered total IgE, OVA-specific IgE, IgG2a, IL-4, and IL-5 in plasma and BALF of OVA-induced asthmatic mice | [100] | |
| Quercetin and isoquercitrin | BALB/c mice | Isoquercitrin 15 mg/kg or quercetin 10 mg/kg | Quercetin and isoquercitrin decrease eosinophilia | [101] | |
| Kaempferol | Embryonic (BEAS-2B cells) | 1-20 mmol/L | Kaempferol reduced NF-kB signaling to decrease TNF-α-induced epithelial inflammation | [102] | |
| Influenza | Resveratrol | Four-week-old female BALB/c mice | 1 mg/kg/day | Prevent nuclear-cytoplasmic translocation of viral ribonucleoproteins (vRNPs) and reduce the production of late viral proteins by inhibiting protein-kinase C and underlying pathways. | [103] |
| Isoquercetin | Female BALB/c mice | female BALB/c mice | Treatment with isoquercetin dramatically decreased lung levels of IFN-, iNOS, and RANTES | [104] | |
| Baicalin | Male BALB/c mice | (10 -120 mg/kg/day | NS1 protein encoded by the IAV is modulated by baicalin to provide antiviral actions | [105] | |
| Curcumin | 30 μM | Anti-infective HA activity may be used to diminish influenza virus (H1N1 and H6N1) infection. | [106] | ||
| Acute Respiratory Distress Syndrome (ARDS)/Acute Lung Injury (ALI) | Naringin | KM mice | 30, 60, 120 mg/kg | Protects against ALI and fibrosis, suppresses oxidative stress and inflammation in the lungs, and increases antioxidant enzymes such as SOD, GSH-Px, and HO-1 | [97] |
| Pinocembrin | Male BALB/c mice | 20 or 50 mg/kg, i.p. | By inhibiting p38MAPK and JNK activation, pinocembrin decreased LPS-induced lung damage | [96] | |
| Rutin, Quercetin, Icarisid II, Isorhamnetin, Chlorogenic Acid | Female C57BL/6 mice | 0.15% or 0.6% Inonotus sanghuang (extract)ISE | An anti-inflammatory and anti-oxidation imbalance is corrected in the lungs in part because of the regulation of NF-κB signaling, which reduces inflammation. | [107] | |
| Punicalagin | Male BALB/c mice | 12.5, 25, and 50 mg/kg | TLR4-mediated NF-κB signaling pathways may be a contributing factor | [98] | |
| Curcumin | Male C57BL/6 mice | intraperitoneal injection of 50 μl (20 mg/ml) | By stimulating CD4+ T cell development into FOXP3+ CD25+ Tregs, curcumin lessens the severity of ALI in mice. | [94] | |
| Kaempferol | Male BALB/c mice | 25, 50 or 100 mg/kg body weight | Antioxidant and anti-inflammatory effects of kaempferol on LPS-induced ALI may be mediated via reduction of MAPKs and NF-κB signaling pathways | [95] | |
| Tuberculosis | Curcumin | Tamm Horsfall Protein (THP-1) cells in a lab dish | 10, 30, and 50 μM | Both apoptosis and autophagy might be mechanisms by which curcumin modulates human macrophages to increase the clearance of intracellular M. tuberculosis. | [87] |
| Epigallocatechin-3-gallate | THP-1 and Jurkat cell lines | Administration of EGCG inhibits the transcription of the TACO gene in a dose-dependent manner. | [108] | ||
| Silymarin | Male Wistar rats | 10 mg/kg | Safely and effectively return ALT, AST, and ALP enzyme concentrations to normal. | [109] | |
| COPD | Casticin | Male Wistar rats | 10, 20, and 30 mg/kg) | Casticin inhibits TLR4 activation in Western blot analysis as well as the phosphorylation of NF-κB and IB. | [50] |
| Phloretin | Male BALB/c mice | 10, 20 mg/kg | It has been shown that phloretin protects against CS-related airway mucus hypersecretion and inflammation, where the EGFR, ERK, and P38 may be involved. | [110] | |
| Hesperidin | C57BL/6 mice | 25, 50 mg/kg | Antioxidant stress and inflammation were decreased in CES-induced COPD model mice when the SIRT1/PGC-1a/NF-jB signaling pathway was activated. | [111] | |
| Baicalin | Rat model | 20 mg/kg, 40 mg/kg, 80 mg/kg | Blocking NF-kB has an anti-inflammatory impact | [55] | |
| Oroxylin A | BEAS-2B and RAW264.7 cells | 15, 30, and 60 mg/kg | The Nrf2 signaling pathway was activated by Oroxylin A to reduce CS-induced oxidative damage. | [56] | |
| Silymarin | BALB/c mouse model in vitro in A549 cells | 20, 40 and 80 mg/Kg | Improved pulmonary function, reduced inflammation, and suppressed the production of pro-inflammatory cytokines. | [112] | |
| Fisetin | NCI-H292 and HEK293T cells | - | TRAF2 in TNF-RSC is inhibited by binding fisetin to PKC | [57] | |
| Curcumin | CS-induced rat model | 100 mg/kg | Curcumin's ability to prevent alveolar epithelial damage in rats with COPD may be in part due to the downregulation of P66Shc. | [113] | |
| Naringenin | Modeling BALB/c mice using A549 cells | 20, 40 and 80 mg/Kg | Naringenin administration improved lung function, decreased inflammation, and inhibited the production of inflammatory cytokines such as TNF- and MMP9. | [114] | |
| Severe acute respiratory syndrome coronavirus (SARS-CoV) | Luteolin | Vero E6 cells | EC50 10 µM | The antiviral action of luteolin may be achieved by disrupting the fusion of virus cells. | [115] |
| Emodin | Vero E6 cells | IC50 200 µM | As the dosage increased, the S protein and ACE2 interaction was significantly inhibited, as was expected. | [116] | |
| Chalcones isolated from Angelica keiskei | In silico | 3CLpro and PLpro inhibitory activity with IC50 values of 11.4 and 1.2 mM | While the SARS-CoV PLpro demonstrated noncompetitive inhibition, the chalcones showed competitive inhibition of the SARS-CoV 3CLpro | [117] | |
| Forsythoside A | CEK cells infected with IBV | 0.16 mM, 0.32 mM, and 0.64 mM | (i) dose-dependent viral load reduction, (ii) IBV nucleocapsid protein expression reduction, and (iii) dose-dependent inhibition of IBV infection | [118] | |
| MERS-CoV infection | Resveratrol | Vero E6 cells infected with MERS-CoV | 250-7.8125µM | (i) ↓cell death, (ii) ↓ viral RNA replication inhibition, (iii) ↓ viral titer, (iv) ↓ nucleocapsid protein expression, (v) ↓ apoptosis | [119] |
| Pulmonary fibrosis | Resveratrol, Quercetin, Mangiferin, Dihydroquercetin (DHQ) | Male CD-1 (CD1(ICR)) mice | 50 mg/kg, 10 mg/kg, 10 mg/kg, 10 mg/kg respectively | 1. Anti-inflammatory effects of resveratrol and quercetin 2. Protective properties of exogenous administration of mangiferin and DHQ | [120] |
| 6-Gingerol | C57BL/6 mice | 100 or 250 mg/kg | 6-gingerol has been shown to reduce lung fibrosis by activating SIRT | [121] | |
| Pulmonary hypertension | Resveratrol | Sprague-Dawley rats | 3 mg/kg | ↓ pulmonary hypertension caused by MCTs | [122] |
| Trimethoxystilbene (TMS) | Sprague-Dawely rats | 5 or 10 mg/kg per day | Inhibits the NOX/VPO1 pathway, which causes oxidative stress and inflammation | [123] | |
| Lung Cancer | Hesperidin | Swiss albino mice | 25 mg/kg | Changing COX-2, MMP-2, and MMP-9 expressions provides anti-carcinogenic effects against lung cancer. | [124] |
| Baicalein | Swiss Albino mice | 12 mg/ kg, 50 mg/kg | Degradation is prevented in TCA cycle enzymes and electron transport chain complexes in lung cancer-bearing mice. | [125] | |
| EGCG | Mice | EGCG's anti-cancer properties are influenced by miRNA-mediated regulation, which is implicated in all of the primary elements of its action | [126] | ||
| Quercetin | A110L human lung cancer cell line | ↓ invasion activity by directly suppressing MMP activities and by inhibiting monocarboxylate transporter activity | [127] | ||
| Peonidin-3-glucoside | in vitro | 10-40 µM | Preventing cancer cell invasion, migration, and the production of MMPs and u-PA by cancer cells | [128] | |
| Anthocyanidins | Mouse | 0.5 mg/mouse | ↓ tumor growth | [129] | |
| Xanthohumol | A549 cancer cells | 14-42 µM | Inducing apoptosis and cell cycle arrest | [130] | |
| Procyanidin C1 | A549 cancer cells | 1.25-40 µg/mL | ↓ TGF-β-induced EMT | [131] | |
| Naringenin | A549 cancer cells | 100 µM | ↑ TRAIL-mediated apoptosis | [132] | |
| Apigenin | in vitro | 40-160 µM | Inducing apoptosis and DNA damage | [133] | |
| Chrysin | A549 cancer cells | 10 µM | Inducing apoptosis, AMPK activation, ROS | [134] | |
| Luteolin | in vivo | 10-30 mg/kg | ↓ tumor growth | [135] | |
| Quercetin | A549 cancer cells | 8.4 mg/kg | ↓ tumor growth | [136] | |
| Kaempferol | in vitro | 10-50 µM | ↓ TGF-β1-induced EMT and migration | [82] | |
| Genistein | H446 cancer cells | 25-75 µM | The apoptosis and cell cycle arrest of cancer cells, as well as a reduction in proliferation and migration, are all achieved by using this treatment. | [137] | |
| Resveratrol | In vitro | 0, 25, 50 and 100 µM of resveratrol for 6 h | ↓ cell proliferation and apoptosis in the study subjects | [78] | |
| Non-Small-Cell Lung Cancer | Resveratrol | H1703 and H1975 human NSCLC cell lines | - | ↓ XRCC1 expression in NSCLC cells may increase the efficacy of etoposide treatment | [75] |
| Curcumin | H446 human SCLC cell line | - | Curcumin enhanced apoptosis in human SCLC NCI-H446 cells through a ROS-mediated mitochondrial mechanism. | [138] | |
| Quercetin | A549 human NSCLC cell line and xenograft model | - | Lung cancer cells can be suppressed by quercetin as an aurora B inhibitor | [139] | |
| Fisetin | A549 and H1792 human NSCLC cell lines | 5-20 µM | Fisetin suppressed PI3K/Akt and mTOR signaling in NSCLC cells | [140] |
Punicalagin can also relieve LPS-induced ARDS. Dexamethasone (5 mg/kg) and punicalagin (12.5, 25, and 50 mg/kg) were administered by intraperitoneal injection one hour before LPS (20 mg/kg) intranasal administration to induce lung injury. In addition, punicalagin has been given to the patients to minimize myeloperoxidation and neutrophil and macrophagic infiltration in the lungs. Moreover, punicalagin has been found to decrease Toll-like receptor 4 (TLR4) expression and NF-κB activation, with NF-κB activation and proinflammatory cytokine production potentially inhibited by lower TLR4 expression [98].
