10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2024; 20(10):3773-3783. doi:10.7150/ijbs.96828 This issue Cite
Review
Ferroptosis: the balance between death and survival in colorectal cancer
1. Clinical Medical College, Yangzhou University, Yangzhou, 225000, P. R. China.
2. School of Medicine, Chongqing University, Chongqing, 400030, P. R. China.
3. Department of General Surgery, Institute of General Surgery, Northern Jiangsu People's Hospital Affiliated to Yangzhou University, Yangzhou, 225000, P. R. China.
#These authors contributed equally to this work.
Received 2024-3-31; Accepted 2024-6-20; Published 2024-7-2
Abstract
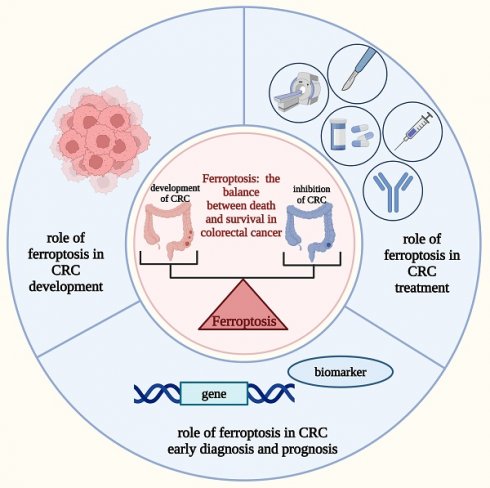
Colorectal cancer (CRC) is a common malignant tumor associated with high morbidity and mortality. Despite an increase in early screening and treatment options, people with CRC still have a poor prognosis and a low 5-year survival rate. Therefore, mining more therapeutic targets and developing means of early diagnosis and determining prognosis are now imperative in the clinical treatment of CRC. Ferroptosis is a recently identified type of regulated cell death (RCD) characterized, which is identified by the accumulation of iron-dependent lipid peroxidation, thereby causing membrane damage and cell death. Recent studies have shown that ferroptosis is associated with tumors, including CRC, and can be involved in CRC progression; however, the underlying mechanisms are complex and heterogeneous and have not been thoroughly summarized. Therefore, this study reviewed the roles of ferroptosis in CRC progression to target ferroptosis-related factors for CRC treatment. The significance of ferroptosis-related biomarkers and genes in the early diagnosis and prognosis of CRC was also investigated. Furthermore, the limitations of ferroptosis studies in the current treatment of CRC, as well as future research perspectives, are discussed.
Keywords: ferroptosis, colorectal cancer, lipid peroxidation, anticancer treatment
Introduction
Colorectal cancer (CRC), a malignant tumor of the digestive system, is marked by the uncontrolled proliferation and survival of aberrant cells in the colon or rectum. As the disease progresses to an advanced stage, the tumor foci can migrate into other normal tissues or sites, which is associated with high morbidity and mortality [1, 2]. In 2020, CRC accounted for 10% of new cancer cases worldwide [3]. The 5-year relative survival of patients with CRC in America is 91% for localized disease and 14% for distant disease [4]. Currently, the primary treatments for CRC are surgical resection, radiotherapy, chemotherapy, immunotherapy, and targeted therapy [5]. However, the therapeutic targets for CRC are limited owing to the many unidentified intermediate molecules involved in CRC pathogenesis, which hinders the clinical effectiveness of treatments [6]. Therefore, exploring the key molecules involved in CRC progression as potential therapeutic targets is crucial to increase the survival rate. In addition, early diagnosis can significantly increase the survival rate of patients with CRC [7]. However, owing to the complex biological characteristics of CRC and a shortage of highly sensitive and specific biomarkers, early screening for CRC still relies on invasive examinations such as endoscopy [7]. Consequently, identifying new biomarkers is critical for developing novel approaches to the early, non-invasive diagnosis and prognosis of CRC.
Apoptosis can be induced in CRC cells by elevating reactive oxygen species (ROS) levels, lowering antioxidant glutathione (GSH) levels, or deactivating glutathione peroxidase 4 (GPX4), all of which are also central to or associated with ferroptosis [8-10]. The concept of ferroptosis was first proposed by Dixon in 2012, and its essence is the impaired metabolism of intracellular lipid oxides, which in turn is abnormally metabolized under the catalysis of iron ions, generating large amounts of lipids, disrupting intracellular redox homeostasis, attacking biomolecules, and triggering cell death, which is a type of iron-ion-dependent non-apoptotic cell necrosis [11, 12]. Activating ferroptosis aids in CRC treatment, whereas inhibiting ferroptosis may induce CRC development or the emergence of drug resistance [9, 13]. As a result, manipulating ferroptosis could be useful in CRC treatment. Recognizing the significance of early diagnosis and accurate prognosis in improving the efficacy of CRC treatment, scientists have identified ferroptosis-related proteins and genes as potential biomarkers for the diagnosis and prognosis of CRC [14, 15]. This review focused on the role and mechanism of ferroptosis in CRC development. Additionally, the significance of targeting ferroptosis core regulators in CRC treatment and the role of ferroptosis-related molecules in early diagnosis and prognosis monitoring were discussed.
Overview of ferroptosis
Core concepts and the three elements of ferroptosis
In the presence of iron, ROS in cells converts polyunsaturated fatty acids (PUFAs) on oxidized lipid membranes into lipid peroxides, causing membrane damage and cell death [16]. This process is termed ferroptosis, a newly discovered regulated cell death (RCD) [16]. RCD is a death mode that occurs in a physiological state or upon failing to adapt to stress, and is under active and orderly control by the cells [17]. Currently, the identified RCD modes include autophagy, apoptosis, necroptosis, pyroptosis, and ferroptosis [18]. Various morphological, biochemical, immunological, and genetic characteristics set it apart from other types of RCD [19].
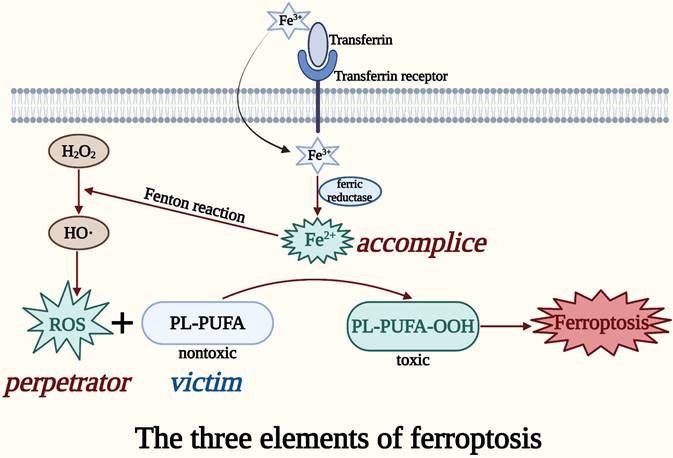
The primary cause of ferroptosis-mediated cell death is the imbalance between intracellular lipid ROS generation and degradation [16]. The reduced antioxidant capability of cells causes excess iron to trigger ferroptosis by producing deadly ROS through the Fenton reaction [20]. ROS can carry extremely unstable energy and are prone to uncontrolled energy loss leading to cell death, and this is the root cause of the harmful effects of ROS on organisms [21]. Iron ions (Fe2+/Fe3+), which are major inducers of ferroptosis, can contribute to the formation of ROS through enzymatic or non-enzymatic reactions [22]. Iron from plasma typically enters cells as Fe3+ through transferrin and its receptor, where it is reduced to Fe2+ by ferric reductase [23]. Fe2+ catalyzes the Fenton reaction, an important cause of rapid and dramatic ferroptosis, to break the peroxide bond from H2O2 and produces the highly oxidized hydroxyl radical, which is the most potent oxidant in ROS [24, 25]. Subsequently, ROS or lipoxygenase (LOX) oxidizes the nontoxic phospholipids containing PUFAs (PL-PUFA) to the toxic peroxidized lipid, PL-PUFA-OOH [26]. PUFAs, the most easily peroxidized lipids among the cell membrane components, incorporated into the membrane to form the PUFA-containing phospholipid, PL-PUFA [26]. PL-PUFA-OOH converted from PL-PUFA is the lipid peroxide in ferroptosis [27]. Eventually, the accumulation of PL-PUFA-OOH damages the cell membrane, leading to ferroptosis [27]. This is the basic process of ferroptosis.
