Impact Factor
ISSN: 1449-2288
Int J Biol Sci 2024; 20(10):4098-4113. doi:10.7150/ijbs.97076 This issue Cite
Review
Exosomes: Emerging Insights into the Progression of Pancreatic Cancer
1. Department of Gastroenterology, Affiliated Hospital of Jiangsu University, Zhenjiang 212001, China.
2. Department of Laboratory Medicine, Wujin Hospital Affiliated with Jiangsu University, Changzhou, 213000, China.
3. Department of Hepatobiliary Pancreas Surgery, Aoyang Hospital Affiliated to Jiangsu University, Suzhou, 215000, China.
4. Jiangsu University School of Medicine, Jiangsu University, Zhenjiang 212001, China.
Received 2024-4-8; Accepted 2024-7-16; Published 2024-8-1
Abstract
Pancreatic cancer is a very aggressive and fatal malignancy with few therapeutic choices and a poor prognosis. Understanding the molecular pathways that drive its growth is critical for developing effective therapeutic strategies. Exosomes, small extracellular vesicles secreted by numerous cell types, have recently emerged as essential intercellular communication mediators, with implications for tumor growth and metastasis. In this article, we present a review of current knowledge about exosomes and their role in pancreatic cancer progression We discuss the biogenesis and characteristics of exosomes, as well as their cargo and functional significance in tumor growth, immune evasion, angiogenesis, invasion, and metastasis. We further emphasize the potential of exosomes as diagnostic biomarkers and therapeutic targets for pancreatic cancer. Finally, we discuss the challenges and future perspectives in using exosomes to improve patient outcomes in pancreatic cancer.
Keywords: exosomes, pancreatic cancer, biomarker, progression
Introduction
Cancer is a severe threat to human health, killing approximately 600,000 individuals per year [1]. Among them, pancreatic ductal adenocarcinoma stands out as a highly dangerous tumor of the digestive tract, ranking as the fourth leading cause of cancer-related deaths worldwide. Unfortunately, the five-year survival rate for patients diagnosed with this type of cancer is less than 9% [2]. Currently, surgical treatment remains the primary approach for treating pancreatic cancer. However, the lack of early specific methods has resulted in missed opportunities for optimal timing. [3]. Both environmental and genetic factors play crucial roles in the development of pancreatic cancer (PC) [4]. In fact, during the progression of PC, various factors can contribute to its malignant behavior. Understanding the interactions between these factors is a prominent focus in the study of PC's malignant behavior and related mechanisms.
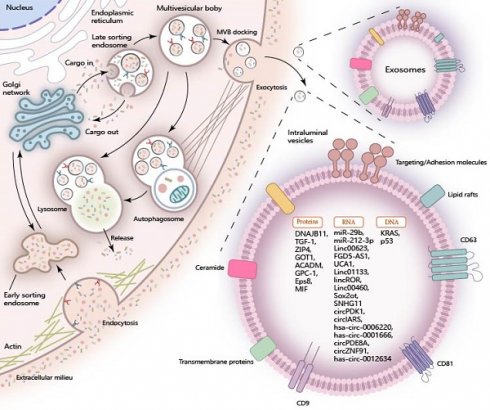
Extracellular vesicles, emitted by a variety of cellular sources, are typically categorized into three distinct types: apoptotic bodies, microvesicles, and exosomes. This classification is based on the differences in their size, molecular content, and the mechanisms by which they are formed [5]. Exosomes, in particular, stand out among other extracellular vesicles for their role in mediating cell-to-cell communication, playing a pivotal part in the exchange of signals and substances between cells [6]. The inward folding of the plasma membrane gives rise to a cup-shaped structure, which aids in the creation of an early-sorting endosome (ESE). This ESE undergoes maturation and develops into late-sorting endosomes (LSEs), which produce multivesicular bodies (MVBs). Intraluminal vesicles (ILVs) are enclosed within these MVBs' lumens before being released as exosomes [7, 8]. These exosomes have a diameter ranging from 30 to 150nm and contain proteins, nucleic acids, and lipids [9]. The unique lipid bilayer structure of exosomes originating from the inward folding of the plasma membrane allows them to protect their contents from external factors while still easily crossing cell membranes. This enables the substances carried by exosomes to be more efficiently absorbed by recipient cells [10].
At present, the application of exosomes as potential early molecular markers and drug delivery vehicles has garnered significant interest among researchers. The ability to isolate and purify exosomes is essential for successful research and development. Luckily, with advanced methodologies such as sequential ultracentrifugation, gradient ultracentrifugation, and ultrafiltration, this process has been greatly facilitated [11]. Furthermore, electroporation enables exosomes to encapsulate small molecule substances and therapeutic agents, thereby broadening the clinical applications of engineered extracellular vesicles [11]. In the context of pancreatic cancer (PC), both cancer-derived and non-cancer-derived exosomes play a significant role in promoting the progression of the disease. They can worsen the prognosis of patients by influencing PC cell behavior, modifying the tumor microenvironment, and increasing invasiveness and resistance. Notably, exosomes serve as a biomarker in liquid biopsy for patients with pancreatic cancer, enabling early prediction before the manifestation of significant symptoms. Additionally, the engineering exosomes facilitate more efficient drug delivery to pancreatic cancer tissues. Therefore, understanding the role of exosomes in PC research can provide valuable insights and pave the way for future investigations in this field.
Cargo Composition of Exosomes in Pancreatic Cancer
Exosomes are formed through the invagination of the plasma membrane and transport cell-surface proteins, soluble proteins, and small molecules like lipids, nucleic acids, and proteins. The encapsulated cargoes play a crucial role in regulating diverse metabolic processes within pancreatic cancer cells when they are internalized by recipient cells through exosomal uptake (Figure 1).
Exosomal RNAs in Pancreatic Cancer
Exosomal RNA plays a pivotal role in intercellular communications, particularly in the context of pancreatic cancer. Specifically, exosomal miRNAs, lncRNAs, and circRNAs play a crucial role in regulating the progression of pancreatic cancer. MiRNA is a class of noncoding transcripts comprising 18-23 nucleotides, which govern gene protein expression by binding to the 3′UTRs of mRNA molecules [12]. These exosomal miRNAs from PC cells are actively transferred to recipient cells, thus exerting a profound influence on the development of pancreatic cancer. For instance, exosomal miR-29b is delivered into human umbilical vein endothelial cells (HUVECs) and attenuates angiogenesis in pancreatic cancer [13]. Similarly, exosomal miR-212-3p is delivered to dendritic cells (DCs) and inhibits the expression of RFXAP, thereby disrupting the immune tolerance of dendritic cells [14]. LncRNA is a diverse class of transcripts longer than 200 nucleotides that possess minimal or no protein-coding ability [15]. Similar to miRNAs, exosomal lncRNAs function as crucial regulators in the progression of pancreatic cancer. Notable examples include exosomal LINC00623 [16], FGD5-AS1 [17], UCA1 [18], LINC01133 [19], linc-ROR [20], LINC00460 [21], Sox2ot [22], SNHG11 [23]. CircRNAs represent a class of non-coding RNAs characterized by their covalently closed loop structure, which confers enhanced stability and resistance to degradation [24]. Within exosomes derived from pancreatic cancer (PC) cells, circRNAs are found in high abundance and are known to perform a myriad of functions. Notable examples include circPDK1 [25], circ-IARS [26], hsa_circ_0006220, has_circ_0001666 [27], circ-PDE8A [28], circZNF91 [29], and has_circ_0012634 [30], each contributing uniquely to the complex regulatory network in PC. These exosomal circRNAs have been implicated in regulating the proliferation and migration of pancreatic cancer.
Exosomal Proteins in Pancreatic Cancer
Exosomal proteins in pancreatic cancer can be classified into two main categories. The first category comprises exosomal structural proteins, which include components of the cytoskeleton, as well as proteins involved in membrane fusion and transport. These proteins are strategically located on the exosomal surface and within the exosomal lumen, playing crucial roles in the exosome's structure and function. Characteristic proteins such as Annexins, Flotillin, Alix, and TSG101 [31] are frequently utilized for the identification and characterization of exosomes, given their presence is indicative of exosomal composition and function. The other proteins are directly involved in the regulation of proliferation, migration, and invasion through multiple signal pathways in pancreatic cancer. Notably, certain exosomal proteins have been identified for their specific roles in pancreatic cancer progression. For instance, exosomal DNAJB11 has been reported to regulate the development of pancreatic cancer via the EGFR/MAPK pathway [32]. Exosomal TGF-β1 is implicated in the induction of epithelial-mesenchymal transition (EMT) in pancreatic cancer [33]. Furthermore, exosomal ZIP4 enhances the proliferation of pancreatic cancer [34]. The upregulated expression of GOT1 in pancreatic cancer tissues is remarkable, as exosomal GOT1 has been found to suppress ferroptosis in pancreatic cancer by activating the Nrf2/HO-1 axis [35]. Moreover, exosomal proteins are associated with chemotherapy resistance and hold potential as biomarkers for pancreatic cancer. For instance, exosomal ACADM has been correlated with gemcitabine sensitivity and may serve as a predictive biomarker for chemotherapy response in patients [35]. Glypican-1 (GPC1) is found to be overexpressed in the exosomes of pancreatic cancer patients compared to those of healthy individuals and individuals with benign pancreatic diseases, suggesting that exosomal GPC1 could potentially serve as a biomarker to aid in the diagnosis and stratification of pancreatic cancer [36]. Eps8 has been observed to be highly expressed in exosomes from pancreatic cancer cells with metastatic characteristics, metastasis‑derived, indicating that exosomal Eps8 holds promise as a biomarker for pancreatic cancer metastasis [37]. Macrophage migration inhibitory factor (MIF) is also reported to be highly expressed in the PC-derived exosomes. Exosomal MIF favors the liver for metastasis and may serve as a prognostic marker for the development of PDAC liver metastasis [38].
Cargo of Exosomes in Pancreatic Cancer. During the biogenesis of exosomes, the inward folding of the plasma membrane and the sequential development of early-sorting endosomes (ESE), late-sorting endosomes (LSEs), and multivesicular bodies (MVBs) are pivotal steps. These processes enable the sequestration of a wide array of molecular constituents, including proteins, RNA, and DNA, into the exosomal lumen. Once internalized by target recipient cells, these encapsulated cargo molecules exert significant influence over a variety of cellular processes, thereby participating in intercellular communication and function regulation.