Current progress toward clinical applications
Polyphenols are bioactive chemical compounds that are found mainly in plants such as cocoa, tea, and coffee and fruits such as grapes, pomegranates, and apples and were found to be beneficial in extensive clinical studies [141-144]. Polyphenols are exogenous antioxidants that play a vital role in protecting cells [145]. They are a vast family of therapeutically active phytochemicals, including several that are currently being tested in preclinical and clinical studies, and have shown promising outcomes for treating respiratory disorders such as asthma, COPD, pneumonia, lung cancer, and influenza. This section illustrates some of the clinical studies on polyphenols to understand their future potential better.
Influenza
The influenza viruses cause widespread annual epidemics and pandemics in humans and animals, resulting in significant mortality and morbidity [146]. Despite the vast repertoire of pharmacotherapeutic options available for suppressing specific influenza pathogenic processes, establishing more efficient therapy options is proving challenging. A literature review by Bahramsoltani et al. found various natural polyphenol extracts, including pomegranate, Cistus incanus, lychee fruit, Glycyrrhiza uralensis juice, Aronia melanocarpa, tea, cranberry, and phenolic compounds such as quercetin, resveratrol, and caffeic acid affected influenza viral infection [147]. C. incanus extract has favorable therapeutic benefits in modulating inflammatory biomarkers in respiratory tract diseases such as influenza [148]. In a randomized, placebo-controlled study, healthcare workers who took tea catechins and theanine capsules had lower rates of influenza infection [149]. Moreover, a study of 124 individuals found a significant decrease in the incidence of influenza in those taking polyphenols as adjuvant therapy [150]. These clinical studies demonstrate the importance of natural polyphenols as adjuvant therapy for managing influenza with conventional drugs.
COPD
COPD is a chronic inflammatory lung condition with deficiently reversible airflow restriction where, in most cases, the inflammatory stimulus is initiated primarily by inhalation of noxious gases, mainly CS [151]. TNF-α and IL-6 are critical proinflammatory mediators that, along with matrix metalloproteinases (MMPs), are strongly associated with lung injury and healing in COPD patients. These inflammatory mediators and MMPs function interdependently. Resveratrol is a natural polyphenol found in various plants, including nuts and fruits, but is particularly prevalent in red wine and grapes. Several previous studies have shown that resveratrol has diverse anti-inflammatory properties [152-154].
A study with 34 COPD patients and 30 healthy individuals placed into four treatment groups (Table 2) found that nuclear translocation of NF-κB and secretion of MMP9 and TNF-α were elevated in the COPD patients compared to healthy individuals. In contrast, genistein and resveratrol inhibited the nuclear translocation of NF-κB and reduced the secretion of MMP9 and TNF-α. Therefore, these findings show that genistein and resveratrol may be promising medicinal candidates for COPD management [58].
Lung cancer
With roughly 80% of lung cancer patients histologically categorized with NSCLC, it has become the leading cause of mortality among human malignancies. It is incurable in several cases because of resistance to many anticancer drugs [155]. The therapeutic modalities available for the treatment of lung cancer include surgical intervention, chemotherapy, radiation therapy, and targeted pharmacotherapy. [156]. For over two decades, combination therapy with cisplatin has been the most effective therapy for NSCLC. However, the long-term survival rate continues to be low [157]. Ginger and green tea are both abundant in antineoplastic and antioxidant polyphenols. Green tea contains epicatechin (EC), epigallocatechin (EGC), EGCG, epicatechin-3-gallate (ECG), and theaflavin, which are collectively known as green tea polyphenols (GTPs) [158]. Green tea and ginger, known for their health advantages, include polyphenols, plant-based chemicals with antioxidants, and other beneficial properties. Green tea mainly contains catechins, whereas ginger is rich in gingerols and shogaols, in addition to some catechins [159]. Studies indicate that polyphenols in these ingredients may enhance their health advantages and perhaps have synergistic effects when ingested together [22]. Ginger contains various polyphenols, including gingerol and shogaol, which have also been shown to have anti-cancer properties, including inhibiting the proliferation of lung cancer cells [160].
Clinical studies on the use of polyphenols against respiratory diseases.
| Polyphenols | Diseases | Model/Method | Outcomes | Ref. |
|---|---|---|---|---|
| CYSTUS052 | Upper respiratory tract infection (Bacterial infection and influenza) | A randomized, placebo-controlled clinical study with 160 patients, including 56 men and 104 women aged between 7 and 81 years, suffered from an upper respiratory tract infection by medical signs. Throat Swabs were collected and placed in an appropriate bacterial culture medium to determine the infection's pathogen | 129 patients finished the study out of 160 participants After four days of treatment, a significant decrease in the symptoms with inflammatory markers, including CRP and FVIII, was seen | [148] |
| Gallic acid, Catechin, Epigallocatechin gallate; Epicatechin; Epicatechin gallate | Common cold | A prospective, randomized, placebo-controlled, double-blinded, multi-centric clinical study with 100 patients (age 20 -65 years): Patients were told to intake 2 to 250 mL of the test beverage or placebo beverage twice per day for ten days | 41.9 percent of patients receiving test beverages reported being complaint-free on the evening of research day 7, compared to 5 percent of patients in the placebo group | [180] |
| Quercetin | Lung cancer | A case-control study with a large population (EAGLE) was conducted in Italy. An investigation was done with 1822 incident lung cancer cases and 1991 frequency-matched controls from the Environment and Genetics in Lung cancer Etiology study. | Consumption of combination fruits and vegetables, alone fruits, and only vegetables were related to a 30, 21, and 24% decreased risk of lung cancer, respectively, in a large population-based case-control research from Northern Italy. A diet high in quercetin-rich foods was associated with a 53% decreased incidence of lung cancer. Women and men, ever smokers, showed the inverse association with quercetin-rich diets, which were highest among the heaviest smokers | [9] |
| Quercetin and Naringin | Lung cancer | Five hundred eighty-two individuals with incident lung cancer were subjected to a population-based, case-control study. Patients were diagnosed with primary lung cancer at all major medical centers of the study region between January 1, 1992, and January 1, 1997 | Inverse associations between lung cancer and polyphenol food sources were shown to be statistically significant, with a 40%-50% decreased risk of lung cancer, with the patients having the highest intake of polyphenols compared with the lowest category. | [162] |
| Resveratrol and genistein | COPD | Lymphocytes were extracted for NF‑κB immunocytochemical staining and analysis of TNF‑α and MMP‑9 concentration levels from 30 healthy people and 34 COPD patients, then placed into four study groups with dexamethasone, resveratrol, and genistein. | The translocation of NF‑κB was inhibited by resveratrol and genistein, and they also reduced TNF‑α and MMP‑9 concentration levels. | [58] |
| Naringenin | Bronchial pneumonia in children | One hundred eighty patients were randomly allocated to one of two groups: naringenin (NAR) and azithromycin (AZI). During the clinical intervention, all individuals were advised to follow a five-day oral dosing protocol, and their blood cytokine levels were analyzed. | Naringenin was able to minimize the frequencies of bronchial pneumonia complications and related adverse responses, as well as enhance the health of the patients, by inhibiting inflammation and shortening the time it took for clinical signs to vanish. | [189] |
| Cranberry polyphenols | Colds and influenza | A randomized, double-blinded, placebo-controlled intervention study to see if cranberry polyphenols might affect immunity, particularly γδ -T cell proliferation. For this purpose, a total number of 54 healthy individuals, including 17 men and 37 women, aged between 21 to 50 years old and with BMI between 18 to 30 kg/m^2, were studied. 45 subjects (83%) out of 54 completed the study | After ten weeks of cranberry beverage intake, the levels of γδ-T cell proliferation were nearly five times higher, with reducing number of symptoms related to colds and influenza. | [179] |
| Catechin | Influenza | A study with 124 individuals (age limit- at least 65 years) was conducted in which 76 out of 124 subjects, including 24 men and 52 women, gargled with tea catechin extract, whereas 48 subjects gargled without tea catechin extracts and were divided into catechin group and control group consecutively. | The incidence of influenza infection decreased significantly from 1.3 percent in the catechin group to 10 percent in the control group. | [150] |
| Theanine and green Tea catechins. | Influenza | During a 5-month randomized, placebo-controlled, double-blinded experiment of 200 healthcare professionals, 98 were given green tea and theanine capsules, and 99 were given a placebo | When compared to the placebo group, the catechin/theanine group had a significantly lower incidence of both clinically and laboratory-confirmed influenza infection | [149] |
| Carvacrol | Asthma | Forty individuals with mild to severe asthma for two months | Improvements in respiratory symptoms and PFT readings were seen as a consequence of the study | [199] |
| Pomegranate juice (Ellagitannins) | COPD | 30 patients for five weeks | Adding pomegranate juice to existing COPD treatment does not improve outcomes | [200] |
