10
Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2024; 20(10):3911-3922. doi:10.7150/ijbs.93697 This issue Cite
Review
Using Combination therapy to overcome diverse challenges of Immune Checkpoint Inhibitors treatment
1. Department of Molecular Microbiology and Biotechnology, The Shmunis School of Biomedicine and Cancer Research, The George S. Wise Faculty of Life Sciences, Tel-Aviv University, Tel-Aviv 69978, Israel.
2. Cancer Biology Research Center, Tel Aviv University, Tel-Aviv 69978, Israel.
3. The Tel Aviv University Center for Combatting Pandemics, Tel-Aviv University, Tel-Aviv 69978, Israel.
Received 2023-12-28; Accepted 2024-6-29; Published 2024-7-15
Abstract
Immune checkpoint inhibitors (ICIs) have heralded a new era in immunotherapy, representing a pivotal breakthrough in cancer treatment. Their impact is profound, with ICIs standing as some of the most prescribed anticancer therapies today. Notably, their ability to induce long-term remission even after treatment cessation provides genuine hope for achieving durable cures. However, despite these strides, challenges persist in the landscape of oncology, including resistance phenomena, immune-related adverse events, and suboptimal response rates.
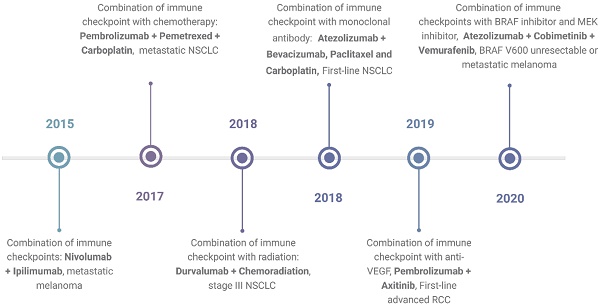
In response to these challenges, combination therapy emerges as a promising approach, poised to enhance treatment outcomes and address limitations inherent to single-agent ICI therapy. By synergistically targeting multiple pathways, combination therapy holds the potential to augment therapeutic efficacy while mitigating toxicity and impeding the emergence of resistance mechanisms. Understanding the intricacies underlying resistance development and adverse events is paramount in devising novel and refined combination strategies. A timeline showing FDA approvals of ICIs combination is shown in Figure 1.
This review aims to provide a comprehensive and up-to-date examples of different combined therapy strategies that can be used to overcome various challenges regarding ICI treatment. Through the exploration of innovative therapeutic combinations, we aim to provide clinicians and researchers with actionable knowledge to optimize patient outcomes and propel the field of immuno-oncology forward.
Introduction
In cancer treatment, immunotherapy represents a strategic approach that harnesses various elements of the immune system to combat cancerous cells. This therapeutic paradigm involves the utilization of diverse pharmacological agents to target specific proteins found on the surface of either cancer cells or immune cells1,2. Central to this strategy are immune checkpoint inhibitors (ICIs), a subclass of immunotherapeutic agents designed to provoke an immune response against tumors by targeting key regulatory proteins. Among the most prominent ICIs are monoclonal antibodies that selectively bind to and inhibit immune checkpoint proteins, notably cytotoxic T lymphocyte-associated protein 4 (CTLA-4), programmed cell death 1 (PD-1) receptors, and its ligand PD-L1. These proteins are pivotal in modulating T cell activation and when targeted by ICIs, can potentiate an anti-tumor immune response3.
The approval of immune checkpoint modulators by the FDA in 2011 marked a significant breakthrough in immunotherapy. Since then, ICIs have catalyzed a paradigm shift in cancer treatment, emerging as cornerstone therapies across a spectrum of malignancies. Notably, anti-PD-1/PD-L1 antibodies have earned recognition as standard-of-care treatments for more than 50 cancer types4. Despite the remarkable advancements facilitated by ICIs in oncology, several significant challenges persist within the treatment landscape5. These challenges include primary and secondary resistance to therapy6, immune-related adverse events (irAEs)2, and suboptimal treatment responses, leading to the inability to achieve desired treatment outcomes. Recognizing the complexity of cancer and the multifaceted nature of therapeutic resistance, combination therapy (CT) has emerged as a promising approach to surmount these obstacles and enhance treatment efficacy. The rationale behind exploring multifaceted targeting strategies lies in the acknowledgment that common diseases, such as cancer, are inherently multifactorial and thus unlikely to be effectively controlled or contained by single-pathway interventions alone7. By concurrently targeting distinct biological pathways or mechanisms, CT holds the potential to synergistically augment therapeutic efficacy while alleviating adverse effects and slow down the emergence of drug resistance. This comprehensive approach aims to optimize treatment outcomes by leveraging the complementary actions of multiple agents.
In this article, we intend to conduct a critical review of the diverse strategies employed in combined therapy and to address the challenges associated with ICI treatment. By exploring various combination approaches, we aim to provide insights into novel therapeutic paradigms that hold promise in overcoming the complexities of immunotherapy resistance and enhancing patient responses to treatment.
The Immune Checkpoint Inhibitors era - remarkable contributions alongside ongoing challenges
Immune checkpoints constitute a crucial regulatory mechanism in the immune system, comprising a network of receptors and ligands tasked with maintaining immune balance. Their primary function is to prevent excessive immune activation and protect normal cells from collateral damage during immune responses unrelated to pathology. Immune checkpoints play a crucial role in maintaining self-tolerance by dampening the activation of T cells, preventing harmful immune reactions from occurring at inappropriate times or locations. Regrettably, cancer cells may exploit these regulatory pathways to evade immune surveillance, hijacking immune checkpoint signaling to suppress anti-tumor immune responses. In response, monoclonal antibodies targeting immune checkpoints have emerged as potent therapeutic agents capable of reactivating the host immune system. By disrupting co-inhibitory signaling pathways, these antibodies unleash an anti-cancer immune response and provide new possibilities for cancer therapy8.
Timeline of FDA-approved combination immunotherapies as of April 2024. The timeline shows the first times the FDA approved a certain type of combination and the indication for which the combination was approved. The data is based on the FDA database. https://www.fda.gov/drugs/resources-information-approved-drugs/oncology-cancer-hematologic-malignancies-approval-notifications. Created with BioRender.com.
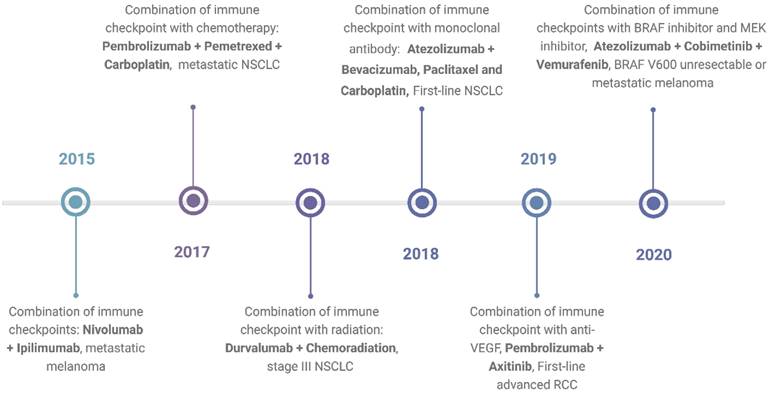
The approval of ipilimumab in 2011 marked a significant milestone in cancer treatment9. Targeting the CTLA-4 molecule, ipilimumab swiftly became a cornerstone therapy for advanced melanoma9. Subsequently, other agents blocking additional immune checkpoint pathways, such as the programmed cell death 1 (PD-1) and its ligand PD-L1, have also been approved10,11. PD-1 inhibitors include nivolumab, pembrolizumab, and cemiplimab, while PD-L1 inhibitors include atezolizumab, durvalumab, and avelumab12. ICIs have become the standard treatment for various types of solid tumors, including melanoma, non-small cell lung cancer, renal cell carcinoma, head and neck cancer, and gastrointestinal malignancies13-15. In hematological malignancies, nivolumab and pembrolizumab have been approved for treating relapsed and refractory classical Hodgkin lymphoma and primary mediastinal large B cell lymphoma16. These approvals underscore the expanding role of ICIs in reshaping therapeutic paradigms and offering hope to patients across diverse oncological settings.