Exosomal DNAs in Pancreatic Cancer
Similar to other substances found in exosomes, exosomal DNAs have shown greater potential in diagnostic applications. These DNAs can be detected more easily in the circulatory system when carried by exosomes, serving as biomarkers for diagnosis and prognosis. Among the most common mutant genes in pancreatic cancer, KRAS mutations are frequently observed. In the serum exosomes of pancreatic cancer patients, KRAS mutations in exosomes have been detected in 43.6% of early-stage cases [39]. TP53 mutation, another common mutation in pancreatic cancer has also been identified in exosomes derived from pancreatic cancer, highlighting its potential as a biomarker for this disease [40, 41]. Furthermore, the application of engineered exosomes has facilitated the impact of exosomal DNA on the progression of pancreatic cancer. Researchers have documented the innovative application of non-autologous exosomes for the delivery of CRISPR/Cas9 plasmid DNA, specifically aimed at targeting the mutant Kras G12D oncogenic allele within pancreatic cancer cells. This approach has demonstrated the potential to effectively suppress tumor growth, offering a promising therapeutic strategy in the treatment of pancreatic cancer [42]. However, further exploration is needed to fully understand and optimize this approach.
Functional Implications of Exosomes in the Progression of Pancreatic Cancer
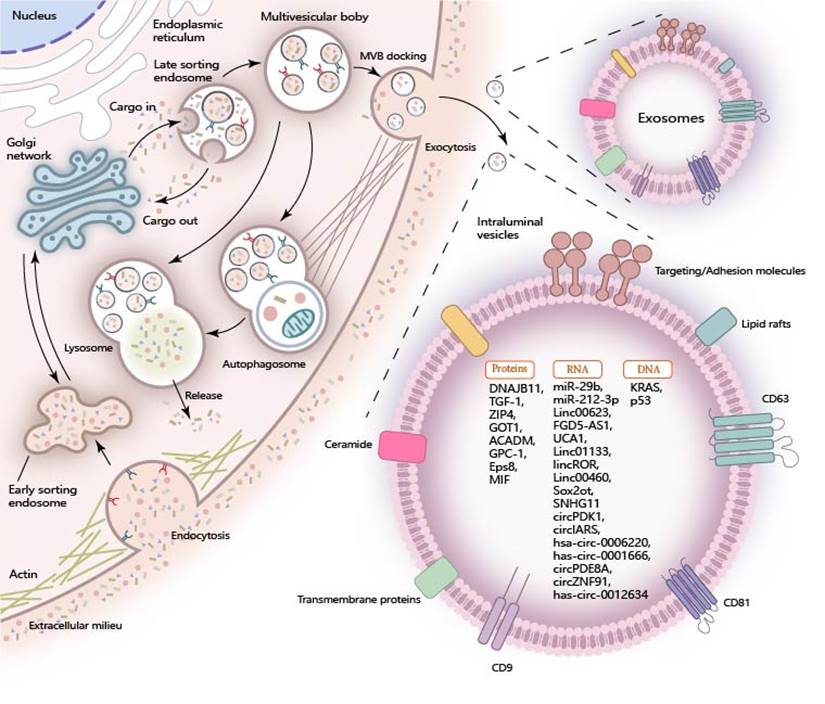
The advancement of pancreatic cancer (PC) encompasses a complex interplay of processes, including the proliferation, invasion, stem-like characteristics, and drug resistance of malignant cells. These phenomena are orchestrated by a multitude of regulatory factors that influence the trajectory and severity of the disease. Exosomes from various sources directly or indirectly favor these malignant tumor behaviors. They can be roughly divided into two types. One type is derived from cancer cells, and these exosomes are consumed by their cancer cells or neighboring cancer cells after being released, thus causing effects. The other types of exosomes come from non-cancer cells, including stromal cells, immune cells, and endothelial cells. These exosomes not only impact the cancer cells themselves but also influence the biological behavior of surrounding cells, thereby modifying the tumor microenvironment to enhance tumor growth. Exosomes act as critical mediators of intercellular communication, linking tumor cells with their surrounding microenvironmental cells. This interaction, as illustrated in Figure 2, plays a pivotal role in the promotion of tumorigenesis by modulating the local environment to support cancer cell growth and progression.
Modulation of the tumor microenvironment
The tumor microenvironment plays an important role in tumor progression. In the case of pancreatic cancer, different types of cells within the tumor immune microenvironment interact with each other, such as immune cells, endothelial cells, and fibroblasts. These interactions lead to the development of unique immunosuppressive properties within the pancreatic cancer microenvironment. Additionally, the tumor microenvironment also has a regulatory effect on the malignant behavior of PC cells. Exosomes, which act as carriers for intercellular message transmission, play an important role in regulating the PC microenvironment. Exosomes derived from pancreatic cancer are responsible for activating various cells within the microenvironment, and these activated stromal cells support the progression of tumor cells.
Exosomes and PC macrophages
Macrophages are an important type of stromal cells found in the PC microenvironment. These cells are derived from progenitor cells in bone marrow. Macrophages can be divided into two subtypes: M1 and M2. Polarized M1 macrophages, which are induced by IFN-γ or lipopolysaccharides, play a role in the immune response of type I helper T cells to different pathogens. They produce pro-inflammatory cytokines and can phagocytose tumor cells. On the other hand, M2 macrophages undergo polarization in response to cytokines such as IL-4, IL-10, or IL-13. They express a distinct set of molecules including arginase 1, IL-6, and angiogenic factors, all of which contribute significantly to the processes of tumorigenesis by creating a supportive microenvironment for tumor growth and progression [43]. Exosomes derived from PC cells deliver FGD5-AS1 into macrophages, leading to the polarization of M2 macrophages and promoting the growth of pancreatic cancer. FGD5-AS1 interacts with p300, resulting in the acetylation and activation of STAT3, as well as the activation of the STAT3/NF-κB pathway [17]. Additionally, ICAM-1, which is enriched in pancreatic cancer cells and their exosomes, interacts with CD11c on macrophages to regulate them. Exosomal ICAM-1 increases the levels of surface markers that indicate polarization towards an immunosuppressive M2-like phenotype.[44]. The expression of LINC00460 is increased in both PC cells and tissues. Exosomal LINC00460 promotes the polarization of M2 macrophages and supports the growth of tumor cells [21]. In a hypoxic state, miR-301a-3p is highly expressed in PC cells. Hypoxic exosomal miR-301a-3p from PC cells induces the polarization of M2 macrophages through the activation of the PTEN/PI3Kγ signaling pathway [45]. In conclusion, exosomes derived from PC cells play a role in the polarization of M2 macrophage. Interestingly, M2 macrophages secret exosomes containing miR-193b-3p [46] and lncRNA SBF2-AS1 [47]. These exosomes have been reported to suppress the malignant growth of PC cells, despite M2 macrophages typically being considered as immune cells that promote cancer. Further research is needed to explore whether exosomes derived from M2 macrophages can be a breakthrough in the treatment of PC.
Exosomes and Vascular Endothelial Cells in PC
The enriched vascular system is a key factor in the metastasis of pancreatic cancer [48]. Human vascular endothelial cells (HUVECs) play a crucial role in promoting the growth of new blood vessels of PC. Pancreatic cancer-derived exosomes are received by HUVECs, enhancing the ability of the tube formation of HUVECs and facilitating the angiogenesis around pancreatic cancer. Exosomal lncRNA SNHG11 is involved in the proliferation and angiogenesis of PC [23]. Moreover, circ-IARS is up-regulated in PC tissues and plasma exosomes from patients. Exosomal circ-IRAS increases the permeability of the blood vessel lining, thereby promoting vascular metastasis of tumor cells [26]. Additionally, MiR-27a plays a role in promoting the growth of multiple cancers. Specifically, exosomes derived from pancreatic cancer cells containing miR-27a have been found to enhance the proliferation, invasion, and angiogenesis in human umbilical vein endothelial cells (HUVECs). This effect is achieved through the negative regulation of a protein called BTG2[49]. Under hypoxic conditions, miR-30b-5p is significantly enriched in PC cell-derived exosomes. These exosomes promote tube formation and the migration of endothelial cells by miR-30b-5p mediating the downregulation of gap junction protein GJA1 [50]. Exosomal lncRNA UCA1 is transferred into HUVECs, promoting the migration and tube formation of recipient cells. Further research has revealed that lncRNA UCA1 acts as a competing endogenous RNA (ceRNA) for miR-96-5p, leading to the upregulation of AMOTL2 and p-ERK1/2 in HUVECs [18]. It is important to note that not all exosomes derived from pancreatic cancer cells promote angiogenesis. For example, MiR-29b is significantly downregulated in BxPC3 and AsPC-1 cells. After transfecting miR-29b mimics into BxPC3 and AsPC-1 cells, exosomal miR-29b can reduce angiogenesis by targeting ROBO1 and SRGAP2[13]. These exosomes are capable of delivering biological molecules into HUVECs and activating their ability to form tube-like structures.
Exosomes and Stellate Cells in PC
Cancer-associated fibroblasts (CAFs) are crucial components of the tumor microenvironment. They originate from stellate cells in the PC and play a significant role in the progression of PC. These CAFs create an immunosuppressive microenvironment for PC, making the tumor resistant to external drug treatments [51]. Research has shown that exosomes derived from pancreatic stellate cells carry miR-5703 into PC cells. This miRNA downregulates CMTM4 and activates the PI3K/Akt pathway, promoting the proliferation of PC cells [52]. In hypoxic conditions, exosomal miR-4465 and miR-616-3p from pancreatic stellate cells (PSCs) target PTEN and activate AKT signaling in PC cells [53]. Additionally, exosomes from PSCs carry miR‑21, which induces EMT through the activation of the Ras/ERK signaling pathway [54]. Once stellate cells transform into CAFs, exosomes from CAFs carry different substances that regulate the progression of PC. For instance, CAF-derived exosomes deliver miR-421 into PC cells, targeting SIRT3 and regulating the SIRT3/H3K9Ac/HIF-1α axis to enhance the proliferation, migration, and invasion abilities of PC cells [55]. Interestingly, exosomes derived from PC cells themselves also promote the conversion of PSCs to CAFs. This creates a positive feedback loop between PC cells and CAFs through exosomal communication, thereby resulting in rapid remodeling of the PC microenvironment and further promoting tumor progression.