| EGCG, EGC, ECG, EC, and catechin gallate | Influenza infection | 76 adult persons for three months | They had a reduced rate of influenza infection in the catechin-treated group compared to the control group | [150] |
| EGCG | Esophagus Cancer | 51 patients | EGCG solution may be effective in treating ARIE in esophageal cancer patients receiving radiation therapy, potentially acting as an ARIE-reliever without compromising radiation therapy efficacy. | [163] |
Catechins affect various molecular and cellular targets associated with cell death and survival, inhibiting cell proliferation and regulatory pathways associated with invasion, growth factor-related proliferation, and angiogenesis [161]. A population-based study performed with 582 lung cancer patients in Hawaii [162] found an inverse relationship between lung cancer and the intake of polyphenols such as quercetin and naringin from food sources such as white grapefruit, apples, and onions, with a statistically significant 40-50% decrease in lung cancer risk in patients with high polyphenol intake compared to those with low polyphenol intake. This finding is supported by other polyphenol studies showing positive results with lung cancer [9]. Phase II research was carried out to confirm the effectiveness and safety of EGCG in treating ARIE. Participants from the Shandong Cancer Hospital and Institute in China were recruited for the research. EGCG was given during the first occurrence of ARIE and weeks after the completion of radiation. The patients were observed for dysphagia, RTOG score, and discomfort associated to esophagitis. The tumor response rate was 86.3%, whereas the overall survival rates were 74.5%, 58%, and 40.5%. Administering EGCG solution orally seems to be a viable treatment for acute radiation-induced esophagitis in patients with esophageal cancer undergoing radiation therapy [163]. A Chinese phase I study shown that EGCG might be a potential treatment for acute radiation-induced esophagitis (ARIE), a common side effect of thoracic irradiation. 37 patients with stage III lung cancer participated in the experiment, receiving either concurrent or sequential chemo-radiation or radiotherapy alone. EGCG was given at a dosage of 440μmol/L once Acute Radiation-Induced Esophagitis (ARIE) developed, and continued for two weeks post-radiotherapy. After administering EGCG and radiation, there was a significant reduction in RTOG score and pain ratings. The experiment verified that oral EGCG is a successful and secure approach for managing ARIE, and a phase III randomized controlled trial is anticipated to validate its efficacy [164].
Asthma
Asthma is a severe airway condition characterized by airway inflammation, hyper-reactivity, and restricted airflow with airway remodeling [165]. The development of asthma is connected with two possibly new genes, SETDB1 and ZNF8, as determined by gene-smoking interaction analysis conducted on different cohorts of the KoGES collaboration [166]. Previous studies regarding the in vivo and in vitro anti-asthmatic and anti-allergic features of flavonoids strongly support the use of flavonoids as a dietary treatment or preventive strategy for asthma and other allergic human diseases [167-170]. Moreover, recent clinical studies on flavonoids found that they can reverse allergic rhinitis effects [171-174]. Pycnogenol is an extract of water-soluble bioflavonoids from maritime pine containing proanthocyanidins that were found to be beneficial for asthma in a randomized, placebo-controlled, double-blinded study of asthmatic individuals with different levels of severity [175].
Similarly, another study of 76 asthma patients suggested that the anti-inflammatory properties of pycnogenol could be beneficial when used in conjunction with inhaled corticosteroids (ICS), reducing ICS dosage and frequency [176]. Moreover, other studies have found pycnogenol to be effective in asthma management [176,177] and collectively support the use of pycnogenol as an adjunct therapy for severe asthma. A study in a community-based trial at a single center discovered that berry fruit extract might decrease chronic airway inflammation and alter airway remodeling in models of lung inflammation generated by allergens. The trial included 28 mild asthmatics who had not used steroids before and had Feno levels of more than 40 ppb. Of them, 25 participants completed both therapies. Participants were randomly allocated to receive either 100 mg of berry fruit polyphenolic extract (BFPE) or a placebo for four weeks, with a 4-week break between the treatments. The primary variable assessed was the FeNO level after four weeks. The study found that BFPE did not have an impact on FeNO levels, which is a biomarker of eosinophilic airway inflammation, in steroid-naïve individuals with moderate asthma and high FeNO levels. It is essential to be cautious when assuming that the improvements shown in mice with lung eosinophilia would translate to effective treatment in asthma patients [178].
Common cold
The most common infectious disease in humans is the acute upper respiratory tract virus infection, generally called the common cold. Polyphenols were effective against cold and flu viruses in some ex vivo tests with γδ-T cells [179]. In addition, clinical trials have also assessed the beneficial effects of polyphenols (Gallic acid, catechin, epigallocatechin gallate, epicatechin, epicatechin gallate, and ascorbic acid) on the common cold. A randomized, placebo-controlled, double-blinded clinical study (Table 2) of 100 patients aged 20 to 65 with at least one cold-related local finding (e.g., throat) who scored at least 5 points based on the severity of five cold symptoms were instructed to drink 2 to 250 mL of a test or placebo beverage twice a day for ten days. Clinical examinations were scheduled prior to the start of treatment (baseline), 3-6 days later after the start of therapy (second), and 7-10 days after the start of treatment (third). By the third examination, 19 of the 49 patients in the test beverage group (38.8%) were free of symptoms compared to just 4 of the 47 patients in the placebo group (8.3%). The difference was magnified in the patient evaluations, where 41.9% of patients in the test beverage group reported being symptom-free on the evening of day 7, compared to just 5% of patients in the placebo group. This study clearly demonstrates that individuals suffering from common cold symptoms can benefit from drinking polyphenol-rich beverages, with symptoms disappearing faster than those who do not [180].
Pneumonia
Bronchial or lobular pneumonia is a frequent infection affecting children, particularly toddlers, and newborns [181]. Bronchial pneumonia in children is caused by a pathogenic bacterial, mold, or viral infection [182,183]. The polyphenol naringenin has been found to have free radical scavenging, anti-atherosclerotic, anti-oxidative, anti-inflammatory, and cell protection properties and pharmacological functions such as anti-microbial and anti-cancer activity and inflammation resolution [184-187]. In addition, multiple animal studies have demonstrated the usefulness of naringenin for treating respiratory diseases such as asthma and COPD [188]. A clinical study with 180 patients randomly placed into one of two groups, naringenin and azithromycin (AZI; Table 2), assessed their blood-borne cytokine levels following a five-day oral dosing protocol and compared the time required for clinical symptoms to subside following therapy and recurrence of complications. They found naringenin to minimize the frequency of pneumonia complications and other adverse responses. It improved patient health by inhibiting inflammation, providing new insights into the potential medical applications for naringenin [189].
Polyphenol research is unquestionably a promising and exciting scientific area. However, several missing connections must be addressed regarding their efficacy for various allergic illnesses before their widespread use in disease prevention and treatment. Firstly, approaches are required to increase polyphenol bioavailability and, eventually, its beneficial effects [146,190,191]. Secondly, cutting-edge biochemical techniques are required to identify active components within various polyphenol extracts to ascertain which polyphenol is associated with a given anti-allergic activity [192-194]. Thirdly, the administration route (oral vs. topical) and intervention window (prevention vs. treatment) of polyphenols require further study for various allergy symptoms. Finally, the long-term estimated risk of therapeutic and dietary polyphenol use in different age groups (children, adults, and older people) must be assessed. These studies are required to confirm the general assumption that polyphenols are harmless since their content and tolerability from different extract sources can differ [195-198].
Concluding remarks and future directions
Various polyphenol-related beneficial effects on lung disease are associated with the polyphenol content of almost all plant-based foods (fruits and vegetables). Preliminary research has shown that metabolic syndromes, such as diabetes, hypercholesterolemia, hypertension, neurodegeneration, other dementias, and various cancers, may provide new lines of preclinical research into strategies to prevent the development of various lung diseases. Nonetheless, this study focuses on the ability of EGCG, proanthocyanidin, and other biochemical compounds to treat various lung diseases. Since polyphenols are abundant in nature, it would be advantageous to study their largely unknown effects. Despite encouraging preclinical findings, only one clinical study into the efficacy of polyphenols in treating lung disease in human patients has been performed. However, it only included a small number of participants, and their follow-up period was short. The therapeutic potential of polyphenols will be better understood using larger sample sizes and longer follow-ups.