One of the most significant breakthroughs achieved by ICIs is the potential for long-term remission even after discontinuation of treatment, instilling genuine hope for a curative approach in some patients4. However, this outcome remains out of reach for many, emphasizing the complexities of ICI therapy and the variability in patient responses. Despite being hailed as a transformative intervention in cancer care, ICIs are not universally effective, with a significant proportion of patients failing to derive substantial benefit17. Indeed, clinical data reveal that approximately 60-70% of patients with melanoma and lung cancer treated with ICIs do not exhibit a desirable response18. Within this cohort, some individuals demonstrate primary resistance to ICIs, while others acquire resistance following an initial positive response. Understanding the diverse mechanisms underpinning resistance is vital for enhancing treatment outcomes. To confront the challenge of resistance, novel combination strategies have emerged, involving the integration of ICIs with various agents such as radiotherapy, chemotherapy, targeted therapies, or next-generation immune modulators. These synergistic or additive approaches aim to amplify anti-tumor immune responses while circumventing mechanisms of resistance19.
Additionally, a subset of patients receiving ICI therapy may experience severe irAEs resembling autoimmune disorders, including autoimmune thyroiditis and inflammatory bowel diseases4,6. A large meta-analysis reported all-grade incidence of irAEs is about 83% with CTLA-4 inhibitors, 72% with PD-1 inhibitors, and 60% with PD-L1 inhibitors20. These adverse events stem from the fundamental action of ICIs in "releasing the brakes" on immune regulation21. irAEs can affect any organ system or tissue, however, the gastrointestinal tract, endocrine glands, skin, and liver are most commonly affected22. Although these side effects can be severe and even life-threatening, close monitoring and prompt intervention can enable the continuation of ICI therapy. Effective management often necessitates collaboration between oncologists and specialists across various medical disciplines23. Despite the potential severity of irAEs, emerging evidence suggests a paradoxical association between these adverse events and therapeutic efficacy, suggesting a complex interplay between treatment response and immune dysregulation24. In this review, we discuss some of the main combination strategies beyond ICI-ICI pairings, aiming to broaden therapeutic insights and enhance clinical outcomes in the pursuit of effective cancer immunotherapy25,26.
Overcoming primary and secondary resistance
Resistance to ICIs can manifest as either primary or acquired, stemming from the complex and dynamic interplay among cancer cells, the tumor microenvironment, and the immune system27,28. The heterogeneous response rates observed across various tumor types highlight the complexity of ICI therapy, with only a subset of patients exhibiting favorable responses29. Broadly, patients' responses to ICI treatment can be categorized into three distinct populations: responders, characterized by a sustained positive response to the treatment; innate resistance, where patients show no initial response; and acquired resistance, whereby initial responders eventually lose responsiveness. To be able to understand how primary and secondary resistance evolve, a comprehensive grasp of the underlying mechanisms governing each stage of the ICI response model is crucial30. By understanding the different resistance mechanisms to immune checkpoint blockade, combination therapies can be developed to overcome resistance and treatment failure. When discussing how to overcome resistance to ICI, it's important to understand the terms "hot" and "cold" tumors. "Hot tumors" are characterized by high T-cell infiltration, increased interferon-γ (IFN-γ) signaling, high PD-L1 expression levels, and high tumor mutational burden (TMB)31. These types of tumors, such as melanoma and lung cancer, tend to be more responsive to ICIs32. In contrast, “cold tumors” are characterized by the lack of T-cell infiltration, low TMB, low major histocompatibility complex (MHC) class I expression, and low PD-L1 expression31. These types of tumors are much less responsive to ICIs treatment33. Combination therapy with ICIs has been suggested as a way to convert “cold tumors” into “hot tumors” which may make these tumors more responsive to ICI therapy. These treatment combinations are focused on promoting T-cell activation, T-cell expansion, and T-cell infiltration34.
It is generally accepted that the reasons for failure of ICI therapy can be divided into three main categories: the first category is failure in generating effective anti-tumor T cells, such failure can result from low levels of adequate neoantigens and deficiencies in neoantigen presentation35. It has been shown that high TMB and elevated neoantigen expression, have an important role in antitumor immune response36,37. The second category of ICI therapy failure is insufficient function of tumor-specific T cells, this category is attributable to both intrinsic and extrinsic factors within the tumor microenvironment (TME)38. The TME, comprising a diverse array of immune cells, fibroblasts, and signaling molecules, harbors immune-suppressive elements such as regulatory T cells and inhibitory cytokines that thwart the anti-tumor immune response39,40. Lastly, the third category is impaired formation of effector memory T cells that can arise from profound T cell exhaustion or epigenetic alterations within T cells. These impairments compromise the generation of robust, long-lasting anti-tumor immune responses critical for sustained treatment efficacy41,42. By delineating these resistance mechanisms, novel therapeutic approaches can be tailored to counteract treatment resistance and enhance patient outcomes. Addressing the multifaceted challenges posed by ICI therapy demands a comprehensive understanding of the intricate immune landscape and the dynamic interactions within the tumor microenvironment.
Several combination strategies involving ICIs are undergoing clinical evaluation, with several already approved for clinical use. A summary of the combination strategies mentioned in this section are listed in Table 1. One particularly effective combination strategy involves pairing ICIs with chemotherapy. Chemotherapy can interact with different components of the immune system, enhancing immunogenicity while dampening immunosuppressive features within the TME. Certain chemotherapeutic agents augment tumor infiltration and increase the activity of effector cells such as cytotoxic T lymphocytes, dendritic cells, and natural killer cells. Additionally, other chemotherapies have the potential to deplete immune-suppressive cell populations like regulatory T cells. For instance, the combination of pembrolizumab with pemetrexed and platinum chemotherapy has demonstrated significant improvements in overall survival (OS) and progression-free survival (PFS) in metastatic non-squamous non-small cell lung cancer (NSCLC) 43,44. Platinum compounds have been shown to recruit dendritic cells, induce their maturation, and enhance antigen presentation within the TME45. Similarly, combining pembrolizumab with carboplatin and paclitaxel or nab-paclitaxel has yielded notable OS and PFS benefits in metastatic squamous NSCLC, with nab-paclitaxel selectively depleting myeloid-derived suppressor cells44.
Overcoming primary and secondary resistance:
| Drug combination | Target | Mechanism/Rational | Outcomes | Indications | Reference |
|---|---|---|---|---|---|
| Pembrolizumab Pemetrexed-Platinum chemotherapy | PD-1 Key proteins that are abundant in cancer cells | Platinum compounds recruit dendritic cells, induce their maturation, and enhance antigen presentation within the TME | Improved OS and PFS compared with placebo plus pemetrexed-platinum | NSCLC | 43, 44 |
| Pembrolizumab carboplatin and paclitaxel/nab-paclitaxel | PD-1 DNA, Tubulin beta-1 chain | carboplatin and paclitaxel/nab-paclitaxel can lead to depletion of myeloid-derived suppressor cells | Significantly improved OS and PFS in comparison to chemotherapy alone | NSCLC | 44 |
| Pembrolizumab Datopotamab deruxtecan | PD-1 Trophoblast cell surface protein 2 | ADCs combine the potency of strong chemotherapy drugs with the specificity of mAbs | Phase Ib trial (TROPION-Lung02) showed promising clinical activity in patients with advanced or metastatic NSCLC | NSCLC | 48, 49 |
| Atezolizumab Vemurafenib with Cobimetinib | PD-L1 BRAF MEK1 | Vemurafenib can enhance antigen processing and increase the expression of major MHC molecules | Improved PFS | Unresectable or metastatic melanoma | 51, 52 |
| Pembrolizumab Lenvatinib | PD-1 VEGFR | Antiangiogenic drugs can increase intra-tumoral effector cells, decrease PDL1 expression, and reduce infiltration of MDSCs and regulatory T cells | Increase in PFS compared to sunitinib alone | Renal cell carcinoma, Endometrial carcinoma | 53, 54, 55, 56 |
| Ipilimumab Rituximab | CTLA-4 CD20 | Depletion of tumor-associated B cells that have been implicated in drug resistance and the secretion of pro-tumorigenic factors | Median survival exceeding 1 year in patients with multi-treated metastatic melanoma | Metastatic melanoma | 57 |
Abbreviations: PD-1 programmed cell death protein 1, PD-L1 programmed death-ligand 1, CTLA-4 cytotoxic-T-lymphocyte-associated protein 4, TME tumor microenvironment, MDSC myeloid-derived suppressor cells, OS overall survival, PFS progression-free survival, MHC major histocompatibility complex, NSCLC non-small cell lung cancer.