Exosomes and Bone Marrow Mesenchymal Stem Cell in PC
There is a strong connection between bone marrow mesenchymal stem cells (BM-MSCs) and pancreatic cancer. BM-MSCs play a role in the development of pancreatic cancer through various methods, including the release of exosomes [56]. Currently, BM-MSC-derived exosomes have been confirmed to inhibit the malignancy of pancreatic cancer. According to Yao et al., exosomes from BM-MSCs carry circ_0030167 into pancreatic cancer cells and regulate miR-338-5p. This regulation leads to enhanced expression of Wif1, which inhibits the Wnt8/β-catenin pathway. Ultimately, exosomal circ_0030167 suppresses the invasion, migration, proliferation, and stemness of PC cells [57]. Another study also supports this conclusion, stating that BM-MSC-derived exosomal circ_6790 facilitates the nuclear translocation of chromobox 7 (CBX7), leading to an increase in the DNA methylation of S100A11 and inhibition of its transcription. As a result, exosomal circ_6790 inhibits the growth, metastasis, and immune escape of PC cells [58]. Additionally, miR-1231 has been found to have a potential inhibitory effect on the progression of PC. Exosomal miR-1231 derived from BM-MSCs inhibits the proliferation, migration, invasion, and adhesion to the matrix of PC cells [59]. In summary, exosomes derived from BM-MSCs have an inhibitory effect on the development of PC. If these exosomes can be engineered to deliver drugs for PC therapy, they would have advantages over exosomes from other sources. In addition to the inhibitory effect of the drug itself on the progression of PC, the substances present in exosomes would further hinder the progression of PC, thereby improving the effectiveness of engineered exosome-loaded drugs.
Exosomes and Lymphocytes in PC
The lymphocyte is one of the main cells to resist the progression of PC. These cells regulate the inflammatory response around PC cells by secreting various active molecules. These immune cells with anti-tumor effects inhibit pancreatic cancer progression by secreting exosomes. Natural killer (NK) cells play a crucial role in the immune system's defense against tumor growth and metastasis. These cells secrete exosomes that are capable of transferring a variety of microRNAs, including miR-16-5p, miR-342-3p, miR-24-3p, miR-92a-3p, and let-7b-5p, into pancreatic cancer cells. Notably, let-7b-5p has been shown to exert an antitumor effect by specifically targeting the gene CDK6, thereby inhibiting cell cycle progression and promoting a tumor-suppressive response. This mechanism highlights the potential of NK cell-derived exosomes as a therapeutic strategy for modulating the tumor microenvironment [60]. In another study, miR-3607-3p is found enriched in the exosomes from NK cells and transmitted to PC cells, which will suppress the progression of PC cells via direct targeting of IL-26[61]. However, recent studies suggest that the content of various types of lymphocytes in the pancreatic cancer microenvironment is low, which also leads to the immunosuppressive properties of pancreatic cancer. T lymphocytes are one of the main cells that carry out anti-tumor effects. According to Tao et al., exosomes from pancreatic cancer are taken up by T lymphocytes to activate p38 MAPK, and then induce endoplasmic reticulum stress-mediated apoptosis [62]. In general, the secretion of exosomes by lymphocytes can inhibit the progression of pancreatic cancer, but at the same time, exosomes originating from PC cells can mediate the death of lymphocytes. Eventually, immunosuppression was observed in the TME.
Proliferation of PC cells
The malignant behavior of tumor cells is caused by the abnormal growth of cells. When tumor cells grow rapidly and uncontrollably, they take away essential nutrients from neighboring cells that are necessary for their survival. Cancer-derived exosomes (CDE) and some non-cancer-derived exosomes (nCDE) contribute to the uncontrolled growth of prostate cancer cells. Recent research indicates that exosomes derived from cancer carry various types of genetic material into cancer cells, fueling their proliferation. For instance, CircPDK1 is abundantly expressed in exosomes derived from both pancreatic cancer cells and the serum of patients. The uptake of exosomal CircPDK1 enhances tumor cell proliferation, migration, and glycolysis by sponging miR-628-3p and activating the BPTF/c-myc axis.[25]. Similarly, lncRNA SNHG11 is also highly expressed in the serum of PC patients. Exosomal SNHG11 promotes cell proliferation, migration, and angiogenesis by sponging miR-324-3p and regulating the expression of VEGFA [23]. Additionally, exosomal lncRNA 01133 activates the Wnt/β-catenin pathway, leading to the proliferation, migration, invasion, and epithelial-mesenchymal transition (EMT) of PC cells [19].
In addition, exosomes also contain various proteins that promote the proliferation of PC cells. One such protein is ZIP4, a membrane-located zinc ion transporter that regulates, intracellular zinc homeostasis and is upregulated in exosomes from PC cells. Exosomal ZIP4 can significantly promote PC growth [34]. Asparaginyl endopeptidase (AEP), highly expressed in solid tumors, is also detected in exosomes from pancreatic cancer. Exosomal AEP contributes to the activation of the phosphoinositide 3-kinase/RAC‑α serine/threonine-protein kinase signaling pathway in PC cells, thereby promoting cell proliferation [63]. Lastly, GOT1 is upregulated in PC tissues, and exosomal GOT1 suppresses tumor cell ferroptosis, accelerating PC progression through activating Nrf2/HO-1 axis and upregulating CCR2 expression [35]. Notably, these molecules are associated with TNM staging and poor prognosis in PC patients and may serve as early biomarkers for the disease. However, more research is needed to provide additional information and clarify the topic.
Pancreatic cancer cells regulate microenvironmental remodeling by secreting exosomes. There are a variety of stromal cells in the microenvironment of pancreatic cancer, such as CAF, macrophages, endothelial cells, etc. Pancreatic cancer cells interact with these cells by secreting exosomes and regulating related biological functions. In addition, other stromal cells also affect tumor cells through the secretion of exosomes, which promotes the remodeling of the pancreatic cancer microenvironment.
Migration of Pancreatic Cancer Cells
In the early stage of pancreatic tumor metastasis, tumor cells undergo a series of changes in their biological behavior. These changes enhance the metastatic ability and anti-apoptosis of tumor cells [64, 65]. Therefore, cancer cells can break away from the primary tumor, invade surrounding tissues, and enter the circulatory system, becoming circulating tumor cells (CTCs) [66]. One important process in this progression is called epithelial-mesenchymal transition (EMT). EMT is a reversible process in which epithelial cells lose their polarity and intercellular connections and acquire a more mesenchymal shape [67]. During the metastasis and invasion of pancreatic cancer (PC), EMT occurs.[68]. Exosomes, which are small extracellular vesicles, play a role in transmitting information in the tumor microenvironment. Both cancer-derived and non-cancer-derived exosomes can induce changes in the biological functions of tumor cells upon being received. Research has shown that exosomes from pancreatic cancer cells deliver TGF-β1 [33] and lncRNA 01133 [19] into cancer cells, leading to the induction of EMT. Exosomal lncSox2ot from highly invasive PC cells promotes tumor invasion and metastasis in vitro and in vivo by enhancing EMT and stem cell-like properties of PC cells [22]. MiR-125b-5p is also upregulated in highly invasive PC cells and their exosomes. These exosomes activate MEK2/ERK2 signaling pathway, inducing EMT [69]. Linc-ROR, which is highly expressed in PC cells and their exosomes, facilitates EMT in PC cells by activating the HIF1α/ZEB1 signaling pathway and interleukin-1β (IL-1β) [20]. Further investigation is needed to determine if these exosomes can serve as biomarkers for the occurrence of EMT in PC.
Maintenance of Stemness of PC Cells
Tumor stemness maintenance refers to the process of maintaining the characteristics of cancer stem cells. This enables cancer cells to exhibit enhanced migration, proliferation, and resistance to chemotherapy. Exosomes derived from PC cells have been found to promote the stemness of cancers. Wang et al. have discovered that hypoxia-induced cancer-derived exosomes transport into tumor cells, thereby promoting the stemness of tumor cells. These exosomal lncROR inhibit the activation of the Hippo/Yes-associated protein (Hippo/YAP) pathway specifically in pancreatic cancer [70]. Exosomal lncRNA-Sox2ot has also been reported to promote the stemness of PC cells by acting as a competing ceRNA [22]. However, in the progression of PC, some exosomes induce PC stem cell differentiation and suppress stemness maintenance. For instance, miR-21a-5p is upregulated in exosomes derived from M2 macrophages. These exosomal miR-21a-5p molecules specifically target KLF3 and regulate pancreatic stem cells [71]. Further research is needed to investigate whether these exosomes, which indicate a strong ability to maintain stemness in PC cells, can serve as biomarkers for predicting the chemoresistance of cancer cells.