We now have a better understanding of how polyphenols are absorbed, digested, and transported throughout the body, enabling more effective clinical trials through increased knowledge about appropriate dosing. Polyphenols can be used to treat and prevent neurodegenerative conditions, where their activity results from various interconnected processes, such as their ability to scavenge free radicals and chelate metals. An additional important factor is the bioavailability of polyphenols. Multiple studies on the safety and toxicity of polyphenols have shown that high tannin levels can be harmful. Since polyphenols are present in a wide variety of foods, more research is required on their effects on humans. We believe that a proper risk-benefit analysis will accelerate the use of polyphenols as a therapy for the prevention and treatment of both acute and chronic lung disease.
Acknowledgements
The authors are thankful to the Deanship of Research and Graduate Studies, King Khalid University, Abha, Saudi Arabia, for financially supporting this work through the Large Research Group Project under Grant no. R.G.P.2/44/45.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2020R1I1A2066868), the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1A5A2019413), and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HF20C0038).
Authors contributions
Talha Bin Emran: Conceptualization, Formal analysis, Investigation, Writing - Original draft, Supervision; Project administration; Taslima Akter Eva: Conceptualization, Formal analysis, Editing, Visualization; Mehrukh Zehravi: Conceptualization, Formal analysis, Investigation, Writing - Original draft, Supervision, Fahadul Islam: Formal analysis, Resources, Editing; Jishan Khan: Resources, Writing - Review & Editing, Visualization, Writing - Original draft; Shaik Kareemulla: Formal analysis, Investigation, Writing - Review & Editing; Uppuluri Varuna Naga Venkata Arjun: Investigation, Writing - Review & Editing; Anitha Balakrishnan: Formal analysis, Investigation, Writing - Review & Editing; Poonam Popatrao Taru: Formal analysis, Investigation, Writing - Review & Editing; Firzan Nainu: Formal analysis, Investigation, Writing - Review & Editing, Emil Salim: Formal analysis, Investigation, Writing - Review & Editing, Safia Obaidur Rab: Resources, Formal analysis, Writing - Review & Editing, Validation, Visualization; Mohamed H. Nafady: Resources, Formal analysis, Writing - Review & Editing, Visualization. Polrat Wilairatana: Formal analysis, Investigation, Writing - Review & Editing, Moon Nyeo Park: Formal analysis, Writing - Review & Editing, Visualization, Funding acquisition, Bonglee Kim: Formal analysis, Writing - Review & Editing, Visualization, Funding acquisition, Supervision.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Dua K, Löbenberg R, Luzo ÂCM, Shukla S, Satija S. Targeting Cellular Signalling Pathways in Lung Diseases. Springer. 2021
2. Mandlik DS, Mandlik SK. New perspectives in bronchial asthma: pathological, immunological alterations, biological targets, and pharmacotherapy. Immunopharmacol Immunotoxicol. 2020;42(6):521-44
3. Cha SR, Jang J, Park SM, Ryu SM, Cho SJ, Yang SR. Cigarette Smoke-Induced Respiratory Response: Insights into Cellular Processes and Biomarkers. Antioxidants. 2023;12(6):1210
4. Hadzic S, Wu CY, Avdeev S, Weissmann N, Schermuly RT, Kosanovic D. Lung epithelium damage in COPD - An unstoppable pathological event? Cell Signal. 2020;68:109540
5. Agusti A, Böhm M, Celli B, Criner GJ, Garcia-Alvarez A, Martinez F. et al. GOLD COPD DOCUMENT 2023: a brief update for practicing cardiologists. Clin Res Cardiol. 2023;113:195-204
6. Iqbal I, Wilairatana P, Saqib F, Nasir B, Wahid M, Latif MF. et al. Plant polyphenols and their potential benefits on cardiovascular Health: A Review. Molecules. 2023;28(17):6403
7. Bié J, Sepodes B, Fernandes PCB, Ribeiro MHL. Polyphenols in Health and Disease: Gut Microbiota, Bioaccessibility, and Bioavailability. Compounds. 2023;3(1):40-72
8. Le Marchand L, Murphy SP, Hankin JH, Wilkens LR, Kolonel LN. Intake of flavonoids and lung cancer. J Natl Cancer Inst. 2000;92(2):154-60
9. Lam TK, Rotunno M, Lubin JH, Wacholder S, Consonni D, Pesatori AC. et al. Dietary quercetin, quercetin-gene interaction, metabolic gene expression in lung tissue and lung cancer risk. Carcinogenesis. 2010;31(4):634-42
10. Li J, Feng Z, Lu B, Fang X, Huang D, Wang B. Resveratrol alleviates high glucose-induced oxidative stress and apoptosis in rat cardiac microvascular endothelial cell through AMPK/Sirt1 activation. Biochem Biophys Reports. 2023;34:10144
11. Jiang Z, Han Z, Zhu M, Wan X, Zhang L. Effects of thermal processing on transformation of polyphenols and flavor quality. Curr Opin Food Sci. 2023;51:101014
12. Wang Z, Yang L. The Therapeutic Potential of Natural Dietary Flavonoids against SARS-CoV-2 Infection. Nutrients. 2023;15(15):3443
13. Wang Z, Yang L. Chinese herbal medicine: Fighting SARS-CoV-2 infection on all fronts. J Ethnopharmacol. 2021;270:113869
14. Dragovic-Uzelac V, Levaj B, Mrkic V, Bursac D, Boras M. The content of polyphenols and carotenoids in three apricot cultivars depending on stage of maturity and geographical region. Food Chem. 2007;102(3):966-75
15. Santhakumar AB, Battino M, Alvarez-Suarez JM. Dietary polyphenols: Structures, bioavailability and protective effects against atherosclerosis. Food Chem Toxicol. 2018;113:49-65
16. Singla RK, Dubey AK, Garg A, Sharma RK, Fiorino M, Ameen SM. et al. Natural polyphenols: Chemical classification, definition of classes, subcategories, and structures. J AOAC Int. 2019;102(5):1397-400
17. Zagoskina N V, Zubova MY, Nechaeva TL, Kazantseva V V, Goncharuk EA, Katanskaya VM. et al. Polyphenols in plants: structure, biosynthesis, abiotic stress regulation, and practical applications. Int J Mol Sci. 2023;24(18):13874
18. Vieira IRS, Conte-Junior CA. Nano-delivery systems for food bioactive compounds in cancer: Prevention, therapy, and clinical applications. Crit Rev Food Sci Nutr. 2024;64(2):381-406
19. Vieira IRS, Tessaro L, Lima AKO, Velloso IPS, Conte-Junior CA. Recent progress in nanotechnology improving the therapeutic potential of polyphenols for cancer. Nutrients. 2023;15(14):3136
20. Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2:270-8
21. Makarewicz M, Drożdż I, Tarko T, Duda-Chodak A. The interactions between polyphenols and microorganisms, especially gut microbiota. Antioxidants. 2021;10(2):188
22. Duda-Chodak A, Tarko T. Possible Side Effects of Polyphenols and Their Interactions with Medicines. Molecules. 2023;28(6):2536
23. Rodríguez-García C, Sánchez-Quesada C, Gaforio JJ. Dietary flavonoids as cancer chemopreventive agents: An updated review of human studies. Antioxidants. 2019;8(5):137
24. Rees A, Dodd GF, Spencer JPE. The effects of flavonoids on cardiovascular health: A review of human intervention trials and implications for cerebrovascular function. Nutrients. 2018;10(12):1852
25. Koes R, Verweij W, Quattrocchio F. Flavonoids: a colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci. 2005;10(5):236-42
26. Ciumărnean L, Milaciu MV, Runcan O, Vesa Ștefan C, Răchișan AL, Negrean V. et al. The effects of flavonoids in cardiovascular diseases. Molecules. 2020;25(18):4320
27. Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci. 2016;5:e47
28. Ullah A, Munir S, Badshah SL, Khan N, Ghani L, Poulson BG. et al. Important flavonoids and their role as a therapeutic agent. Molecules. 2020;25(22):5243
29. D'Amelia V, Aversano R, Chiaiese P, Carputo D. The antioxidant properties of plant flavonoids: their exploitation by molecular plant breeding. Phytochem Rev. 2018;17:611-25
30. Dias MC, Pinto DCGA, Silva AMS. Plant flavonoids: Chemical characteristics and biological activity. Molecules. 2021;26(17):5377
31. Narayana KR, Reddy MS, Chaluvadi MR, Krishna DR. Bioflavonoids classification, pharmacological, biochemical effects and therapeutic potential. Indian J Pharmacol. 2001;33(1):2-16
32. Laoué J, Fernandez C, Ormeño E. Plant flavonoids in mediterranean species: A focus on flavonols as protective metabolites under climate stress. Plants. 2022;11(2):172
33. Sharma A, Sharma P, Tuli HS, Sharma AK. Phytochemical and pharmacological properties of flavonols. eLS. 2018;2018:1-12
34. Popiolek-Kalisz J, Fornal E. The Impact of Flavonols on Cardiovascular Risk. Nutrients. 2022;14(9):1973
35. Bhat IUH, Bhat R. Quercetin: a bioactive compound imparting cardiovascular and neuroprotective benefits: scope for exploring fresh produce, their wastes, and by-products. Biology (Basel). 2021;10(7):586
36. Xu D, Hu MJ, Wang YQ, Cui YL. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules. 2019;24(6):1123
37. Peterson JJ, Dwyer JT, Beecher GR, Bhagwat SA, Gebhardt SE, Haytowitz DB. et al. Flavanones in oranges, tangerines (mandarins), tangors, and tangelos: a compilation and review of the data from the analytical literature. J Food Compos Anal. 2006;19:S66-73
38. Testai L, Calderone V. Nutraceutical value of citrus flavanones and their implications in cardiovascular disease. Nutrients. 2017;9(5):502
39. Çetinkaya S, Akça KT, Süntar I. Flavonoids and anticancer activity: Structure-activity relationship. Stud Nat Prod Chem. 2022;74:81-115