An interesting innovation in the field of chemotherapy are Antibody-Drug Conjugates (ADCs). ADCs are an advanced class of potent anti-cancer compounds that unlike conventional chemotherapies, combine the potency of anti-cancer drugs with the specificity of mAbs46,47. Several preclinical and clinical trials support combination therapy with ADCs and ICIs for treating NSCLC. Initial results from the TROPION-Lung02 trial showed encouraging efficacy and safety when combining datopotamab deruxtecan (an anti-trophoblast cell surface protein 2 (TROP2) mAb, conjugated to a potent DNA topoisomerase I inhibitor) and pembrolizumab with or without platinum chemotherapy48,49.
A class of small-molecule-drugs with a unique mechanism of action are the epigenetic agents (epidrugs). These are drugs that modulate epigenetic modifications such as DNA methylation (such as DNMT inhibitors), histone modifications (such as HDAC inhibitors) and the involvement of ncRNAs, which are believed to comprise the primary regulators of pathophysiological progression. The pivotal roles of epigenetic mechanisms in cancer initiation and progression, as well as in cell fate and functioning of the immune system, led to studies of epidrugs as anti-cancer drugs. In several such studies, combinations of epidrugs with ICIs have been studies as well. To date, combinations of epidrugs with ICIs were evaluated in small patient groups in phase I or II clinical trials, where patients suffering from hematological malignancies and from various carcinomas were treated. Such studies provided promising evidence that combinatorial strategies employing epigenetic drugs along with ICIs provide a novel option for cancer treatment. Further investigations are required to assess the clinical efficacy of combining epigenetic drugs with ICIs. In this way, it will be possible to develop new and improved rationale-based combinational strategies that will significantly enhance the practice of cancer immunotherapy in the future50.
Another potent combination approach that can be used to overcome resistance involves the combination of ICIs with targeted agents such as small molecule kinase inhibitors. For instance, the combination of vemurafenib (a BRAF inhibitor) and cobimetinib (a MEK1 inhibitor) with atezolizumab (a PD-L1 inhibitory mAb) has shown improved PFS in patients with unresectable or metastatic melanoma51. Vemurafenib has been observed to enhance antigen processing and increase the expression of major MHC molecules52. An additional strategy is to combine ICIs with antiangiogenic drugs targeting vascular endothelial growth factor (VEGF) or its receptor (VEGFR). VEGF secretion within the TME blocks T cell development and infiltration while promoting the proliferation of immune-suppressive cells53. This combination therapy has shown efficacy in numerous clinical trials, leading to FDA approvals for several combinations. The administration of antiangiogenic drugs can reverse immune suppression by increasing intra-tumoral effector cells, decreasing PD-L1 expression, and reducing infiltrating myeloid-derived suppressor cells (MDSCs) and regulatory T cells54,55. For instance, combination of pembrolizumab with the multiple receptor tyrosine kinase inhibitor lenvatinib has been evaluated in the treatment of renal carcinoma in patients with favorable risk or intermediate/poor risk and in endometrial carcinoma. The authors suggested that the use of non-chemotherapy containing regimens may spare patients from extended durations of myelosuppression and reduce the risk of infection. Additionally, pembrolizumab with lenvatinib demonstrates efficacy as a first line treatment in clear cell renal carcinoma, second line in endometrial carcinoma, and several potential uses on the horizon56.
Furthermore, the combination of ICIs with anti-CD20 monoclonal antibodies like rituximab has demonstrated promise in overcoming resistance mechanisms, particularly in melanoma. Tumor-associated B cells have been implicated in drug resistance and the secretion of pro-tumorigenic factors such as IGF-1 secretion57, making them viable targets for therapeutic intervention. Data from case series showed median survival exceeding 1 year in patients with multi-treated metastatic melanoma receiving rituximab58.
Overcoming severe irAEs and toxicity
In the context of irAEs, the ICI treatment can be described as a double-edged sword. Releasing the physiological brakes of the immune system to induce a strong anti-tumor immune response is the treatment's mechanism of action. However, the same strong immune response can often result in off-target effects and lead to immune-mediated adverse events, which can resemble autoimmune disorders59. The incidence of irAEs spans a wide spectrum, ranging from 10% to 90%, with severe irAEs (grade 3 or higher) affecting 2.5% to 18% of patients receiving ICIs60. Typically, irAEs manifest within the initial weeks of treatment initiation, although they can emerge at any time point, including after treatment cessation. Alarmingly, up to 19% of patients in ICI clinical trials have had to discontinue treatment due to irAEs61-63. Notably, the incidence of irAEs varies among different ICIs, with anti-CTLA-4 inhibitors associated with the highest occurrence64.
Predictably, irAEs differ markedly from adverse events linked to conventional cytotoxic or targeted therapies, presenting clinicians with a distinct set of clinical challenges. While dermatitis and thyroiditis are common irAEs, more serious manifestations such as pneumonitis, colitis, and hepatitis can occur, albeit less frequently23. Importantly, patients with pre-existing autoimmune diseases pose a unique management challenge, as they are at heightened risk of experiencing disease flares while undergoing ICI therapy65. Treating patients with pre-existing autoimmune diseases can be challenging, however, evidence is accumulating regarding ICI use in these patients, with reassuring safety data reported in several case reports and in several retrospective series66-68. Managing the treatment of patients with irAEs, whether it is "de novo” induced irAEs or a flare of pre-existing autoimmune disease, requires efficient and multidisciplinary monitoring.
Despite the exact pathophysiology of irAEs remaining elusive, several mechanisms are proposed, including the activation of self-reactive T cells69, antigenic overlap between cancer cells and affected tissues70, direct toxicity to organs expressing immune checkpoint proteins, dysregulation of inflammatory cytokines and chemokines71 and decreased levels of regulatory T cells72. Several guidelines on the management of irAEs have been published73-75. These guidelines provide key recommendations for managing immunotherapy-related toxicity and include assessment and treatment algorithms according to the grade of toxicity. As a first line approach, irAEs can be treated with Glucocorticoids such as prednisolone or parenteral methylprednisolone76. However, severe, and life-threatening irAEs are more challenging to diagnose and treat. Since there is a lack of prospective trials on drug immunosuppression in the setting of severe irAEs, information on how to manage such irAEs is collected based on a small series of studies and case reports. It has been suggested that novel biological agents targeting key inflammatory components can be used based on the immunopathological patterns of each patient77. A summary of the combination strategies mentioned in this section are listed in Table 2.
For instance, colitis induced by ICIs can be effectively managed with a single dose of infliximab, a chimeric monoclonal antibody targeting tumor necrosis factor alpha (TNFα). This approach has demonstrated efficacy in treating corticosteroid-refractory ICI-related colitis78. Alternative anti-TNFα agents such as etanercept, adalimumab, certolizumab, and golimumab may also be considered. In severe cases of ICI-induced polyarthritis, adalimumab has shown success in alleviating joint inflammation79. Another therapeutic approach involves blockade of interleukin-1 (IL-1) using agents like anakinra or canakinumab, which have shown promise in managing various irAEs including myasthenia gravis, encephalitis, severe arthritis, and psoriasis80. IL-1 inhibition is particularly beneficial in ICI-related irAEs due to its prominent role in acute inflammation, with no negative impact on cancer response81. Similarly, interleukin-6 (IL-6) blockade with tocilizumab, an IL-6 receptor inhibitory monoclonal antibody, holds potential for managing steroid-refractory irAEs. Aside from the anti-inflammatory benefits, IL-6 is also known to promote cancer development and metastasis. Thus, by targeting IL-6 while treating with ICI, a synergistic effect could be achieved82,83. Possible indications for anti-IL-6 therapy include severe irAEs such as severe arthritis, uveitis, Graves' orbitopathy, myocarditis, large-vessel vasculitis, severe pneumonitis, and myasthenia gravis84-86.