Clinical Application of Exosomes in Pancreatic Cancer
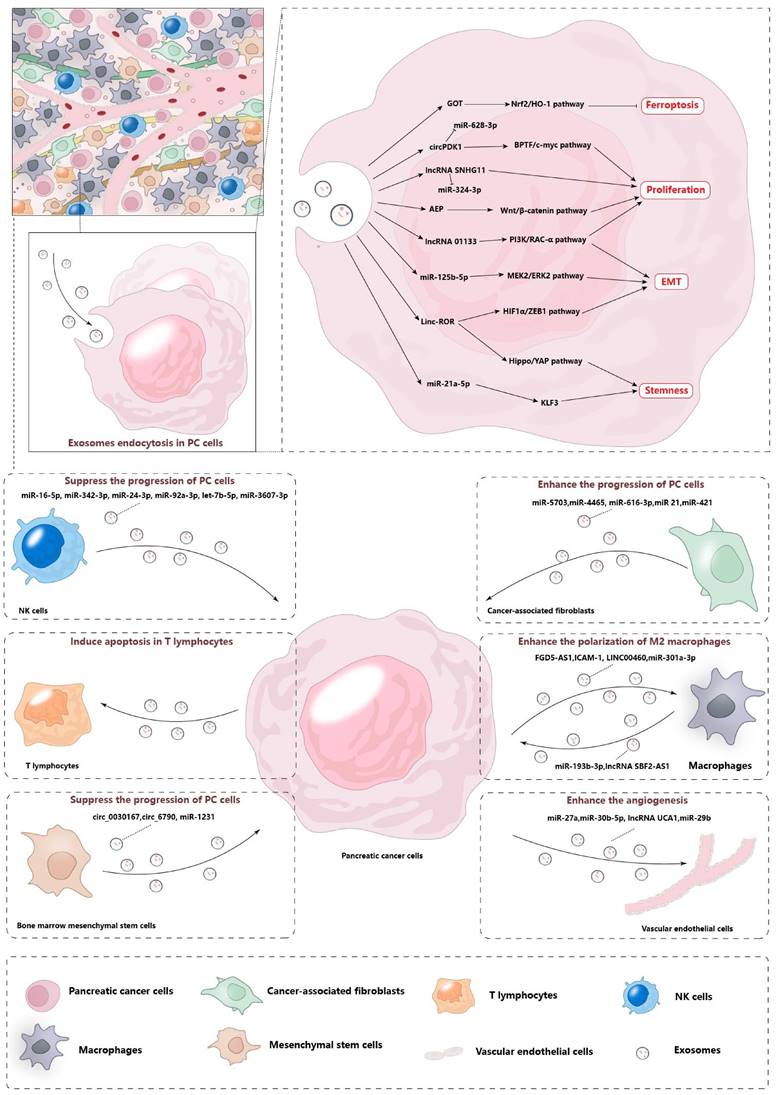
Exosomes hold exceptional promise for clinical applications, as depicted in Figure 3. Their unique bilayer membrane structure endows them with the capacity to safeguard their cargo, rendering them resilient to degradation by external factors. The extraction of exosomes from serum, urine, ascites, and other bodily fluids allows for their utilization as diagnostic and prognostic biomarkers. Furthermore, when cells are transfected with small molecules, the exosomes they secrete encapsulate these molecules, offering a vehicle for potential therapeutic agents. By scrutinizing the specific exosomes present in the circulatory system and monitoring the variations in their molecular cargo, the early detection of pancreatic cancer can be achieved. Additionally, the analysis of exosomes provides a valuable tool for assessing the efficacy of treatments, offering a non-invasive and sensitive approach to monitoring disease progression and response to therapy.
Diagnostic and Prognostic Potential of Exosomes
Early diagnosis plays a crucial role in the treatment of pancreatic cancer. Unfortunately, due to the absence of precise identification methods in the initial stages of the disease, patients often do not receive the most effective treatment when diagnosed. However, there is ongoing research on the potential use of exosomes, which are early indicators, as biomarkers for pancreatic cancer. These exosomes can be found in various bodily fluids and may hold promise for early detection and diagnosis of the disease. Research has investigated that some proteins in exosomes can be detectable in PC.
Exosomes containing specific proteins are often used as biomarkers in pancreatic cancer. For instance, Glypican-1 (GPC1) is a membrane-anchored protein in a variety of cancers including PC. Detecting GPC1-positive circulating exosomes (crExos) is a reliable way to identify early-stage pancreatic cancer, and these GPC1-positive crExos are even more effective as a prognostic marker compared to the current serum biomarker CA 19-9 [72]. Additionally, combining CD63-GPC1-positive exosomes with CA19-9 shows promising potential for diagnosing resectable pancreatic cancer [73]. Another diagnostic biomarker is exosomal ALIX, when combined with CA199, has the potential to detect pancreatic cancer and differentiate between early and advanced stages [74]. Exosomal ZIP4 has been found to promote PC growth and serum exosomal. Also, ZIP4 has been identified as a novel diagnostic biomarker for pancreatic cancer [34]. Similarly, exosomal-EphA2 has been reported to facilitate cell migration in pancreatic cancer, and levels of serum exo-EphA2 are significantly higher in pancreatic cancer patients compared to those with benign pancreatic disease or healthy controls [75]. Additionally, high level of exo-EphA2 expression in pancreatic cancer is linked to a shorter overall survival. This finding indicates that exo-EphA2 can serve as a negative prognostic factor for patients with pancreatic cancer [76, 77]. Furthermore, recent research by Alexander Lux et al. has shown that the exosomes derived from the serum of pancreatic cancer patients express c-Met and PD-L1. Patients with positive for c-Met and PD-L1 have shown a significantly shorter survival time after surgery [78]. Further research has revealed that the levels of PD-L1 in serum-derived exosomes are higher in patients with metastatic pancreatic cancer compared to those with locally advanced disease. Additionally, higher levels of exosomal PD-L1 in patients with advanced pancreatic cancer are associated with worse survival outcomes [79].
Clinical application of exosomes in pancreatic cancer. The unique double-layer membrane of exosomes protects the biomolecules carried in exosomes so that these exosomes carrying special molecules can be maintained in various body fluids for a long time and can be detected and extracted. These exosomes can also be engineered to have a wide range of clinical applications.
The expression of miR-125b-3p, miR-122-5p, and miR-205-5p in plasma exosomes has been investigated as a potential biomarker for PC. A study has confirmed that these miRNAs were overexpressed in the plasma exosomes of PC patients compared to healthy controls [80]. Moreover, plasma-derived exosomal miR-19b holds promise as a diagnostic marker for pancreatic cancer [81]. Jia Chen et al., have discovered that the expression of miR-451a in serum-derived exosomes from PC patients is significantly upregulated compared to those from patients with pancreatic benign disease and healthy individuals. Furthermore, exosomal miR-451a has been shown to have a significant association with clinical stage and distant metastasis in PC [82]. Exosomal miR-1226-3p is another biomarker of PC. The expression of serum exosomal miRNA-1226-3p is downregulated in PDACs compared to benign pancreatic lesions [83]. Xiaofan Pu et al. have demonstrated that exosomal miR-21 from the plasma of patients with PC is higher compared to the control group. Additionally, exosomal miR-21 can differentiate between patients patients with early-stage PC and healthy controls [84]. According to the miRNA microarray analyses, miR-451a has been shown to have the highest upregulation in PC patients at stage II who experienced recurrence after surgery. And exosomal miR-451a shows a significant association with tumor size and stage [85]. Another study has indicated that exosomal miR-4525, miR-451a, and miR-21 levels are upregulated in the portal vein blood [86]. Research from Lun Wu et al. has also reported that exosomal miRNA-21 and miRNA-210 are significantly higher in patients with PC compared to patients with chronic pancreatitis [87]. MiR-483-3p is overexpressed in PC and PanIN tissues compared to normal pancreatic duct cells. Circulating miR-483-3p levels are significantly elevated in the serum and serum exosomes of PC patients compared to healthy controls [88]. The expression of miR‑23b‑3p is consistently higher in serum exosomes from PC patients as compared to those from healthy controls [89]. The expression of exosomal miR-191, miR-21, and miR-451a are significantly up-regulated in patients with PC and IPMN compared to the controls [90]. Plasma exosome-derived circRNAs are also investigated to function as biomarkers. Compared to healthy controls, hsa_circ_0006220 and hsa_circ_0001666 are highly expressed in exosomes in the plasma of PC patients, suggesting that these could be utilized as new biomarkers for the diagnosis and treatment of PC [27].
Furthermore, besides using serum-derived exosomes as biomarkers, researchers have also investigated exosomes from other bodily fluids. In patients with advanced pancreatic cancer, it has been observed that exosomes from ascites have a high expression of CD133. However, more research is needed to determine if exosomal CD133 can be used as a biomarker [91]. Additionally, in urine exosomes, there is an increased ratio of miR-3940-5p to miR-8069 in pancreatic cancer cases.
According to the research, exosomes from the culture media of PC have shown a significantly higher ratio of miR-3940-5p/miR-8069, and this ratio is higher in urine exosomes than in serum exosomes [92]. Another research suggests that messenger RNAs (mRNAs: ARF6 and WASF2) and small nucleolar RNAs (snoRNAs: SNORA25 and SNORA74A) in the serum of patients with PC may be useful for the early detection of PC [93]. The presence of miR-196a and miR-1246 in PC exosomes, as determined by qRT-PCR analysis, could potentially serve as indicators of localized PC [94]. Exosomal alkaline phosphatase placental-like 2 (ALPPL2), a glycosyl phosphatidyl inositol (GPI)-anchored membrane protein, has also been identified as a potential biomarker for early diagnosis of PC [95]. Additionally, cytoskeleton-associated protein 4 (CKAP4), a novel Dickkopf1 (DKK1) receptor, is being investigated as a candidate for PC diagnosis and therapy. CKAP4 is secreted with SEVs from PC cells and the serum CKAP4 levels are higher in patients with PC compared to healthy individuals [96]. However, these studies have lacked relevant epidemiological data, and further research is needed.
Inhibition of exosome release and uptake in Pancreatic Cancer
Pancreatic cancer employs a strategy to promote its progression by releasing exosomes that modulate the functions of various stromal cells, immune cells, and endothelial cells within the tumor microenvironment. This, in turn, facilitates the remodeling of the surrounding microenvironment. Disrupting the release and uptake of exosomes in pancreatic cancer cells presents a promising approach for the treatment of this disease. Yang et al. discovered that ZIP4 activates the CREB-regulated expression of RAB27B, which is crucial for the release of exosomes from pancreatic cancer cells. Targeting these pathways may effectively reduce the proliferation of pancreatic cancer cells [97]. Additionally, PRKD1 has been identified as a suppressor of motility in pancreatic cancer. Loss of PRKD1 results in decreased phosphorylation of cortactin in pancreatic cancer cells, leading to increased F-actin at the plasma membrane and enhanced exosome release [98].
Wang et al. reported that the expression of DUSP2 is significantly suppressed in pancreatic cancer cells, leading to increased secretion of extracellular vesicle-associated VEGF-C [99]. In addition, hypoxia has been found to enhance the release and size distribution of exosomes from pancreatic cancer cells which, HIF-1α is a key factor in this process [100]. Targeting these molecules could offer new strategies for the treatment of pancreatic cancer.