Taken together, the perpetrator ROS, the accomplice iron, and the victim PL-PUFA might be considered the three elements of ferroptosis (Figure 1). The accumulation of the three elements of ferroptosis in an organism can be used as susceptibility factors for the occurrence of ferroptosis, which is important for the treatment and diagnosis of CRC [24].
Primary defense pathways in ferroptosis
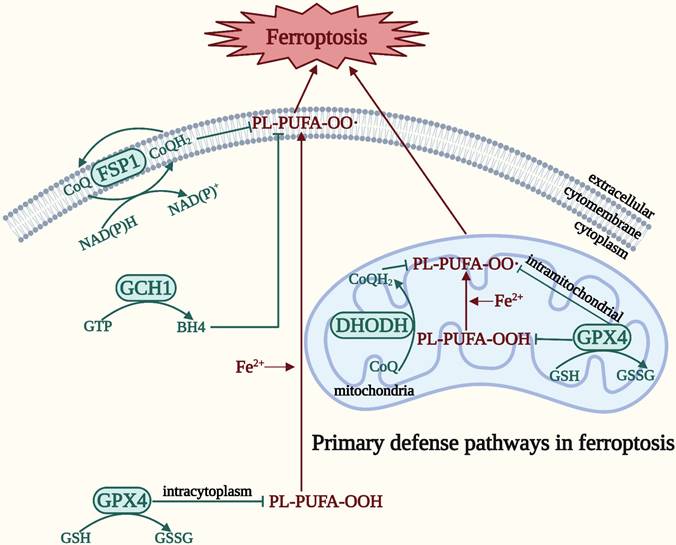
In order to survive against ferroptosis, the body has developed a defense system. There are four main defense pathways in ferroptosis: the GPX4-GSH, ferroptosis suppressor protein 1 (FSP1)-coenzyme Q (CoQ), dihydroorotate dehydrogenase (DHODH)-CoQ, and guanosine 5′-triphosphate cyclohydrolase-1 (GCH1)-tetrahydrobiopterin (BH4) signaling pathways [10] (Figure 2). In contrast to the relatively static, slow process in ferroptosis represented by the three elements, the defense pathway is a relatively dynamic expression of the process of ferroptosis, and is the remedy that prevents the cell from moving toward eventual death after ferroptosis has occurred [10]. GPX4 is a selenoprotein that can break down both relatively complex lipid peroxides and small molecule peroxides, and can also convert cytotoxic lipid hydroperoxides into nontoxic lipid alcohols, preventing the generation and accumulation of deadly ROS in order to protect the integrity of the membrane [28, 29]. GPX4 employs GSH, a tripeptide antioxidant comprising glutamate, cysteine, and glycine, as a cofactor to degrade hydroperoxide [28, 30]. An indirect method of inactivating GPX4 is GSH depletion, which further lowers cellular antioxidant capability and enhances the buildup of lipid ROS and the consequent ferroptosis [30]. Thus, ferroptosis can be induced by impeding GSH synthesis and absorption or hastening its breakdown. In addition, CoQ10 is an endogenous antioxidant that protects cells from ferroptosis by blocking the propagation of lipid peroxides [31]. FSP1 has been classified as a new GSH-independent ferroptosis suppressor that catalyzes the regeneration of CoQ10, thereby inhibiting lipid peroxidation [32]. Therefore, the FSP1-CoQ signaling pathway exists as an independent parallel system that synergistically inhibits phospholipid peroxidation and ferroptosis with the GPX4-GSH signaling pathway [32]. The DHODH-CoQ signaling pathway blocks mitochondrial lipid peroxidation and thus ferroptosis [33]. DHODH, which is located on the outer surface of the inner mitochondrial membrane and operates in parallel with mitochondrial GPX4 (but independently of cytoplasmic GPX4 or FSP1), inhibits ferroptosis in the inner mitochondrial membrane by reducing CoQ to panthenol, a free-radical trapping antioxidant with anti-ferroptosis activity [33]. The GCH1-BH4 signaling pathway is the primary GPX4 non-dependent ferroptosis regulatory system [34]. BH4 biosynthesis requires GCH1 catalysis, thus inducing lipid remodeling and inhibits ferroptosis by selectively preventing depletion of phospholipids with two polyunsaturated fatty acyl tails [34, 35]. The degree of cellular resistance to ferroptosis is substantially determined by the expression level of GCH1. Reduced BH4 resulting from genetic or pharmacological suppression of GCH1 can increase lipid peroxidation and ferroptosis [34]. Conversely, overexpression of GCH1 selectively increases BH4 biosynthesis and reduces ROS production [36]. Moreover, BH4 can convert phenylalanine to tyrosine, which can subsequently be converted into 4-OH-benzoate, a precursor of CoQ10, facilitating the production of CoQ10 to inhibit ferroptosis [35]. Accordingly, these processes coordinate and precisely control ferroptosis by linking the GCH1-BH4 signaling pathway to the FSP1-CoQ signaling pathway.
Taken together, these four ferroptosis defense pathways are interconnected yet regulate ferroptosis relatively independently, making the ferroptosis defense network increasingly complete. However, unidentified signaling molecules in these pathways need to be discovered, and other ferroptosis inhibitory pathways still require investigation.
The three elements of ferroptosis. ROS in cells produces lipid peroxides from PUFAs on oxidized lipid membranes in the presence of iron, thereby causing membrane damage and cell death, which is called ferroptosis. The perpetrator ROS, the accomplice iron, and the victim PL-PUFA can be regarded as the three elements of ferroptosis.
Four main defense pathways in ferroptosis. The GPX4-GSH signaling pathway is primarily located in the cytoplasm and mitochondria. The DHODH-CoQ signaling pathway is primarily located in the mitochondria. The FSP1-CoQ signaling pathway and the GCH1-BH4 signaling pathway are primarily located in the cytoplasm.