While the pathogenic role of T cells in irAEs is well-established, emerging evidence suggests a contribution from B cells as well87,88. ICI-related encephalitis, an uncommon yet severe manifestation of irAEs, is associated with the presence of anti-neural autoantibodies89. Rituximab, an anti-CD20 monoclonal antibody, has shown impressive neurological improvement in patients with ICI-related encephalitis. In these cases, the patients were first treated with corticosteroids and intravenous immunoglobulins (IVIG) without success90,91. IVIG, derived from pooled immunoglobulins of healthy donors, serves as a versatile therapeutic option for various immunodeficiency states and inflammatory conditions, including ICI-related irAEs. Standard IVIG protocols have demonstrated impressive efficacy in managing irAEs92. IVIG can be used in cases of immune thrombocytopenia which is a rare and life-threatening form of irAE that are corticosteroids-refractory (approximately 25% of patients)93. The judicious selection of immunomodulatory agents tailored to the specific irAE profile is paramount in achieving optimal outcomes while minimizing treatment-related toxicity. As our understanding of irAEs continues to evolve, personalized therapeutic approaches will play an increasingly pivotal role in navigating the complexities of ICIs therapy.
Improving the treatment outcomes to ICI and increasing the indication range
The emergence of ICIs has heralded a paradigm shift in cancer immunotherapy, yet the quest for novel strategies to enhance anti-tumor responses and amplify treatment success continues. Among these strategies, combination therapy stands out, with extensive research endeavors aimed at identifying synergistic or additive regimens to augment ICI efficacy and expand its indications. One promising strategy involves combining ICIs with radiotherapy, a standard treatment modality for over 50% of patients suffering from tumors94. In this context, radiotherapy acts synergistically with ICIs by enhancing the expression of leukocyte adhesion molecules on endothelial cells and promoting the secretion of chemokines that attract CD8+ T cells32,95. Moreover, the combination of radiotherapy with targeting ICIs could provoke cytotoxic T cells-mediated anti-tumor immunity by inducing somatic mutations that can generate neoantigens, and further augmenting immune responses96. Clinical studies have demonstrated the therapeutic benefits of combining ICIs with radiotherapy in melanoma, NSCLC, renal cell carcinoma (RCC), and ongoing studies explore its applicability across diverse malignancies97,98. Recently, the combination of ICIs with radiotherapy was evaluated in hepatocellular carcinoma (HCC). That study demonstrated the advantage of combining ICI with radiotherapy compared to the combination of ICIs and antiangiogenic therapy, the inclusion of radiotherapy improved the disease control rate and survival outcomes in patients with advanced-stage HCC. The safety profile of this triple therapy was satisfactory99.
Another promising approach involves leveraging targeted small molecule drugs, which have become mainstream cancer therapies by targeting various cellular pathways including kinases, epigenetic regulators, DNA repair enzymes, and proteasomes100. Several studies have demonstrated the synergistic potential of these agents with ICIs to enhance treatment outcomes. Notably, the combination of ICIs with targeted therapy drugs inhibiting rapidly accelerated fibrosarcoma (RAF) and mitogen-activated protein kinase kinases (MEK) is the most widely studied combination of small molecule drugs and ICIs. In melanoma patients harboring the BRAF V600E mutation, combining BRAF and MEK inhibitors significantly enhances treatment efficacy. BRAF inhibition reduces anti-inflammatory cytokine levels that suppress tumor-infiltrating lymphocytes (TILs). Moreover, PD-L1 is upregulated in patients resistant to BRAF inhibitors101,102. This synergistic interplay between ICIs and BRAF inhibitors has yielded impressive outcomes, exemplified by the combination of dabrafenib (a BRAF inhibitor), trametinib (a MEK inhibitor), and spartalizumab (an anti-PD-1 mAb), which demonstrated a remarkable 100% response rate and reduced relapse compared to BRAF-MEK inhibitor monotherapy in melanoma patients103-105.
Exploring multiple targeting strategies for ICIs serves another crucial purpose: expanding the spectrum of indications to encompass a wider array of cancer types. Initially, breast cancer was not among the cancer subtypes investigated with immune checkpoint inhibitor. Early clinical trials investigating PD-1/PD-L1 monoclonal antibodies as monotherapy for triple-negative breast cancer (TNBC) yielded modest to negligible clinical benefits106-108. However, in 2019, atezolizumab received accelerated approval for first-line treatment of metastatic TNBC in combination with nanoparticle albumin-bound (nab)-paclitaxel107.
Overcoming severe irAEs and toxicity:
| Drug combination | Target | Mechanism/Rational | Outcomes | Indications | Reference |
|---|---|---|---|---|---|
| ICI anti-TNFα agents | PD-1, PD-L1, CTLA-4 TNFα | TNFα is a potent pro-inflammatory cytokine that play an important role in the pathogenesis of chronic inflammatory diseases, such as colitis and arthritis. | Success in managing the inflammation and alleviating symptoms | Corticosteroid-refractory ICI-induced polyarthritis and ICI-induced colitis | 78, 79 |
| ICI Anakinra or Canakinumab | PD-1, PD-L1, CTLA-4 IL-1 | IL-1 inhibition is beneficial in ICI-related irAEs due to its prominent role in acute inflammation, with no negative impact on cancer response. | Alleviation of disease symptoms | Myasthenia gravis, encephalitis, severe arthritis, and psoriasis | 80, 81 |
| ICI Tocilizumab | PD-1, PD-L1, CTLA-4 IL-6 | Aside from the anti-inflammatory benefits, IL-6 is also known to promote cancer development and metastasis. Thus, by targeting IL-6 while treating with ICI, a synergistic effect could be achieved | Alleviation of disease symptoms | Severe arthritis, uveitis, graves' orbitopathy, myocarditis, large-vessel vasculitis, severe pneumonitis, and myasthenia gravis | 82, 83, 84, 85, 86 |
| ICI Rituximab | PD-1, PD-L1, CTLA-4 CD20 | ICI-related encephalitis is associated with the presence of anti-neural autoantibodies | Impressive neurological improvement in patients with ICI-related encephalitis | ICI-related encephalitis | 87, 88, 89, 90, 91 |
| ICI IVIG | PD-1, PD-L1, CTLA-4 Various components of the immune system | Neutralization of pathogenic autoantibodies, prevention of B-cells proliferation, suppression of pro-inflammatory cytokines production | Alleviation of disease symptoms | Pre-existing paraneoplastic neuromuscular diseases in cancer patients, corticosteroids-refractory immune thrombocytopenia | 92, 93 |
Abbreviations: ICI immune checkpoint inhibitor, PD-1 programmed cell death protein 1, PD-L1 programmed death-ligand 1, CTLA-4 cytotoxic-T-lymphocyte-associated protein 4, TNFɑ tumor necrosis factor alpha, IL-1 Interleukin-1, IL-6 Interleukin-6, IVIG intravenous immunoglobulin.
Despite this approval, metastatic TNBC is still one of the most challenging cancer types to treat, prompting ongoing efforts to identify novel combinational regimens to enhance response rates. One of the options that are under clinical development is targeting the human epidermal growth factor receptor 2 (HER-2) oncogene. HER-2-targeted therapy such as trastuzumab, have revolutionized the prognosis for patients with HER2-positive breast cancer109. However, a subset of patients develops resistance to trastuzumab, characterized by upregulated PD-1/PD-L1 expression within the TME110. Consequently, clinical trials evaluating the combination of trastuzumab with pembrolizumab have been undertaken in patients with advanced or metastatic HER2-positive breast cancer refractory to trastuzumab. Preliminary data from these trials have demonstrated promising therapeutic outcomes, including a 15% response rate in PD-L1-positive tumors and a 12-month progression-free survival rate of 13%111. Building upon these advancements, in 2021, the FDA granted accelerated approval to pembrolizumab in combination with trastuzumab and fluoropyrimidine-and platinum-containing chemotherapy for first-line treatment of patients with locally advanced, unresectable, or metastatic HER2-positive gastric adenocarcinoma112. A summary of the combination strategies mentioned in this section are listed in Table 3.
Conclusion
Undoubtedly, ICIs have become a cornerstone in the management of malignancy, affording many cancer patients improved outcomes and extended survival. Yet, primary and secondary resistance, along with the occurrence of severe side effects and low response rates, underlines the ongoing challenges associated with ICI therapy. In response, clinicians and researchers are actively exploring various ways to enhance the efficacy of ICIs, with combination therapy standing out as a promising approach.