Exosomes as Biomarkers in PC.
| Category | Source | Content | AUC | Sensitivity | Specificity | Function | Ref. |
|---|---|---|---|---|---|---|---|
| Protein | serum | GPC1 | 1.000 | 100% | 100% | Diagnostic biomarker for PC | [72] |
| Protein | serum | ALIX | 0.730 | 53.1% | 83.9% | Diagnostic biomarker for PC | [74] |
| serum | ALIX+CA199 | 0.910 | 90.6% | 83.9% | |||
| Protein | serum | ZIP4 | 0.893 | Diagnostic biomarker for PC | [34] | ||
| Protein | serum | EphA2 | Independent risk factor for PC | [76] | |||
| Protein | serum | c-Met | 0.779 | 70% | 85% | Negative prognostic factors for PC | [78] |
| Protein | serum | PD-L1 | 0.491 | 14% | 94% | ||
| miRNA | serum | miR-125b-3p | 0.736 | Diagnostic biomarker for PC | [80] | ||
| miRNA | serum | miR- 122-5p | 0.726 | ||||
| miRNA | serum | miR-205-5p | 0.829 | ||||
| miRNA | serum | miR-19b | 0.942 | 85.48% | 90.57% | Diagnostic biomarker for PC | [81] |
| miRNA | serum | miR-451a | Diagnostic biomarker for PC | [82] | |||
| miRNA | serum | miR-1226-3p | 0.740 | Negative biomarker for PC | [83] | ||
| miRNA | serum | miR-21 | 0.717 | Diagnostic biomarker for PC | [84] | ||
| miRNA | serum | miR-10b | 0.654 | Diagnostic biomarker for PC | |||
| miRNA | serum | miR-21+ miR-10b | 0.791 | Diagnostic biomarker for PC | |||
| miRNA | serum | miR-451a | Diagnostic biomarker for PC | [85] | |||
| miRNA | serum | miR-4525 | Independent prognostic factors for PC | [86] | |||
| miRNA | serum | miR-21 | 0.869 | 80.00% | 90% | Biomarkers and therapeutic targets for PC | [87] |
| miRNA | serum | miR-210 | 0.823 | 83.00% | 90% | ||
| miRNA | serum | miR-483-3p | 0.830 | 85.7% | 72.7% | Diagnostic biomarker for PC | [88] |
| miRNA | serum | miR‑23b‑3p | Biomarkers and therapeutic targets for PC | [89] | |||
| miRNA | serum | miR-191 | 0.788 | 71.9% | 84.2% | Diagnostic biomarker for PC | [90] |
| miRNA | serum | miR-21 | 0.826 | 80.7% | 81.0% | ||
| miRNA | serum | miR-451a | 0.789 | 65.6% | 86.7% | ||
| circRNA | serum | circ_0006220 | 0.782 | 77.42% | 72.58% | Diagnostic biomarker for PC | [27] |
| circRNA | serum | circ_0001666 | 0.806 | 96.77% | 51.61% | ||
| serum | circ_0006220+ circ_0001666 | 0.884 | 74.20% | 87.10% | |||
| Protein | ascite | CD133 | Diagnostic biomarker for PC | [91] | |||
| miRNA | urine | miR-3940-5p/miR-8069 ratio | 0.732 | 58.1 | 89.2 | Diagnostic biomarker for PC | [92] |
| mRNA | serum | WASF2 | 0.943 | Diagnostic biomarker for PC | [93] | ||
| mRNA | serum | ARF6 | 0.940 | ||||
| snoRNA | serum | SNORA74A | 0.909 | ||||
| snoRNA | serum | SNORA25 | 0.903 | ||||
| mRNA | serum | miR-196a | Diagnostic biomarker for PC | [94] | |||
| mRNA | serum | miR-1246 | |||||
| Protein | Cell supernatant | ALPPL2 | Diagnostic biomarker for PC | [95] | |||
| Protein | Cell supernatant | CKAP4 | Diagnostic biomarker for PC | [96] |
Exosomes and the Chemoresistance
Currently, FOLFIRINOX is the primary chemotherapy treatment for pancreatic cancer that has spread locally or metastasized. Modified FOLFIRINOX and combinations of gemcitabine with other drugs are also commonly used [101]. Research has shown that exosomes can impact the resistance of pancreatic cancer cells to chemotherapy by regulating their proliferation, EMT, and stemness. According to the research from Mikamori et al., long-term exposure to GEM will increase the miR-155 expression in PC cells and exosomes, which leads to anti-apoptotic activity and GEM resistance in other PC cells [102]. Specifically, exosome-derived panc-1 cells that are resistant to gemcitabine (GEM) have been found to increase the GEM resistance of MIA PaCa-2 and BxPC-3 cells. This effect is believed to be mediated by Ephrin type-A receptor 2 (EphA2), a protein that is overexpressed in panc-1 cells [103]. Another protein, matrix metalloproteinase 14 (MMP14), a transmembrane Zn2+-dependent MMP, has been found to play a role in promoting recipient cells' resistance to gemcitabine. Exosomal MMP14 is transferred to recipient cells, promoting their sphere formation and migration by enhancing the stability of CD44. As a result, the recipient cells become more resistant to gemcitabine [104]. Additionally, exosomes derived from hypoxic pancreatic cancer cells have been shown to promote gemcitabine resistance in normoxic pancreatic cancer cells. This is due to a hypoxia-induced exosomal circular RNA called circZNF91, which competitively binds to miR-23b-3p. This binding leads to enhanced stability of HIF-1α protein and increased glycolysis, both of which contribute to gemcitabine resistance in normoxic cells [29]. Hypoxia-induced tumor-derived exosomal lncROR has been shown to enhance stemness and promote chemoresistance in pancreatic cancer cells treated with GEM through the Hippo signaling pathway [70]. Interestingly, there is a reciprocal relationship between exosomes and chemoresistance. Prolonged exposure to GEM increases the release of exosomes, which in turn further enhances the chemoresistance in PC [102]. Non-oncogenic exosomes derived from cancer-associated fibroblasts (CAFs) also play a role in regulating chemoresistance. These exosomes carry miR-3173-5p into PC cells and promote chemoresistance to GEM by suppressing ferroptosis through the inhibition of ACSL4 [105]. Moreover, during GEM treatment, certain microRNAs, such as miR-21, miR-181a, miR-221, miR-222, and miR-92a, are highly expressed in exosomes derived from CAFs. These exosomal microRNAs downregulate PTEN expression, leading to increased proliferation and chemoresistance in PC cells [106]. CAFs are also involved in the regulation of exosome release by influencing the stiffness of the extracellular matrix [107]. As mentioned earlier, exosomes play a critical role in mediating GEM chemoresistance. These molecules are packaged into the exosomes and transferred from chemoresistant cells to sensitive cells, conferring resistance to GEM treatment. According to the research conducted by Ella Rimmer and her colleagues, they have stated that the mentioned effect can be counteracted when GW4869, an inhibitor of exosome biogenesis and release, is present [108]. However, it is still necessary to conduct further studies to determine whether the removal of extracellular vesicles during treatment would enhance the response of cells to GEM.
Engineering Exosomes in Pancreatic Cancer
Engineering exosomes refers to the modification of exosomes by physical and chemical means so that the modified exosomes carry small molecules without changing the structure and characteristics of exosomes. According to the research from Kamerkar et al., exosomes derived from normal fibroblast-like mesenchymal cells were engineered to carry short interfering RNA or short hairpin RNA specific to oncogenic KrasG12D. In the mouse models of pancreatic cancer, overall survival is significantly increased after treatment with these exosomes [109]. Another research also reports that non-autologous exosomes can deliver CRISPR/Cas9 plasmid DNA which targets the mutant Kras G12D into recipient pancreatic cancer cells in syngeneic subcutaneous and orthotopic models and suppresses proliferation and tumor growth in these models [42]. Chemotherapy drugs can also be transfected into exosomes. Li et al. report that GEM is loaded into autologous exosomes which is uptaken by pancreatic cancer cells and significantly suppresses tumor growth in tumor-bearing mice [110].
Exosomes from human umbilical cord mesenchymal stem cells (HUMSCs) are reported to suppress the malignant behavior of tumor cells after being pre-conditioned with exogenous molecules. The expression of Galectin-3 is higher in the pancreatic cancer cells. Xie et al. transfected hsa-miRNA-128-3p into HUCMSCs and collected the exosomes from these cells. According to the research, HUCMSC-derived exosomes with hsa-miRNA-128-3p inhibited the proliferation, invasion, and migration of PANC-1 cells in vitro by targeting Galectin-3[111]. Another research by Ding et.al, they used HUMSCs exosomes to deliver exogenous miR-145-5p, which suppressed PDAC cell proliferation and invasion and increased apoptosis and cell cycle arrest [112]. In the research from Klimova et al., exosomes from human dental pulp MSCs (HD-MSCs) can carry GEM and inhibit the cell growth of pancreatic cancer cells in vitro. DP-MSCs were simultaneously engineered to express a suicide gene yCD: UPRT, which will generate 5-fluorouracil (5-FU) in the recipient cancer cells and further suppress the growth of pancreatic cancer cells [113]. In brief, engineered exosomes have shown promising application prospects in both in vivo and in vitro experiments, but there is a lack of relevant clinical trials, and specific research needs to be further carried out.
Challenges and Perspectives
So far, we have discussed the role of exosomes in pancreatic cancer and their potential clinical application. These exosomes play a pivotal role in regulating the progression of pancreatic cancer cells and remodeling the surrounding microenvironment. Targeting these exosomes offers promising avenues for the treatment of the treatment of pancreatic cancer. However, the study and utilization of pancreatic cancer exosomes face several challenges. Firstly, there is a lack of standard isolation and characterization protocols for pancreatic exosomes [114]. The extraction and separation of exosomes are often accompanied by issues such as loss of activity, contamination with non-vesicular substances, and a time-consuming separation process, which are yet to be effectively addressed [115]. Moreover, when examining exosomes using electron microscopy, grouping exosomes can lead to overlap among the subsets and other particles, resulting in inaccuracies in experimental results [116]. Looking ahead, addressing these challenges will be vital for advancing our understanding and utilization of pancreatic cancer exosomes. Developing standardized protocols, improving isolation and purification techniques, and utilizing more accurate methods for exosome characterization will all contribute to the future success of pancreatic cancer exosome research. In addition, utilizing exosomes as biomarkers for the diagnosis and prognosis of pancreatic cancer poses several challenges. Non-invasive methods, such as extracting exosomes from patient serum, urine, or ascites, offer convenience. However, this approach often requires substantial time and cost investment [117]. Moreover, conducting a detailed analysis of specific components within these exosomes becomes more challenging. Loss of exosomes during extraction and contamination with non-vesicular substances further decreases the efficiency of detection. Therefore, developing a cost-effective method for exosome extraction is necessary.