40. Zhuang WB, Li YH, Shu XC, Pu YT, Wang XJ, Wang T. et al. The Classification, Molecular Structure and Biological Biosynthesis of Flavonoids, and Their Roles in Biotic and Abiotic Stresses. Molecules. 2023;28(8):3599
41. Ortiz A de C, Fideles SOM, Reis CHB, Bellini MZ, Pereira E de SBM, Pilon JPG. et al. Therapeutic effects of citrus flavonoids neohesperidin, hesperidin and its aglycone, hesperetin on bone health. Biomolecules. 2022;12(5):626
42. Bhoooshan Pandey K, Ibrahim Rizvi S. Plant polyphenols as dietary antioxidants in humanhealth and disease. Oxid Med Cell Longev. 2009;5(2):270-8
43. Panche AN, Diwan AD, Chandra SR. Flavonoids: An overview. J Nutr Sci. 2016 5
44. Leis K, Gałązka P, Kazik J, Jamrozek T, Bereznicka W, Czajkowski R. Resveratrol in the treatment of asthma based on an animal model. Postep Dermatologii i Alergol. 2022;39(3):433-8
45. Townsend EA, Emala CW. Quercetin acutely relaxes airway smooth muscle and potentiates β-agonist-induced relaxation via dual phosphodiesterase inhibition of PLCβ and PDE4. Am J Physiol - Lung Cell Mol Physiol. 2013;305(5):L396-403
46. Cory H, Passarelli S, Szeto J, Tamez M, Mattei J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front Nutr. 2018;5:87
47. Decramer M, Janssens W. Chronic obstructive pulmonary disease and comorbidities. Lancet Respir Med. 2013;1(1):73-83
48. Kazer MW, Murphy K. Nursing case studies on improving health-related quality of life in older adults. Springer Publishing Company. 2015
49. Lin CH, Cheng SL, Chen CZ, Chen CH, Lin SH, Wang HC. Current progress of COPD Early detection: key points and novel strategies. Int J Chron Obstruct Pulmon Dis. 2023;18:1511-24
50. Li J, Qiu C, Xu P, Lu Y, Chen R. Casticin improves respiratory dysfunction and attenuates oxidative stress and inflammation via inhibition of NF-ĸB in a chronic obstructive pulmonary disease model of chronic cigarette smoke-exposed rats. Drug Des Devel Ther. 2020;14:5019-27
51. Tuder RM, Petrache I. Pathogenesis of chronic obstructive pulmonary disease. J Clin Invest. 2012;122(8):2749-55
52. Roth-Walter F, Adcock IM, Benito-Villalvilla C, Bianchini R, Bjermer L, Caramori G. et al. Metabolic pathways in immune senescence and inflammaging: Novel therapeutic strategy for chronic inflammatory lung diseases. An EAACI position paper from the Task Force for Immunopharmacology. Allergy Eur J Allergy Clin Immunol. 2024;79:1089-1122
53. Han MK, Barreto TA, Martinez FJ, Comstock AT, Sajjan US. Randomised clinical trial to determine the safety of quercetin supplementation in patients with chronic obstructive pulmonary disease. BMJ Open Respir Res. 2020;7(1):e000392
54. Wang D, Li Y. Pharmacological effects of baicalin in lung diseases. Front Pharmacol. 2023;14:1188202
55. Lixuan Z, Jingcheng D, Wenqin Y, Jianhua H, Baojun L, Xiaotao F. Baicalin attenuates inflammation by inhibiting NF-κB activation in cigarette smoke induced inflammatory models. Pulm Pharmacol Ther. 2010;23(5):411-9
56. Li J, Tong D, Liu J, Chen F, Shen Y. Oroxylin A attenuates cigarette smoke-induced lung inflammation by activating Nrf2. Int Immunopharmacol. 2016;40:524-9
57. Lee S, Ro H, In HJ, Choi JH, Kim MO, Lee J. et al. Fisetin inhibits TNF-α/NF-κB-induced IL-8 expression by targeting PKCδ in human airway epithelial cells. Cytokine. 2018;108:247-54
58. Liu X, Bao H, Zeng X, Wei J. Effects of resveratrol and genistein on nuclear factor-κB, tumor necrosis factor-α and matrix metalloproteinase-9 in patients with chronic obstructive pulmonary disease. Mol Med Rep. 2016;13(5):4266-72
59. Hirano T, Higa S, Arimitsu J, Naka T, Shima Y, Ohshima S. et al. Flavonoids such as luteolin, fisetin and apigenin are inhibitors of interleukin-4 and interleukin-13 production by activated human basophils. Int Arch Allergy Immunol. 2004;134(2):135-40
60. Park J, Kim SH, Kim TS. Inhibition of interleukin-4 production in activated T cells via down-regulation of NF-AT DNA binding activity by apigenin, a flavonoid present in dietary plants. Immunol Lett. 2006;103(2):108-14
61. Das M, Ram A, Ghosh B. Luteolin alleviates bronchoconstriction and airway hyperreactivity in ovalbumin sensitized mice. Inflamm Res. 2003;52:101-6
62. Leemans J, Cambier C, Chandler T, Billen F, Clercx C, Kirschvink N. et al. Prophylactic effects of omega-3 polyunsaturated fatty acids and luteolin on airway hyperresponsiveness and inflammation in cats with experimentally-induced asthma. Vet J. 2010;184(1):111-4
63. Salachas EN, Laopodis NT, Kouretas GP. The bank-lending channel and monetary policy during pre-and post-2007 crisis. J Int Financ Mark Institutions Money. 2017;47:176-87
64. Li RR, Pang LL, Du Q, Shi Y, Dai WJ, Yin KS. Apigenin inhibits allergen-induced airway inflammation and switches immune response in a murine model of asthma. Immunopharmacol Immunotoxicol. 2010;32(3):364-70
65. Wu MY, Hung SK, Fu SL. Immunosuppressive effects of fisetin in ovalbumin-induced asthma through inhibition of NF-κB activity. J Agric Food Chem. 2011;59(19):10496-504
66. Park HH, Lee S, Oh JM, Lee MS, Yoon KH, Park BH. et al. Anti-inflammatory activity of fisetin in human mast cells (HMC-1). Pharmacol Res. 2007;55(1):31-7
67. Attoub S, Hassan AH, Vanhoecke B, Iratni R, Takahashi T, Gaben AM. et al. Inhibition of cell survival, invasion, tumor growth and histone deacetylase activity by the dietary flavonoid luteolin in human epithelioid cancer cells. Eur J Pharmacol. 2011;651(1-3):18-25
68. Thandra KC, Barsouk A, Saginala K, Aluru JS, Barsouk A. Epidemiology of lung cancer. Contemp Oncol Onkol. 2021;25(1):45-52
69. Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T, Alizadeh-Navaei R, Allen C. et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: a systematic analysis for the global burden of disease study. JAMA Oncol. 2018;4(11):1553-68
70. Smeltzer MP, Wynes MW, Lantuejoul S, Soo R, Ramalingam SS, Varella-Garcia M. et al. The International Association for the Study of Lung Cancer Global Survey on Molecular Testing in Lung Cancer. J Thorac Oncol. 2020;15(9):1434-48
71. Osann KE. Epidemiology of lung cancer. Curr Opin Pulm Med. 1998;4(4):198-204
72. Niedzwiecki A, Roomi MW, Kalinovsky T, Rath M. Anticancer efficacy of polyphenols and their combinations. Nutrients. 2016;8(9):552
73. Chimento A, De Luca A, D'Amico M, De Amicis F, Pezzi V. The Involvement of Natural Polyphenols in Molecular Mechanisms Inducing Apoptosis in Tumor Cells: A Promising Adjuvant in Cancer Therapy. Int J Mol Sci. 2023;24(2):1680
74. Amararathna M, Johnston MR, Rupasinghe HPV. Plant polyphenols as chemopreventive agents for lung cancer. Int J Mol Sci. 2016;17(8):1352
75. Ko J, Syu J, Chen J, Wang T, Chang P, Chen C. et al. Resveratrol Enhances Etoposide-Induced Cytotoxicity through Down-Regulating ERK 1/2 and AKT-Mediated X-ray Repair Cross-Complement Group 1 (XRCC 1) Protein Expression in Human Non-Small-Cell Lung Cancer Cells. Basic Clin Pharmacol Toxicol. 2015;117(6):383-91
76. Luo H, Yang A, Schulte BA, Wargovich MJ, Wang GY. Resveratrol induces premature senescence in lung cancer cells via ROS-mediated DNA damage. PLoS One. 2013;8(3):e60065
77. Lucas IK, Kolodziej H. Trans-resveratrol induces apoptosis through ROS-triggered mitochondria-dependent pathways in A549 human lung adenocarcinoma epithelial cells. Planta Med. 2015;81:1038-44
78. Shi Q, Geldenhuys W, Sutariya V, Bishayee A, Patel I, Bhatia D. CArG-driven GADD45α activated by resveratrol inhibits lung cancer cells. Genes Cancer. 2015;6(5-6):220
79. Wu F, Sun H, Kluz T, Clancy HA, Kiok K, Costa M. Epigallocatechin-3-gallate (EGCG) protects against chromate-induced toxicity in vitro. Toxicol Appl Pharmacol. 2012;258(2):166-75
80. Li YB, Zhong ZF, Chen MW, Bao JL, Wu GS, Zhang QW. et al. Bisdemethoxycurcumin increases Sirt1 to antagonize t-BHP-induced premature senescence in WI38 fibroblast cells. Evidence-Based Complement Altern Med. 2013;2013:851714
81. Wang C, Li X, Jin L, Zhao Y, Zhu G, Shen W. Dieckol inhibits non-small-cell lung cancer cell proliferation and migration by regulating the PI3K/AKT signaling pathway. J Biochem Mol Toxicol. 2019;33(8):e22346
82. Jo E, Park SJ, Choi YS, Jeon WK, Kim BC. Kaempferol suppresses transforming growth factor-β1-induced epithelial-to-mesenchymal transition and migration of A549 lung cancer cells by inhibiting Akt1-mediated phosphorylation of Smad3 at threonine-179. Neoplasia. 2015;17(7):525-37
83. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424
84. Natarajan A, Beena PM, Devnikar A V, Mali S. A systemic review on tuberculosis. Indian J Tuberc. 2020;67(3):295-311
85. Khalif Ali M, Karanja S, Karama M, Kenyatta J. Factors associated with tuberculosis treatment outcomes among tuberculosis patients attending tuberculosis treatment centres in 2016-2017 in Mogadishu, Somalia. Pan Afr Med J [Internet]. 2017;28(1):197 Available from: https://www.ajol.info/index.php/pamj/article/view/167299
86. Narayanan S, Ramesh K V. Epigallocatechin Gallate, a Green Tea Polyphenol Inhibits Mycobacterium smegmatis: In silico and In vitro Studies. Indian J Pharm Sci. 2017;79(4):625-632
87. Chan ED, Oberley-Deegan RE, McGibney M, Ovrutsky A, Bai X. Curcumin enhances macrophage killing of mycobacterium tuberculosis. In: B48 Tuberculosis and non-tuberculous mycobacterium: treatment outcome studies and case reports. American Thoracic Society. 2010: p. A3171-A3171.
88. Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2(8):611-20
89. Guan T, Zhou X, Zhou W, Lin H. Regulatory T cell and macrophage crosstalk in acute lung injury: future perspectives. Cell Death Discov. 2023;9(1):9
90. Ma M. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol. 2005;33:319-27
91. Aman J, van der Heijden M, van Lingen A, Girbes ARJ, van Nieuw Amerongen GP, van Hinsbergh VWM. et al. Plasma protein levels are markers of pulmonary vascular permeability and degree of lung injury in critically ill patients with or at risk for acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2011;39(1):89-97
92. Grotberg JC, Reynolds D, Kraft BD. Management of severe acute respiratory distress syndrome: a primer. Crit Care. 2023;27(1):289
93. Hu L, Chen Z, Li L, Jiang Z, Zhu L. Resveratrol decreases CD45+ CD206- subtype macrophages in LPS-induced murine acute lung injury by SOCS3 signalling pathway. J Cell Mol Med. 2019;23(12):8101-13
94. Chai Y sen, Chen Y qing, Lin S hui, Xie K, Wang C jiang, Yang Y zheng. et al. Curcumin regulates the differentiation of naïve CD4+ T cells and activates IL-10 immune modulation against acute lung injury in mice. Biomed Pharmacother. 2020;125:109946
95. Chen X, Yang X, Liu T, Guan M, Feng X, Dong W. et al. Kaempferol regulates MAPKs and NF-κB signaling pathways to attenuate LPS-induced acute lung injury in mice. Int Immunopharmacol. 2012;14(2):209-16
96. Soromou LW, Chu X, Jiang L, Wei M, Huo M, Chen N. et al. In vitro and in vivo protection provided by pinocembrin against lipopolysaccharide-induced inflammatory responses. Int Immunopharmacol. 2012;14(1):66-74
97. Chen Y, Nie Y chu, Luo Y long, Lin F, Zheng Y fang, Cheng G hua. et al. Protective effects of naringin against paraquat-induced acute lung injury and pulmonary fibrosis in mice. Food Chem Toxicol. 2013;58:133-40
98. Peng J, Wei D, Fu Z, Li D, Tan Y, Xu T. et al. Punicalagin ameliorates lipopolysaccharide-induced acute respiratory distress syndrome in mice. Inflammation. 2015;38:493-9
99. Iwamura C, Shinoda K, Yoshimura M, Watanabe Y, Obata A, Nakayama T. Naringenin chalcone suppresses allergic asthma by inhibiting the type-2 function of CD4 T cells. Allergol Int. 2010;59(1):67-73
100. Lee M, Kim S, Kwon OK, Oh SR, Lee HK, Ahn K. Anti-inflammatory and anti-asthmatic effects of resveratrol, a polyphenolic stilbene, in a mouse model of allergic asthma. Int Immunopharmacol. 2009;9(4):418-24
101. Rogerio AP, Kanashiro A, Fontanari C, Da Silva EVG, Lucisano-Valim YM, Soares EG. et al. Anti-inflammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma. Inflamm Res. 2007;56:402-8
102. Gong JH, Shin D, Han SY, Kim JL, Kang YH. Kaempferol suppresses eosionphil infiltration and airway inflammation in airway epithelial cells and in mice with allergic asthma. J Nutr. 2012;142(1):47-56
103. Palamara AT, Nencioni L, Aquilano K, De Chiara G, Hernandez L, Cozzolino F. et al. Inhibition of influenza A virus replication by resveratrol. J Infect Dis. 2005;191(10):1719-29
104. Kim Y, Narayanan S, Chang KO. Inhibition of influenza virus replication by plant-derived isoquercetin. Antiviral Res. 2010;88(2):227-35
105. Nayak MK, Agrawal AS, Bose S, Naskar S, Bhowmick R, Chakrabarti S. et al. Antiviral activity of baicalin against influenza virus H1N1-pdm09 is due to modulation of NS1-mediated cellular innate immune responses. J Antimicrob Chemother. 2014;69(5):1298-310
106. Chen DY, Shien JH, Tiley L, Chiou SS, Wang SY, Chang TJ. et al. Curcumin inhibits influenza virus infection and haemagglutination activity. Food Chem. 2010;119(4):1346-51
107. Su X, Liu K, Xie Y, Zhang M, Wang Y, Zhao M. et al. Protective effect of a polyphenols-rich extract from Inonotus Sanghuang on bleomycin-induced acute lung injury in mice. Life Sci. 2019;230:208-17
108. Anand PK, Kaul D, Sharma M. Green tea polyphenol inhibits Mycobacterium tuberculosis survival within human macrophages. Int J Biochem Cell Biol. 2006;38(4):600-9
109. Wali AF, Pillai JR, Al Dhaheri Y, Rehman MU, Shoaib A, Sarheed O. et al. Crocus sativus L. extract containing polyphenols modulates oxidative stress and inflammatory response against anti-tuberculosis drugs-induced liver injury. Plants. 2020;9(2):167
110. Wang H, Yang T, Wang T, Hao N, Shen Y, Wu Y. et al. Phloretin attenuates mucus hypersecretion and airway inflammation induced by cigarette smoke. Int Immunopharmacol. 2018;55:112-9
111. Wang S, He N, Xing H, Sun Y, Ding J, Liu L. Function of hesperidin alleviating inflammation and oxidative stress responses in COPD mice might be related to SIRT1/PGC-1α/NF-κB signaling axis. J Recept Signal Transduct. 2020;40(4):388-94
112. Li D, Hu J, Wang T, Zhang X, Liu L, Wang H. et al. Silymarin attenuates cigarette smoke extract-induced inflammation via simultaneous inhibition of autophagy and ERK/p38 MAPK pathway in human bronchial epithelial cells. Sci Rep. 2016;6(1):37751
113. Zhang M, Xie Y, Yan R, Shan H, Tang J, Cai Y. et al. Curcumin ameliorates alveolar epithelial injury in a rat model of chronic obstructive pulmonary disease. Life Sci. 2016;164:1-8
114. Liu J, Yao J, Zhang J. Naringenin attenuates inflammation in chronic obstructive pulmonary disease in cigarette smoke induced mouse model and involves suppression of NF-κB. J Microbiol Biotechnol. 2018
115. Yi L, Li Z, Yuan K, Qu X, Chen J, Wang G. et al. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J Virol. 2004;78(20):11334-9
116. Ho TY, Wu SL, Chen JC, Li CC, Hsiang CY. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antiviral Res. 2007;74(2):92-101
117. Ko JA, Kim YM, Ryu YB, Jeong HJ, Park TS, Park SJ. et al. Mass production of rubusoside using a novel stevioside-specific β-glucosidase from Aspergillus aculeatus. J Agric Food Chem. 2012;60(24):6210-6
118. Li H, Wu J, Zhang Z, Ma Y, Liao F, Zhang Y. et al. Forsythoside a inhibits the avian infectious bronchitis virus in cell culture. Phyther Res. 2011;25(3):338-42
119. Lin SC, Ho CT, Chuo WH, Li S, Wang TT, Lin CC. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect Dis. 2017;17(1):1-10
120. Impellizzeri D, Talero E, Siracusa R, Alcaide A, Cordaro M, Zubelia JM. et al. Protective effect of polyphenols in an inflammatory process associated with experimental pulmonary fibrosis in mice. Br J Nutr. 2015;114(6):853-65
121. Liu L, Yu N, Leng W, Lu Y, Xia X, Yuan H. 6-Gingerol, a functional polyphenol of ginger, reduces pulmonary fibrosis by activating Sirtuin1. Allergol Immunopathol (Madr). 2022;50(2):104-14
122. Paffett ML, Lucas SN, Campen MJ. Resveratrol reverses monocrotaline-induced pulmonary vascular and cardiac dysfunction: a potential role for atrogin-1 in smooth muscle. Vascul Pharmacol. 2012;56(1-2):64-73
123. Liu B, Luo XJ, Yang ZB, Zhang JJ, Li TB, Zhang XJ. et al. Inhibition of NOX/VPO1 pathway and inflammatory reaction by trimethoxystilbene in prevention of cardiovascular remodeling in hypoxia-induced pulmonary hypertensive rats. J Cardiovasc Pharmacol. 2014;63(6):567-76
124. Kamaraj S, Anandakumar P, Jagan S, Ramakrishnan G, Devaki T. Modulatory effect of hesperidin on benzo(a)pyrene induced experimental lung carcinogenesis with reference to COX-2, MMP-2 and MMP-9. Eur J Pharmacol. 2010;649(1-3):320-7
125. Naveenkumar C, Raghunandakumar S, Asokkumar S, Binuclara J, Rajan B, Premkumar T. et al. Mitigating role of baicalein on lysosomal enzymes and xenobiotic metabolizing enzyme status during lung carcinogenesis of S wiss albino mice induced by benzo (a) pyrene. Fundam Clin Pharmacol. 2014;28(3):310-22