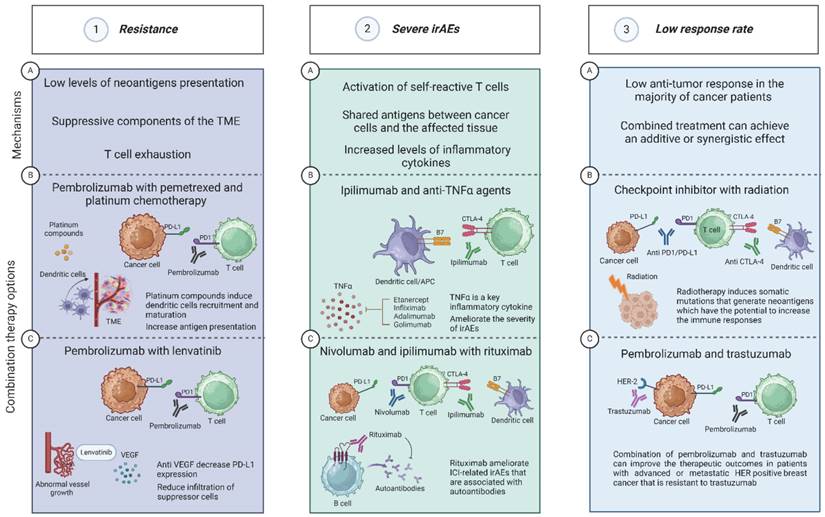
The combination of ICIs with other drugs can significantly contribute to achieving the treatment goals, whether the goal is to overcome resistance, reduce serious and life-threatening side effects, or improve the overall response to the treatment. The judicious selection and integration of appropriate combination therapies tailored to each patient's unique circumstances are vital. In this review, we discussed how different combination therapies can be used for different purposes depending upon the specific drugs utilized. Furthermore, we elucidate the distinct mechanisms of action underlying each therapeutic combination, providing valuable insights into their rationale and potential clinical utility. A summary of the motivation rationales to combine ICIs with other drugs, and some examples of such combinations are shown in Figure 2.
As for future prospects of the field, it is important to note that some combination therapies designed to enhance treatment response or reduce resistance may inadvertently increase the risk of irAEs. Thus, a comprehensive understanding of the underlying mechanisms implicated in each treatment challenge is and will remain crucial for making informed and rational decisions regarding the selection of appropriate combination therapies that maximize patient benefit while minimizing risks. Moving forward, artificial intelligence (AI) may have the potential to open new possibilities in the field of immunotherapy. Machine learning methods have been proven as powerful tools to predict potential anti-cancer synergistic drug combinations, especially as the availability of large datasets has grown113. Continued research efforts and clinical trials are required to identify novel and refined combination strategies tailored to specific pathological conditions.
Improving treatment outcomes to ICI and increasing the indication range
| Drug combination | Target | Mechanism/Rational | Outcomes | Indications | Reference |
|---|---|---|---|---|---|
| ICI Radiotherapy | PD-1, PD-L1, CTLA-4 DNA | Induction of somatic mutations that can generate neoantigens, promotion of chemokines secretion that attract CD8+ T cells | Improved disease control and survival outcomes | NSCLC, RCC, HCC | 32, 95, 96, 97, 98, 99 |
| Spartalizumab Dabrafenib with Trametinib | PD-1 BRAF MEK1 | BRAF inhibition reduces anti-inflammatory cytokine levels that suppress tumor-infiltrating lymphocytes (TILs) | High response rate and reduced relapse compared to BRAF-MEK inhibitor monotherapy | Melanoma | 100, 101, 102, 103-105 |
| Pembrolizumab Trastuzumab | PD-1 HER-2 | Resistance to trastuzumab is characterized by upregulated PD-1/PD-L1 expression, thus, combination of trastuzumab with pembrolizumab can be beneficial | 15% response rate in PD-L1-positive tumors and a 12-month PFS rate of 13% | Metastatic HER2-positive breast cancer, HER2-positive gastric adenocarcinoma | 109, 110, 111, 112 |
Abbreviations: ICI immune checkpoint inhibitor, PD-1 programmed cell death protein 1, PD-L1 programmed death-ligand 1, CTLA-4 cytotoxic-T-lymphocyte-associated protein 4, NSCLC non small cell lung cancer, RCC renal cell carcinoma, HCC hepatocellular carcinoma, HER-2 human epidermal growth factor receptor 2, PFS progression-free survival.
Different drug combinations with ICI can be used for different purposes. Panel 1. Resistance. 1A. The main mechanisms that can lead to the development of ICI-resistance are low levels of neoantigens presentation, suppressive components in the TME, and T cell exhaustion35,38,41. 1B. The combination of pembrolizumab with pemetrexed and platinum compounds can be used to overcome ICI-resistance. Platinum compounds induce dendritic cells recruitment and maturation and increase antigen presentation43-45. 1C. Pembrolizumab and anti VEGF such as Lenvatinib. Lenvatinib can decrease the levels of PD-L1 expression and reduce the infiltration of suppressor cells such as myeloid-derived suppressor cells (MDSCs) and regulatory T cells54,55. Panel 2. Severe irAEs. 2A. The main mechanisms that can lead to the development of irAEs are activation of self-reactive T cells, shared antigens between cancer cells, and increased production of inflammatory cytokines and chemokines69-71. 2B. The combination of ipilimumab and anti-TNFα agent such as infliximab was shown to be effective for treating corticosteroid-refractory ICI-related colitis78. In addition, adalimumab was successful in ameliorating the severity of ICI-related joints inflammation79. 2C. AntI-CD20 agents such as rituximab were effective in ameliorating ICI-related irAEs that are associated with the detection of autoantibodies such as encephalitis89. Panel 3. Low response rate. 3A. There is an ongoing effort to find new strategies to enhance the anti-tumor response since most cancer patients will not achieve the treatment goals17. One of these strategies is to gain an additive or synergistic effect by using combination therapy. 3B. Combining ICIs with radiotherapy has the potential to improve the treatment outcome. Following radiotherapy, some patients may express somatic mutations that generate neoantigens which have the potential to increase the immune responses97,98. 3C. Another significant motivation to explore new CT with ICIs is to increase the range of indications for additional types of cancer, such TNBC. The combination of trastuzumab (an anti-HER-2 mAb) with pembrolizumab has been evaluated in clinical trials in patients with advanced or metastatic HER positive breast cancer that is resistant to trastuzumab. The data gathered from this trial showed promising therapeutic outcomes including 15% response rates in PD-L1-positive tumors with 13% 12-month PFS111. Created with BioRender.com.