Similarly, engineering exosomes for drug delivery also requires addressing cost and quality issues. The process of loading drugs into extracellular vesicles can lead to greater loss of these vesicles, necessitating accurate assessments of their quality [118]. Additionally, further research is needed to determine whether engineered exosomes offer superior advantages over other drug carriers, such as liposomes.
In summary, overcoming these challenges will be crucial for harnessing the full diagnostic and therapeutic potential of exosomes in pancreatic cancer. Developing efficient and cost-effective methods for exosome extraction and engineering, while ensuring their quality, will pave the way for enhanced clinical applications in the future.
Conclusion
Exosomes, which function as messengers between cells, play a crucial role in regulating the biological processes of cells. This review highlights the significance of exosomes derived from pancreatic cancer cells in the development of pancreatic cancer. Firstly, exosomes derived from pancreatic cancer cells can control the proliferation, EMT, and stenness maintenance of pancreatic cancer cells, and they also impact on surrounding cells. These surrounding cells, in turn, release exosomes that influence pancreatic cancer cells, thus promoting the malignant behavior of pancreatic cancer. Secondly, exosomes derived from pancreatic cancer cells have a regulatory effect on the surrounding tumor microenvironment, creating a suitable environment for the survival and progression of pancreatic cancer cells. Lastly, by examining specific exosome molecules, we can determine if they can be used as potential early markers or prognostic indicators for pancreatic cancer. Pancreatic cancer is an extremely malignant digestive tract tumor, and further exploration of the role of exosomes in this context is warranted.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: a cancer journal for clinicians. 2020;70:7-30
2. Wood LD, Canto MI, Jaffee EM, Simeone DM. Pancreatic Cancer: Pathogenesis, Screening, Diagnosis, and Treatment. Gastroenterology. 2022;163:386-402.e1
3. Goral V. Pancreatic Cancer: Pathogenesis and Diagnosis. Asian Pacific journal of cancer prevention: APJCP. 2015;16:5619-24
4. Saba H, Goggins M. Familial Pancreatic Cancer. Gastroenterology clinics of North America. 2022;51:561-75
5. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373-83
6. Kimiz-Gebologlu I, Oncel SS. Exosomes: Large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. Journal of controlled release: official journal of the Controlled Release Society. 2022;347:533-43
7. van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nature reviews Molecular cell biology. 2018;19:213-28
8. Kahlert C, Kalluri R. Exosomes in tumor microenvironment influence cancer progression and metastasis. Journal of molecular medicine (Berlin, Germany). 2013;91:431-7
9. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science (New York, NY). 2020;367:eaau6977
10. Huang G, Zhao Q, Li W, Jiao J, Zhao X, Feng D. et al. Exosomes: A new option for osteoporosis treatment. Medicine. 2022;101:e32402
11. Yang D, Zhang W, Zhang H, Zhang F, Chen L, Ma L. et al. Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics. Theranostics. 2020;10:3684-707
12. Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends in molecular medicine. 2014;20:460-9
13. Wang L, Yang L, Zhuang T, Shi X. Tumor-Derived Exosomal miR-29b Reduces Angiogenesis in Pancreatic Cancer by Silencing ROBO1 and SRGAP2. Journal of immunology research. 2022;2022:4769385
14. Ding G, Zhou L, Qian Y, Fu M, Chen J, Chen J. et al. Pancreatic cancer-derived exosomes transfer miRNAs to dendritic cells and inhibit RFXAP expression via miR-212-3p. Oncotarget. 2015;6:29877-88
15. Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nature reviews Genetics. 2016;17:47-62
16. Feng Z, Li K, Qin K, Liang J, Shi M, Ma Y. et al. The LINC00623/NAT10 signaling axis promotes pancreatic cancer progression by remodeling ac4C modification of mRNA. Journal of hematology & oncology. 2022;15:112
17. He Z, Wang J, Zhu C, Xu J, Chen P, Jiang X. et al. Exosome-derived FGD5-AS1 promotes tumor-associated macrophage M2 polarization-mediated pancreatic cancer cell proliferation and metastasis. Cancer letters. 2022;548:215751
18. Guo Z, Wang X, Yang Y, Chen W, Zhang K, Teng B. et al. Hypoxic Tumor-Derived Exosomal Long Noncoding RNA UCA1 Promotes Angiogenesis via miR-96-5p/AMOTL2 in Pancreatic Cancer. Molecular therapy Nucleic acids. 2020;22:179-95
19. Liu Y, Tang T, Yang X, Qin P, Wang P, Zhang H. et al. Tumor-derived exosomal long noncoding RNA LINC01133, regulated by Periostin, contributes to pancreatic ductal adenocarcinoma epithelial-mesenchymal transition through the Wnt/β-catenin pathway by silencing AXIN2. Oncogene. 2021;40:3164-79
20. Sun Z, Sun D, Feng Y, Zhang B, Sun P, Zhou B. et al. Exosomal linc-ROR mediates crosstalk between cancer cells and adipocytes to promote tumor growth in pancreatic cancer. Molecular therapy Nucleic acids. 2021;26:253-68
21. Yao J, Gao R, Luo M, Li D, Guo L, Yu Z. et al. Exosomal LINC00460/miR-503-5p/ANLN positive feedback loop aggravates pancreatic cancer progression through regulating T cell-mediated cytotoxicity and PD-1 checkpoint. Cancer cell international. 2022;22:390
22. Li Z, Jiang P, Li J, Peng M, Zhao X, Zhang X. et al. Tumor-derived exosomal lnc-Sox2ot promotes EMT and stemness by acting as a ceRNA in pancreatic ductal adenocarcinoma. Oncogene. 2018;37:3822-38
23. Fang X, Cai Y, Xu Y, Zhang H. Exosome-mediated lncRNA SNHG11 regulates angiogenesis in pancreatic carcinoma through miR-324-3p/VEGFA axis. Cell biology international. 2022;46:106-17
24. Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nature reviews Genetics. 2019;20:675-91
25. Lin J, Wang X, Zhai S, Shi M, Peng C, Deng X. et al. Hypoxia-induced exosomal circPDK1 promotes pancreatic cancer glycolysis via c-myc activation by modulating miR-628-3p/BPTF axis and degrading BIN1. Journal of hematology & oncology. 2022;15:128
26. Li J, Li Z, Jiang P, Peng M, Zhang X, Chen K. et al. Circular RNA IARS (circ-IARS) secreted by pancreatic cancer cells and located within exosomes regulates endothelial monolayer permeability to promote tumor metastasis. Journal of experimental & clinical cancer research: CR. 2018;37:177
27. Hong L, Xu L, Jin L, Xu K, Tang W, Zhu Y. et al. Exosomal circular RNA hsa_circ_0006220, and hsa_circ_0001666 as biomarkers in the diagnosis of pancreatic cancer. Journal of clinical laboratory analysis. 2022;36:e24447
28. Li Z, Yanfang W, Li J, Jiang P, Peng T, Chen K. et al. Tumor-released exosomal circular RNA PDE8A promotes invasive growth via the miR-338/MACC1/MET pathway in pancreatic cancer. Cancer letters. 2018;432:237-50
29. Zeng Z, Zhao Y, Chen Q, Zhu S, Niu Y, Ye Z. et al. Hypoxic exosomal HIF-1α-stabilizing circZNF91 promotes chemoresistance of normoxic pancreatic cancer cells via enhancing glycolysis. Oncogene. 2021;40:5505-17
30. Wang L, Wu X, Ruan Y, Zhang X, Zhou X. Exosome-transmitted hsa_circ_0012634 suppresses pancreatic ductal adenocarcinoma progression through regulating miR-147b/HIPK2 axis. Cancer biology & therapy. 2023;24:2218514
31. Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ. et al. Reassessment of Exosome Composition. Cell. 2019;177:428-45.e18
32. Liu P, Zu F, Chen H, Yin X, Tan X. Exosomal DNAJB11 promotes the development of pancreatic cancer by modulating the EGFR/MAPK pathway. Cellular & molecular biology letters. 2022;27:87
33. Nakayama F, Miyoshi M, Kimoto A, Kawano A, Miyashita K, Kamoshida S. et al. Pancreatic cancer cell-derived exosomes induce epithelial-mesenchymal transition in human pancreatic cancer cells themselves partially via transforming growth factor β1. Medical molecular morphology. 2022;55:227-35
34. Jin H, Liu P, Wu Y, Meng X, Wu M, Han J. et al. Exosomal zinc transporter ZIP4 promotes cancer growth and is a novel diagnostic biomarker for pancreatic cancer. Cancer science. 2018;109:2946-56
35. Guo Y, Chen T, Liang X, Gou S, Xiong J, Cui J. et al. Tumor Cell Derived Exosomal GOT1 Suppresses Tumor Cell Ferroptosis to Accelerate Pancreatic Cancer Progression by Activating Nrf2/HO-1 Axis via Upregulating CCR2 Expression. Cells. 2022;11:3893
36. Moutinho-Ribeiro P, Batista IA, Quintas ST, Adem B, Silva M, Morais R. et al. Exosomal glypican-1 is elevated in pancreatic cancer precursors and can signal genetic predisposition in the absence of endoscopic ultrasound abnormalities. World journal of gastroenterology. 2022;28:4310-27
37. Ohshima K, Hatakeyama K, Kanto K, Ide T, Watanabe Y, Moromizato S. et al. Comparative proteomic analysis identifies exosomal Eps8 protein as a potential metastatic biomarker for pancreatic cancer. Oncology reports. 2019;41:1019-34
38. Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK. et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nature cell biology. 2015;17:816-26