126. Zhou H, Chen JX, Yang CS, Yang MQ, Deng Y, Wang H. Gene regulation mediated by microRNAs in response to green tea polyphenol EGCG in mouse lung cancer. BMC Genomics. 2014;15:1-10
127. Izumi H, Takahashi M, Uramoto H, Nakayama Y, Oyama T, Wang K. et al. Monocarboxylate transporters 1 and 4 are involved in the invasion activity of human lung cancer cells. Cancer Sci. 2011;102(5):1007-13
128. Ho ML, Chen PN, Chu SC, Kuo DY, Kuo WH, Chen JY. et al. Peonidin 3-glucoside inhibits lung cancer metastasis by downregulation of proteinases activities and MAPK pathway. Nutr Cancer. 2010;62(4):505-16
129. Kausar H, Jeyabalan J, Aqil F, Chabba D, Sidana J, Singh IP. et al. Berry anthocyanidins synergistically suppress growth and invasive potential of human non-small-cell lung cancer cells. Cancer Lett. 2012;325(1):54-62
130. Yong WK, Ho YF, Abd Malek SN. Xanthohumol induces apoptosis and S phase cell cycle arrest in A549 non-small cell lung cancer cells. Pharmacogn Mag. 2015;11(Suppl 2):S275
131. Kin R, Kato S, Kaneto N, Sakurai H, Hayakawa Y, Li F. et al. Procyanidin C1 from Cinnamomi Cortex inhibits TGF-β-induced epithelial-to-mesenchymal transition in the A549 lung cancer cell line. Int J Oncol. 2013;43(6):1901-6
132. Jin C, Park C, Hwang HJ, Kim G, Choi BT, Kim W. et al. Naringenin up-regulates the expression of death receptor 5 and enhances TRAIL-induced apoptosis in human lung cancer A549 cells. Mol Nutr Food Res. 2011;55(2):300-9
133. Lu HF, Chie YJ, Yang MS, Lu KW, Fu JJ, Yang JS. et al. Apigenin induces apoptosis in human lung cancer H460 cells through caspase-and mitochondria-dependent pathways. Hum Exp Toxicol. 2011;30(8):1053-61
134. Shao J jie, Zhang A ping, Qin W, Zheng L, Zhu Y fan, Chen X. AMP-activated protein kinase (AMPK) activation is involved in chrysin-induced growth inhibition and apoptosis in cultured A549 lung cancer cells. Biochem Biophys Res Commun. 2012;423(3):448-53
135. Hong Z, Cao X, Li N, Zhang Y, Lan L, Zhou Y. et al. Luteolin is effective in the non-small cell lung cancer model with L 858 R/T 790 M EGF receptor mutation and erlotinib resistance. Br J Pharmacol. 2014;171(11):2842-53
136. Zheng SY, Li Y, Jiang D, Zhao J, Ge JF. Anticancer effect and apoptosis induction by quercetin in the human lung cancer cell line A-549. Mol Med Rep. 2012;5(3):822-6
137. Tian T, Li J, Li B, Wang Y, Li M, Ma D. et al. Genistein exhibits anti-cancer effects via down-regulating FoxM1 in H446 small-cell lung cancer cells. Tumor Biol. 2014;35:4137-45
138. Yang CL, Ma YG, Xue YX, Liu YY, Xie H, Qiu GR. Curcumin induces small cell lung cancer NCI-H446 cell apoptosis via the reactive oxygen species-mediated mitochondrial pathway and not the cell death receptor pathway. DNA Cell Biol. 2012;31(2):139-50
139. Xingyu Z, Peijie M, Dan P, Youg W, Daojun W, Xinzheng C. et al. Quercetin suppresses lung cancer growth by targeting Aurora B kinase. Cancer Med. 2016;5(11):3156-65
140. Khan N, Afaq F, Khusro FH, Mustafa Adhami V, Suh Y, Mukhtar H. Dual inhibition of phosphatidylinositol 3-kinase/Akt and mammalian target of rapamycin signaling in human nonsmall cell lung cancer cells by a dietary flavonoid fisetin. Int J cancer. 2012;130(7):1695-705
141. Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev. 1998;56(11):317-33
142. Hounsome N, Hounsome B, Tomos D, Edwards-Jones G. Plant metabolites and nutritional quality of vegetables. J Food Sci. 2008;73(4):R48-65
143. Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79(5):727-47
144. Scalbert A, Johnson IT, Saltmarsh M. Polyphenols: antioxidants and beyond. Am J Clin Nutr. 2005;81(1):215S-217S
145. Bešlo D, Golubić N, Rastija V, Agić D, Karnaš M, Šubarić D. et al. Antioxidant Activity, Metabolism, and Bioavailability of Polyphenols in the Diet of Animals. Antioxidants. 2023;12(6):1141
146. Miller M, Viboud C, Simonsen L, Olson DR, Russell C. Mortality and morbidity burden associated with A/H1N1pdm influenza virus: Who is likely to be infected, experience clinical symptoms, or die from the H1N1pdm 2009 pandemic virus? PLoS Curr. 2009;1:RRN1013
147. Bahramsoltani R, Sodagari HR, Farzaei MH, Abdolghaffari AH, Gooshe M, Rezaei N. The preventive and therapeutic potential of natural polyphenols on influenza. Expert Rev Anti Infect Ther. 2016;14(1):57-80
148. Kalus U, Grigorov A, Kadecki O, Jansen JP, Kiesewetter H, Radtke H. Cistus incanus (CYSTUS052) for treating patients with infection of the upper respiratory tract: a prospective, randomised, placebo-controlled clinical study. Antiviral Res. 2009;84(3):267-71
149. Matsumoto K, Yamada H, Takuma N, Niino H, Sagesaka YM. Effects of green tea catechins and theanine on preventing influenza infection among healthcare workers: a randomized controlled trial. BMC Complement Altern Med. 2011;11(1):1-7
150. Yamada H, Takuma N, Daimon T, Hara Y. Gargling with tea catechin extracts for the prevention of influenza infection in elderly nursing home residents: a prospective clinical study. J Altern Complement Med. 2006;12(7):669-72
151. Barnes PJ, Karin M. Nuclear factor-κB—a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336(15):1066-71
152. Park EJ, Pezzuto JM. The pharmacology of resveratrol in animals and humans. Biochim Biophys Acta (BBA)-Molecular Basis Dis. 2015;1852(6):1071-113
153. Barnes PJ. Cellular and molecular mechanisms of chronic obstructive pulmonary disease. Clin Chest Med. 2014;35(1):71-86
154. Tung BT, Rodríguez-Bies E, Talero E, Gamero-Estévez E, Motilva V, Navas P. et al. Anti-inflammatory effect of resveratrol in old mice liver. Exp Gerontol. 2015;64:1-7
155. Nishio K, Nakamura T, Koh Y, Suzuki T, Fukumoto H, Saijo N. Drug resistance in lung cancer. Curr Opin Oncol. 1999;11(2):109
156. Lahiri A, Maji A, Potdar PD, Singh N, Parikh P, Bisht B. et al. Lung cancer immunotherapy: progress, pitfalls, and promises. Mol Cancer. 2023;22(1):1-37
157. Wang G, Reed E, Li QQ. Molecular basis of cellular response to cisplatin chemotherapy in non-small cell lung cancer. Oncol Rep. 2004;12(5):955-65
158. Mokra D, Joskova M, Mokry J. Therapeutic Effects of Green Tea Polyphenol (-)-Epigallocatechin-3-Gallate (EGCG) in Relation to Molecular Pathways Controlling Inflammation, Oxidative Stress, and Apoptosis. Int J Mol Sci. 2023;24(1):340
159. Imran A, Arshad MU, Sherwani H, Shabir Ahmad R, Arshad MS, Saeed F. et al. Antioxidant capacity and characteristics of theaflavin catechins and ginger freeze-dried extract as affected by extraction techniques. Int J Food Prop. 2021;24(1):1097-116
160. Mao QQ, Xu XY, Cao SY, Gan RY, Corke H, Beta T. et al. Bioactive compounds and bioactivities of ginger (zingiber officinale roscoe). Foods. 2019;8(6):185
161. Adhami VM, Ahmad N, Mukhtar H. Molecular targets for green tea in prostate cancer prevention. J Nutr. 2003;133(7):2417S-2424S
162. Sindhoor SM, Naveen NR, Rao GSNK, Gopan G, Chopra H, Park MN. et al. A spotlight on alkaloid nanoformulations for the treatment of lung cancer. Front. Oncol. 2022;12:994155
163. Li X, Xing L, Zhang Y, Xie P, Zhu W, Meng X. et al. Phase II Trial of Epigallocatechin-3-Gallate in Acute Radiation-Induced Esophagitis for Esophagus Cancer. J Med Food. 2020;23(1):43-9
164. Zhao H, Xie P, Li X, Zhu W, Sun X, Sun X. et al. A prospective phase II trial of EGCG in treatment of acute radiation-induced esophagitis for stage III lung cancer. Radiother Oncol. 2015;114(3):351-6