Author contributions
All authors made a significant contribution to this review article, whether that is in the conception, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising and critically reviewing the article. All authors gave final approval of the version to be published and agree to be accountable for all aspects of the work. All authors have read and agreed to the published version of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Sanmamed MF, Chen L. A Paradigm Shift in Cancer Immunotherapy: From Enhancement to Normalization. Cell. 2018;175(2):313-326 doi:10.1016/j.cell.2018.09.035
2. Ramos-Casals M, Brahmer JR, Callahan MK. et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6(1):38 doi:10.1038/s41572-020-0160-6
3. Shiravand Y, Khodadadi F, Kashani SMA. et al. Immune Checkpoint Inhibitors in Cancer Therapy. Current Oncology. 2022;29(5):3044-3060 doi:10.3390/curroncol29050247
4. Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun. 2020;11(1):3801 doi:10.1038/s41467-020-17670-y
5. Thuny F, Naidoo J, Neilan TG. Cardiovascular complications of immune checkpoint inhibitors for cancer. Eur Heart J. 2022;43(42):4458-4468 doi:10.1093/eurheartj/ehac456
6. Walsh RJ, Sundar R, Lim JSJ. Immune checkpoint inhibitor combinations—current and emerging strategies. Br J Cancer. 2023;128(8):1415-1417 doi:10.1038/s41416-023-02181-6
7. Lehár J, Zimmermann GR, Krueger AS. et al. Chemical combination effects predict connectivity in biological systems. Mol Syst Biol. 2007;3:80 doi:10.1038/msb4100116
8. Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50(12):1-11 doi:10.1038/s12276-018-0191-1
9. Ledford H. Melanoma drug wins US approval. Nature. 2011;471(7340):561 doi:10.1038/471561a
10. Littman DR. Releasing the Brakes on Cancer Immunotherapy. Cell. 2015;162(6):1186-90 doi:10.1016/j.cell.2015.08.038
11. Lonberg N, Korman AJ. Masterful antibodies: Checkpoint blockade. Cancer Immunol Res. 2017;5(4):275-281 doi:10.1158/2326-6066.CIR-17-0057
12. Liebl MC, Hofmann TG. Identification of responders to immune checkpoint therapy: which biomarkers have the highest value? Journal of the European Academy of Dermatology and Venereology. 2019;33(S8):52-56 doi:10.1111/jdv.15992
13. Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8(9):1069-1086 doi:10.1158/2159-8290.CD-18-0367
14. Motzer RJ, Tannir NM, McDermott DF. et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. New England Journal of Medicine. 2018;378(14):1277-1290 doi:10.1056/nejmoa1712126
15. Reck M, Rodríguez-Abreu D, Robinson AG. et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. New England Journal of Medicine. 2016;375(19):1823-1833 doi:10.1056/nejmoa1606774
16. Hradska K, Hajek R, Jelinek T. Toxicity of Immune-Checkpoint Inhibitors in Hematological Malignancies. Front Pharmacol. 2021;12. doi:10.3389/fphar. 2021 733890
17. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science (1979). 2018;359(6382):1350-1355 doi:10.1126/science.aar4060
18. Vafaei S, Zekiy AO, Khanamir RA. et al. Combination therapy with immune checkpoint inhibitors (ICIs); a new frontier. Cancer Cell Int. 2022;22(1):2 doi:10.1186/s12935-021-02407-8
19. Morganti S, Curigliano G. Combinations using checkpoint blockade to overcome resistance. Ecancermedicalscience. 2020;14:1148 doi:10.3332/ECANCER.2020.1148
20. Song P, Zhang D, Cui X, Zhang L. Meta-analysis of immune-related adverse events of immune checkpoint inhibitor therapy in cancer patients. Thorac Cancer. 2020;11(9):2406-2430 doi:10.1111/1759-7714.13541
21. Smithy JW, Faleck DM, Postow MA. Facts and Hopes in Prediction, Diagnosis, and Treatment of Immune-Related Adverse Events. Clinical Cancer Research. 2022;28(7):1250-1257 doi:10.1158/1078-0432.CCR-21-1240
22. Perdigoto AL, Kluger H, Herold KC. Adverse events induced by immune checkpoint inhibitors. Curr Opin Immunol. 2021;69:29-38 doi:10.1016/j.coi.2021.02.002
23. Wang DY, Salem JE, Cohen J V. et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018;4(12):1721-1728 doi:10.1001/jamaoncol.2018.3923
24. Singh N, Hocking AM, Buckner JH. Immune-related adverse events after immune check point inhibitors: Understanding the intersection with autoimmunity. Immunol Rev. 2023;318:81-88 doi:10.1111/imr.13247
25. Willsmore ZN, Coumbe BGT, Crescioli S. et al. Combined anti-PD-1 and anti-CTLA-4 checkpoint blockade: Treatment of melanoma and immune mechanisms of action. Eur J Immunol. 2021;51(3):544-556 doi:10.1002/eji.202048747
26. Meyers BM, Knox JJ, Liu DM. et al. The evolution of immune checkpoint inhibitor combinations in advanced hepatocellular carcinoma - A systematic review. Cancer Treat Rev. 2023;118:102584 doi:10.1016/j.ctrv.2023.102584
27. O'Meara CH, Jafri Z, Khachigian LM. Immune Checkpoint Inhibitors, Small-Molecule Immunotherapies and the Emerging Role of Neutrophil Extracellular Traps in Therapeutic Strategies for Head and Neck Cancer. Int J Mol Sci. 2023;24(14):11695 doi:10.3390/ijms241411695
28. Chen H, Chen G. Dissecting Immunosuppressive Cell Communication Patterns Reveals JunB Proto-Oncogene (JUNB) Shaping a Non-Inflamed Tumor Microenvironment. Front Genet. 2022;13:883583 doi:10.3389/fgene.2022.883583
29. Fares CM, Van Allen EM, Drake CG, Allison JP, Hu-Lieskovan S. Mechanisms of Resistance to Immune Checkpoint Blockade: Why Does Checkpoint Inhibitor Immunotherapy Not Work for All Patients? American Society of Clinical Oncology Educational Book. 2019(39):147-164 doi:10.1200/edbk_240837
30. Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer. 2018;118(1):9-16 doi:10.1038/bjc.2017.434
31. Hegde PS, Karanikas V, Evers S. The where, the when, and the how of immune monitoring for cancer immunotherapies in the era of checkpoint inhibition. Clinical Cancer Research. 2016;22(8):1865-74 doi:10.1158/1078-0432.CCR-15-1507
32. Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18(3):197-218 doi:10.1038/s41573-018-0007-y
33. Herbst RS, Soria JC, Kowanetz M. et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563-7 doi:10.1038/nature14011
34. Liu YT, Sun ZJ. Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics. 2021;11(11):5365-5386 doi:10.7150/thno.58390
35. Anagnostou V, Smith KN, Forde PM. et al. Evolution of neoantigen landscape during immune checkpoint blockade in non-small cell lung cancer. Cancer Discov. 2017;7(3):264-276 doi:10.1158/2159-8290.CD-16-0828
36. Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science (1979). 2015;348(6230):69-74 doi:10.1126/science.aaa4971
37. Riaz N, Morris L, Havel JJ, Makarov V, Desrichard A, Chan TA. The role of neoantigens in response to immune checkpoint blockade. Int Immunol. 2016;28(8):411-9 doi:10.1093/intimm/dxw019
38. Pitt JM, Vétizou M, Daillère R. et al. Resistance Mechanisms to Immune-Checkpoint Blockade in Cancer: Tumor-Intrinsic and -Extrinsic Factors. Immunity. 2016;44(6). doi:10.1016/j. immuni. 2016 06.001
39. Del Prete A, Schioppa T, Tiberio L, Stabile H, Sozzani S. Leukocyte trafficking in tumor microenvironment. Curr Opin Pharmacol. 2017;35:40-47 doi:10.1016/j.coph.2017.05.004
40. Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014-22 doi:10.1038/ni.2703
41. Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell. 2017;168(4):707-723 doi:10.1016/j.cell.2017.01.017
42. O'Donnell JS, Long G V, Scolyer RA, Teng MWL, Smyth MJ. Resistance to PD1/PDL1 checkpoint inhibition. Cancer Treat Rev. 2017;52:71-81 doi:10.1016/j.ctrv.2016.11.007
43. Gadgeel S, Rodríguez-Abreu D, Speranza G. et al. Updated analysis from KEYNOTE-189: Pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. Journal of Clinical Oncology. 2020 38(14). doi:10.1200/JCO.19.03136
44. Gandhi L, Rodríguez-Abreu D, Gadgeel S. et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. New England Journal of Medicine. 2018;378(22):2078-2092 doi:10.1056/nejmoa1801005