Ferroptosis in CRC
Factors that increase CRC cell susceptibility to ferroptosis
Recently, ferroptosis has attracted much attention in the cancer research community, in part because it is a unique form of cell death with its own mechanism and morphology [37]. The close relationship between ferroptosis and CRC has been confirmed by the presence of multiple ferroptosis-inducing factors in CRC cells [38]. The level of ROS, the perpetrator in ferroptosis, is usually higher in CRC cells than in normal counterparts, and hence CRC cells are more susceptible to ferroptosis [39, 40]. In addition, as the necessary component for the transfer of the accomplice iron in the ferroptosis three elements, transferrin receptor 1 (TfR1) is also overexpressed in CRC tissues, which is a type II transmembrane glycoprotein commonly expressed on the cell surface [41]. It is a crucial protein involved in controlling iron intake and cell growth, as well as a major regulator of cellular iron homeostasis [41]. Therefore, CRC cells containing excess iron and TfR1 are theoretically more prone to ferroptosis. Moreover, CRC cells also contain ferroptosis-inducing factors that promote the transformation of the victim in ferroptosis. For example, CRC cells expressed high levels of high levels of LOX, which can oxidize the victim PL-PUFA to the toxic lipid peroxide PL-PUFA-OOH [42]. LOX is thought to be a central player in ferroptosis, as pharmacological inhibition of LOX has been observed to be cytoprotective, so high levels of LOX should make CRC cells more susceptible to ferroptosis [43].
However, CRC cells overexpress the aforementioned ferroptosis susceptibility factors, certainly, not for self-attack, but because these factors can be beneficial for their proliferation or invasion in the first place. Although ROS is regarded as the perpetrator in ferroptosis, higher-than-normal levels of ROS in CRC can lead to cellular damage, DNA mutations, and inflammation, which can promote the proliferation and migration of CRC cells [39, 44]. Additionally, CRC cells proliferation also requires large amounts of iron [45]. The increased need for iron uptake leads to high TfR1 expression, and hence higher levels of TfR1 are primarily for survival rather than for ferroptosis [46]. Furthermore, while high levels of LOX make CRC cells susceptible to ferroptosis, there is evidence that blocking LOX inhibits CRC progression [42, 43]. LOX inhibitors enhanced phosphatase and tensin homolog deleted on chromosome 10 (PTEN) activity to inhibit the phosphatidylinositol 3-kinase (PI3K)/ protein kinase B (AKT) pathway, thus promoting cell survival and inhibiting apoptosis, which slows CRC progression [42]. This suggests that high levels of LOX contribute to the development of CRC. These studies indicated that ferroptosis susceptibility factors can be viewed as a double-edged sword in CRC survival. It is possible that the level of expression of these ferroptosis susceptibility factors causes the different outcomes of CRC cell survival or death: the level of these factors expressed in CRC cells may not be sufficient to cause ferroptosis yet, but can facilitate the growth of CRC tissues. However, the above speculations have not yet been confirmed experimentally, which may be a future research direction with far-reaching implications for targeting ferroptosis for the treatment of CRC.
Factors influencing CRC cell to ferroptosis
However, CRC cells do not undergo ferroptosis as assumed, not only because of the double-edged-sword-like susceptibility factor, but also largely because the defense pathway for ferroptosis is unusually active in CRC cells, which prevents CRC cells from undergoing ferroptosis [47, 48]. For instance, GPX4, the key factor in the GPX4-GSH signaling pathway, is highly expressed in CRC tissues and high GPX4 expression is strongly associated with a poor prognosis in CRC [47]. The active ferroptosis defense system prevents ferroptosis and ultimately leads to CRC progression.
In addition, potential negative regulators of ferroptosis are expressed in CRC cells. The expression of the TP53-induced glycolysis and apoptosis regulator (TIGAR) is significantly higher in CRC tissues than in neighboring normal tissues [49]. Erastin-induced ferroptosis in CRC cells was significantly increased upon the knockdown of TIGAR, indicating that low TIGAR levels make CRC cells more susceptible to erastin-induced ferroptosis and that TIGAR may function as a ferroptosis inhibitor during CRC development [50]. Low levels of TIGAR increased the production of lipid peroxidation and promoted the accumulation of lipid peroxidation product malondialdehyde (MDA), but no significant change was observed in iron levels, suggesting that TIGAR is a potential target for ferroptosis-based CRC treatment by regulating ROS [50]. Similarly, cytochrome P450 1B1 (CYP1B1) is overexpressed in CRC, and CYP1B1 promotes CRC cells resistance to ferroptosis via alleviating lipid peroxidation, resulting in a poor prognosis in patients with CRC [51]. These highly-expressed negative regulators of ferroptosis facilitate CRC development.
To summarize, the presence of ferroptosis-resistant factors in CRC cells, including the abnormally active ferroptosis defense signaling pathway and the presence of ferroptosis-negative regulatory molecules, allows CRC cells to evade ferroptosis to continue proliferating. Therefore, targeting ferroptosis-resistant factors has the potential to broaden the pathway for the treatment of CRC. Existing research has shown the essential function of ferroptosis in the development of CRC, with a view to providing more emerging targets for the clinical treatment of CRC, but more experiments are still needed to explore specific mechanisms.
Target ferroptosis for the treatment of CRC
CRC is still one of the diseases that pose the greatest risk to human health [52]. Patients with CRC typically experience rectal bleeding and abdominal pain, which have a significant impact on their quality of life [53]. CRC is the second leading cause of cancer death in both men and women, trailing only breast cancer in women and lung cancer in men [54]. In response to the current dilemma of conventional treatments, scientists have attempted to develop effective therapeutic alternatives. They discovered that ferroptosis is essential for preventing CRC progression and, thus, can be a target for future anticancer treatment [55].
Increased iron concentration is a crucial characteristic of cells that may undergo ferroptosis, because in accordance with the three elements of ferroptosis, iron is an accomplice in the occurrence of ferroptosis [56]. Iron is strongly associated with the development of several tumors, the most significant of which being CRC [57]. Studies have shown that while iron-deficient CRC patients have a worse prognosis and a lower response to treatment, excess gut luminal iron contributes to the development and progression of CRC [58]. Elevated or depleted levels of unstable intracellular iron induce complete growth arrest and segregation of different CRC cell types [59]. Therefore, balancing optimal iron intake to avoid iron deficiency and iron overload may be a way to improve the prognosis of patients with CRC [60]. Currently, the main forms of iron supplementation include intravenous iron and oral iron, with intravenous iron achieving better clinical outcomes [61]. Besides, increasing the level of ROS, the perpetrator of ferroptosis, in CRC cells to induce cancer cell death is also a promising approach to inhibit CRC progression [62]. For example, activation of p38 mitogen-activated protein kinase (MAPK) by cetuximab inhibits a major regulator of antioxidant transcription factors, nuclear factor erythroid 2-related factor 2 (Nrf2), thereby increasing Ras-selective lethal small molecule 3 (RSL3)-induced lipid ROS, leading to ferroptosis in CRC cells [62]. This finding is expected to contribute to the development of attractive therapeutic strategies for patients with KRAS-mutated CRC: cetuximab combined with ferroptosis inducers [62]. However, it is worth noting that a moderate amount of ROS contributes to tumorigenesis and progression by regulating numerous signaling pathways, and it is an excessive amount of ROS can cause ferroptosis and other forms of programmed cell death [63]. In summary, it is necessary to monitor specific iron intake or intracellular ROS levels when utilizing iron or ROS to treat CRC, in order to prevent counterproductive effects.