165. Cruz AA. Global surveillance, prevention and control of chronic respiratory diseases: a comprehensive approach. World Health Organization. 2007
166. Cha J, Choi S. Gene-Smoking Interaction Analysis for the Identification of Novel Asthma-Associated Genetic Factors. Int J Mol Sci. 2023;24(15):12266
167. Kawai M, Hirano T, Higa S, Arimitsu J, Maruta M, Kuwahara Y. et al. Flavonoids and related compounds as anti-allergic substances. Allergol Int. 2007;56(2):113-23
168. Tanaka T, Higa S, Hirano T, Kotani M, Matsumoto M, Fujita A. et al. Flavonoids as potential anti-allergic substances. Curr Med Chem Anti-Allergy Agents. 2003;2(1):57-65
169. Tanaka T. Flavonoids, natural inhibitors of basophil activation. Basophil Granulocytes. 2011:61-72
170. Tanaka T, Takahashi R. Flavonoids and asthma. Nutrients. 2013;5(6):2128-43
171. Takano H, Osakabe N, Sanbongi C, Yanagisawa R, Inoue K ichiro, Yasuda A. et al. Extract of Perilla frutescens enriched for rosmarinic acid, a polyphenolic phytochemical, inhibits seasonal allergic rhinoconjunctivitis in humans. Exp Biol Med. 2004;229(3):247-54
172. Kishi K, Saito M, Saito T, Kumemura M, Okamatsu H, Okita M. et al. Clinical efficacy of apple polyphenol for treating cedar pollinosis. Biosci Biotechnol Biochem. 2005;69(4):829-32
173. Enomoto T, Nagasako-Akazome Y, Kanda T, Ikeda M, Dake Y. Clinical effects of apple polyphenols on persistent allergic rhinitis: a randomized double-blind placebo-controlled parallel arm study. J Investig Allergol Clin Immunol. 2006;16(5):283
174. Segawa S, Takata Y, Wakita Y, Kaneko T, Kaneda H, Watari J. et al. Clinical effects of a hop water extract on Japanese cedar pollinosis during the pollen season: a double-blind, placebo-controlled trial. Biosci Biotechnol Biochem. 2007;71(8):1955-62
175. Hosseini S, Pishnamazi S, Sadrzadeh SMH, Farid F, Farid R, Watson RR. Pycnogenol® in the management of asthma. J Med Food. 2001;4(4):201-9
176. Belcaro G, Luzzi R, Cesinaro Di Rocco P, Cesarone MR, Dugall M, Feragalli B. et al. Pycnogenol® improvements in asthma management. Panminerva Med. 2011;53(3 Suppl 1):57-64
177. Lau BHS, Riesen SK, Truong KP, Lau EW, Rohdewald P, Barreta RA. Pycnogenol® as an adjunct in the management of childhood asthma. J Asthma. 2004;41(8):825-32
178. Power S, Williams M, Semprini A, Munro C, Caswell-Smith R, Pilcher J. et al. RCT of the effect of berryfruit polyphenolic cultivar extract in mild steroid-naive asthma: A cross-over, placebo-controlled study. BMJ Open. 2017 7(3)
179. Nantz MP, Rowe CA, Muller C, Creasy R, Colee J, Khoo C. et al. Consumption of cranberry polyphenols enhances human γδ-T cell proliferation and reduces the number of symptoms associated with colds and influenza: a randomized, placebo-controlled intervention study. Nutr J. 2013;12(1):1-9
180. Schütz K, Saß M, Graubaum HJ, Grünwald J. Immune-modulating efficacy of a polyphenol-rich beverage on symptoms associated with the common cold: a double-blind, randomised, placebo-controlled, multi-centric clinical study. Br J Nutr. 2010;104(8):1156-64
181. You C, Ran G, Wu X, Wang Y, Tian H, Fan J. et al. High immunoglobulin E level is associated with increased readmission in children with bronchopneumonia. Ther Adv Respir Dis. 2019;13:1753466619879832
182. Peto L, Nadjm B, Horby P, Ngan TTD, van Doorn R, Kinh N Van. et al. The bacterial aetiology of adult community-acquired pneumonia in Asia: a systematic review. Trans R Soc Trop Med Hyg. 2014;108(6):326-37
183. Woodhead M. Community-acquired pneumonia in Europe: causative pathogens and resistance patterns. Eur Respir J. 2002;20(36 suppl):20s-27s
184. Martinez RM, Pinho-Ribeiro FA, Steffen VS, Caviglione C V, Vignoli JA, Barbosa DS. et al. Naringenin inhibits UVB irradiation-induced inflammation and oxidative stress in the skin of hairless mice. J Nat Prod. 2015;78(7):1647-55
185. Lu W, Yu CR, Lien H, Sheu G, Cherng S. Cytotoxicity of naringenin induces Bax-mediated mitochondrial apoptosis in human lung adenocarcinoma A549 cells. Environ Toxicol. 2020;35(12):1386-94
186. Lather A, Sharma S, Khatkar A. Naringenin derivatives as glucosamine-6-phosphate synthase inhibitors: synthesis, antioxidants, antimicrobial, preservative efficacy, molecular docking and in silico ADMET analysis. BMC Chem. 2020;14:1-15
187. Tutunchi H, Naeini F, Ostadrahimi A, Hosseinzadeh-Attar MJ. Naringenin, a flavanone with antiviral and anti-inflammatory effects: A promising treatment strategy against COVID-19. Phyther Res. 2020;34(12):3137-47
188. Xue N, Wu X, Wu L, Li L, Wang F. Antinociceptive and anti-inflammatory effect of Naringenin in different nociceptive and inflammatory mice models. Life Sci. 2019;217:148-54
189. Yao W, Zhang X, Xu F, Cao C, Liu T, Xue Y. The therapeutic effects of naringenin on bronchial pneumonia in children. Pharmacol Res Perspect. 2021;9(4):e00825
190. Scheepens A, Tan K, Paxton JW. Improving the oral bioavailability of beneficial polyphenols through designed synergies. Genes Nutr. 2010;5:75-87
191. Alonso-Salces RM, Ndjoko K, Queiroz EF, Ioset JR, Hostettmann K, Berrueta LA. et al. On-line characterisation of apple polyphenols by liquid chromatography coupled with mass spectrometry and ultraviolet absorbance detection. J Chromatogr A. 2004;1046(1-2):89-100
192. Biasutto L, Marotta E, Garbisa S, Zoratti M, Paradisi C. Determination of quercetin and resveratrol in whole blood—implications for bioavailability studies. Molecules. 2010;15(9):6570-9
193. Çelik SE, Özyürek M, Güçlü K, Apak R. Determination of antioxidants by a novel on-line HPLC-cupric reducing antioxidant capacity (CUPRAC) assay with post-column detection. Anal Chim Acta. 2010;674(1):79-88
194. Medina-Remón A, Barrionuevo-González A, Zamora-Ros R, Andres-Lacueva C, Estruch R, Martínez-González MÁ. et al. Rapid Folin-Ciocalteu method using microtiter 96-well plate cartridges for solid phase extraction to assess urinary total phenolic compounds, as a biomarker of total polyphenols intake. Anal Chim Acta. 2009;634(1):54-60
195. Álvarez P, Alvarado C, Mathieu F, Jiménez L, De la Fuente M. Diet supplementation for 5 weeks with polyphenol-rich cereals improves several functions and the redox state of mouse leucocytes. Eur J Nutr. 2006;45:428-38
196. Aviram M, Rosenblat M, Gaitini D, Nitecki S, Hoffman A, Dornfeld L. et al. Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin Nutr. 2004;23(3):423-33
197. Akazome Y, Kametani N, Kanda T, Shimasaki H, Kobayashi S. Evaluation of safety of excessive intake and efficacy of long-term intake of beverages containing apple polyphenols. J Oleo Sci. 2010;59(6):321-38
198. Mennen LI, Walker R, Bennetau-Pelissero C, Scalbert A. Risks and safety of polyphenol consumption. Am J Clin Nutr. 2005;81(1):326S-329S
199. Alavinezhad A, Khazdair MR, Boskabady MH. Possible therapeutic effect of carvacrol on asthmatic patients: A randomized, double blind, placebo-controlled, Phase II clinical trial. Phyther Res. 2018;32(1):151-9
200. Cerdá B, Soto C, Albaladejo MD, Martinez P, Sanchez-Gascon F, Tomás-Barberán F. et al. Pomegranate juice supplementation in chronic obstructive pulmonary disease: a 5-week randomized, double-blind, placebo-controlled trial. Eur J Clin Nutr. 2006;60(2):245-53
Author contact
![]() Corresponding authors: talha_bin_emranedu (TBE); mahrukh.zehravicom (MZ); bongleekimac.kr (BK).
Corresponding authors: talha_bin_emranedu (TBE); mahrukh.zehravicom (MZ); bongleekimac.kr (BK).

 Global reach, higher impact
Global reach, higher impact