39. Allenson K, Castillo J, San Lucas FA, Scelo G, Kim DU, Bernard V. et al. High prevalence of mutant KRAS in circulating exosome-derived DNA from early-stage pancreatic cancer patients. Annals of oncology: official journal of the European Society for Medical Oncology. 2017;28:741-7
40. Yang S, Che SP, Kurywchak P, Tavormina JL, Gansmo LB, Correa de Sampaio P. et al. Detection of mutant KRAS and TP53 DNA in circulating exosomes from healthy individuals and patients with pancreatic cancer. Cancer biology & therapy. 2017;18:158-65
41. Kahlert C, Melo SA, Protopopov A, Tang J, Seth S, Koch M. et al. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. The Journal of biological chemistry. 2014;289:3869-75
42. McAndrews KM, Xiao F, Chronopoulos A, LeBleu VS, Kugeratski FG, Kalluri R. Exosome-mediated delivery of CRISPR/Cas9 for targeting of oncogenic Kras(G12D) in pancreatic cancer. Life science alliance. 2021;4:e202000875
43. Genin M, Clement F, Fattaccioli A, Raes M, Michiels C. M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC cancer. 2015;15:577
44. Linton SS, Abraham T, Liao J, Clawson GA, Butler PJ, Fox T. et al. Tumor-promoting effects of pancreatic cancer cell exosomes on THP-1-derived macrophages. PloS one. 2018;13:e0206759
45. Wang X, Luo G, Zhang K, Cao J, Huang C, Jiang T. et al. Hypoxic Tumor-Derived Exosomal miR-301a Mediates M2 Macrophage Polarization via PTEN/PI3Kγ to Promote Pancreatic Cancer Metastasis. Cancer research. 2018;78:4586-98
46. Zhang K, Li YJ, Peng LJ, Gao HF, Liu LM, Chen H. M2 macrophage-derived exosomal miR-193b-3p promotes progression and glutamine uptake of pancreatic cancer by targeting TRIM62. Biology direct. 2023;18:1
47. Yin Z, Zhou Y, Ma T, Chen S, Shi N, Zou Y. et al. Down-regulated lncRNA SBF2-AS1 in M2 macrophage-derived exosomes elevates miR-122-5p to restrict XIAP, thereby limiting pancreatic cancer development. Journal of cellular and molecular medicine. 2020;24:5028-38
48. Ruscetti M, Morris J, Mezzadra R, Russell J, Leibold J, Romesser P. et al. Senescence-Induced Vascular Remodeling Creates Therapeutic Vulnerabilities in Pancreas Cancer. Cell. 2020;181:424-41.e21
49. Shang D, Xie C, Hu J, Tan J, Yuan Y, Liu Z. et al. Pancreatic cancer cell-derived exosomal microRNA-27a promotes angiogenesis of human microvascular endothelial cells in pancreatic cancer via BTG2. Journal of cellular and molecular medicine. 2020;24:588-604
50. Chen K, Wang Q, Liu X, Wang F, Yang Y, Tian X. Hypoxic pancreatic cancer derived exosomal miR-30b-5p promotes tumor angiogenesis by inhibiting GJA1 expression. International journal of biological sciences. 2022;18:1220-37
51. Papait A, Romoli J, Stefani FR, Chiodelli P, Montresor MC, Agoni L. et al. Fight the Cancer, Hit the CAF!. Cancers. 2022;14:3570
52. Li M, Guo H, Wang Q, Chen K, Marko K, Tian X. et al. Pancreatic stellate cells derived exosomal miR-5703 promotes pancreatic cancer by downregulating CMTM4 and activating PI3K/Akt pathway. Cancer letters. 2020;490:20-30
53. Cao W, Zeng Z, He Z, Lei S. Hypoxic pancreatic stellate cell-derived exosomal mirnas promote proliferation and invasion of pancreatic cancer through the PTEN/AKT pathway. Aging. 2021;13:7120-32
54. Ma Q, Wu H, Xiao Y, Liang Z, Liu T. Upregulation of exosomal microRNA-21 in pancreatic stellate cells promotes pancreatic cancer cell migration and enhances Ras/ERK pathway activity. International journal of oncology. 2020;56:1025-33
55. Zhou B, Lei JH, Wang Q, Qu TF, Cha LC, Zhan HX. et al. Cancer-associated fibroblast-secreted miR-421 promotes pancreatic cancer by regulating the SIRT3/H3K9Ac/HIF-1α axis. The Kaohsiung journal of medical sciences. 2022;38:1080-92
56. Błogowski W, Bodnarczuk T, Starzyńska T. Concise Review: Pancreatic Cancer and Bone Marrow-Derived Stem Cells. Stem cells translational medicine. 2016;5:938-45
57. Yao X, Mao Y, Wu D, Zhu Y, Lu J, Huang Y. et al. Exosomal circ_0030167 derived from BM-MSCs inhibits the invasion, migration, proliferation and stemness of pancreatic cancer cells by sponging miR-338-5p and targeting the Wif1/Wnt8/β-catenin axis. Cancer letters. 2021;512:38-50
58. Gao G, Wang L, Li C. Circ_0006790 carried by bone marrow mesenchymal stem cell-derived exosomes regulates S100A11 DNA methylation through binding to CBX7 in pancreatic ductal adenocarcinoma. American journal of cancer research. 2022;12:1934-59
59. Shang S, Wang J, Chen S, Tian R, Zeng H, Wang L. et al. Exosomal miRNA-1231 derived from bone marrow mesenchymal stem cells inhibits the activity of pancreatic cancer. Cancer medicine. 2019;8:7728-40
60. Di Pace AL, Pelosi A, Fiore PF, Tumino N, Besi F, Quatrini L. et al. MicroRNA analysis of Natural Killer cell-derived exosomes: the microRNA let-7b-5p is enriched in exosomes and participates in their anti-tumor effects against pancreatic cancer cells. Oncoimmunology. 2023;12:2221081
61. Sun H, Shi K, Qi K, Kong H, Zhang J, Dai S. et al. Natural Killer Cell-Derived Exosomal miR-3607-3p Inhibits Pancreatic Cancer Progression by Targeting IL-26. Frontiers in immunology. 2019;10:2819
62. Shen T, Huang Z, Shi C, Pu X, Xu X, Wu Z. et al. Pancreatic cancer-derived exosomes induce apoptosis of T lymphocytes through the p38 MAPK-mediated endoplasmic reticulum stress. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2020;34:8442-58
63. Yan Q, Yuan WB, Sun X, Zhang MJ, Cen F, Zhou SY. et al. Asparaginyl endopeptidase enhances pancreatic ductal adenocarcinoma cell invasion in an exosome-dependent manner and correlates with poor prognosis. International journal of oncology. 2018;52:1651-60
64. Zheng HC, Xue H, Zhang CY. REG4 promotes the proliferation and anti-apoptosis of cancer. Frontiers in cell and developmental biology. 2022;10:1012193
65. Chen W, Zhang Z, Yung KK, Ko JK. MUC1 is responsible for the pro-metastatic potential of calycosin in pancreatic ductal adenocarcinoma. American journal of cancer research. 2022;12:3242-58
66. Tang HD, Wang Y, Xie P, Tan SY, Li HF, Shen H. et al. The Crosstalk Between Immune Infiltration, Circulating Tumor Cells, and Metastasis in Pancreatic Cancer: Identification of HMGB3 From a Multiple Omics Analysis. Frontiers in genetics. 2022;13:892177
67. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. The Journal of clinical investigation. 2009;119:1420-8
68. Beuran M, Negoi I, Paun S, Ion AD, Bleotu C, Negoi RI. et al. The epithelial to mesenchymal transition in pancreatic cancer: A systematic review. Pancreatology: official journal of the International Association of Pancreatology (IAP) [et al]. 2015;15:217-25
69. Wu M, Tan X, Liu P, Yang Y, Huang Y, Liu X. et al. Role of exosomal microRNA-125b-5p in conferring the metastatic phenotype among pancreatic cancer cells with different potential of metastasis. Life sciences. 2020;255:117857
70. Wang H, Min J, Xu C, Liu Y, Yu Z, Gong A. et al. Hypoxia-elicited Exosomes Promote the Chemoresistance of Pancreatic Cancer Cells by Transferring LncROR via Hippo Signaling. Journal of Cancer. 2023;14:1075-87
71. Chang J, Li H, Zhu Z, Mei P, Hu W, Xiong X. et al. microRNA-21-5p from M2 macrophage-derived extracellular vesicles promotes the differentiation and activity of pancreatic cancer stem cells by mediating KLF3. Cell biology and toxicology. 2022;38:577-90
72. Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J. et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177-82
73. Buscail E, Chauvet A, Quincy P, Degrandi O, Buscail C, Lamrissi I. et al. CD63-GPC1-Positive Exosomes Coupled with CA19-9 Offer Good Diagnostic Potential for Resectable Pancreatic Ductal Adenocarcinoma. Translational oncology. 2019;12:1395-403
74. Yang J, Zhang Y, Gao X, Yuan Y, Zhao J, Zhou S. et al. Plasma-Derived Exosomal ALIX as a Novel Biomarker for Diagnosis and Classification of Pancreatic Cancer. Frontiers in oncology. 2021;11:628346
75. Wei Q, Zhang J, Li Z, Wei L, Ren L. Serum Exo-EphA2 as a Potential Diagnostic Biomarker for Pancreatic Cancer. Pancreas. 2020;49:1213-9
76. Wei Q, Li Z, Feng H, Ren L. Serum Exosomal EphA2 is a Prognostic Biomarker in Patients with Pancreatic Cancer. Cancer management and research. 2021;13:3675-83
77. Wei Q, Wei L, Zhang J, Li Z, Feng H, Ren L. EphA2-enriched exosomes promote cell migration and are a potential diagnostic serum marker in pancreatic cancer. Molecular medicine reports. 2020;22:2941-7
78. Lux A, Kahlert C, Grützmann R, Pilarsky C. c-Met and PD-L1 on Circulating Exosomes as Diagnostic and Prognostic Markers for Pancreatic Cancer. International journal of molecular sciences. 2019;20:3305
79. Park SJ, Park JY, Shin K, Hong TH, Lee M, Kim Y. et al. Clinical significance of serum-derived exosomal PD-L1 expression in patients with advanced pancreatic cancer. BMC cancer. 2023;23:389
80. Marin AM, Mattar SB, Amatuzzi RF, Chammas R, Uno M, Zanette DL. et al. Plasma Exosome-Derived microRNAs as Potential Diagnostic and Prognostic Biomarkers in Brazilian Pancreatic Cancer Patients. Biomolecules. 2022;12:769