In addition, targeting the ferroptosis defense signaling pathway is a novel strategy for CRC treatment. GSH, as an important component in the GPX4-GSH signaling pathway, is observed the chemotherapy resistance resulting from its elevated levels in human CRC cell lines [64]. GSH shortage is a key characteristic of ferroptosis, and cancer cells may be more sensitive to the effects of anticancer drugs if the GSH antioxidant defense system is impaired [65]. Therefore, blocking the ferroptosis defense pathway becomes an important strategy to inhibit CRC. It was found that the use of sodium butyrate on CRC cell lines decreased intracellular GSH concentration and caused apoptosis in CRC cells [66]. GPX4 in the GPX4-GSH signaling pathway is another important factor in the modulation of ferroptosis [67]. Since the cofactors of GPX4 are not restricted to GSH, direct targeting GPX4 may be more effective than GSH-disrupting therapy [68]. Application of mollugin, a phytochemical isolated from Rubia cordifolia L., in CRC cell lines reduced GPX4 to inhibit CRC cell proliferation and displayed favorable anticancer outcomes [69]. The ferroptosis inducer RSL3 drives ferroptosis by inactivating GPX4 in CRC, leading to CRC cell death [70]. These results provide further evidence that GPX4 expression reduction and ferroptosis induction can both effectively hinder the progression of CRC. Targeting other signaling pathways, such as the FSP-CoQ, DHODH-CoQ, and GCH1-BH4 signaling pathways, in addition to the GPX4-GSH system, may also be effective for CRC treatment [34, 71]. For example, the combination of the GCH1 inhibitor and the ferroptosis inducer erastin can synergistically inhibit CRC growth in vivo [34]. When considered collectively, targeting the ferroptosis defense system may affect the growth of CRC, offering novel therapeutic options for CRC management. Some of the studies targeting the ferroptosis defense signaling pathway to inhibit CRC progression are listed in Table 1. However, current therapeutic strategies targeting the ferroptosis defense signaling pathway mainly focus on the GPX4-GSH signaling pathway, with the other signaling pathways rarely engaged. Moreover, many drug targets are not limited to molecules in the ferroptosis defense signaling pathway, and the inhibition of CRC progression is the result of the joint action of multiple pathways, implying that we should focus on the combination of multiple targets in order to achieve better therapeutic outcomes.
Ferroptosis can also influence CRC progression and therapeutic efficacy by orchestrating tumor immunity [72]. Activated CD8+ T cells secrete high levels of interferon-γ (IFN-γ) to induce ferroptosis in tumor cells [73]. However, IFN-γ-mediated ferroptosis is triggered at low levels in tumor cells due to limited IFN-γ secretion by CD8+ T cells in the immunosuppressive tumor microenvironment [73]. Research has indicated that ferroptosis contributes to immune-supportive responses in CRC, with IFN-γ playing a crucial role [72]. Therefore, targeting IFN-γ may be another potential therapeutic approach to trigger ferroptosis in CRC cells and thus improve the prognosis of CRC patients, but extensive experiments are still needed to validate this. Of note, the liver, an immune organ, is one of the most common organs for CRC metastasis and colonization, so the role of ferroptosis in the hepatic immune microenvironment may provide novel strategies for the treatment of CRC liver metastasis (CRLM) [74-76]. In liver metastases, ferroptosis induces activation and infiltration of CD8 T cells, triggering a tumor-suppressive CD8+ T cell response [77]. However, ferroptosis can similarly induce immunosuppression by stimulating myeloid-derived suppressor cells (MDSCs) recruitment, thus suggesting that combining ferroptosis induction with MDSCs blockade could be a promising therapeutic tool for CRLM [77]. Subsequent in vivo experiments confirmed the effectiveness of this combination therapy for CRLM [77]. The preceding experimental results illustrate the complex interaction between ferroptosis and tumor immunity and provide a theoretical framework for targeting ferroptosis to treat CRC primary tumors or metastases.
Taken together, different molecules in ferroptosis can be used as potential therapeutic targets for CRC treatment, providing more possibilities for current therapeutic approaches. However, further research is needed on whether targeting these molecules to induce ferroptosis in CRC cells will also damage normal cells or tissues and how to minimize the damage.
Selected studies targeting ferroptosis defense signaling pathways to inhibit CRC progression in the last five years.
| Drug | Target | Mechanism and result | Research type | References |
|---|---|---|---|---|
| NaB | GSH | NaB can reduce intracellular GSH concentration to induce apoptosis in CRC cells. | Cell experiment | [66] |
| GRh3 | GSH | GRh3 leads to the depletion of GSH and thus induces ferroptosis in CRC cells, effectively inhibiting the proliferation of CRC cells. | Cell and animal experiment | [78] |
| Mollugin | GPX4 | Mollugin can reduce GPX4 to inhibit the proliferation of CRC cells, showing favorable anticancer effects. | Cell experiment | [69] |
| curcumin and andrographis | GPX4 | Curcumin and andrographis combination therapy exhibited anticancer effects in CRC cells by activating ferroptosis via inhibiting GPX4. | Cell experiment | [79] |
| RSV | GPX4 | RSV can promote ferroptosis and effectively inhibit the growth of CRC cells by down-regulating the expression of GPX4. | Cell and animal experiment | [80] |
| β-elemene and cetuximab | GSH and GPX4 | The combination of β-elemene, a natural product isolated from Chinese herbs, and cetuximab induces ferroptosis by targeting ferroptosis-associated molecules, such as GSH and GPX4, to inhibit KRAS-mutant CRC growth and metastasis. | Cell and animal experiment | [81] |
| leflunomide | DHODH | Leflunomide inhibits DHODH and DHODH depletion significantly reduces CRC liver metastasis colonization. | Animal experiment | [71] |
| DAHP | GCH1 | Inhibition of the GCH1/BH4 signaling pathway by DAHP, a specific inhibitor of GCH1, promoted erastin-induced ferroptosis, suggesting that the combination of a GCH1 inhibitor and erastin is a novel therapeutic strategy for the treatment of CRC. | Cell and animal experiment | [34] |
Abbreviations: DAHP, 2,4-diamina-6-hydroxypyrimidine; GRh3, Ginsenoside Rh3; KRAS, Kirsten rat sarcoma virus; NaB, sodium butyrate; RSV, resveratrol.
Combination of targeting ferroptosis with other non-operative therapies for CRC treatment
In addition to the potential of targeting ferroptosis alone in CRC treatment, combining targeting ferroptosis with non-operative therapies, including chemotherapy, radiotherapy, and immunotherapy, can improve the effectiveness of non-operative therapies, opening the door to a new world for comprehensive CRC management [82].
Oxaliplatin is a widely used chemotherapeutic agent in patients with advanced CRC, but frequent resistance limits its therapeutic efficacy [83]. Cyclin-dependent kinase 1 (CDK1) was found to be a key factor in oxaliplatin resistance because CDK1 mediates the degradation of Acyl-CoA synthetase long-chain family 4 (ACSL4) to block the process of ACSL4, inhibiting lipid peroxidation and ferroptosis, which leads to drug resistance [84]. Furthermore, in vivo and in vitro experiments demonstrated that treatment with ferroptosis inhibitors reduced the enhanced sensitivity of CRC cells to oxaliplatin, which is achieved through CDK1 inhibition [84]. This suggests that the combination of ferroptosis inducers with chemotherapy may be another means of CRC treatment.
Moreover, radiotherapy is also often limited by the occurrence of radioresistance [85]. The overexpression of long non-coding RNA ovarian tumor domain-containing 6B-antisense RNA1 (lncRNA OTUD6B-AS1) stabilizes the tripartite motif 16 (TRIM16) mRNA by binding to the RNA-binding protein human antigen R and increases TRIM16 mRNA levels, promoting GPX4-mediated ferroptosis and inhibiting radioresistance in CRC cells; however, inhibiting ferroptosis attenuates the inhibitory effect of overexpressed lncRNA OTUD6B-AS1 in CRC radioresistance [86]. This finding promises to eliminate the limitations of CRC radiotherapy and enhance the therapeutic effect.