45. Lesterhuis WJ, Punt CJA, Hato S V. et al. Platinum-based drugs disrupt STAT6-mediated suppression of immune responses against cancer in humans and mice. Journal of Clinical Investigation. 2011;121(8):3100-88 doi:10.1172/JCI43656
46. Fu Z, Li S, Han S, Shi C, Zhang Y. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Transduct Target Ther. 2022;7(1):93 doi:10.1038/s41392-022-00947-7
47. Mckertish CM, Kayser V. Advances and limitations of antibody drug conjugates for cancer. Biomedicines. 2021;9(8):872 doi:10.3390/biomedicines9080872
48. Vanmeerbeek I, Sprooten J, De Ruysscher D. et al. Trial watch: chemotherapy-induced immunogenic cell death in immuno-oncology. Oncoimmunology. 2020;9(1):1703449 doi:10.1080/2162402X.2019.1703449
49. Goto Y, Su WC, Levy BP. et al. TROPION-Lung02: Datopotamab deruxtecan (Dato-DXd) plus pembrolizumab (pembro) with or without platinum chemotherapy (Pt-CT) in advanced non-small cell lung cancer (aNSCLC). Journal of Clinical Oncology. 2023;41(16_suppl). doi:10.1200/jco. 2023 41.16_suppl.9004
50. Guo R, Li J, Hu J. et al. Combination of epidrugs with immune checkpoint inhibitors in cancer immunotherapy: From theory to therapy. Int Immunopharmacol. 2023;120:110417 doi:10.1016/j.intimp.2023.110417
51. Nathan P, Dummer R, Long GV. et al. LBA43 Spartalizumab plus dabrafenib and trametinib (Sparta-DabTram) in patients (pts) with previously untreated BRAF V600-mutant unresectable or metastatic melanoma: Results from the randomized part 3 of the phase III COMBI-i trial. Annals of Oncology. 2020;31. doi:10.1016/j. annonc. 2020 08.2273
52. Sabbatino F, Wang Y, Scognamiglio G. et al. Antitumor activity of BRAF inhibitor and IFN×alpha; Combination in BRAF-mutant melanoma. J Natl Cancer Inst. 2016;108(7):djv435 doi:10.1093/jnci/djv435
53. Gabrilovich D, Ishida T, Oyama T. et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92(11):4150-66 doi:10.1182/blood.v92.11.4150
54. Ko JS, Zea AH, Rini BI. et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clinical Cancer Research. 2009;15(6):2148-57 doi:10.1158/1078-0432.CCR-08-1332
55. Elamin YY, Rafee S, Toomey S, Hennessy BT. Immune Effects of Bevacizumab: Killing Two Birds with One Stone. Cancer Microenvironment. 2015;8(1):15-21 doi:10.1007/s12307-014-0160-8
56. Eisinger C, Muluneh B. Combined use of pembrolizumab and lenvatinib: A review. Journal of Oncology Pharmacy Practice. 2023;29(6):1461-1466 doi:10.1177/10781552231178461
57. Somasundaram R, Zhang G, Fukunaga-Kalabis M. et al. Tumor-associated B-cells induce tumor heterogeneity and therapy resistance. Nat Commun. 2017;8(1):607 doi:10.1038/s41467-017-00452-4
58. Winkler JK, Schiller M, Bender C, Enk AH, Hassel JC. Rituximab as a therapeutic option for patients with advanced melanoma. Cancer Immunology, Immunotherapy. 2018;67(6):917-924 doi:10.1007/s00262-018-2145-9
59. Boutros C, Tarhini A, Routier E. et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13(8):473-86 doi:10.1038/nrclinonc.2016.58
60. Yoest J. Clinical features, predictive correlates, and pathophysiology of immune-related adverse events in immune checkpoint inhibitor treatments in cancer: a short review. Immunotargets Ther. 2017;6:73-82 doi:10.2147/itt.s126227
61. Borghaei H, Paz-Ares L, Horn L. et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. New England Journal of Medicine. 2015;373(17):1627-39 doi:10.1056/nejmoa1507643
62. Brahmer J, Reckamp KL, Baas P. et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. New England Journal of Medicine. 2015;373(2):123-35 doi:10.1056/nejmoa1504627
63. Ribas A, Puzanov I, Dummer R. et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): A randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16(8):908-18 doi:10.1016/S1470-2045(15)00083-2
64. Gelao L, Criscitiello C, Esposito A, Goldhirsch A, Curigliano G. Immune checkpoint blockade in cancer treatment: A double-edged sword cross-targeting the host as an “innocent bystander.” Toxins (Basel). 2014;6(3). doi:10.3390/toxins6030914
65. Abdel-Wahab N, Shah M, Lopez-Olivo MA, Suarez-Almazor ME. Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease: A systematic review. Ann Intern Med. 2018;168(2):121-130 doi:10.7326/M17-2073
66. Tison A, Garaud S, Chiche L, Cornec D, Kostine M. Immune-checkpoint inhibitor use in patients with cancer and pre-existing autoimmune diseases. Nat Rev Rheumatol. 2022;18(11):641-656 doi:10.1038/s41584-022-00841-0
67. Gulati N, Celen A, Johannet P. et al. Preexisting immune-mediated inflammatory disease is associated with improved survival and increased toxicity in melanoma patients who receive immune checkpoint inhibitors. Cancer Med. 2021;10(21):7457-7465 doi:10.1002/cam4.4239
68. Brown LJ, Weppler A, Bhave P. et al. Combination anti-PD1 and ipilimumab therapy in patients with advanced melanoma and pre-existing autoimmune disorders. J Immunother Cancer. 2021;9(5):e002121 doi:10.1136/jitc-2020-002121
69. Subudhi SK, Aparicio A, Gao J. et al. Clonal expansion of CD8 T cells in the systemic circulation precedes development of ipilimumab-induced toxicities. Proc Natl Acad Sci U S A. 2016;113(42):11919-11924 doi:10.1073/pnas.1611421113
70. Flatz L, Berner F, Bomze D. et al. Association of Checkpoint Inhibitor-Induced Toxic Effects with Shared Cancer and Tissue Antigens in Non-Small Cell Lung Cancer. JAMA Oncol. 2019;5(7):1043-1047 doi:10.1001/jamaoncol.2019.0402
71. Lim SY, Lee JH, Gide TN. et al. Circulating cytokines predict immune-related toxicity in melanoma patients receiving anti-PD-1-based immunotherapy. Clinical Cancer Research. 2019;25(5):1557-1563 doi:10.1158/1078-0432.CCR-18-2795
72. Grigoriou M, Banos A, Hatzioannou A. et al. Regulatory T-cell transcriptomic reprogramming characterizes adverse events by checkpoint inhibitors in solid tumors. Cancer Immunol Res. 2021;9(7):726-734 doi:10.1158/2326-6066.CIR-20-0969
73. Haanen J, Obeid M, Spain L. et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Annals of Oncology. 2022;33(12):1217-1238 doi:10.1016/j.annonc.2022.10.001
74. Puzanov I, Diab A, Abdallah K. et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):95 doi:10.1186/s40425-017-0300-z
75. Li A, Altergott M. Management of Hospital Admissions for Checkpoint Inhibitor Immune-Related Adverse Events at a Regional Cancer Center. Oncol Issues. 2021;36(1):34-41 doi:10.1080/10463356.2020.1849902
76. Morgado M, Plácido A, Morgado S, Roque F. Management of the adverse effects of immune checkpoint inhibitors. Vaccines (Basel). 2020;8(4):575 doi:10.3390/vaccines8040575
77. Martins F, Sykiotis GP, Maillard M. et al. New therapeutic perspectives to manage refractory immune checkpoint-related toxicities. Lancet Oncol. 2019 20(1). doi:10.1016/S1470-2045(18)30828-3
78. Cheng R, Cooper A, Kench J. et al. Ipilimumab-induced toxicities and the gastroenterologist. Journal of Gastroenterology and Hepatology (Australia). 2015;30(4):657-66 doi:10.1111/jgh.12888
79. Oke AR, Wheater M, Karydis I, Wallis D. Successful use of adalimumab in immune checkpoint inhibitor-associated inflammatory arthritis. Rheumatol Adv Pract. 2018;2(1):rky001 doi:10.1093/rap/rky001
80. Harrison SR, McGonagle D, Nizam S. et al. Anakinra as a diagnostic challenge and treatment option for systemic autoinflammatory disorders of undefined etiology. JCI Insight. 2016;1(6):e86336 doi:10.1172/jci.insight.86336
81. Cappelli LC, Shah AA, Bingham CO. Immune-Related Adverse Effects of Cancer Immunotherapy— Implications for Rheumatology. Rheumatic Disease Clinics of North America. 2017;43(1):65-78 doi:10.1016/j.rdc.2016.09.007