81. Wang L, Wu J, Ye N, Li F, Zhan H, Chen S. et al. Plasma-Derived Exosome MiR-19b Acts as a Diagnostic Marker for Pancreatic Cancer. Frontiers in oncology. 2021;11:739111
82. Chen J, Yao D, Chen W, Li Z, Guo Y, Zhu F. et al. Serum exosomal miR-451a acts as a candidate marker for pancreatic cancer. The International journal of biological markers. 2022;37:74-80
83. Wang C, Wang J, Cui W, Liu Y, Zhou H, Wang Y. et al. Serum Exosomal miRNA-1226 as Potential Biomarker of Pancreatic Ductal Adenocarcinoma. OncoTargets and therapy. 2021;14:1441-51
84. Pu X, Ding G, Wu M, Zhou S, Jia S, Cao L. Elevated expression of exosomal microRNA-21 as a potential biomarker for the early diagnosis of pancreatic cancer using a tethered cationic lipoplex nanoparticle biochip. Oncology letters. 2020;19:2062-70
85. Takahasi K, Iinuma H, Wada K, Minezaki S, Kawamura S, Kainuma M. et al. Usefulness of exosome-encapsulated microRNA-451a as a minimally invasive biomarker for prediction of recurrence and prognosis in pancreatic ductal adenocarcinoma. Journal of hepato-biliary-pancreatic sciences. 2018;25:155-61
86. Kawamura S, Iinuma H, Wada K, Takahashi K, Minezaki S, Kainuma M. et al. Exosome-encapsulated microRNA-4525, microRNA-451a and microRNA-21 in portal vein blood is a high-sensitive liquid biomarker for the selection of high-risk pancreatic ductal adenocarcinoma patients. Journal of hepato-biliary-pancreatic sciences. 2019;26:63-72
87. Wu L, Zhou WB, Zhou J, Wei Y, Wang HM, Liu XD. et al. Circulating exosomal microRNAs as novel potential detection biomarkers in pancreatic cancer. Oncology letters. 2020;20:1432-40
88. Shao H, Zhang Y, Yan J, Ban X, Fan X, Chang X. et al. Upregulated MicroRNA-483-3p is an Early Event in Pancreatic Ductal Adenocarcinoma (PDAC) and as a Powerful Liquid Biopsy Biomarker in PDAC. OncoTargets and therapy. 2021;14:2163-75
89. Chen D, Wu X, Xia M, Wu F, Ding J, Jiao Y. et al. Upregulated exosomic miR-23b-3p plays regulatory roles in the progression of pancreatic cancer. Oncology reports. 2017;38:2182-8
90. Goto T, Fujiya M, Konishi H, Sasajima J, Fujibayashi S, Hayashi A. et al. An elevated expression of serum exosomal microRNA-191, - 21, -451a of pancreatic neoplasm is considered to be efficient diagnostic marker. BMC cancer. 2018;18:116
91. Sakaue T, Koga H, Iwamoto H, Nakamura T, Ikezono Y, Abe M. et al. Glycosylation of ascites-derived exosomal CD133: a potential prognostic biomarker in patients with advanced pancreatic cancer. Medical molecular morphology. 2019;52:198-208
92. Yoshizawa N, Sugimoto K, Tameda M, Inagaki Y, Ikejiri M, Inoue H. et al. miR-3940-5p/miR-8069 ratio in urine exosomes is a novel diagnostic biomarker for pancreatic ductal adenocarcinoma. Oncology letters. 2020;19:2677-84
93. Kitagawa T, Taniuchi K, Tsuboi M, Sakaguchi M, Kohsaki T, Okabayashi T. et al. Circulating pancreatic cancer exosomal RNAs for detection of pancreatic cancer. Molecular oncology. 2019;13:212-27
94. Xu YF, Hannafon BN, Zhao YD, Postier RG, Ding WQ. Plasma exosome miR-196a and miR-1246 are potential indicators of localized pancreatic cancer. Oncotarget. 2017;8:77028-40
95. Shin HS, Jung SB, Park S, Dua P, Lee DK. ALPPL2 Is a Potential Diagnostic Biomarker for Pancreatic Cancer-Derived Extracellular Vesicles. Molecular therapy Methods & clinical development. 2019;15:204-10
96. Kimura H, Yamamoto H, Harada T, Fumoto K, Osugi Y, Sada R. et al. CKAP4, a DKK1 Receptor, Is a Biomarker in Exosomes Derived from Pancreatic Cancer and a Molecular Target for Therapy. Clinical cancer research: an official journal of the American Association for Cancer Research. 2019;25:1936-47
97. Yang J, Zhang Z, Zhang Y, Ni X, Zhang G, Cui X. et al. ZIP4 Promotes Muscle Wasting and Cachexia in Mice With Orthotopic Pancreatic Tumors by Stimulating RAB27B-Regulated Release of Extracellular Vesicles From Cancer Cells. Gastroenterology. 2019;156:722-34.e6
98. Armacki M, Polaschek S, Waldenmaier M, Morawe M, Ruhland C, Schmid R. et al. Protein Kinase D1, Reduced in Human Pancreatic Tumors, Increases Secretion of Small Extracellular Vesicles From Cancer Cells That Promote Metastasis to Lung in Mice. Gastroenterology. 2020;159:1019-35.e22
99. Wang CA, Chang IH, Hou PC, Tai YJ, Li WN, Hsu PL. et al. DUSP2 regulates extracellular vesicle-VEGF-C secretion and pancreatic cancer early dissemination. Journal of extracellular vesicles. 2020;9:1746529
100. Patton MC, Zubair H, Khan MA, Singh S, Singh AP. Hypoxia alters the release and size distribution of extracellular vesicles in pancreatic cancer cells to support their adaptive survival. Journal of cellular biochemistry. 2020;121:828-39
101. Pan Y, Tang H, Li Q, Chen G, Li D. Exosomes and their roles in the chemoresistance of pancreatic cancer. Cancer medicine. 2022;11:4979-88
102. Mikamori M, Yamada D, Eguchi H, Hasegawa S, Kishimoto T, Tomimaru Y. et al. MicroRNA-155 Controls Exosome Synthesis and Promotes Gemcitabine Resistance in Pancreatic Ductal Adenocarcinoma. Scientific reports. 2017;7:42339
103. Fan J, Wei Q, Koay EJ, Liu Y, Ning B, Bernard PW. et al. Chemoresistance Transmission via Exosome-Mediated EphA2 Transfer in Pancreatic Cancer. Theranostics. 2018;8:5986-94
104. Li X, Li K, Li M, Lin X, Mei Y, Huang X. et al. Chemoresistance Transmission via Exosome-Transferred MMP14 in Pancreatic Cancer. Frontiers in oncology. 2022;12:844648
105. Qi R, Bai Y, Li K, Liu N, Xu Y, Dal E. et al. Cancer-associated fibroblasts suppress ferroptosis and induce gemcitabine resistance in pancreatic cancer cells by secreting exosome-derived ACSL4-targeting miRNAs. Drug resistance updates: reviews and commentaries in antimicrobial and anticancer chemotherapy. 2023;68:100960
106. Richards KE, Xiao W, Hill R. On Behalf Of The Usc Pancreas Research T. Cancer-Associated Fibroblasts Confer Gemcitabine Resistance to Pancreatic Cancer Cells through PTEN-Targeting miRNAs in Exosomes. Cancers. 2022;14:2812
107. Xiao W, Pahlavanneshan M, Eun CY, Zhang X, DeKalb C, Mahgoub B. et al. Matrix stiffness mediates pancreatic cancer chemoresistance through induction of exosome hypersecretion in a cancer associated fibroblasts-tumor organoid biomimetic model. Matrix biology plus. 2022;14:100111
108. Rimmer E, Rashid S, Kraev I, Miralles F, Elia A. Extracellular Vesicles Inhibit the Response of Pancreatic Ductal Adenocarcinoma Cells to Gemcitabine and TRAIL Treatment. International journal of molecular sciences. 2022;23:7810
109. Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA. et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498-503
110. Li YJ, Wu JY, Wang JM, Hu XB, Cai JX, Xiang DX. Gemcitabine loaded autologous exosomes for effective and safe chemotherapy of pancreatic cancer. Acta biomaterialia. 2020;101:519-30
111. Xie X, Ji J, Chen X, Xu W, Chen H, Zhu S. et al. Human umbilical cord mesenchymal stem cell-derived exosomes carrying hsa-miRNA-128-3p suppress pancreatic ductal cell carcinoma by inhibiting Galectin-3. Clinical & translational oncology: official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2022;24:517-31
112. Ding Y, Cao F, Sun H, Wang Y, Liu S, Wu Y. et al. Exosomes derived from human umbilical cord mesenchymal stromal cells deliver exogenous miR-145-5p to inhibit pancreatic ductal adenocarcinoma progression. Cancer letters. 2019;442:351-61
113. Klimova D, Jakubechova J, Altanerova U, Nicodemou A, Styk J, Szemes T. et al. Extracellular vesicles derived from dental mesenchymal stem/stromal cells with gemcitabine as a cargo have an inhibitory effect on the growth of pancreatic carcinoma cell lines in vitro. Molecular and cellular probes. 2023;67:101894
114. van Niel G, Carter DRF, Clayton A, Lambert DW, Raposo G, Vader P. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nature reviews Molecular cell biology. 2022;23:369-82
115. Ramirez MI, Amorim MG, Gadelha C, Milic I, Welsh JA, Freitas VM. et al. Technical challenges of working with extracellular vesicles. Nanoscale. 2018;10:881-906
116. Veerman RE, Teeuwen L, Czarnewski P, Güclüler Akpinar G, Sandberg A, Cao X. et al. Molecular evaluation of five different isolation methods for extracellular vesicles reveals different clinical applicability and subcellular origin. Journal of extracellular vesicles. 2021;10:e12128
117. Ju Y, Hu Y, Yang P, Xie X, Fang B. Extracellular vesicle-loaded hydrogels for tissue repair and regeneration. Materials today Bio. 2023;18:100522
118. Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11:3183-95
Author contact
![]() Corresponding authors: Min Xu, Department of Gastroenterology, Affiliated Hospital of Jiangsu University, Zhenjiang 212001, China; xumin1974edu.cn; Wenrong Xu, Jiangsu University School of Medicine, Jiangsu University, Zhenjiang 212001, China; iclsedu.cn.
Corresponding authors: Min Xu, Department of Gastroenterology, Affiliated Hospital of Jiangsu University, Zhenjiang 212001, China; xumin1974edu.cn; Wenrong Xu, Jiangsu University School of Medicine, Jiangsu University, Zhenjiang 212001, China; iclsedu.cn.

 Global reach, higher impact
Global reach, higher impact