In 2017, immune checkpoint therapy received regulatory approval for the treatment of a small fraction of patients with CRC (microsatellite instability high or mismatch repair deficient) [87]. For most other types of CRC, the immune checkpoint inhibitors (ICIs), programmed cell death 1 (PD1)-, or programmed cell death 1 ligand 1 (PDL1)-blocking antibodies, are ineffective [87]. Apolipoprotein L3 (APOL3) overexpression can enhance RSL3-induced ferroptosis and improve the therapeutic efficacy of PDL1 inhibitors [88]. Additionally, CYP1B1 overexpression can promote ACSL4 ubiquitination and degradation, making CRC cells resistant to ferroptosis and anti-PD1 therapy, whereas CYP1B1 inhibition promotes ferroptosis, making CRC cells susceptible to anti-PD1 antibodies [51]. These studies may broaden the application of ICIs in CRC treatment.
In summary, combining ferroptosis and other non-operative therapies can reverse drug resistance or broaden the scope of application to compensate for the limitations of the existing approaches, providing a novel concept for comprehensive CRC treatment.
Role of ferroptosis-related biomarkers or genes in CRC early diagnosis and prognosis
Although surgical and pharmacological treatments have improved the prognosis of CRC patients, the 5-year survival rate of patients is still unsatisfactory [89]. The time of the diagnosis and the stage at which the disease is discovered are critical factors in determining CRC prognosis: Stage I has a 5-year survival rate of up to 90%, whereas that for stage IV is less than 10% [7]. Accordingly, it is important to improve the effectiveness of early diagnosis.
Nowadays, imaging and stool-based tests are the primary techniques of CRC screening, including colonoscopy, stool-based tests, Cologuard (a stool DNA test), flexible sigmoidoscopy, and computed tomographic colonography [7]. Due to variations in sensitivity, specificity, cost, time, and patient tolerance, different tests cannot be used routinely in all patients [90]. Therefore, further studies should be conducted to investigate novel biomarkers that can be applied in the early detection and diagnosis of CRC.
Given the role of ferroptosis in the progression and treatment of CRC, its potential in the early diagnosis and prognosis of CRC is being progressively explored [14, 91]. Many studies have provided evidence that ferroptosis-related biomarkers may be useful in the early diagnosis of CRC [14]. The transferrin dipstick appears to be a highly sensitive test for detecting not just cancer but also precancerous lesions, providing an additional tool for CRC screening with an overall accuracy of 76.4% for detecting CRC and precancerous lesions [14]. Ferritin, a major intracellular iron storage protein complex, is another protein linked to ferroptosis and may serve as a biomarker for CRC diagnosis [92, 93]. However, its diagnostic specificity is low and needs to be used in combination with other serum markers to diagnose early CRC [93]. These molecules may have potential in the early diagnosis of CRC, and additional research is required to confirm this possibility and improve their diagnostic specificity and sensitivity.
Many recent studies have focused on the development of prognostic profiles of ferroptosis-related genes (FRGs) to predict the prognosis and treatment response of CRC patients [91, 94]. For instance, Shao et al. established the 10 FRGs signature (TFAP2C, SLC39A8, NOS2, HAMP, GDF15, FDFT1, CDKN2A, ALOX12, AKR1C1, and ATP6V1G2) that may accurately predict the prognosis and survival time of CRC patients [95]. Table 2 summarizes the role of FRGs signature in CRC prognosis. These models may offer useful information to predict the prognosis of CRC patients, although the roles of individual FRG in these models are not fully understood. In addition, the aberrant expression of a single FRG may affect the disease prognosis [76, 96]. The expression of metallothionein-1G (MG1T) reduced significantly in CRC tissues [96]. Patients with CRC with high MT1G levels had a worse prognosis and aberrantly expressed MT1G affected the immune response [96]. Consequently, FRGs have the potential to predict the prognosis of patients with CRC.
In summary, the discovery of ferroptosis-related biomarkers and genes may improve the prognosis and survival of patients with CRC. Future research should focus on establishing an ideal prognostic model and identifying key biomarkers, which will be crucial for accurately predicting CRC prognosis and early diagnosis.
Role of FRGs signature in CRC prognosis.
| Model | FRGs | Role in the prognosis of CRC | References |
|---|---|---|---|
| 3-gene prognostic model | CDKN2A, FDFT1, and ACSL6 | Predict prognosis of CRC and assess immune response | [97] |
| 3-gene prognostic model | ACACA, GSS, and NFS1 | Improve individual prognostic monitoring and provide new ferroptosis-related treatment strategies for CRC patients | [98] |
| 3-gene prognostic model | ATG7, MAPK9, and MMD | Predict immunotherapy responses and help determine CRC treatment strategies | [99] |
| 4-gene prognostic model based on EMT and FRGs | MMP7, YAP1, PCOLCE, and HOXC11 | Recognize metastatic COAD | [100] |
| 10-gene prognostic model | TFAP2C, SLC39A8, NOS2, HAMP, GDF15, FDFT1, CDKN2A, ALOX12, AKR1C1, and ATP6V1G2 | Effectively predict the prognosis and survival time of CRC patients, and provide clinical therapeutic benefits for targeted therapy or immunotherapy | [95] |
| 10-gene prognostic model | ATG7, DUOX1, NOX4, PGD, TP63, ATP6V1G2, DRD4, JDP2, SLC2A3, and VEGFA | Serve as an individualized and more accurate survival prediction tool for CRC patients | [101] |
Abbreviations: COAD, colon adenocarcinoma; EMT, epithelial-mesenchymal transition.
Conclusion
Ferroptosis, a newly discovered form of cell death, has received widespread attention from the scientific community and is becoming a hot topic in oncology and anticancer therapeutic research. The link between the occurrence and treatment of CRC, a common type of malignant tumor, and ferroptosis is being widely explored. Scientists have investigated the role of ferroptosis in CRC progression using its three elements and defense signaling pathways. Moreover, a number of drugs targeting ferroptosis have been found to treat CRC and improve patient outcomes. Molecules associated with ferroptosis have also been explored for their potential in treating CRC and are likely to serve as therapeutic targets. The discovery of multiple biomarkers and the development of predictive models have also aided in the early diagnosis and prognosis of patients with CRC. However, some unresolved questions remain about the regulatory mechanism of ferroptosis and its applicability in CRC: (1) Ferroptosis susceptibility factors exist as a double-edged sword in CRC cells, and their up-regulation may cause ferroptosis while simultaneously providing CRC cells with extra raw materials needed for proliferation. Therefore, it is insufficient to target these factors; instead, we should target the intermediate molecules that cause these two different outcomes, necessitating a more in-depth examination of the mechanisms involved. (2) When targeting ferroptosis for CRC treatment, the uncertainty of the effective targets of ferroptosis should be resolved by choosing the appropriate targets among multiple potential ferroptosis-related molecules and clarifying the appropriate drug dosage. (3) The acceleration of translating basic ferroptosis research findings into clinical applications is crucial for enabling early diagnosis, individualized treatment, and precise prognosis.