82. Laird BJA, Fallon M, Hjermstad MJ. et al. Quality of life in patients with advanced cancer: Differential association with performance status and systemic inflammatory response. Journal of Clinical Oncology. 2016;34(23):2769-75 doi:10.1200/JCO.2015.65.7742
83. Montfort A, Filleron T, Virazels M. et al. Combining Nivolumab and Ipilimumab with Infliximab or Certolizumab in Patients with Advanced Melanoma: First Results of a Phase Ib Clinical Trial. Clinical Cancer Research. 2021;27(4):1037-1047 doi:10.1158/1078-0432.CCR-20-3449
84. Muselier A, Bielefeld P, Bidot S, Vinit J, Besancenot JF, Bron A. Efficacy of tocilizumab in two patients with anti-TNF-alpha refractory uveitis. Ocul Immunol Inflamm. 2011;19(5):382-3 doi:10.3109/09273948.2011.606593
85. Pérez-Moreiras JV, Álvarez-López A, Gómez EC. Treatment of active corticosteroid-resistant Graves' orbitopathy. Ophthalmic Plast Reconstr Surg. 2014;30(2):162-7 doi:10.1097/IOP.0000000000000037
86. Jonsson DI, Pirskanen R, Piehl F. Beneficial effect of tocilizumab in myasthenia gravis refractory to rituximab. Neuromuscular Disorders. 2017;27(6):565-568 doi:10.1016/j.nmd.2017.03.007
87. Osorio JC, Ni A, Chaft JE. et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Annals of Oncology. 2017;28(3):583-589 doi:10.1093/annonc/mdw640
88. Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med. 2014;6(230):230ra45 doi:10.1126/scitranslmed.3008002
89. Papadopoulos KP, Romero RS, Gonzalez G, Dix JE, Lowy I, Fury M. Anti-Hu-Associated Autoimmune Limbic Encephalitis in a Patient with PD-1 Inhibitor-Responsive Myxoid Chondrosarcoma. Oncologist. 2018;23(1):118-120 doi:10.1634/theoncologist.2017-0344
90. Ito M, Fujiwara S, Fujimoto D. et al. Rituximab for nivolumab plus ipilimumab-induced encephalitis in a small-cell lung cancer patient. Annals of Oncology. 2017;28(9):2318-2319 doi:10.1093/annonc/mdx252
91. Williams TJ, Benavides DR, Patrice KA. et al. Association of autoimmune encephalitis with combined immune checkpoint inhibitor treatment for metastatic cancer. JAMA Neurol. 2016;73(8):928-33 doi:10.1001/jamaneurol.2016.1399
92. Xiong G, Young RB, Chow H. et al. Intravenous immunoglobulin is safe and effective in controlling pre-existing paraneoplastic neuromuscular diseases in cancer patients treated with immune checkpoint inhibitors: two case reports and literature review. Front Oncol. 2023;13:1199195 doi:10.3389/fonc.2023.1199195
93. Kopecký J, Trojanová P, Kubeček O, Kopecký O. Treatment possibilities of ipilimumab-induced thrombocytopenia-case study and literature review. Jpn J Clin Oncol. 2015;45(4):381-4 doi:10.1093/jjco/hyu222
94. Trommer M, Yeo SY, Persigehl T. et al. Abscopal effects in radio-immunotherapy-response analysis of metastatic cancer patients with progressive disease under anti-PD-1 immune checkpoint inhibition. Front Pharmacol. 2019;10:511 doi:10.3389/fphar.2019.00511
95. Choi C, Yoo GS, Cho WK, Park HC. Optimizing radiotherapy with immune checkpoint blockade in hepatocellular carcinoma. World J Gastroenterol. 2019;25(20):2416-2429 doi:10.3748/wjg.v25.i20.2416
96. Ashrafizadeh M, Farhood B, Eleojo Musa A, Taeb S, Rezaeyan A, Najafi M. Abscopal effect in radioimmunotherapy. Int Immunopharmacol. 2020;85:106663 doi:10.1016/j.intimp.2020.106663
97. Voronova V, Lebedeva S, Sekacheva M, Helmlinger G, Peskov K. Quantification of Scheduling Impact on Safety and Efficacy Outcomes of Brain Metastasis Radio- and Immuno-Therapies: A Systematic Review and Meta-Analysis. Front Oncol. 2020;10:1609 doi:10.3389/fonc.2020.01609
98. Lehrer EJ, Peterson J, Brown PD. et al. Treatment of brain metastases with stereotactic radiosurgery and immune checkpoint inhibitors: An international meta-analysis of individual patient data. Radiotherapy and Oncology. 2019;130:104-112 doi:10.1016/j.radonc.2018.08.025
99. Ning C, Zhang X, Wang Y. et al. Radiation Therapy With Combination Therapy of Immune Checkpoint Inhibitors and Antiangiogenic Therapy for Hepatocellular Carcinoma. Int J Radiat Oncol Biol Phys. Published online. 2023 doi:10.1016/j.ijrobp.2023.07.001
100. Lee YT, Tan YJ, Oon CE. Molecular targeted therapy: Treating cancer with specificity. Eur J Pharmacol. 2018;834:188-196 doi:10.1016/j.ejphar.2018.07.034
101. Sullivan RJ, Hamid O, Gonzalez R. et al. Atezolizumab plus cobimetinib and vemurafenib in BRAF-mutated melanoma patients. Nat Med. 2019;25(6):929-935 doi:10.1038/s41591-019-0474-7
102. Chowdhury PS, Chamoto K, Honjo T. Combination therapy strategies for improving PD-1 blockade efficacy: a new era in cancer immunotherapy. J Intern Med. 2018;283(2):110-120 doi:10.1111/joim.12708
103. Dummer R, Lebbé C, Atkinson V. et al. Combined PD-1, BRAF and MEK inhibition in advanced BRAF-mutant melanoma: safety run-in and biomarker cohorts of COMBI-i. Nat Med. 2020;26(10):1557-1563 doi:10.1038/s41591-020-1082-2
104. Ribas A, Lawrence D, Atkinson V. et al. Combined BRAF and MEK inhibition with PD-1 blockade immunotherapy in BRAF-mutant melanoma. Nat Med. 2019;25(6):936-940 doi:10.1038/s41591-019-0476-5
105. Eggermont AMM, Robert C, Ribas A. The new era of adjuvant therapies for melanoma. Nat Rev Clin Oncol. 2018;15(9):535-536 doi:10.1038/s41571-018-0048-5
106. Schmid P, Cruz C, Braiteh FS. et al. Abstract 2986: Atezolizumab in metastatic TNBC (mTNBC): Long-term clinical outcomes and biomarker analyses. Cancer Res. 2017 77(13_Supplement). doi:10.1158/1538-7445.am2017-2986
107. Dirix L, Takacs I, Nikolinakos P. et al. Abstract S1-04: Avelumab (MSB0010718C), an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: A phase Ib JAVELIN solid tumor trial. Cancer Res. 2016 76(4_Supplement). doi:10.1158/1538-7445.sabcs15-s1-04
108. Tolba MF, Omar HA. Immunotherapy, an evolving approach for the management of triple negative breast cancer: Converting non-responders to responders. Crit Rev Oncol Hematol. 2018;122:202-207 doi:10.1016/j.critrevonc.2018.01.005
109. Tahover E, Sonnenblick A, Peretz T, Katz D. [Update: adjuvant trastuzumab in HER2 positive breast cancer]. Harefuah. 2012;151(1):37-42 61
110. Stagg J, Loi S, Divisekera U. et al. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc Natl Acad Sci U S A. 2011;108(17):7142-7 doi:10.1073/pnas.1016569108
111. Loi S, Giobbie-Hurder A, Gombos A. et al. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b-2 trial. Lancet Oncol. 2019;20(3):371-382 doi:10.1016/S1470-2045(18)30812-X
112. Coutzac C, Funk-Debleds P, Cattey-Javouhey A. et al. Targeting HER2 in metastatic gastroesophageal adenocarcinomas: What is new? Bull Cancer. 2023;110(5):552-559 doi:10.1016/j.bulcan.2022.08.013
113. Fan K, Cheng L, Li L. Artificial intelligence and machine learning methods in predicting anti-cancer drug combination effects. Brief Bioinform. 2021;22(6):bbab271 doi:10.1093/bib/bbab271
Author contact
![]() Corresponding author: Professor Itai Benhar, Ph.D., Department of Molecular Microbiology and Biotechnology, The Shmunis School of Biomedicine and Cancer Research, the George S. Wise Faculty of Life Sciences, Green Building room 202, Tel-Aviv University, Tel Aviv 69978, Israel, Phone: 972-3-6407511, Cell: 972-52-2524651, Fax: 972-3-6405829, Web: https://en-lifesci.tau.ac.il/profile/benhar, ORCID: https://orcid.org/0000-0002-0824-7177; Email: benhartau.ac.il.
Corresponding author: Professor Itai Benhar, Ph.D., Department of Molecular Microbiology and Biotechnology, The Shmunis School of Biomedicine and Cancer Research, the George S. Wise Faculty of Life Sciences, Green Building room 202, Tel-Aviv University, Tel Aviv 69978, Israel, Phone: 972-3-6407511, Cell: 972-52-2524651, Fax: 972-3-6405829, Web: https://en-lifesci.tau.ac.il/profile/benhar, ORCID: https://orcid.org/0000-0002-0824-7177; Email: benhartau.ac.il.

 Global reach, higher impact
Global reach, higher impact