Abbreviations
ACSL4: Acyl-CoA synthetase long-chain family 4; AKT: protein kinase B; APOL3: apolipoprotein L3; BH4: tetrahydrobiopterin; CDK1: cyclin-dependent kinase 1; CoQ: coenzyme Q; CRC: colorectal cancer; CRLM: CRC liver metastasis; CYGB: cytoglobin; CYP1B1: cytochrome P450 1B1; DHODH: dihydroorotate dehydrogenase; FRGs: ferroptosis-related genes; FSP1: ferroptosis suppressor protein 1; GCH1: guanosine 5'-triphosphate cyclohydrolase-1; GPX4: glutathione peroxidase 4; GSH: antioxidant glutathione; ICIs: immune checkpoint inhibitors; IFN-γ: interferon-γ; lncRNA OTUD6B-AS1: long non-coding RNA ovarian tumor domain containing 6B-antisense RNA1; LOX: lipoxygenase; MAPK: mitogen-activated protein kinase; MDA: malondialdehyde; MDSCs: myeloid-derived suppressor cells; MG1T: metallothionein-1G; Nrf2: nuclear factor erythroid 2-related factor 2; PD1: programmed cell death 1; PDL1: programmed cell death 1 ligand 1; PI3K: phosphatidylinositol 3-kinase; PTEN: phosphatase and tensin homolog deleted on chromosome 10; PUFAs: polyunsaturated fatty acids; RCD: regulated cell death; ROS: reactive oxygen species; RSL3: Ras-selective lethal small molecule 3; TfR1: Transferrin receptor 1; TIGAR: TP53-induced glycolysis and apoptosis regulator; TRIM16: tripartite motif 16.
Acknowledgements
The Figures were created by BioRender (Biorender.com). We thank Home for Researchers editorial team (www.home-for-researchers.com) for language editing service.
Funding
This work was supported by grants from the Graduate Research- Innovation Project in Jiangsu province (SJCX22_1816), the Graduate Research and Practice Innovation Plan of Graduate Education Innovation Project in Jiangsu Province (N0. SJCX211644), Social development project of key R & D plan of Jiangsu Provincial Department of science and technology (BE2022773), and Hospital level management project of Subei People's Hospital YYGL202228, the Social Development-Health Care Project of Yangzhou, Jiangsu Province [No. YZ2021075].
Author contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. All authors have read and agreed to the published version of the manuscript.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Zhu YJ, Li X, Chen TT. et al. Personalised neoantigen-based therapy in colorectal cancer. Clin Transl Med. 2023;13:e1461
2. Shin AE, Giancotti FG, Rustgi AK Metastatic colorectal cancer. mechanisms and emerging therapeutics. Trends Pharmacol Sci. 2023;44:222-236
3. Sung H, Ferlay J, Siegel RL. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249
4. Siegel RL, Wagle NS, Cercek A. et al. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73:233-254
5. Koukourakis IM, Kouloulias V, Tiniakos D. et al. Current status of locally advanced rectal cancer therapy and future prospects. Crit Rev Oncol Hematol. 2023;186:103992
6. Ciardiello F, Ciardiello D, Martini G. et al. Clinical management of metastatic colorectal cancer in the era of precision medicine. CA: A Cancer Journal for Clinicians. 2022;72:372-401
7. Zhang Y, Wang Y, Zhang B. et al. Methods and biomarkers for early detection, prediction, and diagnosis of colorectal cancer. Biomed Pharmacother. 2023;163:114786
8. Huang Z, Gan S, Zhuang X. et al. Artesunate Inhibits the Cell Growth in Colorectal Cancer by Promoting ROS-Dependent Cell Senescence and Autophagy. Cells. 2022;11:2472
9. Huang Y, Yang W, Yang L. et al. Nrf2 inhibition increases sensitivity to chemotherapy of colorectal cancer by promoting ferroptosis and pyroptosis. Sci Rep. 2023;13:14359
10. Liu Y, Lu S, Wu LL. et al. The diversified role of mitochondria in ferroptosis in cancer. Cell Death Dis. 2023;14:519
11. Dixon SJ, Lemberg KM, Lamprecht MR. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060-72
12. Jiang X, Stockwell BR, Conrad M Ferroptosis. mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22:266-282
13. Zhang Q, Deng T, Zhang H. et al. Adipocyte-Derived Exosomal MTTP Suppresses Ferroptosis and Promotes Chemoresistance in Colorectal Cancer. Adv Sci (Weinh). 2022;9:e2203357
14. Sheng JQ, Li SR, Wu ZT. et al. Transferrin dipstick as a potential novel test for colon cancer screening: a comparative study with immuno fecal occult blood test. Cancer Epidemiol Biomarkers Prev. 2009;18:2182-5
15. Liu Y, Xu L, Hao C. et al. Identification and Validation of Novel Immune-Related Alternative Splicing Signatures as a Prognostic Model for Colon Cancer. Front Oncol. 2022;12:866289
16. Chen X, Li J, Kang R. et al. Ferroptosis: machinery and regulation. Autophagy. 2021;17:2054-2081
17. Dhaouadi N, Vitto VAM, Pinton P. et al. Ca(2+) signaling and cell death. Cell Calcium. 2023;113:102759
18. Tang D, Kang R, Berghe TV. et al. The molecular machinery of regulated cell death. Cell Res. 2019;29:347-364
19. Gao W, Wang X, Zhou Y. et al. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct Target Ther. 2022;7:196
20. Yang J, Ma S, Xu R. et al. Smart biomimetic metal organic frameworks based on ROS-ferroptosis-glycolysis regulation for enhanced tumor chemo-immunotherapy. J Control Release. 2021;334:21-33
21. Yang S and Lian G Correction to. ROS and diseases: role in metabolism and energy supply. Mol Cell Biochem. 2020;467:13
22. Xu M, Zha H, Han R. et al. Cyclodextrin-Derived ROS-Generating Nanomedicine with pH-Modulated Degradability to Enhance Tumor Ferroptosis Therapy and Chemotherapy. Small. 2022;18:e2200330
23. Kazan HH, Urfali-Mamatoglu C, Gunduz U. Iron metabolism and drug resistance in cancer. Biometals. 2017;30:629-641
24. Wang Y, Zhang Z, Sun W. et al. Ferroptosis in colorectal cancer: Potential mechanisms and effective therapeutic targets. Biomed Pharmacother. 2022;153:113524
25. Henning Y, Blind US, Larafa S. et al. Hypoxia aggravates ferroptosis in RPE cells by promoting the Fenton reaction. Cell Death Dis. 2022;13:662
26. Stockwell BR, Friedmann Angeli JP, Bayir H. et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell. 2017;171:273-285
27. Fei W, Chen D, Tang H. et al. Targeted GSH-exhausting and hydroxyl radical self-producing manganese-silica nanomissiles for MRI guided ferroptotic cancer therapy. Nanoscale. 2020;12:16738-16754
28. Xie Y, Kang R, Klionsky DJ. et al. GPX4 in cell death, autophagy, and disease. Autophagy. 2023;19:2621-2638
29. Zhang W, Gong M, Zhang W. et al. Correction: Thiostrepton induces ferroptosis in pancreatic cancer cells through STAT3/GPX4 signalling. Cell Death Dis. 2022;13:789
30. Rochette L, Dogon G, Rigal E. et al. Lipid Peroxidation and Iron Metabolism: Two Corner Stones in the Homeostasis Control of Ferroptosis. Int J Mol Sci. 2022;24:449
31. Bersuker K, Hendricks JM, Li Z. et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688-692
32. Doll S, Freitas FP, Shah R. et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575:693-698
33. Mao C, Liu X, Zhang Y. et al. Author Correction: DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature. 2021;596:E13
34. Hu Q, Wei W, Wu D. et al. Blockade of GCH1/BH4 Axis Activates Ferritinophagy to Mitigate the Resistance of Colorectal Cancer to Erastin-Induced Ferroptosis. Front Cell Dev Biol. 2022;10:810327
35. Kraft VAN, Bezjian CT, Pfeiffer S. et al. GTP Cyclohydrolase 1/Tetrahydrobiopterin Counteract Ferroptosis through Lipid Remodeling. ACS Cent Sci. 2020;6:41-53
36. Xue J, Yu C, Sheng W. et al. The Nrf2/GCH1/BH4 Axis Ameliorates Radiation-Induced Skin Injury by Modulating the ROS Cascade. J Invest Dermatol. 2017;137:2059-2068
37. Lei G, Zhuang L, Gan B. Targeting ferroptosis as a vulnerability in cancer. Nat Rev Cancer. 2022;22:381-396
38. Hu B, Yin Y, Li S. et al. Insights on Ferroptosis and Colorectal Cancer: Progress and Updates. Molecules. 2022;28:243
39. Lin S, Li Y, Zamyatnin AA Jr. et al. Reactive oxygen species and colorectal cancer. J Cell Physiol. 2018;233:5119-5132
40. Zheng D, Liu J, Piao H. et al. ROS-triggered endothelial cell death mechanisms: Focus on pyroptosis, parthanatos, and ferroptosis. Front Immunol. 2022;13:1039241
41. Cui C, Cheng X, Yan L. et al. Downregulation of TfR1 promotes progression of colorectal cancer via the JAK/STAT pathway. Cancer Manag Res. 2019;11:6323-6341
42. Chang J, Tang N, Fang Q. et al. Inhibition of COX-2 and 5-LOX regulates the progression of colorectal cancer by promoting PTEN and suppressing PI3K/AKT pathway. Biochem Biophys Res Commun. 2019;517:1-7
43. Shah R, Shchepinov MS, Pratt DA. Resolving the Role of Lipoxygenases in the Initiation and Execution of Ferroptosis. ACS Cent Sci. 2018;4:387-396
44. Zeng J, Li M, Xu JY. et al. Aberrant ROS Mediate Cell Cycle and Motility in Colorectal Cancer Cells Through an Oncogenic CXCL14 Signaling Pathway. Front Pharmacol. 2021;12:764015
45. Xue X, Shah YM. Intestinal iron homeostasis and colon tumorigenesis. Nutrients. 2013;5:2333-51
46. Feng H, Schorpp K, Jin J. et al. Transferrin Receptor Is a Specific Ferroptosis Marker. Cell Rep. 2020;30:3411-3423 e7
47. Liu XS, Yang JW, Zeng J. et al. SLC2A1 is a Diagnostic Biomarker Involved in Immune Infiltration of Colorectal Cancer and Associated With m6A Modification and ceRNA. Front Cell Dev Biol. 2022;10:853596
48. Zhao W, Hao L, Jia L. et al. TAFs contributes the function of PTPN2 in colorectal carcinogenesis through activating JAK/STAT signaling pathway. Am J Cancer Res. 2021;11:3085-3097
49. Al-Khayal K, Abdulla M, Al-Obeed O. et al. Identification of the TP53-induced glycolysis and apoptosis regulator in various stages of colorectal cancer patients. Oncol Rep. 2016;35:1281-6
50. Liu MY, Li HM, Wang XY. et al. TIGAR drives colorectal cancer ferroptosis resistance through ROS/AMPK/SCD1 pathway. Free Radic Biol Med. 2022;182:219-231
51. Chen C, Yang Y, Guo Y. et al. CYP1B1 inhibits ferroptosis and induces anti-PD-1 resistance by degrading ACSL4 in colorectal cancer. Cell Death Dis. 2023;14:271
52. Housini M, Dariya B, Ahmed N. et al. Colorectal cancer: Genetic alterations, novel biomarkers, current therapeutic strategies and clinical trials. Gene. 2024;892:147857
53. Cercek A, Chatila WK, Yaeger R. et al. A Comprehensive Comparison of Early-Onset and Average-Onset Colorectal Cancers. J Natl Cancer Inst. 2021;113:1683-1692
54. Klimeck L, Heisser T, Hoffmeister M. et al. Colorectal cancer: A health and economic problem. Best Pract Res Clin Gastroenterol. 2023;66:101839
55. Yan H, Talty R, Johnson CH. Targeting ferroptosis to treat colorectal cancer. Trends Cell Biol. 2023;33:185-188
56. Stockwell BR Ferroptosis turns 10. Emerging mechanisms, physiological functions, and therapeutic applications. Cell. 2022;185:2401-2421
57. Torti SV and Torti FM Iron and cancer. more ore to be mined. Nat Rev Cancer. 2013;13:342-55
58. Phipps O, Brookes MJ, Al-Hassi HO. Iron deficiency, immunology, and colorectal cancer. Nutr Rev. 2021;79:88-97
59. Sornjai W, Nguyen Van Long F, Pion N. et al. Iron and hepcidin mediate human colorectal cancer cell growth. Chem Biol Interact. 2020;319:109021
60. Aksan A, Farrag K, Aksan S. et al. Flipside of the Coin: Iron Deficiency and Colorectal Cancer. Front Immunol. 2021;12:635899
61. Keeler BD, Dickson EA, Simpson JA. et al. The impact of pre-operative intravenous iron on quality of life after colorectal cancer surgery: outcomes from the intravenous iron in colorectal cancer-associated anaemia (IVICA) trial. Anaesthesia. 2019;74:714-725
62. Yang J, Mo J, Dai J. et al. Cetuximab promotes RSL3-induced ferroptosis by suppressing the Nrf2/HO-1 signalling pathway in KRAS mutant colorectal cancer. Cell Death Dis. 2021;12:1079
63. Perillo B, Di Donato M, Pezone A. et al. ROS in cancer therapy: the bright side of the moon. Exp Mol Med. 2020;52:192-203
64. Polimeni M, Voena C, Kopecka J. et al. Modulation of doxorubicin resistance by the glucose-6-phosphate dehydrogenase activity. Biochem J. 2011;439:141-9
65. Niu B, Liao K, Zhou Y. et al. Application of glutathione depletion in cancer therapy: Enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials. 2021;277:121110
66. Bian Z, Sun X, Liu L. et al. Sodium Butyrate Induces CRC Cell Ferroptosis via the CD44/SLC7A11 Pathway and Exhibits a Synergistic Therapeutic Effect with Erastin. Cancers (Basel). 2023;15:423
67. Li FJ, Long HZ, Zhou ZW. et al. System X(c) (-)/GSH/GPX4 axis: An important antioxidant system for the ferroptosis in drug-resistant solid tumor therapy. Front Pharmacol. 2022;13:910292
68. Wei Y, Lv H, Shaikh AB. et al. Directly targeting glutathione peroxidase 4 may be more effective than disrupting glutathione on ferroptosis-based cancer therapy. Biochim Biophys Acta Gen Subj. 2020;1864:129539
69. Wang W, Zhou L, Zhang X. et al. Mollugin suppresses proliferation and drives ferroptosis of colorectal cancer cells through inhibition of insulin-like growth factor 2 mRNA binding protein 3/glutathione peroxidase 4 axis. Biomed Pharmacother. 2023;166:115427
70. Sui X, Zhang R, Liu S. et al. RSL3 Drives Ferroptosis Through GPX4 Inactivation and ROS Production in Colorectal Cancer. Front Pharmacol. 2018;9:1371
71. Yamaguchi N, Weinberg EM, Nguyen A. et al. PCK1 and DHODH drive colorectal cancer liver metastatic colonization and hypoxic growth by promoting nucleotide synthesis. Elife. 2019;8:e52135
72. Lv Y, Feng QY, Zhang ZY. et al. Low ferroptosis score predicts chemotherapy responsiveness and immune-activation in colorectal cancer. Cancer Med. 2023;12:2033-2045
73. He H, Du L, Xue H. et al. Triple Tumor Microenvironment-Responsive Ferroptosis Pathways Induced by Manganese-Based Imageable Nanoenzymes for Enhanced Breast Cancer Theranostics. Small Methods. 2023;7:e2300230
74. Kubes P, Jenne C. Immune Responses in the Liver. Annu Rev Immunol. 2018;36:247-277
75. Wang Y, Zhong X, He X. et al. Liver metastasis from colorectal cancer: pathogenetic development, immune landscape of the tumour microenvironment and therapeutic approaches. J Exp Clin Cancer Res. 2023;42:177
76. Sun R, Lin Z, Wang X. et al. Correction: AADAC protects colorectal cancer liver colonization from ferroptosis through SLC7A11-dependent inhibition of lipid peroxidation. J Exp Clin Cancer Res. 2022;41:313
77. Conche C, Finkelmeier F, Pesic M. et al. Combining ferroptosis induction with MDSC blockade renders primary tumours and metastases in liver sensitive to immune checkpoint blockade. Gut. 2023;72:1774-1782
78. Wu Y, Pi D, Zhou S. et al. Ginsenoside Rh3 induces pyroptosis and ferroptosis through the Stat3/p53/NRF2 axis in colorectal cancer cells. Acta Biochim Biophys Sin (Shanghai). 2023;55:587-600
79. Miyazaki K, Xu C, Shimada M. et al. Curcumin and Andrographis Exhibit Anti-Tumor Effects in Colorectal Cancer via Activation of Ferroptosis and Dual Suppression of Glutathione Peroxidase-4 and Ferroptosis Suppressor Protein-1. Pharmaceuticals (Basel). 2023;16:383
80. Zhang Z, Ji Y, Hu N. et al. Ferroptosis-induced anticancer effect of resveratrol with a biomimetic nano-delivery system in colorectal cancer treatment. Asian J Pharm Sci. 2022;17:751-766
81. Chen P, Li X, Zhang R. et al. Combinative treatment of beta-elemene and cetuximab is sensitive to KRAS mutant colorectal cancer cells by inducing ferroptosis and inhibiting epithelial-mesenchymal transformation. Theranostics. 2020;10:5107-5119
82. Wang G, Wang JJ, Zhi-Min Z. et al. Targeting critical pathways in ferroptosis and enhancing antitumor therapy of Platinum drugs for colorectal cancer. Sci Prog. 2023;106:368504221147173
83. Li Y, Gan Y, Liu J. et al. Downregulation of MEIS1 mediated by ELFN1-AS1/EZH2/DNMT3a axis promotes tumorigenesis and oxaliplatin resistance in colorectal cancer. Signal Transduct Target Ther. 2022;7:87
84. Zeng K, Li W, Wang Y. et al. Inhibition of CDK1 Overcomes Oxaliplatin Resistance by Regulating ACSL4-mediated Ferroptosis in Colorectal Cancer. Adv Sci (Weinh). 2023;10:e2301088
85. Zhou Y, Shao Y, Hu W. et al. A novel long noncoding RNA SP100-AS1 induces radioresistance of colorectal cancer via sponging miR-622 and stabilizing ATG3. Cell Death Differ. 2023;30:111-124
86. Zhang Z, Ye B, Lin Y. et al. LncRNA OTUD6B-AS1 overexpression promoted GPX4-mediated ferroptosis to suppress radioresistance in colorectal cancer. Clin Transl Oncol. 2023;25:3217-3229
87. Ganesh K, Stadler ZK, Cercek A. et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. 2019;16:361-375
88. Lv Y, Tang W, Xu Y. et al. Apolipoprotein L3 enhances CD8+ T cell antitumor immunity of colorectal cancer by promoting LDHA-mediated ferroptosis. Int J Biol Sci. 2023;19:1284-1298
89. Biller LH and Schrag D Diagnosis and Treatment of Metastatic Colorectal Cancer. A Review. JAMA. 2021;325:669-685
90. Shaukat A and Levin TR Author Correction. Current and future colorectal cancer screening strategies. Nat Rev Gastroenterol Hepatol. 2022;19:551
91. Wang X, Xu Y, Dai L. et al. A novel oxidative stress- and ferroptosis-related gene prognostic signature for distinguishing cold and hot tumors in colorectal cancer. Front Immunol. 2022;13:1043738
92. Hou W, Xie Y, Song X. et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12:1425-8
93. Wild N, Andres H, Rollinger W. et al. A combination of serum markers for the early detection of colorectal cancer. Clin Cancer Res. 2010;16:6111-21
94. Chen Y and Li H Prognostic and Predictive Models for Left- and Right- Colorectal Cancer Patients. A Bioinformatics Analysis Based on Ferroptosis-Related Genes. Front Oncol. 2022;12:833834
95. Shao Y, Jia H, Huang L. et al. An Original Ferroptosis-Related Gene Signature Effectively Predicts the Prognosis and Clinical Status for Colorectal Cancer Patients. Front Oncol. 2021;11:711776
96. Peng B, Peng J, Kang F. et al. Ferroptosis-Related Gene MT1G as a Novel Biomarker Correlated With Prognosis and Immune Infiltration in Colorectal Cancer. Front Cell Dev Biol. 2022;10:881447
97. Yan L, Chen X, Bian Z. et al. A ferroptosis associated gene signature for predicting prognosis and immune responses in patients with colorectal carcinoma. Front Genet. 2022;13:971364
98. Du S, Zeng F, Sun H. et al. Prognostic and therapeutic significance of a novel ferroptosis related signature in colorectal cancer patients. Bioengineered. 2022;13:2498-2512
99. Hu D, Zhou Z, Wang J. et al. Screening of ferroptosis-related genes with prognostic effect in colorectal cancer by bioinformatic analysis. Front Mol Biosci. 2022;9:979854
100. Shi C, Xie Y, Li X. et al. Identification of Ferroptosis-Related Genes Signature Predicting the Efficiency of Invasion and Metastasis Ability in Colon Adenocarcinoma. Front Cell Dev Biol. 2021;9:815104
101. Yang C, Huang S, Cao F. et al. Role of ferroptosis-related genes in prognostic prediction and tumor immune microenvironment in colorectal carcinoma. PeerJ. 2021;9:e11745
Author contact
![]() Corresponding author: Dong Tang, M.D., Ph.D. Address: Department of General Surgery, Institute of General Surgery, Northern Jiangsu People's Hospital Affiliated to Yangzhou University, Yangzhou, 225000, P. R. China. Tel.: +86-18952783556; E-mail: 83392785com.
Corresponding author: Dong Tang, M.D., Ph.D. Address: Department of General Surgery, Institute of General Surgery, Northern Jiangsu People's Hospital Affiliated to Yangzhou University, Yangzhou, 225000, P. R. China. Tel.: +86-18952783556; E-mail: 83392785com.

 Global reach, higher impact
Global reach, higher impact